Abstract
Aims:
Over-the-counter mouthwash comprises part of routine oral care for many; however, potential adverse effects of the long-term daily use have not been evaluated. Most mouthwash contain antibacterial ingredients, which could impact oral microbes critical for nitric oxide formation, and in turn predispose to metabolic disorders including diabetes. Our aim was to evaluate longitudinally the association between baseline over-the-counter mouthwash use and development of pre-diabetes/diabetes over a 3-year follow-up.
Materials and methods:
The San Juan Overweight Adults Longitudinal Study (SOALS) recruited 1206 overweight/obese individuals, aged 40 – 65, and free of diabetes and major cardiovascular diseases; 945 with complete follow-up data were included in the analyses. We used Poisson regression models adjusting for baseline age, sex, smoking, physical activity, waist circumference, alcohol consumption, pre-hypertension/hypertension status; time between visits was included in the models as an offset.
Results:
Many participants (43%) used mouthwash at least once daily and 22% at least twice daily. Participants using mouthwash ≥ twice daily at baseline, had a significantly elevated risk of pre-diabetes/diabetes compared to less frequent users (multivariate IRR = 1.55, 95% CI: 1.21–1.99), or non-users of mouthwash (multivariate IRR = 1.49; 95% CI: 1.13–1.95). The effect estimates were similar after adding income, education, oral hygiene, oral conditions, sleep breathing disorders, diet (processed meat, fruit, and vegetable intake), medications, HOMA-IR, fasting glucose, 2hr post load glucose or CRP to the multivariate models. Both associations were also significant among never-smokers and obese individuals. Mouthwash use lower than twice daily showed no association, suggesting a threshold effect at twice or more daily.
Conclusions:
Frequent regular use of over-the-counter mouthwash was associated with increased risk of developing pre-diabetes/diabetes in this population.
Keywords: Prediction and prevention of type 2 diabetes, Human, Epidemiology, Over-the-counter, Mouth rinse, Mouthwash, Nitric oxide, Microbes, Antibacterial, Oral hygiene
1. Introduction
Bacteria present in the dental plaque are implicated in the pathogenesis of oral diseases, such as dental caries, periodontal diseases, and halitosis (bad breath). Effective supra-gingival plaque control is therefore important for preventing these conditions. Mouthwash has been gaining popularity as an adjunct to mechanical plaque control for reducing oral bacteria and improving oral health [1]. Mouthwash is commonly available in many countries, and its use is often promoted or prescribed by dental health care professionals to help manage specific oral conditions. The American Dental Association gives its seal of acceptance on some commonly used over-the-counter as well as prescription antibacterial mouthwash based on its evaluation of the postulated oral effects for gingivitis, halitosis or dental caries [2]. The efficacy of mouthwash use is well established for reducing gingivitis [3] and halitosis [4] (although halitosis may often be from systemic causes), but there is not much evidence for reducing periodontitis [5–7]. Despite this, around 60% of the US population use mouthwash one or more times weekly and a third use it daily [8].
Regardless of their indicated clinical uses, 90% of commonly used mouthwash products contain some antibacterial ingredient, such as chlorhexidine, triclosan, cetylpyridinium chloride, alcohol, essential oils, fluoride and peroxide [9–11]. Depending on the concentration, these ingredients can be either bacteriostatic or bactericidal. Notably, the antibacterial ingredients in mouthwash do not target specific oral pathogens, but act on a broad spectrum of targets, which may overlap with more specific antibiotic targets, leading to cross-resistance [12,13]. Therefore, frequent use of mouthwash may lead to the overgrowth of pathogenic or resistant bacteria, and may reduce the clinical efficacy of antibiotics. While toxicology is evaluated as part of the criteria for the seal of acceptance, studies to date are short-term, and the effect of chronic mouthwash use on the oral ecology as well as systemic impact have not been evaluated.
The human oral cavity includes more than 700 bacterial species [14]. An increasing number of metagenomic studies are beginning to shed light on possible associations of the oral microbiome with systemic conditions, such as, blood pressure regulation and diabetes [15,16]. Small short-term clinical trials suggest that twice daily use of chlorhexidine decreases systemic nitrate/nitrite concentrations, likely through destruction of oral microbes responsible for catalyzing nitrate reduction to nitrite [17]. Loss of this pathway may lead to decreased NO-bioavailability and increased blood pressure [18–21]. Kapil et al. found that chlorhexidine mouthwash reduced oral nitrite production and plasma nitrite levels and increased blood pressure in healthy volunteers [19]. An alcohol-free mouthwash containing cetylpyridinium chloride significantly reduced oral bacteria for up to 12 hours [22], and reduced plasma and salivary nitrite, while increasing systolic blood pressure among males [23]. Another antibacterial mouthwash also showed detrimental effects on NO bioavailability and blood pressure after a treadmill test challenge [21]. Hence, over-the-counter antibacterial mouthwash may have a detrimental impact on NO bioavailability and blood pressure regulation. These observations are raising important new concerns regarding the need to evaluate the impact of long-term use of mouthwash on the systemic health.
Recent metagenomic studies have shown oral bacteria, particularly of the phylum Actinobacteria, to be inversely associated with type 2 diabetes risk [15]. Mouthwash use abolished the beneficial impact of nitrate rich foods on insulin resistance in obese adults in a recent small clinical trial, suggesting that nitrate reduction by oral bacteria may have a protective role in diabetes risk [24]. Inhibition of endothelial nitric oxide synthases (eNOS) and subsequent decreased NO bioavailability is a central feature of metabolic syndrome and diabetes. Moreover, nitrate or nitrite protect against diabetes or hypercholesterolemia associated vascular dysfunction and inflammation in both human and animal models [25,26].
Based on this information, we hypothesized that regular long-term use of antibacterial mouthwash could increase the risk for pre-diabetes/diabetes. Although clinical trials show a detrimental impact of short-term prescription antibacterial mouthwash use on systemic nitric oxide bioavailability [18,20,21], long-term use of over-the-counter mouthwash has not been evaluated in relation to diabetes risk. Therefore, our objective was to evaluate longitudinally the hypothesis that regular over-the-counter mouthwash use was associated with increased risk of pre-diabetes/diabetes over a three-year period.
2. Materials and methods
Adults aged 40 – 65 years were recruited for the San Juan Overweight Adults Longitudinal Study (SOALS) by advertising (e.g flyers, word of mouth). The study was approved by the University of Puerto Rico Human Research Subjects Protection Office Institutional Review Board and is reported following STROBE guidelines. Recruitment and baseline data collection started in 2011 and the 3- year follow-up exam was completed by 2016 [27].
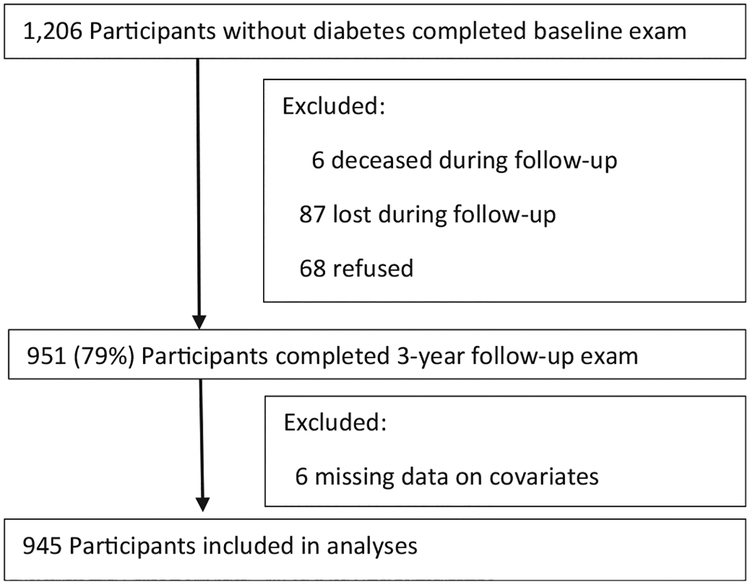
The baseline exclusion criteria were: 1) physician-diagnosed type 1 or type 2 diabetes or taking either insulin or oral anti-hyperglycemic agents; 2) pregnancy; 3) physician-diagnosed hypoglycemia, congenital heart murmurs, heart valve disease, congenital heart disease, endocarditis, rheumatic fever, and hemophilia or bleeding disorders; 4) active dialysis treatment; 5) having undergone procedures related to cardiovascular disease; 6) severe health conditions or psychological or physical disabilities that would interfere with participation in the study; or 7) plans on moving away in the next three-year period. The cohort consists of individuals who reported their race as White (25%), Black (14%), and Mixed race (61%). Retention efforts included phone calls, letters and small tokens. Seventy-nine percent (951/1206) completed the three-year follow-up examination (Fig. 1); of the remaining 255, six were deceased, 68 refused to participate in the follow-up exam, 87 were not reached, 41 moved out of Puerto Rico, and 53 were unable to come. Six participants with missing data on covariates were excluded from these analyses and the remaining 945 participants with complete data were included.
Fig. 1.
Flow chart of the San Juan adults longitudinal study (SOALS) participants’ tracking.
Participants were asked to fast for 10 h prior to their appointment, and through the last blood draw. Blood samples were drawn at fasting, and at 30-mins, 1-h and 2-h after the consumption of a glucose drink containing 75 g dextrose, using a standard protocol and silicone coated sterile blood collection tubes (Becton Dickinson Vacutainer Systems, NJ). Blood was centrifuged to separate RBC and serum/plasma, and EDTA tubes for plasma samples and serum were stored at −80°C. Fasting blood was processed for glucose, insulin, HbA1c and C-reactive protein (hs-CRP). Glucose was assessed using a SIRRUS analyzer (intra-assay coefficient of variation 1.21%; inter-assay 3.06%). Insulin was determined using Chemiluminescence assays with TOSOH analyzer (intra-assay coefficient of variation 1.49%; inter-assay 4.42%). HOMA-IR was calculated as (Fasting glucose × Fasting insulin)/405. HbA1c was assessed using a latex immunoagglutination inhibition method (intra-assay coefficient of variation 2.89% and inter-assay 1.88%). Hs-CRP values were measured by high sensitive latex turbidimetric method (intra-assay coefficient of variation 1.63% and inter-assay 1.75%) by Beckman Coulter AU5421 K-assay (Beckman Coulter, Inc., 250 S. Kraemer Blvd. Brea, CA 92821, USA). We classified people as having diabetes detected from the baseline or follow-up examination tests based on fasting plasma glucose ≥ 7 mmol/l (126 mg/dl), 2-h oral glucose tolerance ≥ 11.1 mmol/l (200 mg/dl), or HbA1c > 6.5% (48 mmol/mol). People were classified as having pre-diabetes if they had fasting glucose 100 – 126 mg/dl, 2-h post load glucose of 140 – 200 mg/dl or HbA1c 5.7% – 6.5% (39 – 48 mmol/mol), or as having normal glycemia if all these values were below the mentioned thresholds for pre-diabetes. Participants were excluded if they had glucose levels above the threshold for diabetes at the baseline exam. Progression to pre-diabetes/diabetes was defined as progression from normal glycemia to pre-diabetes or diabetes, or from pre-diabetes to diabetes based on study assessments, or reported physician diagnosed diabetes during the follow-up period.
Interviewer administered questionnaires were used to assess frequency of oral hygiene aids including mouthwash use. We were interested in the overall risk of over-the-counter mouthwash use, rather than any specific type or brand. Hence, we did not distinguish different types of mouthwash. We evaluated baseline mouthwash use two or more times a day as the primary exposure, as this was consistent with most published literature from clinical trials [19 – 21]. The questionnaire collected information on important covariates including age, gender, smoking, alcohol intake, physician diagnosed hypertension, high blood pressure medication use, and sleep breathing disorders, and time and frequency of physical activity during a typical week. Each activity was assigned a metabolic equivalent (MET) score based on the intensity, and the total MET score was computed for each individual as MET hours/week. The questionnaire also inquired about how often they ate specific food items pertinent to diabetes risk. The categories provided for responses were: never, <1 time per month, 1 time per month, 2 – 3 times per month, 1 time per week, 2 times per week, 3 – 4 times per week, 5 – 6 times per week and 1 time per day, and 2 times per week or more; and we converted these into times per week. Anthropometric measurements were taken according to the NHANES III procedures, and replicate measures were averaged. Blood pressure was measured following the gold standard Korotkoff auscultatory method after 5 min of rest [28], with intervals of 1 min in between 3 measures, and averaged. Participants were classified as hypertensive if they had physician diagnosis of hypertension, and/or if they reported taking high blood pressure medication, and/or had baseline systolic blood pressure (SBP) ≥140 or diastolic blood pressure (DBP) ≥90 mmHg; they were classified as pre-hypertensive if they were not classified as hypertensive and SBP was 120–139 and/or DBP was 80–89 mmHg. High sensitivity C-reactive protein (hs-CRP) values were measured by the high sensitive latex turbidimetric method AU5421 K-assay. Full-mouth oral exams including periodontal disease [29], dental plaque scores [30], gingivitis evaluated as bleeding on probing (BOP) [31], and caries were assessed using a modified version of the NHANES procedures, with examiners trained and calibrated by the NHANES Chief Dental Examiner.
2.1. Statistical analyses
Development of pre-diabetes/diabetes during the three-year follow-up was modeled as the outcome using Stata 13.1. We evaluated Poisson regression models to evaluate the association between baseline mouthwash use and development of pre-diabetes/diabetes. Time between baseline and the follow-up visits was included in the models as an offset. Since we analyzed a binary outcome using Poisson models, we obtained 95% confidence intervals for the Incidence Rate Ratios using robust standard errors for the parameter estimates [32]. Major risk factors for diabetes from the literature including age, sex, smoking, physical activity, waist circumference, alcohol consumption, and pre-hypertension/hypertension status were adjusted for in the analyses. Additional factors including oral conditions, and dietary factors (processed meat, fruit, and vegetable intake, coffee, whole-grain, whole grain bread and sugar sweetened beverages) were considered as potential confounders and evaluated using change in estimate procedures, with a threshold of >10% change as the criteria for inclusion of the confounder in the final model.
3. Results
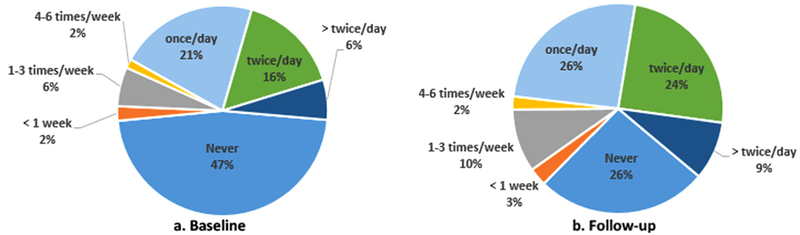
The median follow-up time for this cohort was 2.96 years (interquartile range 2.88 – 3.01). Table 1 shows that baseline characteristics of participants retained and included in these analyses are similar to the overall study sample at baseline. Table 2 shows baseline descriptive statistics by diabetes status and by mouthwash use. Compared to individuals with normal glycemia, people with pre-diabetes at baseline seem on average older (49 vs. 52 years), and less likely to smoke (22 vs. 16%), but more likely to be obese (59 vs. 68%) and hypertensive (41 vs. 52%). Individuals with pre- diabetes showed higher insulin resistance, higher hs-CRP levels, moderate or severe periodontal disease, and more sites with BOP. Individuals who used mouthwash ≥ twice a day (Table 2) were more likely to be female, to not have completed high school, to have sleep breathing disorders and had higher dental plaque scores and drank less alcohol than individuals who used mouthwash less than twice a day. Baseline pre-diabetes, HOMA-IR, hs-CRP, BOP and periodontal disease did not differ across the mouthwash groups. At baseline, six percent used mouthwash more than twice a day, 16% used twice daily, 21% used mouthwash once daily, 10% used less frequently, and 47% did not use mouthwash; mouthwash use was higher at follow-up (Fig. 2a and b). Two hundred and twelve participants developed pre-diabetes/diabetes over the follow-up. Among normo-glycemic individuals at baseline, 37% progressed to pre-diabetes/diabetes, among people with pre-diabetes at baseline 11.4% developed diabetes. Among people using mouthwash twice or more daily, 30% progressed to pre-diabetes/diabetes, and among people using mouthwash less frequently, 20% progressed.
Table 1.
Comparison of Baseline characteristics (mean ± SD or %) between participants retained for these analyses versus the other baseline participants and overall.
| In Mouthwash analyses (N = 945) | SOALS baseline (N = 1206) | |
|---|---|---|
| Age (years) | 50.6 ± 6.8 | 50.4 ± 6.8 |
| Female | 74.2 | 72.7 |
| Current smoker | 18.3 | 19.2 |
| Income (<$20,000) | 54.1 | 56.1 |
| Education | ||
| < High School | 10.8 | 12.2 |
| High School Diploma | 42.8 | 43.0 |
| ≥ Some College | 46.5 | 44.8 |
| Physical activity: METs | 22.1 ± 40.1 | 21.1 ± 38.4 |
| Obese | 63.7 | 63.7 |
| Waist circumference (cm) | 106.0 ±14.2 | 106.3 ± 14.4 |
| Alcohol consumption (g/day) | 2.2 ± 5.6 | 2.3 ± 5.8 |
| Sleep breathing disorder | 17.0 | 15.9 |
| Hypertension status | ||
| Pre-hypertension | 30.7 | 31.3 |
| Hypertensiona | 47.2 | 45.9 |
| Pre-diabetes | 56.7 | 57.5 |
| C-reactive protein (mmol/L) | 5.7 ± 6.2 | 5.7 ± 6.3 |
| HOMA-IR | 2.4 ± 1.7 | 2.5 ± 1.7 |
| Plaque score | 0.8 ± 0.6 | 0.8 ± 0.6 |
| No. of sites bleeding on probing | 12.4±12.1 | 13.0±12.3 |
| bPeriodontal disease (moderate/severe) | 63.7 | 65.8 |
| Active Caries | 1.2 ±4.2 | 1.3±4.3 |
Hypertension if reported physician diagnosis of hypertension, and/or high blood pressure medication, and/or high blood pressure at the baseline exam with systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 (mmHg); and pre-hypertension if no hypertension, and SBP between 80 and 140 and/or DBP between 80 and 90 (mmHg).
Periodontitis was classified using the CDC-AAP definition.
Table 2.
Baseline descriptive data by glycemic status (mean ± SD or %).
| Normoglycemic (N = 409) | Pre-diabetes (N = 536) | Mouthwash < Twice a Day (N = 738) | Mouthwash ≥ Twice a Day (N = 207) | |
|---|---|---|---|---|
| Age (years) | 49.2 ± 6.6 | 51.6 ± 6.8 | 50.6 ± 6.9 | 50.5 ± 6.6 |
| Female | 74.6 | 73.9 | 73.3 | 77.3 |
| Current smoker | 22.0 | 15.5 | 18.2 | 18.8 |
| Income (<$20,000 per year) | 52.8 | 55.1 | 53.3 | 56.8 |
| Education | ||||
| < High School | 10.3 | 11.2 | 9.6 | 15.0 |
| High School | 43.5 | 42.2 | 42.7 | 43.0 |
| ≥ Some College | 46.2 | 46.6 | 47.7 | 42.0 |
| Physical activity: METs | 22.4 ± 36.8 | 21.8 ± 42.5 | 22.2 ± 41.1 | 21.4 ± 36.3 |
| Obese | 58.7 | 67.5 | 63.1 | 65.7 |
| Waist circumference (cm) | 103.9±13.6 | 107.6±14.4 | 106.1 ± 14.5 | 105.8 ± 13.3 |
| Alcohol consumption (g/day) | 2.3 ± 6.3 | 2.1 ± 5.1 | 2.4 ± 6.0 | 1.7 ± 4.3 |
| Sleep breathing disorder | 16.3 | 17.5 | 15.6 | 21.8 |
| Hypertension statusa | ||||
| Pre-hypertension | 30.3 | 31.0 | 31.3 | 28.5 |
| Hypertension | 40.6 | 52.2 | 47.8 | 44.9 |
| Pre-diabetes | 57.9 | 52.7 | ||
| Mouthwash ≥ Twice a Day | 24.0 | 20.3 | ||
| C-reactive protein (mmol/L) | 5.0 ± 5.5 | 6.3 ± 6.6 | 5.8 ± 6.3 | 5.5 ± 5.9 |
| HOMA-IR | 1.9±1.2 | 2.9 ± 1.9 | 2.4 ± 1.7 | 2.4 ± 1.6 |
| Progression to | 36.9 | 11.4 | 20.2 | 30.4 |
| pre-diabetes/diabetes | ||||
| Plaque score | 0.81 ± 0.59 | 0.80 ± 0.60 | 0.79 ± 0.58 | 0.85 ± 0.64 |
| No. of sites bleeding on probing | 11.8±11.7 | 12.8±12.4 | 12.6±12.1 | 11.7±12.2 |
| Periodontal disease (moderate/severe) | 58.8 | 67.4 | 63.5 | 64.6 |
| Active caries | 1.3±5.2 | 1.1 ±3.3 | 1.3 ± 4.7 | 0.85 ± 1.6 |
Hypertension if reported physician diagnosis of hypertension, and/or high blood pressure medication, and/or high blood pressure at the baseline exam with systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 (mmHg); and pre-hypertension if no hypertension, and SBP between 80 and 140 and/or DBP between 80 and 90 (mmHg).
Fig. 2.
Distribution of Mouthwash Use*: Baseline (a) and follow-up (b).
*Limited to participants who reported mouthwash use frequency at both time points.
Table 3 shows results from Poisson regression models. Mouthwash use ≥ twice daily was associated with a significantly elevated risk of pre-diabetes/diabetes progression (age, sex and smoking adjusted IRR = 1.54, 95% CI: 1.20 – 1.97; multivariate (b) IRR 1.55, 95% CI: 1.21–1.99), compared to less frequent users; the crude incidence rate ratio (not shown in tables) was 1.52 (95% CI: 1.18 – 1.95). People who used mouthwash twice a day or more had a significantly increased risk (IRR 1.49; 95% CI: 1.13–1.95) compared to people who did not use mouthwash in the multivariate model. Models controlled for age, sex, smoking, physical activity (METs), waist circumference, alcohol consumption, and pre-hypertension/hypertension status. Additional adjustment for oral hygiene, and oral conditions including plaque, gingivitis (BOP), periodontal disease, caries, visits to a dentist/hygienist and reasons for the visits, and reported periodontal treatments at baseline and follow-up did not change the effect estimates. Similarly, addition of income, education, HOMA-IR, fasting glucose, any medication use, hs-CRP, sleep breathing disorders, processed meat, fruit intake and/or vegetable intake, coffee, whole-grain, whole grain bread and sugar sweetened beverages to the model also did not change the estimates. Hence these variables were not included in the final model. Results were similar when adjusted for alternative physical activity definitions to meeting WHO recommendations instead.
Table 3.
Incidence Rate Ratios relating mouthwash use and diabetes status progression.
| Diabetes status progression | ||
|---|---|---|
| Mouthwash use ≥ twice a day vs less frequent use | IRRa | 95% CI |
| Adjusted for age, sex, and smoking | 1.54* | 1.20–1.97 |
| Multivariateb | 1.55* | 1.21–1.99 |
| Mouthwash use ≥ twice a day vs no use | IRRa | 95% CI |
| Adjusted for age, sex, and smoking | 1.47 | 1.12–1.93 |
| Multivariateb | 1.49* | 1.13–1.95 |
Statistically significant at p < 0.05.
Time between visit was included in the models as an offset.
Adjusted for age (years), sex, smoking (never, former, current), physical activity (meeting WHO guidelines), waist circumference (cm), grams of alcohol per day and hypertension status: hypertension if reported physician diagnosis of hypertension, and/or high blood pressure medication, and/or high blood pressure at the baseline exam with systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 (mmHg); and pre-hypertension if no hypertension, and SBP between 80 and 140 and/or DBP between 80 and 90 (mmHg).
We also explored the association within subgroups defined by baseline smoking, metabolic syndrome, physical activity, BMI categories, HOMA-IR, pre-diabetes and periodontitis, and there was no major effect modification (Table 4). Associations in all subgroups evaluated ranged from 1.35 to 2.11, with no significant effect modification or interactions. The association between mouthwash use and development of pre-diabetes/diabetes remained significant when limited to never smokers (IRR 1.68; 95% CI: 1.21–2.33). Association between mouthwash use and pre-diabetes/diabetes progression are similar among baseline non-diabetic individuals with IRR = 1.54 (95% CI: 1.19–1.98, n = 409), and baseline pre-diabetic individuals with IRR =1.36 (95% CI: 0.79–2.35, n = 536).
Table 4.
Incidence Rate Ratios relating Mouthwash use and Diabetes status progression by subgroups based on baseline characteristics.
| Mouthwash > twice a day vs less frequent use | n | IRRa | 95% CI | |
|---|---|---|---|---|
| Smoking | Never | 605 | 1.68* | 1.21–2.33 |
| Ever | 340 | 1.37 | 0.93–2.01 | |
| Metabolic Syndrome | No | 482 | 1.78* | 1.27–2.50 |
| Yes | 463 | 1.35 | 0.93–1.96 | |
| Physical Activityb | Yes | 521 | 1.43* | 1.03–1.99 |
| No | 424 | 1.63* | 1.10–2.40 | |
| BMI | Overweight | 343 | 1.63* | 1.07 – 2.46 |
| Obese | 602 | 1.50* | 1.10–2.04 | |
| HOMA-IR | <2.5 | 615 | 1.35 | 0.99–1.85 |
| ≥2.5 | 330 | 2.11* | 1.38–3.22 | |
| Pre-diabetes | No | 409 | 1.54* | 1.19–1.98 |
| Yes | 536 | 1.36 | 0.79–2.35 | |
| Periodontitis | No/Mild | 342 | 1.72* | 1.19–2.49 |
| Moderate/Severe | 600 | 1.49* | 1.06–2.11 |
Statistically significant at p < 0.05.
Adjusting for age, gender, smoking status, physical activity (METS), waist circumference, alcohol consumption (g/day), and pre-hypertension/hypertension status; except for the stratifying variable for the smoking and physical activity subgroups. Time between visits was included in the models as an offset.
Complying with WHO Physical activity recommendations.
To explore dose response, mouthwash users were evaluated by different frequency of use in similar multivariate models (data not shown in tables). Compared to no use, mouthwash use less than once a week (N = 466) showed IRR = 1.03 (95% CI: 0.44 – 2.41); 1–3 times a week (N = 502) had IRR = 0.58 (95% CI: 0.30 – 1.13); 4 – 6 times a week (N = 458) had IRR = 1.21 (95% CI: 0.47 – 3.11); once a day (N = 647) had IRR = 0.97 (95% CI: 0.70–1.34); twice a day (N = 594) had IRR = 1.55 (95% CI: 1.15–2.07); and > twice a day (N = 503) had IRR = 1.30 (95% CI: 0.82–2.05). In additional analyses, participants who use mouthwash ≥ once a day showed a small but significant association with HR = 1.27 (95% CI: 1.00–1.61) compared to less frequent users.
4. Discussion
Oral bacteria are implicated in the pathogenesis of oral diseases including dental caries and periodontal disease, as well as systemic diseases, such as bacterial endocarditis, and hemorrhagic stroke. However, several studies also highlight the possible beneficial impact of the oral microbiome on systemic health, including the potential to play a role in controlling NO-bioavailability and, consequently, in key physiological processes pertinent to the cardiometabolic health. These include blood pressure lowering, limiting coagulation and protecting against inflammatory tissue injury [33]. To our knowledge, this study evaluates, for the first time, whether mouthwash use is a risk factor for pre-diabetes/diabetes.
Participants who used mouthwash ≥ twice daily had 55% significantly increased risk of developing pre-diabetes or diabetes over a 3-year follow-up compared to less frequent users, and 49% higher risk compared to non-users of mouthwash. These associations controlled for major established risk factors for diabetes (baseline age, sex, smoking, physical activity, waist circumference, alcohol consumption, and hypertension), and were independent of these and several additional potential confounders that were evaluated. Socioeconomic and lifestyle factors were not related with baseline diabetes or mouthwash use. Mouthwash users were more likely to have sleep breathing disorders; however, it was not a confounder in this study, despite it being associated with diabetes [34]. Adding HOMA-IR to the model did not change the effect estimate; therefore, it is unlikely to be a confounder or mediator in this study. The associations persist within several subgroups suggesting robustness of the findings and independence from confounding by factors such as smoking and physical activity. We cannot exclude the possibility of potential confounding by other unmeasured factors. Importantly, participants were free of major cardiovascular disease and diabetes at baseline and the associations seem independent of major risk factor for diabetes.
Based on these results, and on recent metagenomic studies linking common oral bacteria to reduced risk for type 2 diabetes [15], we hypothesize that the increased risk for diabetes among mouthwash users in our study is due to direct effects on the oral microbiome. Commensal oral bacteria are central to reducing nitrate to nitrite and its important physiological role in systemic NO-signaling and cardiometabolic health inferred from multiple preclinical and human studies. The role of NO in diabetes is complex, with both promoting and preventing disease mechanisms described, dependent in part, to the concentration and setting of NO formation. In general, NO generated from eNOS prevents diabetes associated cardiovascular morbidities. Similarly, emerging data indicate that nitrate also prevents diabetes, with the proposed mechanisms being reduction to nitrite, mediated at least in part by oral microbes. A recent clinical trial demonstrated that nitrate ingestion could lead to short-term improvement in glucose tolerance in obese adults, and that this beneficial effect could be abolished by disruption of oral nitrate metabolism by mouthwash use [24]; also, nitrate supplementation prevented development of metabolic syndrome in eNOS-deficient mice [25]. Our data demonstrate for the first time a significant long-term impact of mouthwash on diabetes risk. However, our study was limited in not evaluating measures of NO bioavailability, such as nitrate/nitrite levels, nitrate reducing oral bacteria. Hence, we cannot establish that the increased risk for the development of diabetes or pre-diabetes in the mouthwash users was due to the impact on the oral NO production pathways, and we cannot exclude the possibility that other mechanisms could be involved. Further studies are needed to directly evaluate the mechanisms by which routine mouthwash use may impact diabetes development.
Many mouthwash products are recommended for twice daily rinsing, consistent with oral hygiene recommendations of brushing twice daily. Our findings suggest a threshold effect with at least twice daily mouthwash increasing risk, rather than dose response. This is not surprising given the small quantity and short time (usually under 1 min) that mouthwash generally remains in the mouth, and that several mouthwash products are advertised to be effective for up to 12 h. Our study did not collect data on duration of use at baseline. However, this is unlikely to be an important limitation as the majority of participants in our study were long-term mouthwash users; of those who used mouthwash twice or more daily at baseline, 85% used mouthwash at least once a day at follow-up, and 62% were continuing to use mouthwash twice daily at follow-up. We also, did not collect data on type of mouthwash and reasons for use. This information was collected only among a small subsample consisting of 132 mouthwash users as part of an ancillary study to SOALS. The majority of mouthwash use reported in this subgroup was for freshening the breath, or for cleaning the teeth. It was hard to decipher the specific mouthwash used from the reported mouthwash type, since brand names were generally reported; each brand includes a variety of mouthwash types for different indications with different formulations, which may impact the ecological balance of the oral microbiome in a variety of ways. Importantly, majority of mouthwash include antibacterial ingredients; hence we observe significant associations without restricting analyses to antibacterial mouthwash, which would likely have shown stronger associations.
An important strength of our study was the high retention rate (78%), which minimizes bias from loss to follow-up. Moreover, the baseline characteristics for the 945 participants included in these analyses were similar to the initial 1206 subjects (Table 1), suggesting minimal bias from loss to follow-up. Our study population comprised overweight/obese mostly Hispanic individuals, which may limit generalizability. However, our findings show that the associations between mouthwash use and pre-diabetes/diabetes are similar for overweight and obese individuals. In addition, previous clinical trials including normal weight individuals among non-Hispanic populations showed a detrimental impact of mouthwash use on NO bioavailability and/or blood pressure. Hence, it seems likely that the associations found in our study would also be similar among normal weight and non-Hispanic individuals.
The pros and cons of mouthwash use need to be considered. Cosmetic mouthwash may temporarily control bad breath and leave behind a pleasant taste, but have no chemical or biological application beyond their temporary benefit. Antibacterial mouthwash has been shown to be clinically effective in reducing dental plaque and gingivitis [3]. However, its clinical effectiveness against the more serious forms of periodontal disease and dental caries is questionable, probably because the bacteria responsible for peridontal disease and dental caries are organized in a thick, multispecies biofilm (‘dental plaque”) [5], which is much more resistant to antibacterial agents than planktonic bacteria [35,36]. At the same time, potentially beneficial bacteria, such as those responsible for nitrate reduction are mostly concentrated on the posterior tongue, soft tissues and saliva [37] and they could be much more severely affected by mouthwash use. Furthermore, dental caries and periodontal disease are not typical “infectious” diseases because they are not caused by specific bacteria; they are induced by ecological shifts in the dental biofilm that promote the development of pathogenic bacterial consortia (“dysbiosis”) [38]. Consistent with this concept, it is becoming widely recognized that effective management of dental caries and periodontal disease should not be focused on the complete elimination of the dental biofilm, but rather on approaches that aim to manipulate this biofilm towards an ecological and metabolic balance that is more compatible with oral health [1]. Despite these limitations, over-the-counter mouthwash is advertised and used daily as part of routine oral hygiene procedures, sometimes for purely cosmetic reasons, unlike prescription mouthwash that is used short-term and is evidence based for addressing specific conditions.
The indiscriminate routine use of antibacterial mouthwash products may cause more harm than good, in light of recent studies, and further supported by findings from this study, suggesting potential detrimental impact of mouthwash use on NO bioavailability, blood pressure, and development of diabetes. Mouthwash use may also have a detrimental impact on diabetes control and possible complications, as these share some common NO-mediated pathways with blood pressure and diabetes. These observations highlight the important need for additional studies to advance our understanding of how commonly used oral hygiene practices might influence the progression of diabetes. Additional randomized and prospective studies will be required to corroborate our findings, to generalize to other populations, and to better understand the relevant metabolic pathways and mechanisms.
Until more studies establish the pros and cons of mouthwash use, we recommend evaluating the possible impact of their antibacterial ingredients on the oral microbial ecology and possible consequences for the systemic health before giving a Seal of Acceptance, endorsing or recommending routine chronic use of mouthwash products. The delicate balance between controlling pathogenic oral bacteria without eliminating the potentially beneficial systemic effects of the commensal oral flora must be considered before these products are prescribed or recommended for routine use [39]. The decision to use or recommend/endorse mouthwash should be based on weighing the potential beneficial and detrimental impact on both oral and systemic conditions.
Acknowledgements
The authors acknowledge Dr. Oelisoa M. Andriankaja, Dr. Maribel Campos, Ms. Tania Ginebra, Ms. Carla León, Dr. Lopez-Candales, Ms. Yashira Maldonado, Dr. Sasha Martinez, Ms. Xiomara O’Farrill, Ms. Samantha Ordaz, Dr. Cynthia Perez, Dr. Margarita Ramirez-Vick, Ms. Elaine Rodriguez, Ms. Rosalyn Roman, Mr. Rafael Ruiz, Ms. Yadiris Santaella, Ms. Grace Velez, Mr. Jose Vergara, Ms. Lay Wah, Mr. Jeanpaul Fernández and PRCTRC laboratory personnel (Ms. Aracelis Arroyo and Ms. Nilda Gonzalez) who contributed to the conduct/oversight/planning of data collection and their help with the study/manuscript. The authors have no conflicts of interest. Part of this work was presented as an abstract in the American Diabetes Association (ADA) 76th Scientific Sessions.
Funding
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research Grant R01DE020111, the National Institute on Minority Health and Health Disparities Grants U54MD007587 and S21MD001830 of the National Institutes of Health.
References
- [1].Marsh PD, Contemporary perspective on plaque control, Br. Dent. J 212 (12) (June 22 2012) 601–606. [DOI] [PubMed] [Google Scholar]
- [2].ADA. ADA: How to Earn the ADA Seal Acceptance, http://www.ada.org/en/science-research/ada-seal-of-acceptance/how-to-earn-the-ada-seal, Accessed 4 October 2016.
- [3].Prasad M, Patthi B, Singla A, et al. , The clinical effectiveness of post-brushing rinsing in reducing plaque and gingivitis: a systematic review, J. Clin. diagnostic Res. JCDR 10 (5) (May 2016) ZE01–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blom T, Slot DE, Quirynen M, Van der Weijden GA, The effect of mouthrinses on oral malodor: a systematic review, Int. J. Dent. Hyg 10 (3) (August 2012) 209–222. [DOI] [PubMed] [Google Scholar]
- [5].Osso D, Kanani N, Antiseptic mouth rinses: an update on comparative effectiveness, risks and recommendations, J. Dent. Hyg. JDH 87 (1) (February 2013) 10–18. [PubMed] [Google Scholar]
- [6].Rashed HT, Evaluation of the effect of hydrogen peroxide as a mouthwash in comparison with chlorhexidine in chronic periodontitis patients: a clinical study, J. Int. Soc. Prev. Community Dent 6 (3) (May-Jun 2016) 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].da Costa LF, Amaral CD, Barbirato DD, Leao AT, Fogacci MF, Chlorhexidine mouthwash as an adjunct to mechanical therapy in chronic periodontitis: a meta-analysis, J. Am. Dent. Assoc 148 (2017) 308–318. [DOI] [PubMed] [Google Scholar]
- [8].CDC. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (Or Examination Protocol, or Laboratory Protocol), U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Hyattsville, MD, 2011–2012. https://wwwn.cdc.gov/Nchs/Nhanes/Search/variablelist.aspx?Component=Questionnaire&CycleBeginYear=2011. [Google Scholar]
- [9].Perala SR, Bhupathiraju P, Efficacy of four fluoride mouth rinses on Streptococcus mutans in high caries risk children - a randomized controlled trial, J. Clin. diagnostic Res. JCDR 10 (9) (September 2016) ZC56–ZC60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Statista, Statista: Sales of the Leading Mouthwash/dental Rinse Brands in the United States in 2015, 2016. http://www.statista.com/statistics/195543/sales-of-leading-us-mouthwash-brands-in-2012-and-2013/. (Accessed 4 October 2016).
- [11].Marsh PD, Controlling the oral biofilm with antimicrobials, J. Dent 38 (Suppl1) (June 2010) S11–S15. [DOI] [PubMed] [Google Scholar]
- [12].Saleem HG, Seers CA, Sabri AN, Reynolds EC. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant, BMC Microbiol. 16 (September 15 2016) 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yazdankhah SP, Scheie AA, Hoiby EA, et al. , Triclosan and antimicrobial resistance in bacteria: an overview, Microb. Drug Resist 12 (2) (Summer 2006) 83–90. [DOI] [PubMed] [Google Scholar]
- [14].Dewhirst FE, Chen T, Izard J, et al. , The human oral microbiome, J. Bacteriol 192 (19) (October 2010) 5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Long J, Cai Q, Steinwandel M, et al. , Association of oral microbiome with type 2 diabetes risk, J. Periodontal Res 52 (3) (February 2017) 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sampaio-Maia B, Caldas IM, Pereira ML, Perez-Mongiovi D, Araujo R, The oral microbiome in health and its implication in oral and systemic diseases, Adv. Appl. Microbiol 97 (2016) 171–210. [DOI] [PubMed] [Google Scholar]
- [17].Alderton WK, Cooper CE, Knowles RG, Nitric oxide synthases: structure, function and inhibition, Biochem. J 357 (Pt 3) (August 1 2001) 593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Govoni M, Jansson EA, Weitzberg E, Lundberg JO, The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash, Nitric Oxide : Bio. Chem./Official J. Nitric Oxide Soc. 19 (4) (December 2008) 333–337. [DOI] [PubMed] [Google Scholar]
- [19].Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A, Physiological role for nitrate-reducing oral bacteria in blood pressure control, Free Radic. Biol. Med 55 (February 2013) 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bondonno CP, Liu AH, Croft KD, et al. , Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women, Am. J. Hypertens 28 (5) (May 2015) 572–575. [DOI] [PubMed] [Google Scholar]
- [21].McDonagh ST, Wylie LJ, Winyard PG, Vanhatalo A, Jones AM, The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure, Int. J. sports Med 36 (14) (December 2015) 1177–1185. [DOI] [PubMed] [Google Scholar]
- [22].He S, Wei Y, Fan X, Hu D, Sreenivasan PK, A clinical study to assess the 12- hour antimicrobial effects of cetylpyridinium chloride mouthwashes on supragingival plaque bacteria, J. Clin. Dent 22 (6) (2011) 195–199. [PubMed] [Google Scholar]
- [23].Woessner M, Smoliga JM, Tarzia B, Stabler T, Van Bruggen M, Allen JD, A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load, Nitric oxide Biol. Chem./official J. Nitric Oxide Soc. 54 (April 01 2016) 1–7. [DOI] [PubMed] [Google Scholar]
- [24].Beals JW, Binns SE, Davis JL, et al. , Concurrent beet juice and carbohydrate ingestion: influence on glucose tolerance in obese and nonobese adults, J. Nutr. Metab 2017 (2017) 6436783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Carlstrom M, Larsen FJ, Nystrom T, et al. , Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice, Proc. Natl. Acad. Sci. U. S. A 107 (41) (October 12 2010) 17716–17720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mohler ER 3rd, Hiatt WR, Gornik HL, et al. , Sodium nitrite in patients with peripheral artery disease and diabetes mellitus: safety, walking distance and endothelial function, Vasc. Med 19 (1) (February 2014) 9–17. [DOI] [PubMed] [Google Scholar]
- [27].Andriankaja OM, Jimenez JJ, Munoz-Torres FJ, Perez CM, Vergara JL, Joshipura KJ, Lipid-lowering agents use and systemic and oral inflammation in overweight or obese adult Puerto ricans: the san juan overweight adults longitudinal study (SOALS), J. Clin. Periodontol 42 (12) (December 2015) 1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pickering TG, Hall JE, Appel LJ, et al. , Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research, Circulation 111 (5) (February 8 2005) 697–716. [DOI] [PubMed] [Google Scholar]
- [29].CDC. CDC: NHANES 2011–2012: Oral Health Examiners Manual, 2011. http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Oral_Health_Examiners_Manual.pdf. (Accessed 4 October 2016).
- [30].Silness J, Loe H, Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condtion, Acta odontol. Scand 22 (February 1964) 121–135. [DOI] [PubMed] [Google Scholar]
- [31].Chaves ES, Wood RC, Jones AA, Newbold DA, Manwell MA, Kornman KS, Relationship of “bleeding on probing” and “gingival index bleeding” as clinical parameters of gingival inflammation, J. Clin. Periodontol 20 (2) (February 1993) 139–143. [DOI] [PubMed] [Google Scholar]
- [32].Zou G, A modified poisson regression approach to prospective studies with binary data, Am. J. Epidemiol 159 (7) (2004) 702–706. [DOI] [PubMed] [Google Scholar]
- [33].Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A, Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health, Free Radic. Biol. Med 105 (April 2017) 48–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Manin G, Pons A, Baltzinger P, et al. , Obstructive sleep apnoea in people with Type 1 diabetes: prevalence and association with micro- and macrovascular complications, Diabet. Med 32 (1) (January 2015) 90–96. [DOI] [PubMed] [Google Scholar]
- [35].Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F, Resistance of bacterial biofilms to disinfectants: a review, Biofouling. 27 (9) (October 2011) 1017–1032. [DOI] [PubMed] [Google Scholar]
- [36].Filoche SK, Soma K, Sissons CH, Antimicrobial effects of essential oils in combination with chlorhexidine digluconate, Oral Microbiol. Immunol 20 (4) (August 2005) 221–225. [DOI] [PubMed] [Google Scholar]
- [37].Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP, Evaluation of bacterial nitrate reduction in the human oral cavity, Eur. J. Oral Sci. 113 (1) (February 2005) 14–19. [DOI] [PubMed] [Google Scholar]
- [38].Marsh PD, The commensal microbiota and the development of human disease - an introduction, J. oral Microbiol. 7 (2015) 29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marsh PD, Head DA, Devine DA, Ecological approaches to oral biofilms: control without killing, Caries Research. 49 (Suppl 1) (2015) 46–54. [DOI] [PubMed] [Google Scholar]