Abstract
Aflatoxins are mycotoxins that are produced by several species of filamentous fungi. In the European Union, the concentration limits for this group of mycotoxins in food and feed products are very low (on the order of parts per billion). Thus, relatively high amounts of these substances in their pure forms are required as reference standards. Chromatographic techniques based on solid stationary phases are generally used to purify these molecules; however, liquid–liquid chromatographic separations may be a promising alternative. Therefore, this study proposes a liquid–liquid chromatographic method for the separation of four aflatoxins and impurities. To optimise the method, numerous biphasic solvent systems (chloroform-, acetone- and acetic acid-based systems) were tested and evaluated in terms of their effectiveness at partitioning aflatoxins; the toluene/acetic acid/water (30:24:50, v/v/v/%) system was found to be the most efficient for application in centrifugal partition chromatographic instrument. Using liquid–liquid instrumental separation, the four aflatoxins, namely B1 (400 mg), B2 (34 mg), G1 (817 mg) and G2 (100 mg), were successfully isolated with 96.3%–98.2% purity from 4.5 L of Aspergillus parasiticus fermented material in a 250 mL centrifugal partition chromatography column. The identities and purities of the purified components were confirmed, and the performance parameters of each separation step and the whole procedure was determined. The developed method could be effectively used to purify aflatoxins for analytical applications.
Keywords: aflatoxin purification, centrifugal partition chromatography, ternary system, separation
1. Introduction
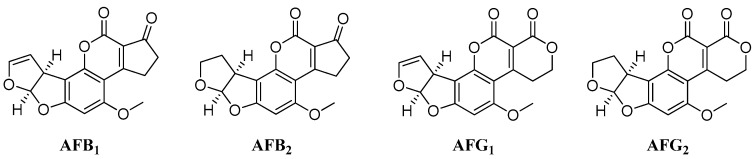
Aflatoxins (AFs) are mycotoxins produced as secondary metabolites mainly by two Aspergillus species, namely A. parasiticus and A. flavus. Four main AFs, B1, G1, B2 and G2 exist (Figure 1), all of which are toxic to both humans and animals [1,2,3]. Consuming food contaminated with AFs could lead to serious health problems, including acute hepatic necrosis, acute liver failure and liver cancer [4,5]. Among these compounds, AFB1 is the most toxic; it is associated with teratogenic, mutagenic and carcinogenic effects [6] and may cause lesions mainly in the liver [7] but also in other exposed organs systems [8]. Because of the dangerous nature of AFs, very low limits (on the order of parts per billion) for these compounds in various food products and commodities are set in the European Union and around the world; the maximum residual limits for total AF contamination range from 4 to 10 µg/kg [9].
Figure 1.
Structures of the four main aflatoxins.
Numerous methods for determining AF concentrations using various matrices have been proposed [10,11,12]. However, high performance liquid chromatography coupled with and ultraviolet detector (HPLC-UV) has also been used to determine AF concentrations in food products [13], the most popular technique is based on the use of a fluorescence detector regarding the existed fluorophores and additional derivatizations [14,15,16,17]. Furthermore, HPLC can be coupled with mass spectrometry (MS) [18] to be used as alternative methods, including HPLC-MS/MS [19], time of flight mass detector (HPLC-TOF) [20,21] and HPLC-Orbitrap MS [22,23,24,25]. Orbitrap MS technique is the most sensitive of these previously proposed methods [24].
The isolation and preparation of AFs were first reported by Sargeant et al. in 1961 [26]. Toxic extracts of Brazilian groundnuts were purified via column chromatography on alumina, yielding a nearly colorless crystalline material. This material was first referred to as AFs G and B by Nesbitt et al. in 1962, who purified the hot methanol extract of A. flavus on silica gel and further purified the AF mixture with a counter-current distribution to separate B and G groups of AFs [27]. One year later, the four main AFs (AFG1, AFG2, AFB1 and AFB2) were described for the first time and separated on silica gel with purities above 90%; moreover, the unsaturation of the furan ring in AFG1 and AFB1 and the saturation of the same ring in AFG2 and AFB2 were reported [28]. Later, Stubblefield et al. separated the crude mixture of AFs extracted from wheat and rice using a process of successive separations with a silica gel mesh as the stationary phase and chloroform/ethanol (99:1, v/v%), and chloroform/acetone/ethanol (97.25:2:0.75, v/v/v%) as eluents; the purity of each obtained AF was above 90% [29]. De Jesus et al. successfully purified three of the four AFs (AFB1, AFB2 and AFG1) from the solid culture of A. flavus extracted from both the media and the mycelia using consecutive normal-phase chromatography steps with different ratios of chloroform and acetone as the eluents; 1.09 g of AFB1, 360 mg of AFB2 and 2.1 of AFG1 were obtained from 21 g of crude broth and 9.7 g of crude mycelial extract [30].
Centrifugal partition chromatography (CPC) is an extensively studied technique but it is less well-known and not as commonly used as solid–liquid chromatography. In CPC, a solvent from a biphasic solvent system is immobilised by a centrifugal force in a stacked and interconnected rotating-discs (the stationary phase), while another phase (the mobile phase) is pumped through this channel and the components injected in a mixture into the mobile phase are eluted from the column according to their partition coefficients [31]. However, for the successful CPC separation, firstly the biphasic solvent system should be selected such that the sample components have different partition coefficients in a range of 0.5–2.0 in each phase [32]. CPC further allows for rapid and inexpensive method development, higher throughput, higher yields and reduced costs compared to typical preparative HPLC techniques [33]. Based on these unique advantages and the high resolution offered by CPC, this technique has been applied for the separation of numerous bioactive natural products including sesamin, catharanthine and vindoline [34,35,36,37,38] as well as certain mycotoxins [39,40,41,42,43]. For example, fumonisin B1 was separated from the crude extract of Fusarium verticillioides by CPC in a single 70-min run with a purity above 98% and total yield of 68% [39]. B-type fumonisins have also been purified by several consecutive separation steps with pentane/tert-butyl methyl ether/butan-1-ol/ethanol/1% formic acid in water and pentane/butan-1-ol/ethanol/1% formic acid in water as the solvent systems; as a result, a total of 500 mg of fumonisin B1, B2 and B3 was obtained with a purity above 98% from 1 kg of maize culture [40]. In another study, nivalenol and fusarenon-X were purified by passing a crude acetonitrile extract from Fusarium graminearum through silica gel then running two consecutive CPC separations: the first with water as the stationary phase and butanol as the mobile phase and the second with a chloroform/methanol/water (65:65:40, v/v/v%) solvent system; from 1 kg of pressed barley culture, 340 mg nivalenol and 600 mg fusarenon-X were obtained with yields of 44% and 68%, respectively [41]. Another liquid–liquid chromatographic separation with a 1:1 ethyl acetate/water solvent system (by volume) was used to purify deoxynivalenol from a rice culture of F. graminearum, resulting in 95% purity after several consecutive runs [42]. Similarly, a 7.5:2.5:10 hexane/ethyl acetate/pH 4 acetic acid solution (by volume) was used as the solvent system to purify the mycotoxin patulin produced by Penicillium expansum that was cultivated in sterilised apple juice and apple cider; the compound was purified with a maximum purity of 98.6% and a recovery of 86.2% [43].
In the present study, for the first time, CPC is applied to isolate all four AFs and completely separate them from impurities following extraction and liquid–liquid chromatographic separation procedures.
2. Results and Discussion
2.1. Cultivation and Extraction
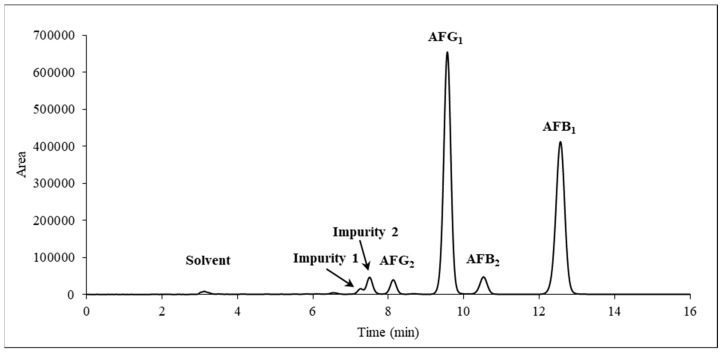
A. parasiticus SZMC 2473 strain, an ex-type strain of the species [44], was selected from Szeged Microbiology Collection (SZMC) for cultivation because it has been reported to produce both AFGs and AFBs in relatively high amounts [45]. After seven days of cultivation, the AFs were extracted in a three-step process using first dichloromethane was used, followed by a hexane/methanol/water ternary system [46] to remove the fat and other non-polar components produced by the fungus. At this stage AFs were distributed into the aqueous phase, which was partitioned again with dichloromethane. An HPLC-UV analysis (Figure 2) revealed that the A. parasiticus produced AFG1 (47.5%, Rt = 9.564 min) and AFB1 (42.6%, Rt = 12.560 min) as the major products and AFG2 (3.0%, Rt = 8.109 min) and AFB2 (4.2%, Rt = 10.523 min) in smaller quantities; the total impurity content was 2.7% (Rt = 7.255 min and Rt = 7.493 min).
Figure 2.
Chromatogram of the crude extract measured with high performance liquid chromatography coupled with an ultraviolet detector (HPLC-UV) at λ = 365 nm.
2.2. Selection of Solvent Systems
For the separation of AFs by liquid–liquid chromatography, several ternary systems, each comprising a “bridge” solvent was used in combination with more polar and less polar partitioning solvents, were created in accordance with the “best solvent” approach [47]. Here, one protic and two aprotic solvents were selected as the best solvents. To form a non-aqueous ternary system, chloroform was used as the best solvent and was paired with hexane and acetonitrile; to form an aqueous ternary system, acetone was used with either hexane and heptane or toluene and water (Table 1). Furthermore, the protic best solvent was acetic acid, which was used in combination with diethyl ether, chloroform or toluene as the non-polar solvent and water as the polar solvent to form a ternary system (Table 2). Thus, a total of 63 biphasic systems based on these seven compositions were investigated.
Table 1.
Tested ternary systems and the corresponding p-values.
| Solvent System | Volume Ratio | PImp1 | PImp2 | PAFG2 | PAFG1 | PAFB2 | PAFB1 | |
|---|---|---|---|---|---|---|---|---|
| 1 | hexane/chloroform/acetonitrile | 55:5.5:39.5 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| 2 | 55:3.8:41.2 | 0.15 | 0.13 | 0.14 | 0.15 | 0.14 | 0.15 | |
| 3 | 77.7:3.2:19.1 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 4 | 77.7:5:17.3 | 0.19 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 5 | 42:9:49 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 6 | 34:8:58 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 7 | 55:7:38 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 8 | 63:10:27 | <0.10 | <0.10 | <0.10 | <0.10 | 0.11 | <0.10 | |
| 9 | 62:9:29 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 10 | hexane/acetone/water | 36:39:25 | 0.14 | <0.10 | <0.10 | <0.10 | 0.14 | <0.10 |
| 11 | 10:50:40 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 12 | 9:39:52 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 13 | 15:60:25 | 0.14 | 0.13 | 0.19 | 0.24 | 0.26 | 0.29 | |
| 14 | 56:24:20 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 15 | 66:24:10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 16 | 50:40:10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 17 | 30:60:10 | <0.10 | <0.10 | <0.10 | 0.11 | 0.13 | 0.15 | |
| 18 | 40:50:10 | 0.16 | 0.14 | 0.23 | 0.24 | 0.29 | 0.32 | |
| 19 | 32:63:5 | 0.13 | 0.13 | 0.19 | 0.21 | 0.24 | 0.26 | |
| 20 | 23:77:10 | 0.53 | 0.61 | 0.62 | 0.64 | 0.66 | 0.68 | |
| 21 | 20:70:10 | <0.10 | <0.10 | 0.14 | 0.14 | 0.18 | 0.20 | |
| 22 | heptane/acetone/water | 44:29:27 | 0.17 | 0.15 | 0.16 | 0.19 | 0.18 | 0.20 |
| 23 | 8:65:27 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 24 | 27:57:16 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 25 | 30:60:10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 26 | 40:20:40 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 27 | 20:10:70 a | - | - | - | - | - | - | |
| 28 | 55:25:20 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 29 | 80.5:9.5:10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 30 | 23:57:20 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 31 | 22:38:40 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | |
| 32 | toluene/acetone/water | 39:26:35 | 1.81 | 2.98 | 5.77 | 9.80 | 11.28 | 22.67 |
| 33 | 14:26:60 | 2.16 | 3.42 | 6.26 | 9.11 | 11.58 | 18.53 | |
| 34 | 10:40:50 | 8.33 | 38.64 | 6.46 | 4.11 | 2.66 | 58.49 | |
| 35 | 14:51:35 | 6.59 | 3.26 | 2.80 | 3.58 | 3.39 | 5.14 | |
| 36 | 29:55:16 | 1.39 | 1.83 | 2.86 | 3.86 | 5.00 | 5.03 | |
| 37 | 30:10:60 | 0.87 | 2.04 | 4.21 | 9.03 | 22.75 | 19.06 | |
| 38 | 6:74:20 b | - | - | - | - | - | - | |
| 39 | 18:12:70 | 0.86 | 1.72 | 2.27 | 6.16 | 13.11 | 12.32 | |
| 40 | 12:67:21 | 1.21 | 1.26 | 1.40 | 1.51 | 1.53 | 1.60 | |
| 41 | 17:67:16 | 1.08 | 1.11 | 1.16 | 1.21 | 1.25 | 1.25 | |
| 42 | 8:67:25 | 1.21 | 1.26 | 1.43 | 1.63 | 1.67 | 1.75 | |
| 43 | 70:10:20 | 0.99 | 1.78 | 2.90 | 5.38 | 9.92 | 9.42 | |
| 44 | 35:55:10 | 1.36 | 1.64 | 2.46 | 3.24 | 4.13 | 4.00 | |
| 45 | 15:55:30 | 1.38 | 1.58 | 2.19 | 2.83 | 2.84 | 3.36 | |
| 46 | 6:55:39 | 1.40 | 1.62 | 2.20 | 2.84 | 3.02 | 3.41 |
a Could not dissolve the sample as it was too polar. b Did not form a biphasic system.
Table 2.
Tested ternary systems with acetic acid as the best solvent and the corresponding p-values.
| Solvent System | Volume Ratio | PImp1 | PImp2 | PAFG2 | PAFG1 | PAFB2 | PAFB1 | |
|---|---|---|---|---|---|---|---|---|
| 1 | diethyl ether/acetic acid/water | 75:5:20 | 0.70 | 0.86 | 1.67 | 1.75 | 3.40 | 3.43 |
| 2 | 60:20:20 | 0.26 | 0.32 | 0.46 | 0.53 | 0.66 | 4.00 | |
| 3 | 30:10:60 | 0.10 | 0.11 | 0.21 | 0.22 | 0.25 | 0.43 | |
| 4 | 45:15:40 | <0.10 | 0.15 | 0.19 | 0.20 | 0.33 | 0.41 | |
| 5 | chloroform/acetic acid/water | 26:24:50 | 0.42 | 0.37 | <0.10 | <0.10 | <0.10 | <0.10 |
| 6 | 36:34:30 | 0.47 | 0.93 | 0.18 | 0.16 | 0.11 | <0.10 | |
| 7 | 35:45:20 | 1.03 | 1.15 | 0.58 | 0.57 | 0.29 | 0.84 | |
| 8 | toluene/acetic acid/water | 80:6:14 | <0.10 | <0.10 | 0.31 | 0.62 | 1.56 | 1.88 |
| 9 | 20:10:70 | 0.14 | 0.43 | 0.73 | 1.70 | 3.18 | 4.57 | |
| 10 | 30:10:60 | <0.10 | 0.4 | 0.63 | 1.36 | 1.73 | 3.33 | |
| 11 | 20:20:60 | <0.10 | 0.3 | 0.3 | 0.63 | 0.94 | 2.65 | |
| 12 | 30:24:50 | 0.04 | 0.14 | 0.18 | 0.36 | 0.54 | 1.21 | |
| 13 | 20:30:50 | <0.10 | 0.12 | 0.16 | 0.28 | 0.56 | 0.92 | |
| 14 | 40:30:30 | <0.10 | <0.10 | <0.10 | 0.16 | 0.42 | 0.47 | |
| 15 | 63:30:7 | <0.10 | <0.10 | <0.10 | 0.10 | 0.23 | 0.20 | |
| 16 | 40:42:18 | <0.10 | 0.11 | 0.11 | <0.10 | 0.23 | 0.22 | |
| 17 | 20:55:25 | <0.10 | <0.10 | <0.10 | <0.10 | 0.31 | 0.21 |
2.2.1. Chloroform and Acetone as Best Solvents
The results with the ternary systems containing chloroform as the best solvent showed that the P value was below 0.1 in most systems, indicating that the examined components remained mainly in the lower, chloroform-rich phase (Table 1). The two-phase region in the ternary diagram shows that it is existed only in a narrow range of chloroform contents (1–12%); therefore, these tested ternary systems covered almost the entire two-phase region of the system [31]. Hence, it can be concluded that none of the examined components could have been transferred to the upper phase; therefore, this ternary system cannot be applied for the CPC separation of AFs.
The application of the next set of ternary systems based on acetone led to similar observations. In the systems containing hexane and heptane, most of p-values were below 0.1, indicating that the components remained in the aqueous (lower) phase (Table 1). Examining the hexane/acetone/water ternary systems, in certain cases, the p-values were closer to one, indicating the successful partitioning of the AFs.
Because none of the previous two ternary systems based on chloroform or acetone fulfilled to the requirements of the CPC application, an organic phase with higher polarity was needed, leading to the use of toluene. Using toluene as the third solvent the p-values were higher than one (Table 1), indicating that the AFs were mainly contained in the upper phase. In the biphasic systems containing more water than toluene, the AFs and the impurities were all transferred completely into the upper phase (Table 1). Furthermore, in the ternary system containing around 65% acetone, the p-values varied within narrow range around one and stagnated around it (Table 1); this was likely due to the shallower slopes of the tie lines in this ternary system [31]. When the amount of toluene was 70%, the AFs are concentrated mostly in the upper phase and the p-values were within the acceptable range (0.9–9.9).
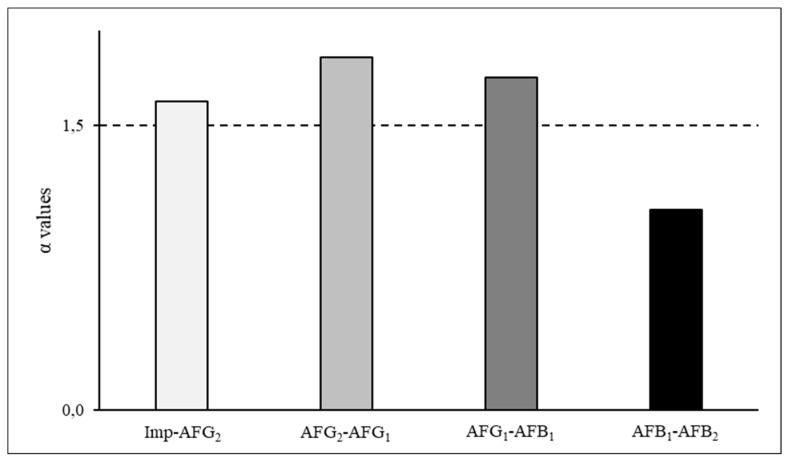
Furthermore, based on the quotients (α values) of the ordered p-values, the system composed of 70:10:20 toluene/acetone/water was able to separate the impurities from the AFs. Furthermore, using this system, AFG1 and AFG2 could be purified separately although the coelution of AFB1 and AFB2 could be expected during the CPC separation due to low α value (1.05) between these components (Figure 3).
Figure 3.
Separation factors of the components partitioned in the ternary system of 70:10:20 toluene/acetone/water. Imp: impurity; AFG1, AFG2, AFB1 and AFB2: Aflatoxins G1, G2, B1 and B2.
2.2.2. Acetic Acid as Best Solvent
Acetic acid was also tested as the best solvent because it has been shown to dissolve the components very well [48,49]. In addition to the acetic acid and water, diethyl ether, chloroform and toluene were tested as non-polar components (Table 2).
Of the diethyl ether/acetic acid/water systems, those with compositions of 30:10:60 and 45:15:40 were associated with low p-values for the AFs. The P value increased when the diethyl ether/water ratio was increased (e.g. to diethyl ether contents of 60% and 75%), but the calculated α values remained below 1.5. The above mentioned four tested compositions covered the full range of acetic acid percentages of the biphasic part of ternary diagram and due to the inappropriate partitions of the components obtained with diethyl ether, the non-polar component of the system was changed to chloroform. In these systems, the partition of the components shifted toward the lower (non-polar) phase with low p-values with low acetic acid concentrations (Table 2). As the tie lines of these ternary systems lean to the left, increasing the volumetric ratio of acetic acid is expected to cause its concentration in the polar phase to increase proportionally. Therefore, the acetic acid concentration was increased, causing the components to shift into the upper (polar) phase (Table 2) while maintaining α values under the acceptable limit for most component pairs. With the system containing 45% acetic acid, the components should theoretically (based on α values) be eluted in three groups: AFB2, AFG1+AFG2 and Imp1+Imp2+AFB1.
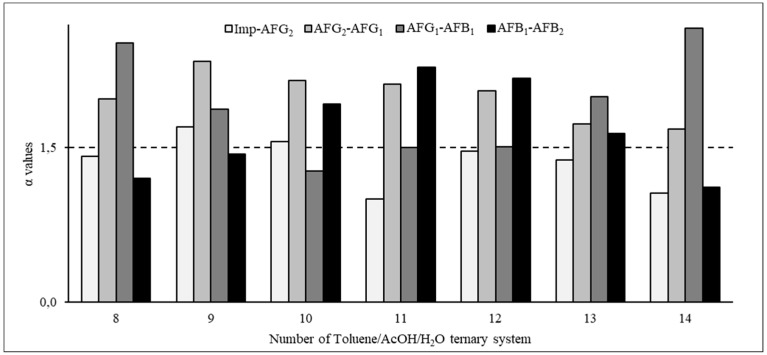
When the non-polar solvent was changed to toluene, the ratio of ascending p-values changed significantly (Table 2), indicating that the components tended to remain in the upper phase in these systems. The α values corresponding to these systems are shown in Figure 4. Based on these calculations, the system of 20:10:70 toluene/acetic acid/water (Table 2, system 9) could separate the AFs and the impurities but the range of the p-values was remarkably wide, necessitating a very long chromatographic run. When the toluene/acetic acid/water ratios were 20:20:60 (Table 2, system 11) and 20:30:50 (Table 2, system 13), the α values between the impurities and AFG2 were low; similarly, when this ratio was 40:30:30 (Table 2, system 14), the α value between AFB1 and AFB2 was not high enough (Table 2, Figure 4). However, when the toluene/acetic acid/water ratio was 30:24:50 (Table 2, system 12), the p-values were in the most suitable range (0.04 to 1.82), allowing for a CPC run that can be completed within an acceptable time and the α values were appropriate (above 1.5 for all AFs), indicating that perfect resolution can be expected (Table 2, Figure 4). Therefore, this system was selected as the optimal system for the final liquid–liquid chromatographic separation.
Figure 4.
Separation factors in the toluene/acetic acid/water ternary system.
2.3. Optimisation of the CPC Method
To determine the mode (ascendant or descendant) for the liquid–liquid separation, the elution volume (Ve) was calculated in each mode while using the optimal theoretical mobile phase/stationary phase ratio (20:80 by volume). The retention volumes for AFB1, AFB2, AFG1 and AFG2 were predicted as 159, 304, 426 and 842 mL, respectively in ascendant mode and 1605, 1045, 560 and 243 mL, respectively, in descendant mode. Hence, the lower phase was used as the stationary phase and the upper phase was used as the mobile phase in subsequent experiments, leading to a normal-phase-like separation.
To set up the instrument for the separation, the column was filled with the stationary phase at a rotor speed of 500 rpm and flow rate of 50 mL/min. Then, the mobile phase was pumped through the system at a flow rate of 10 mL/min with an initial rotor speed of 2200 rpm, which was decreased gradually according to the observed pressure, which should be below 100 bar. Only 10 mL of the stationary phase was extruded (4:96 by volume) when the rotor speed was decreased to 2000 rpm and approximately 25 mL was extruded after reaching a stable pressure when the rotor speed was decreased further to 1800 rpm. Hence, these parameters were subsequently used for the first chromatographic run.
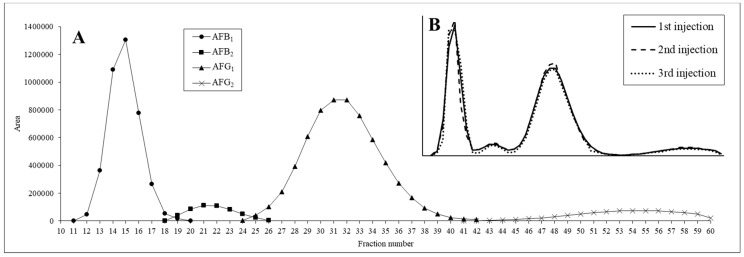
However, this separation process proved to be considerably long due to the high retention of AFG2, which was only eluted from the column after 120 min. To shorten the retention time of this slowly eluted component, the flow rate was increased to 15 mL/min in the final method. The pressure of this system was only 46 bar, which is far below the maximum limit (100 bar) and the extruded volume of the stationary phase increased to 60 mL (24 v%). 90 mg of same dried AF extract, as used for the solvent system selection, was dissolved in 4 mL of each the upper and lower phases and injected to the instrument; the sample was dissolved well to preserve the integrity of the liquid–liquid system. The pressure was stable throughout the run, indicating the stability of the selected biphasic system. Using this method, the four AFs and the impurities were separated successfully: the AFs were eluted completely from the column while the impurities were retained in the system during the 75 min run time and, thus, were not included in the fractogram (Figure 5A).
Figure 5.
Fractograms of (A) the optimized centrifugal partition chromatographic (CPC) run and (B) repeated CPC runs.
After confirming that the separation was successful, the stability and repeatability of the system were evaluated by repeating the same separation process three times with fresh eluents for both the stationary and mobile phases and the same amount of crude AF sample (90 mg). The fractograms of the three consecutive runs (Figure 5B) shows that the composition of the collected fractions during the separation are fit to each other and the relative standard deviations of the AF contents in corresponding fractions were under 10%. Thus, it was concluded that the proposed separation process is repeatable.
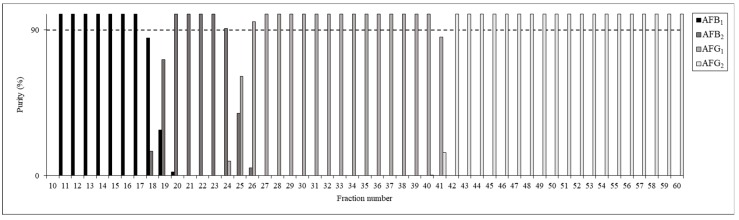
Sixty fractions were collected in total during each run, beginning immediately after the sample injection and proceeding until the 9 mL was eluted. The compositions of the fractions were evaluated by HPLC-UV (Figure 6); the results showed that most fractions had purities above 90%. Fractions 11–17 were pooled to obtain the AFB1 sample (24 mg), fractions 21–23 were pooled for the AFB2 (2 mg), fractions 27–40 were pooled for the AFG1 (49 mg) and fractions 44–59 were pooled for AFG2 (6 mg). The rest of the fractions containing mixtures of more than one AF were then pooled, neutralized and evaporated to dryness for further analyses and purification. For example, AFB2-rich fractions 18–20 and 24–26 contained 3 mg AFB2 as well as 2 mg AFB1 and 1 mg AFG1.
Figure 6.
Composition and purity (%) of the fractions obtained in the optimized CPC run.
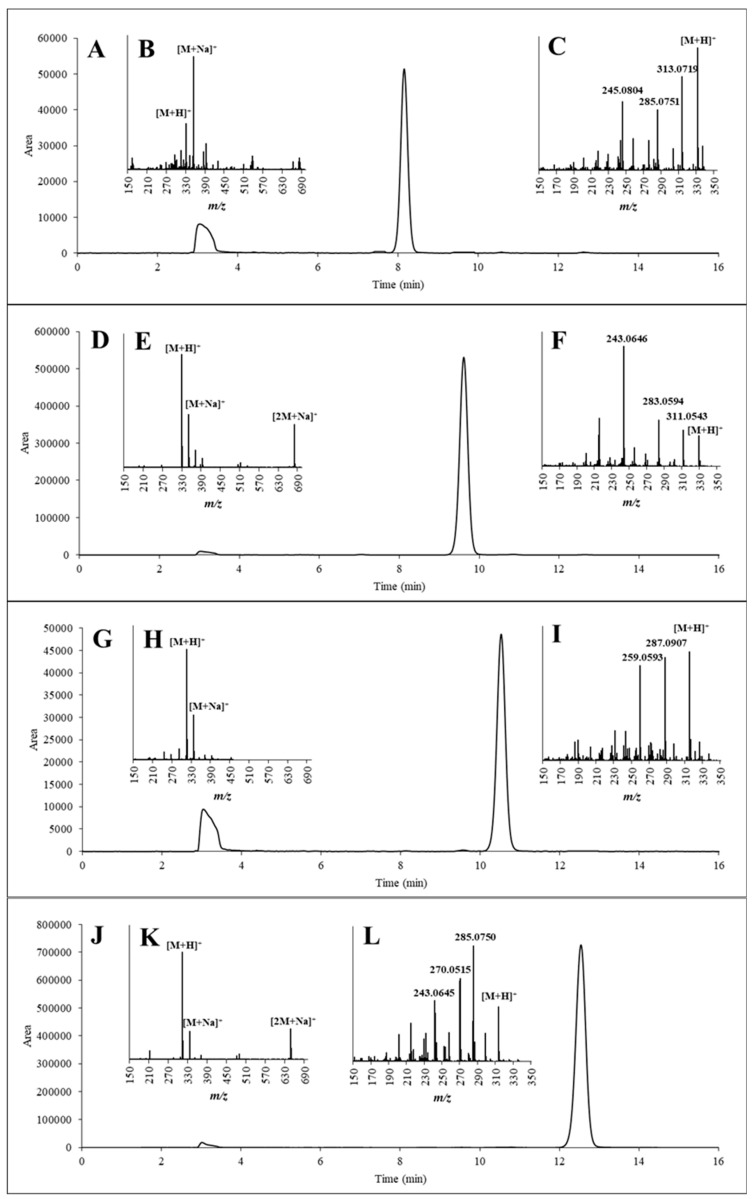
2.4. HPLC-UV and HR-MS to Verify Identity and Determine Purity
To verify the structures of the purified compounds, each sample of them was injected into an HPLC-UV and into an UHPLC-QExactive system containing both quadrupole and orbitrap analysers via flow injection. According to the HPLC-UV chromatograms (Figure 7A,D,G,J), none of the separated AFs contained major impurities and the retention times for all AFs were consistent with those of the reference standards. The adduct ions (the [M + H]+ and [M + Na]+ in all samples and [2M + Na]+ in AFB1 and AFG1) were detected in the full-scan measurements (Figure 7B,E,H,K). Further, after the fragmentation of [M + H]+ as a precursor ion, the three most intense fragment ions were selected to confirm the identities in the MS2 evaluations (Figure 7C,F,I,L).
Figure 7.
HPLC-UV chromatograms of the purified AFG2 (A), AFG1 (D), AFB2 (G) and AFB1 (J); Mass spectra of the purified AFG2 (B), AFG1 (E), AFB2 (H) and AFB1 (K); and MS2 spectra of the [M + H]+ adduct ions of the purified AFG2 (C), AFG1 (F), AFB2 (I) and AFB1 (L).
The MS and MS2 spectra of the purified AFs were compared with those of the corresponding AF standards. The results, which are summarized in Table 3, show that the measured mass values were consistent with the m/z values of the reference compounds with a maximum deviation of only 2.55 ppm. Furthermore, the ratios between the fragment ions were only slightly different (differences were within the range of 0–5%) compared with (Table 3).
Table 3.
Comparisons of the masses and intensity ratios of the detected ion forms with those of the reference standards by via full-scan and MS2 high-resolution mass spectrometry to confirm the identities of the AFs.
| AFB1 | AFB2 | AFG1 | AFG2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
c Ref. (m/z) |
d Mass dev. (ppm) |
e Ratio dev. (%) |
c Ref. (m/z) |
d Mass dev. (ppm) |
e Ratio dev. (%) |
c Ref. (m/z) |
d Mass dev. (ppm) |
e Ratio dev. (%) |
c Ref. (m/z) |
d Mass dev. (ppm) |
e Ratio dev. (%) |
|
| a Full scan | 313.0699 | 1.25 | 0 | 315.0849 | 0.99 | 0 | 329.0646 | 1.43 | 0 | 331.0811 | 1.17 | 0 |
| 335.0504 | 1.56 | 0 | 337.0669 | 1.18 | 0 | 351.0464 | 1.26 | 0 | 353.0631 | 1.66 | 0 | |
| 647.1142 | 2.02 | 0 | - | - | - | 679.1039 | 2.54 | 0 | - | - | - | |
| b MS2 | 285.0750 | 1.99 | 5 | 287.0907 | 1.37 | 1 | 311.0543 | 2.57 | 1 | 313.0719 | 2.03 | 0 |
| 270.0515 | 1.04 | 3 | 259.0593 | 1.18 | 2 | 283.0594 | 1.79 | 3 | 285.0751 | 1.98 | 1 | |
| 243.0645 | 2.30 | 1 | - | - | - | 243.0646 | 2.01 | 4 | 245.0804 | 2.13 | 1 | |
a The full-scan m/z values are consistent with the molecular ion adducts including [M + H]+, [M + Na]+ and [2M + Na]+. b The presented product ions originated from the fragmentation of the protonated molecular ions. c The m/z values of the reference standards. d Mass deviation of the purified compounds from the corresponding ion reference standards determined with the high-resolution mass spectrometer. e Differences between the ratios of the formed ion intensities measured in the reference standards and the purified components.
To test the purity of the separated and fractionated AFs, MS was also applied due to its high sensitivity. Hence, even considering the presence of the [M + H]+ molecular ions of AFs other than the purified one, the m/z values remained under 0.05% in all cases.
2.5. Yield of the Entire Purification Procedure
From the 4.5 L of liquid culture of A. parasiticus SZMC 2473 strain, a total of 1351 mg of AFs was obtained by the proposed CPC method including 442 mg of AFB1, 43 mg of AFB2, 817 mg of AFG1 and 100 mg of AFG2 with purities of 98.2%, 96.3%, 98.1% and 97.0%, respectively (Table 4). At these yields, the recovery rates from the entire procedure were 90.5%, 85.3%, 98.7% and 96.0% for AFB1, AFB2, AFG1 and AFG2, respectively, based on the original concentrations in the crude extracts as measured by HPLC-UV. Furthermore, the overall AF recovery rate was 92.6% (Table 4), indicating that the applied extraction steps did not cause any remarkable loss of AFs.
Table 4.
Purification efficiencies of the AFs from A. parasiticus liquid culture in each separation step.
| AFB1 | AFB2 | AFG1 | AFG2 | Total AFs | ||
|---|---|---|---|---|---|---|
| Crude extract (culture medium → dichloromethane) |
Yield (mg) | 442 | 40 | 827 | 105 | 1414 |
| Purity (%) | 39 | 4.0 | 45.4 | 2.9 | 91.3 | |
| Second extract (dichloromethane → hexane/methanol/water) |
Yield (mg) | 442 | 40 | 827 | 105 | 1414 |
| Purity (%) | 41.7 | 4.0 | 46.9 | 3.0 | 95.6 | |
| Recovery (%) | 100 | 100 | 100 | 100 | 100 | |
| Third extract (hexane/methanol/water → dichloromethane) |
Yield (mg) | 442 | 40 | 827 | 105 | 14141 |
| Purity (%) | 42.6 | 4.2 | 47.5 | 3.0 | 97.3 | |
| Recovery (%) | 100 | 100 | 100 | 100 | 100 | |
| CPC separation (final product) (30:24:50 toluene/acetic acid/water) |
Yield (mg) | 400 | 34 | 817 | 100 | 1351 |
| Purity (%) | 98.2 | 96.3 | 98.1 | 97.0 | 97.3 | |
| Recovery (%) | 90.5 | 85.3 | 98.7 | 96.0 | 92.6 | |
| Whole procedure | Recovery (%) | 90.5 | 85.3 | 98.7 | 96.0 | 92.6 |
Note: AFB1, AFB2, AFG1 and AFG2: aflatoxins B1, B2, G1 and G2; Total AF: sum of the amounts of the four detected AFs; CPC: centrifugal partition chromatography.
3. Conclusions
In this study, we developed an effective liquid–liquid chromatography method to separate the four AFs (B1, B2, G1 and G2) produced by a filamentous fungus, A. parasiticus. After evaluating many systems, the ternary system of 30:24:50 toluene/acetic acid/water was shown to provide an ideal range of p-values and an acceptable resolution for instrumental applications. Using this biphasic system, successful separation was achieved by injecting 90 mg of crude extract onto a 250 mL CPC column. Hence, approximately 1.5 g crude AF mixture could be extracted from 4.5 L ferment broth in one 75 min CPC run, yielding a total of 90 mg of crude extract. The total AF yield of the whole procedure was 92% based on the initial total AF concentration in the crude extract. The resulting recovery rates and purities were found to be sufficiently high to allow for cost-effective reference standard preparations, which is essential for the precise analytical measurements of food and feed products and keeping their mycotoxin contents within tolerable ranges.
4. Materials and Methods
4.1. Chemicals and Solvents
The following microbiological media components were used: Bacto malt extract was purchased from Becton Dickinson (Környe, Hungary) and yeast extract, NaCl and sucrose were purchased from Molar Chemicals (Halásztelek, Hungary). All solvents used for extraction were purchased from Molar Chemicals Hungary and were of analytical grade. The solvents used for the various solvent systems and HPLC-UV measurements were of gradient grade or super gradient grade and were purchased from VWR International (Debrecen, Hungary). The AF standard mixture (including AFB1, AFG1, AFB2 and AFG2) was purchased from Sigma-Aldrich (Budapest, Hungary).
4.2. The Producer Strain and Culture Conditions
Aspergillus parasiticus strain SZMC 2473 (CBS 260.67; GenBank Accession number for ITS: MG662400) was originally isolated in Japan and is the ex-type of the species [44]. To cultivate the strain, 500 mL liquid medium was prepared in 1.2 L Roux-flasks (30 g Bacto malt extract, 20 g yeast extract, 200 g sucrose in 1 L distilled water). The flasks were capped with a cotton cork and sterilised in an autoclave for 30 min at 115 °C. Each flask was inoculated with a 5 mL conidial suspension prepared in a sterilised saline solution and incubated in a horizontal position in the dark at 25 °C for 7 days.
4.3. Aflatoxin Extraction
AFs were extracted from fermented broth in four steps: First, extraction was performed on one litre of broth with 500 mL followed by 250 mL of dichloromethane. The organic phases were combined and evaporated to water; the volume of aqueous residue was then measured. Next, methanol and hexane were added such that the water/methanol/hexane ratio was 45:50:120 (by volume). After the phases were separated, the upper phase was removed and further extraction was performed on that phase with water/methanol (45:50 by volume). The separated water/methanol phases were then combined and dichloromethane was added until two phases formed and extracted in two repetitions. The combined organic phases were dried over MgSO4, membrane filtered and evaporated to dryness.
4.4. Testing of the Solvent Systems
The dried crude extract was dissolved in dichloromethane at a concentration of 1.8 mg/mL and split into 1-mL aliquots; the dichloromethane was then evaporated. The constituents of the solvent systems to be tested (Table 1 and Table 2) were mixed in tubes and vortexed for 10 s. When the phases were let to separate and 500 µL from both phases was added to the previously aliquoted and dried crude extract. After the phase-separation 300 µL of each phase was transferred into new vials and evaporated to dryness and subsequently dissolved in 500 µL of acetic acid prior to HPLC injection.
4.5. HPLC-UV Measurements
Analyses were performed using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) equipped with a DGU-14A degasser, an LC-20AD binary pump, a SIL-20A autosampler, a CTO-10ASvp column thermostat, an SPD-10Avp UV-VIS detector and a CBM-20A system controller. Class VP ver. 6.2 software was used for data acquisition and evaluation. The separation of AFs was performed on an injected sample volume of 5 µL for 16 min in a Phenomenex Gemini C18, 250 mm × 4.6 mm, 5-µm column (Phenomenex, California, USA) with the mobile phase comprising water (A) and a 1:1 mixture of methanol and acetonitrile (B) combined in an A/B ratio of 60:40 by volume. The flow rate was 1 mL/min and the column temperature was maintained at 40 °C. The peaks of the AFs and the impurities were detected at λ = 365 nm. This method was also applied for the purity determination of the fractions and the final products.
4.6. Evaluation of the Separation
The partition coefficient (P) of each compound was calculated by dividing the area under the peaks detected in the upper and lower phases. The separation factor (α) for each solvent system was calculated by dividing the greatest P value by the smallest one arranged in the ascending series. The elution volume (Ve) was calculated as follows: Ve = VS + P(VC − VS), where VS is the volume of the stationary phase and VC is the volume of the column.
4.7. Centrifugal Partition Chromatography
Preparative-scale separations were performed using a laboratory-scale 250 mL CPC column (Gilson, Saint-Ave, France) coupled with a PLC 2250 flash/prep hybrid instrument (Gilson, Saint-Ave, France) containing an UV/Vis detector, fraction collector, electronically actuated injector valve with a 10 mL sample loop and an electronically actuated four-way two-position ascendant/descendant valve. Gilson Glider Prep (Ver. 5.1) software (Gilson, Saint-Ave, France) was used to control the instrument and acquire data.
Before the instrumental analysis, the selected system was prepared in a 5 L separation funnel and shaken to saturate the phases. The phases were then separated and transferred into glass bottles. At the beginning of the instrumental analysis, the column was filled with the stationary phase. Then the mobile phase was pumped with decreased flow through the column until the system reached to hydrodynamic equilibrium. The volume of the extruded stationary phase was measured using a graduated cylinder and the ratio between the volumes of phases was calculated.
The final optimised chromatographic run was carried out in ascendant mode at 1800 rpm with a flow rate of 15 mL/min for the biphasic system comprising toluene/acetic acid/water in a volumetric ratio of 30:24:50. The separation was carried out for 75 min and 20 mL fractions were collected during the whole run, while the UV detector was set to 366 nm. The pH of each fraction fractions was neutralised by adding a saturated NaHCO3 solution. The AF content in each fraction was measured by HPLC-UV. The fractions containing pure AFs (> 95%) were pooled and the solvent was evaporated. The resulting white powder containing the four AFs was used for subsequent analyses.
4.8. HR-MS Analyses
The identities of the purified AFs were analysed using a Dionex Ultimate 3000 UHPLC system (Thermo Fischer Scientific, Waltham, USA) coupled with a Q-Exacitve Focus Orbitrap mass spectrometer (Thermo Fischer Scientific, Waltham, Massachusetts, USA) using flow injection with an isocratic eluent (20:80 water/methanol mixture by volume with 0.1% acetic acid). The capillary temperature of the heated electrospray interface (HESI) and the heater temperature were set to 250 °C. The sheath gas flow rate was 30 a.u. and the auxiliary gas flow rate was 15 a.u. The capillary voltage was set to 4.5 kV. To achieve the fragmentation of the examined molecules, the HESI capillary and the auxiliary gas were heated to 350 °C, the normalised collision energy in the collision cell of the instrument was set to 70 a.u. and N2 was used as the collision gas. From the purified AF solution solved in acetic acid (100 µg/mL), 5 µL was injected for analysis.
Author Contributions
Conceptualization, C.V. and A.S.; methodology, G.E. and Z.H.; software, G.E. and Z.H.; formal analysis, B.Š; investigation, G.E., Z.H. and A.T.; writing—original draft preparation, G.E.; writing—review and editing, C.V. and A.S.; supervision, A.S.; funding acquisition, C.V. and A.S. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This work was supported by GINOP-2.3.2-15-2016-00012 and by OTKA K115690. The infrastructural background was established with the support of GINOP-2.3.3-15-2016-00006 grant. BS participation was supported through the bilateral project TÉT_16-1-2016-0148.
Conflicts of Interest
The authors declare no conflicts of interest.
Key Contribution
Liquid–liquid extraction of aflatoxins from liquid cultures of Aspergillus parasiticus was optimised. A biphasic system to successfully partition all four aflatoxins was developed. This study included a demonstration of the first effective application of centrifugal partition chromatography for aflatoxin purification.
References
- 1.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaton D.L., Gallagher E.P. Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- 3.Varga J., Baranyi N., Chandrasekaran M., Vágvölgyi Cs., Kocsubé S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015;59:151–167. [Google Scholar]
- 4.Nakai V.K., Rocha L.O., Goncalez E., Fonseca H., Ortega E.M.M., Corrêa B. Distribution of fungi and aflatoxins in a stored peanut variety. Food Chem. 2008;106:285–290. doi: 10.1016/j.foodchem.2007.05.087. [DOI] [Google Scholar]
- 5.Rustom I.Y.S. Aflatoxin in food and feed: Occurrence, legislation and inactivation by physical methods. Food Chem. 1997;59:57–67. doi: 10.1016/S0308-8146(96)00096-9. [DOI] [Google Scholar]
- 6.Calderari T.O., Iamanaka B.T., Frisvad J.C., Pitt J.I., Sartori D., Pereira J.L., Fungaro M.H., Taniwaki M.H. The biodiversity of Aspergillus section Flavi in brazil nuts: From rainforest to consumer. Int. J. Food Microbiol. 2013;160:267–272. doi: 10.1016/j.ijfoodmicro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Johnsson P., Linbald M., Thim M., Johnsson N., Vargas E.A., Medeiros N.L., Brabet C., Quaresma M., Olsen M. Growht of aflatoxigenic moulds and aflatoxin formation in Brazil nuts. World Mycotoxin J. 2008;1:127–137. doi: 10.3920/WMJ2008.1033. [DOI] [Google Scholar]
- 8.Bakırdere S., Bora S., Bakırdere E.G., Aydın F., Arslan Y., Komesli O.T., Aydın I., Yıldırım E. Aflatoxin species: Their health effects and determination methods in different foodstuffs. Cent. Eur. J. Chem. 2012;10:675–685. doi: 10.2478/s11532-012-0009-2. [DOI] [Google Scholar]
- 9.Alexander J., Benford D., Cockburn A., Cravedi J.P., Dogliotti E., Dmenico A.D. Effects on public health of an increase of the levels for aflatoxin total form 4 µg/kg to 10 µg/kg for tree nuts other than almonds, hazelnuts and pistachios. J. EFSA. 2009;1168:1–11. [Google Scholar]
- 10.Hepsag F., Golge O., Kabak B. Quantitation of aflatoxins in pistachios and groundnuts using HPLC-FLD method. Food Control. 2014;38:75–81. doi: 10.1016/j.foodcont.2013.10.005. [DOI] [Google Scholar]
- 11.Golge O., Hepsag F., Kabak B. Determination of aflatoxins in walnut sujuk and Turkish delight by HPLC-FLD method. Food Control. 2016;59:731–736. doi: 10.1016/j.foodcont.2015.06.035. [DOI] [Google Scholar]
- 12.Roma A.D., Rossini C., Riteni A. A survey on the aflatoxin M1 occurrence and seasonal variation in buffalo and cow milk from southern Italy. Food Control. 2017;81:30–33. doi: 10.1016/j.foodcont.2017.05.034. [DOI] [Google Scholar]
- 13.Herzallah S.M. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009;114:1141–1146. doi: 10.1016/j.foodchem.2008.10.077. [DOI] [Google Scholar]
- 14.Campos W.E.O., Rosas L.B., Neto A.P. Extended validation of a sensitive and robust method for simultaneous quantification of aflatoxins B1, B2, G1 and G2 in Brazil nuts by HPLC-FLD. J. Food Compos. Anal. 2017;60:90–96. doi: 10.1016/j.jfca.2017.03.014. [DOI] [Google Scholar]
- 15.Kabak B. Aflatoxins in hazelnuts and dried figs: Occurrence and exposure assessment. Food Chem. 2016;211:8–16. doi: 10.1016/j.foodchem.2016.04.141. [DOI] [PubMed] [Google Scholar]
- 16.Shiran A.Y., Tan G.H., Wong R.C.S. Method validation in the determination of aflatoxins in noodle samples using the QuEChERS method (Quick, Easy, Cheap, Effective, Rugged and Safe) and high performance liquid chromatography coupled to a fluorescence detector (HPLC-FLD) Food Control. 2011;22:1807–1813. [Google Scholar]
- 17.Kong W., Wei R., Logrieco A.F. Occurrence of toxigenic fungi and determination of mycotoxins by HPLC-FLD in functional foods and species in China markets. Food Chem. 2014;146:320–326. doi: 10.1016/j.foodchem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Saldan N.C., Almeida R.T.R., Avíncola A. Development for an analytical method for identification of Aspergillus flavus based on chemical markers using HPLC-MS. Food Chem. 2018;241:113–121. doi: 10.1016/j.foodchem.2017.08.065. [DOI] [PubMed] [Google Scholar]
- 19.Beltrán E., Ibáñez M., Sancho J.V. UHPLC-MS/MS highly sensitive determination of aflatoxins, the aflatoxin metabolite M1 and ochratoxin A in baby food and milk. Food Chem. 2011;126:737–744. doi: 10.1016/j.foodchem.2010.11.056. [DOI] [Google Scholar]
- 20.Catharino R.R., Marques L.A., Santos L.S. Aflatoxin screening my MALDI-TOF mass spectrometry. Anal. Chem. 2005;77:8155–8157. doi: 10.1021/ac051111p. [DOI] [PubMed] [Google Scholar]
- 21.Luo X., Wang R., Wang L. Analyses by UPLC Q-TOF MS of products of aflatoxin B1 after ozone treatment. Food Addit. Contam. Part A. 2014;31:105–110. doi: 10.1080/19440049.2013.853323. [DOI] [PubMed] [Google Scholar]
- 22.Mao J., Zheng N., Wen F. Multi-mycotoxin analysis in raw milk by ultra high performance liquid chromatography coupled to quadrupole orbitrap mass spectrometry. Food Control. 2018;84:305–311. doi: 10.1016/j.foodcont.2017.08.009. [DOI] [Google Scholar]
- 23.Martínez-Domínguez G., Romero-González R., Frenich A.G. Multi-class methodology to determine pesticides and mycotoxins in green tea and royal jelly supplements by liquid chromatography coupled to Orbitrap high resolution mass spectrometry. Food Chem. 2016;197:907–915. doi: 10.1016/j.foodchem.2015.11.070. [DOI] [PubMed] [Google Scholar]
- 24.Reinholds I., Pugajeva I., Bartkevics V. A reliable screening of mycotoxins and pesticide residues in paprika using ultra high performance liquid chromatography coupled to high resolution Orbitrap mass spectrometry. Food Control. 2016;60:683–689. doi: 10.1016/j.foodcont.2015.09.008. [DOI] [Google Scholar]
- 25.Jia W., Chu X., Ling Y. Multi-mycotoxin analysis in dairy products by liquid chromatography coupled to quadrupole orbitrap mass spectrometry. J. Chromatogr. A. 2014;1345:107–114. doi: 10.1016/j.chroma.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Sargenat K., Sheridan A., O’Kelly J. Toxicity associated with certain samples of groundnuts. Nature. 1961;192:1096–1097. doi: 10.1038/1921096a0. [DOI] [Google Scholar]
- 27.Nesbitt B.F., O’Kelly J., Sargeant K. Aspergillus flavus and Turkey X disease: Toxic metabolites of Aspergillus flavus. Nature. 1962;195:1062–1063. doi: 10.1038/1951062a0. [DOI] [PubMed] [Google Scholar]
- 28.Hartley R.D., Nesbitt B.F., O’Kelly J. Toxic metabolites of Aspergillus flavus. Nature. 1963;198:1056–1058. doi: 10.1038/1981056a0. [DOI] [PubMed] [Google Scholar]
- 29.Stubblefield R.D., Shotwell O.L., Shannon G.M. Aflatoxins B1, B2, G1 and G2: Separation and purification. J. Am. Oil Chem. Soc. 1968;45:686–688. doi: 10.1007/BF02541258. [DOI] [PubMed] [Google Scholar]
- 30.De Jesus A.E., Gorst-Allman C.P., Horak R.M. Large-scale purification of the mycotoxins aflatoxin B1, B2 and G1. J. Chromatogr. 1988;450:101–104. doi: 10.1016/S0021-9673(00)90719-2. [DOI] [PubMed] [Google Scholar]
- 31.Foucault A.P. Centrifugal Partition Chromatography. Marcel Dekker; New York, NY, USA: 1995. [Google Scholar]
- 32.Friesen J.B., Pauli G.F. G.U.E.S.S.—A generally useful estimate of solvent systems for CCC. J. Liq. Chrom. Relat. Technol. 2005;28:2777–2806. doi: 10.1080/10826070500225234. [DOI] [Google Scholar]
- 33.DeAmicis C., Edwards N.A., Giles M.B. Comparison of preparative reversed phase liquid chromatography and countercurrent chromatography for the kilogram scale purification of crude spinetoram insecticide. J. Chromatogr. A. 2011;1218:6122–6127. doi: 10.1016/j.chroma.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 34.Jeon J.S., Park C.L., Syed A.S. Preparative separation of sesamin and sesamolin from defatted sesame meal via centrifugal partition chromatography with consecutive sample injection. J. Chromatogr. B. 2016;1011:108–113. doi: 10.1016/j.jchromb.2015.12.062. [DOI] [PubMed] [Google Scholar]
- 35.Kotland A., Chollet S., Diard C. Industrial case study on alkaloids purification by pH-zone refining centrifugal partition chromatography. J. Chromatogr. A. 2016;1474:59–70. doi: 10.1016/j.chroma.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 36.Lee J.H., Ko J.Y., Oh J.Y. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014;158:433–437. doi: 10.1016/j.foodchem.2014.02.112. [DOI] [PubMed] [Google Scholar]
- 37.Amarouche N., Boudesocque L., Sayagh C. Purification of a modified cyclosporine A by co-current centrifugal partition chromatography: Process development and intensification. J. Chromatogr. A. 2013;1311:72–78. doi: 10.1016/j.chroma.2013.08.053. [DOI] [PubMed] [Google Scholar]
- 38.Bouju E., Berthold A., Faure K. Carnosol purification. Scaling up centrifugal partition chromatography purifications. J. Chromatogr. A. 2016;1466:59–66. doi: 10.1016/j.chroma.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Szekeres A., Lorántfy L., Bencsik O. Rapid purification method for fumonisin B1 using centrifugal partition chromatography. Food Addit. Contam. Part A. 2013;30:147–155. doi: 10.1080/19440049.2012.729161. [DOI] [PubMed] [Google Scholar]
- 40.Hübner F., Harrer H., Fraske A. Large scale purification of B-type fumonisins using centrifugal partition chromatography (CPC) Mycotoxin Res. 2012;28:37–43. doi: 10.1007/s12550-011-0114-7. [DOI] [PubMed] [Google Scholar]
- 41.Onji Y., Aoki Y., Yamazoe Y. Isolation of nivalenol and fusarenon-x from pressed barley culture by centrifugal partition chromatography. J. Liq. Chromatogr. 1988;12:2537–2546. doi: 10.1080/01483918808076745. [DOI] [Google Scholar]
- 42.He J., Yang R., Zhou T. Purification of deoxynivalenol from Fusarium graminearum rice culture and mouldy corn by high-speed countercurrent chromatography. J. Chromatogr. A. 2007;1151:187–192. doi: 10.1016/j.chroma.2007.01.112. [DOI] [PubMed] [Google Scholar]
- 43.He J., Tsao R., Yang R. Purification of patulin from Penicillim expansum culture: High-speed counter-current chromatography (HSCCC) versus preparative high-performance liquid chromatography (prep-HPLC) Food Addit. Contam. Part A. 2009;29:101–107. doi: 10.1080/02652030802273148. [DOI] [PubMed] [Google Scholar]
- 44.Frisvad J.C., Hubka V., Ezekiel C.N. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019;93:1–63. doi: 10.1016/j.simyco.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gömöri Cs., Nacsa-Farkas E., Kerekes E.B. Effect of essential oil vapours on aflatoxin production of Aspergillus parasiticus. World Mycotoxin J. 2018;11:579–588. doi: 10.3920/WMJ2017.2260. [DOI] [Google Scholar]
- 46.Shih C.N., Marth E.H. A procedure for rapid recovery of aflatoxins from cheese and other foods. J. Food Prot. 1971;34:119–123. doi: 10.4315/0022-2747-34.3.119. [DOI] [Google Scholar]
- 47.Foucault A.P., Chevolot L. Counter-current chromatography: Instrumentation, solvent selection and some recent applications to natural product purification. J. Chromatogr. A. 1998;808:3–22. doi: 10.1016/S0021-9673(98)00121-6. [DOI] [Google Scholar]
- 48.Garcia M., Blanco J.L., Suarez G. Aflatoxins B1 and G1 solubility in standard solutions and stability during storage. Mycotoxin Res. 1994;10:97–100. doi: 10.1007/BF03192258. [DOI] [PubMed] [Google Scholar]
- 49.Beaver R.W. Degradation of Aflatoxins in common HPLC solvents. J. High Res. Chromatogr. 1990;13:833–835. doi: 10.1002/jhrc.1240131208. [DOI] [Google Scholar]