Abstract
Purpose.
Children with Hutchinson-Gilford progeria syndrome (HGPS), a rare premature aging disease, exhibit extraskeletal calcifications detected by radiographic analysis and on physical examination. The aim of this study was to describe the natural history and pathophysiology of these abnormal calcifications in HGPS, and to determine whether medications and/or supplements tested in clinical trials alter their development.
Methods.
Children from two successive clinical trials administering 1) lonafarnib (n=26) and 2) lonafarnib + pravastatin + zoledronic acid (n=37) were studied at baseline (pre-therapy), one year on therapy, and at end-of-therapy (3.3–4.3 years after the baseline visit). Calcium supplementation (oral calcium carbonate) was administered during the first year of the second trial and was subsequently discontinued. Information on calcifications was obtained from physical examinations, radiographs, and serum and urinary biochemical measures. The mineral content of two skin-derived calcifications was determined by x-ray diffraction.
Results.
Extraskeletal calcifications were detected radiographically in 12/39 (31%) patients at baseline. The odds of exhibiting calcifications increased with age (p=0.045). The odds were unaffected by receipt of lonafarnib, pravastatin, and zoledronate therapies. However, administration of calcium carbonate supplementation, in conjunction with all three therapeutic agents, significantly increased the odds of developing calcifications (p=0.009), with the odds plateauing after the supplement’s discontinuation. Composition analysis of calcinosis cutis showed hydroxyapatite similar to bone. Although serum calcium, phosphorus, and parathyroid hormone (PTH) were within normal limits at baseline and on-therapy, PTH increased significantly after lonafarnib initiation (p<0.001). Both the urinary calcium/creatinine ratio and tubular reabsorption of phosphate (TRP) were elevated at baseline in 22/39 (56%) and 31/37 (84%) evaluable patients, respectively, with no significant changes while on-therapy. The mean calcium × phosphorus product (Ca × Pi) was within normal limits, but plasma magnesium decreased over both clinical trials. Fibroblast growth factor 23 (FGF23) was lower compared to age-matched controls (p=0.03).
Conclusions.
Extraskeletal calcifications increased with age in children with HGPS and were composed of hydroxyapatite. The urinary calcium/creatinine ratio and TRP were elevated for age while FGF23 was decreased. Magnesium decreased and PTH increased after lonafarnib therapy which may alter the ability to mobilize calcium. These findings demonstrate that children with HGPS with normal renal function and an unremarkable Ca × Pi develop extraskeletal calcifications by an unidentified mechanism that may involve decreased plasma magnesium and FGF23. Calcium carbonate accelerated their development and is, therefore, not recommended for routine supplementation in these children.
Keywords: Progeria, extraskeletal calcifications, magnesium, parathyroid hormone, HGPS, lamin, aging, laminopathy
Introduction
There are in vitro, murine, and human studies that support a dysfunction in calcium handling in Hutchinson-Gilford progeria syndrome (HGPS)1–3, a rare premature aging disease characterized by early atherosclerosis and cardiovascular death at a mean age of 14.6 years5. Progerin is a mutant form of lamin A that is responsible for disease manifestations6, 7, including calcification within atherosclerotic plaques and cardiac valves, skeletal dysplasia, among other features1, 2, 4, 8, 9. HGPS is one of a few rare inherited monogenic diseases associated with arterial and cardiac valvular calcification.
Abnormal extraskeletal calcifications have been detected in subjects with HGPS on standard radiographs and by dermatologic examination1. The mechanisms underlying the ectopic and intravascular calcification in HGPS are unclear. In an in vitro calcification model, it has been shown that calcium/phosphate deposition can occur even in the presence of normal serum calcium and phosphate concentrations10.
The current study aimed to describe the natural history of these findings in greater detail, explore potential underlying causal mechanisms, and determine whether medications and/or supplements tested in clinical trials in children with HGPS (e.g., lonafarnib, zoledronic acid, pravastatin, and calcium carbonate) impacted the appearance of extraskeletal calcifications detected in these patients (radiographically and on physical examination).
Methods:
Participants were two years of age and older with clinically and genetically confirmed c.1824 C>T, p.Gly608Gly classic HGPS, adequate organ and marrow function, and able to travel for regular study visits. Age-matched healthy controls were enrolled for whom a blood sample for FGF23 was obtained. Control participants had no chronic disease and were not receiving medications known to influence bone and mineral status. Written informed consent was obtained and the protocols were approved by the Boston Children’s Hospital Committee on Clinical Investigation. Age-appropriate assent was also obtained from participants ages ≥ 8 years. Histories, physical examinations, and all efficacy testing were performed at Boston Children’s Hospital.
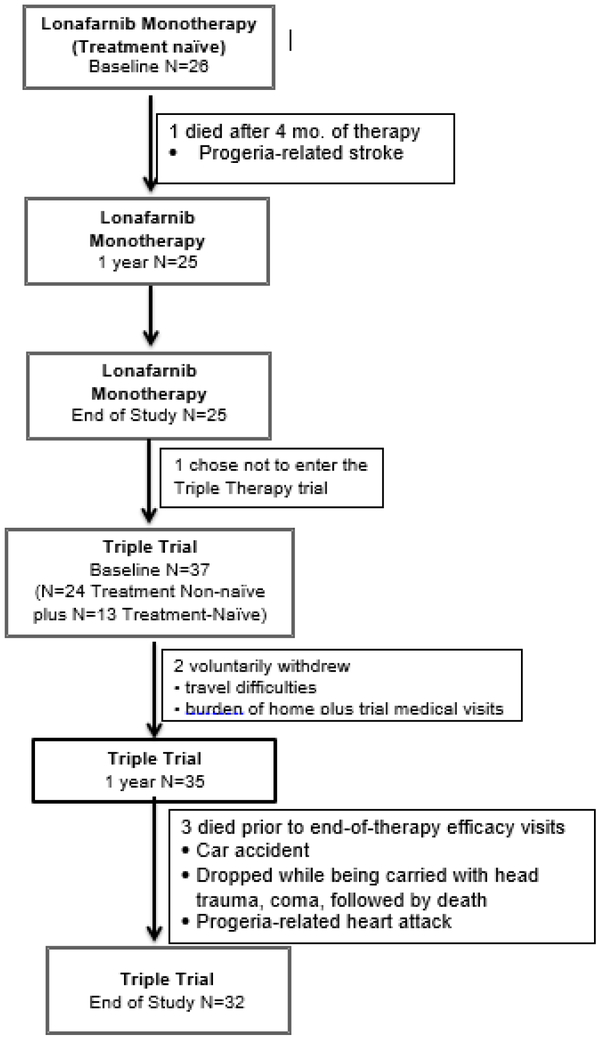
Study drug dosing and administration have been previously reported in detail11, 12.Figure 1 provides details about each clinical trial. All trials were registered with ClinicalTrials.gov: NCT009167471, NCT00879034, and NCT00916747. Briefly, in both trials, subjects were prescribed the oral farnesyltransferase inhibitor, lonafarnib (Merck & Co., Inc.), either by capsule or liquid suspension dispersed in Ora-Blend SF or Ora-Plus (Paddock Laboratories, Inc.) every 12 ± 2 hours. In Trial 1 ( NCT00425607), lonafarnib monotherapy was initiated at 115 mg/m2 for 4 months, when doses were escalated to 150 mg/m2 for a total of 24 months of therapy. Subsequent to the completion of Trial 1, a three-drug combination therapy, consisting of lonafarnib, pravastatin (Pravachol, Bristol-Meyers Squibb), and zoledronic acid (Novartis, Inc.), was initiated (henceforth, the Triple Trial). In the Triple Trial, lonafarnib dosing was initiated at 150 mg/m2. Subjects experiencing drug-related grade 3 or 4 toxicity were dose-reduced to 115 mg/m2. Once reduced, subjects were permitted to increase the dose of lonafarnib back to 150 mg/m2 as tolerated. Oral pravastatin dosing was 5 mg for subjects weighing under 10 kg, and 10 mg for subjects weighing over 10 kg, once every 24 ± 2 hours. Zoledronic acid was administered intravenously over 30 minutes, at baseline, months 6, 12, and 18, and at the end-of-therapy. The initial infusion was 0.0125 mg/kg body weight; all other infusions were 0.05 mg/kg body weight. Serum calcium was measured immediately after and at 1–2 days post-infusion. Oral calcium carbonate (500 mg) and vitamin D (cholecalciferol, 1000 IU) were supplemented daily to avoid hypocalcemia after receipt of intravenous bisphosphonate therapy and vitamin D deficiency, respectively. Calcium supplementation was discontinued after 12 months. Triple Trial medications were administered for 3.3–4.3 years.
Figure 1.
Consort (Consolidated Standards of Reporting Trials) Diagram of Trials Inclusion Total treatment-naïve patients completing baseline evaluations was N=39; 25 from Lonafamib Monotherapy trial and 13 from Triple Trial
For the FGF23 measurement, the study was approved by the Institutional Review Board of Hasbro Children’s Hospital, Providence, RI. Healthy pediatric control subjects who matched at least one HGPS child by age (±6 months) and gender had a blood sample drawn at Hasbro Children’s Hospital after they and/or their parents gave informed assent and consent, respectively.
2.1. Radiographs:
Images included a lateral view of the skull, AP/PA and lateral images of the chest, an AP supine image of the abdomen, AP supine and frog-leg lateral images of the pelvis, AP and lateral images of the humeri, forearms, and lower legs, and a PA image of each hand. All images were interpreted by a pediatric radiologist (RHC or MW).
2.2. Dermatologic Examination and Photographs:
Skin examinations for calcinosis cutis were performed by a single dermatologist (MGL). Location, size and character were noted. A separate photographic consent was obtained from all participants.
2.3. Biochemical Data:
Serum concentrations of calcium, phosphate, alkaline phosphatase, parathyroid hormone (PTH), magnesium, and 25-hydroxyvitamin D (25OHD) were measured. Random urinary concentrations of calcium, phosphorus and creatinine were also measured and urinary spot ratios of calcium/creatinine (Ca/Cr), tubular reabsorption of phosphate (TRP), and the calcium × phosphate product (Ca × Pi) were calculated.
2.4. FGF23
Plasma FGF23 was measured in duplicate using human FGF23 (intact) ELISA kits (Quidel Corporation, CA). Data analysis was performed via GraphPad Prism 7.
2.5. Tissue samples:
Mineral characterization of skin-derived calcifications was performed at the Hospital for Special Surgery and Weill-Cornell Medical College, New York, NY (laboratory of the late A. Boskey, PhD). Samples were analyzed by x-ray diffraction, representing the gold standard technique for phase identification from which calculation of the crystal size of the apatite crystals can be performed in microns. Aliquots of lyophilized fresh-frozen tissue from two patients, ground in a liquid nitrogen–cooled freezer mill (Spex, Metuchen, NJ), were analyzed by wide-angle x-ray diffraction using a Bruker AX-8 diffractometer (Bruker, Madison, WI) with Cu Kα radiation, as previously described13. Scans were recorded from 20° to 40° 2θ, the line-width of the 002 peak measured, and c-axis crystallite size determined based on the standard x-ray line broadening equation as previously described14, using manufacturer-supplied software (AXS Toas P, version 2; Bruker). In addition, samples were analyzed by attenuated total reflection (ATR) Fourier transform infrared spectroscopy on a Nicolet 4700 spectrometer. Spectral data were acquired from 500 – 2000 cm−1, and the mineral: matrix ratio calculated as the ratio of the integrated areas of the phosphate absorbance (900–1200 cm−1) to the protein amide I absorbance (1700–1600 cm−1).
2.6. Statistics:
Descriptive statistics included mean, standard deviation, median, and quartiles for continuous variables, and counts and percentages for categorical variables. Assessments of changes over time were based on General Estimating Equation (GEE) logistic and linear regression for dichotomous and continuous variables, respectively. Logistic models that assessed changes in presence of calcification were adjusted for age and sex; linear regression models that assessed changes in continuous variables were adjusted for age, sex, and baseline value of the continuous variable. Individual patient changes in laboratory measures were summarized using shift tables, and significant changes were based on the test of symmetry. Differences in plasma FGF23 between matched HGPS children and healthy controls were assessed using a linear GEE model that adjusted for age and sex. To account for non-normality, log-transformed FGF23 concentration was used as the outcome. P-values presented are two-sided and are considered significant at the 0.05 level. They do not reflect adjustment for multiple comparisons and should only be interpreted descriptively. All statistical analyses were carried out using SAS version 9.4.
RESULTS
3.1. Patient demographics:
Twenty-six children from 16 countries with classic HGPS enrolled in the initial clinical study, a lonafarnib monotherapy treatment trial (Trial 1)12. One child died after four months on study. The Triple Trial was conducted serially to Trial 1, whereby 24 out of the 25 surviving Trial 1 participants enrolled in the Triple Trial (during their final study site visit for Trial 1). Within the Triple Trial, these children are identified as “treatment-non-naïve” due to receipt of prior lonafarnib monotherapy. In addition, 13 “treatment-naïve” children with classic HGPS, originating from 11 countries, who had not previously received lonafarnib were enrolled in the Triple Trial11. The total number of children included in the baseline analysis is thus 39. Table 1 details patient ages and enrollment totals.
Table 1:
Prevalence of Radiographic Extraskeletal Calcifications at Baseline (pre-treatment)
| Variable | Male (n= 16) | Female (n= 23) | All (n= 39) | |
|---|---|---|---|---|
| Age (years) | Mean ± SD (n) | 6.81 ± 4.29 (16) | 5.74 ± 3.24 (23) | 6.18 ± 3.69 (39) |
| Median (IQR) | 8.00 (3.00, 9.50) | 5.00 (3.00, 8.00) | 6.00 (3.00, 8.00) | |
| Calcium Deposit Seen on X-ray, n (%) | Yes | 3 (19) | 9 (39) | 12 (31) |
| No | 13 (81) | 14 (61) | 27 (69) | |
| Number of Ca Deposits, n (%) | 0 | 13 (81) | 14 (61) | 27 (69) |
| 1 | 2 (13) | 6 (26) | 8 (20.5) | |
| 2 | 0 (0) | 3 (13) | 3 (8) | |
| 3 | 1 (6) | 0 (0) | 1 (2.5) | |
| Area of largest Ca deposit (mm2) for positive patients | Mean ± sd (n) | 46.3 ± 70.7 (3) | 7.6 ± 6.8 (9) | 17.3 ± 35.4 (12) |
| Median (IQR) | 7.0 (4.0, 128.0) | 6.00 (3.6, 8.2) | 6.50 (3.8, 8.9) | |
| Location of largest Ca deposit for positive patients (n (%) | Tuft | 2 (12.5) | 8 (35) | 10 (26) |
| Abdomen | 0 (0) | 1 (4) | 1 (2.5) | |
| Tibia | 1 (6) | 0 (0) | 1 (2.5) | |
3.2. Radiographic data:
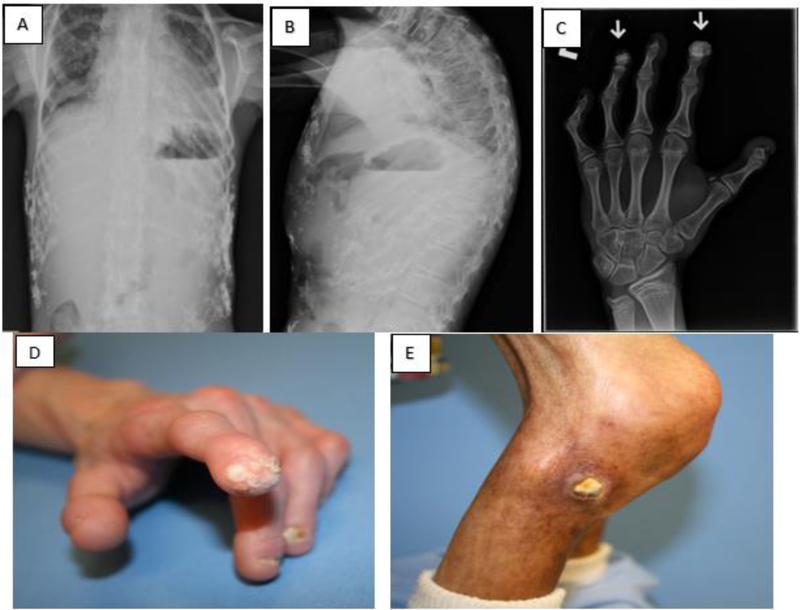
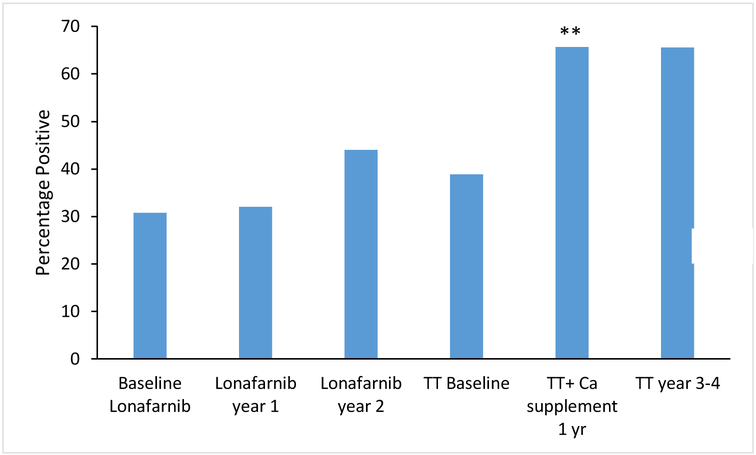
The principle data of interest were extraskeletal calcifications detected radiographically and assessed longitudinally from the beginning of the monotherapy trial to the end of the Triple Trial. Table 1 details the extraskeletal calcifications detected in treatment-naive patients at baseline for both clinical trials, collectively. Extraskeletal calcifications were detected in 12/39 (31%) of patients at baseline. Of these, 8/12 (67%) had a single deposit and 10/12 (83%) were located at the distal phalanges (Figure 1 C–D, supplemental table). Deposits were also noted at the abdomen and tibia (Figure 2 A, B and C). At baseline, there was a positive association between age and presence or absence of deposits (odds ratio for presence of calcification = 1.28 per year, p=0.045, 95% CI 1.01, 1.62). Thus, for each one-year increase in age, the odds of having at least one extraskeletal calcium deposit detected radiographically increased by 28%, after adjusting for sex. There was no significant association between sex and presence or absence of deposits at baseline (odds ratio for presence of calcification = 4.70 for females, p=0.09, 95% CI 0.79, 28.06), after adjusting for age. Although sex was not significantly associated with presence or absence of deposits, all analyses were adjusted for both age and sex given the importance of these clinical variables. Figure 3 depicts the percentage of patients with calcium deposits detected radiographically at baseline for each clinical trial, followed by the percentage at each trial visit. There was no significant change during Trial 1. During the Triple Trial, there was a significant increase in frequency of calcifications during year 1, when calcium supplementation was incorporated into the treatment regimen (p=0.009). From year 1 of the Triple Trial to the end of the Triple Trial, calcium supplementation had been discontinued, and the rate of increase plateaued.
Figure 2 —
Photographs and radiographs of calcifications in children with HGPS A) Radiograph: PA chest; B) lateral chest and C) Tufts of digits (arrows); D) Photograph: Calcifications at tuft of digit; and E) Calcified lesion at the lateral knee.
Figure 3:
Percentage of patients with calcium deposits detected radiographically at baseline for each trial, followed by the percentage of patients with calcium deposits at each trial visit. ** indicates that percent of patients significantly (p<0.05) increased between visits.
3.3. Cutaneous calcifications:
Overall, calcinosis cutis was noted at the distal digits, heel, trunk, upper and lower leg, chest and abdomen, with a predilection for distal digits (Figure 2). Within the lonafarnib monotherapy trial, at the baseline visit, 2/26 (8%) participants with calcifications were noted to have cutaneous calcifications on physical examination, with three additional patients developing this finding at the 1-year study and one at end-of study examination, for a positive study total of 4 of the 26 participants (15%). During the Triple Trial, one additional patient was positive for cutaneous calcifications at baseline, one after one year of therapy, and another three at the end-of-study examination, for a positive study total of 9 participants (24%). There was no association between cutaneous calcinosis and age (p=0.30) or sex (p=0.94) at baseline. Although one child exhibited more extensive involvement (i.e., larger and more extensive areas of ectopic calcification, see Figure 2F–G) than others, the median size of calcifications was 3 mm2 (range 3–36 mm2).
3.4. Calcinosis Cutis Composition Analysis:
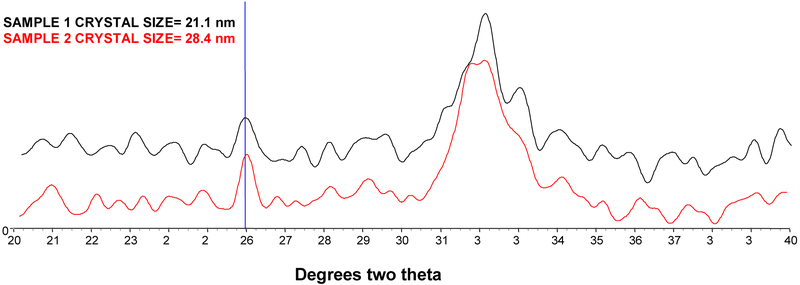
To investigate the composition of the calcinosis cutis, two samples were analyzed. The x-ray diffraction data showed that there was bone-like hydroxyapatite in both (Figure 4). The apatite was similar in size and crystallinity (crystal size and perfection) to bone hydroxyapatite, in the range of 10 – 30 nm in the c-axis direction15. Further, the FTIR-determined mineral matrix ratios for the two samples, 9.7:1 and 7.8:1, were also in the range of that of biological bone tissue.
Figure 4–
X-ray diffraction data from two cutaneous samples from patients with HGPS. Crystal size was calculated from the line broadening of the 002 reflection, which is noted with the vertical line at 26° 2θ.
3.5. Biochemical Data:
3.5.1. Evaluations in Untreated Patients:
Serum and urinary factors relevant to bone formation were examined at baseline before treatment initiation. Thirty-eight of 39 patients submitted serum samples for the items evaluated in Table 2. Additionally, PTH and TRP were evaluated in 37/39 patients. Descriptive statistics, including fractions of patients in the abnormal range for these factors, are presented in Table 2. Mean serum calcium, phosphorus, magnesium, PTH, and Ca × Pi were within normal limits. However, mean values for both urinary Ca/Cr and TRP were higher than the normal range for age. In addition 31/39 (79%) and 22/37 (59%) patients exhibited high urinary Ca/Cr and TRP, respectively.
Table 2:
Prevalence of Biochemical Abnormalities at Baseline (Pre-treatment)
| Variable | Mean ± SD (n) |
Normal Range | Patients with Abnormally High Valuesa N (%) |
|---|---|---|---|
| Calcium (mg/dL) | 10.09±0.35 (38) | < 18y.o.=
8.0–10.5 > 18y.o. = 8.4–10.5 |
2 (5) |
| Phosphorus (mg/dL) | 4.99±0.52 (38) | 1–13y.o. =
3.0–5.7 >13y.o. = 2.7–4.9 |
2 (5) |
| Mg | 2.25±0.16 (38) | 1.5–2.5 | 2 (5) |
| Ca × Pi | 50.44±6.21 (38) | 60 | 3 (8) |
| PTH (pg/mL) | 13.51±8.83 (37) | 10.0–65.0 | 0 (0) |
| Alkaline phosphatase (IU/L) | 149.9±57.1 (38) | 0–3y = 110–400; 3y-10y = 100–320; 10y-13y Female = 60–335; 13y-15y Female = 50–165; >15y Female = 30–120; 10y-13y Male = 40–360; 13y-18y Male = 70–390 |
1 (3) |
| TRP (%) | 93.65 ±3.64 (37) | 78–91% | 31 (84) |
| Urinary Ca/Cr | 0.38±0.25 (39) | ≥ 5 y.o < 0.2; < 5 y.o.< 0.5 |
22 (56) |
Serum for these studies was not obtained for 1/39 patients. PTH and TRP were not determined for one additional patient.
3.5.2. Mean Values With Treatment:
Changes in mean values between study site visits were assessed in response to different treatment regimens (Table 3).
Table 3:
Changes in Biochemical Data with Clinical Trial Therapy
| Baseline Lonafarnib Monotherapy Mean ± SD (n) | Lonafarnib Monotherapy 1 year Mean ±SD (n) | Lonafarnib Monotherapy 2 years Mean ±SD (n) | Baseline Triple Trial Mean ± SD (n) | Triple Therapy with calcium supplement (1 year) Mean ±SD (n) | Triple Therapy only (2–4 years) Mean ±SD (n) | |
|---|---|---|---|---|---|---|
| Calcium (mg/dL) | 9.98±0.32 (25) | 10.2312.13 (25) | 9.89±0.47 (25) | 10.05±0.46 (37) | 9.69±0.47 (35) | 9.67±0.44 (32) |
| Change from prior column (a P value) | - | 0.25±2.12 (0.55) (24) | −0.34±2.11 (0.40) (25) | - | −0.35±0.43 (<0.001) (35) | −0.04±0.57 (0.67) (32) |
| Phosphorus (mg/dL) | 4.97±0.56 (25) | 4.96±0.63 (25) | 4.4811.13 (25) | 4.78±0.65 (37) | 4.23±0.71 (35) | 4.35±0.63 (32) |
| Change from prior column (a P value) | - | −0.01±0.61 (0.94) (24) | −0.49±1.23 (0.06) (25) | - | −0.52±0.77 (<0.001) (35) | 0.21±0.61 (0.14) (32) |
| Ca × Pi. | 49.68±6.29 (25) | 51.02±13.99 (25) | 44.16±11.13 (25) | 48.05±7.19 (37) | 40.86±6.36 (35) | 42.09±6.91 (32) |
| Change from prior column (a P value) | - | 1.32±14.33 (0.64) (24) | −6.86±17.54 (0.06) (25) | - | −6.91±8.26 (<0.001) (35) | 1.99±6.74 (0.22) (32) |
| PTH (pg/mL) | 12.22±6.18 (25) | 29.57±17.83 (24) | 22.09±15.82 (25) | 19.54±14.66 (36) | 35.69±28.06 (35) | 33.45±21.29 (30) |
| Change from prior column (a P value) | - | 18.39±16.73 (<0.001) (23) | −8.03±23.76 (0.14) (24) | - | 16.88±23.91 (<0.001) (34) | −0.31±31.41 (0.77) (30) |
| Magnesium (mg/dL) | 2.30±0.17 (25) | 2.20±0.20 (25) | 2.10±0.19 (25) | 2.12±0.17 (37) | 2.03±0.14 (35) | 2.04±0.19 (32) |
| Change from prior column (a P value) | - | −0.11±0.15 (<0.001) (24) | −0.09±0.16 (0.002) (25) | −0.09±0.13 (<0.001) (35) | 0.01±0.15 (0.79) (32) | |
| Alkaline phosphatas e (IU/L) | 143.5±46.9 (25) | 154.1 ±46.1 (25) | 153.0±41.9 (25) | 156.2±54.7 (37) | 122.6±35.6 (35) | 127.4±38.1 (32) |
| Change from prior column (a P value) | - | 14.0±21.8 (0.002) (24) | −1.1±21.6 (0.94) (25) | - | −33.7±50.2 (<0.001) (35) | 2.0±31.6 (0.66) (32) |
| TRP (%) | 94.57±2.70 (25) | ND | 91.16±9.98 (22) | 91.36±8.41 (34) | 92.30±8.31 (35) | 91.17±8.76 (28) |
| Change from prior column (a P value) | - | - | −3.43±9.62 (0.07) (22) | 1.24±12.44 (0.75) (33) | −1.11±10.03 (0.62) (28) | |
| Urinary Ca/Cr | 0.38±0.22 (26) | 0.48±0.31 (25) | 0.48±0.40 (25) | 0.45±0.37 (37) | 0.56±0.44 (35) | 0.61±0.39 (27) |
| Change from prior column (a P value) | - | 0.11±0.31 (0.05) (25) | 0.02±0.46 (0.92) (25) | - | 0.10±0.57 (0.25) (35) | 0.06±0.50 (0.39) (27) |
P-values based on GEE model adjusting for baseline, age and sex; number of patients included in changes from prior column include only patients with data at both examinations. ND= not determined
3.5.3. Calcium, Phosphorus, and Ca × Pi product:
Serum total calcium, phosphorus, and the Ca × Pi product did not change during the lonafarnib monotherapy trial. However, concentrations of all decreased significantly during year one of the Triple Trial (calcium −0.35 mg/dL, phosphorus −0.52 mg/dL, and Ca × Pi −6.91; all P<0.001) and did not change significantly from year 1 to end-of-study. Of note, the mean Ca × Pi product never exceeded a high threshold of 60 over the course of both trials.
3.5.4. PTH:
PTH significantly increased from baseline to year 1 of the lonafarnib monotherapy trial (+18.39, p<0.001). A similar increase occurred during year one of the Triple Trial (+16.88, p<0.001).
3.5.5. Alkaline phosphatase:
There was an increase in alkaline phosphatase during year 1 of the lonafarnib monotherapy trial (+14.0 IU/L, p=0.002), but a significant decrease during year 1 of the Triple Trial (−33.7, <0.001).
3.5.6. Magnesium:
Magnesium significantly decreased over the course of both trials. In the monotherapy trial, the change was −0.11 at one year, p<0.001 and −0.09 at end of study, p=0.002. During the Triple Trial, the change was −0.09 at 1 year, p<0.001 and 0.01 at end of study, p=0.79.
Comparisons Between Patients With and Without Extraskeletal Calcifications:
At all trial visits, there were no significant differences in levels of serum creatinine, calcium, phosphorous, alkaline phosphatase, PTH, TRP, and Ca × Pi product between those patients with versus without detected extraskeletal calcifications (all p>0.05).
3.5.7. Tubular reabsorption of phosphate (TRP) and Urinary Ca/Cr:
TRP was calculated at baseline and end of study for Trial 1 and at all site visits for the Triple Trial. During Trial 1, there was a trend towards a decrease in mean TRP (p=0.07), However, there were no significant changes noted during the Triple Trial.
3.5.8. FGF23:
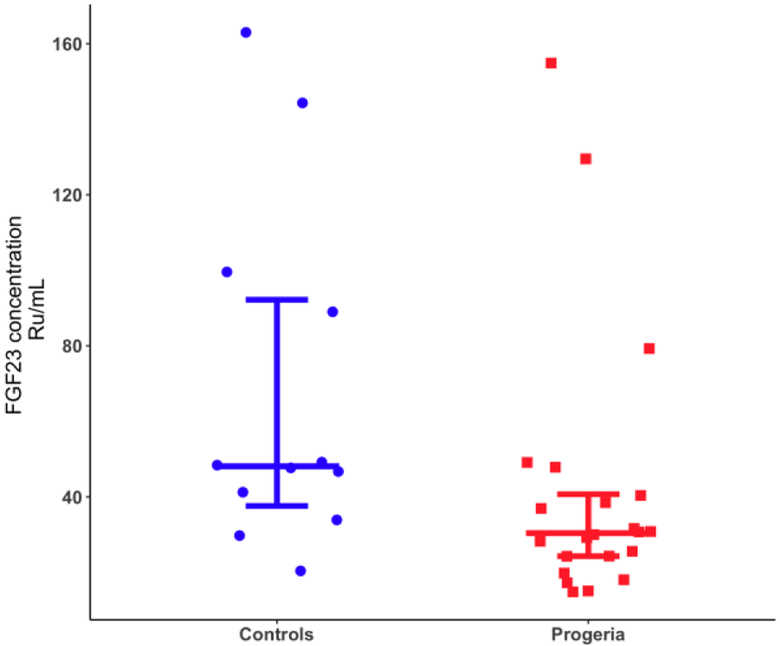
Intact FGF23 was measured at baseline of Trial 1 and compared with pediatric reference levels. Median (IQR) FGF23 was 30.4 (24.3, 40.7) RU/mL for HGPS (N=22) and 48.1 (37.6, 92.2) RU/mL for healthy controls (N=12). FGF23 concentrations were significantly lower in children with HGPS when compared to a cohort of unaffected children (p=0.03; Figure 5).
Figure 5:
Serum FGF23 concentrations in children with progeria compared to healthy control subjects. Each circle represents one control participant and each square, a child with HGPS. Long line is median; shorter lines are 25th and 75th percentiles.
DISCUSSION:
Calcium dysfunction has been previously understudied in HGPS and may underlie the skeletal abnormalities, as well as excessive vascular calcification seen in affected patients1, 2, 8, 9. A better understanding of the cutaneous and radiologic abnormalities associated with HGPS and improved insight into the biology of the pathogenic molecule, , progerin, will advance care for these children. . Extraskeletal calcifications noted on both radiographs and physical examination increased with age. Additionally, a significant increase in the odds of developing these calcifications was noted after administration of daily oral calcium carbonate; this observation plateaued after its discontinuation. Serum variables that were monitored over the course of the trials showed a decrease in ionized calcium and the Ca × Pi product, consistent with vigorous bone mineralization in these children, and an increase in PTH coincident with the decrease in calcium. Since these patients were receiving calcium supplementation with pharmacologic agents, a decrease or suppression of PTH was expected, and a decrease was seen over the course of both trials. These data are consistent with a reorganization of bone structure in HGPS with after recipt of therapies, as is reflected in the dramatic improvement in bone structure noted on peripheral quantitative tomography assessments, reported previously in both trials2, 11, 12.
The calcinosis cutis tissue samples obtained from two children with progeria were particularly informative. Each revealed hydroxyapatite to be present, and the crystals were almost identical in size and crystallinity to bone hydroxyapatite. The proportion of mineral in the organic matrix was slightly higher than healthy trabecular bone in children and adults (9.7:1 and 7.8:1), but not as high as that seen in the dystrophic calcifications of myositis ossificans or tumoral calcinosis, each where mineral/matrix ratios can be as high as 20:116, 17. The observation that the mineral derived cutaneous calcifications are bone-like suggests that the formation is cell-mediated rather than physiochemical (i.e., that stemming from an elevated Ca × Pi). Our data showing a normal mean Ca × Pi product is also consistent with that conclusion.
A number of comparator diseases displaying extraskeletal calcifications are informative for the study of HGPS. Patients with scleroderma, a more common diagnosis than HGPS, frequently exhibit cutaneous calcifications18. In one study, hydroxyapatite was the only crystalline material identified in skin lesions from these patients. However, hydroxyapatite was the minor component and most samples contained more than 50% organic (vs. osteogenic) material.
Another comparator is Werner syndrome (WS), an adult premature aging disorder caused by mutations in the WRN gene, a member of the RecQ DNA helicase family19. WS and HGPS share some progeroid features including atherosclerosis. Soft-tissue calcification seen on radiographs, especially in the Achilles tendon, and/or dermal calcifications, is a common WS disease manifestation. Similar to our findings for HGPS, patients with WS exhibit normal serum calcium, phosphate, and PTH concentrations. One study showed that overexpression of Pit-1, a transmembrane type III Na-Pi cotransporter, may play a key role in the formation of soft tissue calcification WS. Lastly, hyperphosphatemic familial tumoral calcinosis (hFTC) is a rare disorder of phosphate metabolism defined by hyperphosphatemia and ectopic calcifications20. These patients have recessive mutations in genes involved in phosphate metabolism: FGF23, GALNTs, and α-Klotho. The mutations result in inadequate FGF23 secretion or resistance to FGF23 activation at the fibroblast growth factor receptor - α-Klotho complex. These patients may have an increased TRP, but unlike HGPS, exhibit increased production of 1,25-dihydroxyvitamin D and hyperphosphatemia. While ectopic calcifications in HGPS are generally painless, in hFTC, the lesions can be painful and debilitating.
The radiographic features of HGPS bear a striking resemblance to tumoral calcinosis, a rare familial condition characterized by painless, periarticular, subcutaneous or soft tissue calcified masses21. Patients with tumoral calcinosis often present with systemic signs of increased inflammation (e.g., increased C-reactive protein levels). Interestingly, children with HGPS do not have evidence of increased inflammation despite the radiographic similarity4, 22. Also, unlike our cohort with HGPS, hyperphosphatemia is typically seen with tumoral calcinosis, due to decreased action of FGF23 resulting in increased renal phosphate reabsorption. There is a markedly increased Ca × Pi product which leads to the characteristic tissue calcifications that were not seen in our current cohort of children with HGPS21.
Fibrodysplasia ossificans progressiva is a rare disorder in which endochondral bone formation occurs in muscles, tendons, ligaments, and other connective tissue23. The ectopic bone can form plate-like bridges that restrict movement. This autosomal dominant condition can be caused by mutations in the gene encoding the BMP receptor ACVR1, and biochemical assessments interestingly are often normal. Given that ACVR1 has emerged as a therapeutic target to inhibit BMP signaling and ACVR1 inhibitors are currently in development24, future investigation of whether ACVR1 is associated with abnormal calcification in HGPS is of interest.
While there are reports suggesting an increase in atherosclerotic plaque formation and subsequent cardiovascular events in the elderly after receipt of calcium and vitamin D supplementation25–27, many whom were also receiving a bisphosphonate for osteoporosis and a statin for hypercholesterolemia, a recent large study (i.e., 502,637 men and women aged 40 to 69 years) found this association not to be upheld28. In that population based cohort from the UK, supplementation with calcium/vitamin D was self-reported and the association with incident ischemic heart disease, myocardial infarction, and subsequent death was not found. However, because young patients with HGPS may have abnormalities in calcium handling, the assumption of caution from the earlier reports suggesting the link between calcium supplementation and cardiovascular events would be the most prudent position at this time until more information is available in these children..
Although there are published articles supporting the use of intravenous bisphosphonates to decrease calcifications, the current study does not support zoledronate having this effect children with HGPS29. During year 1 of the Triple trial, rates of extraskeletal calcification increased. However, these rates subsequently plateaued after oral calcium carbonate supplementation was discontinued. We surmise that supplementation with calcium carbonate, given prophylactically to avoid hypocalcemia in the face of bisphosphonate therapy and to augment bone health, may have been partly responsible for the increased extraskeletal calcifications noted. This supplementation, a seemingly benign intervention to augment nutrition, may have further aggravated calcium dysfunction in children with HGPS, thus leading to an acceleration of calcifications (radiographically and cutaneously).. Given this potential concern, careful provision of calcium via dietary means is likely the safest intake strategy for these young patients.
During both trials, serum magnesium levels decreased with treatment. Magnesium is an inhibitor of ectopic calcification30. Decreased magnesium is also associated with an increased propensity to form hydroxyapatite in vivo31. Therefore, given that our subjects with HGPS had a normal Ca × Pi product and renal function, the development of extraskeletal calcifications may involve decreased plasma magnesium. In this regard, magnesium is a well-known inhibitor of ectopic mineralization and increased dietary magnesium is known to suppress ectopic calcifications in murine models of Pseudoxanthoma elasticum (PXE), a monogeneic calcification disorder32.
Limitations merit consideration. A number of potential factors were not assessed that could be part of the mechanistic underpinnings of the calcium dysfunction in these children. Examples include the potential role of bone morphogenetic proteins 2 and −7 (BMP-2 and −7), both integrally involved with osteoblast differentiation, and bone formation and repair33. Quantitating isoforms in plasma from these patients could help determine whether the calcifications occur through a connection between pyrophosphate (PPi) and the BMP pathways.
Defective synthesis of pyrophosphate has also been proposed as a risk factor for vascular calcification3. Studies of other disorders suggest that a disturbed serum phosphate/pyrophosphate ratio is a major force triggering arterial and cardiac valve calcification34, the specifics of which are not yet understood in HGPS. Additional hormones that play into the Pi / PPi balance include FGF23 and tissue nonspecific alkaline phosphatase (TNAP)35, 36. We found serum FGF23 to be low in our cohort, a finding that merits further study in the face of normal serum phosphorus concentrations. TNAP levels are elevated in an HGPS mouse model3 and should be explored in future studies of children with HGPS. Lastly, future studies should consider the potential relationship between extraskeletal calcifications and cardiovascular disease in HGPS, as well as whether phalangeal calcifications are associated with less severe clinical consequences versus those at other sites.
In summary, our data suggest that the development of extraskeletal calcifications in HGPS is an age-related phenomenon, with these lesions increasing as these children get older which triggers the osteogenic changes that are involved in the formation of a well-organized, calcified crystalline structure10. Even a seemingly benign maneuver such as low-dose oral calcium carbonate supplementation appeared tooffset the metabolic balance in these patients. This supplementation is also likely not needed as we now understand that nutritional deficiency is not typically a problem in these children2, 12. After discontinuing calcium supplementation, no hypocalcemia or other adverse effects of bisphosphonates were seen and the rate of development of extraskeletal calcifications plateaued. However, as all participants were also receiving bisphosphonate and statin therapy, the independent effect of calcium carbonate could not be evaluated. The downward trend of magnesium noted during both trials is noteworthy as it may suggest a mechanism by which patients with HGPS have a propensity to calcify. An enhanced understanding of bone and mineral status in these patients will lead to more evidence-based clinical recommendations for patients with this ultra-rare disease.
Highlights:
Extraskeletal calcifications have been noted in children with HGPS with unclear pathophysiologic significance in this disease.
Calcifications were noted radiographically at baseline in almost one-third of patients and increased with age.
Cutaneous calcifications consisted primarily of hydroxyapatite.
The appearance of calcifications radiographically and on physical examination accelerated after administration of calcium carbonate supplementation and then plateaued after discontinuation. Other trial medications were not associated with the accelerated appearance of calcifications.
Plasma magnesium decreased over time during all clinical trials and may be mechanically linked to the development of these calcifications.
Acknowledgments
We gratefully acknowledge the children with progeria and their families for participation in this study, Dr. Adele Boskey and Dr. Nancy Pleshko for their expert assistance with the x-ray diffraction analyses, and funding from The Progeria Research Foundation (PRF2002-CB, PRFCLIN2007–02 and PRFCLIN2009–03), National Heart, Lung, and Blood Institute grant 1RC2HL101631–0, and The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Author LBG is the parent of a child who participated in the study.
REFERENCES
- 1.Cleveland RH, Gordon LB, Kleinman ME, et al. A prospective study of radiographic manifestations in Hutchinson-Gilford progeria syndrome. Pediatric radiology. 2012; 42: 1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon CM, Gordon LB, Snyder BD, et al. Hutchinson-Gilford progeria is a skeletal dysplasia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011; 26: 1670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villa-Bellosta R, Rivera-Torres J, Osorio FG, et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of hutchinson-gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation. 2013; 127: 2442–51. [DOI] [PubMed] [Google Scholar]
- 4.Merideth MA, Gordon L, Clauss S, et al. Phenotype and Course of Hutchinson-Gilford Progeria Syndrome. New England Journal of Medicine. 2008; 358: 592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon LB, Massaro J, D’Agostino RB Sr., et al. Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation. 2014; 130: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson M, Brown WT, Gordon L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003; 423: 293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin A Truncation in Hutchinson-Gilford Progeria. Science. 2003; 300: 2055. [DOI] [PubMed] [Google Scholar]
- 8.Gerhard-Herman M, Smoot LB, Wake N, et al. Mechanisms of premature vascular aging in children with Hutchinson-Gilford progeria syndrome. Hypertension. 2012; 59: 92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash A, Gordon LB, Kleinman ME, et al. Cardiac Abnormalities in Patients With Hutchinson-Gilford Progeria Syndrome. JAMA Cardiol. 2018; 3: 326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villa-Bellosta R and Sorribas V. Calcium Phosphate Deposition With Normal Phosphate Concentration. Circulation Journal. 2011; 75: 2705–10. [DOI] [PubMed] [Google Scholar]
- 11.Gordon LB, Kleinman ME, Massaro J, et al. Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children With Hutchinson-Gilford Progeria Syndrome. Circulation. 2016; 134: 114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon LB, Kleinman ME, Miller DT, et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012; 109: 16666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pachman LM, Veis A, Stock S, et al. Composition of calcifications in children with juvenile dermatomyositis: association with chronic cutaneous inflammation. Arthritis and rheumatism. 2006; 54: 3345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pleshko N, Boskey A and Mendelsohn R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophysical journal. 1991; 60: 786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querido W, Ailavajhala R, Padalkar M and Pleshko N. Validated Approaches for Quantification of Bone Mineral Crystallinity Using Transmission Fourier Transform Infrared (FTIR), Attenuated Total Reflection (ATR) FT-IR, and Raman Spectroscopy. Appl Spectrosc. 2018; 72: 1581–93. [DOI] [PubMed] [Google Scholar]
- 16.Ibarra M, Chou PM, Pachman LM, Zhao YD and Boskey AL. Calcification in a case of circumscribed myositis ossificans. The Journal of rheumatology. 2010; 37: 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke B Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008; 3 Suppl 3: S131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu VM, Emge T and Schlesinger N. X-ray diffraction analysis of spontaneously draining calcinosis in scleroderma patients. Scandinavian journal of rheumatology. 2017; 46: 118–21. [DOI] [PubMed] [Google Scholar]
- 19.Honjo S, Yokote K, Fujimoto M, et al. Clinical outcome and mechanism of soft tissue calcification in Werner syndrome. Rejuvenation research. 2008; 11: 809–19. [DOI] [PubMed] [Google Scholar]
- 20.Folsom LJ and Imel EA. Hyperphosphatemic familial tumoral calcinosis: genetic models of deficient FGF23 action. Current osteoporosis reports. 2015; 13: 78–87. [DOI] [PubMed] [Google Scholar]
- 21.Ramnitz MS, Gourh P, Goldbach-Mansky R, et al. Phenotypic and Genotypic Characterization and Treatment of a Cohort With Familial Tumoral Calcinosis/Hyperostosis-Hyperphosphatemia Syndrome. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016; 31: 1845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon LB, Campbell SE, Massaro JM, et al. Survey of plasma proteins in children with progeria pre-therapy and on-therapy with lonafarnib. Pediatr Res. 2018; 83: 982–92. [DOI] [PubMed] [Google Scholar]
- 23.Bouvard B, Masson C, Legrand E and Audran M. Fibrodysplasia ossificans progressiva. A case report and focus on the BMP signaling pathway. Morphologie : bulletin de l’Association des anatomistes. 2016; 100: 250–5. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Alsamarah A, Zhang K and Hao J. Development of New Therapeutic Agents for Fibrodysplasia Ossificans Progressiva. Current molecular medicine. 2016; 16: 4–11. [DOI] [PubMed] [Google Scholar]
- 25.Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008; 336: 262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010; 341: c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolland MJ, Grey A, Avenell A, Gamble GD and Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011; 342: d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey NC, D’Angelo S, Paccou J, et al. Calcium and Vitamin D Supplementation Are Not Associated With Risk of Incident Ischemic Cardiac Events or Death: Findings From the UK Biobank Cohort. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018; 33: 803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canas CA, Bonilla-Abadia F, Mejia M and Tobon GJ. Recovery of Severe Muscular and Fascial Calcinosis After Treatment With Bisphosphonates in a Child With Juvenile Dermatomyositis. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2015; 21: 267–9. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Q and Uitto J. Restricting dietary magnesium accelerates ectopic connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(−/−)). Experimental dermatology. 2012; 21: 694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu L, Feyerabend F, Schilling AF, Willumeit-Romer R and Luthringer BJC. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta biomaterialia. 2015; 27: 294–304. [DOI] [PubMed] [Google Scholar]
- 32.Kupetsky-Rincon EA, Li Q and Uitto J. Magnesium reduces carotid intima-media thickness in a mouse model of pseudoxanthoma elasticum: a novel treatment biomarker. Clinical and translational science. 2012; 5: 259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao X and Chen D. The BMP signaling and in vivo bone formation. Gene. 2005; 357: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nitschke Y and Rutsch F. Inherited Arterial Calcification Syndromes: Etiologies and Treatment Concepts. Current osteoporosis reports. 2017. [DOI] [PubMed] [Google Scholar]
- 35.Murali SK, Andrukhova O, Clinkenbeard EL, White KE and Erben RG. Excessive Osteocytic Fgf23 Secretion Contributes to Pyrophosphate Accumulation and Mineralization Defect in Hyp Mice. PLoS biology. 2016; 14: e1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savinov AY, Salehi M, Yadav MC, Radichev I, Millan JL and Savinova OV. Transgenic Overexpression of Tissue-Nonspecific Alkaline Phosphatase (TNAP) in Vascular Endothelium Results in Generalized Arterial Calcification. Journal of the American Heart Association. 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]