Abstract
More than 80% of infectious bacteria form biofilm, which is a bacterial cell community surrounded by secreted polysaccharides, proteins and glycolipids. Such bacterial superstructure increases resistance to antimicrobials and host defenses. Thus, to control these biofilm-forming pathogenic bacteria requires antimicrobial agents with novel mechanisms or properties. Pseudomonas aeruginosa, a Gram-negative opportunistic nosocomial pathogen, is a model strain to study biofilm development and correlation between biofilm formation and infection. In this study, a recombinant hemolymph plasma lectin (rHPLOE) cloned from Taiwanese Tachypleus tridentatus was expressed in an Escherichia coli system. This rHPLOE was shown to have the following properties: (1) Binding to P. aeruginosa PA14 biofilm through a unique molecular interaction with rhamnose-containing moieties on bacteria, leading to reduction of extracellular di-rhamnolipid (a biofilm regulator); (2) decreasing downstream quorum sensing factors, and inhibiting biofilm formation; (3) dispersing the mature biofilm of P. aeruginosa PA14 to improve the efficacies of antibiotics; (4) reducing P. aeruginosa PA14 cytotoxicity to human lung epithelial cells in vitro and (5) inhibiting P. aeruginosa PA14 infection of zebrafish embryos in vivo. Taken together, rHPLOE serves as an anti-biofilm agent with a novel mechanism of recognizing rhamnose moieties in lipopolysaccharides, di-rhamnolipid and structural polysaccharides (Psl) in biofilms. Thus rHPLOE links glycan-recognition to novel anti-biofilm strategies against pathogenic bacteria.
Keywords: rhamnose-binding protein, anti-biofilm, quorum sensing factor, anti-infection
1. Introduction
Part of the reasons for bacterial resistance to antibiotics are due to the formation of biofilm, composed of secreted polysaccharides, proteins, glycolipids and small molecules in the bacterial microenvironment. Recent development of antimicrobial agents with novel molecular mechanisms to control bacterial infectious diseases has drawn much attention [1]. The inhibition of bacterial biofilm formation by non-microbicidal mechanisms is an example of anti-pathogenic approaches [2,3]. One nosocomial infectious bacteria, Pseudomonas aeruginosa, is responsible for various infections, particularly in immuno-compromised individuals [4], and it forms biofilms to make antibiotic treatments inefficient and therefore promotes acute infections, for example, at upper airway, skin, wound, urinary tract, enteric and lung [5]. P. aeruginosa PA14 is a clinically isolated and highly virulent strain representing the most common clonal group worldwide [6]. The PA14 genome showing a high degree of conservation compared to that of the P. aeruginosa strain PAO1 contains two specific pathogenicity islands. The PA14 islands carried several genes implicated in virulence that are absent in PAO1, including genes encoding effectors of the type III secretion system for secreting virulent factors [7,8]. These characteristics make PA14 a threat to public health, but a good model for P. aeruginosa infection studies.
Biofilm development is associated with changes in bacteria phenotype and metabolic pathways [9]. During biofilm development, physiological changes of bacterial cells are regulated by a chemical signaling mechanism involving cell-to-cell communication, such as quorum-sensing (QS) signaling. The process of P. aeruginosa biofilm development is mainly regulated by three interconnected QS systems: Two use acyl-l-homoserine lactones (AHLs) and the third uses aquinolone [10]. In the AHL QS system, LasI-synthase generates N-(3-oxododecanoyl)-l-homoserine lactone [11] and RhlI synthase generates N-butyryl-l-homoserine lactone [12]. The former is a key factor in the maturation stage of the biofilm [13], and the latter is responsible for production of another biofilm regulator, di-rhamnolipid [14]. Numerous molecules with anti-QS property have been reported to inhibit biofilm formation of P. aeruginosa [15]. AHL analogues have inhibitory activities on biofilm formation in P. aeruginosa by down-regulating LasR-based QS system (LasR, a transcriptional regulator response for AHL). Some modified AHL analogues can also down-regulate pyocyanin, a virulence factor with elastase activity [16]. Certain enzymes are secreted by mammalian cells such as paraoxonases, which lactonase activity can degrade P. aeruginosa AHLs and further inhibit QS and biofilm formation. Most of these AHL pathway inhibitors efficiently work under 10 μM.
There already exist many different anti-biofilm agents from various resources. For example, cultured broth from certain marine cold adapted bacteria destabilized biofilm of Pseudomonas aeruginosa [17] and some essential oils from Mediterranean plants or selected exopolysaccharide from marine bacteria acts as anti-QS factors to inhibit biofilm formation of Pseudomonas aeruginosa [18,19]. Although AHL pathway inhibitors already exist, they still cannot completely inhibit the biofilm produced by P. aeruginosa [15], indicating that biofilm development is not only controlled by the AHL pathway but also other pathways that might partially complement to develop biofilm. This hypothesis leads us to focus on factors that may directly regulate biofilm development. Di-rhamnolipid, as a glycolipid secreted by P. aeruginosa, has been implicated in each of the following phases of biofilm development: (i) Forming microcolonies [20], (ii) regulating both cell-to-cell and cell-to-surface interactions [21], (iii) creating and maintaining fluid channels for water and oxygen flow around the base of the biofilm [21] and (iv) facilitating 3-D mushroom-shaped structure formation [22]. Accordingly, di-rhamnolipid seems to be a regulator that might directly interact with biofilms. The influence of di-rhamnolipid to biofilm development was observed, but the interaction of di-rhamnolipid with other QS factors remains unclear. To develop anti-biofilm reagents directly targeting di-rhamnolipid might be a novel anti-biofilm approach.
Rhamnose binding proteins (RBPs) are mainly isolated from eggs, ovary cells of fish and invertebrates with l-rhamnose (Rha) or d-galactose binding specificities [23,24]. RBPs are typically located in immune-related tissues or cells [25], suggesting that RBPs may be relevant to self-defense mechanisms. RBPs may interact and agglutinate Gram-negative and Gram-positive bacteria by recognizing lipopolysaccharide (LPS) and lipoteichoic acid (LTA), respectively [26]. RBPs can recognize some O-antigens and bind to glycolipids and glycoproteins of fish pathogens [27]. The RBP receptor is expressed on peritoneal macrophages of fish after an inflammatory stimulation [28]. The tissue specificity of expression and ability to interact with bacteria indicates that RBPs are naturally related to the innate immune system in animals as a pathogen recognition element.
An RBP possessing specific binding of l-rhamnose was discovered from horseshoe crab plasma and recently engineered for expression in the Escherichia coli system [29]. This recombinant horseshoe crab plasma lectin (rHPL) possesses a very low sequence identity with known RBPs and does not have conserved domains. Interestingly, rHPL binds to bacteria or pathogen-associated molecular patterns (PAMPs) by recognizing rhamnose moieties and inhibits the growth of P. aeruginosa [29]. Unlike other RBLs, rHPL only binds to l-rhamnose and rhamnobiose but not to galactose or mannose [30]. This high substrate specificity makes rHPL a prospective candidate to bind rhamnose-containing components in biofilm of P. aeruginosa and examine the biological functions of these bindings.
2. Results
2.1. rHPLOE Was Expressed in E. coli and Purified by Affinity Chromatography
rHPL was successfully expressed in E. coli in 2014. The yield of rHPL purified using a nickel-affinity column was ~8 mg/L, and the purity was 93% [29]. To improve the productivity and solubility of rHPL, the codon usage of synthetic hpl (hploe) was optimized for E. coli, and recombinant HPLOE was co-expressed with chaperones. The expression product, rHPLOE, was purified by fast protein liquid chromatography (FPLC) equipped with a HisTrap™ affinity column following purification scheme in supplementary data showed in Table S1. rHPLDM, representing rHPL with two artificial mutations Y88A and F145A, which showed no binding activities to a bacterial cell- (Figure S1A) or pathogen-associated molecular pattern (PAMP; Figure S1B), was applied as a negative control in this study. Figure S2 illustrated the purification chromatogram and SDS polyacrylamide gel electrophoresis analysis data. The purified rHPLOE was desalted and concentrated using an Amicon protein concentrator (10 kDa cut-off) and subjected to further analysis. Figure S3 showed the secondary structure of rHPLOE determined by circular dichroism (CD) spectroscopy in comparison to those of rHPL and rHPLDM, indicating that both rHPLOE and rHPLDM possessed similar structures to rHPL [29]. A yield of 11.33 mg/L rHPLOE with a comparable purity. The yield of rHPLOE was 1.4-fold higher than that of rHPL, suggesting that the chaperone improved the solubility and stability of rHPL in the E. coli system.
2.2. rHPLOE Bound to Cell-Free Biofilm Matrix from P. aeruginosa PA14 via Recognizing Rhamnose
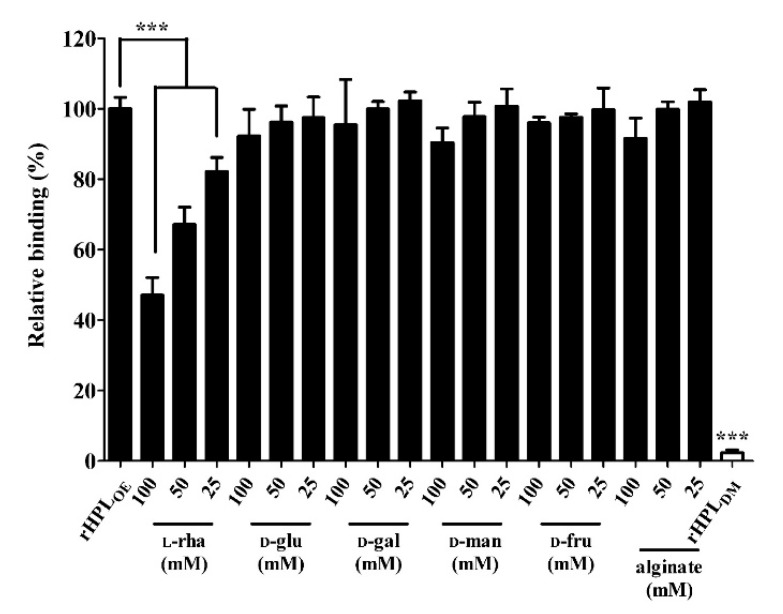
Our hypothesis is that rHPLOE might bind to rhamnose-containing components in the biofilm and further interrupt bacterial biofilm development. First, binding capacity of rHPLOE to the cell-free biofilm matrix from P. aeruginosa PA14 was tested. Here, a mature P. aeruginosa PA14 biofilm was extracted using a NaCl solution to give a cell-free biofilm matrix. In the extracted PA14 biofilm, the total protein was 0.19 mg/mL and polysaccharide, 0.32 mg/mL. Di-rhamnolipid, an important QS-factor and putative binding target of rHPLOE, was extracted using chloroform, and was 0.66 μg/mL by methylene blue method. The interaction between rHPLOE and the cell-free biofilm matrix was measured by ELISA, and the inhibitory effect of monosaccharides and alginate on rHPLOE-biofilm interaction was determined using competitive ELISA. rHPLOE at a final concentration of 0.1 μM was mixed with l-rhamnose and loaded into microplate wells coated separately with extracted P. aeruginosa PA14 biofilm. rHPLOE mixed with buffer only was used as a positive control. rHPLDM without rhamnose binding activity served as a negative control. As shown in Figure 1, addition of 25, 50, and 100 mM of l-rhamnose effectively reduced binding of rHPLOE to the extracted biofilm, in comparison with the positive control. The inhibitory constant (Ki) of l-rhamnose to rHPLOE interaction with the biofilm of P. aeruginosa PA14 was 98 mM. The addition of 100 Mm of d-mannose, d-glucose, d-fructose, d-galactose and alginate could not reduce the binding between rHPLOE and the extracted biofilm. These results indicated that rHPLOE specifically bound to rhamnose containing components in P. aeruginosa PA14 biofilm.
Figure 1.
Inhibitory effects of monosaccharides on the recombinant hemolymph plasma lectin (rHPLOE)-biofilm interaction. The binding activity of rHPLOE to the PA14 biofilm and inhibitory effects of monosaccharides or alginate on this binding were determined by competitive ELISA. rHPLDM was applied as a negative control. *** p < 0.001 versus the rHPLOE only group (positive control).
2.3. Synthetic Rhamnobiosides and a Pentasaccharide Inhibited rHPLOE-Biofilm Interaction
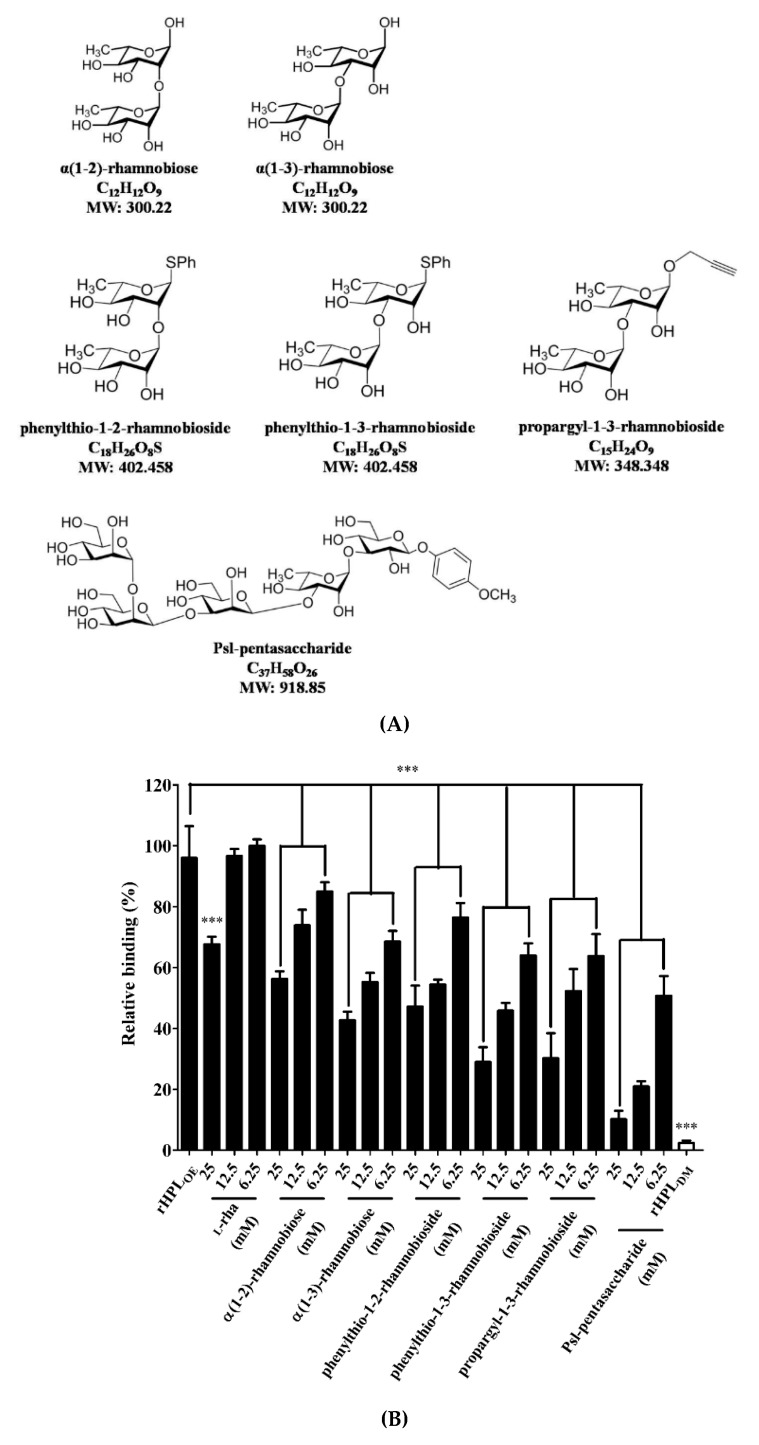
To confirm that binding of rHPLOE to the P. aeruginosa PA14 biofilm was via molecular recognition of rhamnose moiety, synthetic rhamno-oligosaccharides (Figure 2A,B) were used as competitors in a competitive ELISA. The chemical synthesis of phenylthio-rhamnobiosides and the Psl-pentasaccharide will be published elsewhere. The characterization of the new oligosaccharides used in this study is given in the Supplementary Information (Figures S4–S6).
Figure 2.
Structures of synthesized rhamnobioses and the rhamnose-containing Psl-pentasaccharide and inhibitory effects of thereof on the rHPLOE-biofilm interaction. (A) Structures of the synthesized rhamnosyl di- and pentasaccharides: α(1-2)-rhamnobiose, α(1-3)-rhamnobiose, phenylthio-1-2-rhamnobioside, phenylthio-1-3-rhamnobioside, propargyl-1-3-rhamnobioside and Psl-pentasaccharide. (B) The binding activity of rHPLOE on the biofilm from Pseudomonas aeruginosa PA14 and the inhibitory effects of rhamnose or rhamnobiosides on this binding were determined by competitive ELISA. *** p < 0.001 versus the rHPLOE-only group.
Figure 2 indicated that all synthetic compounds competed with the rHPLOE-biofilm interaction. The disaccharides are better inhibitor than monosaccharides, and (1-3)-linked rhamnosides were better than its (1-2)-linked counterparts. Psl-pentasaccharide showed the strongest competition presumably due to longer glycan chain length. The competitive effects of rhamnobiosides were stronger than those of rhamnobioses, indicating either a fixed anomeric configuration or hydrophobicity of aglycones slightly favors binding.
2.4. rHPLOE Bound to Di-Rhamnolipid via Recognizing Rhamnose Portion
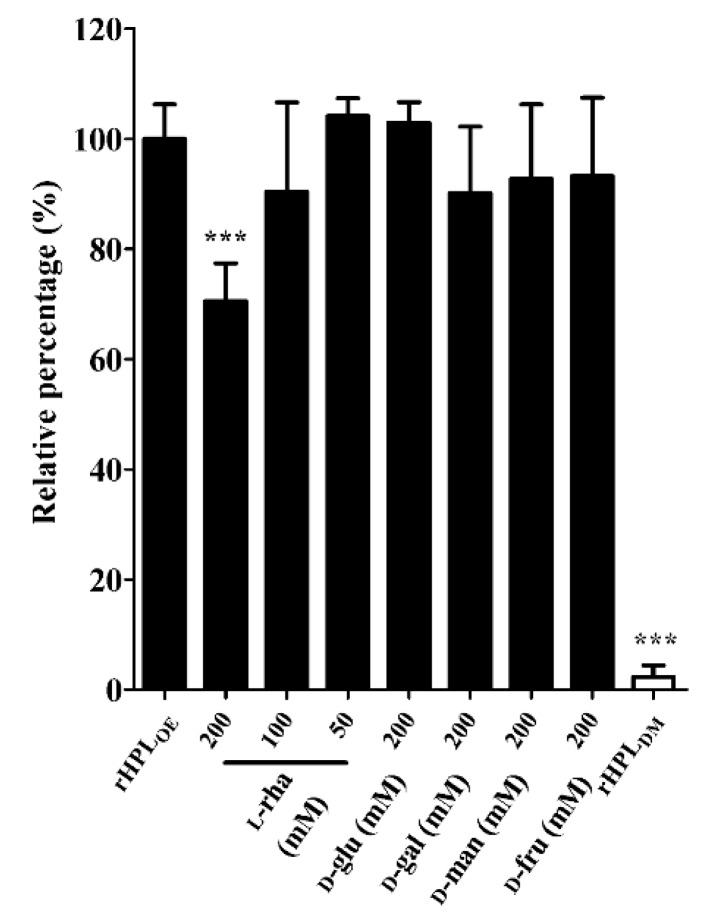
After confirming the binding activity of rHPLOE to synthetic rhamnobiosides, direct binding activity of rHPLOE to di-rhamnolipid from P. aeruginosa PA14 biofilm was measured by ELISA. Further competitive ELISA indicated that rHPLOE binding to di-rhamnolipid could be competed more effectively by l-rhamnose compared to other monosaccharides (Figure 3).
Figure 3.
Inhibitory effects of monosaccharides on the rHPLOE-di-rhamnolipid interaction. The binding activity of rHPLOE for di-rhamnolipid and inhibitory effects of monosaccharides on this binding was determined by competitive ELISA (competed with by l-rhamnose, d-glucose, d-galactose, d-mannose and d-fructose). *** p < 0.001 versus the rHPLOE-only group.
2.5. rHPLOE Inhibited Biofilm Formation and Dispersed the Preformed Biofilm of P. aeruginosa PA14
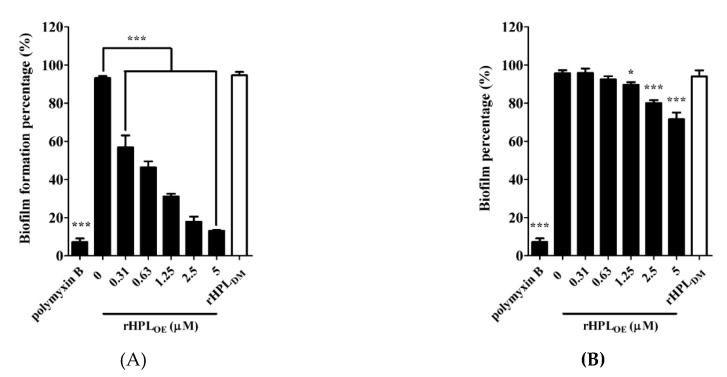
This experiment was conducted in M63 broth, a minimal low-osmolarity medium to slow down the growth rate of bacteria and mimic nutrient-depleted conditions for biofilm growth [31]. rHPLDM was applied as a negative control. As shown in Figure 4A, 0.31 µM and higher concentrations up to 5 µM of rHPLOE significantly inhibited the biofilm formation of P. aeruginosa PA14. Treatment with 0.31, 0.63, 1.25, 2.5 and 5 µM rHPLOE significantly reduced biofilm formation to 49%, 51%, 39%, 18% and 13%, respectively. As expected, rHPLDM did not inhibit biofilm formation. rHPLOE was also evaluated for its biofilm dispersion activity with preformed biofilm. The mature biofilm of P. aeruginosa PA14 was dispersed by 16% and 24% upon treatment with 2.5 and 5 µM rHPLOE for 24 h, respectively (Figure 4B). However, rHPLDM did not disperse the mature biofilm. These results indicated that rHPLOE inhibited biofilm formation and dispersed the mature biofilm of P. aeruginosa PA14 and that both activities perhaps are correlated with the rhamnose binding activity.
Figure 4.
Inhibitory and dispersion effect on the biofilm of P. aeruginosa PA14 by rHPLOE. Quantification of crystal violet staining associated with (A) the biofilm of P. aeruginosa PA14 after treatment with rHPLOE at the indicated concentration for 24 h and (B) after biofilm formation for 24 h and treatment with rHPLOE at the indicated concentration for a further 24 h. rHPLDM was applied as a negative control. The buffer-treated group was set as 100% (mock). * p < 0.05 and *** p < 0.001 versus the buffer-treated group.
2.6. rHPLOE Inhibited Swarming Activity and Decreased Secreted Rhamnolipids of P. aeruginosa PA14
Swarming motility is positively correlated with the amount of extracellular rhamnolipids, leading to rapid bacterial translocation that promotes efficient colonization of bacterial cells on a surface [32]. Under optimal growth conditions, P. aeruginosa cell population would spread along a solid surface and cover a large area. If swarming were inhibited, the bacterial cell population would gather together to form a colony. The swarming area of P. aeruginosa PA14 was significantly reduced upon treatment with rHPLOE but not rHPLDM (Figure S7A). The swarming area of PA14 was reduced by 33%, 43%, 48% and 46%, respectively, upon treatment with 0.63, 1.25, 2.5 and 5 µM rHPLOE for 72 h (Figure S7B). Figure 5 showed that the extracellular rhamnolipid levels of PA14 (11.7 ± 0.4 μg/mL in the control treated with buffer) reduced by 44%, 50%, 54%, 64% and 59% upon treatment with 0.31, 0.63, 1.25, 2.5 and 5 µM rHPLOE, respectively. However, the 5 µM rHPLDM treatment did not affect the extracellular rhamnolipid levels of PA14. This result was consistent with the swarming assay, indicating that the binding of rHPLOE to biofilm inhibited PA14 swarming activity by down-regulating the levels of extracellular di-rhamnolipid.
Figure 5.
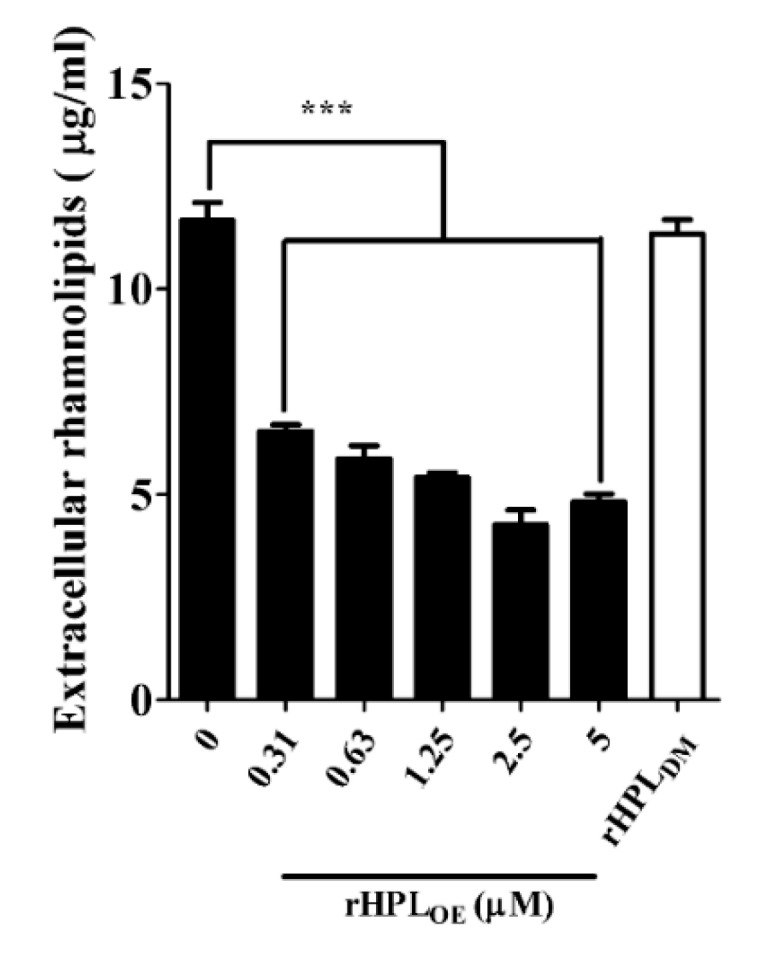
Down-regulation effect on extracellular rhamnolipids of P. aeruginosa PA14 by rHPLOE or rHPLDM. Extracellular rhamnolipids of P. aeruginosa PA14 treated by rHPLOE or rHPLDM were measured by the chloroform–methyl blue method. *** p < 0.001 versus the buffer-treated group.
2.7. rHPLOE Attenuated QS-Associated Factors
The development of biofilms appears to be regulated by the QS system. Most reagents that attenuate biofilm development work through inhibiting production of QS-factors or disrupting function of QS-factors by direct binding to QS-factors [33,34,35]. Among these agents, pyoverdine and pyocyanin are unique toxic molecules related to the virulence of P. aeruginosa PA14. To understand the attenuation mechanism of P. aeruginosa biofilm by rHPLOE, the effect of rHPLOE on QS-mediated azocasein-degrading protease activity and the secretion of two virulent factors, pyoverdine and pyocyanin were evaluated. Bacterial extracellular proteases degrade proteins in host cells (infected tissue), thereby facilitating bacterial invasion and growth [36]. Interestingly, bacterial protease secretion by P. aeruginosa PA14 was down-regulated by rHPLOE treatment (Figure S8A). The azocasein-degrading assay showed that 1.25 and 2.5 µM rHPLOE respectively decreased 16% and 12% of total proteolytic activities. Treatment with 0.31, 0.63, 1.25 and 2.5 µM rHPLOE decreased the pyoverdine levels by 12%, 8%, 17% and 21%, respectively (Figure S8B). Treatment with the same concentrations of rHPLOE decreased the pyocyanin levels by 41%, 56%, 45% and 40%, respectively (Figure S8C).
These results indicated that the binding of rHPLOE to the biofilm down-regulated the protease activities and QS-factors (Table 1). Consistent with previous observations, 2.5 µM rHPLDM did not show an inhibitory effect. Relatively weak inhibition of rHPLOE on protease activity and pyoverdine expression indicated that these effects might be indirect effects. The effect of rHPLOE on pyocyanin production was detectable but not concentration-dependent. A possible alternate reason for this may be that the inhibition effect of rHPLOE on pyocyanin production was compensated by other regulatory pathways.
Table 1.
Inhibition percentage (%) of QS-factors in rHPLOE treated P. aeruginosa PA14.
| rHPLOE(μM) | 0.31 | 0.63 | 1.25 | 2.5 | |
|---|---|---|---|---|---|
| QS-factors | |||||
| Activities of extracellular proteases | 7 ± 1.4 | 4.8 ± 0.8 | 15.9 ± 2.2 | 12.4 ± 1.4 | |
| Pyoverdine | 12.3 ± 0.2 | 8.2 ± 0.2 | 16.7 ± 0.2 | 21.2 ± 0.2 | |
| Pyocyanin | 40.8 ± 13.9 | 56.1 ± 4 | 45.4 ± 9.2 | 40.5 ± 24.2 | |
2.8. Combination Treatment with rHPLOE Improved Efficacies of Antibiotics on P. aeruginosa with Preformed Biofilms
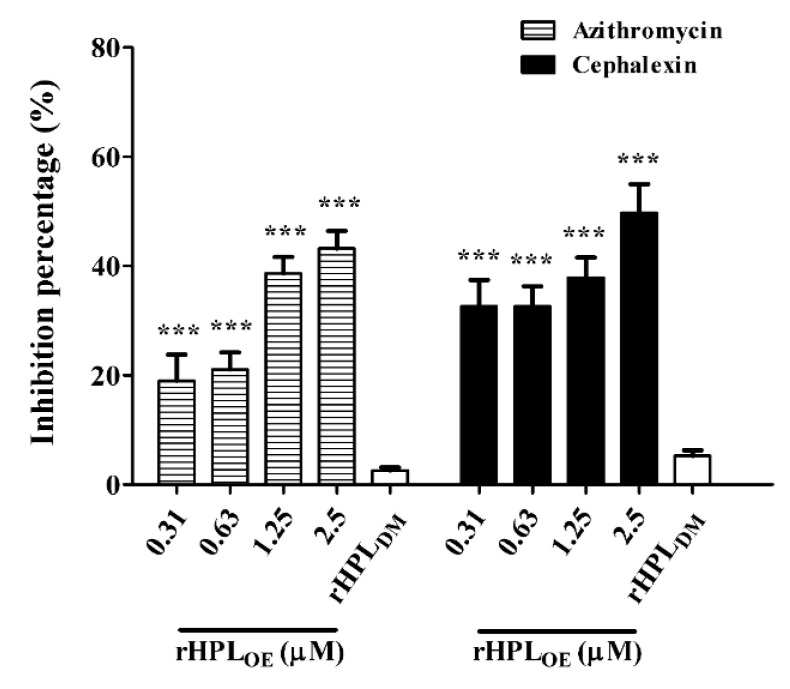
Since rHPLOE exhibited dispersion of P. aeruginosa PA14 biofilm, we hypothesized that rHPLOE might increase the activities of antibiotics against P. aeruginosa PA14 in mature biofilms. Total protein assay showed that the combination of rHPLOE with either azithromycin (hydrophobic) or cephalexin (hydrophilic) significantly attenuated the total protein levels in the biofilm. The IC50 of azithromycin and cephalexin to P. aeruginosa PA14 with pre-formed biofilm was 27.3 ± 1.4 and 27.7 ± 0.9 μg/mL, respectively. In the presence of 25 μg/mL azithromycin, in combination with 0.31, 0.63, 1.25 and 2.5 µM rHPLOE reduced the total protein in the biofilm by 19%, 21%, 39% and 43%, respectively, compared to the antibiotic-only control (Figure 6).
Figure 6.
Synergistic effect of rHPLOE with IC50 doses of azithromycin and cephalexin on P. aeruginosa. Quantity of the percentage of biofilm total protein inhibition (with respect to the antibiotic-only control) of P. aeruginosa PA14. *** p < 0.001 versus the control group.
In the presence of 25 μg/mL cephalexin, combination treatment with the same concentrations of rHPLOE reduced the total protein in biofilms by 33%, 33%, 38% and 50%, respectively, compared to the antibiotic-only control (Figure 6). This observation suggested that rHPLOE might facilitate antibiotics to kill P. aeruginosa PA14 by partially interfering with biofilm regulation and thus destroying the structure of the mature biofilm.
2.9. rHPLOE Inhibited Infection of P. aeruginosa in Mammalian Cells and Zebrafish Embryos
During P. aeruginosa infection, rhamnolipids and pyocyanin are the important virulence factors causing cytotoxicity. Since rHPLOE inhibited rhamnolipid and pyocyanin production in P. aeruginosa PA14, we predicted that rHPLOE could also reduce P. aeruginosa PA14 infection inhuman lung cells. To evaluate this hypothesis, cell death of human lung cell line A549 by P. aeruginosa PA14 infection in the presence of rHPLOE was examined. As shown in Figure 7, the PA14-infected A549 cell death was reduced by 14%, 21%, 57% and 73% in the presence of 0.31, 0.63, 1.25 and 2.5 µM rHPLOE, respectively.
Figure 7.
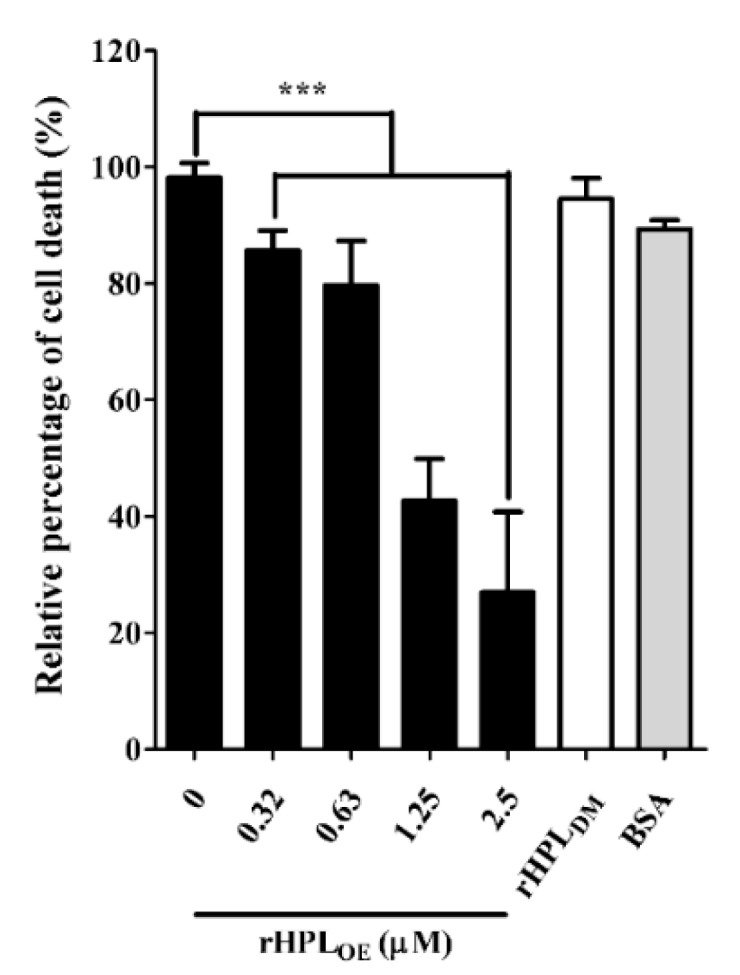
Inhibitory activities of rHPLOE on P. aeruginosa PA14 infection of human A549 lung cells. The percent cell death was quantified by the alamarBlue cell viability assay and normalized to cells in the absence of rHPLOE, for which the cell death was set as 100%. *** p < 0.001 versus the control group.
Regarding the negative control, 2.5 µM BSA or 2.5 µM rHPLDM did not influence A549 cell death. These assays clearly demonstrated that the mortality rate of A549 cells killed by P. aeruginosa PA14 decreased due to the presence of rHPLOE. Therefore, rHPLOE attenuated P. aeruginosa PA14 infection in human lung A549 cells through direct interaction between rHPLOE and rhamnose-containing bacterial compounds.
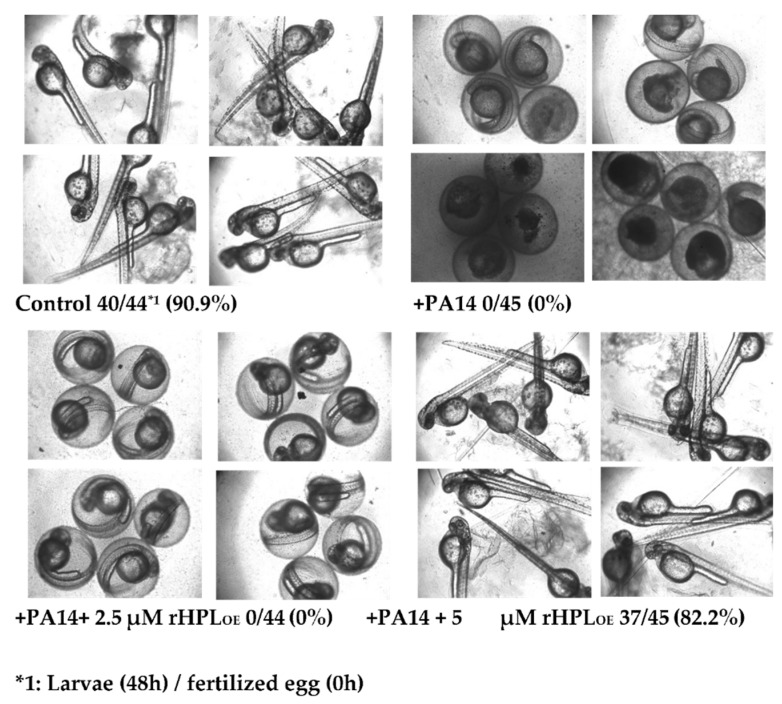
Zebrafish (Danio rerio) has become an important vertebrate animal model for many disease studies including pathogen infection [37] in recent years. Here fertilized eggs from zebrafish were used as an animal model to evaluate whether rHPLOE attenuated P. aeruginosa PA14 infection in vivo. Zebrafish embryos showed normal development in the absence of P. aeruginosa PA14, while the development of most embryos was significantly delayed or even died at 48 h following infection with P. aeruginosa PA14 (Figure 8).
Figure 8.
Inhibitory activity of rHPLOE on P. aeruginosa PA14 infection of zebrafish embryos. Zebrafish embryos were infected with P. aeruginosa PA14 in the presence or absence of rHPLOE and imaged after 48 h of infection. All of the pictures were taken with an inverted microscope at 40×.
However, when P. aeruginosa PA14 was pre-incubated with 5 μM rHPLOE, 82.2% of embryos developed normally as compared to control embryos showing a development rate of 90.9%. Zebrafish embryos treated with 2.5 μM rHPLOE still developed slowly, but almost all of the embryos survived. These results indicated that rHPLOE could protect zebrafish embryos from P. aeruginosa PA14 infection at concentrations higher than 2.5 μM.
3. Discussion
To study the carbohydrate components of the biofilm, carbohydrate-binding proteins, such as plant lectins, have been widely applied. Previously many different neutral carbohydrates including N-acetyl-d-glucosamine, N-acetyl-d-galactosamine, d-glucose and d-mannose were identified in the exopolymeric matrix of the biofilm [38]. Lectins with various specificities show an interaction with the carbohydrate components of biofilms. For example, concanavalin A from Canavalia ensiformis seeds is specific for d-glucose and d-mannose [39], and lectin from Triticum vulgaris germs is specific for N-acetyl-d-glucosamine and sialic acid [40]. l-Rhamnose as a unique sugar in bacteria and plants is a common component of the cell wall and capsule of many pathogenic bacteria including Gram-negative P. aeruginosa [41], Salmonella typhimurium [42], Vibrio cholera [43,44] as well as Mycobacterium tuberculosis [45]. P. aeruginosa produces at least three distinct exopolysaccharides that contribute to biofilm development and architecture: Alginate, Pel and Psl [46]. Alginate consists of only uronic acids, Pel is a glucose-rich polysaccharide and Psl consists of a repeating pentasaccharide containing d-mannose, d-glucose and l-rhamnose [47]. Psl serves as a primary structural scaffold for biofilm development. In addition, Psl is involved in early stages of biofilm formation and promoting cell-to-cell interactions [48,49]. Another rhamnose-containing component in the biofilm was the QS-factor di-rhamnolipid. A mutant strain (P. aeruginosa PAO1C-rhlAB) that could not produce rhamnolipids lost its swarming activity, which agreed with our observation in rHPLOE-treated PA14 [50]. rHPLOE bound to both Psl and di-rhamnolipid through targeting rhamnose moiety and synthetic rhamnobiosides-competitive ELISA showed that rHPLOE preferred to bind with α(1-3)-rhamnobiose rather than α(1-2)-rhamnobiose. The linkage of the rhamnose of Psl was reported to be α(1-3) [47] while that of di-rhamnolipid was α(1-2) [51], perhaps favoring that rHPLOE binding to rhamnose component in biofilm was governed by recognition of unique structure feature of rhamnosyl moiety. Although rHPLOE binding to Psl still required more abundant glycan for detail characterization, rHPLOE binding to di-rhamnolipid down-regulated the expression of di-rhamnolipid and QS-factors of P. aeruginosa and further inhibited biofilm formation.
The most important finding of this study was that the binding of rHPLOE to the P. aeruginosa PA14 biofilm inhibited biofilm development and disrupted the mature biofilm. Our data clearly showed that rHPLOE caused these bio-effects by interacting with components in the biofilm including structural polysaccharides or di-rhamnolipid, largely due to the down-regulation of QS-factors including di-rhamnolipid, pyocyanin, pyoverdine and extracellular proteases by interrupting the functions of di-rhamnolipid.
Based on the biofilm dispersion activity of rHPLOE, we proposed that rHPLOE possessed synergistic effects with antibiotics on P. aeruginosa. Two antibiotics commonly applied for treating P. aeruginosa infections, azithromycin, a hydrophobic azalide that kills bacteria by decreasing protein production and cephalexin, a hydrophilic beta-lactam that kills bacteria by inhibiting cell wall synthesis, were used in this study in combination with rHPLOE. The results showed that rHPLOE significantly improved the bactericidal activity of both antibiotics, strongly suggesting that rHPLOE was useful as a biofilm dispersion reagent for deconstructing the biofilm and improving the activities of antibiotics.
Many studies reported that P. aeruginosa infection could be inhibited by down-regulating QS-factors. Importantly, these anti-QS reagents (allicin, triazolyldihydrofuranone and baicalin hydrate) are effective against multidrug-resistant P. aeruginosa [33,34,35]. Studies in the past decade revealed that these anti-QS reagents also inhibited many human infections caused by biofilm-producing bacteria [52,53]. This fact is important for fighting human pathogenic bacteria, as biofilms are found to be involved in over 80% of microbial infections in humans [54]. Since rHPLOE could reduce the levels of pyoverdine and pyocyanin, we speculate that rHPLOE might also inhibit P. aeruginosa infection. It was found that rHPLOE significantly reduced the cytotoxicity towards A549 cells and neutralized toxicity (leading to development retardation or death) of the zebrafish embryo caused by P. aeruginosa PA14.
4. Conclusions
Binding and down-regulation of di-rhamnolipid has not attracted much attention so far. In this study, we optimized the production of rHPL in E. coli using chaperone co-expression. rHPLOE, a highly specific rhamnose binding protein, bound to not only bacterial cells and PAMPs [29] but also extracted cell-free biofilm from P. aeruginosa. In addition, such interaction inhibited biofilm formation and dispersed mature biofilm through down-regulating secretion of di-rhamnolipid in biofilm and further down-regulating other QS-factors including extracellular proteases, pyoverdine and pyocyanin. As a biofilm dispersion reagent, rHPLOE increased the antibiotic activity against P. aeruginosa PA14 with pre-formed biofilm. Therefore, rHPLOE promised to be an effective anti-biofilm agent for combination therapy. At cellular and animal levels, rHPLOE inhibited the infection and toxicity of P. aeruginosa PA14 towards human lung epithelial cells and zebrafish embryos. These results indicated that rHPLOE served as a novel anti-biofilm agent by targeting rhamnose-containing components in biofilm, which in turn linked glycan-recognition to novel anti-biofilm strategies against pathogenic bacteria.
5. Materials and Methods
5.1. Bacteria Strains, Growth Medium and Plasmid
Escherichia coli TOP10F’ (Invitrogen, Waltham, MA, USA) was used as a host for vector construction and DNA manipulation. E. coli BL21(DE3) (Invitrogen, Waltham, MA, USA) was used as a host for protein expression. Pseudomonas aeruginosa PA14 (serotype O19) was kindly provided by Dr. Hwan-You Chang (Institute of Molecular Medicine, National Tsing Hua University, Hsinchu, Taiwan). The vector pET-23a (+) (Novagen, Burlington, MA, USA) with a T7 promoter was used for recombinant protein expression in E. coli cells and sequence analysis. Takara’s Chaperone Plasmid Set (#3340, TaKaRa, Shiga, Japan) was used for chaperone co-expression. All other buffers and reagents were of the highest commercial purity.
5.2. Expression and Purification of rHPLOE
Chloramphenicol (≥98%, #C0378), ampicillin (96%–102%, #A9393), isopropyl β-d-1-thiogalactopyranoside (IPTG, ≥99%, #I6758), Tris base (ACS reagent, ≥99.8%, #252859), NaCl (ACS reagent, ≥99%, #746398), imidazole (≥99%, #I5513), HCl (#320331) and phenylmethylsulfonyl fluoride (PMSF, ≥99%, # 78830) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Luria-Bertani (LB, #244610) was purchased from BD (Franklin lake, NJ, USA). rHPLOE was the chaperone co-expressed product of the synthesized sequence (hplOE) and the codon of hplOE was optimized for E. coli expression. To generate a co-expression clone, pET23a-hplOE was transformed into E. coli BL21(DE3) competent cells containing pG-KJE8 (#3340, TaKaRa, Shiga, Japan), and transformants were selected by an LB plate with 20 μg/mL chloramphenicol and 50 μg/mL ampicillin. After induction with a final concentration of 0.1 mM IPTG at 16 °C for 16 h, cells were harvested by centrifugation (KUBOTA, Osaka, Japan), and the residues were suspended in equilibrium buffer (20 mM Tris-HCl, 200 mM NaCl and 5 mM imidazole, pH 7.4) supplemented with a protease inhibitor (1 mM PMSF) and disrupted by three passages through a cell homogenizer (Sonicator 3000, Misonix, Farmingdale, NY, USA) at 15,000 psi. The recombinant proteins were purified using a HisTrap™ HP immobilized metal ion affinity column (#17524701, GE Healthcare, Little Chalfont, Buckinghamshire, UK) and ÄKTA start system (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Purified proteins were then concentrated and buffer-exchanged to the Tris buffer (20 mM Tris-HCl and 200 mM NaCl, pH 7.4) using a 15 kDa cut off Amicon Ultra centrifugal filter unit (#UFC901096, Millipore, Burlington, MA, USA).
5.3. Biofilm Formation and Extraction
Bacterial biofilm extraction was as described by Chibaet et al. [55]. P. aeruginosa PA14 grown on LB plates was inoculated in LB medium and incubated overnight at 37 °C with 250 rpm circular shaking. A portion of the overnight culture was 1000-fold diluted in 10 mL LB medium and incubated at 37 °C to an A600nm of 1. Bacterial cells with biofilm were harvested from incubated solution by centrifugation at 8000× g for 10 min at 25 °C (KUBOTA, Osaka, Japan). The harvested pellet was re-suspended with 1 mL of 1.5 M NaCl to extract a cell-free biofilm component. The suspensions were centrifuged at 5000× g for 10 min at 25 °C (KUBOTA, Osaka, Japan), and the supernatants containing the biofilm fraction were collected.
5.4. Polysaccharide Quantification in the Extracted Biofilm
Absolute ethanol (≥99.8%, #32221), phenol (#P1037) and sulfuric acid (95%–98%, #435589) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Polysaccharide in biofilm was measured as described by Tribedi and Sil [56]. Extracted biofilm solution was mixed with 2.2 volumes of chilled absolute ethanol, incubated at −20 °C for 1 h and centrifuged at 3500× g for 20 min at 4 °C (KUBOTA, Osaka, Japan). The pellet containing exopolysaccharides was dissolved in sterile water and measured by the phenol-sulfuric acid method. Fifty microliters of re-suspended sample was mixed with 200 μL phenol, and then, 750 μL sulfuric acid was added. The solution was left standing for 40 min, and vigorously shaken. After shaking, the A490nm was measured using a spectrophotometer (U3310, HITACHI, Tokyo, Japan), and the amount of total sugar (excluding amino sugars) was calculated with a standard curve established using glucose.
5.5. Binding Activity of rHPLOE to Biofilm or Di-Rhamnolipid
Di-rhamnolipid (95%, #R95DD), phosphate buffer saline (PBS, #P3813), Tween-20 (#P1379), l-rhamnose (≥99%, #R3875), d-glucose (≥99.5%, #G8270), d-galactose (≥99%, #G0750), d-fructose (≥99%, #F0127), d-mannose (≥99%, #M8574) and sodium alginate (#W201502) was purchased from Sigma-Aldrich (St. Louis, MO, USA). For competitive enzyme-linked immunosorbent assays (ELISA), the extracted biofilm or prepared di-rhamnolipid solution (120 μg/mL in ddH2O) was diluted in 10× the volume of the coating solution (#5150−0014, SeraCare KPL, Milford, MA, USA), and 50 μL of the mixture was added to each well of the 96-well microplate (#442404, Thermo Fisher, Waltham, MA, USA) and incubated at 4 °C overnight. After blocking with a blocking reagent (#10057177103, Roche, Basel, Switzerland) at 37 °C for 1 h, the plates were washed with PBST (PBS with 0.05% Tween-20) three times. To the washed wells, 25 μL of 0.2 μM rHPLOE was mixed with 25 μL of two-fold the indicated concentration of l-rhamnose, d-glucose, d-galactose, d-fructose, d-mannose or sodium alginate and the mixture was added to the wells, which were maintained at 37 °C for 1 h. Fifty microliters of 0.1 μM purified rHPLOE was added in parallel as a positive control, and Tris-buffer (20 mM Tris-HCl and 200 mM NaCl, pH 7.4) was added in parallel as a negative control. After washing three times with PBST, the microplates were incubated with monoclonal anti-His-tag (1:5000; #631212, Clontech, Mountain View, CA, USA) in PBST at 37 °C for 1 h. Subsequently, horseradish peroxidase-conjugated anti-mouse IgG (1:5000; #AB2338512, Jackson ImmunoResearch, West Grove, PA, USA) in PBST was added to the microplates, and after washing three times with PBST, the plates were incubated at 37 °C for 1 h. After being washed three times with PBST, 100 μL of 3,3’,5,5’-tetramethylbenzidine substrate (#5120-0077, SeraCare KPL, Milford, MA, USA) was added to each well and incubated at 37 °C for exactly 15 min. Finally, the reaction was terminated by the addition of 100 μL of 2 N H2SO4. The A450nm was read using a microplate spectrophotometer (iMark Microplate Absorbance Reader, Bio-Rad, Hercules, CA, USA). The inhibitory constant (Ki) was analyzed with GraphPad 5.0 software (GraphPad Software, La Jolla, CA, USA; fitted with a Binding-Competitive/Onesite-Fit Ki).
5.6. Anti-Biofilm Assay
KH2PO4 (≥99%, #P5655), K2HPO4 (≥99%, #P3768), (NH4)2SO4 (≥99%, #A4418), MgSO4 (≥99.5%, #M7506), L-arginine (≥98%, #A5006), polymyxin B (meets USP testing specifications, #P0972) and crystal violet (≥90%, #C0775) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Biofilm formation was described by O’Toole [57]. Briefly, overnight culture of the P. aeruginosa PA14 in LB medium was diluted with fresh M63 medium (22 mM KH2PO4, 40 mM K2HPO4, 15 mM (NH4)2SO4, 1 mM MgSO4 and 0.4% l-arginine). A total of 1 × 106 CFU/well bacterial cells were then cultured with various concentrations of rHPLOE in a final volume of 100 µL M63 medium onto a 96-well clear round bottom polystyrene microplate (#3788, Thermo Fisher, Waltham, MA, USA) and incubated at 37 °C for 24 h without shaking. A standard antibiotic polymyxin B (50 µg/mL) often used to kill P. aeruginosa in other studies was applied as a positive control, and M63 only was used as a negative control. The suspended culture was then discarded, and the plate was washed with ddH2O twice to remove any remaining suspended cells in the microtiter wells. The biofilm was then stained with 125 µL of 0.1% crystal violet for 15 min, after which the stained biofilm was washed with ddH2O five times to remove any unbound dye. The crystal violet bound to the biofilm was solubilized with 125 µL 30% (v/v) acetic acid and quantified by measuring the A595nm using the microplate spectrophotometer (iMark Microplate Absorbance Reader, Bio-Rad, Hercules, CA, USA). For the biofilm dispersion test, biofilm cultures were grown statically in 80 µL of M63 medium for 24 h, followed by the addition of 20 µL of the indicated concentration of rHPLOE in M63 medium and incubation for an additional 24 h. The addition of polymyxin B (50 µg/mL) to the culture before biofilm formation was used as a positive control.
5.7. Swarming Motility Measurement
The swarming motility of P. aeruginosa PA14 was investigated in plates containing swarming motility media (LB with 0.5% (wt) glucose and 0.6% (wt) agar). Agar was purchased from OXOID (#LP0011, Basingstoke, Hampshire, UK). An aliquot of 2 μL of motility media containing 1 × 106 CFU/mL bacterial cells was inoculated in plates with different concentrations of rHPLOE. Subsequently, spots were dired for 20 min at room temperature and incubated at 37 °C for 48 h. The diameter of the circular bacterial growth was measured [50].
5.8. Extracellular Rhamnolipid Quantification
Methylene blue (≥82%, #M9140), sodium borate (ACS reagent, ≥99.5%, #S9640) and chloroform (≥99.5%, #C2432) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Rhamnolipid was quantified as described by Pinzon et al. [58]. The supernatant of the anti-biofilm test was collected, and bacterial cells were removed by centrifugation (KUBOTA, Osaka, Japan). The pH of the cell-free supernatant was first adjusted to 2.3 ± 0.2 using 1 N HCl. Two hundred microliters of acidified sample was then extracted with the same volume of chloroform five times. One milliliter of the chloroform extract was carefully removed and mixed with 40 μL of 0.1% methylene blue (freshly prepared and pH adjusted to 8.6 ± 0.2 with 50 mM borate buffer) and 960 μL of distilled water in a 2 mL tube. After being vigorously shaken for 4 min, the samples were left to stand for 15 min. The chloroform phase was transferred into a quartz cuvette and value of A638 nm was measured by spectrophotometer (U3310, HITACHI, Tokyo, Japan). The values were converted to rhamnolipid concentrations using a calibration curve established by applying the same procedure to standard rhamnolipid solutions of different concentrations.
5.9. Azocasein Degradation Assay
The proteolytic activity was determined in the cell-free culture supernatant according to the method by Kessler et al. [59]. The amount of protease released by P. aeruginosa treated by rHPLOE, the supernatant of the anti-biofilm test was collected by centrifugation (KUBOTA, Osaka, Japan). One hundred and fifty microliters of supernatant were added to 0.3% of 1 mL azocasein (#A2765, Sigma-Aldrich St. Louis, MO, USA) in 50 mM Tris-HCl (pH 7.5) and incubated at 37 °C for 15 min. The reaction was stopped by the addition of 0.5 mL 10% trichloroacetic acid, and the mixture was centrifuged at 8000× g for 5 min (KUBOTA, Osaka, Japan) to obtain a clear supernatant. The absorbance of the clear supernatant was then measured at 400 nm (U3310, HITACHI, Tokyo, Japan) [60].
5.10. Pyoverdine Quantification Assay
P. aeruginosa was incubated with different concentrations of rHPLOE at 37 °C for 24 h. Thereafter, the cultured liquid was centrifuged at 8000× g for 15 min (KUBOTA, Osaka, Japan), and the cell-free supernatant was used for the pyoverdine measurement. The relative concentrations of pyoverdine in all of the treated supernatants with respect to the control was measured through a fluorescence microplate spectrophotometer (Victor2, PerkinElmer, Waltham, MA, USA) at an excitation wavelength of 405 nm and an emission wavelength of 465 nm [61].
5.11. Pyocyanin Quantification Assay
The pyocyanin quantification assay was performed according to the method described by Essar et al. [62]. P. aeruginosa was incubated with different concentrations of rHPLOE at 37 °C for 24 h, and the cell-free supernatant was collected by centrifugation (KUBOTA, Osaka, Japan). Five milliliters of the supernatant were extracted with 3 mL of chloroform and the chloroform layer was re-extracted with 1 mL of 0.2 N HCl to produce an orange yellow to pink solution, and the chloroform phase was transferred into a quartz cuvette and value of A520 nm was measured by a spectrophotometer (U3310, HITACHI, Tokyo, Japan).
5.12. Synergistic Effect of rHPLOE with Antibiotics
The experiment design was according to the method described by Das et al. [63]. P. aeruginosa with a mature biofilm was treated with rHPLOE in combination with either azithromycin (≥95%, #75199, Sigma-Aldrich, St. Louis, MO, USA) [63] or cephalexin (≥95%, #C4895, Sigma-Aldrich, St. Louis, MO, USA) [64]. Solutions of rHPLOE in 0.3125 µM, 0.625 µM, 1.25 µM and 2.5 µM in combination with different concentrations of antibiotics were directly added to the cultured bacterial liquid with a mature biofilm and held at 37 °C for 24 h. The total protein and amount of biofilm were measured to validate the synergistic antibacterial effect of rHPLOE and antibiotics.
5.13. Total Protein Concentration Measurement in the Biofilm
Concentration of the extractable protein was determined as a measure of the P. aeruginosa biofilm population density. The microbial population density in the biofilm is assumed to be directly proportional to the extractable protein concentration [56]. After incubation, planktonic P. aeruginosa cells were removed, and adhered cells remained in the biofilm were gently washed with sterile PBS and boiled for 30 min in 5 mL of 0.5 N NaOH (ACS reagent, ≥97%, #221465, Sigma-Aldrich, St. Louis, MO, USA) to extract the protein. After that, the suspension was centrifuged at 8000× g for 5 min (KUBOTA, Osaka, Japan), and the resulting clear supernatant was collected. The supernatant protein concentration was then measured by the bicinchoninic acid (BCA) protein assay kit (#23225, Thermo Fisher, Waltham, MA, USA).
5.14. Anti-A549 Infection Assay
The Roswell Park Memorial Institute (RPMI)-1640 medium (#10-040 CMS) and Antibiotics–Antimycotic solution (PSA, #30-004-CIS) was purchased from Corning (Corning, NY, USA). Characterized fetal bovine serum (FBS, #SH30071) was purchased from HyClone Laboratory (Logan, UT, USA). The A549 infection model was modified from method described by Chi [65]. A549 cells (ATCC® number: CCL-185™, BCRC, Hsinchu, Taiwan), adenocarcinomic human lung cells, were seeded in 96-well tissue culture plates at 2 × 104 containing 100 µL of RPMI-1640 medium supplemented with 10% (v/v) fetal FBS and 1% (v/v) PSA and allowed to grow at 37 °C for 16 to 18 h. Culture supernatants were removed, the monolayer was washed once with PBS buffer, and then 50 µL of serum-free RPMI-1640 containing two times the indicated concentration of rHPLOE was added to cells and incubated for 30 min, followed by inoculation with P. aeruginosa PA14. RPMI-1640 with 5 µM BSA or rHPLDM was used as negative controls. For inoculation, the fresh bacterial cells cultured in LB broth were washed with PBS, re-suspended and diluted in RPMI-1640 medium to a concentration of 1 × 108 CFU/mL. Thereafter, 50 µL of the bacterial dilution was applied to the rHPLOE-treated A549 cells at a multiplicity of infection (MOI) of 50 (i.e., 1 × 106 CFU/50 µL/well). The blank group was A549 cells treated by 2.5 μM rHPLOE without bacterial infection. After infection for 16 h at 37 °C, the A549 cell viability was determined by the AlamarBlue cell viability assay (BUF012B, Bio-Rad, Hercules, CA, USA). It should be noted that 50 µg/mL polymyxin B should be added to alamarBlue reagent to prevent a survival signal from residual bacteria. The level of viability, expressed as a percentage, was calculated as follows: % viability = [OD of assay cells/OD of control cells] × 100.
5.15. Anti-Zebrafish Infection Assay
Zebrafish embryos were purchased from Gendanio Biotech Inc. (New Taipei City, Taiwan). Healthy, transparent and regular embryos were selected and aliquoted into 96-well plates with four embryos per well, followed by incubation at 28 °C in 100 µL embryo water. Thereafter, 100 µL of P. aeruginosa (1 × 108 CFU/mL) pre-incubated with 5 mM rHPLOE for 1 h was added to the embryos at 24 h post-fertilization (hpf). The zebrafish embryos were further incubated at 28 °C, and the development of each embryo in 24 h and 48 h was observed using an inverted microscope (TS100, NIKON, Tokyo, Japan) equipped with a digital camera. The number of larva at 48 h in each group was counted, and the rate of successful development was calculated. All zebrafish related experiments were conducted in accordance with the ethical guidelines of Council of Agriculture, Executive Yuan and Ministry of Science and Technology.
5.16. Statistical Analyses
All statistical analyses were carried out using GraphPad Prism version 5.01 for Windows (GraphPad Software, La Jolla, CA, USA). Each value was the average of three measurements, where the presented data is the mean ± SD. All means were compared by one-way ANOVA.
Acknowledgments
We thank Hwan-You Chang for providing P. aeruginosa PA14. We also thank Yung-Jen Chuang for directing the experiments about zebrafish, as well as for providing valuable comments.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/6/355/s1. The supplementary materials contained: 1. Table S1 FPLC purification scheme of rHPLOE, 2. Figure S1. Bacterial cell or PAMP binding activity of rHPLOE and rHPLDM, 3. Figure S2. Purification and characterization of rHPLOE with HisTrap™ HP immobilized metal ion affinity chromatography, 4. Figure S3. Secondary structure analysis of rHPLOE, rHPLDM and rHPL using circular dichroism (CD), 5. Figure S4. Structure and NMR spectra (1H and 13C) of pehnylthio-1-2-rhamnobioside, 6. Figure S5. Structure and NMR spectra (1H and 13C) of phenylthio-1-3-rhamnobioside, 7. Figure S6. Structure and NMR spectra (1H and 13C) of Psl-pentasaccharide. 4-Methoxyphenyl α-d-mannopyranosyl-(1→2)-β-d-mannopyranosyl-(1→3)-β-d-mannopyranosyl-(1→3)-α-l-rhamnopyranosyl-(1→3)-β-d-glucopyranoside, 8. Figure S7. Inhibitory effect on the swarming motility of P. aeruginosa PA14 by rHPLOE or rHPLDM, 9. Figure S8. Down-regulation effect on the extracellular protease activities or QS-factors of P. aeruginosa PA14 by rHPLOE.
Author Contributions
Conceptualization, T.-K.F. S.-K.N. and Y.-C.L.; Methodology, T.-K.F., S.-K.N, A.B., C.-Y.L., C.-H.C, C.-L.C and Y.-C.L.; Resources, F.D., M.H. and A.B.; Experiments were carried out by T.-K.F., S.-K.N., F.D. and Y.-E.C.; Writing—original draft preparation, T.-K.F.; writing—review and editing, S.-K.N., A.B., C.-H.C, C.-L.C., C.-Y.L., Y.-C.L. and M.D.-T.C.; Funding acquisition, project administration and supervision, M.D.-T.C. and Y.-C.L.; All authors read and approved the final manuscript.
Funding
This study was funded by Ministry of Science and Technology (MOST) grants (MOST 103-2627-M-007-006 and MOST 107-0210-01-19-04) to Dr. Margaret Dah-Tsyr Chang, as well as by Simpson Biotech Co., Ltd. (Taiwan). The research was also supported by the EU and co-financed by the European Regional Development Fund under the projects GINOP-2.3.2-15-2016-00008 (A. B.) and by the ÚNKP-18-3 New National Excellence Program of the Ministry of Human Capacities of Hungary (F. D.).
Conflicts of Interest
The authors declare no conflict of interest. The funders (Simpson Biotech Co., Ltd.) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Cegelski L., Marshall G.R., Eldridge G.R., Hultgren S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008;6:17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalia V.C., Purohit H.J. Quenching the quorum sensing system: Potential antibacterial drug targets. Crit. Rev. Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 3.Sharma G., Rao S., Bansal A., Dang S., Gupta S., Gabrani R. Pseudomonas aeruginosa biofilm: Potential therapeutic targets. Biologicals. 2014;42:1–7. doi: 10.1016/j.biologicals.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Deep A., Chaudhary U., Gupta V. Quorum sensing and bacterial pathogenicity: From molecules to disease. J. Lab. Physicians. 2011;3:4–11. doi: 10.4103/0974-2727.78553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS. Suppl. 2013:1–51. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 6.Wiehlmann L., Wagner G., Cramer N., Siebert B., Gudowius P., Morales G., Kohler T., Van Delden C., Weinel C., Slickers P., et al. Population structure of Pseudomonas aeruginosa. Proc. Nat. Acad. Sci. USA. 2007;104:8101–8106. doi: 10.1073/pnas.0609213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J., Baldini R.L., Deziel E., Saucier M., Zhang Q., Liberati N.T., Lee D., Urbach J., Goodman H.M., Rahme L.G. The broad host range pathogen Pseudomonas aeruginosa strain PA14 carries two pathogenicity islands harboring plant and animal virulence genes. Proc. Nat. Acad. Sci. USA. 2004;101:2530–2535. doi: 10.1073/pnas.0304622101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikkelsen H., Ball G., Giraud C., Filloux A. Expression of Pseudomonas aeruginosa CupD fimbrial genes is antagonistically controlled by RcsB and the EAL-containing PvrR response regulators. PLoS ONE. 2009;4:e6018. doi: 10.1371/journal.pone.0006018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watnick P., Kolter R. Biofilm, city of microbes. J. Bacteriol. 2000;182:2675–2679. doi: 10.1128/JB.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobsen T.H., Bjarnsholt T., Jensen P.O., Givskov M., Hoiby N. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: Current and emerging inhibitors. Future Microbiol. 2013;8:901–921. doi: 10.2217/fmb.13.57. [DOI] [PubMed] [Google Scholar]
- 11.Gambello M.J., Iglewski B.H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochsner U.A., Koch A.K., Fiechter A., Reiser J. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 1994;176:2044–2054. doi: 10.1128/jb.176.7.2044-2054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies D.G., Parsek M.R., Pearson J.P., Iglewski B.H., Costerton J.W., Greenberg E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 14.Pearson J.P., Pesci E.C., Iglewski B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasamiravaka T., Labtani Q., Duez P., El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 2015;2015:759348. doi: 10.1155/2015/759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith K.M., Bu Y., Suga H. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 2003;10:563–571. doi: 10.1016/S1074-5521(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 17.Papa R., Selan L., Parrilli E., Tilotta M., Sannino F., Feller G., Tutino M.L., Artini M. Anti-biofilm activities from marine cold adapted bacteria against staphylococci and Pseudomonas aeruginosa. Front. Microbiol. 2015;6:1333. doi: 10.3389/fmicb.2015.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artini M., Patsilinakos A., Papa R., Bozovic M., Sabatino M., Garzoli S., Vrenna G., Tilotta M., Pepi F., Ragno R., et al. Antimicrobial and antibiofilm activity and machine learning classification analysis of essential oils from different mediterranean plants against Pseudomonas aeruginosa. Molecules. 2018;23:482. doi: 10.3390/molecules23020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S., Liu G., Jin W., Xiu P., Sun C. Antibiofilm and anti-infection of a marine bacterial exopolysaccharide against Pseudomonas aeruginosa. Front. Microbiol. 2016;7:102. doi: 10.3389/fmicb.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pamp S.J., Tolker-Nielsen T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007;189:2531–2539. doi: 10.1128/JB.01515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davey M.E., Caiazza N.C., O’Toole G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003;185:1027–1036. doi: 10.1128/JB.185.3.1027-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glick R., Gilmour C., Tremblay J., Satanower S., Avidan O., Deziel E., Greenberg E.P., Poole K., Banin E. Increase in rhamnolipid synthesis under iron-limiting conditions influences surface motility and biofilm formation in Pseudomonas aeruginosa. J. Bacteriol. 2010;192:2973–2980. doi: 10.1128/JB.01601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naganuma T., Ogawa T., Hirabayashi J., Kasai K., Kamiya H., Muramoto K. Isolation, characterization and molecular evolution of a novel pearl shell lectin from a marine bivalve, Pteria penguin. Mol. Divers. 2006;10:607–618. doi: 10.1007/s11030-006-9051-3. [DOI] [PubMed] [Google Scholar]
- 24.Tateno H. SUEL-related lectins, a lectin family widely distributed throughout organisms. Biosci. Biotechnol. Biochem. 2010;74:1141–1144. doi: 10.1271/bbb.100086. [DOI] [PubMed] [Google Scholar]
- 25.Tateno H., Yamaguchi T., Ogawa T., Muramoto K., Watanabe T., Kamiya H., Saneyoshi M. Immunohistochemical localization of rhamnose-binding lectins in the steelhead trout (Oncorhynchus mykiss) Dev. Comp. Immunol. 2002;26:543–550. doi: 10.1016/S0145-305X(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 26.Tateno H., Ogawa T., Muramoto K., Kamiya H., Saneyoshi M. Rhamnose-binding lectins from steelhead trout (Oncorhynchus mykiss) eggs recognize bacterial lipopolysaccharides and lipoteichoic acid. Biosci. Biotechnol. Biochem. 2002;66:604–612. doi: 10.1271/bbb.66.604. [DOI] [PubMed] [Google Scholar]
- 27.Booy A., Haddow J.D., Olafson R.W. Isolation of the salmonid rhamnose-binding lectin STL2 from spores of the microsporidian fish parasite Loma salmonae. J. Fish Dis. 2005;28:455–462. doi: 10.1111/j.1365-2761.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 28.Sugawara S., Hosono M., Ogawa Y., Takayanagi M., Nitta K. Catfish egg lectin causes rapid activation of multidrug resistance 1 P-glycoprotein as a lipid translocase. Biol. Pharm. Bull. 2005;28:434–441. doi: 10.1248/bpb.28.434. [DOI] [PubMed] [Google Scholar]
- 29.Ng S.K., Huang Y.T., Lee Y.C., Low E.L., Chiu C.H., Chen S.L., Mao L.C., Chang M.D. A recombinant horseshoe crab plasma lectin recognizes specific pathogen-associated molecular patterns of bacteria through rhamnose. PLoS ONE. 2014;9:e115296. doi: 10.1371/journal.pone.0115296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herczeg M., Mezo E., Molnar N., Ng S.K., Lee Y.C., Dah-Tsyr Chang M., Borbas A. Inhibitory effect of multivalent rhamnobiosides on recombinant horseshoe crab plasma lectin interactions with Pseudomonas aeruginosa PAO1. Chem. Asian J. 2016;11:3398–3413. doi: 10.1002/asia.201601162. [DOI] [PubMed] [Google Scholar]
- 31.Mah T.F., O’Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/S0966-842X(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 32.Kumar L., Chhibber S., Harjai K. Zingerone inhibit biofilm formation and improve antibiofilm efficacy of ciprofloxacin against Pseudomonas aeruginosa PAO1. Fitoterapia. 2013;90:73–78. doi: 10.1016/j.fitote.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Hentzer M., Wu H., Andersen J.B., Riedel K., Rasmussen T.B., Bagge N., Kumar N., Schembri M.A., Song Z., Kristoffersen P., et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim B., Park J.S., Choi H.Y., Yoon S.S., Kim W.G. Terrein is an inhibitor of quorum sensing and c-di-GMP in Pseudomonas aeruginosa: A connection between quorum sensing and c-di-GMP. Sci. Rep. 2018;8:8617. doi: 10.1038/s41598-018-26974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subhadra B., Kim D.H., Woo K., Surendran S., Choi C.H. Control of biofilm formation in healthcare: Recent advances exploiting quorum-sensing interference strategies and multidrug efflux pump inhibitors. Materials. 2018;11:1676. doi: 10.3390/ma11091676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musthafa K.S., Saroja V., Pandian S.K., Ravi A.V. Antipathogenic potential of marine Bacillus sp. SS4 on N-acyl-homoserine-lactone-mediated virulence factors production in Pseudomonas aeruginosa (PAO1) J. Biosci. 2011;36:55–67. doi: 10.1007/s12038-011-9011-7. [DOI] [PubMed] [Google Scholar]
- 37.Goldsmith J.R., Jobin C. Think small: Zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012;2012:817341. doi: 10.1155/2012/817341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purish L.M., Asaulenko L.G., Abdulina D.R., Voitchuk S.I., Iutinskaia G.A. Lectin-binding analysis of the biofilm exopolymeric matrix carbohydrate composition of corrosion-aggressive bacteria. Prikl. Biokhim. Mikrobiol. 2013;49:450–456. doi: 10.7868/s0555109913050103. [DOI] [PubMed] [Google Scholar]
- 39.Sumner J.B., Gralen N., Eriksson-Quensel I.B. The molecular weights of urease, canavalin, concanavalin a and concanavalin B. Science. 1938;87:395–396. doi: 10.1126/science.87.2261.395. [DOI] [PubMed] [Google Scholar]
- 40.Wu A.M., Wu J.H., Song S.C., Tsai M.S., Herp A. Studies on the binding of wheat germ agglutinin (Triticum vulgaris) to O-glycans. FEBS Lett. 1998;440:315–319. doi: 10.1016/S0014-5793(98)01469-0. [DOI] [PubMed] [Google Scholar]
- 41.Burrows L.L., Charter D.F., Lam J.S. Molecular characterization of the Pseudomonas aeruginosa serotype O5 (PAO1) B-band lipopolysaccharide gene cluster. Mol. Microbiol. 1996;22:481–495. doi: 10.1046/j.1365-2958.1996.1351503.x. [DOI] [PubMed] [Google Scholar]
- 42.Li Q., Reeves P.R. Genetic variation of dTDP-l-rhamnose pathway genes in Salmonella enterica. Microbiology. 2000;146:2291–2307. doi: 10.1099/00221287-146-9-2291. [DOI] [PubMed] [Google Scholar]
- 43.Chiang S.L., Mekalanos J.J. rfb mutations in Vibrio cholerae do not affect surface production of toxin-coregulated pili but still inhibit intestinal colonization. Infect. Immun. 1999;67:976–980. doi: 10.1128/iai.67.2.976-980.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita Y., Tomihisa K., Nakano Y., Shimazaki Y., Oho T., Koga T. Recombination between gtfB and gtfC is required for survival of a dTDP-rhamnose synthesis-deficient mutant of Streptococcus mutans in the presence of sucrose. Infect. Immun. 1999;67:3693–3697. doi: 10.1128/iai.67.7.3693-3697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma Y., Stern R.J., Scherman M.S., Vissa V.D., Yan W., Jones V.C., Zhang F., Franzblau S.G., Lewis W.H., McNeil M.R. Drug targeting Mycobacterium tuberculosis cell wall synthesis: Genetics of dTDP-rhamnose synthetic enzymes and development of a microtiter plate-based screen for inhibitors of conversion of dTDP-glucose to dTDP-rhamnose. Antimicrob. Agents Chemother. 2001;45:1407–1416. doi: 10.1128/AAC.45.5.1407-1416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryder C., Byrd M., Wozniak D.J. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 2007;10:644–648. doi: 10.1016/j.mib.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrd M.S., Sadovskaya I., Vinogradov E., Lu H., Sprinkle A.B., Richardson S.H., Ma L., Ralston B., Parsek M.R., Anderson E.M., et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 2009;73:622–638. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma L., Conover M., Lu H., Parsek M.R., Bayles K., Wozniak D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009;5:e1000354. doi: 10.1371/journal.ppat.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colvin K.M., Gordon V.D., Murakami K., Borlee B.R., Wozniak D.J., Wong G.C., Parsek M.R. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011;7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris J.D., Hewitt J.L., Wolfe L.G., Kamatkar N.G., Chapman S.M., Diener J.M., Courtney A.J., Leevy W.M., Shrout J.D. Imaging and analysis of Pseudomonas aeruginosa swarming and rhamnolipid production. Appl. Environ. Microbiol. 2011;77:8310–8317. doi: 10.1128/AEM.06644-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel-Mawgoud A.M., Lepine F., Deziel E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Environ. Microbiol. 2010;86:1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manner S., Fallarero A. Screening of natural product derivatives identifies two structurally related flavonoids as potent quorum sensing inhibitors against gram-negative bacteria. Int. J. Mol. Sci. 2018;19:1346. doi: 10.3390/ijms19051346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tshikantwa T.S., Ullah M.W., He F., Yang G. Current trends and potential applications of microbial interactions for human welfare. Front. Microbiol. 2018;9:1156. doi: 10.3389/fmicb.2018.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biel M.A. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol. Biol. 2010;635:175–194. doi: 10.1007/978-1-60761-697-9_13. [DOI] [PubMed] [Google Scholar]
- 55.Chiba A., Sugimoto S., Sato F., Hori S., Mizunoe Y. A refined technique for extraction of extracellular matrices from bacterial biofilms and its applicability. Microb. Biotechnol. 2015;8:392–403. doi: 10.1111/1751-7915.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tribedi P., Sil A.K. Cell surface hydrophobicity: A key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J. Appl. Microbiol. 2014;116:295–303. doi: 10.1111/jam.12375. [DOI] [PubMed] [Google Scholar]
- 57.O’Toole G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011 doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinzon N.M., Ju L.K. Analysis of rhamnolipid biosurfactants by methylene blue complexation. Appl. Microbiol. Biotechnol. 2009;82:975–981. doi: 10.1007/s00253-009-1896-9. [DOI] [PubMed] [Google Scholar]
- 59.Kessler E., Safrin M., Olson J.C., Ohman D.E. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J. Biol. Chem. 1993;268:7503–7508. [PubMed] [Google Scholar]
- 60.Wiegand I., Hilpert K., Hancock R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 61.Adonizio A., Kong K.F., Mathee K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob. Agents Chemother. 2008;52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Essar D.W., Eberly L., Hadero A., Crawford I.P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das M.C., Sandhu P., Gupta P., Rudrapaul P., De U.C., Tribedi P., Akhter Y., Bhattacharjee S. Attenuation of Pseudomonas aeruginosa biofilm formation by Vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016;6:23347. doi: 10.1038/srep23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bassetti M., Vena A., Croxatto A., Righi E., Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chi E., Mehl T., Nunn D., Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect. Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.