Abstract
Worldwide, there is a rise in the prevalence of allergic diseases, and novel efficient therapeutic approaches are still needed to alleviate disease burden. Prostaglandin D2 (PGD2) has emerged as a central inflammatory lipid mediator associated with increased migration, activation and survival of leukocytes in various allergy-associated disorders. In the periphery, the hematopoietic PGD synthase (hPGDS) acts downstream of the arachidonic acid/COX pathway catalysing the isomerisation of PGH2 to PGD2, which makes it an interesting target to treat allergic inflammation. Although much effort has been put into developing efficient hPGDS inhibitors, no compound has made it to the market yet, which indicates that more light needs to be shed on potential PGD2 sources and targets to determine which particular condition and patient will benefit most and thereby improve therapeutic efficacy. In this review, we want to revisit current knowledge about hPGDS function, expression in allergy-associated cell types and their contribution to PGD2 levels as well as beneficial effects of hPGDS inhibition in allergic asthma, rhinitis, atopic dermatitis, food allergy, gastrointestinal allergic disorders and anaphylaxis.
Keywords: hPGDS, hPGDS inhibitor, PGD2, DP receptors, allergic inflammation, eosinophilic inflammation
1. Introduction
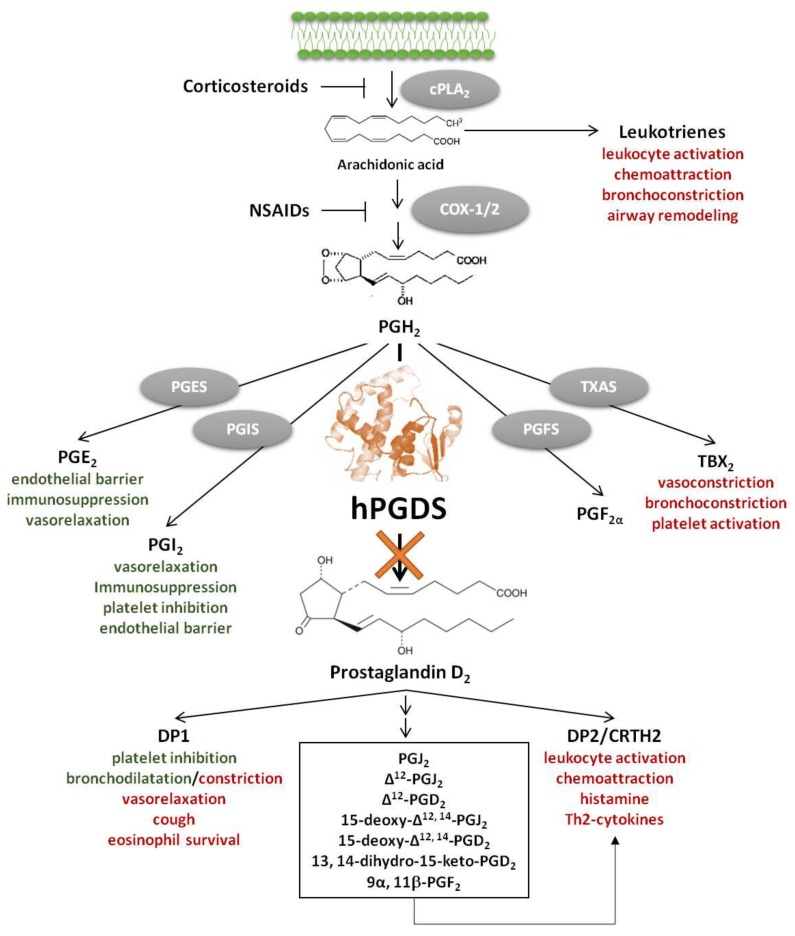
Accumulating evidence suggests a central role of the pro-inflammatory lipid mediator Prostaglandin D2 (PGD2) in allergy development and progression [1,2,3]. PGD2 is a potent pro-inflammatory lipid mediator downstream of the arachidonic acid/cyclooxygenase (COX) pathway. Arachidonic acid-derived lipid mediators including leukotrienes, lipoxins, thromboxane A2, PGD2, prostaglandin E2 (PGE2) and prostacyclin (PGI2) play a central role in allergic inflammation; each of them having specific immunomodulatory functions (Figure 1). Notably, in contrast to COX inhibition, specific inhibition of unfavourable pro-inflammatory PGD2 effects and its metabolites would keep physiological functions of beneficial mediators like PGE2 and prostacyclin intact. In mice, about 90% of the systemic biosynthesis of PGD2 is generated by the hematopoietic PGD synthase (hPGDS)-dependent pathway and only partially through lipocalin-type PGD synthase (LPGDS) [4]. Most prostaglandins are generated by competitive enzymatic interactions, however, it has been suggested that prostaglandins may also be generated from precursor eicosanoids by non-enzymatic conversion [5], which also needs to be taken into account in a therapeutic setting. PGD2 exerts its function by activating two G-protein coupled receptors, d-type prostanoid receptor 1 (DP1) and 2 (DP2), the latter also being referred to as chemoattractant receptor homologous-molecule expressed in Th2 cells (CRTH2) [6]. DP1-mediated responses include inhibition of platelet aggregation, vasorelaxation and bronchodilatation [7], but DP1 antagonists have also been found to ameliorate rhinitis, conjunctivitis and pulmonary inflammation in animal models [8,9,10], while DP1 receptor activation aggravated neutrophil infiltration in acute lung injury [11]. In contrast, DP2/CRTH2 receptor activation has primarily been linked to pro-inflammatory effects including initiation and potentiation of immune cell migration, respiratory burst, type 2 cytokine production and histamine release [3]. PGD2 is a potent modulator of inflammation; apparently, its influence strongly depends on whether it acts in the early or late phase of inflammation. On the one hand, it has been reported that in acute inflammation, i.e., experimental dermatitis [12] and colitis [13], lipopolysaccharide-induced pulmonary inflammation [14] as well as in anaphylactic shock [15], PGD2 seems to have protective effects. On the other hand, in late phase skin inflammation [12], and chronic and allergic inflammation [16,17], PGD2/CRTH2/DP2 activation exacerbates leukocyte migration, activation and survival, while DP1 activation has been linked to increased mucus production and airway hyperreactivity [18]. In addition, some PGD2 metabolites, such as 15-deoxy-Δ12,14-PGJ2 have been shown to exert anti-inflammatory, pro-resolving effects by activating nuclear receptors, e.g., peroxisome proliferator-activated receptors (PPAR)-γ [19] but the physiological relevance thereof is still unclear [20].
Figure 1.
hPGDS as therapeutic target downstream of the arachidonic acid/cyclooxygenase (COX) pathway. Hematopoietic PGDS inhibition specifically targets PGD2 and PGD2 metabolite production—mediators that primarily activate pro-inflammatory DP2/CRTH2 receptor [1]. Non-steroidal anti-inflammatory drugs (NSAIDs) block all lipid mediators downstream of COX-1/2, including potentially beneficial effects of PGE2 and PGI2. Corticosteroids are standard-of-care therapeutics of asthmatic patients that effectively block all downstream products of arachidonic acid including leukotrienes; however, therapy interferes with many physiological processes causing numerous adverse effects. Favorable effects of selected lipid mediators in allergic inflammation highlighted in green; unfavorable effects highlighted in red.
Taken together, both PGD2 receptors, DP1 and DP2/CRTH2, have emerged as potential drug targets for the treatment of allergic diseases and beyond [1,21,22]. However, as an alternative to receptor blockade, great clinical interest has also been attributed to the development of hPGDS inhibitors to nip PGD2 signalling in the bud and thereby attenuate allergic inflammation, and potentially other conditions.
2. hPGDS Structure, Function and Regulation
Two distinct rate-limiting PGD synthases have been described, lipocalin-type PGD synthase (LPGDS) and hematopoietic PGD synthase (hPGDS), which differ vastly in origin, structure, tissue distribution, and functional context. LPGDS is primarily localized in the central nervous system, and reproductive tracts; it is secreted into cerebrospinal fluid and the bloodstream, whereby this enzyme does not need reduced glutathione (GSH) as a co-factor [23]. In contrast, hPGDS is a Sigma-class glutathione transferase expressed in peripheral tissues and catalyzes the isomerization of PGH2 to PGD2 using GSH and Ca2+ or Mg2+ as cofactors [24]. The hPGDS enzyme forms a homodimer with 23 kDa subunits and each subunit is associated with one GSH [25]. Site-directed mutagenesis indicates that Lys112, Cys156, and Lys198 are involved in the binding of PGH2, Trp104 is critical for structural integrity of the catalytic center for GSH-transferase (GST) and PGD synthase activities, and Tyr8 and Arg14 are essential for activation of the thiol group of glutathione [26]. Notably, LPGDS is quite different in terms of catalytic properties, amino acid sequence, tertiary structure, evolutional origin, gene structure, chromosomal localization, cellular localization, tissue distribution and also functional relevance—a good example of functional convergence [23]. A number of naturally occurring hPGDS enzyme variants have been identified, differing in thermal stability and half-life in relation with GST-activity [27]. In this study, they could identify a highly stable hPGDS isoenzyme (Val187Ile) found in African Americans, which seems to be associated with reduced colorectal cancer risk. However, whether different hPGDS variants are associated with allergic disease or other inflammatory disorders has not been clarified to date. Aritake et al. suggest a functional coupling between COX-2 and hPGDS as well as a possible role of hPGDS membrane translocation in modulation of efficient PGD2 synthesis [28]. There is evidence that hPGDS activity is dependent on pH, which has an impact on H+ abstraction from the GSH thiol group [24]. Zhao et al. could show that reactive oxygen species are crucial for proper hPGDS function. Selective inhibition of NADPH oxidase-2 in murine bone marrow-derived macrophages attenuated bacterial lipopolysaccharide (LPS)-induced production of PGD2 but not PGE2 [29]. In line with this, Ishii highlights the importance of the transcription factor nuclear-erythroid-2 p45-related factor (Nrf2) in combination with peroxiredoxin 1 and 6 in regulating PGD2 production by hPGDS in murine bone marrow-derived macrophages [30]. Oxidative stress and activation of the Toll-like receptor 4 trigger nuclear factor-кB signaling and peroxiredoxin 6 phosphorylation, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activity, which in turn activates NADPH Oxidase-2 [31]. This also stresses the importance of NADPH oxidase-2 activation in maintaining cellular glutathione levels to ensure efficient hPGDS function. Interestingly, Nrf2 seems to be involved in a positive feedback induction of LPGDS by hPGDS-derived PGD2 and 15-deoxy-Δ12,14-PGJ2 in murine macrophages, thereby contributing to resolution of LPS-induced lung inflammation in mice [32]. Another study in human eosinophils found that PGD2 synthesis was located at the nuclear envelope and was associated with intracellular lipid bodies [33]. Inhibition of hPGDS with a specific inhibitor (HQL-79) also reduced lipid body formation in eosinophils.
3. Hematopoietic PGDS Expression in Leukocytes and Parenchymal Cells
Hematopoietic PGD synthase is differentially expressed in peripheral tissues, varying between species. In the rat, hPGDS could be detected on protein level in spleen, bone marrow, liver, colon, small intestine, skin and thymus, while the highest expression of hPGDS mRNA in human tissue was found in macrophages, placenta, intestine, adipose tissue and foetal liver [34]. Allergy-associated cell types reported to express hPGDS on mRNA or protein level and their PGD2 production potential have been summarized in Table 1.
Table 1.
Cell type specific hPGDS expression and reported PGD2 release.
| Cell Type | Species | hPGDS Expression | Stimuli | PGD2 Release | Ref. | |
|---|---|---|---|---|---|---|
| Parenchymal Cells | Epithelial cells | ms hu |
mRNA, protein mRNA, protein |
RSV infection Unstimulated IL-13, IL-4 |
1.2 ng/mL 100 pg/mL 25–50 pg/mL |
[43,93] [93] [105] |
| Endothelial cells | hu |
LPGDS-mRNA, protein LPGDS-protein |
sheer stress tumor CM |
150 pg/mL 600 pg/mL |
[101] [102] |
|
| Smooth muscle cells | ms | mRNA, protein | OVA lung inflammation | [103] | ||
| Keratinocytes | hu | protein | antimycotics | 8 ng/106 cells | [97] | |
| Immune Cells | Monocytes | hu | unstimulated | 66 fmol/106 cells | [67] | |
| Macrophages | ms | protein mRNA |
LPS zymosan A -(peritoneal lavage) |
20 ng/mL 800 pg/mouse |
[29,73] [75] |
|
| hu | mRNA | IL-4, HDM | 400 pg/mL | [77] | ||
| rat | protein | [11,63] | ||||
| Dendritic cells | hu | mRNA, protein | LPS, INF-γ | 250 pg/106 cells | [82] | |
| rat, h | protein | [63,83] | ||||
| Eosinophils | ms hu |
mRNA, protein mRNA, protein |
eotaxin/A23187 eotaxin/A23187 |
15 pg/2 × 106 cells 15–80 pg/2 × 106 |
[33] | |
| hu | mRNA, protein | Lysin-Aspirin | 1.5 ng/105 cells | [50] | ||
| Neutrophils | ms | protein | [14] | |||
| Basophils | ms hu |
mRNA, protein protein |
TNP-OVA (IgE) IL-3, anti IgE |
700 pg/5 × 105 cells 100 pg/5 × 105 cells |
[56] [56,57] |
|
| Mast cells | hu rat |
anti-IgE anti-IgE anti-IgE |
40 ng/106 cells 1 ng/104 cells 13 ng/106 cells |
[39] [106] [39] |
||
| ms | protein | [41,42,44] | ||||
| ILC2 | hu | mRNA mRNA |
IL-33, IL-25, TSLP | 1.5 ng/mL | [84] [107] |
|
| Th2 cells | hu | mRNA, protein | OKT3, KOLT-2 | 30 ng/5 × 105 | [88] | |
| hu | protein | PMA, ionomycin | 70 pg/mL | [89] |
Abbreviations: ILC—innate lymphoid cells, Th2—CD4+ type-2 helper T cells, ms—mouse, hu—human, IgE—Immunoglobulin E, TNP—2,4,6-Trinitrophenyl hapten, OVA—ovalbumin, LPS—lipopolysaccharide, TSLP—thymic stromal lymphopoietin, RSV—respiratory syncytial virus, CM—conditioned medium, HDM—house dust mite, PMA—phorbol 12-myristate 13-acetate, OKT-3—anti-CD3 antibody, KOLT-2—anti-CD28 antibody.
3.1. Immune Cells
Mast cells have been characterized most thoroughly as potential PGD2 sources in allergic disease. They are mainly tissue-resident and located in the skin and mucosal surfaces throughout the body, with increased abundance in asthmatic patients, inflammatory bowel disease and atopic dermatitis [35,36,37]. A transcriptional signature approach revealed that the hPGDS transcript is enriched in human and murine tissue-resident mast cells as compared to other cells analyzed [38]. Upon allergen-induced cross-linking of Fcε-bound IgE mast cells produce high local concentrations of PGD2 in the nanomolar range [39]. Baothman et al. observed that PGD2 generation in isolated human lung mast cells largely relied on COX-1 and hPGDS but not LPGDS, while COX-2 was absent both at baseline and after LPS or stem cell factor stimulation [40]. In food antigen-induced mast cell hyperplasia in mice, c-Kit+/FcE-RI+ mast cells in colon sections stained highly positive for hPGDS, and tetranor PGD metabolite, a mast cell-derived PGD2 metabolite, proved to be a specific biomarker for food allergy in patients [41,42]. Higher levels of hPGDS but not LPGDS expression could also be found in the nasal mucosa of patients with allergic rhinitis, where mast cells could be identified as hPGDS+ cells next to infiltrating eosinophils, macrophages and lymphocytes [43]. In mice, hPGDS+ lung tumor-infiltrating c-Kit+/FcE-RI+ mast cells could be identified by immunofluorescence microscopy [40,44].
Granulocytes are short-lived cells of the innate immune system with characteristically lobed nuclei and carry granules with antimicrobial peptides and cytotoxic proteins. Accumulation of eosinophil granulocytes is a hallmark of allergic inflammation where they contribute to airway hyper-reactivity in asthma, mucus production and tissue remodeling [45,46]. Activated eosinophils secrete various cytotoxic proteins and pro-inflammatory mediators that drive Th2-type inflammation including IL-4, IL-5, IL-10 and IL-13 [47]. Both DP receptors are expressed on the cell surface, mediating eosinophil migration and activation by PGD2 [3,48,49]; however, recently it has been shown that eosinophils themselves can act as PGD2 sources. Human peripheral blood eosinophils and mouse bone marrow-derived eosinophils constitutively express hPGDS and release up to 80 pg PGD2/ 2 × 106 cells upon stimulation in a HQL-79-sensitive manner [33]. Similarly, infiltrating eosinophils in the nasal mucosa of patients with allergic rhinitis express hPGDS [43]. Interestingly, Feng et al. observed that human peripheral blood eosinophils from patients with aspirin-exacerbated respiratory disease express significantly higher levels of hPGDS on protein and mRNA level than eosinophils from asthmatic or healthy subjects [50]. Eosinophils from these patients released PGD2 up to 1 ng/105 cells upon stimulation.
Basophils, despite their relatively low numbers in comparison with other effector cells, such as mast cells and eosinophils, have attracted notice in allergic responses including systemic anaphylaxis, atopic dermatitis, allergic rhinitis and asthma [51,52,53,54,55]. Via DP2/CRTH2, PGD2 acts as potent chemoattractant and activator for basophils which may result in basophil accumulation at sites of inflammation [2]. Like mast cells, basophils are activated by IgE-receptor cross-linking, which triggers secretion of various cytokines favoring a type-2 inflammation, including IL-4 [51]. Besides contributing to cytokine levels in allergic inflammation, basophils have also been considered as PGD2 sources. Ugajin et al. showed that murine basophils express hPGDS on mRNA and protein level. Further, sensitization of mouse bone marrow-derived as well as peripheral blood basophils with anti-TNP-IgE and subsequent stimulation with TNP-OVA triggered PGD2 (up to 700 pg PGD2/5 × 105 basophils) and PGE2 release at similar levels [56]. Primary human basophils also released PGD2 (100 pg PGD2/5 × 105 basophils) after priming with IL-3, sensitization and stimulation with anti-human IgE, but interestingly no PGE2 could be detected. The authors suggest that human basophils might favor the hPGDS pathway over the PGES pathway, thereby augmenting allergic inflammation [56]. Recently, another group addressed the role of basophils in systemic lupus erythematosus development and found that autocrine PGD2 production and CXCR4-dependent stimulation of basophils can be reversed by using a specific hPGDS inhibitor (HPGDS inhibitor I, Cayman) [57].
Neutrophils comprise the most abundant cell population of peripheral blood leukocytes and play a central role in acute bacterial infections and type-1 inflammatory reactions [58]. In addition, increased numbers of activated neutrophils in nasal mucosa of allergic rhinitis patients exhibit T-cell priming capacity and facilitate eosinophil migration [59]. Polak et al. elaborated on this and could show that neutrophil phagocytosis and antigen presentation fully activates allergen-specific T-cells in Birch pollen allergy [60,61]. In the context of PGD2, there is evidence of an indirect link between increased PGD2 levels and neutrophil influx mediated by macrophages as neutrophils are not directly attracted or activated by PGD2 [11]. Hematopoietic PGDS expression in neutrophils has been detected in a murine model of LPS-induced lung inflammation [14], however, PGD2 production by neutrophils has not been described to date.
Mononuclear phagocytes are a heterogeneous population of antigen presenting cells with specific, but partly overlapping functions: monocytes primarily serve as precursor cells and cytokine sources, macrophages are highly phagocytic, while dendritic cells are specialized on antigen presentation and activating naïve T-cells [62]. In 1989, Urade et al. reported that antigen-presenting cells, i.e., hPGDS-expressing macrophages and dendritic cells in spleen, thymus, Peyer’s patch of the intestine, submucosal layer of the stomach and in the liver, are likely the major source of PGD2 in the rat [63].
Peripheral blood monocytes are recruited from the blood to inflamed tissue, where they either actively contribute to inflammation or differentiate into monocyte-derived macrophages and dendritic cells [64]. Challenge of selected patients with inhaled allergens caused changes in circulating monocyte populations 24 h post stimulation [65]. Further, infants who developed food allergy within the first year of life displayed higher number of monocytes and reduced numbers of Treg cells in chord blood, which might contribute to Th2-type inflammation [66]. Human monocytes express both DP1 and DP2/CRTH2 receptors but show only minor response to activation [11]. A study looking into changes in prostaglandin production of human monocytes if cultured in vitro reported a release of PGD2 in femtomol-range by unstimulated monocytes, suggesting that those cells should have the machinery to produce PGD2 if activated accordingly [67]. In current literature, monocytes have not been considered as PGD2 sources to date.
Macrophages are distributed throughout the body and play a crucial role in maintaining tissue homeostasis, linking innate and adaptive immune response and are exposed continuously to various kinds of particles, toxins, allergens and infectious agents [68]. Most adult tissue macrophages originate from embryonic cells rather than from circulating monocytes, but during inflammation monocytes are recruited to inflamed tissue and act as precursors for monocyte-derived macrophages [69]. A central role of macrophages as mediators of inflammation has been reviewed vastly in allergic asthma [70], food allergy [71] and atopic dermatitis [72]. Human macrophages express both DP receptors and activation with PGD2 triggers a chemotactic response as well as release of several inflammatory mediators [11]. Numerous studies showed that murine bone marrow-derived macrophages express hPGDS and release up to 20 ng PGD2/mL (1 × 106 cells) upon pro-inflammatory stimuli like LPS or Zymosan A [29,73,74,75]. Further, hPGDS expression in macrophages was reported in the nasal mucosa of allergic rhinitis patients [43], pulmonary macrophages in acute respiratory distress syndrome [11] and human adipose tissue macrophages [76]. One study showed that hPGDS knock-down with specific siRNA but not LPGDS siRNA as well as co-treatment with HQL-79, a hPGDS inhibitor, but not AT-56, a LPGDS inhibitor, attenuated PGD2 production by mouse bone marrow-derived macrophages [29]. Recently, Henkel et al. stressed the importance of altered macrophage function in allergic disease by showing that human monocyte-derived macrophages co-stimulated with IL-4 and house dust mite allergen produce PGD2 [77].
Dendritic cells are highly specified in antigen sampling, processing and presentation to memory and naïve T-cells and they form a strong network of surveillance at sites of antigen entrance such as epithelial and mucosal linings of skin, alimentary and urogenital tract, and airways [78]. Their function is to keep the balance between host defense and tolerance; however, if an allergen is recognized as potentially harmful it is processed and presented to naïve T-cells in lymph nodes leading potentially to a Th2 type inflammatory reaction. PGD2 seems to impact the differentiation and function of human monocyte-derived dendritic cells by affecting antigen processing and presentation which leads to a bias in T-cell maturation towards Th2 cells [79]. This effect was seen to be even more pronounced with a DP1-specific agonist. Another study suggested that elevated PGD2 levels in neonates, e.g., during respiratory viral infections, cause a delay in dendritic cell maturation and migration to lymph nodes by DP1 activation [80]. As prototype of an antigen presenting cell, dendritic cells have been considered as potential PGD2 source as immunohistochemical staining could confirm that these cells express hPGDS [63]. Interestingly, Lee et al. found a relationship between decreased cAMP and increased hPGDS mRNA levels in murine DCs, and in turn a Th2-biased response [81]. Although PGD2 production by mouse bone marrow-derived DCs was not measured, the Th2-bias to the immune response was blunted after transfection of these cells with hPGDS-specific siRNA. Another study found that peripheral blood DCs as well as tissue plasmacytoid and myeloid DCs in atopic dermatitis patients stained positive for hPGDS [82]. They could see a PGD2 release up to 250 pg/ 1 × 106 human monocyte-derived DCs after LPS stimulation, while LPS caused a downregulation of hPGDS expression after 6 h. Therefore, differences between specific DC populations need to be considered as well as effects due to culture conditions. Accordingly, primary synovial fluid DCs express much higher hPGDS levels than monocyte-derived DCs, but hPGDS expression decreases in synovial fluid DCs after two days in culture [83].
Lymphocytes play an important role during induction and maintenance of allergic inflammation. In this context, a dysregulation of the adaptive immune response is initiated which leads to the production of allergen-specific IgE antibodies. It has been reported that PGD2 signaling primarily influences Th2 and group 2 innate lymphoid cell (ILC2) function during allergic reactions [54,84].
Th2 cells have multiple roles in the initiation and maintenance of allergic inflammation including induction of allergen-specific IgE antibody production, type-2 cytokine secretion and promotion of eosinophilic inflammation [85]. The DP2/CRTH2 receptor has been characterized first in Th2 cells which resulted in the synonym chemoattractant receptor homologous molecule expressed on Th2 cells (CRTH2) and stresses the importance of PGD2 for these cells [54]. Increased numbers of DP2/CRTH2+CD4+ Th2 cells could be found in the circulation and skin lesions of atopic dermatitis patients, and transcriptional profiling of Th2 cells from asthmatic patients also revealed several changes in allergy-associated features including genes relevant for prolonged survival and activation [86,87]. Another study demonstrated that inhibition of PGD2 production with an hPGDS inhibitor (HQL-79) as well as blocking DP2/CRTH2 reduced lymphocyte influx in hapten-specific IgE-induced ear swelling [16]. Stimulation of hPGDS+DP2/CRTH2+ Th2 cells from healthy adults with a combination of OKT3 (monoclonal anti-CD3 antibody) and KOLT-2 (monoclonal anti-CD28 antibody) induced up to 30 ng PGD2/mL (5 × 106 cells) [88]. Unlike Th1 cells, hPGDS+CRTH2+ Th2 cells express hPGDS, which was confirmed by flow cytometry, Northern and Western blotting. Further, Mitson-Salazar et al. could identify hPGDS and DP2/CRTH2 double-positive cells as pro-eosinophilic effector Th2 cell population in patients with eosinophilic gastrointestinal disorder [89]. CD161+/hPGDS+ Th2 cells release higher levels of IL-5 and IL-13, thereby favoring a type-2 inflammation.
ILC2s can be found in the lung, skin and gut and are believed to play an important role in development of type 2 inflammation [90]. Comparable to Th2 cells, PGD2 regulates the function, migration and accumulation of ILC2s during pulmonary inflammation and is now established as one of the cell surface markers for this lymphoid cell population [91]. Recently, we demonstrated that of autocrine PGD2 to enable ILC2 function and activation [84]. We found that human ILC2s express hPGDS on mRNA level and release up to 1.5 ng/mL PGD2 (5 × 105 cells) after stimulation with IL-33, IL-25 and TSLP. Disruption of endogenous PGD2 production with a COX-1/2 or hPGDS inhibitor (KMN-698, 100 nM) as well as blockade of DP2/CRTH2 reduced IL-5 and IL-13 secretion, CD25 upregulation and PGD2 production.
3.2. Parenchymal Cells
Epithelial cells align at the surface of various parts of the body including skin, gastrointestinal and respiratory tract where they form a tight barrier to keep out pathogens as well as allergens. Beyond maintaining their barrier function, epithelial cells at mucosal surfaces are now considered to actively participate in the development and progression of allergic disease [92]. In a neonatal mouse model of viral bronchiolitis it could be shown that bronchial epithelial cells constitutively express low levels of hPGDS, while viral infection in combination with cockroach allergen-sensitization triggered the upregulation of hPGDS expression and PGD2 production 10 days post infection [93]. In the same study they could prove that also human bronchial epithelial cells express hPGDS and release up to 1500 pg PGD2/mL 24 h after viral infection. In line with this, Jakiela et al. documented changes in the lipidomic profile of rhinovirus-infected airway epithelial cells including an increase in PGD2 release [94].
The gut epithelium consists of several highly specified cell types while tuft cells are the only cells that constitutively express all enzymes necessary for PGD2 synthesis including hPGDS [95]. The role of tuft cells in diseases is still unclear; however, an increase in tuft cell numbers was associated with gastric inflammation as well as various respiratory inflammatory syndromes [95]. In atopic dermatitis, epidermal keratinocytes have been linked to drive Th2-mediated inflammation by contributing to increased TSLP levels [96]. A combination of the viral mimic poly[I:C] and IL-4 triggered the release of TSLP but also LPGDS-derived PGD2 by human keratinocytes [97]. In this study, however, they claim that an increase in PGD2 or more specifically the PGD2-metabolite 15d-PGJ2 was able to decrease keratinocyte-derived TSLP. In total, there seems to be a strong connection between epithelial-derived PGD2 and viral infections, which could also be important in the development of allergic inflammation.
Endothelial cells line the inner surface of blood vessels and form an active barrier to selectively control extravasation of blood components and circulating cells. Prostaglandins are able to modulate vascular permeability, which needs to be tightly controlled to prevent excessive inflammation [98]. Besides keeping a tight barrier, endothelial cells are part of the innate response to allergens and there is more and more evidence that angiogenesis may be an early step in asthma initiation [99,100]. No studies to date could prove that endothelial cells express hPGDS; however, some studies presented evidence that they express LPGDS and are able to produce PGD2 [101,102]. A recently published article showed that LPGDS knock-out mice but not hPGDS knock-out mice display a cardiovascular phenotype. In the vasculature, an autocrine LPGDS-derived PGD2 effect seems crucial to protect from hypertension and thrombogenesis [4].
Smooth muscle cell hyper-contraction is a characteristic feature of asthmatic airways causing a limitation of gas exchange. In a mouse model of OVA-induced allergic pulmonary inflammation, the COX-2/hPGDS/PGD2 cascade was suggested to be involved in the development of bronchial smooth muscle cell hyper-responsiveness [103]. Microarray, RT-qPCR and Western blot results of this study revealed an increased hPGDS expression in bronchial epithelial cells of allergen-challenged mice suggesting an autocrine pathway. Additionally, DP2/CRTH2 activation promotes smooth muscle cell proliferation, thereby contributing to airway remodeling [104].
4. PGD2 Signaling as Therapeutic Target in Allergic Diseases
4.1. Allergic Asthma and Rhinitis
Asthma is a chronic respiratory condition characterized by airway hyper-responsiveness and airflow obstruction. Despite the availability of reasonably good standard-of-care therapy for most asthmatics, new drugs are needed for severe and poorly controlled asthma to effectively replace oral corticosteroids [108]. PGD2 is found in human airways during acute allergen challenge and in patients with severe asthma. Further, disease severity has been shown to correlate with PGD2 levels [109,110]. PGD2 acts as a chemoattractant and activator of Th2 cells, eosinophils and basophils, primarily initiated by DP2/CRTH2 activation [54]. The role of DP1 in asthma remains elusive; however, in preclinical models of asthma and acute lung injury, DP1 agonism attenuated type 2 inflammation and endothelial cell damage, respectively [8,14]. In contrast, concentrations of PGD2 and the DP1 agonist BW245c in the micromolar range trigger smooth muscle contraction and bronchoconstriction via the thromboxane receptor [111]. A study involving atopic asthmatic patients could show, however, that thromboxane receptor blockade only partially reduced bronchoconstriction in response to PGD2 and PGD2-induced bronchoconstriction potentially involves a vascular component [112]. Further, PGD2-DP1 signalling but not PGD2-DP2/CRTH2 signalling induced cough in guinea pigs by activation of vagal sensory neurons [113]. Also, in comparison to wild-type mice, DP1 KO mice subjected to OVA-induced allergic inflammation showed less mucus-containing cells in the airways as well as reduced airway hyper-reactivity [18]. In contrast, PGD2/CRTH2/DP2 signalling induces smooth muscle cell proliferation [104], which also contributes to airway narrowing in asthma. As a consequence, both PGD2 receptors, DP1 and DP2/CRTH2, have emerged as potential drug targets for the treatment of allergic asthma [22,114,115,116,117].
In combination with allergen exposure and genetic risk factors, lower respiratory viral infections have been associated with a higher likelyhood of developing asthma as well as disease progression and acute asthmatic exacerbations [118]. Recently, it has been shown that PGD2 production is elevated in viral respiratory infections and in turn promotes disease severity via DP2/CRTH2 [93]. This study also showed that hPGDS expressing immune cells were present immediately after infection while at later time points bronchial epithelial cells upregulated hPGDS and PGD2 production. Further, allergic inflammation is often associated with excessive eosinophil influx and activation resulting in ongoing inflammation and tissue injury. Elevated PGD2 levels prolong eosinophil survival via the DP1 receptor and attract more eosinophils and Th2 cells via DP2/CRTH2 from the bloodstream [48,54]. In aspirin-exacerbated respiratory disease, hPGDS-expressing eosinophils seem to contribute to elevated PGD2 levels as well as in allergic inflammation, which could be blocked with the hPGDS inhibitor HQL-79 [33,50]. Besides eosinophils, basophils have also been suggested as potential PGD2 sources in IgE-mediated inflammation [56]. As potent drivers of type-2 inflammation and their role in allergic asthma, ILC-2s have been of great interest lately. Inhibition of autocrine PGD2 production by human ILC-2s with hPGDS inhibitor KMN-698 abolished IL-5 and IL-13 production as well as CD25 upregulation [84]. In allergic rhinitis, hPGDS but not LPGDS expression could be detected in the nasal mucosa, while various hPGDS expressing infiltrating cells were identified [43]. Blockade of hPGDS function by treatment of antigen-challenged guinea pigs with hPGDS inhibitor TAS-204 or TFC-007 suppressed nasal blockage, nasal airway resistance and eosinophil infiltration [119,120]. Treatment with hPGDS inhibitor HQL-79 suppressed OVA-induced allergic airway inflammation in wild types and also in mice overexpressing human hPGDS [28]. HQL-79 has originally been developed as histamine receptor 1 antagonist; however, in the same study they could show that an anti-allergic effect could also be seen in histamine receptor 1 knock-out mice claiming that the beneficial effect was due to reduction of PGD2 synthesis. Severe asthma often involves airway remodelling including smooth muscle cell hyper-proliferation and activity. PGD2 promotes smooth muscle cell proliferation via DP2/CRTH2 activation and contributes to a hyper-responsive phenotype seen in asthmatic patients [103,104]. In summary, therapeutically blocking hPGDS and PGD2 biosynthesis could be beneficial to delay the onset of allergic asthma, reduce eosinophilic and Th2 type inflammation, alleviate symptoms including allergic rhinitis and cough as well as reduce airway remodelling.
4.2. Atopic Dermatitis
Atopic dermatitis is a chronic inflammatory skin disease which affects up to 20% of children and 10% of adults in industrialized countries [121]. Patients suffer from erythema, edema and crusting whereby skin barrier defects and immune hyper-activation are major components of disease development and progression [122]. In atopic dermatitis, Langerhans cells, plasmatoid and myeloid dendritic cells express hPGDS and are able to modulate PGD2 production upon activation with exogenous stimuli like bacterial LPS [82]. In addition, epidermal keratinocytes of atopic dermatitis patients are also able to produce LPGDS-derived PGD2, whereby the PGD2-metabolite 15d-PGJ2 seems to attenuate TSLP production of keratinocytes [97]. Indeed, PGD2 has opposing roles in skin inflammation as it has been shown to suppress inflammation via the DP receptor in the early phase while enhancing inflammation via DP2/CRTH2 receptor in the late phase in croton oil-induced dermatitis in mice [12]. In this study, transgenic hPGDS overexpressing mice showed reduced vascular leakage in the early phase but increased immune cell infiltration in the late phase thereby prolonging inflammation. Similar results were seen in the later phase of hapten-specific IgE-induced skin inflammation: PGD2/DP2/CRTH2 signalling resulted in lymphocyte, eosinophil and basophil infiltration as well as increased levels of macrophage chemokines which was suppressed by DP2/CRTH2 antagonist ramatroban or hPGDS inhibitor HQL-79 [16]. Therefore, blocking mast cell, dendritic cell and macrophage-derived PGD2 production by inhibiting hPGDS could help to reduce eosinophil, basophil and Th2 cell numbers as well as inflammatory cytokine levels in atopic dermatitis lesions.
4.3. Food Allergy and Gastrointestinal Allergic Disorder
The allergic reaction to food proteins can be mediated by IgE-dependent and -independent pathways, sometimes even resulting in life-threatening anaphylactic responses [123]. Food allergies are frequent disorders in most countries and typically occur for the first time during early childhood. Allergic reactions are mostly type-2 cytokine-driven and manifest with various symptoms from skin rashes, eczema, nausea, abdominal pain, respiratory hyper-sensitivity up to anaphylactic shock [124]. Current standard of care primarily suggests to avoid food allergens and treatment of systemic anaphylactic reactions with adrenaline [125]. Specialized preventive and therapeutic options would be of substantial value for effective disease management. Maeda et al. stated that high levels of urinary PGD2 metabolite tetranor-PGDM can be found in patients with food allergy but not in those with other allergic diseases or healthy volunteers [41]. Further, they could see that OVA-induced intestinal allergic inflammation as well as milk-induced food allergy in mice resulted in increased urinary tetranor-PGDM, which reflected the severity of allergic symptoms and intestinal mast cell hyperplasia. Consistently, hPGDS knock-out but not LPGDS knock-out mice had reduced levels of urinary tetranor-PGDM after allergen challenge. Intestinal mast cells express high levels of the hPGDS enzyme and play a key role in IgE-mediated allergic reactions to food allergens. In contrast to the previous study, Nakamura et al. observed an increased OVA-induced intestinal allergic inflammation in mast cell-specific hPGDS knock-out mice [42]. This effect could be minimized by providing hPGDS sufficient bone marrow-derived mast cells. Hematopoietic PGDS expression in mast cells seems to be important for balancing intestinal inflammatory reactions, however, hPGDS expression in other cell types has been associated with pathogenic function. There is evidence that the TSLP/ /hPGDS/PGD2/DP2/CRTH2 axis may potentiate an interaction between a hPGDS+ Th2 subpopulation and ILC2 cells to amplify eosinophilic inflammation [126]. It was shown that TSLP-activated DCs induce hPGDS upregulation in CD4+ Th2 cells [86], which might contribute to increased PGD2 levels, activation of DP2/CRTH2+ memory T cells and enhanced ILC2 function. A pro-eosinophilic, pathogenic human DP2/CRTH2+/hPGDS+ Th2 cell subpopulation was discovered in patients with eosinophilic gastrointestinal disorder (EGID). These patients have characteristically higher numbers of DP2/CRTH2+/hPGDS+ Th2 cells, which display enhanced type-2 cytokine production thereby driving allergic eosinophilic inflammation [89]. PGD2/DP2/CRTH2 interaction is a strong chemotactic stimulus for eosinophils and blocking DP2/CRTH2 with specific antagonists could alleviate symptoms in eosinophilic esophagitis patients [127,128]. In summary, a reduction of PGD2 levels in allergic gastrointestinal inflammation could be beneficial to diminish eosinophilic inflammation, and ILC2 and Th2 cell activation.
4.4. Anaphylactic Reaction
Anaphylaxis is a life-threatening condition resulting from IgE-mediated mast cell and basophil activation followed by the release of inflammatory mediators e.g., histamine and PGD2 into the bloodstream causing systemic inflammation [129]. From a study with 35 anaphylactic patients, the PGD2-derived metabolite 9α, 11β-PGF2 emerged as potential urinary biomarker as it correlated with the severity of the reaction [130]. However, a study in mice revealed that mast cell-derived PGD2 attenuates vascular hyperpermeability [15], which is characteristic for anaphylactic shock, by activation of the DP1 receptor on endothelial cells and strengthening of endothelial barrier function [131]. Additionally, hPGDS knock-out mice exhibit a stronger anaphylactic reaction which could be rescued by additional treatment with DP1 agonist BW 245c [15]. The effect of PGD2 in anaphylactic reactions is still inconclusive; however, the beneficial effects of PGD2/DP1 signalling seen in mouse models warrants further investigation.
5. Patents and Clinical Studies
Several patents and patent applications for hPGDS inhibitors as therapeutic option in allergic inflammation, asthma, chronic obstructive pulmonary disease and other inflammatory diseases have been issued [132,133,134]. Regardless of more and more commercially available compounds (Table 2), only few clinical studies evaluating hPGDS inhibitor safety and efficacy can be found. In 2015, Phase I clinical trial (NCT 02397005) was initiated to evaluate tolerability and pharmacokinetics of the selective and reversible hPGDS inhibitor ZL-2102 for treatment of COPD, asthma and idiopathic pulmonary fibrosis [135]. In this clinical trial, 120 participants were enrolled to perform a randomized, double-blind, placebo-controlled study of ascending single and repeated oral doses of ZL-2102; currently the estimated study completion date is August 2019. While these results will give a first impression of the tolerability of hPGDS inhibitors in patients, considerable effort will have to be put into stratification of patients, i.e., to identify those who might benefit most from hPGDS inhibiton, for future clinical studies evaluating hPGDS inhibitors. Beyond allergic inflammation as the expected indication ofor hPGDS inhibitors, another Phase I clinical trial investigating safety and pharmacokinetics of hPGDS inhibitor TAS-205 in 23 boys with Duchenne’s muscular dystrophy could be completed successfully in 2018 [136]. Their rationale was based on studies showing increased PGD2 levels in myonecrotic areas presumably driving inflammation as well as an increase of urinary tetranor-PGD metabolite in Duchenne’s patients. Hematopoietic PGDS inhibitor TAS-205 was tested over various dose ranges and was deemed safe and tolerable in patients with Duchenne’s muscular dystrophy. Urinary PGD-metabolite was significantly reduced following treatment without interfering with urinary PGE2 metabolite levels—fostering the hypothesis that hPGDS inhibitor TAS-205 in this dosage range is well tolerated with significant biological activity in patients.
Table 2.
hPGDS inhibitors and their cellular targets, effective concentrations and doses, and experimental settings reported in literature.
| Inhibitor | Chemical Structure | Company | Cell Type/Disease | Concentration | Ref. |
|---|---|---|---|---|---|
| HQL-79 |
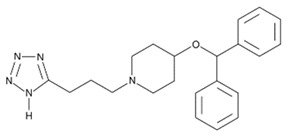
|
Cayman Chemicals | BMDM, eosinophils; OVA-induced lung inflammation |
5–100 µM 10 mg/kg |
[29,33] [28] |
| TFC-007 |
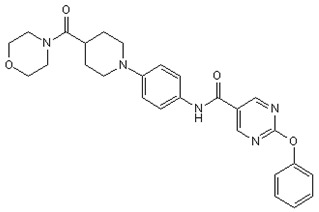
|
Tocris | nasal blockage in guinea pigs | 30 mg/kg | [120] |
| HPGDS inhibitor I |
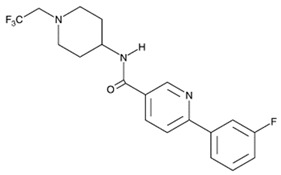
|
Cayman Chemicals | basophils; murine lupus model |
5 mg/kg | [57] |
| TAS-204 |
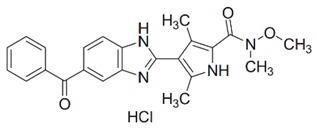
|
Taiho Pharmaceutical Co. Ltd. | nasal blockage in guinea pigs | 15–30 mg/kg | [119] |
| TAS-205 | not reported | Taiho Pharmaceutical Co. Ltd. | Duchenne‘s muscular dystrophy (Phase I) | 1.67–13.33 mg/kg/dose | [136] |
| KMN-698 | not reported | Sanofi Aventis (Cayman Chemicals) |
ILC-2 | 100 nM | [84] |
| ZL-2102 | not reported | Zai Lab Pty. Ltd./Sanofi | healthy males (Phase I) |
5 to 750 mg | [135] |
BMDM—bone marrow-derived macrophage; ILC—innate lymphoid cells; OVA—ovalbumin.
Notably, clinical research on hPGDS inhibitors for allergic and other inflammatory disorders is still in its infancy in contrast to i.e., CRTH2/DP2 antagonists and the therapeutic potential of hPGDS inhibitors will need further exploration to confirm efficacy.
6. Future Perspectives
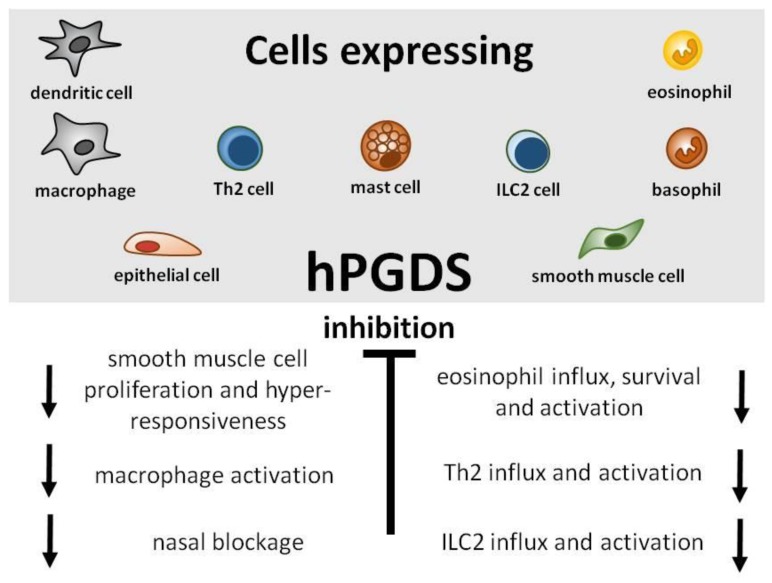
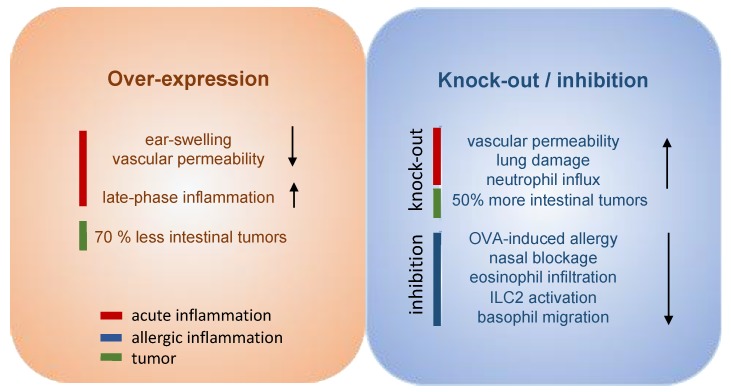
Great efforts have already been put into the development and evaluation of hPGDS inhibitors to diminish PGD2-related exacerbation of inflammation without interfering with LPGDS-derived PGD2 production important for endothelial cell function. Clinically efficient, commercially available hPGDS inhibitors would enable targeting various hPGDS-expressing cell types simultaneously and hence reduce recruitment and activation of many inflammatory cells involved in allergic progression (Figure 2). Therapeutic efficacy of hPGDS inhibitors might strongly depend on disease phenotype, i.e., the source of elevated PGD2 levels, target cells involved in inflammatory reaction. It has been suggested that PGD2 exerts a biphasic effect in inflammation, i.e anti-inflammatory effects were observed in the acute phase of inflammation, whereas pro-inflammatory DP2/CRTH2-mediated effects, i.e., chemotaxis and activation of effector cells, prevail in the late phase; this proposes timing of treatment as another variable yet to consider to successfully select target groups. Hematopoietic PGDS overexpression, knock-out and inhibition studies in mice are summarized in Figure 3 that gives an overview how systemic excess or loss of hPGDS-derived PGD2 influences (patho-)physiological settings. A relevant concern of hPGDS inhibition is the influence on other eicosanoids downstream of cyclooxygenases. The possibility that excess PGH2 will serve as precursor for other eicosanoids arises, thereby causing an imbalance in the arachidonic acid/COX pathway. Interestingly, an increased PGE2 to PGD2 ratio in patients with respiratory disorders has been associated with better prognosis [109], suggesting a positive effect of shunting excess PGH2 into PGE2 production. However, studies investigating HQL-79-induced hPGDS inhibition in mice could show that PGE2 and PGF2 levels did not change upon treatment [28,137]. Indeed, most studies that showed beneficial effects of hPGDS inhibitors were conducted in allergy models (Figure 2 and Figure 3); type-2 inflammatory disorders like allergic asthma, food allergy, EGID and atopic dermatitis involve similar effector cell types that react strongly to PGD2 via DP2/CRTH2 stimulation but also DP1 activation has been linked to induction of cough and eosinophil survival. Increased hPGDS expression and/or PGD2 production has been confirmed in patients with acute exacerbations of asthma. Viral infection upregulated hPGDS in bronchial epithelial cells and increased PGD2 levels were associated with aggravated respiratory inflammation [93]. As in Duchenne’s patients, increased urinary PGD metabolite has been associated with worse prognosis in Aspirin-exacerbated respiratory disease [138]. TAS-205 has been shown to efficiently reduce urinary PGD metabolite in Duchenne’s patients and would potentially be a drug candidate to investigate beneficial effects in this patient group. As already mentioned, patient stratification will be crucial to explore translational potential of novel therapeutic approaches for allergic disorders. To illustrate potential obstacles, CRTH2 inhibitors have been studied extensively in recent years both in animal models and patients, but clinical investigations are still ongoing with no approved drug on the market yet. There are still many factors to consider before clinical efficacy and translatability of hPGDS inhibitors can be explored. However, regarding the extensive number of cell types capable of producing PGD2 and/or being influcenced by PGD2 highlights hPGDS as a promising pharmaceutical target to modulate allergic respiratory inflammation. Whether the concept of inhibiting PGD2 biosynthesis as opposed to blocking PGD2 receptors is valid and clinically superior also remains to be elucidated.
Figure 2.
Potential target cells and beneficial effects of hPGDS inhibition in allergic inflammation. Hematopoietic PGDS expression has been reported in many cell types involved in allergic inflammation. Elevated PGD2 levels have been shown to induce DP2/CRTH2-mediated smooth muscle cell proliferation and hyperresponsiveness [103], macrophage activation [11], nasal blockage [10] as well as influx, activation and survival of eosinophils, ILC2s and Th2 cells. Inhibition of hPGDS-derived PGD2 production would target all above-mentioned pathological phenomena.
Figure 3.
Experimental overexpression, inhibition and knock-out of hPGDS differentially modulates acute and chronic inflammation. Overexpression of transgenic hPGDS in mice resulted in reduced ear swelling and vascular permeability in the acute phase of inflammation, however, it exacerbated leukocyte influx in the late phase; In the same model, hPGDS knock-out potentiated vascular extravasation during acute skin inflammation [12]. Neutrophil influx and lung damage was more prominent in hPGDS knock-out mice [14]. In contrast, hPGDS inhibition proved to be beneficial in experimental models of allergic inflammation [28,119,120]. Interestingly, hPGDS over-expression was able to reduce the number of intestinal tumors, while hPGDS knock-out showed the opposite effect [27].
Acknowledgments
We thank Georg Richtig for his helpful comments on this manuscript.
Author Contributions
S.R. and A.H. wrote and edited the document.
Funding
This work was supported by the Austrian Science Fund FWF (DK MOLIN-W1241), the Medical University of Graz and BioTechMed Graz.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pettipher R. The roles of the prostaglandin D 2 receptors DP 1 and CRTH2 in promoting allergic responses. Br. J. Pharmacol. 2008;153:191–199. doi: 10.1038/sj.bjp.0707488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marone G., Galdiero M.R., Pecoraro A., Pucino V., Criscuolo G., Triassi M., Varricchi G. Prostaglandin D2 receptor antagonists in allergic disorders: safety, efficacy, and future perspectives. Expert Opin. Investig. Drugs. 2018;28:73–84. doi: 10.1080/13543784.2019.1555237. [DOI] [PubMed] [Google Scholar]
- 3.Schuligoi R., Sturm E., Luschnig P., Konya V., Philipose S., Sedej M., Waldhoer M., Peskar B.A., Heinemann A. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology. 2010;85:372–382. doi: 10.1159/000313836. [DOI] [PubMed] [Google Scholar]
- 4.Song W.-L., Ricciotti E., Liang X., Grosser T., Grant G.R., FitzGerald G.A. Lipocalin-Like Prostaglandin D Synthase but Not Hemopoietic Prostaglandin D Synthase Deletion Causes Hypertension and Accelerates Thrombogenesis in Mice. J. Pharmacol. Exp. Ther. 2018;367:425–432. doi: 10.1124/jpet.118.250936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu R., Xiao L., Zhao G., Christman J.W., van Breemen R.B. Competitive Enzymatic Interactions Determine the Relative Amounts of Prostaglandins E2 and D2. J. Pharmacol. Exp. Ther. 2011;339:716–725. doi: 10.1124/jpet.111.185405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hata A.N., Breyer R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Giles H., Leff P., Bolofo M.L., Kelly M.G., Robertson A.D. The classification of prostaglandin DP-receptors in platelets and vasculature using BW A868C, a novel, selective and potent competitive antagonist. Br. J. Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammad H., Kool M., Soullié T., Narumiya S., Trottein F., Hoogsteden H.C., Lambrecht B.N. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J. Exp. Med. 2007;204:357–367. doi: 10.1084/jem.20061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spik I., Brenuchon C., Angeli V., Staumont D., Fleury S., Capron M., Trottein F., Dombrowicz D. Activation of the Prostaglandin D2 Receptor DP2/CRTH2 Increases Allergic Inflammation in Mouse. J. Immunol. 2005;174:3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 10.Arimura A., Yasui K., Kishino J., Asanuma F., Hasegawa H., Kakudo S., Ohtani M., Arita H. Prevention of allergic inflammation by a novel prostaglandin receptor antagonist, S-5751. J. Pharmacol. Exp. Ther. 2001;298:411–419. [PubMed] [Google Scholar]
- 11.Jandl K., Stacher E., Bálint Z., Sturm E.M., Maric J., Peinhaupt M., Luschnig P., Aringer I., Fauland A., Konya V., et al. Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. J. Allergy Clin. Immunol. 2016;137:833–843. doi: 10.1016/j.jaci.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarashina H., Tsubosaka Y., Omori K., Aritake K., Nakagawa T., Hori M., Hirai H., Nakamura M., Narumiya S., Urade Y., et al. Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation. J. Immunol. 2014;192:459–465. doi: 10.4049/jimmunol.1302080. [DOI] [PubMed] [Google Scholar]
- 13.Ajuebor M.N., Singh A., Wallace J.L. Cyclooxygenase-2-derived prostaglandin D 2 is an early anti-inflammatory signal in experimental colitis. Am. J. Physiol. Liver Physiol. 2000;279:G238–G244. doi: 10.1152/ajpgi.2000.279.1.G238. [DOI] [PubMed] [Google Scholar]
- 14.Murata T., Aritake K., Tsubosaka Y., Maruyama T., Nakagawa T., Hori M., Hirai H., Nakamura M., Narumiya S., Urade Y., et al. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc. Natl. Acad. Sci. USA. 2013;110:5205–5210. doi: 10.1073/pnas.1218091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T., Fujiwara Y., Yamada R., Fujii W., Hamabata T., Lee M.Y., Maeda S., Aritake K., Roers A., Sessa W.C., et al. Mast cell–derived prostaglandin D2attenuates anaphylactic reactions in mice. J. Allergy Clin. Immunol. 2017;140:630–632.e9. doi: 10.1016/j.jaci.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Satoh T., Moroi R., Aritake K., Urade Y., Kanai Y., Sumi K., Yokozeki H., Hirai H., Nagata K., Hara T., et al. Prostaglandin D2 Plays an Essential Role in Chronic Allergic Inflammation of the Skin via CRTH2 Receptor. J. Immunol. 2006;177:2621–2629. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
- 17.Fujitani Y., Kanaoka Y., Aritake K., Uodome N., Okazaki-Hatake K., Urade Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. J. Immunol. 2002;168:443–449. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka T., Hirata M., Tanaka H., Takahashi Y., Murata T., Kabashima K., Sugimoto Y., Kobayashi T., Ushikubi F., Aze Y., et al. Prostaglandin D 2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 19.Scher J.U., Pillinger M.H. 15d-PGJ2: The anti-inflammatory prostaglandin? Clin. Immunol. 2005;114:100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Ide T., Egan K., Bell-Parikh L.C., FitzGerald G.A. Activation of nuclear receptors by prostaglandins. Thromb. Res. 2003;110:311–315. doi: 10.1016/S0049-3848(03)00418-3. [DOI] [PubMed] [Google Scholar]
- 21.Jandl K., Heinemann A. The therapeutic potential of CRTH2/DP2 beyond allergy and asthma. Prostaglandins Other Lipid Mediat. 2017;3:42–48. doi: 10.1016/j.prostaglandins.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabashima K., Narumiya S. The DP receptor, allergic inflammation and asthma. Prostaglandins Leukot. Essent. Fat. Acids. 2003;69:187–194. doi: 10.1016/S0952-3278(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 23.Urade Y., Eguchi N. Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat. 2002;68–69:375–382. doi: 10.1016/S0090-6980(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 24.Uchida Y., Urade Y., Mori S., Kohzuma T. UV resonance Raman studies on the activation mechanism of human hematopoietic prostaglandin D2 synthase by a divalent cation, Mg2+ J. Inorg. Biochem. 2010;104:331–340. doi: 10.1016/j.jinorgbio.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Inoue T., Irikura D., Okazaki N., Kinugasa S., Matsumura H., Uodome N., Yamamoto M., Kumasaka T., Miyano M., Kai Y., et al. Mechanism of metal activation of human hematopoietic prostaglandin D synthase. Nat Struct Mol Biol. 2003;10:291–296. doi: 10.1038/nsb907. [DOI] [PubMed] [Google Scholar]
- 26.Pinzar E., Miyano M., Kanaoka Y., Urade Y., Hayaishi O. Structural basis of hematopoietic prostaglandin D synthase activity elucidated by site-directed mutagenesis. J. Biol. Chem. 2000;275:31239–31244. doi: 10.1074/jbc.M000750200. [DOI] [PubMed] [Google Scholar]
- 27.Tippin B.L., Levine A.J., Materi A.M., Song W.-L., Keku T.O., Goodman J.E., Sansbury L.B., Das S., Dai A., Kwong A.M., et al. Hematopoietic prostaglandin D synthase (HPGDS): A high stability, Val187Ile isoenzyme common among African Americans and its relationship to risk for colorectal cancer. Prostaglandins Other Lipid Mediat. 2012;97:22–28. doi: 10.1016/j.prostaglandins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aritake K., Kado Y., Inoue T., Miyano M., Urade Y. Structural and functional characterization of HQL-79, an orally selective inhibitor of human hematopoietic prostaglandin D synthase. J. Biol. Chem. 2006;281:15277–15286. doi: 10.1074/jbc.M506431200. [DOI] [PubMed] [Google Scholar]
- 29.Zhao G., Yu R., Deng J., Zhao Q., Li Y., Joo M., van Breemen R.B., Christman J.W., Xiao L. Pivotal role of reactive oxygen species in differential regulation of lipopolysaccharide-induced prostaglandins production in macrophages. Mol. Pharmacol. 2013;83:167–178. doi: 10.1124/mol.112.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii T. Close teamwork between Nrf2 and peroxiredoxins 1 and 6 for the regulation of prostaglandin D 2 and E 2 production in macrophages in acute inflammation. Free Radic. Biol. Med. 2015;88:189–198. doi: 10.1016/j.freeradbiomed.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee S., Feinstein S.I., Dodia C., Sorokina E., Lien Y.C., Nguyen S., Debolt K., Speicher D., Fisher A.B. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J. Biol. Chem. 2011;286:11696–11706. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim K.H., Sadikot R.T., Xiao L., Christman J.W., Freeman M.L., Chan J.Y., Oh Y.K., Blackwell T.S., Joo M. Nrf2 is essential for the expression of lipocalin-prostaglandin D synthase induced by prostaglandin D2. Free Radic. Biol. Med. 2013;65:1134–1142. doi: 10.1016/j.freeradbiomed.2013.08.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luna-Gomes T., Magalhaes K.G., Mesquita-Santos F.P., Bakker-Abreu I., Samico R.F., Molinaro R., Calheiros A.S., Diaz B.L., Bozza P.T., Weller P.F., et al. Eosinophils as a Novel Cell Source of Prostaglandin D2: Autocrine Role in Allergic Inflammation. J. Immunol. 2011;187:6518–6526. doi: 10.4049/jimmunol.1101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jowsey I.R., Thomson A.M., Flanagan J.U., Murdock P.R., Moore G.B., Meyer D.J., Murphy G.J., Smith S.A., Hayes J.D. Mammalian class Sigma glutathione S-transferases: catalytic properties and tissue-specific expression of human and rat GSH-dependent prostaglandin D2 synthases. Biochem. J. 2001;359:507–516. doi: 10.1042/0264-6021:3590507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balzar S., Fajt M.L., Comhair S.A.A., Erzurum S.C., Bleecker E., Busse W.W., Castro M., Gaston B., Israel E., Schwartz L.B., et al. Mast cell phenotype, location, and activation in severe asthma: Data from the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakami T., Ando T., Kimura M., Wilson B.S., Kawakami Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amin K. The role of mast cells in allergic inflammation. Respir. Med. 2012;106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Dwyer D.F., Barrett N.A., Austen K.F. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat. Immunol. 2016;17:878–887. doi: 10.1038/ni.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis R.A., Soter N.A., Diamond P.T., Austen K.F., Oates J.A., Roberts L.J. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J. Immunol. 1982;129:1627–1631. [PubMed] [Google Scholar]
- 40.Baothman B.K., Smith J., Kay L.J., Suvarna S.K., Peachell P.T. Prostaglandin D2generation from human lung mast cells is catalysed exclusively by cyclooxygenase-1. Eur. J. Pharmacol. 2018;819:225–232. doi: 10.1016/j.ejphar.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Maeda S., Nakamura T., Harada H., Tachibana Y., Aritake K., Shimosawa T., Yatomi Y., Murata T. Prostaglandin D2metabolite in urine is an index of food allergy. Sci. Rep. 2017;7:1–8. doi: 10.1038/s41598-017-17798-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura T., Maeda S., Horiguchi K., Maehara T., Aritake K., Choi B.I., Iwakura Y., Urade Y., Murata T. PGD2deficiency exacerbates food antigen-induced mast cell hyperplasia. Nat. Commun. 2015;6:1–10. doi: 10.1038/ncomms8514. [DOI] [PubMed] [Google Scholar]
- 43.Okano M., Fujiwara T., Sugata Y., Gotoh D., Masaoka Y., Sogo M., Tanimoto W., Yamamoto M., Matsumoto R., Eguchi N., et al. Presence and characterization of prostaglandin D2–related molecules in nasal mucosa of patients with allergic rhinitis. Am. J. Rhinol. 2006;20:342–348. doi: 10.2500/ajr.2006.20.2865. [DOI] [PubMed] [Google Scholar]
- 44.Murata T., Aritake K., Matsumoto S., Kamauchi S., Nakagawa T., Hori M., Momotani E., Urade Y., Ozaki H. Prostagladin D2 is a mast cell-derived antiangiogenic factor in lung carcinoma. Proc. Natl. Acad. Sci. USA. 2011;108:19802–19807. doi: 10.1073/pnas.1110011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fulkerson P.C., Rothenberg M.E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 2013;12:117–129. doi: 10.1038/nrd3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peinhaupt M., Sturm E.M., Heinemann A. Prostaglandins and Their Receptors in Eosinophil Function and As Therapeutic Targets. Front. Med. 2017;4:1–12. doi: 10.3389/fmed.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamkhioued B., Aldebert D., Gounni A.S., Delaporte E., Goldman M., Capron A., Capron M. Synthesis of cytokines by eosinophils and their regulation. Int. Arch. Allergy Immunol. 1995;107:122–123. doi: 10.1159/000236949. [DOI] [PubMed] [Google Scholar]
- 48.Peinhaupt M., Roula D., Theiler A., Sedej M., Schicho R., Marsche G., Sturm E.M., Sabroe I., Rothenberg M.E., Heinemann A. DP1 receptor signaling prevents the onset of intrinsic apoptosis in eosinophils and functions as a transcriptional modulator. J. Leukoc. Biol. 2018;104:159–171. doi: 10.1002/JLB.3MA1017-404R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radnai B., Sturm E.M., Stancic A., Jandl K., Labocha S., Ferreirós N., Grill M., Hasenoehrl C., Gorkiewicz G., Marsche G., et al. Eosinophils contribute to intestinal inflammation via chemoattractant receptor-homologous molecule expressed on Th2 Cells, CRTH2, in experimental crohn’s disease. J. Crohn’s Colitis. 2016;10:1087–1095. doi: 10.1093/ecco-jcc/jjw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng X., Ramsden M.K., Negri J., Baker M.G., Payne S.C., Borish L., Steinke J.W. Eosinophil production of PGD2 in Aspirin-Exacerbated Respiratory Disease. J. Allergy Clin. Immunol. 2016;138:1089–1097. doi: 10.1016/j.jaci.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyake K., Karasuyama H. Emerging roles of basophils in allergic inflammation. Allergol. Int. 2017;66:382–391. doi: 10.1016/j.alit.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Koshino T., Arai Y., Miyamoto Y., Sano Y., Itami M., Teshima S., Hirai K., Takaishi T., Ito K., Morita Y. Airway basophil and mast cell density in patients with bronchial asthma: relationship to bronchial hyperresponsiveness. J. Asthma. 1996;33:89–95. doi: 10.3109/02770909609054536. [DOI] [PubMed] [Google Scholar]
- 53.Kepley C.L., McFeeley P.J., Oliver J.M., Lipscomb M.F. Immunohistochemical detection of human basophils in postmortem cases of fatal asthma. Am. J. Respir. Crit. Care Med. 2001;164:1053–1058. doi: 10.1164/ajrccm.164.6.2102025. [DOI] [PubMed] [Google Scholar]
- 54.Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Satoh T., Ito Y., Miyagishi C., Yokozeki H. Basophils Infiltrate Skin Lesions of Eosinophilic Pustular Folliculitis (Ofuji’s Disease) Acta Derm. Venereol. 2011;91:371–372. doi: 10.2340/00015555-1052. [DOI] [PubMed] [Google Scholar]
- 56.Ugajin T., Satoh T., Kanamori T., Aritake K., Urade Y., Yokozeki H. FcεRI, but Not FcγR, Signals Induce Prostaglandin D2 and E2 Production from Basophils. Am. J. Pathol. 2011;179:775–782. doi: 10.1016/j.ajpath.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pellefigues C., Dema B., Lamri Y., Saidoune F., Chavarot N., Lohéac C., Pacreau E., Dussiot M., Bidault C., Marquet F., et al. Prostaglandin D2amplifies lupus disease through basophil accumulation in lymphoid organs. Nat. Commun. 2018;9:725. doi: 10.1038/s41467-018-03129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosales C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018;9:1–17. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arebro J., Ekstedt S., Hjalmarsson E., Winqvist O., Kumlien Georén S., Cardell L.O. A possible role for neutrophils in allergic rhinitis revealed after cellular subclassification. Sci. Rep. 2017;7:1–9. doi: 10.1038/srep43568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polak D., Samadi N., Vizzardelli C., Acosta G.S., Rosskopf S., Steinberger P., Jahn-Schmid B., Bohle B. Neutrophils promote T-cell-mediated inflammation in allergy. J. Allergy Clin. Immunol. 2018;143:1923–1925. doi: 10.1016/j.jaci.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 61.Polak D., Hafner C., Briza P., Kitzmüller C., Elbe-Bürger A., Samadi N., Gschwandtner M., Pfützner W., Zlabinger G.J., Jahn-Schmid B., et al. A novel role for neutrophils in IgE-mediated allergy: Evidence for antigen presentation in late-phase reactions. J. Allergy Clin. Immunol. 2018;148:1143–1152. doi: 10.1016/j.jaci.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baharom F., Rankin G., Blomberg A., Smed-Sörensen A. Human lung mononuclear phagocytes in health and disease. Front. Immunol. 2017;8:1–16. doi: 10.3389/fimmu.2017.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urade Y., Ujihara M., Horiguchi Y., Ikai K., Hayaishi O. The major source of endogenous prostaglandin D2 production is likely antigen-presenting cells. Localization of glutathione-requiring prostaglandin D synthetase in histiocytes, dendritic, and Kupffer cells in various rat tissues. J. Immunol. 1989;143:2982–2989. [PubMed] [Google Scholar]
- 64.Serbina N.V., Jia T., Hohl T.M., Pamer E.G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kowal K., Møller H.J., DuBuske L.M., Moestrup S.K., Bodzenta-Lukaszyk A. Differential expression of monocyte CD163 in single and dual-asthmatic responders during allergen-induced bronchoconstriction. Clin. Exp. Allergy. 2006;36:1584–1591. doi: 10.1111/j.1365-2222.2006.02573.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y., Collier F., Naselli G., Saffery R., Tang M.L., Allen K.J., Ponsonby A.-L., Harrison L.C., Vuillermin P. Cord blood monocyte–derived inflammatory cytokines suppress IL-2 and induce nonclassic “T H 2-type” immunity associated with development of food allergy. Sci. Transl. Med. 2016;8:321ra8. doi: 10.1126/scitranslmed.aad4322. [DOI] [PubMed] [Google Scholar]
- 67.Norwitz E.R., Bernal A.L., Starkey P.M. Prostaglandin production by human peripheral blood monocytes changes with in vitro differentiation. Prostaglandins. 1996;51:339–349. doi: 10.1016/0090-6980(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 68.Kopf M., Schneider C., Nobs S.P. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 69.Epelman S., Lavine K.J., Randolph G.J. Origin and Functions of Tissue Macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Draijer C., Boorsma C.E., Robbe P., Timens W., Hylkema M.N., Ten Hacken N.H., van den Berge M., Postma D.S., Melgert B.N. Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways. J. Allergy Clin. Immunol. 2017;140:280–283.e3. doi: 10.1016/j.jaci.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 71.Kumar S., Dwivedi P.D., Das M., Tripathi A. Macrophages in food allergy: An enigma. Mol. Immunol. 2013;56:612–618. doi: 10.1016/j.molimm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 72.Kasraie S., Werfel T. Role of macrophages in the pathogenesis of atopic dermatitis. Mediators Inflamm. 2013;2013:1–15. doi: 10.1155/2013/942375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao L., Ornatowska M., Zhao G., Cao H., Yu R., Deng J., Li Y., Zhao Q., Sadikot R.T., Christman J.W. Lipopolysaccharide-Induced Expression of Microsomal Prostaglandin E Synthase-1 Mediates Late-Phase PGE2 Production in Bone Marrow Derived Macrophages. PLoS ONE. 2012;7:e50244. doi: 10.1371/journal.pone.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon Y.S., Lee Y.J., Choi Y.H., Park Y.M., Kang J.L. Macrophages programmed by apoptotic cells inhibit epithelial-mesenchymal transition in lung alveolar epithelial cells via PGE2, PGD2, and HGF. Sci. Rep. 2016;6:1–18. doi: 10.1038/srep20992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kong D., Shen Y., Liu G., Zuo S., Ji Y., Lu A., Nakamura M., Lazarus M., Stratakis C.A., Breyer R.M., et al. PKA regulatory IIα subunit is essential for PGD 2 -mediated resolution of inflammation. J. Exp. Med. 2016;213:2209–2226. doi: 10.1084/jem.20160459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Virtue S., Masoodi M., de Weijer B.A.M., van Eijk M., Mok C.Y.L., Eiden M., Dale M., Pirraco A., Serlie M.J., Griffin J.L., et al. Prostaglandin profiling reveals a role for haematopoietic prostaglandin D synthase in adipose tissue macrophage polarisation in mice and humans. Int. J. Obes. 2015;39:1151–1160. doi: 10.1038/ijo.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henkel F.D.R., Friedl A., Haid M., Thomas D., Bouchery T., Haimerl P., de los Reyes Jiménez M., Alessandrini F., Schmidt-Weber C.B., Harris N.L., et al. House dust mite drives pro-inflammatory eicosanoid reprogramming and macrophage effector functions. Allergy. 2018;74:1090–1101. doi: 10.1111/all.13700. [DOI] [PubMed] [Google Scholar]
- 78.Humeniuk P., Dubiela P., Hoffmann-Sommergruber K. Dendritic Cells and Their Role in Allergy: Uptake, Proteolytic Processing and Presentation of Allergens. Int. J. Mol. Sci. 2017;18:1491. doi: 10.3390/ijms18071491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gosset P., Pichavant M., Faveeuw C., Bureau F., Tonnel A.B., Trottein F. Prostaglandin D2 affects the differentiation and functions of human dendritic cells: Impact on the T cell response. Eur. J. Immunol. 2005;35:1491–1500. doi: 10.1002/eji.200425319. [DOI] [PubMed] [Google Scholar]
- 80.Tumala B., Phelps K.R., Zhang S., Bhattacharya S., Shornick L.P. Prostaglandin D 2 Levels Regulate CD103 + Conventional Dendritic Cell Activation in Neonates During Respiratory Viral Infection. Viral Immunol. 2018;31:658–667. doi: 10.1089/vim.2018.0090. [DOI] [PubMed] [Google Scholar]
- 81.Lee J., Kim T.H., Murray F., Li X., Choi S.S., Broide D.H., Corr M., Lee J., Webster N.J.G., Insel P.A., et al. Cyclic AMP concentrations in dendritic cells induce and regulate Th2 immunity and allergic asthma. Proc. Natl. Acad. Sci. USA. 2015;112:1529–1534. doi: 10.1073/pnas.1417972112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimura C., Satoh T., Igawa K., Aritake K., Urade Y., Nakamura M., Yokozeki H. Dendritic cells express hematopoietic prostaglandin D synthase and function as a source of prostaglandin D2 in the skin. Am. J. Pathol. 2010;176:227–237. doi: 10.2353/ajpath.2010.090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moghaddami M., Ranieri E., James M., Fletcher J., Cleland L.G. Prostaglandin D(2) in inflammatory arthritis and its relation with synovial fluid dendritic cells. Mediators Inflamm. 2013;2013:329494. doi: 10.1155/2013/329494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maric J., Ravindran A., Mazzurana L., Van Acker A., Rao A., Kokkinou E., Ekoff M., Thomas D., Fauland A., Nilsson G., et al. Cytokine-induced endogenous production of PGD2 is essential for human ILC2 activation. J. Allergy Clin. Immunol. 2018;143:2202–2214. doi: 10.1016/j.jaci.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 85.Romagnani S. The role of lymphocytes in allergic disease. J. Allergy Clin. Immunol. 2000;105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y.-H., Ito T., Wang Y.-H., Homey B., Watanabe N., Martin R., Barnes C.J., McIntyre B.W., Gilliet M., Kumar R., et al. Maintenance and Polarization of Human TH2 Central Memory T Cells by Thymic Stromal Lymphopoietin-Activated Dendritic Cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 87.Seumois G., Zapardiel-Gonzalo J., White B., Singh D., Schulten V., Dillon M., Hinz D., Broide D.H., Sette A., Peters B., et al. Transcriptional Profiling of Th2 Cells Identifies Pathogenic Features Associated with Asthma. J. Immunol. 2016;197:655–664. doi: 10.4049/jimmunol.1600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka K., Ogawa K., Sugamura K., Nakamura M., Takano S., Nagata K. Cutting Edge: Differential Production of Prostaglandin D2 by Human Helper T Cell Subsets. J. Immunol. 2000;164:2277–2280. doi: 10.4049/jimmunol.164.5.2277. [DOI] [PubMed] [Google Scholar]
- 89.Mitson-Salazar A., Yin Y., Wansley D.L., Young M., Bolan H., Arceo S., Ho N., Koh C., Milner J.D., Stone K.D., et al. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human TH2 cell subpopulation with enhanced function. J. Allergy Clin. Immunol. 2016;137:907–918. doi: 10.1016/j.jaci.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Tait Wojno E.D., Monticelli L.A., Tran S.V., Alenghat T., Osborne L.C., Thome J.J., Willis C., Budelsky A., Farber D.L., Artis D. The prostaglandin D2 receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015;8:1313–1323. doi: 10.1038/mi.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maric J., Ravindran A., Mazzurana L., Björklund Å.K., Van Acker A., Rao A., Friberg D., Dahlén S.-E., Heinemann A., Konya V., et al. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. J. Allergy Clin. Immunol. 2018;141:1761–1773.e6. doi: 10.1016/j.jaci.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gour N., Lajoie S. Epithelial Cell Regulation of Allergic Diseases. Curr. Allergy Asthma Rep. 2016;16:65. doi: 10.1007/s11882-016-0640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Werder R.B., Lynch J.P., Simpson J.C., Zhang V., Hodge N.H., Poh M., Forbes-Blom E., Kulis C., Smythe M.L., Upham J.W., et al. PGD2/DP2 receptor activation promotes severe viral bronchiolitis by suppressing IFN- production. Sci. Transl. Med. 2018;10:eaao0052. doi: 10.1126/scitranslmed.aao0052. [DOI] [PubMed] [Google Scholar]
- 94.Jakiela B., Gielicz A., Plutecka H., Hubalewska-Mazgaj M., Mastalerz L., Bochenek G., Soja J., Januszek R., Aab A., Musial J., et al. Th2-type cytokine induced mucous metaplasia decreases susceptibility of human bronchial epithelium to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 2014;51:229–241. doi: 10.1165/rcmb.2013-0395OC. [DOI] [PubMed] [Google Scholar]
- 95.Gerbe F., Legraverend C., Jay P. The intestinal epithelium tuft cells: specification and function. Cell. Mol. Life Sci. 2012;69:2907–2917. doi: 10.1007/s00018-012-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soumelis V., Reche P.A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 97.Kanda N., Kano R., Ishikawa T., Watanabe S. The antimycotic drugs itraconazole and terbinafine hydrochloride induce the production of human β-defensin-3 in human keratinocytes. Immunobiology. 2011;216:497–504. doi: 10.1016/j.imbio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 98.Konya V., Üllen A., Kampitsch N., Theiler A., Philipose S., Parzmair G.P., Marsche G., Peskar B.A., Schuligoi R., Sattler W., et al. Endothelial E-type prostanoid 4 receptors promote barrier function and inhibit neutrophil trafficking. J. Allergy Clin. Immunol. 2013;131:532–540. doi: 10.1016/j.jaci.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Asosingh K., Swaidani S., Aronica M., Erzurum S.C. Th1- and Th2-Dependent Endothelial Progenitor Cell Recruitment and Angiogenic Switch in Asthma. J. Immunol. 2014;178:6482–6494. doi: 10.4049/jimmunol.178.10.6482. [DOI] [PubMed] [Google Scholar]
- 100.Asosingh K., Weiss K., Queisser K., Wanner N., Yin M., Aronica M., Erzurum S. Endothelial cells in the innate response to allergens and initiation of atopic asthma. J. Clin. Invest. 2018;128:3116–3128. doi: 10.1172/JCI97720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taba Y., Sasaguri T., Miyagi M., Abumiya T., Miwa Y., Ikeda T., Mitsumata M. Fluid shear stress induces lipocalin-type prostaglandin D(2) synthase expression in vascular endothelial cells. Circ. Res. 2000;86:967–973. doi: 10.1161/01.RES.86.9.967. [DOI] [PubMed] [Google Scholar]
- 102.Omori K., Morikawa T., Kunita A., Nakamura T., Aritake K., Urade Y., Fukayama M., Murata T. Lipocalin-type prostaglandin D synthase-derived PGD 2 attenuates malignant properties of tumor endothelial cells. J. Pathol. 2018;244:84–96. doi: 10.1002/path.4993. [DOI] [PubMed] [Google Scholar]
- 103.Chiba Y., Suto W., Sakai H. Augmented Pla2g4c/Ptgs2/Hpgds axis in bronchial smooth muscle tissues of experimental asthma. PLoS ONE. 2018;13:e0202623. doi: 10.1371/journal.pone.0202623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen G., Zuo S., Tang J., Zuo C., Jia D., Liu Q., Liu G., Zhu Q., Wang Y., Zhang J., et al. Inhibition of CRTH2-mediated Th2 activation attenuates pulmonary hypertension in mice. J. Exp. Med. 2018;215:2175–2195. doi: 10.1084/jem.20171767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jakiela B., Gielicz A., Plutecka H., Hubalewska M., Mastalerz L., Bochenek G., Soja J., Januszek R., Musial J., Sanak M. Eicosanoid biosynthesis during mucociliary and mucous metaplastic differentiation of bronchial epithelial cells. Prostaglandins Other Lipid Mediat. 2013;106:116–123. doi: 10.1016/j.prostaglandins.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 106.Obata T., Nagakura T., Masaki T., Maekawa K., Yamashita K. Eicosapentaenoic acid inhibits prostaglandin D2 generation by inhibiting cyclo-oxygenase-2 in cultured human mast cells. Clin. Exp. Allergy. 1999;29:1129–1135. doi: 10.1046/j.1365-2222.1999.00604.x. [DOI] [PubMed] [Google Scholar]
- 107.Björklund Å.K., Forkel M., Picelli S., Konya V., Theorell J., Friberg D., Sandberg R., Mjösberg J. The heterogeneity of human CD127+ innate lymphoid cells revealed by single-cell RNA sequencing. Nat. Immunol. 2016;17:451–460. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- 108.Barnes P.J. New therapies for asthma: is there any progress? Trends Pharmacol. Sci. 2010;31:335–343. doi: 10.1016/j.tips.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 109.Yoshimura T., Yoshikawa M., Otori N., Haruna S., Moriyama H. Correlation between the Prostaglandin D2/E2 Ratio in Nasal Polyps and the Recalcitrant Pathophysiology of Chronic Rhinosinusitis Associated with Bronchial Asthma. Allergol. Int. 2008;57:429–436. doi: 10.2332/allergolint.O-08-545. [DOI] [PubMed] [Google Scholar]
- 110.Fajt M.L., Gelhaus S.L., Freeman B., Uvalle C.E., Trudeau J.B., Holguin F., Wenzel S.E. Prostaglandin D2 pathway upregulation: Relation to asthma severity, control, and TH2 inflammation. J. Allergy Clin. Immunol. 2013;131:1504–1512.e12. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.al Jarad N., Hui K., Barnes N. Effects of a thromboxane receptor antagonist on prostaglandin D2 and histamine induced bronchoconstriction in man. Br. J. Clin. Pharmacol. 1994;37:97–100. doi: 10.1111/j.1365-2125.1994.tb04249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnston S.L., Freezer N.J., Ritter W., O’Toole S., Howarth P.H. Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. Eur. Respir. J. 1995;8:411–415. doi: 10.1183/09031936.95.08030411. [DOI] [PubMed] [Google Scholar]
- 113.Maher S.A., Birrell M.A., Adcock J.J., Wortley M.A., Dubuis E.D., Bonvini S.J., Grace M.S., Belvisi M.G. Prostaglandin D2 and the role of the DP1, DP2 and TP receptors in the control of airway reflex events. Eur. Respir. J. 2015;45:1108–1118. doi: 10.1183/09031936.00061614. [DOI] [PubMed] [Google Scholar]
- 114.Kostenis E., Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol. Med. 2006;12:148–158. doi: 10.1016/j.molmed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 115.Christ A.N., Labzin L., Bourne G.T., Fukunishi H., Weber J.E., Sweet M.J., Smythe M.L., Flanagan J.U. Development and Characterization of New Inhibitors of the Human and Mouse Hematopoietic Prostaglandin D2 Synthases. J. Med. Chem. 2010;53:5536–5548. doi: 10.1021/jm100194a. [DOI] [PubMed] [Google Scholar]
- 116.Santus P., Radovanovic D. Prostaglandin D2 receptor antagonists in early development as potential therapeutic options for asthma. Expert Opin. Investig. Drugs. 2016;25:1083–1092. doi: 10.1080/13543784.2016.1212838. [DOI] [PubMed] [Google Scholar]
- 117.Kupczyk M., Kuna P. Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives. Drugs. 2017;77:1281–1294. doi: 10.1007/s40265-017-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.James K.M., Gebretsadik T., Escobar G.J., Wu P., Carroll K.N., Li S.X., Walsh E.M., Mitchel E.F., Sloan C., Hartert T.V. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J. Allergy Clin. Immunol. 2013;132:227–229. doi: 10.1016/j.jaci.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kajiwara D., Aoyagi H., Shigeno K., Togawa M., Tanaka K., Inagaki N., Miyoshi K. Role of hematopoietic prostaglandin D synthase in biphasic nasal obstruction in guinea pig model of experimental allergic rhinitis. Eur. J. Pharmacol. 2011;667:389–395. doi: 10.1016/j.ejphar.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 120.Nabe T., Kuriyama Y., Mizutani N., Shibayama S., Hiromoto A., Fujii M., Tanaka K., Kohno S. Inhibition of hematopoietic prostaglandin D synthase improves allergic nasal blockage in guinea pigs. Prostaglandins Other Lipid Mediat. 2011;95:27–34. doi: 10.1016/j.prostaglandins.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 121.Flohr C., Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014;69:3–16. doi: 10.1111/all.12270. [DOI] [PubMed] [Google Scholar]
- 122.Hajar T., Gontijo J.R.V., Hanifin J.M. New and developing therapies for atopic dermatitis. An. Bras. Dermatol. 2018;93:104–107. doi: 10.1590/abd1806-4841.20187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sampson H.A., Muñoz-Furlong A., Campbell R.L., Adkinson N.F., Bock S.A., Branum A., Brown S.G.A., Camargo C.A., Cydulka R., Galli S.J., et al. Second symposium on the definition and management of anaphylaxis: Summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 124.Perry T.T., Pesek R.D. Clinical Manifestations of Food Allergy. Pediatr. Ann. 2013;42:e106–e111. doi: 10.3928/00904481-20130522-09. [DOI] [PubMed] [Google Scholar]
- 125.Yu W., Freeland D.M.H., Nadeau K.C. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016;16:751–765. doi: 10.1038/nri.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wen T., Rothenberg M.E., Wang Y.-H. Hematopoietic prostaglandin D synthase: Linking pathogenic effector CD4+ TH2 cells to proeosinophilic inflammation in patients with gastrointestinal allergic disorders. J. Allergy Clin. Immunol. 2016;137:919–921. doi: 10.1016/j.jaci.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang S., Wu X., Yu S. Prostaglandin D2 receptor D-type prostanoid receptor 2 mediates eosinophil trafficking into the esophagus. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus. 2014;27:601–606. doi: 10.1111/dote.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Straumann A., Hoesli S., Bussmann C., Stuck M., Perkins M., Collins L.P., Payton M., Pettipher R., Hunter M., Steiner J., et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68:375–385. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- 129.Simons F.E.R., Frew A.J., Ansotegui I.J., Bochner B.S., Golden D.B.K., Finkelman F.D., Leung D.Y.M., Lotvall J., Marone G., Metcalfe D.D., et al. Risk assessment in anaphylaxis: current and future approaches. J. Allergy Clin. Immunol. 2007;120:S2–24. doi: 10.1016/j.jaci.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 130.Ono E., Taniguchi M., Mita H., Fukutomi Y., Higashi N., Miyazaki E., Kumamoto T., Akiyama K. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin. Exp. Allergy. 2009;39:72–80. doi: 10.1111/j.1365-2222.2008.03104.x. [DOI] [PubMed] [Google Scholar]
- 131.Kobayashi K., Tsubosaka Y., Hori M., Narumiya S., Ozaki H., Murata T. Prostaglandin D2-DP signaling promotes endothelial barrier function via the cAMP/PKA/Tiam1/Rac1 pathway. Arterioscler. Thromb. Vasc. Biol. 2013;33:565–571. doi: 10.1161/ATVBAHA.112.300993. [DOI] [PubMed] [Google Scholar]
- 132.Sanofi, Paris (FR) Pyrimidine Hydrazide Compounds as PGDS Inhibitors. 8258,130 B2. U.S. Patent. 2012 Sep 4;
- 133.Sanofi, Paris (FR) Phenyloxadialzole Derivatives as PGDS Inhibitors. 9,937,175 B2. U.S. Patent. 2018 Apr 10;
- 134.Astex Therapeutics Limited (UK) GlaxoSmithKline Intelectual (UK) Quinoline-3-Carboxamides as h-pgds Inhibitors. 2017/103851 A1. WO Patent. 2017 Jun 22;
- 135.Zai Lab Pty. Ltd. Study of the Tolerability and Pharmacokinetic of ZL-2102 With an Investigation of Food Effect in Healthy Male Subjects. [(accessed on 7 March 2019)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02397005.
- 136.Takeshita E., Komaki H., Shimizu-Motohashi Y., Ishiyama A., Sasaki M., Takeda S. A phase I study of TAS-205 in patients with Duchenne muscular dystrophy. Ann. Clin. Transl. Neurol. 2018;5:1338–1349. doi: 10.1002/acn3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Farhat A., Philibert P., Sultan C., Poulat F., Boizet-Bonhoure B. Hematopoietic-Prostaglandin D2 synthase through PGD2 production is involved in the adult ovarian physiology. J. Ovarian Res. 2011;4:3. doi: 10.1186/1757-2215-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cahill K.N., Bensko J.C., Boyce J.A., Laidlaw T.M. Prostaglandin D2: A dominant mediator of aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2015;135:245–252. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]