Abstract
Trichosporon spp. are yeast-like microorganisms responsible for skin, urinary, pulmonary, or bloodstream infections. Due to intrinsic resistance to echinocandins, poor susceptibility to polyenes, and preferred occurrence in immunocompromised patients, such infections are often of poor prognosis. Yet no consensual therapeutic guidelines are presently available. Several clinical cases of Trichosporon infections have been successfully treated with azole therapy, including voriconazole which appeared frequently effective against Trichosporon both in vitro and in vivo. However, the low efficacy associated with some Trichosporon genotypes, complex pharmacokinetics, and the side effects of voriconazole represent limitations for its use and has prompted a search for other therapeutic options. Here, we report a case of T. asahii fungemia in a patient with B-cell acute lymphoblastic leukemia which was successfully treated with isavuconazole consecutive to stopping voriconazole therapy due to severe side effects. This observation suggests that isavuconazole with a similar spectrum to voriconazole, fewer pharmacology interactions, and side effects may be considered as a valuable therapeutic option against Trichosporon infections.
Keywords: fungemia, Trichosporon asahii, isavuconazole, acute lymphoid leukemia
Case
Trichosporon species are a common cause of fungemia after Candida species in patients with hematologic malignancies. Among all species, Trichosporon asahii is the most frequently implicated. Several fatal cases of Trichosporon asahii infections have been reported, and early use of an appropriate antifungal appears essential for survival. While Trichosporon spp. exhibits intrinsic resistance to echinocandins and amphotericin B, azoles appear to be the most constantly effective agents, and voriconazole has been successfully used in clinical cases although no well-documented consensus guidelines are presently available.1–3 Here, we report a case of a T.asahii infection which was successfully treated with isavuconazole in a patient suffering from hematologic malignancies.
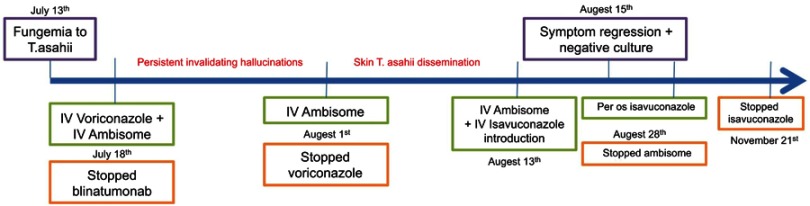
A 66-year-old man of 76 kg was hospitalized in June 2018 with a recurrence of acute lymphoid leukemia. This patient was initially diagnosed with an IgG-kappa type multiple myeloma in 2008. Treatment was based on bortezomib-dexamethasone in association with dorso-lumbar D9-L1 radiotherapy and melphalan® 200 autologous stem cell transplant followed by 33 cycles of lenalidomide maintenance therapy (IFM-2005 clinical trial). Complete remission of myeloma was obtained with this scheme. In March 2015, an acute biphenotypic monosomy 7 and FLT3 negative leukemia was diagnosed and treated by chemotherapy. In February 2018, magnetic resonance imaging revealed hemochromatosis following multiple transfusions. In June 2018, the patient had pancytopenia and a diagnosis of acute pro-B lymphoblastic leukemia was established. The patient was treated with blinatumomab, a bi-specific anti-CD3 and anti-CD19 monoclonal antibody. Due to febrile neutropenia, a large spectrum of antibiotics was initiated on June 26th, 2018. Empiric caspofungin therapy was started on July 6th due to prolonged febrile neutropenia with suspicion of invasive fungal infection. However, fungemia occurred on July 13th. T.asahii was identified by mass spectrometry (maldi biotyper, Bruker) from blood culture. At this date, on July 13th, the patient had leucopenia (0.3 G/L). Minimal inhibitory concentrations (MICs) for T. asahii isolates were determined by E test (Table 1). On July 18th, based on obtained MICs, a combined voriconazole (IV: 6 mg/kg every 12 hrs for 1 day and then, 4 mg/kg every 12 hrs) + amphotericin B therapy (Ambisome: liposomal form 3 mg/kg/day) was started and blinatumomab therapy was stopped. Consecutively to stopping blinatumomab therapy, leucocyte concentration gradually increased from: 0.3 G/L, 2 G/L (polymorphonuclear neutrophils: 1.5 G/L), 2.8 G/L (polymorphonuclear neutrophils: 2.2 G/L), 4.5–5 G/L and finally 10.3 G/L on July 18th, August 1st, August 3rd, August 7th to 16th and August 20th, respectively. Two days consecutively to voriconazole introduction, on July 20th, voriconazole plasma concentration was 4.9 µg/mL, increasing to 6.5 µg/mL on July 26th (N: 1–5µg/mL). Consecutively to this dosage and due to neurological adverse effects, voriconazole dose was decreased from 4 mg/kg to 2 mg/kg every 12 hrs. Four days later, on July 30th voriconazole plasma concentration was within the normal range at 1.1 µg/mL (N: 1–5 µg/ml). On August 1st, the patient had grade III neurological toxicity with persistent invalidating hallucinations and voriconazole therapy was discontinued. Within two days of liposomal amphotericin B monotherapy, erythematous skin lesions appeared on the patient’s right thigh and disseminated in a few days (biopsy confirmed T. asahii infection) even with leucocyte concentrations ranging between 2.8 and 5 G/L. On August 13th, based on MIC results, isavuconazole (IV 200 mg twice a day) was added to liposomal amphotericin B therapy. Two days later, the infection declined with regression of skin lesions and culture-negative results. Isavuconazole (IV) was then used at 200 mg a day for 2 weeks and then, orally at the same dose for 3 months (Figure 1). Finally, the fungemia was successfully treated with an isavuconazole-based therapy. Eleven months later (to date with the current report), the patient is still in complete remission from his underlying pathology.
Table 1.
Sequential susceptibilities of Trichosporon asahii isolates to antifungal agents. Susceptibilities were estimated based on Candida albicans EUCAST breakpoints version 9.0 and provided as “indicative” to clinicians in absence of available breakpoints
| Tested antifungal agent | Isolate 1a | Isolate 2# | ||
|---|---|---|---|---|
| Minimal inhibitory concentrations (mg/L) | Susceptibility | Minimal inhibitory concentrations (mg/L) | Susceptibility | |
| Amphotericin B | 0.75 | Susceptible | 1.5 | Intermediate |
| Voriconazole | 0.023 | Susceptible | 0.023 | Susceptible |
| Isavuconazole | 0.023 | Susceptible | 0.047 | Susceptible |
| Flucytosine | 32 | Resistant | 32 | Resistant |
| Anidulafungin | 32 | Resistant | 32 | Resistant |
| Itraconazole | 0.5 | Resistant | 1.0 | Resistant |
| Posaconazole | 0.094 | Resistant | 0.094 | Resistant |
Notes: aIsolate 1 was obtained from blood culture 7 days after initiation of empiric caspofungin treatment (one month before isavuconazole therapy). Isolate 2 was obtained from cutaneous biopsy the day voriconazole therapy was stopped (12 days before isavuconazole therapy).
Figure 1.
Case study timeline.
In this reported case, we observed both the inefficacy of amphotericin B-based therapy and successful treatment with isavuconazole. Immune restoration probably potentiated the efficacy of isavuconazole. However, it is difficult to attribute recovery to immune restoration alone since the patient had leucocytes ranging from 4.5 to 5 G/L for 6 days before isavuconazole initiation and infection was out of control. In this patient, several previously identified conditions promoting Trichosporon infection were present. In the present case, immunodepression was associated with malignant hemopathies, and the additional role of anti-CD3 and anti-CD19 blinatumomab therapy for the treatment of acute lymphoid leukemia requires further investigation. Moreover, among risk factors our patient presented hemochromatosis. Indeed, in 2010, Shih-Ta Shang et al described Trichosporon fungemia in an immunocompetent patient with hemochromatosis.4 They hypothesized that iron overload decreased T-cell immunity, natural killer cell, and neutrophil activities and monocyte phagocytosis. Additionally, our patient had a vascular catheter that was removed after Trichosporon infection diagnosis and was suspected as the source of fungemia.
No consensus guidelines are currently available for the treatment of Trichosporon infections even if voriconazole is presented as a preferred therapy.3 Trichosporon exhibits natural resistance to echinocandins and inconstant sensitivity to amphotericin B according to species. The azole class of antifungals is the most efficient for the treatment of Trichosporon infections, and several cases of Trichosporon infection have been treated with voriconazole often associated with amphotericin B.2 Voriconazole, however, may interact with several other pharmaceutical agents. In additon, high intra- and inter-individual pharmacokinetic variability is well established and can easily cause over- or underdosing. Consequently, many side effects of voriconazole therapy have been observed such as hallucinations which led to treatment discontinuation in our case. These side effects require a search for alternative therapeutic options. Isavuconazole has exhibited an excellent in vitro activity against Trichosporon.5 In the present clinical case, voriconazole, used in first intention with amphotericin B, was discontinued due to side effects. Isavuconazole administration resulted in improved skin lesions and finally fungemia was controlled in absence of side effects. This observation suggests that isavuconazole with fewer side effects and similar efficiency against Trichosporon spp. compared with voriconazole should be considered as a valuable alternative to voriconazole in Trichosporon infections.
Acknowledgments
The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, and JJ Ballet for their help in editing the manuscript. We are also grateful to laboratory technicians.
Ethic statement
Written informed consent has been provided by the patient to have the case details published.
Transparency declaration
No institutional approval is required to publish the case details.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Vincent Camus report personal fees, non-financial support from ROCHE, personal fees, non-financial support from AMGEN, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Karigane D, Sakurai M, Matsuyama E, et al. Successful treatment of breakthrough disseminated Trichosporon asahii fungemia in a patient with acute myeloid leukemia receiving itraconazole prophylaxis. Med Mycol Case Rep. 2018;20:1–3. doi: 10.1016/j.mmcr.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asada N, Uryu H, Koseki M, Takeuchi M, Komatsu M, Matsue K. Successful treatment of breakthrough Trichosporon asahii fungemia with voriconazole in a patient with acute myeloid leukemia. Clin Infect Dis. 2006;43(4):e39–e41. doi: 10.1086/505970 [DOI] [PubMed] [Google Scholar]
- 3.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O. ESCMID† and ECMM‡ joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20:76–98. doi: 10.1111/1469-0691.12360 [DOI] [PubMed] [Google Scholar]
- 4.Shang S-T, Yang Y-S, Peng M-Y. Nosocomial Trichosporon asahii fungemia in a patient with secondary hemochromatosis: a rare case report. J Microbiol Immunol Infect. 2010;43(1):77–80. doi: 10.1016/S1684-1182(10)60012-6 [DOI] [PubMed] [Google Scholar]
- 5.Thompson GR, Wiederhold NP, Sutton DA, Fothergill A, Patterson TF. In vitro activity of isavuconazole against Trichosporon, Rhodotorula, Geotrichum, Saccharomyces and Pichia species. J Antimicrob Chemother. 2009;64(1):79–83. doi: 10.1093/jac/dkp138 [DOI] [PubMed] [Google Scholar]