Abstract
Recently, patient-derived xenograft (PDX) models of many types of tumors including breast cancer have emerged as a powerful tool for predicting drug efficacy and for understanding tumor characteristics. PDXs are established by the direct transfer of human tumors into highly immunodeficient mice and then maintained by passaging from mouse to mouse. The ability of PDX models to maintain the original features of patient tumors and to reflect drug sensitivity has greatly improved both basic and clinical study outcomes. However, current PDX models cannot completely predict drug efficacy because they do not recapitulate the tumor microenvironment of origin, a failure which puts emphasis on the necessity for the development of the next generation PDX models. In this article, we summarize the advantages and limitations of current PDX models and discuss the future directions of this field.
Keywords: PDX, breast cancer, humanized mice
1. Introduction
Breast cancer is the leading cause of death in women worldwide, though much progress has been made in diagnosis and treatment strategies over the several decades. Tumors, including those of breast cancer, are composed of highly heterogeneous populations [1]; this heterogeneity is the major cause of the difficulty in eradicating them with current therapies. The inter-tumor heterogeneity of breast cancer also makes it difficult to accurately predict drug efficacy with actively used cancer models like cell lines cultured in vitro and in vivo. Patient-derived xenograft (PDX) models have recently been developed to better reflect the heterogeneity of patient tumors of origin. These models are expected to improve therapeutic strategies against breast cancer.
In PDX models, cancer cells or small tumor tissues derived from patients are injected into immune-deficient mice. The PDX models are mainly used to assess the efficiency of anti-tumor drugs or to clarify the characteristics of cancer cells and their microenvironment since the models closely resemble the original tumors of patients. PDX models have been established for many types of tumors including breast cancer [2], colorectal cancer [3], pancreatic cancer [4], B cell lymphoma [5], lung cancer [6], and ovarian cancer [7]. These PDX models have started to be widely used for drug development and pre- or co-clinical trials. Though current PDX models have some limitations and drawbacks, they have contributed to progress in basic and clinical cancer research. In this review, we compare the advantages of PDX and other cancer models, summarize research utilizing PDX models, and discuss some limitations and future directions of PDX models, mainly focusing on breast cancer PDXs.
2. Generation of PDX Models
2.1. Immunodeficient Mice
To establish PDX models, patient-derived tumors have to be injected into highly immunodeficient mice, because the mouse immune system could eradicate transplanted cancer cells and prohibit tumor engraftment. Nude mice or severe combined immunodeficiency (SCID) mice are rarely used for the establishment of PDX models, although they are often used to establish cell line xenograft models. Non-obese diabetic (NOD)-scid, NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJl (NSG), or NOD.Cg-PrkdcscidIl2rgtm1Sug/ShiJic (NOG) mice, which are characterized by a relatively high immunodeficiency due to a decrease or complete lack of natural killer (NK) cell functions, are the major tools for PDX establishment [8,9]. Furthermore, NSG and NOG mice can be a recipient of human hematopoietic stem cells [10]. As described later, immunodeficient mice engrafted with human immune systems have been established as a powerful tool for the next generation of PDX models [11], as well as for infectious disease mouse models [12].
2.2. Patient-Derived Tumors
Tumor samples obtained from patients by surgical resection or biopsy should be maintained in sterile conditions to prevent bacterial contamination. Before implantation, tumors are cut into small pieces and/or digested into single cells [13]. The tissues or the cells can be implanted heterotopically (in most cases subcutaneously) or orthotopically into immunodeficient mice. Heterotopic injection is often chosen because the method is much easier, and the monitoring of tumor size can be done accurately for any type of tumor. However, for the breast cancer PDX, most researchers choose orthotopic implantation into mammary fat pads [14,15,16,17], as it is not technically difficult and can be accurately monitored. In addition, orthotopic implantation has many advantages compared to heterotopic implantation. The microenvironment around the implanted tumor is more closely preserved, which enables tumors to interact with microenvironment components. Orthotopic implantation also increases the incidence of metastases [16], leading to a wide application of PDX models.
3. Current Representative Line of Cancer Models
In order to enable researchers to do cancer research efficiently, many advances have been made in the establishment of cancer models. Each model has several advantages and limitations. Therefore, choosing an appropriate model for their own purpose is very important. The advantages and limitations of four cancer models—cell line (in vitro), cell line xenograft, genetically engineered mouse, and PDX—are summarized below and in Table 1.
Table 1.
Advantages and limitations of each cancer model.
| Advantages | Limitations | Recommendations to Overcome Limitations | |
|---|---|---|---|
| Cell line (cultured in vitro) | ·Maintained inexpensively ·Treated very easily ·Grow infinitely |
·Completely lack the tumor microenvironment ·Can’t maintain original cell properties ⇒Very low predictive value |
·Should be used in basic studies and very early stages of drug development ·Co-culture with cancer associated fibroblasts (CAFs) or immune cells will improve the predictive value |
| Cell line xenograft | ·High take rates ·Slightly recapitulate tumor microenvironment ·Take short time to be established |
·Can’t reproduce heterogeneity ·Can’t maintain the original cell properties ⇒Low predictive value |
·Should be used in the relatively early stages of drug development with a large number of mice, which can reflect the inter-tumor heterogeneity |
| Genetically engineered mouse | ·Recapitulate tumor initiation and early development process ·Gene of interest can be studied in detail ·Can be increased easily after establishment |
·Can’t reproduce heterogeneity of human tumor ⇒Low predictive value ·Take long time to be established |
·Should be used when investigating how a specific gene of interest could contribute to tumor initiation and relapse |
| PDX | ·Partly recapitulate tumor microenvironment ·Maintain histologic and genetic features of origin ⇒High predictive value ·Can be used for metastatic model |
·Low take rate ·Very expensive ·Take long time to be established |
·Development of new immunodeficient mice and/or better methods of tumor transplantation will improve the take rates and the cost |
3.1. Cell line (cultured in vitro)
Cancer cell lines are the most frequently used tool for cancer research [18]. They can be maintained inexpensively and treated very easily, so many researchers use this model for the first screening of newly developed drug candidates, for investigating cancer characteristics, and for many other purposes. However, cancer cell lines cultured in vitro completely lack interaction with the tumor microenvironment, which is the main reason for the difficulties of predicting drug efficacy and in understanding drug resistance mechanisms with this model [19]. Taking these characteristics into consideration, this model should be applied to basic studies and very early stages of drug development in which the large number of samples are needed.
3.2. Cell Line Xenograft Model
To recapitulate the interaction between cancer cells and the tumor microenvironment, cancer cell lines are transplanted into immunodeficient mice. These cell line xenograft models have been widely used since the first development of the soft tissue sarcoma cell line xenograft model [20]. If cancer cells are transplanted orthotopically, the tumor microenvironment resembles the condition in which the original tumor existed in the patient. Cell line xenograft models are often used for obtaining proof of concept in vivo in the relatively early stages of drug development. In addition, this model is frequently used to understand cancer genetics and drug resistance mechanisms. The popularity of cell line xenograft models is due to their high availability, their lesser costs compared to PDX models, and their high take rates. However, cell line xenografts have limited predictive value for drug efficacy [21].
The main factor for this disadvantage is thought to be the change of the cell properties between the original tumor and established cancer cell lines. Cancer cell lines tend to lose the heterogeneous characteristics of original tumors by the selective pressure on cell culture in vitro [22]. In addition, the long term culture with a culture medium can alter the properties of cancer cell lines little by little. Another factor which makes cell line xenograft models inappropriate for drug efficacy prediction is that most cell lines are derived from highly aggressive malignant tumors. This tendency makes it difficult to recapitulate inter-tumor heterogeneity. Therefore, cell line xenograft models are not the best tools for precise medicine and co-clinical trials.
3.3. Genetically Engineered Mouse Model
Genetically engineered mouse models of breast cancer are established by several approaches [23,24]. One approach is the introduction of an exogenous gene of interest (oncogene) by directly injecting DNA into a fertilized egg [25]. Another approach is the knockout of the gene of interest (tumor suppressor gene) by introducing a targeting vector-encoding modified version of the tumor suppressor gene into mouse embryonic stem cells. The development of a Cre/loxP system enabled the conditional knock in and knock out in specific organs, like mammary gland, which made genetically engineering systems more convenient tools.
This type of mouse model has often been used for understanding of the process of tumor initiation, early development, and relapse after therapies [26]. Spontaneous tumor initiation and relapse occur within the appropriate microenvironment in this model. Therefore, this model has been widely used when investigating how a specific gene could contribute to tumor initiation and relapse. For example, by using this mouse model, Goel et al. showed that cyclin D1/Cyclin-dependent kinase 4 (CDK4) mediated resistance to human epidermal growth factor 2 (HER2)-targeted therapy and that CDK4/6 inhibitors delayed a HER2 positive tumor relapse [27]. However, genetically engineered mice are not always thought to be very suitable for pre- or co-clinical studies. This model cannot completely mimic the human tumors because cancer cells are originally derived from mouse cells. Historical models of mouse mammary tumor virus (MMTV)-infected tumors do not histologically show human breast tumor characteristics [28]. Even if driver genes are introduced into the cells and breast tumors are formed, the heterogeneity of human tumors is not completely recapitulated in these models. Furthermore, it takes very long time (~1year or more) to establish the model. After interesting gene mutations are found in tumor patients, it is impossible to establish the new mouse model with such mutations for the process of precision medicine.
3.4. PDX Model
PDX models have strong advantages in the field of pre- and co-clinical studies when compared with cell line xenografts and genetically engineered mouse models. It has been shown that tumors formed in PDX models resemble the original tumors in patients, both histologically and genetically. Furthermore, Zhang et al. clearly proved that PDX tumors show comparable treatment responses to those observed clinically. In their study, 12 of 13 PDX tumors from 13 patients treated with the same drug showed a matched response with the corresponding clinical response [2].
The recent development of highly immunodeficient mice has enabled us to establish PDX models. Despite some disadvantages, including low take rates and high costs, PDX models are becoming more and more popular, especially for the use of drug efficacy prediction. Recently, The Jackson Laboratory (Bar Harbor, Maine, USA) started to distribute PDX models, and many consortiums on PDX have been established, which has made PDX models more accessible for researchers worldwide.
PDX models have also been used for metastatic models [15]. Since modeling tumor metastasis is difficult for genetically engineered mice, PDX metastasis models have the potential to make great progress in metastatic research. However, there are still some challenges to be overcome. The common metastatic sites of breast cancer PDX models are only lymph nodes and lungs, even though breast cancer in patients also frequently metastasizes to the brain and bones [29]. Therefore, an improved strategy for metastatic PDX establishment will be needed to better predict metastatic behavior by PDX models.
4. PDX Models of Each Breast Cancer Subtype
Breast tumors are normally classified into four subtypes to decide the therapeutic strategies—luminal A, luminal B, and human epidermal growth factor 2 (HER2) positive and triple negative [30,31]. Luminal A and luminal B subtype tumors express an estrogen receptor (ER) and/or a progesterone receptor (PR), and most of them are dependent on estrogen for their growth. HER2 positive tumors are driven to grow by the activation of signaling pathways regulated by HER2 homodimerization and heterodimerization with other HER family members—HER1, HER3, and HER4. Triple negative subtypes have the worst prognosis and show no therapeutic targets at the moment. As therapeutic strategies differ among these subtypes, the development of all types of PDX models is needed [2,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] (Table 2).
Table 2.
Applications of each breast cancer subtype patient-derived xenograft (PDXs).
| Authors | Host Mouse | Tissue Source | Subtypes of PDX (Number) | Method | Reference |
|---|---|---|---|---|---|
| Agnoletto and collegues | nude | primary, metastasis | triple negative (7), HER2+ (2), luminal (2) | interscapular | [31] |
| Al-Hajj and collegues | NOD/SCID | primary | triple negative (4) | orthotopic | [32] |
| Arango and collegues | nude | primary | triple negative (5) | orthotopic | [33] |
| Bruna and collegues | NSG | primary, biopsy, plueral effusion | triple negative (24), HER2+ (6), luminal (52) | orthotopic | [34] |
| Castroviejo-Bermejo and collegues | NSG | primary, biopsy | triple negative (8), luminal (5) | orthotopic | [35] |
| Contreras-Zárate and collegues | NSG | metastasis | triple negative (3), HER2+ (5), luminal (1) | orthotopic | [36] |
| Coussy and collegues | nude | primary | triple negative (61) | orthotopic | [37] |
| Cruz and colleagues | nude | primary | triple negative (9), luminal (1) | orthotopic | [38] |
| Dávila-González and collegues | SCID/Bg | primary | triple negative (5) | orthotopic | [39] |
| DeRose and collegues | NOD/SCID | primary, pleural effusion | triple negative (5), HER2+ (2), luminal (5) | orthotopic | [40] |
| Evans and collegues | NOD/SCID, nude | primary | triple negative (24) | orthotopic | [41] |
| Fatima and collegues | NSG | primary | triple negative (2) | orthotopic | [42] |
| Fleming and collegues | NOD/SCID | pleural effusion | - | orthotopic | [43] |
| Formisano and collegues | SCID/Bg | primary | luminal (2) | orthotopic | [44] |
| González-González and collegues | NSG | primary | triple negative (2) | orthotopic | [45] |
| Hsu and collegues | NSG | primary | luminal (2) | orthotopic | [46] |
| Hu and collegues | NOD/SCID | primary | - | orthotopic | [47] |
| Jung and collegues | NOD/SCID | primary | triple negative (24) | orthotopic | [48] |
| Kabos and collegues | NOD/SCID, NSG | primary, metastasis | triple negative (2), luminal (8) | orthotopic | [49] |
| Kanaya and collegues | NSG | primary | luminal (9) | orthotopic | [50] |
| Li and collegues | NOD/SCID | primary, metastasis | triple negative (12), HER2+ (2), luminal (8) | orthotopic | [51] |
| Liu and collegues | NSG | pleural effusion | HER2+ (2), luminal (2) | orthotopic | [52] |
| Ma and collegues | NOD/SCID | primary, metastasis | triple negative (3) | orthotopic | [53] |
| Marangoni and collegues | nude | primary | triple negative (15), HER2+ (2), luminal (1) | orthotopic | [54] |
| Matossian and collegues | SCID/Bg | primary | triple negative (1) | orthotopic | [55] |
| Méndez-Pertuz and collegues | nude | primary, metastasis | luminal (7) | orthotopic, lower flank | [56] |
| Merino and collegues | NSG | primary | triple negative (2) | orthotopic | [57] |
| Pillai and collegues | NOD/SCID | primary | triple negative (3), luminal (2) | orthotopic | [58] |
| Rather and collegues | NSG | primary | triple negative (1) | s.c. in the right flank | [59] |
| Rosato and collegues | NSG | triple negative (5) | orthotopic | [60] | |
| Ruiz de Garibay and collegues | nude | primary | triple negative (1) | orthotopic | [61] |
| Ryu and collegues | NSG, NOG | primary | triple negative (9), HER2+ (7), luminal (4) | orthotopic | [62] |
| Wang and collegues | SCID/Bg | primary | triple negative (2) | orthotopic | [63] |
| Wang and collegues | nude | primary | luminal (1) | orthotopic | [64] |
| Zhang and collegues | SCID/Bg, NSG | primary, pleural effusion | triple negative (12), HER2+ (3), luminal (2) | orthotopic | [2] |
| Zhang and collegues | NOD/SCID | primary, metastasis | triple negative (7) | orthotopic | [65] |
| Zhang and collegues | NSG | biopsy | - | orthotopic | [66] |
4.1. Luminal A and Luminal B Subtypes
Most tumors of these subtypes are efficiently treated by therapeutic strategies targeting ER and estrogen production [68], suggesting that they are strongly dependent on the function of estrogen. Therefore, estrogen pellets are often injected into immunodeficient mice before tumor transplantation when producing luminal-type PDX models [2,50,69]. Kabos et al. showed that the xenografts derived from luminal tumor expressed ER at a similar level to patient tumors of origin [50]. This indicated that the dependency on estrogen did not change after transplantation. Another report using luminal subtype PDX models for pre-clinical purpose made it clear that the B-cell lymphoma 2 (Bcl-2) Homology 3 (BH3) mimetic improved the tumor response to the antiestrogen tamoxifen [70].
4.2. HER2 Positive Subtype
HER2 positive subtype tumors show relatively low take rates, and the number of therapeutic studies using this type of PDX is limited. However, Kang et al. successfully utilized this model and showed that WW-binding protein 2 (WBP2) helped the inhibitory effect of trastuzumab, a monoclonal antibody targeting HER271. They suggested that WBP2 would be useful as a companion diagnostic for the management of HER2 positive breast cancer with trastuzumab-based therapies. In addition, PDX models of HER2 positive breast cancer-derived brain metastases were also developed by Ni et al. [71]. By using these PDX models, they found that the combined inhibition of phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) led to the durable regression of metastasized tumors. The brain-metastasized models of this type of tumor also resembled the parental metastasized tumors of patients histologically, highlighting the ability of PDX models to maintain original features.
4.3. Triple Negative Subtype
This type of breast tumors does not express ER, PR, or HER2. Therefore, they cannot be treated with endocrine therapy or anti-HER2 targeting strategies. The triple negative subtype represents about 15–20% of breast cancer patients and shows the worst prognosis of the major four subtypes due to the lack of effective treatment other than cytotoxic chemotherapy [72,73]. Therefore, the development of new therapeutic strategies is urgently needed. PDX models of triple negative breast cancers are often utilized for such a purpose [66,74,75]. One reason for their popularity is that, in all the subtypes of breast cancer, the triple negative type shows the highest take rate, partly because of its strong aggressiveness [76,77].
Research using a triple negative breast PDX by EI Ayachi et al. showed that the inhibition of Wnt/β-catenin/HMGA2/EZH2 signaling deprived chemo-resistance to doxorubicin in this type of tumor [78]. Based on the results, they proposed Wnt signaling network targeting therapy as a promising strategy for triple negative breast cancers. Furthermore, a pre-clinical rational for developing treatment approaches using the Notch1 monoclonal antibody [79], Wee1 kinase inhibitor [80], Wnt inhibitor [78], and so on, has been established by utilizing these PDX models.
5. Application of PDX Models for Clinical Use
PDX models are superior to cell line xenografts and genetically engineered mouse models, especially in the field of pre- and co-clinical studies, because they have much higher predictive values [81,82,83]. As long as PDXs are maintained in vivo by directly passaging from mouse to mouse, their character closely resembles that of their parental tumors for several generations. Though the take rate of first transplantation from patient to mouse varies dependent on tumor types [77,84,85,86,87], the second and subsequent take rates from mouse (or frozen stock) to mouse are generally high. Therefore, if PDXs are successfully established by first transplantation, researchers can use them for many purposes by passaging formed tumors to a larger number of mice.
5.1. PDX Models for Drug Development
A very large number of novel drug candidates drops in phase Ⅱ of clinical trials, which is the cause of the enormous costs needed for drug development. The major reason for this problem is the poor predictive values of current cancer models (cell line xenograft or genetically engineered mouse) used in pre-clinical studies. PDX models have the potency to improve such a situation by enabling researchers to predict drug efficacy more precisely [88], as suggested by Zhang et al. [2]. Karamboulas et al. established a large collection of PDX models of head and neck squamous cell carcinoma (HNSCC). They showed the efficacy of CDK4 and CDK6 inhibitors for HNSCC with CCND1 and CDKN2A genomic alterations [89,90]. For breast cancer, Grunewald et al. evaluated the activity of a novel fibroblast growth factor receptor (FGFR) inhibitor, rogaratinib, using cancer cell lines and breast cancer PDX models [91]. They found that the inhibitor has a strong efficacy for FGFR overexpressing cells, both in vitro and in vivo. Furthermore, based on these findings, they started clinical trials of rogaratinib for patients with FGFR overexpressing tumors.
5.2. PDX Models for Precision Medicine
Making appropriate therapeutic regimes based on the features of each tumor leads to better treatment responses. Breast cancers are categorized based on the expression levels of ER, PR, and HER2, and many patients have benefitted from the therapeutic strategies developed according to these categorizations. On the other hand, patients with triple negative subtype tumors show a worse prognosis due to the lack of the good therapeutic targets [92]. In order to improve this situation, researchers are trying to further divide triple negative types into some detailed subtypes, such as basal-like 1, basal-like 2, mesenchymal, and luminal androgen receptor [93,94]. PDX models in drug screening tests will accelerate these studies by enabling us to predict drug efficacy on each triple negative tumor subtype.
5.3. PDX Models for Co-Clinical Trials
In order to determine appropriate therapeutic strategies for each patient, co-clinical trials using mouse models are parallelly operated with clinical treatments [95,96]. Originally, genetically engineered mouse models that had the similar genetic abnormalities to patients were used for this purpose [97,98,99]. However, to predict drug efficacy more precisely, PDX models are being used more these days. For some tumor types, including ovarian cancer and head and neck sarcomas, studies to confirm the efficacy of PDX co-clinical trials are now ongoing. Though some reports, including one by Julic et al., support this concept, there are many hurdles that must be crossed to use breast cancer PDX models for co-clinical trials. Firstly, the take rates of breast cancer are very low, which makes breast cancer PDX models unreliable for therapeutic options. Secondly, it takes a long time (3 months–1 year) to establish the model, which could cause delays to determining therapeutic strategies.
6. Limitations of Current PDX Models
6.1. Lack of Immune Cells
In many types of tumors, including breast cancer, immune cells in tumor microenvironments play very important roles for tumor growth and progression [100,101]. However, current PDX models are established by transplanting tumors into highly immunodeficient mice, which lack the majority of an immune system required in order to obtain higher take rates. Therefore, PDX models cannot reproduce the interaction between cancer cells (or other microenvironment components) and immune cells which exist in patient tumors. This may make it difficult to completely predict drug efficacy and to understand drug resistance mechanisms [102,103]. Fortunately, as described later, next generation PDX models have been established to overcome this kind of limitation.
6.2. Low Take Rates
The take rates of transplanted tumors greatly differ among tumor types of origin. In general, the take rates of patient derived breast cancers are very low (approximately 10–25% on average) [21,77], although the development of pre-exposure methods of estrogen has slightly enhanced the take rates of luminal type tumors. The low take rates and long term incubation periods in transplanted mice make it difficult for us to utilize breast PDX models for pre- or co-clinical studies. If further studies develop more suitable mice for PDX models or better methods of tumor transplantation, which contribute to higher rates of breast tumors, breast cancer PDX models will be more popular in clinical studies.
6.3. High Cost
The financial aspect should also be taken into consideration, because highly immunodeficient mice are very expensive. Maintaining those mice in a clean environment also takes a high cost, as it takes long time before tumors are engrafted and begin to grow in PDX models.
In the case of co-clinical trials using PDX models, a whole genome analysis of original tumors will also be needed. There are still some problems to be solved before PDX models show their true value in clinical settings.
7. Next Generation PDX Models with Human Immune System
It has become clear in the last few decades that components of the tumor microenvironment play very important roles for cancer cell growth and maintenance [104]. The tumor microenvironment is composed of very heterogeneous populations: Cancer cells, cancer associated fibroblasts (CAFs), vascular epithelial cells, many kinds of immune cells, and platelets [105]. In order to reproduce more accurate conditions of tumor origins, much progress has been made in PDX establishment methods. One example is to transplant patient-derived CAFs or mesenchymal stem cells (MSCs), along with cancer cells, to recapitulate the interaction between cancer cells and CAFs [106].
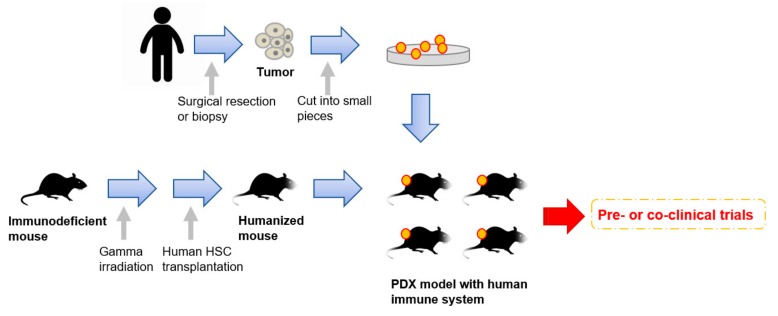
Very recently, humanized mice have started to gather attention as an attractive tool for PDX models [61,64,107] (Figure 1). To enhance the take rates of patient derived tumors, highly immunodeficient mice have been widely used for PDX model establishment. However, mice lacking an immune system cannot recapitulate the interaction between cancer cells and immune cells in the tumor microenvironment. To overcome this disadvantage, mice engrafted with a human immune system are expected to be a promising tool for the next generation PDX models. In 2008, Pearson et al. suggested a protocol for the generation of humanized mice with human immune cells [108]. Adult immune deficient mice like NSG mice are irradiated by 240 cGy whole body gamma irradiation. After four hours, T-cell depleted hematopoietic stem cells (HSCs) containing CD34+ cells are injected into the lateral tail vein. Then, human HSCs are engrafted in the immune deficient mice 10 to 12 weeks after injection [108]. Rosato et al. established triple negative breast cancer PDX models with these humanized mice [61] and provided the evidence supporting the use of humanized PDX models as good models for the pre-clinical investigation of immune-based therapies. In addition to immune-based therapies, humanized PDX models will also improve the predictive values for other types of therapeutic strategies.
Figure 1.
An overall procedure for the generation of PDX models engrafted with a human immune system. Humanized mice are generated by a human hematopoietic stem cells (HSC) transplantation into irradiated immunodeficient mice. Patient-derived tumors obtained by surgical resection or biopsy are sliced into small pieces and then transplanted into the humanized mice.
8. Conclusions
In this review, we discuss the advantages and limitations of current PDX models, usage examples of PDX models derived from all major subtypes of breast cancers, and the development of next generation PDX models with human immunity. Concrete evidence has accumulated to suggest that breast cancer PDX models are valuable tools for predicting drug efficacy in pre- and co-clinical trials because these models well-maintain the heterogeneity and properties of the patient tumors of origin. However, there is a large engraftment bias toward the triple negative subtype. We still have to improve the take rates, particularly of luminal A, B, and HER2 positive subtype tumors, to make good use of PDXs for clinical purposes.
Though current PDX models have some limitations, including the loss of immune systems, researchers are now developing next generation models to overcome such drawbacks. If PDX models, which more accurately reflect the features of the human tumor microenvironment, become popular, cancer research—both in basic and clinical levels—will be highly accelerated. In order to utilize breast cancer PDX models in clinical settings, low take rates and the high cost of PDX establishment are big problems that must be overcome. International collaborative networks may work together to solve these problems. While there are still some obstacles, PDX models have a great potential to improve therapeutic strategies against breast cancer.
Author Contributions
Conceptualization, N.G.; writing—original draft preparation, T.M.; writing—review and editing, N.G.: supervision, N.G.; project administration, N.G.
Funding
This research was funded by AMED grants 16 cm0106120h0001 and 16ck0106194h0001, and Japan Society for the Promotion of Science grants 17K19587 and 18H02679.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Polyak K. Heterogeneity in breast cancer. J. Clin. Investig. 2011;121:3786–3788. doi: 10.1172/JCI60534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X., Claerhout S., Prat A., Dobrolecki L.E., Petrovic I., Lai Q., Landis M.D., Wiechmann L., Schiff R., Giuliano M., et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013;73:4885–4897. doi: 10.1158/0008-5472.CAN-12-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Julien S., Merino-Trigo A., Lacroix L., Pocard M., Goere D., Mariani P., Landron S., Bigot L., Nemati F., Dartigues P., et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin. Cancer Res. 2012;18:5314–5328. doi: 10.1158/1078-0432.CCR-12-0372. [DOI] [PubMed] [Google Scholar]
- 4.Kim M.P., Evans D.B., Wang H., Abbruzzese J.L., Fleming J.B., Gallick G.E. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat. Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapuy B., Cheng H., Watahiki A., Ducar M.D., Tan Y., Chen L., Roemer M.G., Ouyang J., Christie A.L., Zhang L., et al. Diffuse large B-cell lymphoma patient-derived xenograft models capture the molecular and biological heterogeneity of the disease. Blood. 2016;127:2203–2213. doi: 10.1182/blood-2015-09-672352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong X., Guan J., English J.C., Flint J., Yee J., Evans K., Murray N., Macaulay C., Ng R.T., Gout P.W., et al. Patient-derived first generation xenografts of non-small cell lung cancers: Promising tools for predicting drug responses for personalized chemotherapy. Clin. Cancer Res. 2010;16:1442–1451. doi: 10.1158/1078-0432.CCR-09-2878. [DOI] [PubMed] [Google Scholar]
- 7.Weroha S.J., Becker M.A., Enderica-Gonzalez S., Harrington S.C., Oberg A.L., Maurer M.J., Perkins S.E., AlHilli M., Butler K.A., McKinstry S., et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin. Cancer Res. 2014;20:1288–1297. doi: 10.1158/1078-0432.CCR-13-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada S., Vaeteewoottacharn K., Kariya R. Establishment of a Patient-Derived Tumor Xenograft Model and Application for Precision Cancer Medicine. Chem. Pharm. Bull. 2018;66:225–230. doi: 10.1248/cpb.c17-00789. [DOI] [PubMed] [Google Scholar]
- 9.Shultz L.D., Goodwin N., Ishikawa F., Hosur V., Lyons B.L., Greiner D.L. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb. Protoc. 2014;2014:694–708. doi: 10.1101/pdb.top073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt K., Akkina R. Ultra-Sensitive HIV-1 Latency Viral Outgrowth Assays Using Humanized Mice. Front. Immunol. 2018;9:344. doi: 10.3389/fimmu.2018.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shultz L.D., Ishikawa F., Greiner D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 12.Brehm M.A., Wiles M.V., Greiner D.L., Shultz L.D. Generation of improved humanized mouse models for human infectious diseases. J. Immunol. Methods. 2014;410:3–17. doi: 10.1016/j.jim.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth R.A., Perkons N., Dondossola E., Subudhi S.K., Gade T.P., Tam A.L. Patient-Derived Xenograft Tumor Models: Overview and Relevance to IR. J. Vasc. Interv. Radiol. 2018;29:880.e1–882.e1. doi: 10.1016/j.jvir.2018.01.782. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga K., Minato H., Murayama T., Sasahara A., Nishimura T., Kiyokawa E., Kanauchi H., Shimizu S., Sato A., Nishioka K., et al. Semaphorin signaling via MICAL3 induces symmetric cell division to expand breast cancer stem-like cells. Proc. Natl. Acad. Sci. USA. 2019;116:625–630. doi: 10.1073/pnas.1806851116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X., Taftaf R., Kawaguchi M., Chang Y.F., Chen W., Entenberg D., Zhang Y., Gerratana L., Huang S., Patel D.B., et al. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov. 2019;9:96–113. doi: 10.1158/2159-8290.CD-18-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman R.M. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer. 2015;15:451–452. doi: 10.1038/nrc3972. [DOI] [PubMed] [Google Scholar]
- 17.Ni J., Ramkissoon S.H., Xie S., Goel S., Stover D.G., Guo H., Luu V., Marco E., Ramkissoon L.A., Kang Y.J., et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat. Med. 2016;22:723–726. doi: 10.1038/nm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neve R.M., Chin K., Fridlyand J., Yeh J., Baehner F.L., Fevr T., Clark L., Bayani N., Coppe J.P., Tong F., et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imamura Y., Mukohara T., Shimono Y., Funakoshi Y., Chayahara N., Toyoda M., Kiyota N., Takao S., Kono S., Nakatsura T., et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015;33:1837–1843. doi: 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- 20.Hajitou A., Lev D.C., Hannay J.A., Korchin B., Staquicini F.I., Soghomonyan S., Alauddin M.M., Benjamin R.S., Pollock R.E., Gelovani J.G., et al. A preclinical model for predicting drug response in soft-tissue sarcoma with targeted AAVP molecular imaging. Proc. Natl. Acad. Sci. USA. 2008;105:4471–4476. doi: 10.1073/pnas.0712184105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittle J.R., Lewis M.T., Lindeman G.J., Visvader J.E. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. 2015;17:17. doi: 10.1186/s13058-015-0523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaled W.T., Liu P. Cancer mouse models: Past, present and future. Semin. Cell Dev. Biol. 2014;27:54–60. doi: 10.1016/j.semcdb.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Borowsky A.D. Genetically engineering a mouse. Comp. Med. 2003;53:249–250. [PubMed] [Google Scholar]
- 24.Menezes M.E., Das S.K., Emdad L., Windle J.J., Wang X.Y., Sarkar D., Fisher P.B. Genetically engineered mice as experimental tools to dissect the critical events in breast cancer. Adv. Cancer Res. 2014;121:331–382. doi: 10.1016/B978-0-12-800249-0.00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voncken J.W. Genetic modification of the mouse: General technology—Pronuclear and blastocyst injection. Methods Mol. Biol. 2011;693:11–36. doi: 10.1007/978-1-60761-974-1_2. [DOI] [PubMed] [Google Scholar]
- 26.Shibata H., Komura S., Yamada Y., Sankoda N., Tanaka A., Ukai T., Kabata M., Sakurai S., Kuze B., Woltjen K., et al. In vivo reprogramming drives Kras-induced cancer development. Nat. Commun. 2018;9:2081. doi: 10.1038/s41467-018-04449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel S., Wang Q., Watt A.C., Tolaney S.M., Dillon D.A., Li W., Ramm S., Palmer A.C., Yuzugullu H., Varadan V., et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell. 2016;29:255–269. doi: 10.1016/j.ccell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardiff R.D., Rosner A., Hogarth M.A., Galvez J.J., Borowsky A.D., Gregg J.P. Validation: The new challenge for pathology. Toxicol. Pathol. 2004;32(Suppl. 1):31–39. doi: 10.1080/01926230490424662. [DOI] [PubMed] [Google Scholar]
- 29.Eyre R., Alferez D.G., Spence K., Kamal M., Shaw F.L., Simoes B.M., Santiago-Gomez A., Sarmiento-Castro A., Bramley M., Absar M., et al. Patient-derived Mammosphere and Xenograft Tumour Initiation Correlates with Progression to Metastasis. J. Mammary Gland Biol. Neoplasia. 2016;21:99–109. doi: 10.1007/s10911-016-9361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perou C.M., Sorlie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Rees C.A., Pollack J.R., Ross D.T., Johnsen H., Akslen L.A., et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 31.Sorlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agnoletto C., Minotti L., Brulle-Soumare L., Pasquali L., Galasso M., Corra F., Baldassari F., Judde J.G., Cairo S., Volinia S. Heterogeneous expression of EPCAM in human circulating tumour cells from patient-derived xenografts. Biomark. Res. 2018;6:31. doi: 10.1186/s40364-018-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arango N.P., Yuca E., Zhao M., Evans K.W., Scott S., Kim C., Gonzalez-Angulo A.M., Janku F., Ueno N.T., Tripathy D., et al. Selinexor (KPT-330) demonstrates anti-tumor efficacy in preclinical models of triple-negative breast cancer. Breast Cancer Res. 2017;19:93. doi: 10.1186/s13058-017-0878-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruna A., Rueda O.M., Greenwood W., Batra A.S., Callari M., Batra R.N., Pogrebniak K., Sandoval J., Cassidy J.W., Tufegdzic-Vidakovic A., et al. A Biobank of Breast Cancer Explants with Preserved Intra-tumor Heterogeneity to Screen Anticancer Compounds. Cell. 2016;167:260.e22–274.e22. doi: 10.1016/j.cell.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castroviejo-Bermejo M., Cruz C., Llop-Guevara A., Gutierrez-Enriquez S., Ducy M., Ibrahim Y.H., Gris-Oliver A., Pellegrino B., Bruna A., Guzman M., et al. A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. EMBO Mol. Med. 2018:10. doi: 10.15252/emmm.201809172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contreras-Zarate M.J., Ormond D.R., Gillen A.E., Hanna C., Day N.L., Serkova N.J., Jacobsen B.M., Edgerton S.M., Thor A.D., Borges V.F., et al. Development of Novel Patient-Derived Xenografts from Breast Cancer Brain Metastases. Front. Oncol. 2017;7:252. doi: 10.3389/fonc.2017.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coussy F., de Koning L., Lavigne M., Bernard V., Ouine B., Boulai A., El Botty R., Dahmani A., Montaudon E., Assayag F., et al. A large collection of integrated genomically characterized patient-derived xenografts highlighting the heterogeneity of triple-negative breast cancer. Int. J. Cancer. 2019 doi: 10.1002/ijc.32266. [DOI] [PubMed] [Google Scholar]
- 39.Cruz C., Castroviejo-Bermejo M., Gutierrez-Enriquez S., Llop-Guevara A., Ibrahim Y.H., Gris-Oliver A., Bonache S., Morancho B., Bruna A., Rueda O.M., et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann. Oncol. 2018;29:1203–1210. doi: 10.1093/annonc/mdy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davila-Gonzalez D., Choi D.S., Rosato R.R., Granados-Principal S.M., Kuhn J.G., Li W.F., Qian W., Chen W., Kozielski A.J., Wong H., et al. Pharmacological Inhibition of NOS Activates ASK1/JNK Pathway Augmenting Docetaxel-Mediated Apoptosis in Triple-Negative Breast Cancer. Clin. Cancer Res. 2018;24:1152–1162. doi: 10.1158/1078-0432.CCR-17-1437. [DOI] [PubMed] [Google Scholar]
- 41.DeRose Y.S., Wang G., Lin Y.C., Bernard P.S., Buys S.S., Ebbert M.T., Factor R., Matsen C., Milash B.A., Nelson E., et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans K.W., Yuca E., Akcakanat A., Scott S.M., Arango N.P., Zheng X., Chen K., Tapia C., Tarco E., Eterovic A.K., et al. A Population of Heterogeneous Breast Cancer Patient-Derived Xenografts Demonstrate Broad Activity of PARP Inhibitor in BRCA1/2 Wild-Type Tumors. Clin. Cancer Res. 2017;23:6468–6477. doi: 10.1158/1078-0432.CCR-17-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fatima I., El-Ayachi I., Taotao L., Lillo M.A., Krutilina R.I., Seagroves T.N., Radaszkiewicz T.W., Hutnan M., Bryja V., Krum S.A., et al. The natural compound Jatrophone interferes with Wnt/beta-catenin signaling and inhibits proliferation and EMT in human triple-negative breast cancer. PLoS ONE. 2017;12:e0189864. doi: 10.1371/journal.pone.0189864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming J.M., Miller T.C., Meyer M.J., Ginsburg E., Vonderhaar B.K. Local regulation of human breast xenograft models. J. Cell. Physiol. 2010;224:795–806. doi: 10.1002/jcp.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Formisano L., Stauffer K.M., Young C.D., Bhola N.E., Guerrero-Zotano A.L., Jansen V.M., Estrada M.M., Hutchinson K.E., Giltnane J.M., Schwarz L.J., et al. Association of FGFR1 with ERalpha Maintains Ligand-Independent ER Transcription and Mediates Resistance to Estrogen Deprivation in ER(+) Breast Cancer. Clin. Cancer Res. 2017;23:6138–6150. doi: 10.1158/1078-0432.CCR-17-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Gonzalez A., Munoz-Muela E., Marchal J.A., Cara F.E., Molina M.P., Cruz-Lozano M., Jimenez G., Verma A., Ramirez A., Qian W., et al. Activating Transcription Factor 4 Modulates TGFbeta-Induced Aggressiveness in Triple-Negative Breast Cancer via SMAD2/3/4 and mTORC2 Signaling. Clin. Cancer Res. 2018;24:5697–5709. doi: 10.1158/1078-0432.CCR-17-3125. [DOI] [PubMed] [Google Scholar]
- 47.Hsu P.Y., Wu V.S., Kanaya N., Petrossian K., Hsu H.K., Nguyen D., Schmolze D., Kai M., Liu C.Y., Lu H., et al. Dual mTOR Kinase Inhibitor MLN0128 Sensitizes HR(+)/HER2(+) Breast Cancer Patient-Derived Xenografts to Trastuzumab or Fulvestrant. Clin. Cancer Res. 2018;24:395–406. doi: 10.1158/1078-0432.CCR-17-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y., Yague E., Zhao J., Wang L., Bai J., Yang Q., Pan T., Zhao H., Liu J., Zhang J. Sabutoclax, pan-active BCL-2 protein family antagonist, overcomes drug resistance and eliminates cancer stem cells in breast cancer. Cancer Lett. 2018;423:47–59. doi: 10.1016/j.canlet.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 49.Jung J., Jang K., Ju J.M., Lee E., Lee J.W., Kim H.J., Kim J., Lee S.B., Ko B.S., Son B.H., et al. Novel cancer gene variants and gene fusions of triple-negative breast cancers (TNBCs) reveal their molecular diversity conserved in the patient-derived xenograft (PDX) model. Cancer Lett. 2018;428:127–138. doi: 10.1016/j.canlet.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Kabos P., Finlay-Schultz J., Li C., Kline E., Finlayson C., Wisell J., Manuel C.A., Edgerton S.M., Harrell J.C., Elias A., et al. Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res. Treat. 2012;135:415–432. doi: 10.1007/s10549-012-2164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanaya N., Somlo G., Wu J., Frankel P., Kai M., Liu X., Wu S.V., Nguyen D., Chan N., Hsieh M.Y., et al. Characterization of patient-derived tumor xenografts (PDXs) as models for estrogen receptor positive (ER+HER2- and ER+HER2+) breast cancers. J. Steroid Biochem. Mol. Biol. 2017;170:65–74. doi: 10.1016/j.jsbmb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S., Shen D., Shao J., Crowder R., Liu W., Prat A., He X., Liu S., Hoog J., Lu C., et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4:1116–1130. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H., Patel M.R., Prescher J.A., Patsialou A., Qian D., Lin J., Wen S., Chang Y.F., Bachmann M.H., Shimono Y., et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc. Natl. Acad. Sci. USA. 2010;107:18115–18120. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma H.Y., Liu X.Z., Liang C.M. Inflammatory microenvironment contributes to epithelial-mesenchymal transition in gastric cancer. World J. Gastroenterol. 2016;22:6619–6628. doi: 10.3748/wjg.v22.i29.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marangoni E., Vincent-Salomon A., Auger N., Degeorges A., Assayag F., de Cremoux P., de Plater L., Guyader C., De Pinieux G., Judde J.G., et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin. Cancer Res. 2007;13:3989–3998. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- 56.Matossian M.D., Burks H.E., Elliott S., Hoang V.T., Bowles A.C., Sabol R.A., Wahba B., Anbalagan M., Rowan B., Abazeed M.E., et al. Drug resistance profiling of a new triple negative breast cancer patient-derived xenograft model. BMC Cancer. 2019;19:205. doi: 10.1186/s12885-019-5401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendez-Pertuz M., Martinez P., Blanco-Aparicio C., Gomez-Casero E., Belen Garcia A., Martinez-Torrecuadrada J., Palafox M., Cortes J., Serra V., Pastor J., et al. Modulation of telomere protection by the PI3K/AKT pathway. Nat. Commun. 2017;8:1278. doi: 10.1038/s41467-017-01329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merino D., Weber T.S., Serrano A., Vaillant F., Liu K., Pal B., Di Stefano L., Schreuder J., Lin D., Chen Y., et al. Barcoding reveals complex clonal behavior in patient-derived xenografts of metastatic triple negative breast cancer. Nat. Commun. 2019;10:766. doi: 10.1038/s41467-019-08595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pillai S.G., Li S., Siddappa C.M., Ellis M.J., Watson M.A., Aft R. Identifying biomarkers of breast cancer micrometastatic disease in bone marrow using a patient-derived xenograft mouse model. Breast Cancer Res. 2018;20:2. doi: 10.1186/s13058-017-0927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rather G.M., Lin S.Y., Lin H., Banach-Petrosky W., Hirshfield K.M., Lin C.Y., Johnson M.D., Szekely Z., Bertino J.R. Activated matriptase as a target to treat breast cancer with a drug conjugate. Oncotarget. 2018;9:25983–25992. doi: 10.18632/oncotarget.25414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosato R.R., Davila-Gonzalez D., Choi D.S., Qian W., Chen W., Kozielski A.J., Wong H., Dave B., Chang J.C. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018;20:108. doi: 10.1186/s13058-018-1037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruiz de Garibay G., Mateo F., Stradella A., Valdes-Mas R., Palomero L., Serra-Musach J., Puente D.A., Diaz-Navarro A., Vargas-Parra G., Tornero E., et al. Tumor xenograft modeling identifies an association between TCF4 loss and breast cancer chemoresistance. Dis. Model. Mech. 2018:11. doi: 10.1242/dmm.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryu J.S., Sim S.H., Park I.H., Lee E.G., Lee E.S., Kim Y.H., Kwon Y., Kong S.Y., Lee K.S. Integrative In Vivo Drug Testing Using Gene Expression Signature and Patient-Derived Xenografts from Treatment-Refractory HER2 Positive and Triple-Negative Subtypes of Breast Cancer. Cancers. 2019;11:574. doi: 10.3390/cancers11040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang M., Yao L.C., Cheng M., Cai D., Martinek J., Pan C.X., Shi W., Ma A.H., De Vere White R.W., Airhart S., et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 2018;32:1537–1549. doi: 10.1096/fj.201700740R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang D., Xu J., Liu B., He X., Zhou L., Hu X., Qiao F., Zhang A., Xu X., Zhang H., et al. IL6 blockade potentiates the anti-tumor effects of gamma-secretase inhibitors in Notch3-expressing breast cancer. Cell Death Differ. 2018;25:330–339. doi: 10.1038/cdd.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H., Cohen A.L., Krishnakumar S., Wapnir I.L., Veeriah S., Deng G., Coram M.A., Piskun C.M., Longacre T.A., Herrler M., et al. Patient-derived xenografts of triple-negative breast cancer reproduce molecular features of patient tumors and respond to mTOR inhibition. Breast Cancer Res. 2014;16:R36. doi: 10.1186/bcr3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang S., Zhang H., Ghia E.M., Huang J., Wu L., Zhang J., Lam S., Lei Y., He J., Cui B., et al. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc. Natl. Acad. Sci. USA. 2019;116:1370–1377. doi: 10.1073/pnas.1816262116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheang M.C., Chia S.K., Voduc D., Gao D., Leung S., Snider J., Watson M., Davies S., Bernard P.S., Parker J.S., et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl. Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goetz M.P., Kalari K.R., Suman V.J., Moyer A.M., Yu J., Visscher D.W., Dockter T.J., Vedell P.T., Sinnwell J.P., Tang X., et al. Tumor Sequencing and Patient-Derived Xenografts in the Neoadjuvant Treatment of Breast Cancer. J. Natl. Cancer Inst. 2017:109. doi: 10.1093/jnci/djw306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaillant F., Merino D., Lee L., Breslin K., Pal B., Ritchie M.E., Smyth G.K., Christie M., Phillipson L.J., Burns C.J., et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24:120–129. doi: 10.1016/j.ccr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Kang S.A., Guan J.S., Tan H.J., Chu T., Thike A.A., Bernado Morales C., Arribas J., Wong C.Y., Tan P.H., Gudi M., et al. Elevated WBP2 expression in HER2-positive breast cancers correlates with sensitivity to trastuzumab-based neo-adjuvant therapy:A Retrospective and Multicentric Study. Clin. Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-18-3228. [DOI] [PubMed] [Google Scholar]
- 72.Cleator S., Heller W., Coombes R.C. Triple-negative breast cancer: Therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 73.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 74.Leconet W., Chentouf M., du Manoir S., Chevalier C., Sirvent A., Ait-Arsa I., Busson M., Jarlier M., Radosevic-Robin N., Theillet C., et al. Therapeutic Activity of Anti-AXL Antibody against Triple-Negative Breast Cancer Patient-Derived Xenografts and Metastasis. Clin. Cancer Res. 2017;23:2806–2816. doi: 10.1158/1078-0432.CCR-16-1316. [DOI] [PubMed] [Google Scholar]
- 75.Byrd T.T., Fousek K., Pignata A., Szot C., Samaha H., Seaman S., Dobrolecki L., Salsman V.S., Oo H.Z., Bielamowicz K., et al. TEM8/ANTXR1-Specific CAR T Cells as a Targeted Therapy for Triple-Negative Breast Cancer. Cancer Res. 2018;78:489–500. doi: 10.1158/0008-5472.CAN-16-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tentler J.J., Tan A.C., Weekes C.D., Jimeno A., Leong S., Pitts T.M., Arcaroli J.J., Messersmith W.A., Eckhardt S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu J., Qin B., Moyer A.M., Sinnwell J.P., Thompson K.J., Copland J.A., 3rd, Marlow L.A., Miller J.L., Yin P., Gao B., et al. Establishing and characterizing patient-derived xenografts using pre-chemotherapy percutaneous biopsy and post-chemotherapy surgical samples from a prospective neoadjuvant breast cancer study. Breast Cancer Res. 2017;19:130. doi: 10.1186/s13058-017-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Ayachi I., Fatima I., Wend P., Alva-Ornelas J.A., Runke S., Kuenzinger W.L., Silva J., Silva W., Gray J.K., Lehr S., et al. The WNT10B network is associated with survival and metastases in chemoresistant triple-negative breast cancer. Cancer Res. 2018 doi: 10.1158/0008-5472.CAN-18-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu M., Peng Q., Jiang I., Carroll C., Han G., Rymer I., Lippincott J., Zachwieja J., Gajiwala K., Kraynov E., et al. Specific inhibition of Notch1 signaling enhances the antitumor efficacy of chemotherapy in triple negative breast cancer through reduction of cancer stem cells. Cancer Lett. 2013;328:261–270. doi: 10.1016/j.canlet.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 80.Chen X., Low K.H., Alexander A., Jiang Y., Karakas C., Hess K.R., Carey J.P.W., Bui T.N., Vijayaraghavan S., Evans K.W., et al. Cyclin E Overexpression Sensitizes Triple-Negative Breast Cancer to Wee1 Kinase Inhibition. Clin. Cancer Res. 2018;24:6594–6610. doi: 10.1158/1078-0432.CCR-18-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morgan K.M., Riedlinger G.M., Rosenfeld J., Ganesan S., Pine S.R. Patient-Derived Xenograft Models of Non-Small Cell Lung Cancer and Their Potential Utility in Personalized Medicine. Front. Oncol. 2017;7:2. doi: 10.3389/fonc.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott C.L., Becker M.A., Haluska P., Samimi G. Patient-derived xenograft models to improve targeted therapy in epithelial ovarian cancer treatment. Front. Oncol. 2013;3:295. doi: 10.3389/fonc.2013.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Annibali D., Leucci E., Hermans E., Amant F. Development of Patient-Derived Tumor Xenograft Models. Methods Mol. Biol. 2019;1862:217–225. doi: 10.1007/978-1-4939-8769-6_15. [DOI] [PubMed] [Google Scholar]
- 84.Moro M., Bertolini G., Caserini R., Borzi C., Boeri M., Fabbri A., Leone G., Gasparini P., Galeone C., Pelosi G., et al. Establishment of patient derived xenografts as functional testing of lung cancer aggressiveness. Sci. Rep. 2017;7:6689. doi: 10.1038/s41598-017-06912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshikawa T., Kobori G., Goto T., Akamatsu S., Terada N., Kobayashi T., Tanaka Y., Jung G., Kamba T., Ogawa O., et al. An original patient-derived xenograft of prostate cancer with cyst formation. Prostate. 2016;76:994–1003. doi: 10.1002/pros.23188. [DOI] [PubMed] [Google Scholar]
- 86.Bernardo C., Costa C., Sousa N., Amado F., Santos L. Patient-derived bladder cancer xenografts: A systematic review. Transl. Res. 2015;166:324–331. doi: 10.1016/j.trsl.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Li H., Wheeler S., Park Y., Ju Z., Thomas S.M., Fichera M., Egloff A.M., Lui V.W., Duvvuri U., Bauman J.E., et al. Proteomic Characterization of Head and Neck Cancer Patient-Derived Xenografts. Mol. Cancer Res. 2016;14:278–286. doi: 10.1158/1541-7786.MCR-15-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao H., Korn J.M., Ferretti S., Monahan J.E., Wang Y., Singh M., Zhang C., Schnell C., Yang G., Zhang Y., et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015;21:1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 89.Karamboulas C., Bruce J.P., Hope A.J., Meens J., Huang S.H., Erdmann N., Hyatt E., Pereira K., Goldstein D.P., Weinreb I., et al. Patient-Derived Xenografts for Prognostication and Personalized Treatment for Head and Neck Squamous Cell Carcinoma. Cell Rep. 2018;25:1318.e4–1331.e4. doi: 10.1016/j.celrep.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 90.Karamboulas C., Ailles L. Patient-derived xenografts: A promising resource for preclinical cancer research. Mol. Cell. Oncol. 2019;6:1558684. doi: 10.1080/23723556.2018.1558684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grunewald S., Politz O., Bender S., Heroult M., Lustig K., Thuss U., Kneip C., Kopitz C., Zopf D., Collin M.P., et al. Rogaratinib: A potent and selective pan-FGFR inhibitor with broad antitumor activity in FGFR-overexpressing preclinical cancer models. Int. J. Cancer. 2019 doi: 10.1002/ijc.32224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engebraaten O., Vollan H.K.M., Borresen-Dale A.L. Triple-negative breast cancer and the need for new therapeutic targets. Am. J. Pathol. 2013;183:1064–1074. doi: 10.1016/j.ajpath.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 93.Abramson V.G., Lehmann B.D., Ballinger T.J., Pietenpol J.A. Subtyping of triple-negative breast cancer: Implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lehmann B.D., Jovanovic B., Chen X., Estrada M.V., Johnson K.N., Shyr Y., Moses H.L., Sanders M.E., Pietenpol J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clohessy J.G., Pandolfi P.P. Mouse hospital and co-clinical trial project--from bench to bedside. Nat. Rev. Clin. Oncol. 2015;12:491–498. doi: 10.1038/nrclinonc.2015.62. [DOI] [PubMed] [Google Scholar]
- 96.Byrne A.T., Alferez D.G., Amant F., Annibali D., Arribas J., Biankin A.V., Bruna A., Budinska E., Caldas C., Chang D.K., et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer. 2017;17:254–268. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- 97.Gopinathan A., Morton J.P., Jodrell D.I., Sansom O.J. GEMMs as preclinical models for testing pancreatic cancer therapies. Dis. Model. Mech. 2015;8:1185–1200. doi: 10.1242/dmm.021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lunardi A., Pandolfi P.P. A co-clinical platform to accelerate cancer treatment optimization. Trends Mol. Med. 2015;21:1–5. doi: 10.1016/j.molmed.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dutta A., Panja S., Virk R.K., Kim J.Y., Zott R., Cremers S., Golombos D.M., Liu D., Mosquera J.M., Mostaghel E.A., et al. Co-clinical Analysis of a Genetically Engineered Mouse Model and Human Prostate Cancer Reveals Significance of NKX3.1 Expression for Response to 5alpha-reductase Inhibition. Eur. Urol. 2017;72:499–506. doi: 10.1016/j.eururo.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi J., Gyamfi J., Jang H., Koo J.S. The role of tumor-associated macrophage in breast cancer biology. Histol. Histopathol. 2018;33:133–145. doi: 10.14670/HH-11-916. [DOI] [PubMed] [Google Scholar]
- 102.Inoue T., Terada N., Kobayashi T., Ogawa O. Patient-derived xenografts as in vivo models for research in urological malignancies. Nat. Rev. Urol. 2017;14:267–283. doi: 10.1038/nrurol.2017.19. [DOI] [PubMed] [Google Scholar]
- 103.Bartucci M., Ferrari A.C., Kim I.Y., Ploss A., Yarmush M., Sabaawy H.E. Personalized Medicine Approaches in Prostate Cancer Employing Patient Derived 3D Organoids and Humanized Mice. Front. Cell Dev. Biol. 2016;4:64. doi: 10.3389/fcell.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hui L., Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015;368:7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 105.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cassidy J.W., Caldas C., Bruna A. Maintaining Tumor Heterogeneity in Patient-Derived Tumor Xenografts. Cancer Res. 2015;75:2963–2968. doi: 10.1158/0008-5472.CAN-15-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morton J.J., Bird G., Refaeli Y., Jimeno A. Humanized Mouse Xenograft Models: Narrowing the Tumor-Microenvironment Gap. Cancer Res. 2016;76:6153–6158. doi: 10.1158/0008-5472.CAN-16-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pearson T., Greiner D.L., Shultz L.D. Creation of “humanized” mice to study human immunity. Curr. Protoc. Immunol. 2008;81:15–21. doi: 10.1002/0471142735.im1521s81. [DOI] [PMC free article] [PubMed] [Google Scholar]