Abstract
Background:
Pediatric low-grade glioma (pLGG) is the most common central nervous system tumor of childhood. Although overall survival is very good, many children suffer from multiple progressions and functional morbidities. There is no one universally accepted therapy for children with recurrent disease, however, standard cytotoxic chemotherapies are often utilized by most practitioners. The Pediatric Brain Tumor Consortium conducted a multi-institutional phase II study evaluating selumetinib (AZD6244, ARRY-142886), a MAP/ERK Kinase I/II inhibitor, in patients with recurrent, refractory or progressive pLGG assigned to numerous strata. The aim of the study was to evaluate the efficacy of selumetinib in these patients.
Methods:
Eligibility required age 3–21 y/o, a Lansky or Karnofsky performance score greater than 60 and the presence of recurrent, refractory or progressive pLGG after at least one standard therapy. Stratum 1 included children with World Health Organization (WHO) grade I pilocytic astrocytoma (PA) harboring either one of the two most common BRAF aberrations (KIAA1549-BRAF fusion or the BRAFV600E mutation). Stratum 3 included children with any neurofibromatosis type 1 (NF1)-associated pLGG (WHO grades I and II). Selumetinib was provided as capsules given orally at the recommended phase II dose of 25 mg/m2 twice daily. The primary endpoint was stratum-specific objective response rate assessd by the local site and sustained for at least 8 weeks. All responses were reviewed centrally and statistical analyses were done as per protocol. Although the trial ( NCT01089101) is still ongoing in other strata, enrollment and planned follow-up is compete on both strata 1 and 3.
Findings:
Between July 25, 2013, and June 12, 2015, 25 eligible and evaluable children were accrued to stratum 1, and between August 28, 2013, and June 25, 2015, 25 eligible and evaluable children were accrued to stratum 3. On stratum 1, 9/25 (36%) patients achieved a partial response (PR). The median follow-up for the 11 patients who have not yet experienced an event is 36.4 months (4.4–50.5; IQR=23.9). On stratum 3, 10/25 (40%) patients achieved a PR with a median follow-up of 48.6 months (8.6–59.1; IQR=12.2) for the 17 subjects without progressions. All patients evaluable for visual acuity had improved or stable vision. The most common attributable toxicities on both strata were grade 1 and 2 CPK elevation, hypoalbuminemia, dyspnea, rash, duodenal ulcer, anemia, dry skin, fatigue and diarrhea. Rare grade 3 toxicities included elevated CPK (n=5), maculopapular rash (n=5), neutropenia (n=3), nausea (n=3), paronychia (n=3), acneiform rash (n=2), diarrhea (n=2), elevated ALT (n=1), decreased ejection fraction (n=1), gastric hemorrhage (n=1), headache (n=1), skin infection (n=1), tooth infection (n=1) and weight gain (n=1). There was only one grade 4 toxicity, lymphopenia. There were no treatment-realted deaths. Patient reported outcomes and quality of life assessments were not part of the current study.
Interpretation:
Selumetinib is active against recurrent, refractory or progressive PA harboring common BRAF aberrations and NF1-associated pLGG. To our knowledge, this is one of the first prospectively tested and successful molecularly-targeted agents in pLGG. These data not only provide an alternative to standard chemotherapy for these subgroups of patients, but this success has led to an interest in exploring efficacy in patients as a first-line therapy. In fact, these data have directly led to the development of two Children’s Oncology Group phase III studies in newly diagnosed pLGG patients both with and without NF1 comparing standard chemotherapy to selumetinib. The current trial was funded by a National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP) PBTC U01 Grant: 2UM1CA081457 (UM1) and by the American Lebanese Syrian Associated Charities.
Introduction
Pediatric low-grade glioma (pLGG) is the most common central nervous system tumor in children.(1) The mainstay of therapy is a complete surgical resection as this can be curative; however, children for whom a gross total resection is not achievable often require additional therapy.(1, 2) There are multiple first-line chemotherapy regimens including combinations of carboplatin and vincristine (CV), combinations of thioguanine, procarbazine, lomustine and vincristine and vinblastine monotherapy.(3, 4) Five-year overall survival (OS) with chemotherapy on the most recent Children’s Oncology Group (COG) pLGG study, CCG A9952, for children without Neurofibromatosis type 1 (NF1) is excellent (86±2.2%), but the same study showed a 5-year progression-free survival (PFS) of only 45%±3.2%, emphasizing the need for alternative therapies.(3) A separate evaluation of those children on CCG A9952 with NF1 non-randomly assigned to carboplatin and vincristine revealed a 5-year OS and PFS of 98±1% and 69±4%, respectively.(5) In addition to lower PFS outcomes, many children suffer with functional morbidities such as visual disturbances, motor disabilities, poor quality of life (QOL) and neuropsychological deficits.(6–9)
Classic chemotherapy exposes children to toxicities like myelosuppression, allergic reactions, peripheral neuropathy, constipation, secondary malignancies and infertility.(3) Although effective, radiotherapy increases the risk of secondary malignancy, ototoxicity, endocrinopathies and neurocognitive decline.(10, 11) Radiotherapy is often avoided in young children, especially those with NF1 for whom there is even greater risk of secondary malignancy and Moyamoya disease.(2, 12) NF1 is a genetic disorder caused by loss-of-function alterations in neurofibromin 1, a negative regulator of the MAPK pathway. Approximately 15–20% of NF1 patients will develop pLGG, most commonly within the optic pathway and brainstem.(13)
Abnormal MAPK pathway activation is the most common genetic aberration in pLGG.(14–17) This is most often the result of activation of the BRAF oncogene either through a tandem duplication resulting in a KIAA1549-BRAF fusion or an activating point mutation, BRAFV600E.(18) Approximately 80–90% of pilocytic astrocytomas (PA), the most common pLGG, harbor the KIAA1549-BRAF fusion whereas BRAFV600E mutations are identified in approximately 10–20% of all pLGG.(19)
Numerous drugs that target the MAPK pathway have been developed. Selumetinib (AZD6244, ARRY-142886) (Wilmington, DE, USA) is a selective and potent orally-available non-ATP-competitive small molecule inhibitor of MAP/ERK (MEK) Kinase I/II. The Pediatric Preclinical Testing Program showed that selumetinib induced complete regression in a BRAFV600E xenograft glioma model.(20) Recently, the Pediatric Brain Tumor Consortium (PBTC) completed a phase I trial in 38 children with recurrent, refractory or progressive pLGG establishing the recommended phase II dose (RP2D) as 25 mg/m2/dose twice daily.(21) Notably, 5/25 patients treated at the RP2D achieved a partial response (PR).(21) Simultaneously, a phase I trial in patients with NF1-associated plexiform neurofibromas had a 70.8% PR rate.(22) Both trials demonstrated tolerable toxicities and the same RP2D.
These successes led to this prospective PBTC phase II trial evaluating selumetinib efficacy at the RP2D in children with recurrent, refractory or progressive pLGG (World Health Organization [WHO] Grade I and II). Herein, we report the results of strata 1 and 3. Strata 2, 4, 5 and 6 are ongoing.
Methods
Study Design and Participants
This prospective phase II trial was developed and conducted by PBTC investigators in collaboration with the National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP). The trial enrolled children with recurrent, refractory or progressive pLGG to 6 unique strata separated by histology, location, NF1 status and BRAF aberration status. Herein we report on strata 1 and 3 which represent some of the most common patients with recurrent pLGG. Stratum 1 included children with World Health Organization (WHO) grade I pilocytic astrocytoma (PA) harboring either one of the two most common BRAF aberrations (KIAA1549-BRAF fusion or the BRAFV600E mutation). Stratum 3 included children with any neurofibromatosis type 1 (NF1)-associated pLGG (WHO grades I and II). The remaining strata data collection is ongoing and will be reported separately. These included the following: stratum 2, WHO grade I PA not harboring either one of the two most common BRAF aberrations; stratum 4, optic pathway pLGG not associated with NF1; stratum 5, WHO grade 1 and 2 non-pilocytic astrocytoma harboring either one of the two most common BRAF aberrations; and stratum 6, pLGG not associated with NF1 with tissue available for BRAF analyses that could not be classified into stratum 1, 2 or 5 due to inadequate tissue quality or assay failure.
Data was analyzed by the PBTC Operations Biostatistical Data Management Core (OBDMC). The protocol was approved by each site’s local institutional review board (IRB). All patients and/or legal guardians provided written informed consent/assent as required by the local IRB.To meet eligibility criteria, patients had to have recurrent, progressive or refractory disease after at least one standard therapy, including chemotherapy or radiotherapy. Patients must have received their last dose of known myelosuppressive chemotherapy at least 3 weeks prior to study registration and at least 6 weeks prior if a nitrosourea. Patients must have received their last dose of any biologic agent ≥ 7 days prior to study registration. For biologic agents that had a prolonged half-life and for all monoclonal antibodies, at least three half-lives must have elapsed prior to registration. Patients must have had their last fraction of local irradiation to the target tumor ≥12 months prior to registration. Recurrent and progressive disease included either a 25% increase in bi-dimensional tumor size and/or development of new lesions. In the case of patients with optic pathway tumors, vision deterioration thought secondary to the tumor without imaging progression could also deem a patient eligible. A Lansky or Karnofsky performance score greater than 60 was required, and patients with any clinically significant unrelated systemic illness (serious infections or significant cardiac, pulmonary, hepatic or other organ dysfunction) likely to interfere with the study procedures or results were excluded. It was also required that any neurologic deficits were stable for at least one week prior to registration. Patients with uncontrolled seizures were excluded. Patients were required to be 3–21 years old. There is limited data available on the pharmacokinetics of selumetinib in children less than 3 y/o and the study required that a patient swallow capsules whole. For these reasons, those patients less than 3 y/o were excluded. Patients were required to have adequate complete blood counts, liver and renal function, left ventricular ejection fraction ≥53%, and QTc interval <450 milliseconds. Bi-dimensional measurable disease was required. On stratum 1, children with PA (WHO grade 1) were eligible for screening for the KIAA1549-BRAF fusion or BRAFV600E mutation as detailed in the Procedures section below. If screening was positive, the patient was eligible for treatment. Children with imaging characteristics suggestive of a pLGG or a biopsy proven pLGG (WHO grade I and II) and a clinical or genetic diagnosis of NF1 were eligible for stratum 3.
Procedures
Required follow-up assessments included clinical examination, laboratory evaluations, ophthalmologic examination and MRIs. MRIs were obtained every 2 months during the first year and every three months thereafter. Patients whose tumor achieved a radiographc response (CR or PR) as assessed locally underwent central radiographic review at the PBTC Neuroimaging center. Laboratories, including complete blood count, full chemistry panel, liver function tests and CPK were performed every 4 weeks and prior to each subsequent cycle. Echocradiogram was performed every 3 months. Patients with an optic pathway tumor enrolled to stratum 3 had ophthalmologic exams for visual acuity performed every 3 months. Full toxicity assessments were completed every 4 weeks and prior to each subsequent cycle and were graded according to the Common Terminology Criteria for Adverse Events version 4.0. Dosing adherence was monitored through patient diaries and pill counts. Selumetinib was administered as capsules given orally at 25 mg/m2/dose twice daily. Each course was 28 days, and patients could be treated for up to 26 courses in the absence of unacceptable toxicity or tumor progression. Patients were taken off treatment if they met criteria for progressive disease as describe below or had unacceptable toxicity. Up to 2 dose reductions were allowed, however, re-escalation was not permitted. Dose modifying toxicities included any selumetinib-related adverse event that resulted in a delay of treatment > 7 days, any grade 4 non-hematologic toxicity, grade 3 non-hematologic toxicities and any grade 2 non-hematological toxicity that persisted for more than 7 days and was considered medically significant or intolerable by patients to warrant treatment interruption and/or dose reduction. Exceptions to non-hematologic grade 3 dose modifying toxicities included the following: grade 3 nausea and vomiting < 5 days, grade 3 transaminitis that returned to eligibility criteria levels within 7 days of selumetinib cessation, grade 3 fever/infection less than 5 days’ duration, grade 3 electrolyte abnormalities (hypophosphatemia, hypokalemia, hypocalcemia or hypomagnesemia) responsive to oral supplementation and grade 3 asymptomatic CPK elevation. Hematologic dose modifying toxicity included any grade 4 hematologic toxicity (with the exception of lymphopenia) and grade 3 thrombocytopenia with associated bleeding.
All BRAF testing was performed centrally at the Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. Formalin-fixed paraffin-embedded (FFPE) unstained slides were analyzed for 3’BRAF duplication, which leads to KIAA1549-BRAF fusion, by fluorescence in situ hybridization (FISH).(21) Testing was considered positive/present if >15% of nuclei showed an extra copy of 3’BRAF signal. While this is not a direct assessment of KIAA1549-BRAF fusion, it captures the 3’BRAF tandem duplication event leading to KIAA1549-BRAF fusion. The BRAFV600E mutation testing utilized PCR amplification and dye termination sequencing of exon 15 of the BRAF gene using specific primers.(21) Pharmacokinetic (PK) studies evaluated the disposition of selumetinib and its active metabolite N-desmethyl-selumetinib on Day 1, Course 1 in consenting patients. Samples were collected pre-dose, 1, 3, and 8 (±1) hours post-selumetinib administration. A non-compartmental approach determined the maximum concentration (Cmax), time to maximum (tmax) concentration, the area under the plasma concentration versus time curve from 0 to tlast (AUC0–tlast) and the apparent oral clearance (Cl/F).
Pre-trial fresh frozen tumor material and paired blood samples were collected from consenting patients for whole exome and RNA sequencing analyses and performed at the German Cancer Research Center in Heidelberg, Germany, to identify genomic alterations in the MAPK pathway or recurrent genomic alterations in other pathways. When only FFPE tumor was available, DNA methylation array analysis for tumor subgrouping and generation of copy number plots as well as gene panel sequencing using a targeted neuro-oncology-specific gene set were performed.(23),(24) Putative alternative events (e.g. novel BRAF fusions) were detected based on a combination of gene panel data and copy number information. Alternative BRAF fusion genes were detected by integrating RNA sequence data and copy-number data inferred from exome sequencing or DNA methylation array results. Suspected fusions were verified by PCR and Sanger Sequencing testing. The primary goal was to identify predictive biomarkers of response.
Outcomes
The primary endpoint was stratum-specific objective response rate assessd by the local site and sustained for at least 8 weeks. Responses were based on 2-dimensional MRI evaluation. A complete response was defined as complete tumor disappearance on T2/fluid attenuation inversion recovery (FLAIR) images, no new lesions and disappearance of all enhancement on T1 post-contrast imaging. PR was defined as a ≥50% tumor reduction on T2/FLAIR. Stable disease (SD) was defined as neither sufficient increase or reduction to qualify as PR or progressive disease (PD). PD was >25% increase or development of new lesions. These response criteria were used both for local site and central imaging response assessments. As discussed in the phase I selumetinib publication, initial imaging response definitions were based more heavily upon enhancement.(21) Since this is not compatible with historic and current definitions, an amendment using the above response criteria was approved. Secondary endpoints included the following: to estimate the PFS distributions associated with selumetinib treatment separately in each of the 6 separate strata; to explore correlations between BRAF aberrations and treatment response and PFS in patients for whom relevant biologic data are available; to assess MAPK aberrations by a combination of whole-exome and RNA sequencing; and to characterize the inter- and intra-patient variability in selumetinib pharmacokinetics administered at the recommended phase II dose (RP2D). All secondary endpoints as they relate to Strata 1 and 3 are presented here. Similar information for Strata 2, 4, 5 and 6 will be reported elsewhere as the data become available.
Statistical Analyses
Identical Simon’s Minimax designs were used for each stratum; “unacceptable” vs. “desirable” RR were 10% versus 30%, respectively, leading to a sample size of 25 subjects per stratum with 10% type 1 error and 90% power. Interim analysis was planned after 16 subjects and ≥2 responses were required to expand accrual to 25. The final success threshold was ≥5 responses in 25 subjects. All eligible subjects who initiated treatment were evaluable for the efficacy and toxicity objectives which were conducted per protocol. Eligible patients with available assessments or samples were also evaluable for the respective secondary objectives. This study is registered with ClinicalTrials.gov ( NCT01089101). All statistical analyses were conducted in SAS 9.4. Kaplan Meier estimator was utilized for PFS calculations, and if necessary, log-rank test or Cox Hazard model was used for comparisons. PFS was defined as the time from treatment initiation until disease progression or death from any cause or time to last follow-up for patients without these events. Log-rank tests and Cox proportional hazards models were used to explore associations between PFS and various covariates.
Role of Funding Source
The trial was funded by a National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP) PBTC U01 Grant: 2UM1CA081457 (UM1) and by the American Lebanese Syrian Associated Charities (ALSAC). ALSAC provides funding and infrastructure support for the OBDMC personnel but had no involvement in trial design, patient recruitment, data collection, analyses or manuscript preparation. CTEP provided input on study design, approved the trial and its amendments, served as the IND sponsor and reviewed the manuscript prior to submission. CTEP was not directly involved in patient enrollment, data collection, analyses or interpretation. AstraZeneca provided selumetinib and financial support for BRAF testing, CPK lab analyses, echocardiograms and ophthalmologic exams. AstraZeneca reviewed the manuscript; however, they did not have a direct role in trial design, patient recruitment, data collection, analyses or manuscript preparation. Only JF, AO and SW had access to the raw clinical data, and the corresponding author (JF) had full access to all of the data and the final responsibility to submit for publication.
Results
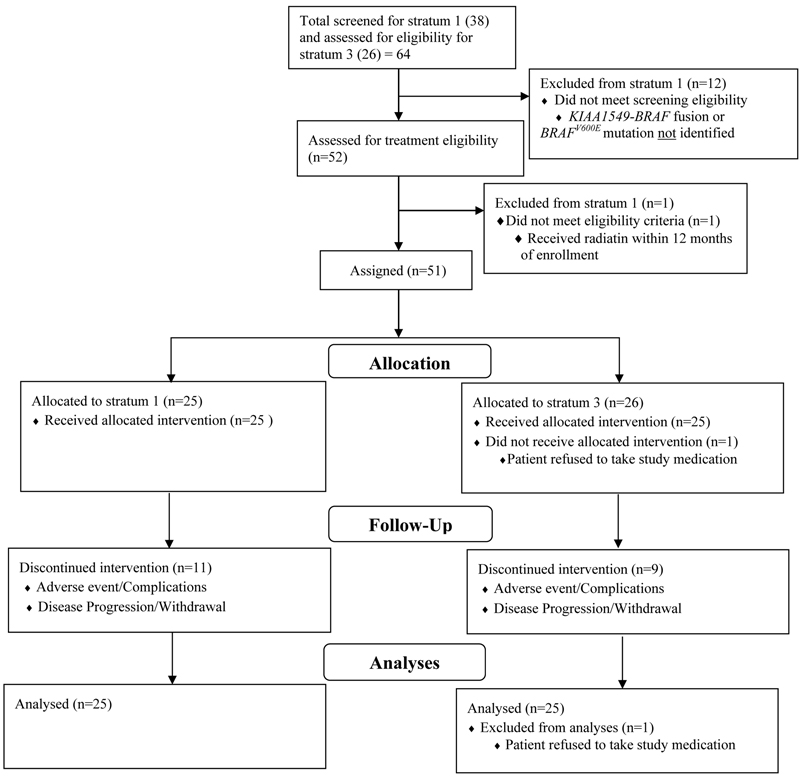
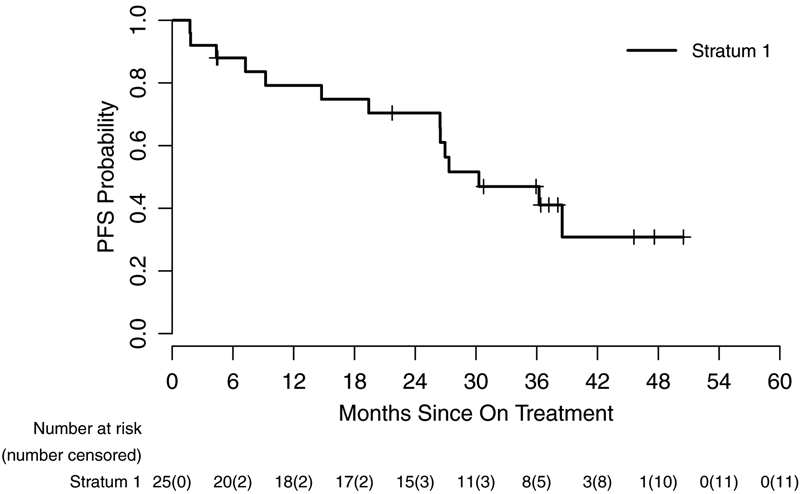
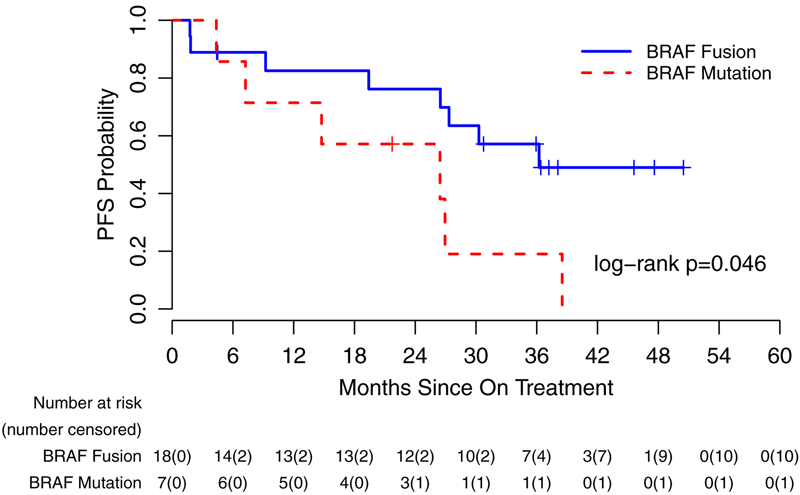
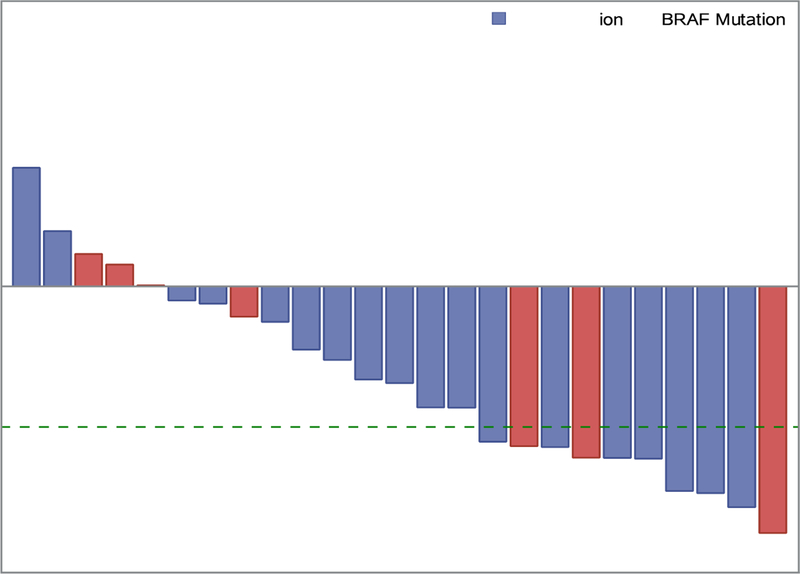
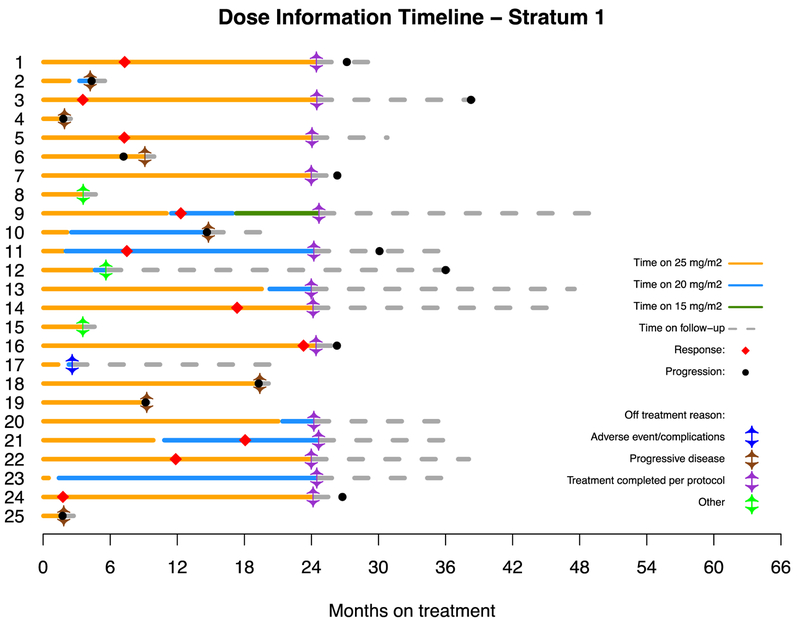
Patients were enrolled at 11 PBTC member institutions located across the US (Appendix, page 7). Thirty-eight patients were screened for possible enrollement on startum 1. Twenty-six of the 38 (68.4%) screened positive for either the KIAA1549-BRAF fusion or the BRAFV600E mutation and were enrolled between July 25, 2013, and June 12, 2015. One of 26 (3.8%) was subsequently found to be ineligible due to receiving radiation therapy within 12 months of registration which was an exclusion criterion, leaving 25 eligible and evaluable patients for analyses. Eighteen of 25 (72%) eligible and evaluable tumors harbored a KIAA1549-BRAF fusion and 7 of 25 (28%) had a BRAFV600E mutation. Table 1 provides demographic data and Figure 1 depicts the CONSORT flow diagram. Twenty-four of 25 (96%) patients on stratum 1 received prior chemotherapy (median 3 prior chemotherapy regimens, range 1–6). Twenty-four of 25 (96%) patients had previous tumor surgeries and 5/25 (20%) had previously received radiotherapy. The institutional assessment of best responses were 9/25 (36%) PR, 9/25 (36%) SD and 7/25 (28%) PD. Eight of 9 PR (88.9%) were confirmed centrally; the ninth had a 43% tumor reduction on cenral review. The median time to a PR was 7.54 months (IQR=7.31–12.40). The 2-year PFS was 70% (95% CI: 47%−85%) (Figure 2A). Among the tumors with a KIAA1549-BRAF fusion, 7/18 (38.9%) had a PR compared to 2/7 (28.6%) of the tumors with BRAFV600E. Fourteen of 25 patients (56%) completed all 26 courses of therapy with stable or responsive disease. Figure 3A is the waterfall plot depicting the greatest percentage change in local site reviewed bi-dimensional measurements. Age at treatment and tumor size were not statistically associated with response or PFS. Specific BRAF aberration was not associated with response, however, it was associated with PFS whereby patients whose tumors harbored a BRAFV600E mutation had a worse PFS as compared to patients whose tumor harbored the KIAA1549-BRAF fusion (p=0.047) (Figure 2C). Fourteen of 25 (56%) patients have progressed; 9/14 (64.2%) while on therapy and 5/14 (35.8%) while off treatment. The median time to progression was 22.93 months (range=1.75–38.51; IQR=7.24–27.34). The median follow-up for all eligible and evaluable patients (n=25) from treatment initiation to these analyses is 26.9 months (IQR=9.2–36.4). Eleven of 25 (44%) patients have not progressed with a median follow-up since treatment initiation of 36.4 months (range=4.4–50.48; IQR=21.72–45.59). In patients who have not progressed, the median follow-up since stopping selumetinib is currently 12.5 months (range=0–25.75; IQR= 6.64–21.38).
Table 1.
Patient demograhic data on both strata 1 and 3
| Stratum 1 (N=25) | Stratum 3 (N=25) | |
|---|---|---|
| AGE (Years) at Diagnosis | ||
| Median/Range/IQR | 4.4/0.3–19.1/2.01–8.45 | 3.1/0.6–12.2/6.63–12.71 |
| AGE (Years) at Study Entry | ||
| Median/Range/IQR | 9.2/3.9–20.8/6.88–12.44 | 10.2/3.5–16.6/7.49–12.90 |
| SEX (Number (%) | ||
| Males | 12 (48) | 15 (60) |
| Females | 13 (52) | 10 (40) |
| ETHNICITY (Number (%) | ||
| Hispanic or Latino | 3 (12) | |
| Non-Hispanic | 22 (88) | 24 (96) |
| Unknown | 1 (4) | |
| PRIOR # of THERAPIES | ||
| Median/Range/IQR | 4/2–9/3–5 | 3/1–9/2–4 |
| RACE (Number (%) | ||
| Asian | 1 (4) | |
| Black | 1 (4) | |
| Unknown | 0 (0) | 3 (12) |
| White, Non-Hispanic | 23 (92) | 22 (88) |
| DIAGNOSIS (Number (%) | ||
| Astrocytoma, NOS | 2 (8) | |
| Glioma, NOS | 19 (76) | |
| Pilocytic Astrocytoma | 25 (100) | 4 (16) |
| LOCATION (Number (%) | ||
| Brain Stem | 6 (24) | 5 (20) |
| Brain, NOS | - | 1 (4) |
| Cerebellum, NOS | 2 (8) | - |
| Cisterna Interpeduncularis | 1 (4) | - |
| Corpus Callosum, NOS | - | 2 (8.0) |
| Cerebral Hemisphere | 2 (8) | 2 (8) |
| Hypothalamus, NOS | 9 (36) | 2 (8.0) |
| Lateral Ventricle, NOS | 1 (4) | - |
| Optic Tract | 1 (4) | 13 (52) |
| Supratentorial Brain, NOS | 1 (4) | - |
| Thalamus, NOS | 2 (8) | - |
Figure 1.
CONSORT diagram depicting patients enrolled, eligible and evaluable.
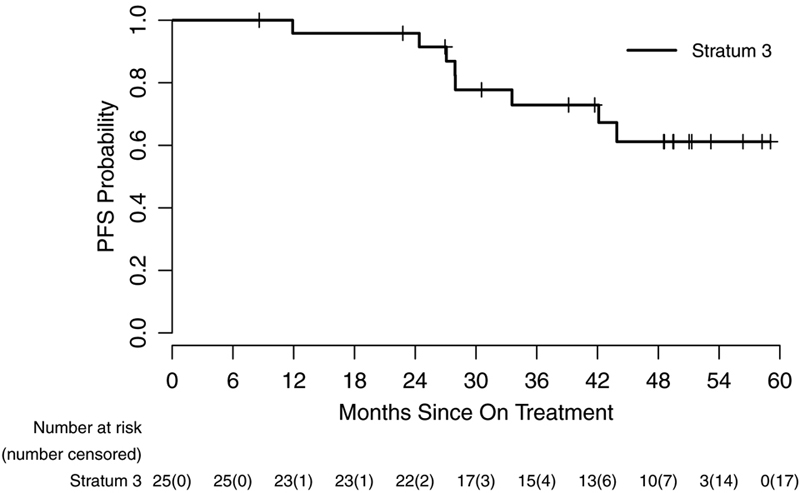
Figure 2.
Kaplan-Meir curve representing progression-free survival of patients from the dates on treatment to dates of tumor progression, death, or last contact (95% CI) for stratum 1 (A), stratum 3 (B) and based on specific BRAF aberration in stratum 1 (C).
Figure 3.
Panel A: Stratum 1 waterfall plot depicting the maximum percentage change of two-dimensional tumor sizes during treatment from baseline by BRAF Status: KIAA1549-BRAF fusion and BRAFV600E mutation. The green dash line shows 50% tumor reduction from baseline which signifies a partial response (PR). Panel B: Stratum 3 waterfall plot depicting the maximum percentage change of two-dimensional tumor sizes during treatment from baseline. The green dash line shows 50% tumor reduction from baseline which signifies a partial response (PR).
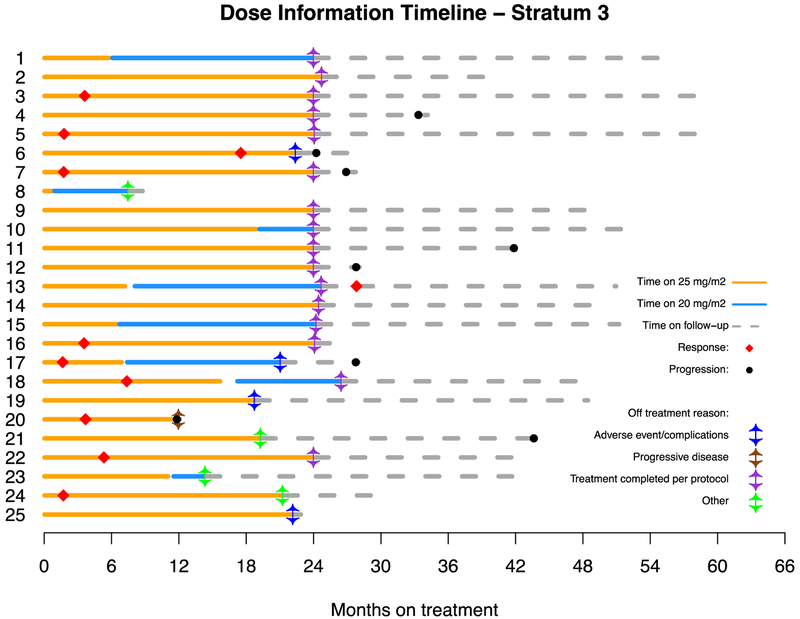
Between August 28, 2013, and June 25, 2015, 26 eligible patients were enrolled on stratum 3. One of 26 (3.8%) eligible patients enrolled was not evaluable due the patient’s refusal to take any study drug, leaving 25 eligible and evaluable patients. Thirteen of 25 (52%) eligible and evaluable patients had an optic pathway glioma (OPG). Three of 25 (12%) patients provided tumor samples and all 3 were negative for BRAF aberrations. Table 1 provides demographic data and Figure 1 depicts the CONSORT flow diagram. All 25 patients on stratum 3 (100%) received prior chemotherapy with a median of 2 prior chemotherapy regimens (range 1–6). Nine of 25 (36%) patients had previous tumor surgeries and only 1/25 (4%) had previously received radiotherapy. The institutional assessment of best responses were 10/25 (40%) PR, 15/25 (60%) SD and 1/25 (4%) PD. Nine of 10 (90%) PRs were confirmed centrally, and the tenth had 49% tumor reduction on central review. The median time to a PR was 3.57 months (IQR=1.76–5.36), which is notably shorter than that seen in stratum 1. The 2-year PFS was 96% (95% CI: 74%−99.4%) (Figure 2B). Sixteen of 25 patients (64%) completed all 26 courses of therapy with either stable or responsive disease. Figure 3B depicts the waterfall plot. Age at treatment and tumor size were not statistically associated with response or PFS on stratum 3. There were insufficient data on BRAF aberration status to assess this as a predictive marker. Eight of 25 (32%) patients have progressed; 1/8 (12.5%) while on therapy and 7/8 (87.5%) while off treatment. The median time to progression was 27.95 months (range=11.9–43.9; IQR=25.74–37.84). The median follow-up for all eligible and evaluable patients (n=25) from treatment initiation to these analyses is 42.1 months (IQR=27.9–59.1). Seventeen of 25 (68%) patients have not progressed with a median follow-up since treatment initiation of 48.6 months (range=8.6–59.07; IQR=39.14–51.31). In patients who have not progressed, the median follow-up since stopping selumetinib is currently 25.49 months (range=0.56–35.01; IQR=14.38–28.83). Figures 4a and 4b represent dosing, duration of response and duration of follow-up on all patients enrolled on both strata. The median time off therapy for all eligible and evaluable patients on both strata that have not yet progressed (n=28) is 20.28 months (range=0–35.01; IQR=10.33–26.68).
Figure 4.
Dosing, duration of treatment, reason off treatment and follow-up on all patients enrolled on both stratum 1 (A) and stratum 3 (B).
On stratum 3, 10/13 (76.9%) patients with an OPG had Snellen visual acuity (VA) comparisons in at least one eye at baseline and 1 year on therapy. Among the 18 evaluable eyes (in 10 patients), there was improvement in VA (≥ 0.2 logMAR improvement) in 2 eyes (11.1%) and stability (neither ≥ 0.2 logMAR improvement nor worsening) in 16 (88.9%) eyes. No patient had worsening (≥ 0.2 logMAR worsening). Therefore, two (20%) patients had improvement in VA and 8 (80%) had stable VA. Based upon Goldmann perimetry testing comparisons at baseline and 1 year, 9 (90%) patients had stable visual fields (VF) and 1 (10%) had improvement. There was no correlation between MRI response and visual acuity.
Twenty-three of 50 patients participated in the optional PK studies. The median Cmax, tmax, and AUC0–8 were 1719 nM (917–6201 nM), 1.4 hr (0.6–2.7 hr), and 3959 nM*hr (2483–8827 nM*hr), respectively. The median apparent oral clearance was 10.9 L/hr (4.5–20.0 L/hr). The median for the N-desmethyl-selumetinib AUC0–8 was 327 nM*hr (172–679 nM*hr). A linear relationship was noted between age and apparent oral selumetinib clearance (Spearman correlation coefficient; r=0.50; p=0.01). The relationship between selumetinib exposure and toxicity could not be thoroughly investigated because only 1 patient experienced a grade 4 toxicity.
Complete DNA methylation profiles were generated for five cases from stratum 1 and one from stratum 3. These data were used to investigate DNA methylation-based tumor subgrouping (Appendix, page 8). Three of five (60%) cases in stratum 1 displayed the typical KIAA1549-BRAF fusion and 1/5 (20%) harbored a BRAFV600E mutation. In the fifth stratum 1 case no BRAF aberration was identified. This patient’s tumor screened positive for the KIAA1549-BRAF fusion, however, the KIAA1549-BRAF fusion was not detected based on DNA panel sequencing/copy number analyses, possibly due to the low tumor purity (high normal tissue content) in this sample. Despite the lack of confirmation, this patients had an imaging PR. No obvious additional pathogenic mutations beyond NF1 were identified in stratum 3 tumors. These small number of samples precluded any formal assessment of correlation with responses.
The most common attributable toxicities on both strata were grade 1 and 2 CPK elevation, hypoalbuminemia, dyspnea, rash, duodenal ulcer, anemia, dry skin, fatigue and diarrhea (Table 2). Rare grade 3 toxicities included elevated CPK (n=5), maculopapular rash (n=5), neutropenia (n=3), nausea (n=3), paronychia (n=3), acneiform rash (n=2), diarrhea (n=2), elevated ALT (n=1), decreased ejection fraction (n=1), gastric hemorrhage (n=1), headache (n=1), skin infection (n=1), tooth infection (n=1) and weight gain (n=1). There was only one grade 4 toxicity, lymphopenia. All attributable toxicities and all grade 3 and 4 adverse events (AEs) on both strata can be seen in the Appendix, pages 1–4. On stratum 1, 10/25 patients (40%) required a dose reduction due to toxicity and 1/25 (4%) required two dose reductions. On stratum 3, 8/25 (32%) required a dose reduction due to toxicity (Figure 4 and Appendix, page 6). There were no reported deaths while on study. On stratum 1, only one patient disconituned drug due to toxicity, a grade 3 rash. On stratum 3, 4 patients discontinued drug due to toxicity, incuding grade 2 intolerable paronychia (n=1), grade 3 gastric hemorrhage (n=1), grade 2 intolerable fatigue (n=1) and grade 2 intolerable dyspnea (n=1). There was no appreciable difference observed in the toxicity profiles between stratum 1 and 3.
Table 2.
Toxicities
| Toxicity^# | Grade | |||||
|---|---|---|---|---|---|---|
| 1&2 | 3 | 4 | ||||
| N | % | N | % | N | % | |
| Abdominal pain | 12 | 24.00 | . | . | . | . |
| Alanine aminotransferase increased | 20 | 40.00 | 1 | 2.00 | . | . |
| Alkaline phosphatase increased | 11 | 22.00 | . | . | . | . |
| Alopecia | 7 | 14.00 | . | . | . | . |
| Anemia | 28 | 56.00 | . | . | . | . |
| Anorexia | 6 | 12.00 | . | . | . | . |
| Aspartate aminotransferase increased | 25 | 50.00 | . | . | . | . |
| CPK increased | 34 | 68.00 | 5 | 10.00 | . | . |
| Constipation | 11 | 22.00 | . | . | . | . |
| Cough | 7 | 14.00 | . | . | . | . |
| Creatinine increased | 7 | 14.00 | . | . | . | . |
| Diarrhea | 27 | 54.00 | 2 | 4.00 | . | . |
| Dizziness | 5 | 10.00 | . | . | . | . |
| Dry skin | 28 | 56.00 | . | . | . | . |
| Duodenal ulcer | 29 | 58.00 | . | . | . | . |
| Dyspnea | 30 | 60.00 | . | . | . | . |
| Edema limbs | 13 | 26.00 | . | . | . | . |
| Ejection fraction decreased | 19 | 38.00 | 1 | 2.00 | . | . |
| Erythema multiforme | 5 | 10.00 | . | . | . | . |
| Fatigue | 28 | 56.00 | . | . | . | . |
| Gastric hemorrhage | . | . | 1 | 2.00 | . | . |
| Headache | 14 | 28.00 | 1 | 2.00 | . | . |
| Hyperglycemia | 10 | 20.00 | . | . | . | . |
| Hypertension | 11 | 22.00 | . | . | . | . |
| Hypoalbuminemia | 31 | 62.00 | . | . | . | . |
| Hypocalcemia | 9 | 18.00 | . | . | . | . |
| Hypokalemia | 12 | 24.00 | . | . | . | . |
| Hypomagnesemia | 18 | 36.00 | . | . | . | . |
| Hyponatremia | 5 | 10.00 | . | . | . | . |
| Hypophosphatemia | 7 | 14.00 | . | . | . | . |
| Lymphocyte count decreased | 19 | 38.00 | . | . | 1 | 2.00 |
| Lymphocyte count increased | 21 | 42.00 | . | . | . | . |
| Metabolism and nutrition disorders - Other, specify | 5 | 10.00 | . | . | . | . |
| Mucositis oral | 7 | 14.00 | . | . | . | . |
| Nausea | 21 | 42.00 | . | . | . | . |
| Neutrophil count decreased | 14 | 28.00 | 3 | 6.00 | . | . |
| Paronychia | 19 | 38.00 | 3 | 6.00 | . | . |
| Pericardial effusion | 20 | 40.00 | . | . | . | . |
| Platelet count decreased | 8 | 16.00 | . | . | . | . |
| Pruritus | 10 | 20.00 | . | . | . | . |
| Rash acneiform | 29 | 58.00 | 2 | 4.00 | . | . |
| Rash maculo-papular | 26 | 52.00 | 5 | 10.00 | . | . |
| Skin and subcutaneous tissue disorders - Other, specify | 25 | 50.00 | . | . | . | . |
| Skin infection | 7 | 14.00 | 1 | 2.00 | . | . |
| Tooth infection | . | . | 1 | 2.00 | . | . |
| Vomiting | 22 | 44.00 | . | . | . | . |
| Weight gain | 5 | 10.00 | 1 | 2.00 | . | . |
| White blood cell decreased | 19 | 38.00 | . | . | . | . |
All grade 1 and 2 attributable toxicities seen in 10% or more of patients combining both strata 1 and 3 are listed. Grade 1 and 2 non-attributable toxicities were not reported.
All grade 3 and 4 toxicities combining both strata are also included.
The highest grade observed within a subject for each toxicity is reported.
There were no grade 5 events reported.
Discussion
Selumetinib is active against recurrent, refractory or progressive PA with common BRAF aberrations and NF1-associated pLGG. Both strata surpassed their statistical predetermined success thresholds based on RR. In stratum 1, the percentage of PRs in tumors harboring KIAA1549-BRAF fusions was slightly higher than those with BRAFV600E mutations (38.9% versus 28.6%), but specific BRAF aberration was not statistically predictive of response. It was, however, predictive for PFS whereby patients with pLGG that harbored a BRAFV600E mutation had worse PFS (Figure 2C). This finding is similar to a recent report showing pLGG with BRAFV600E mutations have worse PFS as compared to both pLGG with wild-type BRAF and KIAA1549-BRAF fusion when treated with chemotherapy and radiotherapy.(25) Interestingly, theses data would be the first to our knowledge showing the same negative prognostic value of BRAFV600E mutation in a homogenous group of pLGG tumors treated prospectively with a MEK inhibitor. For patients with NF1-associated pLGG, 40% showed PR, and only one patient progressed while on therapy. Numerous children in both strata achieved between 1–49% tumor shrinkage and these observed prolonged “stable disease” outcomes are clinically beneficial. Since the vast majority of patients will not succumb to their disease, current opinions are that PFS and functional outcomes are as important as radiographic response.(15, 19)
The 2-year PFS for Stratum 1 and 3 were 70% (95% CI: 47%−85%) and 96% (95% CI: 74%−99.4%), respectively. These PFS and response rates compare favorably to previous trials in children with recurrent pLGG. A phase II study of weekly vinblastine monotherapy in 50 evaluable patients with recurrent and refractory pLGG found a 36% response rate; however, the designation of “minor response” was included as a response for patients with 25–50% reduction in 2-dimensional tumor measurements which would have been categorized as stable disease in our study. If this designation were included on the current study, the overall response rate would increase even further. Five-year OS and EFS with vinblastine were 93.2% ± 3.8%, and 42.3% ± 7.2%, respectively.(26) A PBTC phase II study of bevacizumab and irinotecan in 35 evaluable children with recurrent pLGG showed 2-year PFS of 47.8% (SE ± 9.27%). Two of 35 patients (5.7%) had a documented response and patients received a median of 12 courses of therapy.(27)
Our results for patients with NF1-associated pLGG align with results published for NF1 patients with plexiform neurofibromas. In the phase I plexiform neurofibroma trial, MRI volumetric imaging evaluated response. Approximately 70% of patients were considered partial responders, defined as a ≥20% tumor volume reduction, and the vast majority of patients showed some degree of tumor shrinkage.(22),(28) These volumetric response definitions differ from the classic bi-dimensional 50% shrinkage classically utilized to define a PR in pLGG and used on the current study, perhaps explaining the response variation between the two trials. This common denominator of NF1 MAPK dysregulation seems to harbor these lesions more responsive to selumetinib therapy; however, the exact mechanisms of this increased susceptibility has yet to be elucidated.
As noted, there are patients who did not respond or even progressed while on selumetinib. If additional molecular aberrations were found consistently among these less responsive tumors, combination therapies may be a potential strategy to overcoming this resistance. A limitation of the current study was the lack of tissue available for more advanced molecular testing. Encouragingly, a significant proportion of patients on both arms remain progression-free at a median follow-up of 20.28 months (range=0–35.01; IQR=10.33–26.68) since stopping selumetinib, demonstrating that many pLGG can have prolonged stability even after therapy cessation.
A large retrospective report of 4000 patients with pLGG using Surveillance, Epidemiology and End Results data revealed that the 20-year overall survival was 87±0.8%.(29) They concluded that future treatments should focus upon disease control and minimizing late effects.(29) Also, a pLGG international consensus panel recommended that future studies should incorporate functional outcomes such as visual acuity, quality of life and motor function, since these morbidities are more common than mortality.(19) Therefore, the activity of selumetinib is best evaluated in the context of toxicity, functional outcomes and QOL.
As a MEK-1/2 inhibitor, selumetinib has the benefit of avoiding the adverse event of paradoxical activation of the MAPK pathway seen when BRAF-KIAA1549 aberrant pLGG tumors are treated with direct BRAF inhibitors.(30) Overall, the toxicity profile of selumetinib was manageable with rare grade 3 and 4 toxicities. This compares favorably to both up-front standard pLGG chemotherapy and common chemotherapies used at the time of recurrence. On CCG A9952, 19% of patients developed grade 3/4 vincristine-associated peripheral neuropathy, and 26/137 (18.9%) on the CV arm had grade 3/4 allergic reactions to carboplatin.(3, 5) On A9952, depending upon the treatment arm, 56–68% of patients developed grade 3/4 neutropenia, 15–35% had grade 3/4 thrombocytopenia and 26–29% had grade 3/4 anemia.(3) On the published weekly vinblastine trial, the most common toxicities were hematologic. Eighteen of 50 (36%) patients had grade 4 neutropenia requiring dose reduction and 5 patients required red blood cell transfusions. Five patients with NF1 also had grade 3 peripheral neuropathy.(26) On the PBTC bevaicizumab and irinotecan trial, the most common toxicities were grade 1 and 2 hypertension, fatigue, epistaxis, and grades 1–4 proteinuria.(27) These data contrast with the current study whereby only 6% (3/50) of patients had grade 3 neutropenia; 2% (1/50) had grade 4 lymphopenia and there were no grade 3 or 4 thrombocytopenia or anemia. There was no significant peripheral neuropathy or allergic reactions. As expected, grade 1 and 2 asymtommatic CPK elevation and grade 1 and 2 rashes were some of the most common attributable toxicities seen with selumetinib. Rashes were often mitigated with topical supportive care. There were some patients who did require selumetinib dose reductions (Table 3); however, responses did not appear minimized in those patients requiring dose reductions suggesting the RP2D is not necessary for response. Interestingly, on A9952, NF1 patients who were non-randomly assigned to CV had less toxicity than non-NF1 patients receiving CV.(3, 5) Our selumetinib data does not show this difference.
An adult multi-institutional phase II study of selumetinib in patients with advanced biliary cancer reported that patients receiving selumetinib experienced an average of 5% non-fluid weight gain.(31) On the current study, only 5/50 (10%) patients had grade 1 and 2 weight gain and 1/50 (2%) patient had a grade 3 weight gain. Although there is concern that this adverse event may be unrecognized and thus under reported, future prospective studies will be required to evaluate this more completely in the pediatric population.
A limitation of the current trial is that QOL and patient reported outcomes (PRO) were not assessed; however, there are some notable differences in the patients’ experience between selumetinib and chemotherapy. Selumetinib requires monthly, shorter clinic visits and is taken orally with no requirement for central line placement as compared to weekly CV, bi-monthly bevacizumab/irinotecan and weekly vinblastine visits which often require a central line.
The visual function outcome data in children with OPG revealed stable or improved vision in all patients. Fisher et al. reported on 115 previously untreated NF1 patients with OPG and retrospectively evaluated visual outcomes after chemotherapy. Thirty-two percent of patients had VA improvement, 40% had stable VA, and 28% had worsening VA.(32) Similar to our data, there was no correlation between imaging and visual outcomes.(32) Although smaller numbers, our selumetinib data in recurrent pLGG patients compares favorably to the Fisher et al data following classic chemotherapy in previously untreated patients.(32) A limitation of these data are the small patient numbers and the lack of more standard visual acuity assessments such a Teller acuity cards and HOTV testing.
To our knowledge, aside from the use of mTOR inhibitors in tuberous sclerosis-associated subependymal giant cell astrocytoma which is a very rare entity in the pediatric population, selumetinib is one of the first prospectively-tested and successful molecularly-targeted agents in pLGG. These data provide a new alternative for patients with multiply recurrent pLGG. These data have also directly led to the development of two large multi-institutional prospective randomized phase III COG trials in previously untreated patients comparing selumetinib to standard chemotherapy in both NF1-associated and sporadic pLGG, possibly re-defining the standard of care in pLGG. In these forthcoming COG studies, functional outcomes including VA, QOL, neuropsychological and PRO are included as both primary and secondary objectives.
Supplementary Material
Research in Context.
Evidence Before this Study
Prior to initiation of this phase II clinical trial, all relevant pre-clinical and early phase clinical trial research in both adults and children evaluating glioma biology and MAPK pathway inhibition was considered. Between approximately April 2, 2013, and July 5, 2013, a thorough search of PubMed, specifically for pediatric low-grade glioma (pLGG), selumetinib, MAPK pathway, BRAFV600E mutation and KIAA1549-BRAF fusion was undertaken. No date restrictions were employed. The data quality was robust and consistent verifying that abnormal Ras-MAP kinase-signaling is the most common genetic aberration in pLGG and that targeting of this pathway in cell lines and animal models led to tumor regression. Phase I clinical trials in both children and adults supported safety and preliminary efficacy of selumetinib, a non-ATP-competitive small molecule inhibitor of MAP/ERK (MEK) Kinase I/II. These data led to the development of this phase II efficacy trial of selumetinib in children with recurrent, refractory or progressive pLGG.
Added Value of this Study
To our knowledge, this is one of the first prospectively studied and successful molecularly-targeted agents in pLGG, providing a new alternative to children with NF1-associated pLGG and those with pLGG harboring the most common BRAF aberrations. These phase II data verified the findings seen in the phase I study and better delineated the specific subpopulations of pLGG patients most sensistive to selumetinib as well as further defined duration of response, visual outcomes and toxicity.
Implication of All the Available Evidence
The implications of these data are significant. These data have led directly to 2 large prospective randomized phase III Children’s Oncology Group studies in both newly diagnosed NF-associated and sporadic pLGG testing efficacy, visual outcomes, quality of life and patient reported outcomes of selumetinib compared to standard chemotherapy. Taken together, these data are shaping the future treatment paradigm for children with pLGG.
Acknowledgements
We would like to thank the Department of Neuropathology, Heidelberg University and the Genomics and Proteomics Core Facility of the German Cancer Research Center (DKFZ) for their excellent technical support as well as one of their funding sources, A Kids’ Brain Tumour Cure PLGA Foundation. We would also like to acknowledge the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Declaration of Interest
RIJ participated in a paid selumetinib Advisory Board for AstraZeneca and was an employee of AstraZeneca from 9/2014–5/2017. RJP serves on a selumetinib plexiform neurofibroma Advisory Committee for AstraZeneca. MJF acted as an unpaid consultant for AstraZeneca and received travel reimbursement to participate in an Advisory Board meeting. All other authors declare no competing interests.
Data Sharing Statement
The Pediatric Brain Tumor Consortium (PBTC) makes de-identified research data, including subject level information as well as a data dictionary, available to other investigators for use in research projects. Interested investigators may submit in writing a description of the research project, the specific data requested, and a list of investigators involved with the project and their affiliated research institutions. Once approved, the responsible investigator will be required to complete a Data Use Agreement as part of the conditions for data release. Requests for data will only be considered once the primary study analyses have been published.
References:
- 1.Chalil A, Ramaswamy V. Low Grade Gliomas in Children. J Child Neurol. 2016;31(4):517–22. [DOI] [PubMed] [Google Scholar]
- 2.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24(11):1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lassaletta A, Scheinemann K, Zelcer SM, Hukin J, Wilson BA, Jabado N, et al. Phase II Weekly Vinblastine for Chemotherapy-Naive Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J Clin Oncol. 2016;34(29):3537–43. [DOI] [PubMed] [Google Scholar]
- 5.Ater JL, Xia C, Mazewski CM, Booth TN, Freyer DR, Packer RJ, et al. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: A report from the Children’s Oncology Group. Cancer. 2016;122(12):1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aarsen FK, Paquier PF, Reddingius RE, Streng IC, Arts WF, Evera-Preesman M, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106(2):396–402. [DOI] [PubMed] [Google Scholar]
- 7.Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. Longitudinal investigation of adaptive functioning following conformal irradiation for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys. 2013;85(5):1301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadighi ZS, Curtis E, Zabrowksi J, Billups C, Gajjar A, Khan R, et al. Neurologic impairments from pediatric low-grade glioma by tumor location and timing of diagnosis. Pediatr Blood Cancer. 2018;65(8):e27063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner CD, Chordas CA, Liptak CC, Rey-Casserly C, Delaney BL, Ullrich NJ, et al. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53(3):417–23. [DOI] [PubMed] [Google Scholar]
- 10.Chadderton RD, West CG, Schuller S, Quirke DC, Gattamaneni R, Taylor R. Radiotherapy in the treatment of low-grade astrocytomas. II. The physical and cognitive sequelae. Childs Nerv Syst. 1995;11(8):443–8. [DOI] [PubMed] [Google Scholar]
- 11.West CG, Gattamaneni R, Blair V. Radiotherapy in the treatment of low-grade astrocytomas. I. A survival analysis. Childs Nerv Syst. 1995;11(8):438–42. [DOI] [PubMed] [Google Scholar]
- 12.Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, Baser ME, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–5. [DOI] [PubMed] [Google Scholar]
- 13.Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12(1):1–11. [DOI] [PubMed] [Google Scholar]
- 14.Hargrave D Paediatric high and low grade glioma: the impact of tumour biology on current and future therapy. Br J Neurosurg. 2009;23(4):351–63. [DOI] [PubMed] [Google Scholar]
- 15.Jones DTW, Kieran MW, Bouffet E, Alexandrescu S, Bandopadhayay P, Bornhorst M, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol. 2018;20(2):160–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DT, Hutter B, Jager N, Korshunov A, Kool M, Warnatz HJ, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packer RJ, Pfister S, Bouffet E, Avery R, Bandopadhayay P, Bornhorst M, et al. Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol. 2017;19(6):750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Neale G, Keir ST, et al. Initial testing (stage 1) of AZD6244 (ARRY-142886) by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;55(4):668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Young Poussaint T, et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 2017;19(8):1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, et al. Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas. N Engl J Med. 2016;375(26):2550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahm F, Schrimpf D, Jones DT, Meyer J, Kratz A, Reuss D, et al. Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol. 2016;131(6):903–10. [DOI] [PubMed] [Google Scholar]
- 25.Lassaletta A, Zapotocky M, Mistry M, Ramaswamy V, Honnorat M, Krishnatry R, et al. Therapeutic and Prognostic Implications of BRAF V600E in Pediatric Low-Grade Gliomas. J Clin Oncol 2017;35(25):2934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–63. [DOI] [PubMed] [Google Scholar]
- 27.Gururangan S, Fangusaro J, Poussaint TY, McLendon RE, Onar-Thomas A, Wu S, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16(2):310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, Barker FG, Connor S, Evans DG, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21 Suppl 1):S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Marcus KJ, et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61(7):1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karajannis MA, Legault G, Fisher MJ, Milla SS, Cohen KJ, Wisoff JH, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol. 2014;16(10):1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011;29(17):2357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.