Abstract
Reading is a learned skill crucial for educational attainment. Children from families of lower socioeconomic status (SES) tend to have poorer reading performance and this gap widens across years of schooling. Reading relies on the orchestration of multiple neural systems integrated via specific white‐matter pathways, but there is limited understanding about whether these pathways relate differentially to reading performance depending on SES background. Kindergarten white‐matter FA and second‐grade reading outcomes were investigated in an SES‐diverse sample of 125 children. The three left‐hemisphere white‐matter tracts most associated with reading, and their right‐hemisphere homologs, were examined: arcuate fasciculus (AF), superior longitudinal fasciculus (SLF), and inferior longitudinal fasciculus (ILF). There was a significant and positive association between SES and fractional anisotropy (FA) in the bilateral ILF in kindergarten. SES moderated the association between kindergarten ILF and second grade reading performance, such that it was positive in lower‐SES children, but not significant in higher‐SES children. These results have implications for understanding the role of the environment in the development of the neural pathways that support reading.
Keywords: SES, fractional anisotropy, brain, pre‐readers, reading development, longitudinal, education, MRI, white matter
1. INTRODUCTION
Reading is a learned skill crucial for successful educational attainment. There are well‐documented socioeconomic status (SES) disparities in reading achievement across development (Peterson & Pennington, 2015; Reardon, 2011). Neural specialization for reading is experientially driven and occurs through utilizing and repurposing distributed brain structures that support vision, audition, and language (Dehaene, 2009). The efficient integration across these spatially disparate brain regions is made possible by long‐range white matter connections that form across development (Wandell, Rauschecker, & Yeatman, 2012). Three white matter tracts in particular have a documented association with reading and reading‐related skills in adults and children as early as preschool (1) arcuate fasciculus (AF), connecting the superior temporal lobe with the inferior frontal gyrus (IFG); (2) superior longitudinal fasciculus (SLF), connecting the inferior parietal with the inferior frontal/premotor regions; and (3) inferior longitudinal fasciculus (ILF), connecting the posterior inferior temporal gyrus with the ventral anterior and medial temporal lobe (Deutsch et al., 2005; Frye et al., 2011; Klingberg et al., 2000; Lebel & Beaulieu, 2009; Myers et al., 2014; Niogi & McCandliss, 2006; Travis, Adams, Kovachy, Ben‐Shachar, & Feldman, 2017; Yeatman, Dougherty, Ben‐Shachar, & Wandell, 2012a; Yeatman et al., 2011; Saygin et al., 2013; Vandermosten, Boets, Wouters, & Ghesquiere, 2012; Wang et al., 2016; Zhao, de Schotten, Altarelli, Dubois, & Ramus, 2016; Supporting Information Figure S1). The left ILF passes in close proximity to the visual word form area (Yeatman, Rauschecker, & Wandell, 2013), a cortical region that becomes left‐lateralized and specialized for word recognition through experience (Cohen et al., 2002; Dehaene & Cohen, 2011). The current study investigated, for the first time, whether there is a relationship between SES and these three white‐matter pathways bilaterally in pre‐reading children and whether SES moderates the links between these pathways and longitudinal reading outcomes.
A confluence of genetic and environmental influences interact reciprocally to affect children's reading development (Ozernov‐Palchik, Yu, Wang, & Gaab, 2016). Hereditary risk is a strong predictor of reading disability, as approximately 40–60% of children who have a parent who is reading‐disabled will have reading problems themselves (Gilger, Hanebuth, Smith, & Pennington, 1996; Snowling, Gallagher, & Frith, 2003). Environmental factors are also significant in determining reading skill and explain up to 30% of individual differences in reading, with parental SES exerting the most influence (Olson, Keenan, Byrne, & Samuelsson, 2014; Petrill, Deater‐Deckard, Thompson, De Thorne, & Schatschneider, 2006; Taylor, Roehrig, Hensler, Connor, & Schatschneider, 2010). SES is a multidimensional construct that encompasses parental education levels, economic resources such as income, and social status (Tomalski & Johnson, 2010). SES is also a proxy for quantifying the quality of the prenatal and postnatal environment to which a child is exposed. Parental education is considered to be a particularly stable indicator of SES and is correlated with parental involvement in children's educational attainment (Bradley & Corwyn, 2002; Friend, DeFries, & Olson, 2008).
Gaps in reading achievement between lower‐ and higher‐SES children are one of the most consistent findings in the educational literature (Reardon, 2011). Lower SES has been associated with lower performance in vocabulary, phonological awareness, single word decoding, reading comprehension, and grammar (Bowey, 1995; Noble & McCandliss, 2005). Children from lower‐SES families are 2.5 times more likely to read below grade level and more likely to meet the criteria for reading disability than children from higher‐SES backgrounds (Peterson & Pennington, 2015). Crucially, these gaps in reading achievement begin even before children enter school (Coley, 2002; Reardon & Portilla, 2016), widen across the years of schooling (Feinstein, 2003), and have been widening over the past decades (Brooks‐Gunn & Duncan, 1997; Reardon, 2011; Reardon & Portilla, 2016). This underscores the need to understand the early impact of SES on reading development and its neurobiology in order to prevent and remediate the spiraling effects of SES on educational achievement.
SES in childhood has profound and widespread implications for brain development (Hackman, Farah, & Meaney, 2010), with language and reading‐related brain structures being particularly affected (Noble & McCandliss, 2005; Noble, Tottenham, & Casey, 2005; Romeo et al., 2017; Rowe & Goldin‐Meadow, 2009). SES is thought to affect brain development, and subsequent behavioral outcomes, through a range of mediating factors such as maternal stress and cognitive stimulation (Hackman et al., 2010). Animal studies have demonstrated the effects of these factors on mechanisms underlying neural development and plasticity such as dendritic branching, gliogenesis, synapotogenesis, neurogenesis, and the integration of newly generated neurons into functional circuits (for a review see Hackman et al., 2010).
Indeed, the association between SES and brain structure and function has been demonstrated in multiple ways (e.g., Betancourt et al., 2015; D'Angiulli, Herdman, Stapells, & Hertzman, 2008; Finn et al., 2017; Hackman & Farah, 2009; Hanson et al., 2013; Jednoróg et al., 2012; Leonard, Mackey, Finn, & Gabrieli, 2015; Luby et al., 2013; Mackey et al., 2015; Noble et al., 2015; Noble, Houston, Kan, & Sowell, 2012; but see Brain Development Cooperative Group, 2012; Eckert, Lombardino, & Leonard, 2001; Lange, Froimowitz, Bigler, Lainhart, & Brain Development Cooperative Group, 2010; Raizada, Richards, Meltzoff, & Kuhl, 2008). Specifically, lower SES has been linked to reduced gray matter volume (Hanson et al., 2011, 2013; Jednoróg et al., 2012; Luby et al., 2013), reduced cortical thickness (Mackey et al., 2015), reduced degree of cortical gyrification (Jednoróg et al., 2012), and reduced surface area (Natalie & Noble, 2014) in bilateral occipito‐temporal, temporo‐parietal, and inferior frontal regions that support reading development (Booth et al., 2001; Martin, Schurz, Kronbichler, & Richlan, 2015; for a review see Ozernov‐Palchik & Gaab, 2016). Functional MRI and electrophysiological studies have also reported decreased specialization for reading and language in task‐relevant regions in children from lower‐SES backgrounds (see a review by Pavlakis, Noble, Pavlakis, Ali, & Frank, 2015).
Few studies to date have investigated the direct association between structural or white‐matter connectivity and SES (Chiang et al., 2011; Noble, Korgaonkar, Grieve, & Brickman, 2013; Piras, Cherubini, Caltagirone, & Spalletta, 2011; Vandermosten et al., 2015), with even fewer studies in children (Chiang et al., 2011; Gullick, Demir‐Lira, & Booth, 2016; Jednoróg et al., 2012; Vandermosten et al., 2015). The results of these investigations in school‐age children are mixed, with some studies finding SES‐related differences in white‐matter pathways including left SLF and ILF (Dufford & Kim, 2017; Gullick et al., 2016; Ursache & Noble, 2016; Vandermosten et al., 2015), but others finding no association between white matter and SES (Chiang et al., 2011; Jednoróg et al., 2012).
Only two studies have examined the relations among SES, white matter, and reading ability (Gullick et al., 2016; Vandermosten et al., 2015). The earlier study, in Dutch children, investigated whether kindergarten white matter properties of two bilateral pathways, the arcuate fasciculus and inferior fronto‐occipital fasciculus (IFOF), mediated parental influences on children's reading abilities in second grade (Vandermosten et al., 2015). The study found that fractional anisotropy of the left IFOF mediated the influence of parental reading history and SES on cognitive and literacy precursors and subsequently, second‐grade reading. The direct association between the neuroanatomical measures and SES was not examined in the study. Instead, the neuroanatomical measures included in the mediation model were selected based on their association with both parental and children's reading skills.
In the later study of older children ages 8–14, higher SES was associated with higher FA in several white matter tracts involved in reading, and SES moderated the association between tract measures and reading ability, such that brain–reading ability links were stronger in lower‐SES children (Gullick et al., 2016). Interpretation of that study is difficult, however, because the commonly observed relation between SES and reading ability (e.g., Reardon, 2011) was not observed in this study. Nevertheless, the findings of the modulatory effects of SES on the brain‐behavior relationship are consistent with prior findings in the reading (Brito, Piccolo, & Noble, 2017; Noble, Wolmetz, Ochs, Farah, & McCandliss, 2006; Romeo et al., 2017) and non‐reading (e.g., Muscatell et al., 2012; Swartz, Knodt, Radtke, & Hariri, 2018) domains. These findings warrant further investigation into the multiplicative effects of low SES on neural variation and the importance of this interaction for reading development.
The present study differs from prior research relating SES, reading, and the brain in two fundamental ways. First, prior studies examined children after multiple years of formal education, which confounds factors related to early home environment and later school quality. We examined children before kindergarten or early in kindergarten. Second, we examined variation in longitudinal reading outcomes in relation to SES and white matter microstructure. We accomplished this by: (i) examining the association between SES and pre‐literacy and literacy performance, (ii) examining whether there were significant SES‐related variations in fractional anisotropy (FA) in the bilateral AF, ILF, and SLF tracts important for reading, and (iii) investigating whether SES moderates the longitudinal association between kindergarten white matter FA and second‐grade reading outcomes. To probe the direction of the modulatory effect, we investigated how kindergarten white matter predicted second‐grade reading separately in lower‐SES and higher‐SES groups. Finally, we conducted an exploratory analysis to test whether the SES effects were specific to the reading circuitry or whether or not the associations between parental SES and FA were widespread and present in other tracts.
Based on prior findings, we hypothesized that lower SES would be associated with significantly poorer pre‐reading and reading skills. We hypothesized that lower SES would also be associated with reduced FA in one or more of the six tracts implicated in reading development. Based on previous findings, we expected modulatory effects on the FA–reading relationship with a stronger association between white‐matter FA and reading outcomes in children from lower‐SES backgrounds. In light of the previous literature demonstrating widespread effects of SES on brain development (Hackman et al., 2010), we expected that the association between FA and SES would expand beyond the reading network.
2. METHODS
2.1. Overview
Children from 20 diverse schools in New England completed a short battery of pre‐reading assessments administered by trained researchers in their schools (for details see Ozernov‐Palchik, et al., 2016). These assessments took place in the spring of pre‐kindergarten or fall of kindergarten. A subset of children were contacted and screened for eligibility, and eligible children participated in a follow‐up visit that took place 1–3 months later. Inclusion criteria, based on parental report, were normal hearing, no neurological or psychiatric disorders, American English as a native language, and a full‐term birth (>36 weeks). All children had KBIT‐2 Matrices (nonverbal IQ) subtest standard scores above 80. The follow‐up visit included a neuroimaging session and additional behavioral assessments. Because the study focused on risk for reading difficulty, children invited for neuroimaging over‐represented children at apparent higher risk due to a family history of reading difficulty or low scores on pre‐reading assessments. Children who participated in the neuroimaging session completed additional behavioral follow‐up sessions at the ends of first and second grades. This study was approved by the institutional review boards at the Massachusetts Institute of Technology and Boston Children's Hospital. Parents gave written consent and children gave verbal assent to participate.
2.2. Participants
Children with complete MRI diffusion and behavioral data (N = 129, 53% female, mean age = 66.98 months, SD = 4.16, range 58–80) were included in the current analysis. Four participants were excluded due to poor quality diffusion data. Parents indicated which ethnic and racial category they identified their child with. The group reported the following racial and ethnic identities: 14% African American, 72% White, 3% Asian, 2% American Indian or Alaska Native, 4% mixed race, 5% did not report race; 90% not Hispanic, and 10% Hispanic.
2.3. Kindergarten behavioral measures
All participants completed a comprehensive psychometric battery assessing cognitive and language skills in kindergarten/pre‐kindergarten (see Ozernov‐Palchik, Yu, et al., 2016). Based on the extensive literature on the importance of these pre‐literacy skills for reading development, we focused on letter knowledge, phonological awareness, rapid naming, and vocabulary (e.g., Ozernov‐Palchik, Norton, et al., 2016; Schatschneider, Fletcher, Francis, Carlson, & Foorman, 2004). Nonverbal IQ was further included to rule out differences due to general cognitive abilities.
2.3.1. Phonological awareness
Three subtests were administered from the Comprehensive Test of Phonological Processing (CTOPP, Wagner, Torgesen, & Rashotte, 1999); (1) Elision: the child repeats a word after removing a given sound; (2) Blending Words: the child blends sounds together to make a real word; and (3) Nonword Repetition: the child repeats a nonsense word. The mean of Elision, Blending Words, and Nonword Repetition standard scores was used as the PA composite score.
2.3.2. Rapid automatized naming
The Colors and Objects subtests of the Rapid Automatized Naming/Rapid Alternating Stimulus (RAN/RAS) tests (Wolf & Denckla, 2005) were administered. The child is asked to name an array of items (color patches, objects) on the page as quickly and accurately as possible. The score was the time to name all items. The mean of Colors and Objects standard scores was used as the RAN composite score.
2.3.3. Letter sound knowledge
The Letter Sound Knowledge subtest from the York Assessment of Reading for Comprehension (YARC, Stothard, Hulme, Clarke, Barmby, & Snowling, 2010) assesses knowledge of letter sounds. The child is asked to produce the sound a grapheme or digraph makes. The raw scores were converted to standard scores based on the full kindergarten sample distribution in the current study (n > 1,200; Ozernov‐Palchik, Yu, et al., 2016).
2.3.4. Nonverbal IQ
The Matrices subtest from the Kaufman Brief Intelligence Test, Second Edition (KBIT‐2, Kaufman & Kaufman, 2004) was administered. It assesses nonverbal matrix reasoning skills, specifically the understanding of relations between either concrete stimuli (pictures of objects) or abstract stimuli (e.g., designs or symbols). The child is asked to decide which of a series of pictures presented to them completes the pattern.
2.3.5. Vocabulary
The Peabody Picture Vocabulary Test, Fourth Edition (PPVT‐4, Dunn & Dunn, 2007) assesses vocabulary knowledge. The experimenter says a word aloud, and the child is asked to identify which of four pictures best illustrates the meaning of the word.
2.4. Second‐grade behavioral measures
An extensive battery of cognitive and reading assessments was administered at the end of second grade. For the current study, the following measures were used to characterize children's second‐grade outcomes:
2.4.1. Untimed single real word and nonword reading
The Word Identification (WID) and Word Attack (WA) subtests of the Woodcock Reading Mastery Tests (WRMT‐III, Woodcock, 2011) assess single‐word reading skills. The child is asked to read aloud as many single words or nonwords of increasing difficulty as possible.
2.4.2. Timed single real word and nonword reading
The Sight Word Efficiency (SWE) and Phonemic Decoding Efficiency (PDE) subtests of the Test of Word Reading Efficiency (TOWRE‐2, Torgesen, Wagner, & Rashotte, 1999) assess rapid word identification and decoding ability. The child is asked to rapidly read aloud single words or nonwords within 45 s.
2.5. Measures of reading outcomes
To first investigate whether SES moderates the relationship between white matter and reading ability, a linear regression analysis with second‐grade reading as the dependent variable was conducted. For this continuous analysis, a mean word‐reading score (WR) of the standard scores of the four second‐grade measures was created.
2.6. Socioeconomic status
The Barratt Simplified Measure of Social Status (BSMSS) Questionnaire (Barratt, 2006) was completed by one of the child's parents. Maternal and paternal years of education were used as measures of socioeconomic status (ranging from a score of 3 for less than seventh‐grade education to a score of 21 for graduate degree). This construct is considered a more stable and objective measure than income or occupation and has been shown to relate to quality of child's home environment (Duncan & Magnuson, 2012; Mayes & Lewis, 2012). In the moderation analysis, based on a median score of 18 children were divided into higher‐SES (≥18) and lower‐SES (<18) groups.
2.7. Home literacy environment
Information about the home literacy environment was collected via a parent report questionnaire. The questionnaire was adapted from Sénéchal (1997) and included the following questions that have been shown to directly contribute to the acquisition of early reading skills (Powers, Wang, Beach, Sideridis, & Gaab, 2016; Sénéchal, 1997; Sénéchal & leFevre, 2002; Sénéchal, LeFevre, Hudson, & Lawson, 1996): number of children's books at home, age of the child when first read to, frequency of the child being read to, frequency of the child looking at books, and direct instruction of writing and the alphabet. A mean composite score (HLE) of the six questions was created for the analysis.
2.8. Parental history of reading difficulties
Parental history of reading difficulties was evaluated using the Adult Reading History Questionnaire (ARHQ, Lefly & Pennington, 2000). The questionnaire is scored by summing the responses to all questions and dividing by the total number of questions for each parent. Greater scores on the ARHQ indicate more reading impairment.
2.9. Imaging procedures
As described by Raschle et al. (2012), children practiced in a mock scanner area at the beginning of each MRI session, with child‐friendly equipment (e.g., pediatric headphones, head padding, etc.) and procedures (e.g., strategies for motion reduction) that were also used during actual data acquisition. MRI sequences were acquired on a Siemens 3T Trio whole‐body MRI scanner at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT, using a standard 32‐channel head coil. The T1‐weighted MPRAGE scan used the following specifications: 176 slices, TR = 2,350 ms; TE = 1.64 ms; flip angle = 9°; FOV = 256 mm; voxel size 1.0 × 1.0 × 1.0 mm. As in Saygin et al. (2013), an online prospective motion correction algorithm was implemented to reduce the effect of motion artifacts during the structural scan, and 10 selective reacquisition time points were acquired and included to replace time points that were affected by head motion (Tisdall et al., 2012). The diffusion‐weighted (DW) MRI scan included 10 non‐diffusion‐weighted volumes (b = 0) and 30 diffusion‐weighted volumes acquired with non‐collinear gradient directions (b =700 s/mm2), all at 128 × 128 base resolution and isotropic voxel resolution of 2.0 mm3. Scans were evaluated for motion and scanner‐induced outliers using DTI prep software (Lui, Hansen, & Kriegstein, 2011). Motion parameters were found by rigidly registering the interleaved subvolumes. The translation threshold was set to 2.0 mm and the rotation threshold to 0.5°. From the original sample of 129 participants, four participants with 10 or more motion outliers were excluded from the analysis. There was no significant association between the number of motion outliers and SES (r [127] = −0.04, p = .7).
2.10. Identification of key white matter tracts
The Automatic Fiber Quantification (AFQ; http://github.com/jyeatman/AFQ) software package (Yeatman, Dougherty, Myall, Wandell, & Feldman, 2012b) was used to identify 20 white matter tracts (see Supporting Information Table S2 for a list), including the six tracts of interest chosen for their documented role in reading development (i.e., bilateral ILF, SLF, and AF), and quantify the diffusion parameters along the tract. The AFQ pipeline includes the following steps (for details see Yeatman, Dougherty, Myall, et al., 2012b): (1) fiber tracts are estimated using a deterministic streamlines tracking algorithm (STT) (Basser, Pajevic, Pierpaoli, Duda, & Aldroubi, 2000; Mori, Crain, Chacko, & Van Zijl, 1999) with an FA threshold of 0.2 and angle threshold of 40°; (2) fiber tracts are segmented using a region of interest (ROI) approach and fiber tract probability maps; (3) fiber groups are cleaned into a compact bundle using an iterative statistical outlier rejection algorithm; and (4) diffusion characteristics are calculated at each node, or spatial location, along the trajectory of the fiber. Each fiber is sampled at 100 equidistant nodes that can be used to compute the FA value at each node along the fiber. AFQ segments the whole‐brain fiber group into 20 white matter tracts that are defined in the white matter atlas (Wakana et al., 2007). Diffusion parameters were computed using a weighted sum of each fiber's value at a given node based on fiber's Mahalanobis distance from the core or mean location of the tract (Johnson et al., 2013). Consistent with previous studies (e.g., Yeatman et al., 2011; Vandermosten et al., 2015) the right AF was not reconstructed in a number of participants (23%), so the association analysis of SES with right AF was conducted in the subset of participants with complete data (n = 96).
Our analyses focused on three bilateral tracts that have been linked to reading ability in young children: AF, SLF, and ILF (Ben‐Shachar, Dougherty, & Wandell, 2007; Saygin et al., 2013; Wang et al., 2016; Yeatman et al., 2011; Yeatman, Dougherty, Ben‐Shachar, et al., 2012a). An exploratory analysis of the association of SES and (pre)reading with FA for all available tracts was also performed and is given in Supporting Information Table S2. Consistent with prior studies, fractional anisotropy (FA) was used as the primary parameter of interest to characterize white‐matter microstructure, but axial diffusivity (AD) and radial diffusivity (RD) were also computed and used in follow‐up analyses of SES differences. FA is a summative measure of the three diffusion directions that characterizes microstructural properties of white matter (Beaulieu, 2002; Pierpaoli & Basser, 1996), whereas AD and RD can inform understanding of the mechanisms of FA differences. AD has been related to axonal properties of white matter, whereas RD has been related to axonal myelination and density (Song et al., 2003, 2005; Tyszka, Readhead, Bearer, Pautler, & Jacobs, 2006; Zhang et al., 2009).
2.11. Statistical analyses
All analyses were executed in the statistical package R (Ihaka & Gentleman, 1996).
2.11.1. Missing data imputation
A subset of participants (n = 19) were missing longitudinal reading data and another subset of participants were missing paternal ARHQ data (n = 35). These occurrences do not occur at random and could be related to home environment as well as academic and cognitive outcomes. Therefore, a complete case analysis approach (i.e., removing cases with missing data), may inadvertently bias model estimation (Enders, 2010; Rubin, 1976). Consequently, missing data were replaced using multiple imputation (mice package in R, Buuren & Groothuis‐Oudshoorn, 2010) as recommended by Snijders and Bosker (2012). Mice uses regression models to produce multiple simulated versions of the complete datasets, which are analyzed separately and are then aggregated (Enders, 2010; Little & Rubin, 2014). This approach was shown to produce low false negative and false positive rates when less than 30% of the data is missing across a sample, as was the case in the current study (Vaden et al., 2012). Missing ARHQ scores were imputed for one of the parents for 39 participants and for longitudinal reading data for 19 participants.
2.11.2. Pre‐literacy and literacy performance by SES
To determine whether our study was consistent with the behavioral literature on the associations between SES and educational skills, we first examined the correlation between SES and kindergarten pre‐literacy as well as second‐grade literacy skills. False discovery rate (FDR) correction was used to adjust for multiple comparisons among these tests (Benjamini, Drai, Elmer, Kafkafi, & Golani, 2001).
2.11.3. Association between SES and tract FA
To test the association between SES and FA in the six tracts, a correlation analysis was conducted separately with maternal and paternal education. The Shapiro–Wilk test of normality was performed on all FA values to determine whether to use the Spearman or Pearson test for each paired comparison. Due to the high degree of correlation among the nodes on a specific tract, the traditional Bonferroni method is overly conservative and could lead to type 2 errors. Instead, permutation‐based multiple correction (the AFQ_MultiCompCorrection function based on Nichols & Holmes, 2002) was applied in Matlab (The Mathworks Inc., 2007) to determine the appropriate p‐value. Significance was set at p = .05 for all analyses and p = .05 for cluster‐based permutation corrections.
To establish the specificity of the FA‐SES relationship and to examine which other factors could explain this relationship, a hierarchical regression model was conducted with mean FA of the clusters demonstrating significant association with SES as a dependent variable and with age, gender, pre‐literacy performance, and parental reading history, home literacy, and parental education as independent predictors.
2.11.4. Multicollinearity assessment
Multicollinearity among the variables in the regression models was evaluated using the variance inflation factor (VIF) and the Farrar–Glauber test (using the mctest package in R, Imdadullah, Aslam & Altaf 2016). Results indicated that although the predictors were moderately correlated (see Supporting Information Table S1), the VIF values for all variables ranged from 1.09 to 1.77, suggesting that multicollinearity is not an issue of concern for the regression models reported (O'brien, 2007). The Farrar–Glauber test also indicated insignificant collinearity. In order to select the most parsimonious model in explaining second grade reading, stepwise regression and variable relative importance analyses were conducted.
2.11.5. Association between SES and AD and RD
To reveal possible mechanisms underlying SES‐related differences in FA, the correlation analysis was repeated to test the association between SES and RD and AD in tracts and nodes that demonstrated significant correlation with FA.
2.11.6. Exploratory analyses of associations with control tracts
To reveal whether SES is associated with FA in other tracts outside the reading circuitry, mean FA was computed for each of the 20 available tracts. A series of bivariate correlations, controlling for gender, was then conducted between each of the tracts and maternal and paternal education. Additional correlations with mean FA were conducted for the pre‐reading and reading measures to further test the specificity of the associations, by examining whether the tracts that demonstrate the association with SES are also related to (pre‐)reading performance.
2.11.7. Moderating effects of SES on the FA–reading relationship (longitudinal)
To test whether SES moderated the association between kindergarten FA and second‐grade reading abilities, after partialling out the variance associated with pre‐literacy skills and parental reading background, a multiple linear regression model was constructed for each of the significant tracts as follows: WR scores = β0 + β1 × IQ + β3 × PA + β4 × LSK + β5 × RAN + β6 × PA + β7 × mom ARHQ + β8 × dad ARHQ + β9 × FA + β10 × SES + β11 × (FA × SES) + ε. The Shapiro–Wilk test was performed to determine whether each variable was normally distributed. The relative importance of each variable was calculated using the relaimpo package in R (Grömping, 2006). Ninety‐five percent bootstrap confidence intervals were computed with 1,000 iterations. A stepwise regression analysis was implemented to identify the optimal set of predictors for later reading abilities. To probe the nature of the moderation effects identified in the regression model, correlations between ILF FA and second‐grade reading were computed separately in each group after controlling for age, IQ, pre‐literacy, and parental ARHQ.
3. RESULTS
See Supporting Information Table S1 for a summary of the behavioral correlations. After FDR adjustment for multiple comparisons, there was a significant positive correlation between maternal education and HLE (r[123] = .24, p = .012). There was also a negative association with maternal (r[123] = −.20, p = .031) ARHQ scores indicating stronger maternal history of reading difficulties in children from lower maternal‐education backgrounds. Paternal education was not significantly associated with age (r[123] = −0.01, p = .96), HLE (r[123] = .14, p = .151), gender (χ2[5] = 9.5, p = .09), maternal ARHQ (r[123] = −0.08, p = .46), or IQ (r[123] = .16, p = .12). There was a significant positive association between paternal education and paternal ARHQ (r[123] = −0.21, p = .05). There were no significant correlations between maternal education and age (r[123] = −0.01, p = .1), paternal ARHQ (r[123] = −0.21, p = .26), gender (χ2(3) = 3.8, p = .3), or IQ (r[123] = .16, p = .124).
3.1. Association between SES and (pre‐)literacy performance
See Supporting Information Figure S2 for a summary of correlations between all behavioral measures. In kindergarten, higher maternal education was associated with significantly better performance on phonological awareness (r[123] = 0.35, p < .001), vocabulary (r[123] = 0.37, p < .001), letter‐sound knowledge (r[123] = 0.33, p < .001), and RAN (r[123] = 0.19, p = .044). In second grade, maternal education was associated with better performance on all of the individual reading measures: WID (r[123] = 0.33, p < .001), WA (r[123] = .24, p = .012), PDE (r[123] = .33, p < .001), SWE (r[123] = .19, p = .037), and the second‐grade WR composite (r[123] = .3, p = .002).
Higher paternal education was associated with better kindergarten performance on phonological awareness (r[123] = 0.43, p < .001), vocabulary (r[123] = .42, p < .001), letter‐sound knowledge (r[123] = .33, p < .001), but not RAN (r[123] = −0.05, p = .96). It was also associated with better performance on second‐grade WID (r[123] = 0.2, p = .03), WA (r[123] = .24, p = .02), and PDE (r[123] = .24, p = .02). There was no significant association with the reading composite WR (r[123] = .19, p = .06) or SWE (r[123] = .02, p = .33).
3.2. Association between SES and tract FA
We examined the association between maternal and paternal education and FA in bilateral ILF, SLF, and AF. There was a significant positive association between maternal education and FA of the left ILF (nodes 4–69) and of the right ILF (nodes 1–33) but not with any of the other tracts. Paternal education was not significantly associated with FA in any of the six tracts. A regression model that included IQ, pre‐literacy measures (LSK, PA, RAN, and Vocab), HLE, ARHQ, and paternal education demonstrated a unique and significant association between maternal education and mean left ILF and right ILF FA for the significant nodes. The Shapiro–Wilk test revealed that the mean FA variables were not normally distributed (W = 0.98, p = .04). Accordingly, the lmPerm package (Wheeler, Torchiano, & Torchiano, 2016) in R was used to calculate permuted linear regression for all analyses. Maternal education accounted for 7.2% of the variance in left ILF FA and the adjusted R‐squared (to account for the multiple predictors in the model) for the overall model was 0.15. The only other significant variable was PA, accounting for 3.7% of the variance in mean left ILF. Similarly, maternal education accounted for 9.1% of unique variance in mean right ILF, after controlling for IQ, pre‐literacy measures, HLE, ARHQ, and paternal education. No other variables accounted for unique variance in right ILF and the overall adjusted R‐squared was 0.13.
3.3. Association between SES and AD and RD
There was a significant association between RD and maternal education in nodes 4–69 of the left ILF (p < .001) and nodes 1–33 of the right ILF (p = .009). There were no significant associations with AD.
3.4. Exploratory analyses of associations with control tracts
As shown in Supporting Information Table S2, a series of correlations revealed that the effects observed between SES and FA were not specific to the reading tracts, but rather extended to tracts that have not been traditionally associated with reading (e.g., left corticospinal tract). Although the left ILF was significantly associated with PA and the right ILF was associated with PA and LSK, some of the control tracts that were associated with SES were not significantly correlated with (pre)reading measures. This confirms the non‐specificity of the SES‐related findings (Figure 1).
Figure 1.
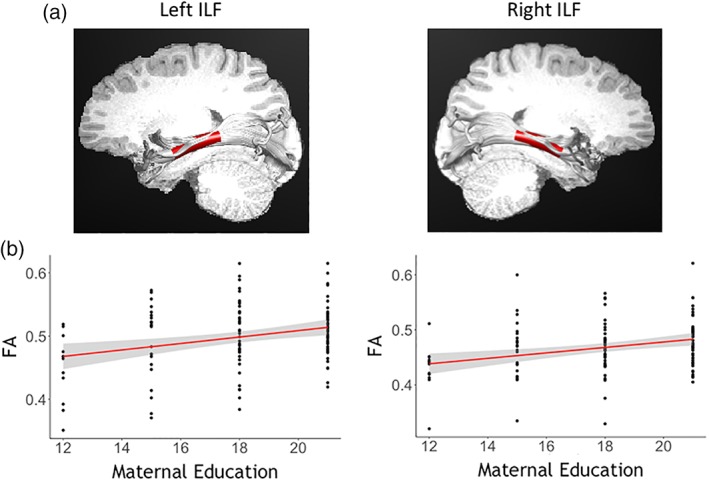
Association between maternal education and fractional anisotropy in left and right ILF. (a) Tract profile of the left ILF depicting left and right ILF nodes that demonstrated significant association with maternal education (marked in red). (b) Scatter plots of FA and maternal education (BSMSS score). The representative node 11 is plotted for left ILF and node 23 for right ILF
3.5. Moderating effects of SES on the FA–reading relationship (longitudinal)
The mean FA for the significant nodes, ARHQ, HLE, IQ, pre‐reading measures, maternal and paternal education, and the FA × maternal education interaction term were combined in a stepwise regression model for the left and right ILF tracts to identify the optimal set of predictors for second‐grade reading (Figure 2). The R 2 for the entire left ILF model was 0.32 (p < .001) and the best set of predictors included the following: (1) PA (contributing 49% of R 2), (2) maternal education × left ILF FA interaction (contributing 19.49% of R 2), (3) maternal education (contributing 17.2% of R 2), (4) RAN (contributing 12.31% of R 2), and (5) left ILF FA (contributing 2% of R 2). The R‐squared for the right ILF model was 0.34 (p < .001) and the best set of predictors included the following: (1) PA (contributing 45.3% of R 2), (2) maternal education × right ILF FA interaction (contributing 24.31% of R 2), (3) maternal education (contributing 15.77% of R 2), (4) RAN (contributing 12.25% of R 2), and (5) right ILF FA (contributing 2.37% of R 2).
Figure 2.
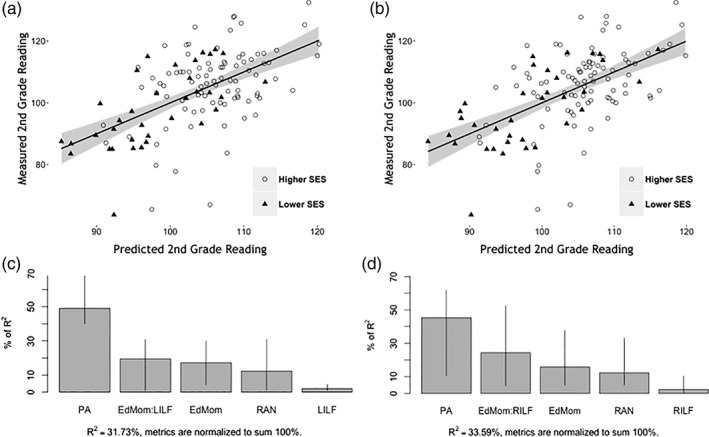
Association with second‐grade reading using stepwise regression analysis to identify the best set of predictors, which includes the combination of pre‐literacy, parental, and FA measures, as well as the FA × maternal education interaction term. (a) and (b) show final prediction models for lower and higher‐SES groups. Actual values versus predicted are plotted, with predicted varying in size. (c) and (d) are the relative importance bar plots with 95% bootstrap confidence intervals for left ILF and right ILF, respectively. Tract names and behavioral measures are abbreviated as follows: PA, phonological awareness; EdMom × LILF or EdMom × RILF, interaction term between maternal education and left or right ILF FA (mean FA of significant nodes); RAN, rapid automatized naming
To probe the significant interaction effect, the association between mean ILF FA bilaterally and WR was examined separately in higher (n = 90) and lower‐SES (n = 35) groups (divided based on the median maternal education score of 18). Bartlett's K‐squared test was used to evaluate differences in variance between lower‐ and higher‐SES groups on reading skills (WR). This test revealed no significant differences in variance [k 2(1) = 0.06, p = .8] between the two samples. Greater FA In the left (R 2 = .15, p = .02) and right ILF (R 2 = 0.13, p = .034) was associated with better reading outcomes in the lower‐SES, but not the higher‐SES group (Left ILF: R 2 = 0.02, p = .3; right ILF: R 2 = 0.004, p = .561) (Figure 3).
Figure 3.
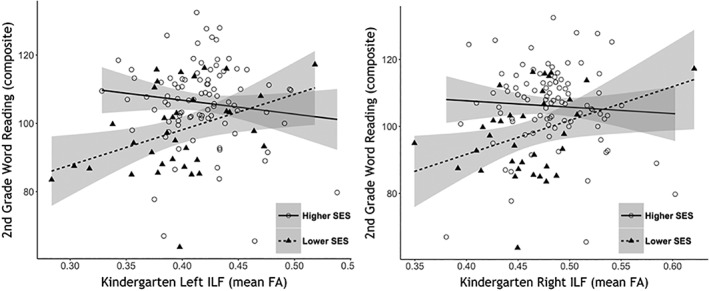
Association between tract FA (mean FA of significant nodes) and reading by SES. Scatter plot depicts the significant positive association between FA and second‐grade word‐reading composite in the lower‐ but not the higher‐SES group. Word reading composite (mean score of WRMT Word Attack and Word ID, and TOWRE Phonemic Decoding and Sight Word Efficiency) is a standard score based around 100
4. DISCUSSION
The present study found the expected relation between SES (operationalized as parental education) and both pre‐literacy kindergarten measures and second‐grade reading, such that children from higher‐SES environments had higher pre‐literacy and reading scores than children from lower‐SES environments. One novel finding was that children from lower‐SES environments—specifically, children whose mothers had fewer years of formal education—appeared to have weaker structural connectivity in the left and right inferior longitudinal fasciculus (ILF) as measured by reduced fractional anisotropy (FA) and higher radial diffusivity (RD) in kindergarten. A second novel finding was the differential relation between white‐matter microstructure in kindergarten and reading outcomes in second grade, such that in children from lower‐SES, but not higher‐SES environments, better reading in second grade was associated with greater FA in the left and right ILF in kindergarten. In combination, these findings reveal that white‐matter characteristics in young kindergartners have a differential relation to long‐term reading outcomes that depend upon their environments.
4.1. Association between SES and (pre‐)literacy performance
Consistent with previous findings (Reardon, 2011), we observed significant associations between SES and reading‐related performance in kindergarten and second grade. All pre‐literacy skills examined, except RAN, were associated with paternal and maternal education. RAN was associated with maternal, but not paternal, education. The achievement gap between lower‐ and higher‐SES children has been one of the most robust patterns in educational research (Reardon, 2011), and the confirmation of this gap in the current sample points to its representativeness.
4.2. ILF tract FA and SES
Maternal, but not paternal, education in the current study was significantly associated with brain measures. This is consistent with previous literature documenting that maternal education, as compared with other components of SES (occupation, income, paternal education), has the strongest association with children's cognitive skills (Harding, Morris, & Hughes 2015; Reardon, 2011). Studies have also shown that maternal education, in particular, predicts the presence of resources within the family that influence children's well‐being such as economic security family structure, and maternal well‐being (McLanahan 2004; Kiernan & Huerta 2008; Meadows, McLanahan, & Brooks‐Gunn 2008).
Overall, the findings of a positive association between SES with left and right ILF FA and a negative association with RD are in line with documented SES‐related differences in neural structure and function across development (Hanson et al., 2013; Jednoróg et al., 2012; Lawson, Duda, Avants, Wu, & Farah, 2013; Mackey et al., 2015; Noble et al., 2012; Noble et al., 2006; Raizada & Kishiyama, 2010). Consistent with the present findings, lower parental SES has been associated with decreased FA in multiple white matter clusters including in the bilateral ILF tract in older children (Gullick et al., 2016). To our knowledge, no studies to date have directly investigated differences in white matter based on SES in younger kindergarten children. By revealing which reading‐related pathways are affected in lower‐SES children very early in reading development, our results speak to the contribution of the early familial environment, rather than that of the quality of schooling, to observed SES‐related disparities.
The ILF pathway is generally considered a pathway through which information is conveyed from the extrastriate occipital cortex to the lateral temporal cortex, parahippocampal gyrus, and amygdala (Catani et al. 2003; Schmahmann et al., 2007). The ILF tract bilaterally has been linked to a range of visual functions such as object recognition, visual learning, and face processing (Catani et al., 2003; Hodgetts et al., 2015; Ortibus et al., 2012; Ross, 2008). The left ILF, specifically, is a component of the ventral reading pathway that transmits information between the visual word form area (VWFA), located within the fusiform gyrus, and the anterior and medial temporal lobe (Epelbaum et al., 2008; Wandell et al., 2012). The VWFA is involved in orthographic processing and mapping printed words to meaning, and develops specialization for print through learning to read a particular orthography (Dehaene & Cohen, 2011; Dehaene, Cohen, Morais, & Kolinsky, 2015; Dehaene et al., 2010; Heim, Pape‐Neumann, van Ermingen‐Marbach, Brinkhaus, & Grande, 2015). This experience dependent specialization has been described as increased engagement of the left fusiform gyrus for print, accompanied by decreased engagement for other visual perceptual functions, such as face recognition (Centanni et al., 2018; Dehaene et al., 2010; Saygin et al., 2016). Specialized patterns of white‐matter connectivity to the VWFA have been shown to predate, and possibly drive, the region's specialization for word recognition (Saygin et al., 2016). Taken together, this suggests that factors associated with being raised in a lower‐SES environment are related to the connectivity patterns of the VFWA in a manner that could constrain reading development.
What could be the mechanisms that link SES and changes in these tracts? Fractional anisotropy is influenced by two opposing and partially environmentally‐driven processes: myelination that increases FA, and pruning of axons that decreases FA (Yeatman, Dougherty, Ben‐Shachar, et al., 2012a), as well as other factors. Studies in animal models have indicated that RD is more sensitive to myelination, whereas AD is more sensitive to axonal degradation (Song et al., 2003; 2005). Findings of increased RD in the lower‐SES group, but absence of differences in AD, therefore suggest that differences in FA are due to increased myelination of the ILF tract in the higher‐SES group. Myelination speeds conduction between distant cortical regions. Across development, myelination of axons is guided both by intrinsic genetic codes and extrinsically driven mechanisms, such as neuronal activity along a particular axon (Emery, 2010; Mount & Monje, 2017). For example, it has been demonstrated that neuronal activity stimulates the proliferation of committed oligodendrocyte progenitor cells, which generate those glial cells, the oligodendrocytes, that form myelin (Mount & Monje, 2017). Developmentally, higher FA in childhood indicates maturation, as FA tends to increase through childhood, reaching a plateau in early‐to‐mid‐adulthood (depending on the tracts) and then starts to decrease (Lebel, Treit, & Beaulieu, 2017). ILF FA was shown to increase most rapidly between ages five and seven, remaining largely stable after the age of seven (Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008). Thus, taken together, evidence suggests that reduced ILF FA in the lower‐SES group reflects decreased myelination and delayed maturation of this pathway.
Our current understanding of how SES affects brain development is modest. It is hypothesized that the quality of parental care varies depending on SES factors and can influence neural development through epigenetic processes, and subsequently, the neural activity that regulates cognition (Hackman et al., 2010). In the present study, there was evidence that children from higher‐SES environments had enhanced home literacy environments (HLE scores) and vocabulary relative to children from lower‐SES environments. Additionally, children from lower‐SES backgrounds had increased familial history of reading impairment. A plethora of factors not measured in the current study but commonly associated with higher SES (e.g., greater cognitive stimulation, better nutrition, less stress), together with enhanced language and pre‐literacy experiences, may spur increased myelination of the ILF, as well as some of the other non‐reading tracts, as measured by higher FA and lower RD values. The contribution of mediators not measured in the current study to SES‐ILF association is supported by the persistence of the association between maternal education and FA, even after controlling for parental background and children's psychometric performance measures. This is consistent with previous findings that SES explained variance in white‐matter microstructure and reading skills independently of parental reading history and home literacy (Vandermosten et al., 2015). There are numerous candidate mechanisms that could explain the association between SES and neurodevelopment. It has been demonstrated, for example, that conversational turn‐taking between a parent and their child, in addition to vocabulary, is associated with functional brain specialization for language and partially accounts for the links between parental education and children's language skills (Romeo et al., 2018). Future studies, therefore, need to include more comprehensive measures of children's linguistic environments.
There is considerable evidence that experience or training can alter white‐matter FA. For example, increased FA in target tracts has been demonstrated in response to juggling training (Scholz, Klein, Behrens, & Johansen‐Berg, 2009), meditation training (Tang et al., 2010), cognitive training (Mackey, Whitaker, & Bunge, 2012), learning to read (Carreiras et al., 2009; Hofstetter, Friedmann, & Assaf, 2017; Thiebaut de Schotten, Cohen, Amemiya, Braga, & Dehaene, 2014), and following reading intervention (Keller & Just, 2009; Huber, Donnelly, Rokem, & Yeatman, 2018; Hofstetter et al., 2017). For example, in a study of adults, learning Morse code was associated with a significant increase in left ILF FA (Schlaffke, Leemans, Schweizer, Ocklenburg, & Schmidt‐Wilcke, 2017). In another study, rapid changes in white matter properties of the left ILF, among other tracts, were demonstrated in children with dyslexia in response to an intensive reading intervention (Huber et al., 2018). Importantly, the effects of parental practices (e.g., quality of nutrition, conflict in the household, and verbal abuse) on white matter structure in early childhood have been shown (Choi, Jeong, Rohan, Polcari, & Teicher, 2009; Dufford & Kim, 2017; Lebel et al., 2016; Ou et al., 2014). Therefore, the present finding of the region's sensitivity to influences related to familial SES is in accordance with its general malleability in response to environmental input and experience.
4.3. Moderating effects of SES on the ILF–reading relationship
Consistent with previous studies that demonstrated modulatory influences of SES on the brain–reading relationship (Brito, Piccolo, & Noble, 2017; Gullick et al., 2016; Noble et al., 2006; Romeo et al., 2017), ILF FA in kindergarten was associated with better second‐grade reading outcomes in lower‐SES but not higher‐SES children. The longitudinal nature of the current findings is a notable difference from other studies that only examined the SES × brain association with reading concurrently. One possible interpretation of these findings is that SES can exaggerate or mitigate the links between early brain connectivity and reading outcomes. For example, higher quality childcare and schools, better housing, and higher quality and quantity of cognitive and linguistic stimulation could lead to successful reading outcomes even in the face of neuroanatomical variations—namely reduced white matter FA of bilateral ILF (Brito et al., 2017). Another possible interpretation is related to differences in heritability of reading across SES groups. It has been shown that environmental differences accounted for more variance in reading performance in lower‐SES children than in higher‐SES children (Friend et al., 2008; but see Kirkpatrick, Legrand, Iacono, & Mcgue, 2011). This raises the possibility that the neurobiological basis of reading varies based on SES, with stronger experiential effects on brain–reading relationships in children from lower‐SES families. Due to the correlational nature of the current study, the causal pathway from SES to brain development and to reading outcomes could not be established. Therefore, a third possibility is that a greater variation in environmental influences in children from lower‐SES backgrounds negatively and independently affected both reading and brain development, with poorer consequences in both domains for the outcomes of these children. Future studies are needed to evaluate these possibilities and determine the precise mechanisms of the modulatory influences of SES on brain–reading links.
4.4. Limitations and future directions
First, although the sample of lower‐SES children in the current study was racially and ethnically diverse, it was not truly representative of the lower segment of the United States population in terms of SES. All mothers and 95% of fathers in the current lower‐SES sample had completed high school, whereas the national high school completion rate stands at 83% (Aud et al., 2012). Nevertheless, the mean SES of the sample is comparable to previous neuroimaging studies of SES in children (e.g., Gullick et al., 2016; Noble et al., 2006; Skoe, Krizman, & Kraus, 2013) and even greater disparities between the two SES groups would be expected were the lower‐SES group less advantaged. Second, because white matter development begins in utero and continues through adolescence, in order to gain a deep, mechanistic understanding of the role of parental SES in a child's brain development and to disentangle hereditary from environmental influences, future work needs to examine these effects in infancy and longitudinally. Third, this study used a correlational approach, which limits our ability to draw strong conclusions regarding the causal nature of the reported SES effects.
DATA AVAILABILITY
The data required to reproduce reported findings is available at https://data.mendeley.com/datasets/jtc5dnykby/draft?a=aa39098f-a536-4f81-a89c-c922064c0b11. The analysis code will be provided upon request.
Supporting information
Supporting Information Figure S1 White matter tracts (arcuate fasciculus‐red, inferior frontal occipital fasciculus‐yellow, and inferior longitudinal fasciculus‐green) investigated in the current study. Note: although the current study investigated these tracts bilaterally, only left hemispheric pathways are depicted [Color figure can be viewed at http://wileyonlinelibrary.com]
Supporting Information Table S1 Correlations between parental education and background and (pre) literacy measures.
Supporting Information Table S2: Correlations of SES and (pre)reading measures with mean FA for all available tracts
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health–National Institute of Child Health and Human Development (Grants R01HD067312 to JDEG and NG and R01HD65762‐01 to NG). We thank Steven Shannon, Sheeba Arnold, Christina Triantafyllou, Atsushi Takahashi and the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT. We thank Kelly Halverson for assistance with data collection. We thank Xi Yu and Ted Turesky for assistance with figure preparation and Satra Ghosh for helpful discussions. We sincerely thank our research testers, school coordinators and principals, and participating families. Participating schools are listed at http://gablab.mit.edu/index.php/READstudy. The authors have no conflicts of interest to report.
Ozernov‐Palchik O, Norton ES, Wang Y, et al. The relationship between socioeconomic status and white matter microstructure in pre‐reading children: A longitudinal investigation. Hum Brain Mapp. 2019;40:741–754. 10.1002/hbm.24407
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: R01HD067312, R01HD65762‐01
REFERENCES
- Aud, S. , Hussar, W. , Johnson, F. , Kena, G. , Roth, E. , Manning, E. , Wang, X. , and Zhang, J. . (2012). The condition of education. Washington, DC: U.S. Department of Education, National Center for Education Statistics.
- Barratt, W. (2006). The Barratt simplified measure of social status (BSMSS). Terre Haute, IN: Indiana State University. [Google Scholar]
- Basser, P. J. , Pajevic, S. , Pierpaoli, C. , Duda, J. , & Aldroubi, A. (2000). In vivo fiber tractography using DT‐MRI data. Magnetic Resonance in Medicine, 44(4), 625–632. [DOI] [PubMed] [Google Scholar]
- Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomedicine, 15(7–8), 435–455. [DOI] [PubMed] [Google Scholar]
- Ben‐Shachar, M. , Dougherty, R. F. , & Wandell, B. A. (2007). White matter pathways in reading. Current Opinion in Neurobiology, 17(2), 258–270. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , Drai, D. , Elmer, G. , Kafkafi, N. , & Golani, I. (2001). Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research, 125(1–2), 279–284. [DOI] [PubMed] [Google Scholar]
- Betancourt, L. M. , Avants, B. , Farah, M. J. , Brodsky, N. L. , Wu, J. , Ashtari, M. , & Hurt, H. (2015). Effect of socioeconomic status (SES) disparity on neural development in female African‐American infants at age 1 month. Developmental Science, 19(6), 947–956. [DOI] [PubMed] [Google Scholar]
- Booth, J. R. , Burman, D. D. , Van Santen, F. W. , Harasaki, Y. , Gitelamn, D. R. , Parrish, T. R. , & Mesulam, M. M. (2001). The development of specialized brain systems in reading and oral language. Child Neuropsychology, 7(3), 119–141. [DOI] [PubMed] [Google Scholar]
- Bowey, J. A. (1995). Socioeconomic status differences in preschool phonological sensitivity and first‐grade reading achievement. Journal of Educational Psychology, 87(3), 476–487. [Google Scholar]
- Bradley, R. H. , & Corwyn, R. F. (2002). Socioeconomic status and child development. Annual Review of Psychology, 2002(53), 371–399. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group . (2012). Total and regional brain volumes in a population‐based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cereb. Cortex, 22, 1–12. 10.1093/cercor/bhr018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito, N. H. , Piccolo, L. R. , & Noble, K. G. (2017). Associations between cortical thickness and neurocognitive skills during childhood vary by family socioeconomic factors. Brain and Cognition, 116, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks‐Gunn, J. , & Duncan, G. J. (1997). The effects of poverty on children. The Future of Children, 7, 55–71. [PubMed] [Google Scholar]
- Buuren, S. V. , & Groothuis‐Oudshoorn, K. (2010). mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 1–68. [Google Scholar]
- Carreiras, M. , Seghier, M. L. , Baquero, S. , Estevez, A. , Lozano, A. , Devlin, J. T. , & Price, C. J. (2009). An anatomical signature for literacy. Nature, 461(7266), 983–986. [DOI] [PubMed] [Google Scholar]
- Catani, M. , Jones, D. K. , Donato, R. , & Ffytche, D. H. (2003). Occipito‐temporal connections in the human brain. Brain, 126(9), 2093–2107. [DOI] [PubMed] [Google Scholar]
- Centanni, T. M. , Norton, E. S. , Park, A. , Beach, S. D. , Halverson, K. , Ozernov‐Palchik, O. , … Gabrieli, J. D. (2018). Early development of letter specialization in left fusiform is associated with better word reading and smaller fusiform face area. Developmental Science, 21, e12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, M.‐C. , McMahon, K. L. , de Zubicaray, G. I. , Martin, N. G. , Hickie, I. , Toga, A. W. , … Thompson, P. M. (2011). Genetics of white matter development: A DTI study of 705 twins and their siblings aged 12 to 29. NeuroImage, 54(3), 2308–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. , Jeong, B. , Rohan, M. L. , Polcari, A. M. , & Teicher, M. H. (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry, 65(3), 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, L. , Lehericy, S. , Chochon, F. , Lemer, C. , Rivaud, S. , & Dehaene, S. (2002). Language‐specific tuning of visual cortex? Functional properties of the visual word form area. Brain, 125(Pt 5), 1054–1069. [DOI] [PubMed] [Google Scholar]
- Coley, R. J. (2002). An uneven start: Indicators of inequality in school readiness. Policy Information Report., Policy Information Center, Educational Testing Service.
- D'Angiulli, A. , Herdman, A. , Stapells, D. , & Hertzman, C. (2008). Children's event‐related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology, 22(3), 293–300. [DOI] [PubMed] [Google Scholar]
- Dehaene, S. (2009). Reading in the brain: The new science of how we read. New York, NY: Penguin. [Google Scholar]
- Dehaene, S. , & Cohen, L. (2011). The unique role of the visual word form area in reading. Trends in Cognitive Sciences, 15(6), 254–262. [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , Cohen, L. , Morais, J. , & Kolinsky, R. (2015). Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nature Reviews Neuroscience, 16(4), 234–244. [DOI] [PubMed] [Google Scholar]
- Dehaene, S. , Pegado, F. , Braga, L. W. , Ventura, P. , Nunes Filho, G. , Jobert, A. , … Cohen, L. (2010). How learning to read changes the cortical networks for vision and language. Science, 330(6009), 1359–1364. [DOI] [PubMed] [Google Scholar]
- Deutsch, G. K. , Dougherty, R. F. , Bammer, R. , Siok, W. T. , Gabrieli, J. D. , & Wandell, B. (2005). Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex, 41(3), 354–363. [DOI] [PubMed] [Google Scholar]
- Dufford, A. J. , & Kim, P. (2017). Family income, cumulative risk exposure, and white matter structure in middle childhood. Frontiers in Human Neuroscience, 11, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, G. J. , & Magnuson, K. (2012). Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science, 3(3), 377–386. [DOI] [PubMed] [Google Scholar]
- Dunn, L. M. , & Dunn, D. M. (2007). Peabody picture vocabulary test, 4th ed. (PPVT‐4). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Eckert, M. A. , Lombardino, L. J. , & Leonard, C. M. (2001). Planar asymmetry tips the phonological playground and environment raises the bar. Child Development, 72(4), 988–1002. [DOI] [PubMed] [Google Scholar]
- Emery, B. (2010). Regulation of oligodendrocyte differentiation and myelination. Science, 330(6005), 779–782. [DOI] [PubMed] [Google Scholar]
- Enders, C. K. (2010). Applied missing data analysis. New York, London: Guilford Press. [Google Scholar]
- Epelbaum, S. , Pinel, P. , Gaillard, R. , Delmaire, C. , Perrin, M. , Dupont, S. , … Cohen, L. (2008). Pure alexia as a disconnection syndrome: New diffusion imaging evidence for an old concept. Cortex, 44(8), 962–974. [DOI] [PubMed] [Google Scholar]
- Feinstein, L. (2003). Inequality in the early cognitive development of British children in the 1970 cohort. Economica, 70(277), 73–97. [Google Scholar]
- Finn, A. S. , Minas, J. E. , Leonard, J. A. , Mackey, A. P. , Salvatore, J. , Goetz, C. , … Gabrieli, J. D. E. (2017). Functional brain organization of working memory in adolescents varies in relation to family income and academic achievement. Developmental Science, 20, e12450. [DOI] [PubMed] [Google Scholar]
- Friend, A. , DeFries, J. C. , & Olson, R. K. (2008). Parental education moderates genetic influences on reading disability. Psychological Science, 19(11), 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye, R. E. , Liederman, J. , Hasan, K. M. , Lincoln, A. , Malmberg, B. , McLean, J., III , & Papanicolaou, A. (2011). Diffusion tensor quantification of the relations between microstructural and macrostructural indices of white matter and reading. Human Brain Mapping, 32(8), 1220–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilger, J. W. , Hanebuth, E. , Smith, S. D. , & Pennington, B. F. (1996). Differential risk for developmental reading disorders in the offspring of compensated versus noncompensated parents. Reading and Writing, 8(5), 407–417. [Google Scholar]
- Grömping, U. (2006). Relative importance for linear regression in R: The package relaimpo. Journal of Statistical Software, 17(1), 1–27. [Google Scholar]
- Gullick, M. M. , Demir‐Lira, Ö. E. , & Booth, J. R. (2016). Reading skill–fractional anisotropy relationships in visuospatial tracts diverge depending on socioeconomic status. Developmental Science, 19(4), 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman, D. A. , & Farah, M. J. (2009). Socioeconomic status and the developing brain. Trends in Cognitive Science, 13, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman, D. A. , Farah, M. J. , & Meaney, M. J. (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11(9), 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, J. L. , Hair, N. , Shen, D. G. , Shi, F. , Gilmore, J. H. , Wolfe, B. L. , & Pollak, S. D. (2013). Family poverty affects the rate of human infant brain growth. PLoS One, 8(12), e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, M. J. , Miller, A. D. , Diamond, K. , Odom, S. , Lieber, J. , Butera, G. , … Fleming, K. (2011). Neighborhood community risk influences on preschool children's development and school readiness. Infants & Young Children, 24(1), 87–100. [Google Scholar]
- Harding, J. F. , Morris, P. A. , & Hughes, D. (2015). The relationship between maternal education and children's academic outcomes: A theoretical framework. Journal of Marriage and Family, 77(1), 60–76. [Google Scholar]
- Heim, S. , Pape‐Neumann, J. , van Ermingen‐Marbach, M. , Brinkhaus, M. , & Grande, M. (2015). Shared vs. specific brain activation changes in dyslexia after training of phonology, attention, or reading. Brain Structure and Function, 220(4), 2191–2207. [DOI] [PubMed] [Google Scholar]
- Hodgetts, C. J. , Postans, M. , Shine, J. P. , Jones, D. K. , Lawrence, A. D. , & Graham, K. S. (2015). Dissociable roles of the inferior longitudinal fasciculus and fornix in face and place perception. Elife, 4, e07902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter, S. , Friedmann, N. , & Assaf, Y. (2017). Rapid language‐related plasticity: Microstructural changes in the cortex after a short session of new word learning. Brain Structure and Function, 222(3), 1231–1241. [DOI] [PubMed] [Google Scholar]
- Huber, E. , Donnelly, P. M. , Rokem, A. , & Yeatman, J. D. (2018). Rapid and widespread white matter plasticity during an intensive reading intervention. Nature Communications, 9(1), 2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihaka, R. , & Gentleman, R. (1996). R: A language for data analysis and graphics. Journal of Computational and Graphical Statistics, 5(3), 299–314. [Google Scholar]
- Imdadullah, M. , Aslam, M. , & Altaf, S. (2016). Mctest: An R package for detection of collinearity among regressors. The R Journal, 8, 2. [Google Scholar]
- Jednoróg, K. , Altarelli, I. , Monzalvo, K. , Fluss, J. , Dubois, J. , Billard, C. , … Ramus, F. (2012). The influence of socioeconomic status on children's brain structure. PLoS One, 7(8), e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. T. , Yeatman, J. D. , Wandell, B. A. , Buonocore, M. H. , Amaral, D. G. , & Nordahl, C. W. (2013). Diffusion properties of major white matter tracts in young, typically developing children. NeuroImage, 88, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, A. S. , & Kaufman, N. L. (2004). KBIT‐2: Kaufman brief intelligence test (2nd ed.). Minneapolis, MN: NCS Pearson. [Google Scholar]
- Keller, T. A. , & Just, M. (2009). Altering cortical connectivity: Remediation‐induced changes in the white matter of poor readers. Neuron, 64(5), 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan, K. E. , & Huerta, M. C. (2008). Economic deprivation, maternal depression, parenting and children's cognitive and emotional development in early childhood. The British Journal of Sociology, 59(4), 783–806. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, R. M. , Legrand, L. N. , Iacono, W. G. , & McGue, M. (2011). A twin and adoption study of reading achievement: Exploration of shared‐environmental and gene–environment‐interaction effects. Learning and Individual Differences, 21(4), 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg, T. , Hedehus, M. , Temple, E. , Salz, T. , Gabrieli, J. D. , Moseley, M. E. , & Poldrack, R. A. (2000). Microstructure of temporo‐parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron, 25(2), 493–500. [DOI] [PubMed] [Google Scholar]
- Lange, N. , Froimowitz, M. P. , Bigler, E. D. , Lainhart, J. E. , & Brain Development Cooperative Group . (2010). Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Developmental Neuropsychology, 35(3), 296–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, G. M. , Duda, J. T. , Avants, B. B. , Wu, J. , & Farah, M. J. (2013). Associations between children's socioeconomic status and prefrontal cortical thickness. Developmental Science, 16(5), 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , & Beaulieu, C. (2009). Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Human Brain Mapping, 30(11), 3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , Treit, S. , & Beaulieu, C. (2017). A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR in Biomedicine, 3, e3778. [DOI] [PubMed] [Google Scholar]
- Lebel, C. , Walker, L. , Leemans, A. , Phillips, L. , & Beaulieu, C. (2008). Microstructural maturation of the human brain from childhood to adulthood. NeuroImage, 40(3), 1044–1055. [DOI] [PubMed] [Google Scholar]
- Lebel, C. , Walton, M. , Letourneau, N. , Giesbrecht, G. F. , Kaplan, B. J. , & Dewey, D. (2016). Prepartum and postpartum maternal depressive symptoms are related to children's brain structure in preschool. Biological Psychiatry, 80(11), 859–868. [DOI] [PubMed] [Google Scholar]
- Lefly, D. L. , & Pennington, B. F. (2000). Reliability and validity of the adult reading history questionnaire. Journal of Learning Disabilities, 33(3), 286–296. [DOI] [PubMed] [Google Scholar]
- Leonard, J. A. , Mackey, A. P. , Finn, A. S. , & Gabrieli, J. D. E. (2015). Differential effects of socioeconomic status on working and procedural memory systems. Frontiers in Neuroscience, 9, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, R. J. , & Rubin, D. B. (2014). Statistical analysis with missing data (Vol. 333). New York, NY: Wiley. [Google Scholar]
- Luby, J. , Belden, A. , Botteron, K. , Marrus, N. , Harms, M. P. , Babb, C. , … Barch, D. (2013). The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167(12), 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui, J. H. , Hansen, D. V. , & Kriegstein, A. R. (2011). Development and evolution of the human neocortex. Cell, 146(1), 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey, A. P. , Finn, A. S. , Leonard, J. A. , Jacoby‐Senghor, D. S. , West, M. R. , Gabrieli, C. F. , & Gabrieli, J. D. (2015). Neuroanatomical correlates of the income‐achievement gap. Psychological Science, 26(6), 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey, A. P. , Whitaker, K. J. , & Bunge, S. A. (2012). Experience‐dependent plasticity in white matter microstructure: Reasoning training alters structural connectivity. Frontiers in Neuroanatomy, 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A. , Schurz, M. , Kronbichler, M. , & Richlan, F. (2015). Reading in the brain of children and adults: A meta‐analysis of 40 functional magnetic resonance imaging studies. Human Brain Mapping, 36(5), 1963–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes L., & Lewis M. (Eds.). (2012). The Cambridge handbook of environment in human development. Cambridge: Cambridge University Press. [Google Scholar]
- McLanahan, S. (2004). Diverging destinies: How children are faring under the second demographic transition. Demography, 41(4), 607–627. [DOI] [PubMed] [Google Scholar]
- Meadows, S. O. , McLanahan, S. S. , & Brooks‐Gunn, J. (2008). Stability and change in family structure and maternal health trajectories. American Sociological Review, 73(2), 314–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, S. , Crain, B. J. , Chacko, V. P. , & Van Zijl, P. (1999). Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology, 45(2), 265–269. [DOI] [PubMed] [Google Scholar]
- Mount, C. W. , & Monje, M. (2017). Wrapped to adapt: Experience‐dependent myelination. Neuron, 95(4), 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell, K. A. , Morelli, S. A. , Falk, E. B. , Way, B. M. , Pfeifer, J. H. , Galinsky, A. D. , … Eisenberger, N. I. (2012). Social status modulates neural activity in the mentalizing network. NeuroImage, 60(3), 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, C. A. , Vandermosten, M. , Farris, E. A. , Hancock, R. , Gimenez, P. , Black, J. M. , … Hoeft, F. (2014). Structural changes in white matter are uniquely related to children's reading development. Psychological Science, 25(10), 1870–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalie, H. , & Noble, K. G. (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, T. E. , & Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroiaging: A primer with examples. Human Brain Mapping, 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi, S. N. , & McCandliss, B. D. (2006). Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia, 44(11), 2178–2188. [DOI] [PubMed] [Google Scholar]
- Noble, K. G. , & McCandliss, B. D. (2005). Reading development and impairment: Behavioral, social, and neurobiological factors. Journal of Developmental and Behavioral Pediatrics, 26(5), 370–378. [DOI] [PubMed] [Google Scholar]
- Noble, K. G. , Houston, S. M. , Brito, N. H. , Bartsch, H. , Kan, E. , Kuperman, J. M. , … Libiger, O. (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18(5), 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, K. G. , Houston, S. M. , Kan, E. , & Sowell, E. R. (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15(4), 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, K. G. , Korgaonkar, M. S. , Grieve, S. M. , & Brickman, A. M. (2013). Higher education is an age‐independent predictor of white matter integrity and cognitive control in late adolescence. Developmental Science, 16(5), 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, K. G. , Tottenham, N. , & Casey, B. (2005). Neuroscience perspectives on disparities in school readiness and cognitive achievement. The Future of Children, 15(1), 71–89. [DOI] [PubMed] [Google Scholar]
- Noble, K. G. , Wolmetz, M. E. , Ochs, L. G. , Farah, M. J. , & McCandliss, B. D. (2006). Brain‐behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science, 9(6), 642–654. [DOI] [PubMed] [Google Scholar]
- O'brien, R. M. (2007). A caution regarding rules of thumb for variance inflation factors. Quality & Quantity, 41(5), 673–690. [Google Scholar]
- Olson, R. K. , Keenan, J. M. , Byrne, B. , & Samuelsson, S. (2014). Why do children differ in their development of reading and related skills? Scientific Studies of Reading, 18(1), 38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortibus, E. L. S. , Verhoeven, J. , Sunaert, S. , Casteels, I. , De Cock, P. , & Lagae, L. (2012). Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: A diffusion tensor imaging study. Developmental Medicine & Child Neurology, 54(1), 38–43. [DOI] [PubMed] [Google Scholar]
- Ou, X. , Andres, A. , Cleves, M. A. , Pivik, R. , Snow, J. H. , Ding, Z. , & Badger, T. M. (2014). Sex‐specific association between infant diet and white matter integrity in 8‐y‐old children. Pediatric Research, 76(6), 535–543. [DOI] [PubMed] [Google Scholar]
- Ozernov‐Palchik, O. , & Gaab, N. (2016). Tackling the ‘dyslexia paradox’: reading brain and behavior for early markers of developmental dyslexia. Wiley Interdisciplinary Reviews: Cognitive Science, 7, 156–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov‐Palchik, O. , Norton, E. S. , Sideridis, G. , Beach, S. D. , Wolf, M. , Gabrieli, J. D. , & Gaab, N. (2016). Longitudinal stability of pre‐reading skill profiles of kindergarten children: Implications for early screening and theories of reading. Developmental Science, 20(5), 12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov‐Palchik, O. , Yu, X. , Wang, Y. , & Gaab, N. (2016). Lessons to be learned: How a comprehensive neurobiological framework of atypical reading development can inform educational practice. Current Opinion in Behavioral Sciences, 10, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis, A. E. , Noble, K. , Pavlakis, S. G. , Ali, N. , & Frank, Y. (2015). Brain imaging and electrophysiology biomarkers: Is there a role in poverty and education outcome research? Pediatric Neurology, 52(4), 383–388. [DOI] [PubMed] [Google Scholar]
- Peterson, R. L. , & Pennington, B. F. (2015). Developmental dyslexia. Annual Review of Clinical Psychology, 11, 283–307. [DOI] [PubMed] [Google Scholar]
- Petrill, S. A. , Deater‐Deckard, K. , Thompson, L. A. , De Thorne, L. S. , & Schatschneider, C. (2006). Reading skills in early readers genetic and shared environmental influences. Journal of Learning Disabilities, 39(1), 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli, C. , & Basser, P. J. (1996). Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine, 36(6), 893–906. [DOI] [PubMed] [Google Scholar]
- Piras, F. , Cherubini, A. , Caltagirone, C. , & Spalletta, G. (2011). Education mediates microstructural changes in bilateral hippocampus. Human Brain Mapping, 32(2), 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, S. J. , Wang, Y. , Beach, S. D. , Sideridis, G. D. , & Gaab, N. (2016). Examining the relationship between home literacy environment and neural correlates of phonological processing in beginning readers with and without a familial risk for dyslexia: an fMRI study. Annals of Dyslexia, 66(3), 337–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada, R. D. , & Kishiyama, M. M. (2010). Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Frontiers in Human Neuroscience, 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada, R. D. , Richards, T. L. , Meltzoff, A. , & Kuhl, P. K. (2008). Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage, 40(3), 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle, N. , Zuk, J. , Ortiz‐Mantilla, S. , Sliva, D. D. , Franceschi, A. , Grant, P. E. , … Gaab, N. (2012). Pediatric neuroimaging in early childhood and infancy: Challenges and practical guidelines. Annals of the New York Academy of Sciences, 1252(1), 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon, S. F. (2011). The widening academic achievement gap between the rich and the poor: New evidence and possible explanations In Murnane R. & Duncan G. (Eds.), Whither opportunity (pp. 91–116). New York, NY: Russell Sage Foundation Press. [Google Scholar]
- Reardon, S. , & Portilla, X. (2016). Recent trends in income, racial, and ethnic school readiness gaps at kindergarten entry. AERA Open, 2(3), 1–18.26942210 [Google Scholar]
- Romeo, R. R. , Christodoulou, J. A. , Halverson, K. K. , Murtagh, J. , Cyr, A. B. , Schimmel, C. , … Gabrieli, J. D. (2017). Socioeconomic status and reading disability: Neuroanatomy and plasticity in response to intervention. Cerebral Cortex, 28(7), 2297–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo, R. R. , Leonard, J. A. , Robinson, S. T. , West, M. R. , Mackey, A. P. , Rowe, M. L. , & Gabrieli, J. D. (2018). Beyond the 30‐Million‐Word Gap: Children's Conversational Exposure Is Associated With Language‐Related Brain Function. Psychological science, 29(5), 700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, E. D. (2008). Sensory‐specific amnesia and hypoemotionality in humans and monkeys: Gateway for developing a hodology of memory. Cortex, 44(8), 1010–1022. [DOI] [PubMed] [Google Scholar]
- Rowe, M. L. , & Goldin‐Meadow, S. (2009). Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science, 323(5916), 951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, D. B. (1976). Inference and missing data. Biometrika, 63(3), 581–592. [Google Scholar]
- Saygin, Z. M. , Norton, E. S. , Osher, D. E. , Beach, S. D. , Cyr, A. B. , Ozernov‐Palchik, O. , … Gabrieli, J. D. (2013). Tracking the roots of reading ability: White matter volume and integrity correlate with phonological awareness in prereading and early‐reading kindergarten children. Journal of Neuroscience, 33(33), 13251–13258. 10.1523/JNEUROSCI.4383-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin, Z. M. , Osher, D. E. , Norton, E. S. , Youssoufian, D. A. , Beach, S. D. , Feather, J. , … Kanwisher, N. (2016). Connectivity precedes function in the development of the visual word form area. Nature Neuroscience, 19(9), 1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatschneider, C. , Fletcher, J. M. , Francis, D. J. , Carlson, C. D. , & Foorman, B. R. (2004). Kindergarten prediction of Reading skills: A longitudinal comparative analysis. Journal of Educational Psychology, 96(2), 265–282. [Google Scholar]
- Schlaffke, L. , Leemans, A. , Schweizer, L. M. , Ocklenburg, S. , & Schmidt‐Wilcke, T. (2017). Learning Morse code alters microstructural properties in the inferior longitudinal fasciculus: A DTI study. Frontiers in Human Neuroscience, 11, 383 10.3389/fnhum.2017.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann, J. D. , Pandya, D. N. , Wang, R. , Dai, G. , D'arceuil, H. E. , de Crespigny, A. J. , & Wedeen, V. J. (2007). Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain, 130(3), 630–653. [DOI] [PubMed] [Google Scholar]
- Scholz, J. , Klein, M. C. , Behrens, T. E. , & Johansen‐Berg, H. (2009). Training induces changes in white‐matter architecture. Nature Neuroscience, 12(11), 1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sénéchal, M. (1997). The differential effect of storybook reading on preschoolers' acquisition of expressive and receptive vocabulary. Journal of Child Language, 24(1), 123–138. [DOI] [PubMed] [Google Scholar]
- Sénéchal, M. , & leFevre, J. (2002). Parental involvement in development of children's reading skills: A five year longitudinal study. Child Development, 73(2), 445–460. [DOI] [PubMed] [Google Scholar]
- Sénéchal, M. , LeFevre, J. A. , Hudson, E. , & Lawson, E. P. (1996). Knowledge of storybooks as a predictor of young children's vocabulary. Journal of Educational Psychology, 88, 520–536. [Google Scholar]
- Skoe, E. , Krizman, J. , & Kraus, N. (2013). The impoverished brain: Disparities in maternal education affect the neural response to sound. Journal of Neuroscience, 33(44), 17221–17231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders, T. A. B. , & Bosker, R. J. (2012). Discrete dependent variables In Snijders T. A. B, Bosker R. J. (Eds.), Multilevel analysis: an introduction to basic and advanced multilevel modeling (pp. 304–307). Los Angeles;London: SAGE. [Google Scholar]
- Snowling, M. J. , Gallagher, A. , & Frith, U. (2003). Family risk of dyslexia is continuous: Individual differences in the precursors of reading skill. Child Development, 74(2), 358–373. [DOI] [PubMed] [Google Scholar]
- Song, S.‐K. , Sun, S.‐W. , Ju, W.‐K. , Lin, S.‐J. , Cross, A. H. , & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage, 20(3), 1714–1722. [DOI] [PubMed] [Google Scholar]
- Song, S.‐K. , Yoshino, J. , Le, T. Q. , Lin, S.‐J. , Sun, S.‐W. , Cross, A. H. , & Armstrong, R. C. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage, 26(1), 132–140. [DOI] [PubMed] [Google Scholar]
- Stothard, S. , Hulme, C. , Clarke, P. , Barmby, P. , & Snowling, M. (2010). YARC York assessment of reading for comprehension (Secondary). Itasca, IL: Riverside Publishing. [Google Scholar]
- Swartz, J. R. , Knodt, A. R. , Radtke, S. R. , & Hariri, A. R. (2018). Post‐secondary maternal education buffers against neural risk for psychological vulnerability to future life stress. Neuropsychologia, 109, 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y.‐Y. , Lu, Q. , Geng, X. , Stein, E. A. , Yang, Y. , & Posner, M. I. (2010). Short‐term meditation induces white matter changes in the anterior cingulate. Proceedings of the National Academy of Sciences of the United States of America, 107(35), 15649–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. , Roehrig, A. , Hensler, B. S. , Connor, C. , & Schatschneider, C. (2010). Teacher quality moderates the genetic effects on early reading. Science, 328(5977), 512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Mathworks Inc . (2007). MATLAB 7.5. Natick, MA: Author. [Google Scholar]
- Thiebaut de Schotten, M. , Cohen, L. , Amemiya, E. , Braga, L. W. , & Dehaene, S. (2014). Learning to read improves the structure of the arcuate fasciculus. Cerebral Cortex, 24(4), 989–995. 10.1093/cercor/bhs383 [DOI] [PubMed] [Google Scholar]
- Tisdall, M. D. , Hess, A. T. , Reuter, M. , Meintjes, E. M. , Fischl, B. , & van der Kouwe, A. J. (2012). Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic Resonance in Medicine, 68(2), 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski, P. , & Johnson, M. H. (2010). The effects of early adversity on the adult and developing brain. Current Opinion in Psychiatry, 23(3), 233–238. [DOI] [PubMed] [Google Scholar]
- Torgesen, J. K. , Wagner, R. K. , & Rashotte, C. A. (1999). TOWRE: Test of word reading efficiency. Austin, TX: PRO‐ED. [Google Scholar]
- Travis, K. E. , Adams, J. N. , Kovachy, V. N. , Ben‐Shachar, M. , & Feldman, H. M. (2017). White matter properties differ in 6‐year old readers and pre‐readers. Brain Structure and Function, 222(4), 1685–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka, J. M. , Readhead, C. , Bearer, E. L. , Pautler, R. G. , & Jacobs, R. E. (2006). Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. NeuroImage, 29(4), 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursache, A. , & Noble, K. G. (2016). Neurocognitive development in socioeconomic context: Multiple mechanisms and implications for measuring socioeconomic status. Psychophysiology, 53(1), 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden, K. I., Jr. , Gebregziabher, M. , Kuchinsky, S. E. , & Eckert, M. A. (2012). Multiple imputation of missing fMRI data in whole brain analysis. NeuroImage, 60(3), 1843–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten, M. , Boets, B. , Wouters, J. , & Ghesquiere, P. (2012). A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience & Biobehavioral Reviews, 36(6), 1532–1552. [DOI] [PubMed] [Google Scholar]
- Vandermosten, M. , Vanderauwera, J. , Theys, C. , De Vos, A. , Vanvooren, S. , Sunaert, S. , … Ghesquière, P. (2015). A DTI tractography study in pre‐readers at risk for dyslexia. Developmental Cognitive Neuroscience, 14, 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, R. K. , Torgesen, J. K. , & Rashotte, C. A. (1999). CTOPP: Comprehensive test of phonological processing. Austin, TX: PRO‐ED. [Google Scholar]
- Wakana, S. , Caprihan, A. , Panzenboeck, M. M. , Fallon, J. H. , Perry, M. , Gollub, R. L. , … Mori, S. (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage, 36(3), 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell, B. A. , Rauschecker, A. M. , & Yeatman, J. D. (2012). Learning to see words. Annual Review of Psychology, 63, 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Mauer, M. V. , Raney, T. , Peysakhovich, B. , Becker, B. L. , Sliva, D. D. , & Gaab, N. (2016). Development of tract‐specific white matter pathways during early Reading development in at‐risk children and typical controls. Cerebral Cortex, 1, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler, B. , Torchiano, M. , & Torchiano, M. M. (2016). Package ‘lmPerm’. R Package Version, 1.1‐2.
- Wolf, M. , & Denckla, M. B. (2005). RAN/RAS: Rapid automatized naming and rapid alternating stimulus tests. Austin, TX: PRO‐ED. [Google Scholar]
- Woodcock, R. (2011). Woodcock reading mastery test (WRMT‐III). San Antonio, TX: Pearson. [Google Scholar]
- Yeatman, J. D. , Dougherty, R. F. , Ben‐Shachar, M. , & Wandell, B. A. (2012a). Development of white matter and reading skills. Proceedings of the National Academy of Sciences of the United States of America, 109(44), E3045–E3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman, J. D. , Dougherty, R. F. , Myall, N. J. , Wandell, B. A. , & Feldman, H. M. (2012b). Tract profiles of white matter properties: Automating fiber‐tract quantification. PLoS One, 7(11), e49790 10.1371/journal.pone.0049790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman, J. D. , Dougherty, R. F. , Rykhlevskaia, E. , Sherbondy, A. J. , Deutsch, G. K. , Wandell, B. A. , & Ben‐Shachar, M. (2011). Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. Journal of Cognitive Neuroscience, 23(11), 3304–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman, J. D. , Rauschecker, A. M. , & Wandell, B. A. (2013). Anatomy of the visual word form area: Adjacent cortical circuits and long‐range white matter connections. Brain and Language, 125(2), 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Dietrich, U. M. , Geng, J.‐G. , Bicknell, R. , Esko, J. D. , & Wang, L. (2009). Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood, 114(19), 4300–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , de Schotten, M. T. , Altarelli, I. , Dubois, J. , & Ramus, F. (2016). Altered hemispheric lateralization of white matter pathways in developmental dyslexia: Evidence from spherical deconvolution tractography. Cortex, 76, 51–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1 White matter tracts (arcuate fasciculus‐red, inferior frontal occipital fasciculus‐yellow, and inferior longitudinal fasciculus‐green) investigated in the current study. Note: although the current study investigated these tracts bilaterally, only left hemispheric pathways are depicted [Color figure can be viewed at http://wileyonlinelibrary.com]
Supporting Information Table S1 Correlations between parental education and background and (pre) literacy measures.
Supporting Information Table S2: Correlations of SES and (pre)reading measures with mean FA for all available tracts
Data Availability Statement
The data required to reproduce reported findings is available at https://data.mendeley.com/datasets/jtc5dnykby/draft?a=aa39098f-a536-4f81-a89c-c922064c0b11. The analysis code will be provided upon request.


