Abstract
The C3-like toxins are single-domain proteins that represent a minimal mono-ADP-ribosyl transferase (mART) enzyme with a simple model scaffold for the entire cholera toxin (CT)-group. These proteins possess a single (A-domain) that modifies Rho proteins. In contrast, C2-like toxins require a binding/translocation partner (B-component) for intoxication. These are A-only toxins that contain the E-x-E motif, modify G-actin, but are two-domains with a C-domain possessing enzymatic activity. The N-domain of the C2-like toxins is unstructured, and its function is currently unknown. A sequence-structure-function comparison was performed on the N-terminal region of the mART domain of the enzymatic component of the CT toxin group in the CATCH fold (3.90.210.10). Special consideration was given to the N-domain distal segment, the α-lobe (α1–α4), and its different roles in these toxin sub-groups. These results show that the role of the N-terminal α-lobe is to provide a suitable configuration (i) of the α2–α3 helices to feature the α3-motif that has a role in NAD+ substrate binding and possibly in the interaction with the protein target; (ii) the α3–α4 helices to provide the α3/4-loop with protein-protein interaction capability; and (iii) the α1-Ntail that features specialized motif(s) according to the toxin type (A-only or A-B toxins) exhibiting an effect on the catalytic activity via the ARTT-loop, with a role in the inter-domain stability, and with a function in the binding and/or translocation steps during the internalization process.
Keywords: mono-ADP-ribosylation toxins, C3-like toxins, C2-like toxins, ADP-ribosylation, CT-toxins, target substrate motifs, N-terminal α-lobe
1. Introduction
Bacterial mono ADP-ribosyl transferase toxins (mART toxins) belong to a family of toxins that catalyzes the covalent transfer of an ADP-ribose moiety from NAD+ to a protein or DNA target in a host cell, changing target activity and impairing target cell function and survival [1]. This toxin family includes diverse members such as exotoxin A (ExoA) from Pseudomonas aeruginosa, pertussis toxin (PT) from Bordetella pertussis, and cholera toxin (CT) from Vibrio cholerae. Members of this family are steadily increasing and are classified as either CT-group (after “Cholera toxin”) or DT-group (after “Diphtheria toxin”).
All bacterial mART toxins share a common structural fold composed of ~100 residues with low sequence homology formed by a core scaffold of two perpendicular β-sheets, flanked by variable helical sub-structures and exposed-loops that constitute a cleft for the binding of the NAD+ substrate. The SCOP2 database [2] assigns mART toxins (SCOP2: 56400) to the α + β class of proteins (SCOP2: 53931) and to the “unusual” ADP-ribosylation fold (SCOP2: 56398). This fold is shared with eukaryotic mART proteins, Ecto-ARTs (SCOP2: 82814), and with the C-terminal domain of poly (ADP-ribose) polymerases known as PARPs (SCOP2: 56398), among others ADP-ribosylating proteins (for a review see Fieldhouse, 2008 [3]).
The CT-group of mART toxins is a large group of A-only toxins (containing the catalytic component only, e.g., C3bot1, Vis, ExoS) [4,5,6], and binary A-B toxins (A: catalytic, B: cell binding/translocation components in distinct proteins, e.g., iota, and CT) [7,8] and A/B (A and B components in the same polypeptide, e.g., Certhrax [9,10,11] with low sequence identity but containing three conserved regions with the consensus (Y/F)R (Underline refers to conserved residues) (Region 1), (Y/F)xSTS (Region 2), and the catalytic (Q/E)xE (Region 3) motifs, with either NAD+-binding or catalytic function (for reviews see Fieldhouse, 2010 [12]). The CT-group of toxins is classically divided into ExoS-like, C2-like, C3-like, and CT/PT-like subgroups of toxins, based mainly on the catalytic motif (Region 3), subunit organization, and macromolecule targets.
Exoenzyme S (ExoS) (UP: Q51448) (UP: UniProtKB accession number) from P. aeruginosa is an A-only, four-domain, bi-functional toxin/effector with a C-terminal mART domain; it requires factor- activating ExoS (FAS) for activation [13]. Three additional ExoS-like toxins have been identified: ExoT toxin (UP: Q9I788) from P. aeruginosa [6], the single-domain VopT toxin (UP: Q87G19) from V. parahaemolyticus [14], and AexT toxin (UP: Q93Q17) from Aeromonas sp. [15]. ExoS-like toxins are secreted into target cells via the type III secretion system (T3SS), possess the ExE catalytic motif and target the RAS family of G-proteins (except for AexT which targets G-actin) (for a review see Barbieri, 2004 [6]).
C2 is a mART toxin (UP: D4N871) from Clostridium botulinum [16] and is an A-B multi-unit exotoxin whereas its C2I mART subunit (A-component) requires the C2II receptor-binding subunit (B-component) to facilitate cell entry of the catalytic A-subunit. The A-component is a bi-domain protein with both halves structurally, but not functionally equivalent. The C-terminal domain harbors the mART activity, while the N-terminal domain interacts with the B-component. The C2- like subgroup includes members such as iota toxin (UP: Q46220, Ia subunit) from Clostridium perfringens type E [17], CST toxin (UP: O06497, SA subunit) from Clostridium spiroforme [18], VIP toxin (UP: G8C882, VIP2 subunit) from Bacillus cereus [19], CDT toxin (UP: Q9KH42, CDTa subunit) from Clostridium difficile [20]; and the newly discovered CPILE toxin (UP: X512D7, CPILE-a subunit) from Clostridium perfringens W5052 strain [21]. C2-like toxins contain the catalytic ExE motif, form an AB7 oligomer to gain cell access, and ADP-ribosylate G-actin (for reviews see Tsuge 2017 [22]).
The C3 exotoxin (UP: P15879) from C. botulinum [23] (C3bot1) is a single-domain (A-only) mART toxin. Eight other members of C3-like toxins have been described so far: C3bot2 (UP: Q00901) from C. botulinum [24], C3lim (UP: Q46134) from C. limosum [25], C3cer (UP: Q8KNY0) from B. cereus [26], C3larvin (UP: W2E3J5) and Plx2A (UP:M9V3B7) from Paenibacillus larvae [27,28], and three isoforms C3stau1(UP: P24121), C3stau2 (UP: Q8GAX6), and C3stau3 (UP: Q8VVU2) produced by Staphyococcus aureus [29,30]. C3-like toxins harbor the catalytic QxE signature and modifies RhoA, B, and C, among others GTPases (for reviews see Vogelsgesang, 2007 [31] and Tsuge, 2017 [22]).
The CT-like subgroup (after cholera/pertussis toxins) is a more heterogenous group in terms of the unit organization, catalytic motif, molecular target, and modified residues. This group includes CT (UP: P01555, A-subunit) and Chelt toxins (UP: A2PU44, A-subunit) from V. cholera [12,32], PT toxin (UP: P04977, A-subunit) from Bacillus. pertussis [33], human LT (UP: P43530, A-subunit) from Eshcerichia coli [34], Scabin toxin (UP: C9Z6T8) from S. scabies [35], among others.
However, there are other mART toxins that do not clearly match with any of the previous classification schemes for the CT group. In these “ungrouped” toxins are the following: SpyA toxin (UP: Q1J858/ Q1JN57) from Streptococcus pyogenes [36], which is structurally similar to C3-like toxins (A-only) and with a secretion signal-sequence, but harbors the ExE motif and ADP-ribosylates vimentin among other substrates [37], and Plx2A toxin (UP: M9V3B7) from P. larvae [28]. These proteins are related to C3-like toxins (single-domain A-component with QxE catalytic motif that ADP-ribosylate Rho proteins) but also show features of C2-like toxins (require a binding/translocation partner, B-component) for intoxication. SpvB toxin (UP: P555220) from Salmonella enterica [38], VahC toxin (UP: Q49TP5) from Aeromonas hydrophila [39], and Photox toxin (UP: Q7N9B1) from Photorhabdus luminescens [40] possess the ExE motif, ADP-ribosylate G-actin, and are two-domains toxins (C-domain has mART activity). AexU toxin (UP: A0FKE5) from A. hydrophila [41] has a domain organization like AexT and is secreted into target cells by using the type III secretion system and harbors the catalytic QxE motif rather than the ExE motif characteristic of ExoS- like toxins. Certhrax toxin (UP: Q4MV79) from B. cereus strain G9241 [9] is a two-domain A/B toxin with its catalytically C-domain homologous to C3-like toxins (QxE motif), but its N-domain is homologous to the PA-binding domain of the anthrax lethal factor from Bacillus anthracis. Vis toxin (UP: A3UNN4) from Vibrio splendidus strain 12B01 [5] is a single-domain A-only toxin homologous to C3-toxins with an N-terminal secretion signal peptide. Vis harbors the ExE motif, although Vis does not covalently modify conventional C2-like nor ExoS-like substrates. Mav toxin (UP: AOQLI5) from Mycobacterium avium strain 104 [12] is a tetra-domain A/B toxin with the mART activity in the C-terminal domain that contains the ExE catalytic motif. EFV toxin (UP: Q838U8) from Enterococcus faecalis strain V8583 [12] is a bi-domain protein with the mART activity in the C-terminal domain and an ExE catalytic motif that targets actin (unpublished results).
The CATH database [42] assigns the closely related codes 3.90.176.10 for C2-, C3-, and ExoS- like subgroups, and 3.90.210.10 for the CT/PT-like subgroup of CT-group of mART toxins. The enzymatic component of the CT-group (CATCH fold, 3.90.176.10) at the N-terminal region, i.e., upstream of the strand β1 of the β-core scaffold, is well structured with a similar fold, albeit not much attention has been given to this section. In the present work, we performed a sequence-structure-function comparison of the N-terminal region of CT-group mART toxins in the CATCH 3.90.176.10 category (non-CT/PT-like toxins), with special emphasis on the N-most distal segment (a putative helix-coil structure) and its different roles in these toxins.
2. Results and Discussion
2.1. The Overall Structure of the N-Terminal α-Lobe
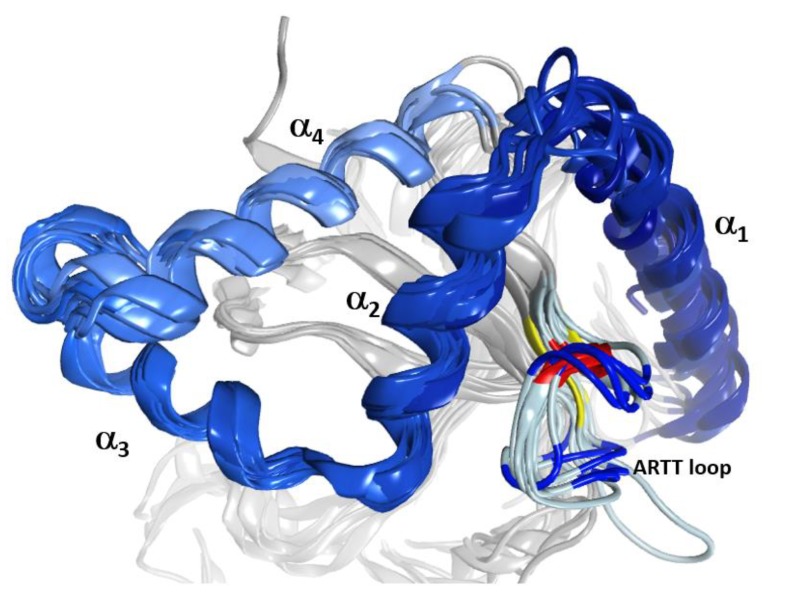
The C3-like toxins are single-domain proteins that represent a minimal mART enzyme with a simple scaffold that serves as the model for the entire CT-group. The N terminus is a helical region formed by four consecutive α-helices, α1–α4 (Figure 1). This region shares a lower average similarity for those residues in structured elements (~68%, from 52% to 88% pairwise) than with the set of β-strand residues that form the β-core superstructure (~73%, from 59% to 99% pairwise). Structurally, this N-terminal region consists of a conserved fold configured within a compact lobe, the “α-lobe”, with a high degree of overlap among the C3-like toxins (average RMSDα = 2.4 Å; Figure 1).
Figure 1.
The α-lobe in C3-like toxins. Superposition of six C3-like toxins showing a compact N-terminal substructure that encompasses four α-helices. The ARTT loop (ADP-ribosyl-turn-turn) is a catalytic region that is responsible for the target substrate recognition and is shown at the bottom right of the overlaid structures. The superposed structures include C3 exotoxin (PDB:1G24) and C3bot2 (PDB:1R45) from C. botulinum, C3lim (PDB:3BW8) from C. limosum, C3cer (PDB:4XSG) from B. cereus, C3larvin (PDB:4TR5) from P. larvae, and C3stau2 (PDB:1OJQ) produced by S. aureus.
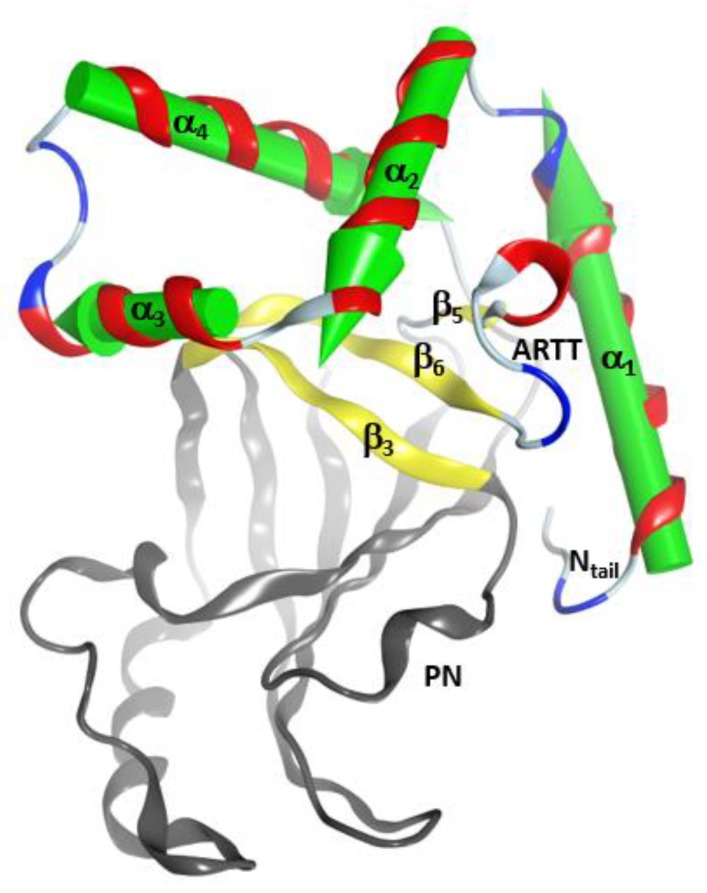
This α-lobe is packed following a V−L−αα-corner topology (Figure 2), which encloses the βII sheet and its connecting loops/turns according to: (i) an open V-shaped α1–α2 superstructure that surrounds the catalytic ARTT loop followed by (ii) an L-shaped α2–α3 superstructure that exposes the α2/3-turn and the key α3-motif (defined later) to the NAD+-binding pocket and to the protein target; and finally, (iii) an α3–α4-corner superstructure. The loop that links helices α3 and α4, the “α3/4-loop”, offers the flexibility required to form an αα-corner superstructure with a longer connection by turning the inertial axis of helix α4 orthogonal to both the α3 and the plane formed by the α2 and α3 axes. As a result, α4 is in a transverse orientation in relation to the βII sheet, increasing the contact surface between the α-lobe and the βII sheet.
Figure 2.
The α-lobe topology in C3-like toxins. Depiction of the V–L–αα-corner topology of the α- lobe. The green arrows correspond to the inertial axes of the α-helices. The βII sheet is shown in yellow ribbons with β3, β5, and β6 strongly interacting with the elements of the αα corner. The PN (phosphate-nicotinamide) loop is an NAD+ substrate-binding loop and it is indicated along with the N-terminus (N-terminal tail) of the domain.
In addition, C3-like toxins are characterized by an unstructured N-terminal segment, the “Ntail”, of variable length (Figure 2). Furthermore, the C3-like α-lobe is a well-structured, globular motif and is topologically identical or similar to: (i) the N-terminal helical region of the single-domain enzymatic component of the A-B binary Plx2 and Larvin toxins (Plx2A, PDB:5URP); (ii) the N- terminal helical region of the catalytic C-domain, “C2C-domain” of the A-component of the binary C2 toxin (Figure 3), C2I (PDB: 2J3Z), and C2-like toxins such as Vip2 (PDB: 1QS1), iota Ia (PDB: 1GIQ), CdtA (PDB: 2WN4), and SA component of the CST toxin (HM2). In these toxins, the N-terminal region of the non-catalytic N-domain, “C2N-domain”, interacts with the binding/translocation component (see later), shows a similar topology and superposes well with the C3-like α-lobes; it also possesses an additional well-defined helix (α4a) connecting α3 and α4; (iii) the helical region of the Certhrax toxin C-terminal domain (PDB: 4GF1)–Certhrax toxin has a significantly longer α3/4-loop than most C3- and C2-like toxins; and (iv) the helical region of the VahC C-terminal domain (PDB: 4FML) and SpvB (PDB: 2GWM). The first two crystallographically ‘solved’ helices in these toxins superpose well with the α2 and α3 helices of the α-lobe. There is also a 20-residue long helix-loop insertion between the second (equivalent to α3) and third (equivalent to α4) solved helices in these toxins. Additionally, the mART domain of Photox toxin reveals a high sequence homology with VahC and SpvB toxins; consequently, homology models of Photox show the canonical α2–α4 topology; and finally, (v) the N- terminal segment of Vis toxin (PDB: 4XZJ) which has a longer α1 helix with a slightly different orientation.
Figure 3.
Topology of the A-component of the C2-like toxins. Structure of C2I toxin (PDB: 2J3Z) as a representative member of the C2-like class, showing the “adaptor” N domain (in cyan ribbons) and the “catalytic” C domain (in fuchsia ribbons). The T-segment of each domain is colored in yellow ribbons.
There is no X-ray structure for any member of the ExoS-like group. However, Sun et al. reported homology models of the N-terminal mART domains of ExoS and ExoT toxins, based on Ia toxin as a template; these models superpose well with the C3-like α-lobe [43]. On the other hand, for the enzymatic component of the CT/PT-subgroup (e.g., CT, LT-A/IIB, PT, and Scabin toxins, among others) there is no structural equivalent to the C3-like α-lobe. Instead, certain coils, helices, and strands (and their connecting loops) of the βII sheet occupy the same location within the α-lobe without significant overlap with other elements—only the backbone structure of the α3-motif is roughly traced by an active-site helix or loop.
2.2. Stability of the α2−α4 Superstructure
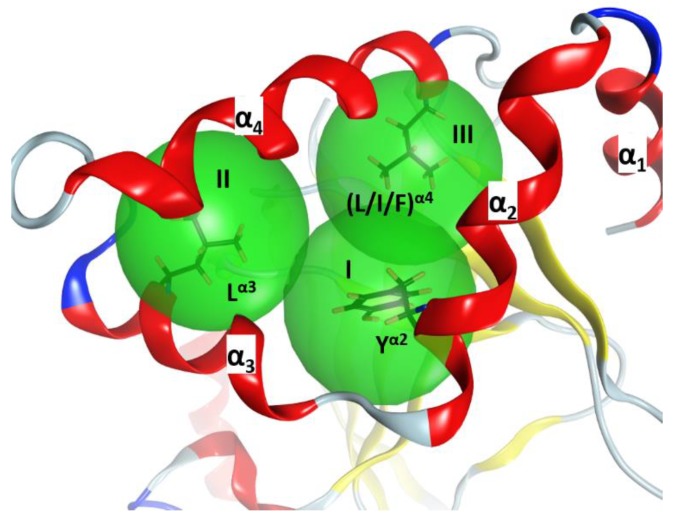
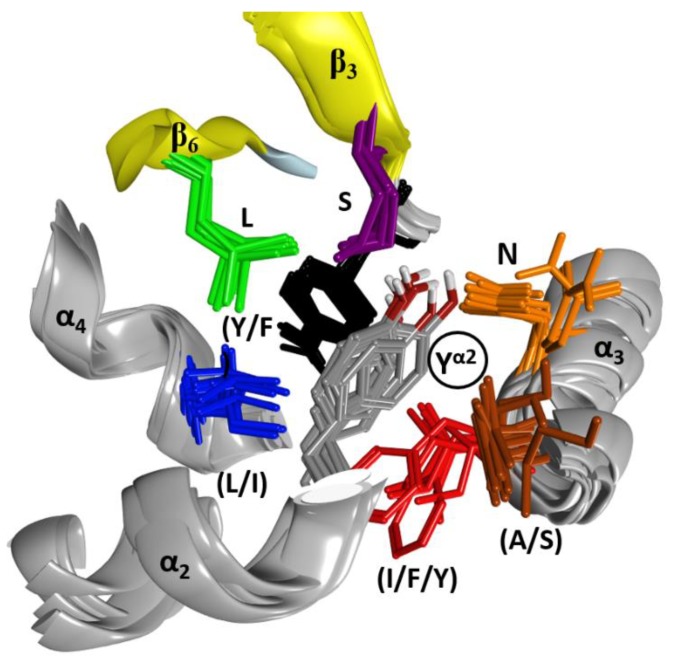
In C3-like toxins, the α2−α4 superstructure of the α-lobe is clustered and is in contact with the rest of the protein by a network of hydrophobic interactions centered in three key residues: Tyr in α2 (Tyrα2), Leu in α3 (Leuα3), and Leu/Ile/Phe in α4—one in each helix (Figure 4). In addition, an aromatic residue is present in the β2/3-turn, (Tyr/Phe)β2/3 (not shown). Effectively, Tyrα2 is the center of a cluster of interactions, “clusterI”, that cements the whole α2−α4 segment with the rest of the structure (Figure 5). The structural relevance of this hydrophobic and polar cluster of residues is evidenced by the large number of conserved and similar residues (including an invariant Leu in β6, see Figure 5) and the significant overlap of their side-chains. The reduced mobility of these residues as reported by their crystallographic B-factors reveals the mutually imposed constraint within the compact structure of the α-lobe. Tyrα2 is conserved in Plx2A in the C2C-domains, in most of the C2N-domains, and even in most of the toxins of the CT-group with known α-lobe topology. Compatible with its structural role, Tyrα2 is conservatively replaced with a Phe in Vis toxin, and with Leu in C2IN and Vip2N domains. However, Tyrα2 might also play an “active-site” role as its side-chain hydroxyl bridges the conserved Serβ3 (part of the STS motif) with Asnα3 (part of the NLR or α3-motif, see later); these are two critical residues involved in NAD+ substrate binding. The substitution of Tyrα2 with Hisα2 in HopU1 toxin is still compatible with the suggested role, particularly considering that HopU1 has a longer α2/3-link that might assist in the binding of NAD+ [44].
Figure 4.
The α-lobe stability. α2–α4 superstructure of C3larvin (PDB: 4TR5), showing three hydrophobic clusters (depicted as green spheres) centered at three key residues. The structural “clusters” for the α2–α4 superstructure are designated as I, II, and III and the superstructure is framed by three α helices (α1, α2, and α3).
Figure 5.
Interactions in ClusterI of the α2–α4 superstructure. The cluster of residues around the conserved Tyrα2 (circled label; grey residue) is shown in this set of six overlaid C3 structures. The side- chains are shown for Tyr/Pheβ2,3 (black), Serβ3 (mauve), Leuβ6 (green), Leu/Ileα4 (blue), Ile/Phe/Tyrα3 (red), Ala/Serα3 (brown), and Asnα3 (orange).
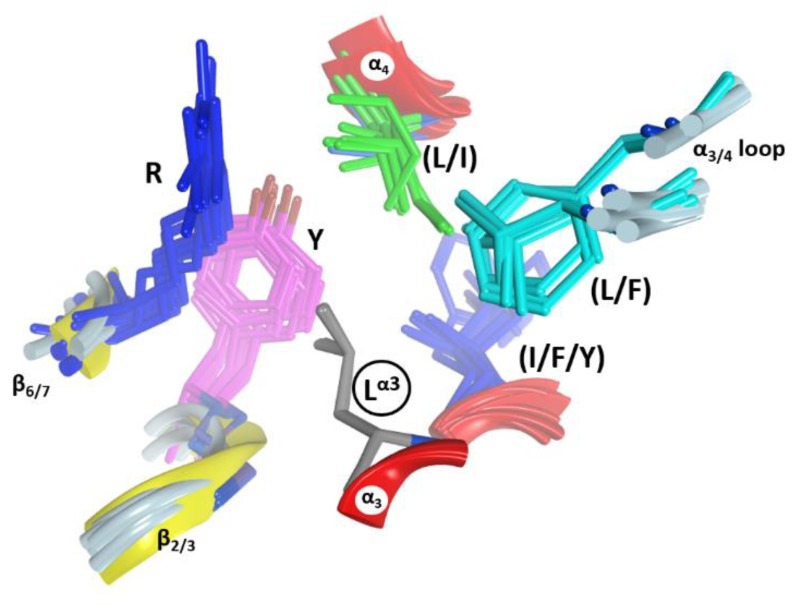
In C3-like toxins, the conserved Leuα3 is part of the (NLR)α3 motif and is the center of a second cluster of hydrophobic interactions, “clusterII” that links α3 and α4 and connects them to other elements (Figure 6). In this cluster, Leuα3 interacts with (Leu/Ile)α4, with some residues in the α3/4-loop, and with two other regions that act as hinges between both β-sheets–the conserved Tyr β2/3 and the conserved Arg β6/7 (part of the (LPR) β6/7 motif). This cluster is structurally well defined in C3-like toxins as evidenced by superposing the structures on Leuα3 (grey residue in Figure 6). It is remarkable how identical this configuration (i.e., same side-chain torsion angles) is for these clustered residues. Leuα3 is conserved in Plx2A, the C2-like toxins (C2C-domains, but not the C2N-domains) and in most of the non-PT-like toxins (except, for example, the TccC3 and TccC5 toxins) [45]. This observation suggests an active role for Leuα3 in the stability of the α3-motif, which is relevant for the binding of the NAD+ substrate, rather than serving a pure structural role in the lobe stability.
Figure 6.
Interactions in ClusterII. The cluster of residues around the conserved Leuα3 (circled label) in ClusterII is shown in this set of six overlaid C3 structures. The side-chains are shown for Arg β6/7 (dark blue), Tyr β2/3 (mauve), Leuα3 (grey), Ile/Phe/Tyrα3 (light blue), Leu/Pheα3,4 (cyan) and Ile/Valα4 (green). The residue nomenclature is as follows: the superscript refers to the alpha or beta secondary structure followed by the strand or helix number in the C3bot1 structure (PDB:1G24).
A hydrophobic residue at the N terminus of α4, either Leu or Ile, is the center of the “clusterIII” in C3-toxins and most non-PT-like toxins (not shown). The central (Leu/Ile)α4 contacts the conserved (Tyr/Phe)β2/3 (Figure 6) and the conserved Leu β6 (Figure 5) in most toxins of the CT-group (an exception is HopU1 toxin) [46]. It is important to highlight (Tyr/Phe)β2/3 (Figure 6); this residue welds (coordinates) the three hydrophobic clusters in all the catalytic domains with the α-lobe configuration, even in the C2N-domains, which reaffirms the important structural role of this aromatic motif. Moreover, the presence of (Tyr/Phe)β2/3 as part of the (Y/F)xSTS motif in the toxins of the CT-group [3,12] reveals a structural role in preserving the N-terminal configuration, regardless of whether this region has the α-lobe topology in contact with the β-scaffold.
2.3. The α3-Helix and the α3-Motif
The α3-helix is defined in all the C2/C3-like toxins and most of the CT-like toxins with the α-lobe topology. In C2/C3-like toxins, the α3 helix harbors the α3-motif, Yα2−(IN−LR)α3 (Figure 5), which includes residues from both the α2 and α3 helices. The spatial orientation of the α3-motif along the α3 inertial axis offers a recognition surface aligned with the long axis of the bound pose of the NAD+ substrate; hence, functionally, the α3-motif binds the NAD+ substrate and contacts the target protein substrate. Thus, the α2/3-loop (N-terminal end), the conserved Asnα3 (at the center), and the semi- conserved Argα3 (at the C-end) of the α3-motif, all point their side-chains towards the binding cavity and contact the bound NAD+ and/or the target protein substrate. In effect, in the complexes of C3bot1 (PDB: 2C8F) and C3stau2 (PDB: 1OJZ) with NAD+, the NH of Asnα3 H-bonds the NAD+ A- phosphate, and Argα3 stacks with the NAD+ adenine ring. The relevance of these two residues in NAD+ binding is evidenced by their absence in the non-catalytic C2N-domains (see later). However, Asnα3 is absolutely conserved in all the non-PT-like toxins, while Argα3 is less conserved (e.g., an Ile residue in certain C2-toxins). The role of Tyrα2 and of Leuα3 in this motif was already discussed above.
2.4. Role of the Ntail-α1 Segment in C3- and C2-Like Toxins
Contrary to the conservation seen in the α2−α4 region, the Ntail-α1 of C3-like toxins is a variable segment. The length of the unstructured Ntail is variable, being non-existent in C3larvin to 20 residues in C3stau(s) and C3cer. Likewise, α1 is 15 residues in C3lim, but is only eight residues in both C3cer (which superposes with the N-terminal half of C3lim α1) and C3larvin (which superposes with the C-terminal half of C3lim α1).
The eight known C3-like toxins do not possess a specialized cell binding/translocation component or domain for access/entry into their target host cells, and the details on the mechanism and molecular determinants involved in the toxin internalization await further characterization (for a recent review see Rohrbeck, 2016 [47]). This is relevant for all C3-like toxins except C3stau toxins (C3stau1, C3stau2 and C3stau3), since S. aureus infects the host cell and releases the toxins into the cell cytoplasm [29]. Thus, C3bot1, C3bot2, C3lim, C3cer, and C3larvin toxins will be referred to collectively as “C3-etoxins” (with “e” after extracellular).
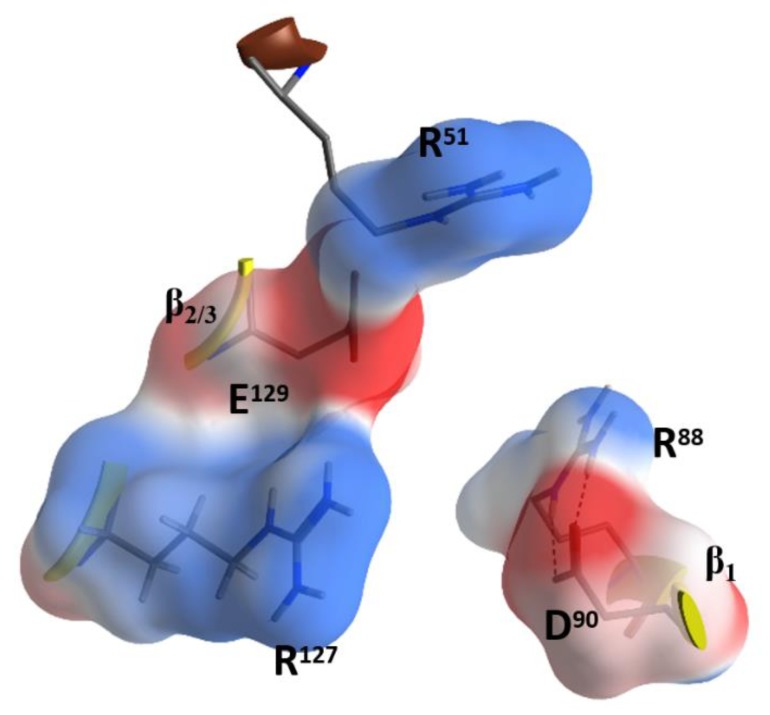
Due to the relatively high concentrations and long incubation times required for C3-etoxins to enter the target host cells, it was previously suggested that these toxins gain host access by non- specific pinocytosis [25]. Also, C3-etoxins have a basic α1 helix; basic peptides have been shown to interact with charged phospholipids on the outer layer of the cell membrane of host cells, causing destabilization of the lipid bilayer [48]. In fact, a short “transport” peptide fused to the C terminus of C3bot1 enabled chimeric toxin entry into the cytoplasm by a receptor-independent mechanism [49]. However, C3bot1 and C3lim may be selectively internalized into the cytoplasm of macrophage-like murine cells likely by a specific endocytotic mechanism [50]. Indeed, Rohrbeck and colleagues [51] identified a membrane partner that binds C3bot1, and vimentin (rod domain) was established as the cellular receptor in neuronal and macrophage cell lines. A more recent study showed the 88-RGD-90 sequence in C3bot1 functions as a vimentin binding-motif in neuronal cells [52]. Effectively the RGD motif is unique in C3-etoxins; however, it should be noted that this Arg residue (at β1) is an invariant residue in all CT-like toxins (the signature “R”) involved in the binding of the NAD+ substrate. The conserved Arg residue is buried in the NAD+-binding pocket and is stabilized by strong salt-bridges with the Asp in this motif in the apo structures. The fact that C3stau harbors RLL, instead of the RGDβ1 motif, and only weakly intoxicates HT22 cells [53], would suggest that there is a concerted participation of the residues involved. In addition, because of the highly buried nature of the RGDβ1 motif (e.g., Arg88 is only partially exposed to solvent), we envisage that other residue(s) that form the NAD+-binding interacting surface might participate in the recognition motif for vimentin. In this sense, the RGDβ1 motif is close to the conserved Rα3 of the signature α3-motif (Arg51 in C3bot1), and to the RxE motif located in the β2 strand (residues 127–129 in C3bot1) of the C3 group, (Figure 7). The RGDβ1 motif along with the RxE β2 motif and Rα3 form an ‘electrostatic clamp’ with complementary charges and H-bond capabilities in C3-etoxins (Figure 7).
Figure 7.
Putative vimentin binding motifs in C3bot1. Depiction of Arg88 and Asp90 of the RGD motif, the Arg127 and Glu129 of the RxE motif, and the conserved Arg51 in the signature α3 motif.
Interestingly, monomeric (soluble) vimentin is reported to be ADP-ribosylated by SpyA in the head domain of the protein [36,37]. This implies that vimentin must not bind into the NAD+-binding pocket of SpyA in order to behave as a protein substrate for the transfer reaction. Incidentally, SpyA lacks the RGDβ1 motif (RYVβ1 instead), lacks Rα3 (Dα3 instead), and lacks the RxEβ2 motif (YxKβ2). Additionally, no C2-like toxin exhibits these motifs without also binding vimentin. Plx2A harbors RGTβ1, Rα3 and LxEβ2 and enters mouse macrophages in the absence of the Plx2B protein [28]. Therefore, the Rα3 and Eβ2 residues may play a key role in the binding of the vimentin rod domain.
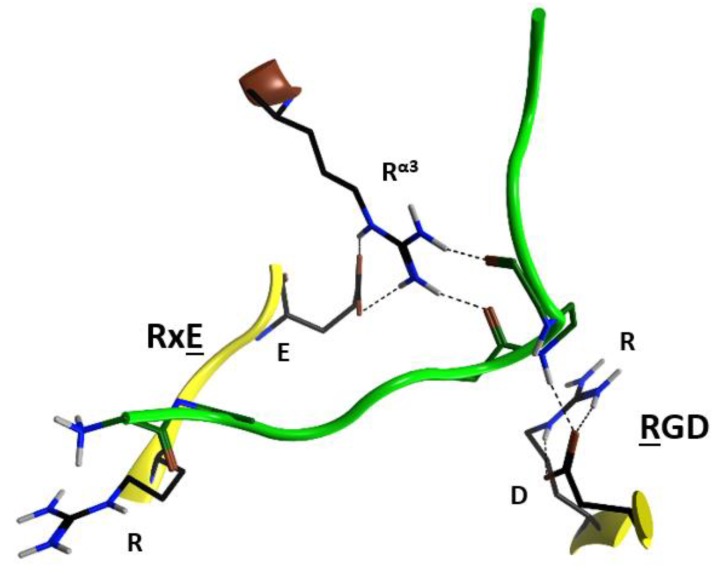
Notably, the RxE motif (TxE in C3cer) is only present in the C3-etoxins. Thus, active-site residues, RGD (and likely also Rα3 and RxEβ2), while important for interaction with NAD+ for catalysis, have the role of binding vimentin on the membrane surface of the host cell. This dual function may be rationalized because these two activities are manifested in different compartments (i.e., the cytoplasm for enzymatic activity and the extracellular space for the vimentin recognition) and in different stages of the intoxication process. Therefore, considering that C3-like toxins correspond to a “minimal” toxin, then Asp90 and Arg127 (in C3bot1) may have evolved to use the invariant Arg51, Glu129, and Arg88 residues to bind a required membrane component for toxin internalization. Validation of the role of these residues in protein–protein interactions lies in the crystal contacts of a C3larvin fragment—of a symmetrically related molecule–docked into the NAD+-binding pocket in the C3larvin crystal structure (PDB: 4TR5) (Figure 8).
Figure 8.
Protein–protein interactions in C3larvin. Depiction of the docking of a segment of C3larvin (green backbone) into the NAD+-binding pocket of another C3larvin molecule in the crystal structure (PDB: 4TR5). The residues correspond to the motifs presented in Figure 7.
There is additional evidence to suggest that the helical α-lobe might be part of the binding machinery with specific membrane component(s). In principle, the Rα3 signature fulfills this proposal since Rα3 is in the α-lobe. However, C3larvin fails to enter vimentin-expressing mouse macrophage cells [27] despite harboring RGDβ1, Rα3, and RTEβ2. Notably, C3larvin possesses a truncated α1; a chimeric construct formed by adding 18 N-terminal residues (Ala1-Trp18) from the α1 helix of C3bot1 achieved cell penetration [27].
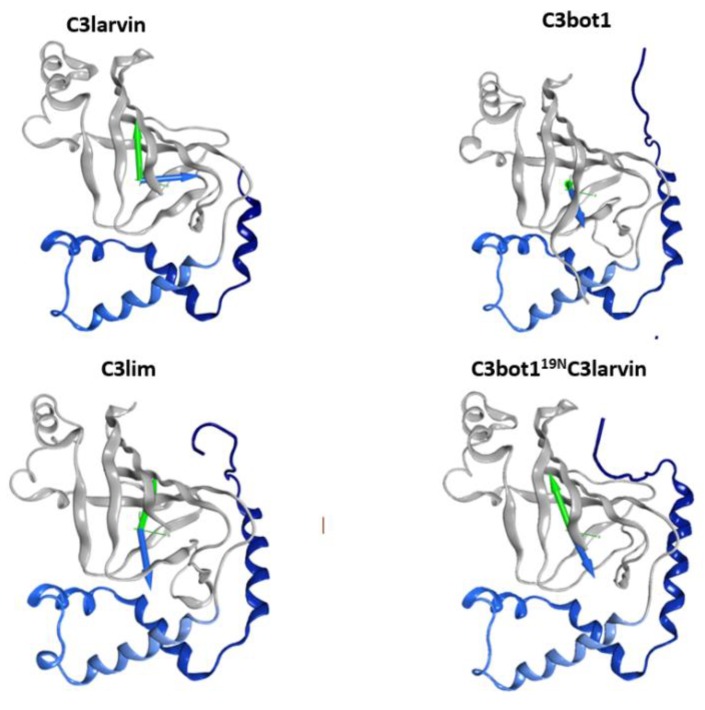
Inspection of the short α1 in C3larvin reveals a negative residue at the N-terminus, Glu2, which is unique for this position (consensus Asn in C3-like toxins and Lys in C2-like toxins) and remarkably represents the only difference with the entire sequence of the Plx2A toxin, which shows excellent cell binding and penetration [28]. This Glu residue at one end and a cluster of basic residues at the other end of α1 confer a significant dipole-moment to the helix and suggest an electrostatic mode of interaction for this element. Notably, the calculated protein dipole moment for C3larvin points in a direction perpendicular to C3bot1 and C3lim dipole vectors (Figure 9); however, the protein dipole moment for the in silico C3bot118NC3larvin chimera is practically aligned to the dipole vectors of C3bot1 and C3lim toxins. Thus, it seems that not only the net charge, but also the charge distribution may be important determinants for cell internalization.
Figure 9.
Dipole and hydrophobic moments for C3 toxins. The arrows represent the vectors of the electric (blue) and hydrophobic (green) dipoles moments for the wild-type (WT) and chimeric (bottom right) proteins.
The apparent role of the Ntail-α1 in mediating toxin cell entry may not be exclusive to C3-etoxins. In the enzymatic component of binary C2-like toxins, the N- (adaptor) and C- (catalytic) domains face one another, so there is an obstruction in the central cleft (NAD+-binding pocket) of the N-domain, rendering it inactive (Figure 3). The N-domain of C2I toxin is considered a specialized structure that interacts with the binding/translocation protein partner (B-component) [54], and studies of chimeric fusion proteins with C2 toxin have revealed the αN-lobe of this toxin mediated cell entry. In effect, a construct formed by fusing the C2IN-domain with the C3lim toxin (C2INC3lim construct) was able to intoxicate mammalian cells in the presence of the activated C2II protein (B-component) [55]. Moreover, the N-terminal α-lobe of the C2IN-domain (residues 1–87) alone was enough to facilitate uptake of the C2IN87NC3bot1 fusion protein [56]. Furthermore, a construct of C2IN without the first 29 residues and the C3bot1 toxin, C2INΔ29NC3bot1, failed to enter into enter HeLa cells [57]. Notably, the C3-like Ntail-α1 element is preserved in the two domains of all C2-like toxins, and it is comparable in structure and relative location in each of them.
Plx2A toxin resembles C3-like toxins in the sense that its A-component is a single domain mART enzyme. Accordingly, the α-lobe of Plx2A is highly like the α-lobe of C3-like toxins, e.g., it possesses a small α3/4-loop (or α4a helix), and the Ntail-α1 is a terminal structure. However, at the same time, Plx2A resembles C2-like toxins because it requires a B-component, Plx2B, as a binding/translocation partner for the cell internalization [28,58]. In this sense, the advent of Plx2A offers an invaluable opportunity to assess the “properties” encoded in the terminal Ntail-α1 segment of the C2- and C3-like toxins.
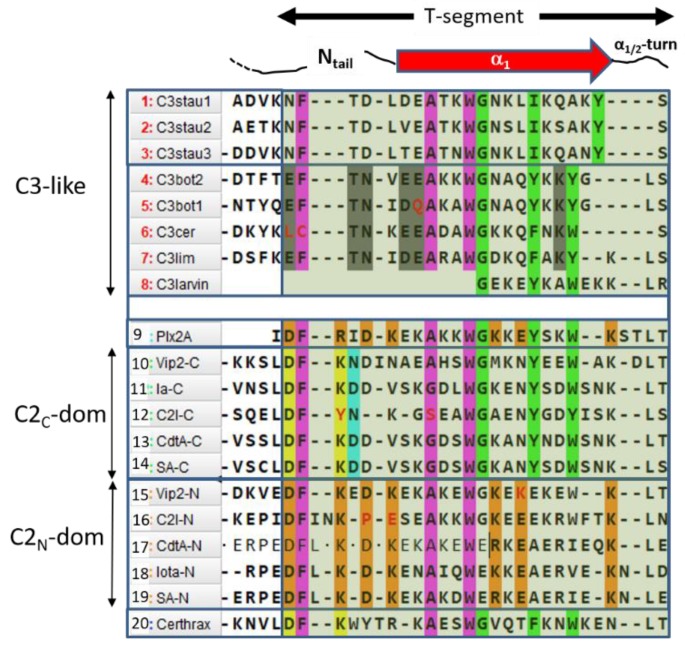
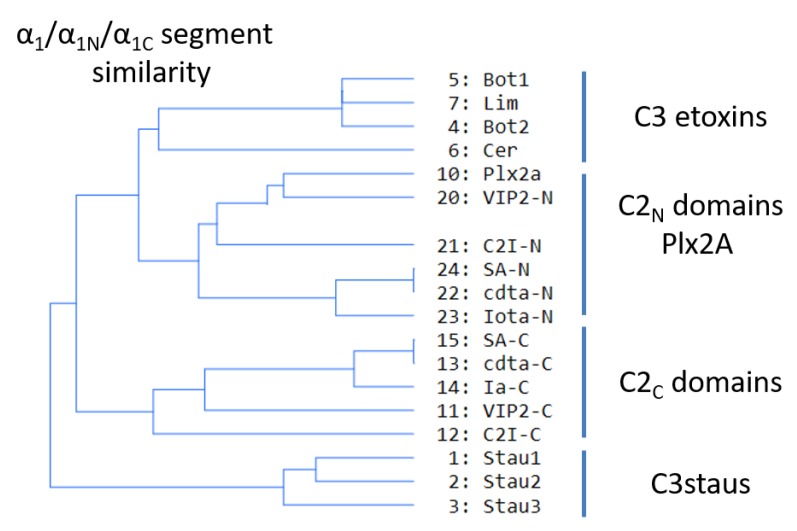
Sequence-function analysis of the T-segment—a structure-based, multiple-sequence-alignment (MSA) enhanced with the pattern of molecular interactions—was performed for the Ntail-α1 segment of the C3-like toxins, Plx2A, and the C2N- and C2C-domains (Figure 10). With the previous alignment and taking Plx2A toxin as the “master” sequence because it has the shortest Ntail among the aforementioned toxins, the “T-segment” (“T” after terminal) was identified that comprises five residues of the Ntail, the α1 helix, and the α1/2-turn (Figure 10). Subsequently, a cluster analysis based on the similarity of the T-segment residues was performed on this toxin group, excluding C3larvin because it lacks the Ntail and has a truncated α1 (Figure 11).
Figure 10.
The T-segment motif. Multiple sequence alignment of the Ntail-α1 segment of C3-like toxins (#1–#8), Plx2A toxin (#9), and C2-like toxins (#10–#19), and definition of the T-segment (translucent green box). The functional motifs are shown in color: purple, S-motif; green, C-motif; yellow and cyan, I-motif; light brown, B-motif; and gray, T-motif. Non-synonymous substitutions are highlighted in red text. Certhrax toxin (#20) is included for comparison.
Figure 11.
T-segment similarity. Cluster analysis of C3-like toxins (except C3larvin), C2-like toxins (N- and C-domains), and Plx2A, based on T-segment similarity.
Three noteworthy features of the T-segment include: (i) C3staus are unique from other domains/toxins; (ii) single-domain C3-etoxins cluster closer with C2N-domains than with the catalytic C2C-domains of C2-like toxins; and (iii) single-domain Plx2A toxin clusters with C2N-domains, rather than with the single-domain C3-etoxins or with the catalytic C2C-domains.
The T-segment in C3-etoxins can be defined by the consensus sequence (The long hyphen “–“ corresponds to any number of residues; like Xn),
TC3-segment: EFITN–(E/D)EA–WIIG–(Y/F)III–KYIV
which comprises four (numbered as I to IV) highly conserved aromatic residues. Likewise, the T-segment of the N-domain of C2-like toxins can be defined by the
TC2N-segment: DFI–K–D–(A/G)–(K/R)–WII–K–E–K
and the T-segment of the C-domain of C2-like toxins can be defined by
TC2C-segment: DFI–K(N/D)D–A–WIIG–YIII–(Y/W)IV−K
Thus, combining the three previous definitions, along with the T-segments of Plx2A, the following consensus sequences arise:
-
(S1)
FI–A–WIIG–(Y/F)III–(Y/W)IV, in Plx2A, and C3-etoxins,
-
(S2)
DFI–D–(A/G)–WIIG–YIII–WIV−K, in Plx2A, and C2C domains, and
-
(S3)
DFI–K–D–(K/R)–A–WII–(K/R)−E–K, in Plx2A, and C2N domains.
In turn, four sequence motifs emerge:
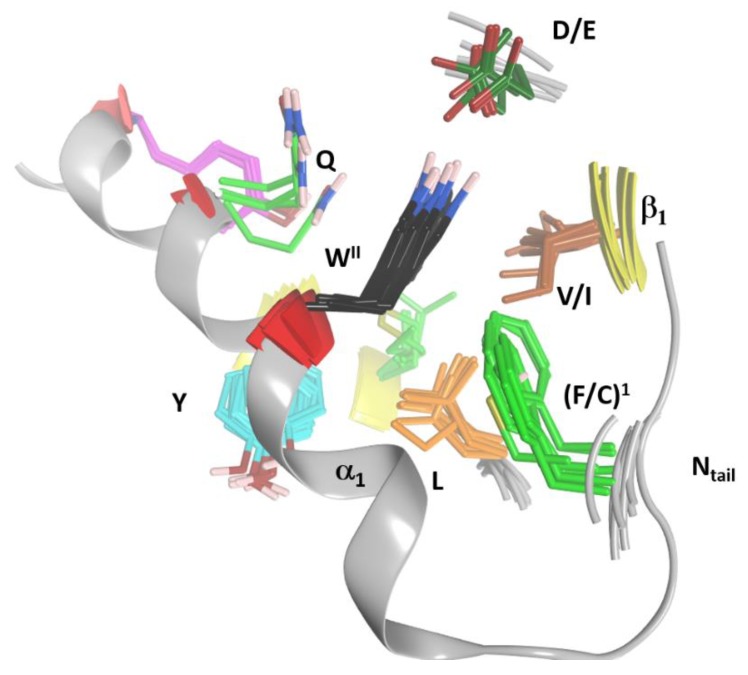
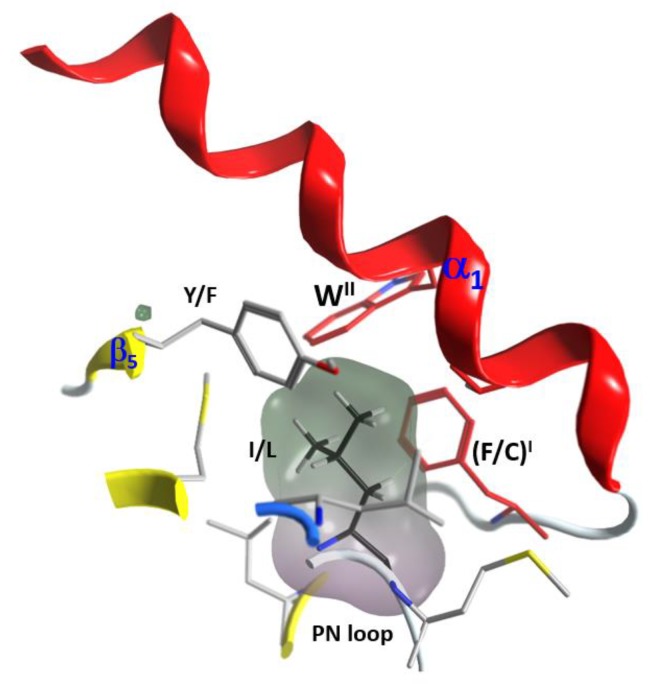
(1) The “S-motif”, FI–(A/G)–WII, which is contained in the three S1−S3 consensus sequences motifs (fuchsia residues in Figure 10). The S-motif appears in all T-segments (including C3staus) and therefore likely participates in stabilizing the T-segment and neighboring regions (“S” after structure). In effect, PheI (Cys in C3cer) stabilizes the coiled Ntail segment with β1 in a β-like configuration. In turn, (Phe/Cys)I along with Ala/Gly and the conserved TrpII orient the α1 helix of the α-lobe by means of a network of hydrophobic and H-bond interactions (Figure 12). In particular, the interaction of the S-motif residues with both semi-conserved (Iso/Leu)PN in the PN-loop, and with (Tyr/Phe) β5 in the ARTT-loop (Figure 13) is relevant and will be discussed later.
Figure 12.
S-motif residues. Important residues that cluster around the two aromatic cornerstone residues of the S-motif. The cornerstone residues include Phe (mid-green) and Trp (black) and are surrounded by a cluster of residues, Gln (light green), Tyr (cyan), Leu (orange), Val/Ile (brown) and Asp/Glu (dark green).
Figure 13.
S-motif key interactions with PN- and ARTT-loops. Depiction of the interactions of the two aromatic residues (FI and WII) of the S-motif with PN- and ARTT-loop residues (Y/F and I/L).
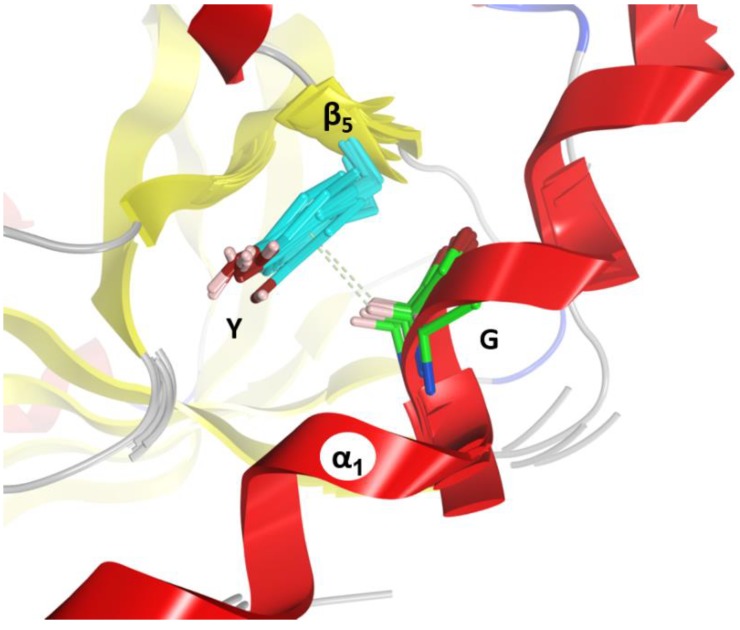
(2) The “C-motif”, G–(Y/F)III–(Y/W)IV is found in the S1 and S2 consensus sequences and features residues not found in the S-motif (green residues in Figure 10). This motif only appears in catalytic domains/toxins, including C3stau’s (with Ile instead of (Y/F)II) and C3larvin, and therefore might be related to the stabilization of catalytic residues (“C” after catalytic). In effect, the conserved Gly residue fits into a small space and interacts/stabilizes the conserved Tyrβ5 (Phe in C2IC) adjacent to the ARTT-loop by a H-Pi-type H-bond (Figure 14). The last two aromatic residues in the motif, (Tyr/Phe)III and (Tyr/Trp)IV, in addition to stabilizing the α1–α2 superstructure, interact with conserved residues at or adjacent to the ARTT-loop (not shown). Thus, the C-motif might have evolved along with the ARTT-loop and any key determinant might have been lost in the co-evolution of the C2N-domains with the “atrophy” of catalytic signatures in the ARTT-loop.
Figure 14.
Conserved α1–ARTT interactions. Depiction of the H Pi-type H-bond between Gly (light green) of the C-motif and the conserved Tyr/Phe residue (cyan) in the ARTT-loop in C3-like toxins.
(3) The “B-motif”, D–(K/R)–D–(K/R)–(K/R)−E−K, appears only in the S3 consensus sequence and includes residues not present in the S- or C- motifs (orange residues in Figure 10). This unique motif appears in the “terminal” T-segment of the binary toxins A-component—Plx2A and C2N-domains. Incidentally, no other basic or acidic residue located in any helix displays this degree of conservation in these toxins/domains (C2N-domains, Plx2A) but shows no consensus in the rest of toxins/domains (C3-like toxins and C2C-domains). The length/flexibility, exposure, electric charge, and H-bond capability of the B-motif residues qualify it as a candidate for the binding of the B-component and/or to mediate the translocation of the complex into the cytoplasm (“B” after binding). The B-motif is remarkable, since it confirms that Plx2A shares elements with binary C2N-domains that might be implicated in the host cell internalization process.
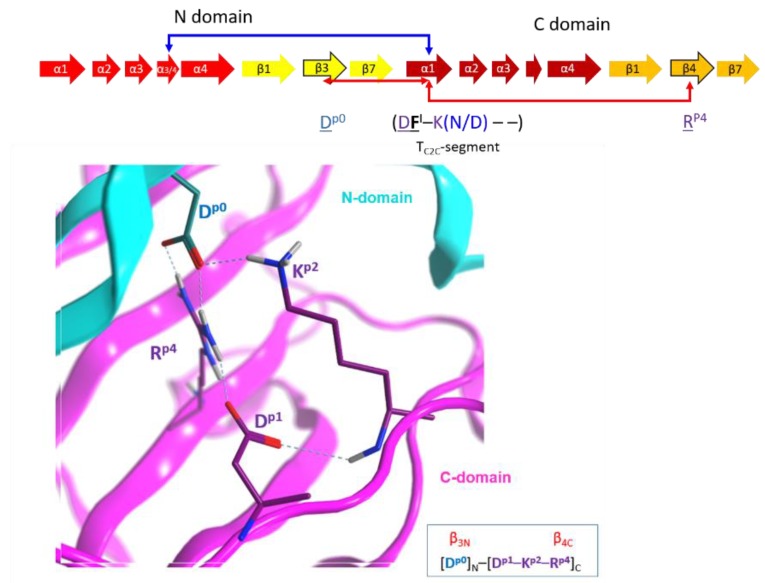
(4) The “I-motif”, D–K(N/D), has residues in the TC2C-segments that are not included in the previous motifs (yellow and cyan residues in Figure 10) and this motif participates in the inter-domain stabilization (“I” after interdomain). This motif can be defined by three positions (p1...p3) according to Dp1–Kp2(N/D)p3. The p1 and p2 positions of the I-motif might have evolved along with a residue in the β3N-strand (additional position p0) and a residue in the β4C-strand (additional position p4), to form a quaternary cluster, [Dp0]N−[Dp1−Kp2−Rp4]C that stabilized both domains (Figure 15). In agreement with the previous assertion, Aspp0 is conserved in β3N and does not appear in β3C; Argp4 is invariant in β4C and does not appear in β4N. Incidentally, Aspp0 and Argp4 are absent in equivalent elements (strands β3 and β4, respectively) of single-domain toxins. In addition, the 3rd position of the I-motif, (N/D)p3, makes effective contacts with the α3/4-loop in the C2N-domains (α3/4N-loop) to stabilize both domains (see the next section).
Figure 15.
Inter-domain interactions. Depiction of the inter-domain interactions between the N-domain (Dp0) and the C-domain (Dp1KP2–Rp4) residues of the I-motif that stabilize the structure of C2-like toxins.
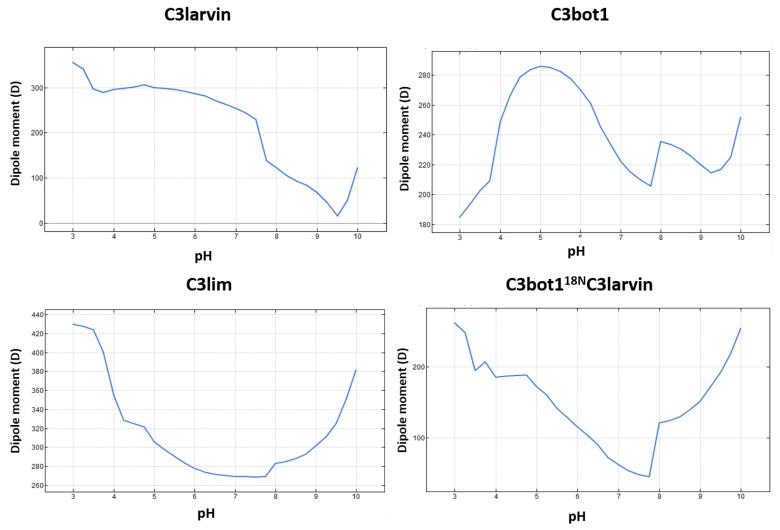
(5) The “T-motif” E–TN–(E/D)E–K contains residues that are unique in the TC3-segments and are not found in the previous motifs (gray residues in Figure 10). The role of this motif might be related to the translocation (“T” after translocation), consistent with the previous section. Accordingly, the increased toxicity observed for C3bot1 and C3lim when the pH is reduced from 7.4 to 5.5 correlates with the increase in both the protein net charge and dipole moment (Figure 16). Furthermore, assessing the pH-dependence of the cellular toxicity, the calculated pKas for Asp90 and Glu129 (the two acidic residues in the proposed binding-motifs of C3bot1) are 2.5 and 2.8, respectively—these are too low to be responsible for the pH response. However, Glu8 and Asp13 (both in the T-motif of C3bot1) have calculated pKa values of 4.6 and 3.8, respectively. C3lim exhibits a higher pH- dependence than C3bot1 in intoxicating macrophage cells [59]. Incidentally, C3lim has four acidic residues in its T-segment, with pKa values that range from 3.7 to 4.0, while the acidic residues of the interacting motifs possess lower pKas of 2.6 and 1.4, respectively. Obviously, the alkaline nature of the T-segment is enhanced by the protonation (neutralization) of the acidic residues.
Figure 16.
pH-dependence of the protein dipole-moment of C3 toxins. The pH profile of the electrical dipole-moment is shown for three C3-like toxins and for the chimeric protein consisting of the fusion of the N-terminus of C3bot1 with C3larvin toxin.
2.5. The Putative Role of the α3/4-Loop
In the single-domain Plx2A, and C3-like toxins, the segment that connects helices α3 and α4, the “α3/4-loop”, is either short and unstructured (e.g., C3bot1) or contains a small 310-helix configuring a loop-helix-loop motif (e.g., C3lim), called the LHL-motif that protrudes from the α-lobe [60]. In the C2C-domains, the α3/4C-loop is also short and unstructured (e.g., Vip2) or harbors a small α-helix (e.g., CdtA). In most of these toxins/domains, there is an abundance of Asn, Gly, and Pro residues, which imply a structural role in allowing the αα-corner that connects α3 with α4 (Gly and Pro allow the turns, while the uncharged, but polar Asn, is either exposed or buried). However, the presence of both charged and hydrophobic residues in the turn of the α3/4-loops of some C3-like toxins (e.g., C3bot1 and C3bot2), along with their solvent exposure, makes the α3/4-loop a probable interacting motif in these toxins. Indeed, even the short LHL-motif of C3bot1 mediates the non-enzymatic interaction of the toxin with the RalA GTPase [60].
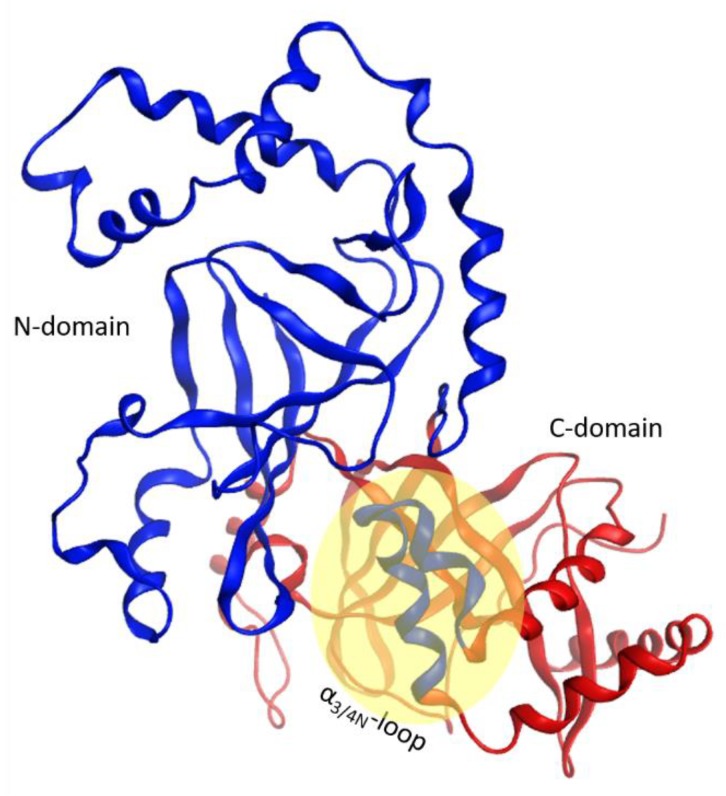
On the other hand, the α3/4N-loop is longer than its counterpart in C3-like toxins and C2C-domains and harbors an invariant Phe as well as other polar and hydrophobic residues. Interestingly, in Ia, CdtA, and SA toxins, the α3/4N-loop and α4N are highly conserved with an average identity of 80.3% (90.2% similarity), and the Ia α3N−α4N superstructure may be involved in the interaction with Ib (iota B-component) [61]. In effect, the segment responsible for the binding/internalization in Ib of iota toxin is not located at the N-terminus (TC2N-segment), likewise in C2I toxin (see previous section). Alternatively, in Ia toxin, this segment is more centrally located (residues 62–257), arising from α3/4N (in the αN-lobe) to α3C (in the αC-lobe) including both elements [61]. This postulate is reasonable if the α3/4N-loop can be considered part of the C2C-domain according to distance and packing criteria (Figure 17), such that the spatial proximity between helix α1C and the α3/4N-loop may have a functional role. Notably, short segments defined by residues 42–177 (which includes α3N- and α4N-helices) and 222–257 (α1C−α2C of the αC-lobe) may control binding to the Ib protein with a C3-like toxin chimera [61]. In addition, the (N/D)p3 residue of the I-motif interacts with the enlarged α3/4N-loop to stabilize the inter-domain architecture (see the previous section) with an invariant Ile residue in α4aN (in α4N for Vip2N).
Figure 17.
Inter-domain motif proximity. Identification of domains based on geometric criteria by the MOE 2018 algorithm, where the α3 and α4a helices of the N-domain (in red ribbons) are part of the C-domain (in blue ribbons) in C2-like toxins. The yellow oval highlights the special proximity of the α1 of the C-domain with the α3/4-loop of the N-domain.
2.6. The T-Segment in Other CT-Toxins
Certhrax toxin from B. cereus (PDB: 4GF1) is a bi-domain A-component toxin with the catalytically C-domain homologous to the C3-like toxin or the C2C-domain, but with the N-domain homologous to the PA-binding domain of anthrax lethal factor from B. anthracis [9]. Accordingly, Certhrax T-segment fulfills the S- and C-motifs of the canonical α1 configuration and catalytic ability (Figure 10). Incidentally, Certhrax T-segment lacks the T-(C3-like translocation) and B-(C2N-like binding/translocation) motifs; it exhibits inter-domain interactions via a modified I-motif. Thus, Certhrax clusters with the C2C-subgroup based on T-segment similarity (not shown), which is compatible with its catalytic non-terminal T-segment.
The X-ray structure of the mART domain of SpvB (PDB: 2GWM), a single-component actin-ADP-ribosylating toxin from Salmonella spp, lacks atomic coordinates of the region corresponding to the T-segment (i.e., upstream in the first “solved” helical structure, α2), and lacks the S- and C-motifs. Accordingly, an estimate of the secondary structure of this segment by the PSIPRED server predicted a coiled structure for this region, and several in-house homology models of the full-length SpvB from S. typhimurium report an extended coil for the T-segment.
Another mART actin-modifying toxin with known structure is VahC from A. hydrophila [39]. Unfortunately, the N-terminal truncated structures (PDBs: 4FML and 3NTS) lack the coordinates corresponding to the T-segment (i.e., upstream α2). The sequence of the VahC T-segment is highly like the corresponding segment in SpvB, and both segments harbor a poly-proline sequence. Likewise, Photox toxin from P. luminescens [40], has a T-segment that is highly similar and unstructured as found in both VahC and SpvB toxins. Interestingly, SpvB, VahC, and Photox lack the conserved Tyrβ5, which is part of the canonical α1 configuration with catalytic ability (i.e., C3-like and C2C-like toxins, including Plx2A, and Certhrax). The phenol side chain of Tyr β5 is the H-acceptor from the conserved Gly (backbone) in the α1-motif (Figure 14). Thus, the G α1−Tyr β5 pair is involved in the mutual stability of α1 and the ARTT-loop. Notably, VahC, SpvB, and Photox do not show GH activity [7,39,40,43] which might be related to an unstructured T-segment that does not enclose the ARTT-loop (see later).
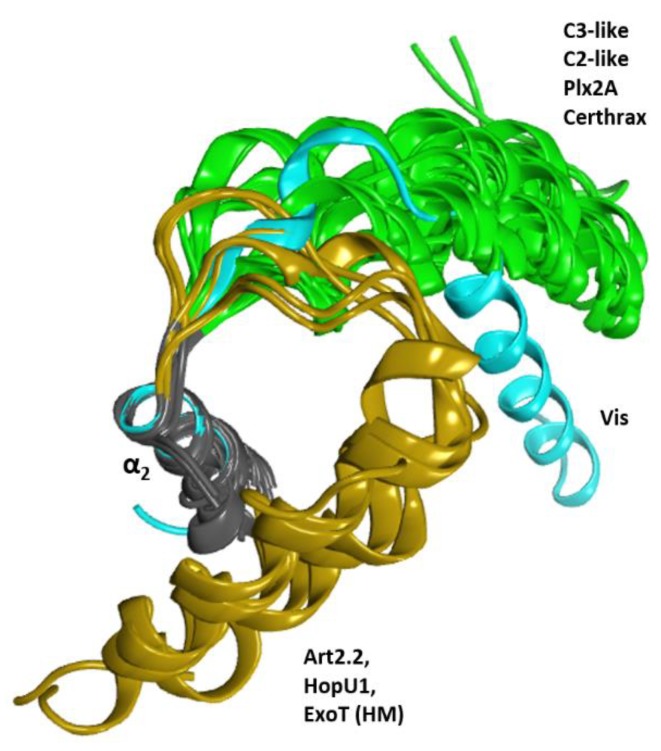
Vis toxin from V. splendidus (PDB: 4XZJ) is a special case. The elongated α1 of Vis toxin follows a V-shaped configuration of the α1−α2 superstructure like C2/C3-toxins, although in a slightly ‘altered’ orientation (Figure 18). Accordingly, Vis has the aromatic WII in its T-segment and does not fully qualify as an S-motif that would stabilize helix α1 in the canonical α-lobe configuration. Rather, the stabilization of α1 is achieved by an Arg residue in the Ntail that forms a salt-bridge with a Glu in the PN-loop and an Asp in the ARTT-loop (see later). Although it possesses some aromatic residues in the T-segment, Vis does not possess a C-motif and is unique in having the small, polar Ser residue replacing the conserved Gly. Notably, this Ser residue interacts in a similar manner with a residue (Thrβ5, also a unique substitution in Vis) proximal to the ARTT-loop as observed for Gly in the C-motif.
Figure 18.
Conformations of the α1 helix. Superposition of several toxins of the CT-group for the α2 helix (gray ribbons) showing three conformation clusters for the α1 helix. These clusters are the canonical conformation in green, the altered conformation of Vis in cyan, and the folded conformation of HopU1, Art2.2, and ExoT (HM) in ochre.
In contrast, the HopU1 toxin from Pseudomonas syringae (PDB: 3UOJ) has α1 with an orientation diametrically opposed to the canonical conformation: an α1−α2 forming an αα-corner rather than the V-shaped configuration (Figure 18). The αα-corner configuration between α1 and α2 is facilitated by a long α1,2-loop (termed L1) [44] and is stabilized by a hydrophobic cluster of aromatic residues in the C-terminus of the T-segment (not shown). Although four aromatic residues are found in its T-segment, all the motifs in the canonical T-segment are absent in this toxin; notably, HopU1 lacks the S-motif that anchors α1 to the βI strand and lacks the catalytic C-motif. Nevertheless, in HopU1 the N-end of the ARTT-loop (the end linked to strand β5) is stabilized by the αα-corner configuration, while the C-end of the ARTT-loop (the end linked to strand β6) is left partially exposed.
3. Conclusions
In summary, the role of the α-lobe is to provide a suitable configuration (location and orientation) of (i) the α2–α3 helices to feature the α3-motif that has a role in NAD+ substrate binding and possibly in the interaction with the protein target; (ii) the α3–α4 helices to provide the α3/4-loop with protein–protein interaction capability; and (iii) the α1-Ntail, defined in the T-segment, that features specialized motif(s) according to the toxin type (A-only or A-B toxins) exhibiting an effect on the catalytic activity via the ARTT-loop, with a role in the inter-domain stability, and with a role in the binding and/or translocation steps during the internalization process.
The canonical (C3-like) α-lobe configuration has the α1 helix forming a V-shape with α2 that surrounds the ARTT-loop. This configuration is stabilized by the ubiquitous S-motif in the T-segment of C3-like, C2-like (N- and C-domains), Plx2A and Certhrax toxin. Accordingly, other non-PT-like toxins (e.g., Vis and HopU1 toxins) with known structures show the canonical α2–α4 configuration. However, the α1 helix in an alternative configuration consistent with the lack of the S-motif in its T-segment.
The presence of the catalytic signature on the ARTT-loop is not enough to guarantee GH- activity—the contrary is true—no catalytic residues in the ARTT-loop means no GH-activity. In non-PT-like toxins, GH-activity requires a stabilized ARTT-loop conformation, and this is achieved in toxins with the canonical α-lobe in the V-shape configuration of the α1–α2 helices, and specifically by the C-motif of the T-segments. In particular, the Gα1−Tyrβ5 pair is involved in the mutual stability of α1 and the ARTT-loop. Incidentally, C3larvin, which has a truncated α1, still has the C-motif and Tyrβ5 and exhibits GH activity [27]; also, Certhrax has the canonical α1 configuration, Tyrβ5, and C-motif and shows GH activity [9]. Accordingly, the alternative α1 conformation of Vis toxin encloses the ARTT-loop and shows GH activity [5]. On the contrary, VahC, SpvB, and Photox lack both the Tyrβ5 and the C-motif (their T-segments are likely unstructured) and do not show GH activity [7,39,40,43]. HopU1 has an alternative α1 conformation and shows GH activity (unpublished data).
The specialization of the A-component of binary toxin classes (i.e., C2-like toxins) likely involves selective forces related to the intoxication mechanism that may dictate the composition of the T-segment. The bi-domain constitution might have arisen by gene duplication of an ancestral ADP-ribosyl transferase [62]; consequently, the TC2N- and TC2C-segments evolved to harbor residues appropriate for their location and roles. The TC2N-segment features the S- and B motifs. In effect, the inability of the C2N domain to bind NAD+ must have forced the atrophy of the C-motif and the ARTT-loop (a required motif in the single-domain catalytic precursor), and instead, the TC2N-segment evolved to interact with the B-component. On the other hand, the TC2C-segment features the S-, C-, and I- motifs. In effect, the TC2C-segment might have evolved according to (a) a higher specialization of its ancestral kinetic role. Accordingly, the C-motif is better defined in the C2C-domains (G–YIII–WIV, with only the YIV variation in C2IC-domain) than in the single-domain toxins with the general definition; and (b) possesses an emerging structural role associated with the bi-domain topology—the I-motif. The I-motif, along with specific substitutions in the β3N and β4C strand, and with the α3/4N-loop, participates in inter-domain stabilization.
It has been reported that the α-lobe of the C2IN domain for C2 toxin and the RxG motif (possibly along with Rα3 and the RxE motif) for C3bot1 may be the minimum sub-structure/motif necessary to stably bind to the membrane component—C2II and vimentin, respectively. In addition, the N-terminal T-segment may also participate in the process of internalization of both toxin groups into the host cell; it be involved in the translocation step from the early endosome to the cytoplasm. Accordingly, C3-like toxins feature the T-motif with acidic residues that might trigger the conformational changes required for membrane translocation; while C2-like toxins feature the B- motif. In this sense, the B-motif may bind to the B-component and/or to mediate the translocation into the cytoplasm. Intoxication experiments that monitor phenotypic alterations of the host cell do not distinguish which event is abolished when working with a toxin. Thus, in agreement with the translocation role of the B-motif, a construct of C2IN without the first 29 residues and the C3bot1 toxin, C2INΔ29NC3bot1, failed to be transported into HeLa cells, although binding of the construct to C2II on cell membranes was still observed [57]. Also, it is feasible that the α3/4N-loop is the sub-structure needed for binding (as has been observed in Ia toxin), while the B-motif is needed for the translocation step.
4. Materials and Methods
4.1. Ensemble of X-Ray Protein Structures
X-ray structures entries were downloaded from the Protein Data Bank (PDB). The datasets include high resolution (1.57 to 2.70 A) X-ray structures mainly of WT proteins in apo forms. However, in some cases proteins in different liganded states and diverse catalytically altered variants were also included for comparative purposes. When multiple molecules were presented in the asymmetric unit of some crystal forms, the most complete molecule was selected.
4.2. Force-Field Settings and Structure Preparation
Protein preparation and molecular mechanics (MM) calculations were performed using the computational suite Molecular Operative Environment (MOE) release 2018.10 (Chemical Computing Group Inc, Montreal, CA, USA). The force field employed was the MOE Amber12: EHT, with AMBER12 parameters set (ff12) for protein, and parameters calculated from the Extended Hückey Theory for the NAD+ molecule and co-solvents. For the implicit solvent model, the Generalized Born-Volume Integral (GB/VI) formalism was employed, with dielectrics for the interior of the protein.
When short sections of X-ray structures were missing in the PDB data files, the peptide segments were crafted by using built-in homology model procedures in MOE. Then, the full X-ray structures were protonated using the MOE Protonate3D module to assign the ionization states and tautomers of protein side-chains and to orient crystallographic water molecules (CWMs) at T = 300 K, pH 7.4 and 0.1 M of ionic strength, along with the GB-VI (Generalized Born-Volume Integral) solvation model and MMFF94 partial charges. The protonated structures were initially geometry-optimized by keeping backbone coordinates fixed, tethering all other heavy atoms with a 100 kcal/mol force constant (0.25 Å buffer), and then energy-minimized until an RMS gradient ≤0.001 kcal/mol/Å2.
4.3. Others
The multiple sequence alignments (MSA) were also performed using MOE 2018 software and based on the overall matching of secondary and tertiary structural elements. For a higher resolution alignment of short segments, the procedure was enhanced from the pattern of common molecular interactions. All protein structures were rendered in MOE 2018 software.
Acknowledgments
We thank K. Heney for excellent technical support.
Author Contributions
Conceptualization, A.R.M.; Data curation, M.R.L.; Formal analysis, M.R.L. and A.R.M.; Funding acquisition, A.R.M.; Project administration, A.R.M.; Resources, A.R.M.; Software, M.R.L.; Writing—original draft, M.R.L.; Writing—review & editing, A.R.M.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada, Discovery grant (#105440) and Strategic grant (#494213) to A. Rod Merrill.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
The role of the α-lobe in CT-group bacterial mono-ADP-ribosyltransferase toxins is to provide a scaffold for (i) the α2–α3 helices that feature the α3-motif involved in NAD+ substrate binding and protein target interaction; (ii) the α3–α4 helices that provide the α3/4-loop with protein–protein interaction capability; and (iii) the α1-Ntail in the T-segment that features specialized motif(s) according to the toxin type (A-only or A-B toxins). These elements participate in the catalytic activity via the ARTT-loop; inter-domain stability; and play a role in the binding and/or translocation steps during the host cell intoxication process.
References
- 1.Simon N.C., Aktories K., Barbieri J.T. Novel bacterial ADP-ribosylating toxins: Structure and function. Nat. Rev. Microbiol. 2014;12:599–611. doi: 10.1038/nrmicro3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreeva A., Howorth D., Chothia C., Kulesha E., Murzin A.G. Investigating protein structure and evolution with SCOP2. Curr. Protoc. Bioinform. 2015;49:1.26.1–1.26.21. doi: 10.1002/0471250953.bi0126s49. [DOI] [PubMed] [Google Scholar]
- 3.Fieldhouse R.J., Merrill A.R. Needle in the haystack: Structure-based toxin discovery. Trends Biochem. Sci. 2008;33:546–556. doi: 10.1016/j.tibs.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Barth H., Fischer S., Moglich A., Fortsch C. Clostridial C3 toxins target monocytes/macrophages and modulate their functions. Front. Immunol. 2015;6:339. doi: 10.3389/fimmu.2015.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravulapalli R., Lugo M.R., Pfoh R., Visschedyk D., Poole A., Fieldhouse R.J., Pai E.F., Merrill A.R. Characterization of vis toxin, a novel ADP-ribosyltransferase from Vibrio splendidus. Biochemistry. 2015;54:5920–5936. doi: 10.1021/acs.biochem.5b00921. [DOI] [PubMed] [Google Scholar]
- 6.Barbieri J.T., Sun J. Reviews of Physiology, Biochemistry and Pharmacology. Volume 152. Springer; Berlin/Heidelberg, Germany: 2004. Pseudomonas aeruginosa ExoS and ExoT; pp. 79–92. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai J., Nagahama M., Hisatsune J., Katunuma N., Tsuge H. Clostridium perfringens iota-toxin, ADP-ribosyltransferase: Structure and mechanism of action. Adv. Enzyme Regul. 2003;43:361–377. doi: 10.1016/S0065-2571(02)00044-4. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez J., Holmgren J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell. Mol. Life Sci. 2008;65:1347–1360. doi: 10.1007/s00018-008-7496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visschedyk D., Rochon A., Tempel W., Dimov S., Park H.W., Merrill A.R. Certhrax toxin, an anthrax-related ADP-ribosyltransferase from Bacillus cereus. J. Biol. Chem. 2012;287:41089–41102. doi: 10.1074/jbc.M112.412809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon N.C., Barbieri J.T. Bacillus cereus Certhrax ADP-ribosylates vinculin to disrupt focal adhesion complexes and cell adhesion. J. Biol. Chem. 2014;289:10650–10659. doi: 10.1074/jbc.M113.500710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon N.C., Vergis J.M., Ebrahimi A.V., Ventura C.L., O’Brien A.D., Barbieri J.T. Host cell cytotoxicity and cytoskeleton disruption by CerADPr, an ADP-ribosyltransferase of Bacillus cereus G9241. Biochemistry. 2013;52:2309–2318. doi: 10.1021/bi300692g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fieldhouse R.J., Turgeon Z., White D., Merrill A.R. Cholera- and anthrax-like toxins are among several new ADP-ribosyltransferases. PLoS Comput. Biol. 2010;6:e1001029. doi: 10.1371/journal.pcbi.1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iglewski B.H., Sadoff J., Bjorn M.J., Maxwell E.S. Pseudomonas aeruginosa exoenzyme S: An adenosine diphosphate ribosyltransferase distinct from toxin A. Proc. Natl. Acad. Sci. USA. 1978;75:3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kodama T., Rokuda M., Park K.S., Cantarelli V.V., Matsuda S., Iida T., Honda T. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type III secretion system 2. Cell. Microbiol. 2007;9:2598–2609. doi: 10.1111/j.1462-5822.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 15.Litvak Y., Selinger Z. Aeromonas salmonicida toxin AexT has a Rho family GTPase-activating protein domain. J. Bacteriol. 2007;189:2558–2560. doi: 10.1128/JB.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aktories K., Barmann M., Ohishi I., Tsuyama S., Jakobs K.H., Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986;322:390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- 17.Vandekerckhove J., Schering B., Barmann M., Aktories K. Clostridium perfringens iota toxin ADP-ribosylates skeletal muscle actin in Arg-177. FEBS Lett. 1987;225:48–52. doi: 10.1016/0014-5793(87)81129-8. [DOI] [PubMed] [Google Scholar]
- 18.Popoff M.R., Milward F.W., Bancillon B., Boquet P. Purification of the Clostridium spiroforme binary toxin and activity of the toxin on HEp-2 cells. Infect. Immun. 1989;57:2462–2469. doi: 10.1128/iai.57.8.2462-2469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakroun M., Banyuls N., Bel Y., Escriche B., Ferre J. Bacterial vegetative insecticidal proteins (vip) from entomopathogenic bacteria. Microbiol. Mol. Biol. Rev. 2016;80:329–350. doi: 10.1128/MMBR.00060-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popoff M.R., Rubin E.J., Gill D.M., Boquet P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 1988;56:2299–2306. doi: 10.1128/iai.56.9.2299-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yonogi S., Matsuda S., Kawai T., Yoda T., Harada T., Kumeda Y., Gotoh K., Hiyoshi H., Nakamura S., Kodama T., et al. BEC, a novel enterotoxin of Clostridium perfringens found in human clinical isolates from acute gastroenteritis outbreaks. Infect. Immun. 2014;82:2390–2399. doi: 10.1128/IAI.01759-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuge H., Tsurumura T., Toda A., Murata H., Toniti W., Yoshida T. Comparative studies of actin- and Rho-specific ADP-ribosylating toxins: Insight from structural biology. Curr. Top. Microbiol. Immunol. 2017;399:69–86. doi: 10.1007/82_2016_23. [DOI] [PubMed] [Google Scholar]
- 23.Aktories K., Rosener S., Blaschke U., Chhatwal G.S. Botulinum ADP-ribosyltransferase C3. Purification of the enzyme and characterization of the ADP-ribosylation reaction in platelet membranes. Eur. J. Biochem. 1988;172:445–450. doi: 10.1111/j.1432-1033.1988.tb13908.x. [DOI] [PubMed] [Google Scholar]
- 24.Nemoto Y., Namba T., Kozaki S., Narumiya S. Clostridium botulinum C3 ADP-ribosyltransferase gene. Cloning, sequencing, and expression of a functional protein in Escherichia coli. J. Biol. Chem. 1991;266:19312–19319. [PubMed] [Google Scholar]
- 25.Just I., Mohr C., Schallehn G., Menard L., Didsbury J.R., Vandekerckhove J., Van Damme J., Aktories K. Purification and characterization of an ADP-ribosyltransferase produced by Clostridium limosum. J. Biol. Chem. 1992;267:10274–10280. [PubMed] [Google Scholar]
- 26.Wilde C., Vogelsgesang M., Aktories K. Rho-specific Bacillus cereus ADP-ribosyltransferase C3cer cloning and characterization. Biochemistry. 2003;42:9694–9702. doi: 10.1021/bi034583b. [DOI] [PubMed] [Google Scholar]
- 27.Krska D., Ravulapalli R., Fieldhouse R.J., Lugo M.R., Merrill A.R. C3larvin toxin, an ADP-ribosyltransferase from Paenibacillus larvae. J. Biol. Chem. 2015;290:1639–1653. doi: 10.1074/jbc.M114.589846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebeling J., Funfhaus A., Knispel H., Krska D., Ravulapalli R., Heney K.A., Lugo M.R., Merrill A.R., Genersch E. Characterization of the toxin Plx2A, a RhoA-targeting ADP-ribosyltransferase produced by Paenibacillus larvae. Environ. Microbiol. 2017;19:5100–5116. doi: 10.1111/1462-2920.13989. [DOI] [PubMed] [Google Scholar]
- 29.Wilde C., Just I., Aktories K. Structure-function analysis of the Rho-ADP-ribosylating exoenzyme C3stau2 from Staphylococcus aureus. Biochemistry. 2002;41:1539–1544. doi: 10.1021/bi015809i. [DOI] [PubMed] [Google Scholar]
- 30.Evans H.R., Sutton J.M., Holloway D.E., Ayriss J., Shone C.C., Acharya K.R. The crystal structure of C3stau2 from Staphylococcus aureus and its complex with NAD. J. Biol. Chem. 2003;278:45924–45930. doi: 10.1074/jbc.M307719200. [DOI] [PubMed] [Google Scholar]
- 31.Vogelsgesang M., Pautsch A., Aktories K. C3 exoenzymes, novel insights into structure and action of Rho-ADP-ribosylating toxins. Naunyn Schmiedebergs Arch. Pharmacol. 2007;374:347–360. doi: 10.1007/s00210-006-0113-y. [DOI] [PubMed] [Google Scholar]
- 32.Gill D.M., Meren R. Adp-ribosylation of membrane proteins catalyzed by cholera toxin: Basis of the activation of adenylate cyclase. Proc. Natl. Acad. Sci. USA. 1978;75:3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farizo K.M., Fiddner S., Cheung A.M., Burns D.L. Membrane localization of the S1 subunit of pertussis toxin in Bordetella pertussis and implications for pertussis toxin secretion. Infect. Immun. 2002;70:1193–1201. doi: 10.1128/IAI.70.3.1193-1201.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taxt A., Aasland R., Sommerfelt H., Nataro J., Puntervoll P. Heat-stable enterotoxin of enterotoxigenic Escherichia coli as a vaccine target. Infect. Immun. 2010;78:1824–1831. doi: 10.1128/IAI.01397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyons B., Ravulapalli R., Lanoue J., Lugo M.R., Dutta D., Carlin S., Merrill A.R. Scabin, a novel DNA-acting ADP-ribosyltransferase from Streptomyces scabies. J. Biol. Chem. 2016;291:11198–11215. doi: 10.1074/jbc.M115.707653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coye L.H., Collins C.M. Identification of SpyA, a novel ADP-ribosyltransferase of Streptococcus pyogenes. Mol. Microbiol. 2004;54:89–98. doi: 10.1111/j.1365-2958.2004.04262.x. [DOI] [PubMed] [Google Scholar]
- 37.Icenogle L.M., Hengel S.M., Coye L.H., Streifel A., Collins C.M., Goodlett D.R., Moseley S.L. Molecular and biological characterization of Streptococcal SpyA-mediated ADP-ribosylation of intermediate filament protein vimentin. J. Biol. Chem. 2012;287:21481–21491. doi: 10.1074/jbc.M112.370791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edgar R.J., Chen J., Kant S., Rechkina E., Rush J.S., Forsberg L.S., Jaehrig B., Azadi P., Tchesnokova V., Sokurenko E.V., et al. SpyB, a small heme-binding protein, affects the composition of the cell wall in Streptococcus pyogenes. Front. Cell. Infect. Microbiol. 2016;6:126. doi: 10.3389/fcimb.2016.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shniffer A., Visschedyk D.D., Ravulapalli R., Suarez G., Turgeon Z.J., Petrie A.A., Chopra A.K., Merrill A.R. Characterization of an actin-targeting ADP-ribosyltransferase from Aeromonas hydrophila. J. Biol. Chem. 2012;287:37030–37041. doi: 10.1074/jbc.M112.397612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visschedyk D.D., Perieteanu A.A., Turgeon Z.J., Fieldhouse R.J., Dawson J.F., Merrill A.R. Photox, a novel actin-targeting mono-ADP-ribosyltransferase from Photorhabdus luminescens. J. Biol. Chem. 2010;285:13525–13534. doi: 10.1074/jbc.M109.077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sha J., Wang S.F., Suarez G., Sierra J.C., Fadl A.A., Erova T.E., Foltz S.M., Khajanchi B.K., Silver A., Graf J., et al. Further characterization of a type III secretion system (T3SS) and of a new effector protein from a clinical isolate of Aeromonas hydrophila—Part I. Microb. Pathog. 2007;43:127–146. doi: 10.1016/j.micpath.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Greene L.H., Lewis T.E., Addou S., Cuff A., Dallman T., Dibley M., Redfern O., Pearl F., Nambudiry R., Reid A., et al. The CATH domain structure database: New protocols and classification levels give a more comprehensive resource for exploring evolution. Nucleic Acids Res. 2007;35:D291–D297. doi: 10.1093/nar/gkl959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J., Maresso A.W., Kim J.J., Barbieri J.T. How bacterial ADP-ribosylating toxins recognize substrates. Nat. Struct. Mol. Biol. 2004;11:868–876. doi: 10.1038/nsmb818. [DOI] [PubMed] [Google Scholar]
- 44.Jeong B.R., Lin Y., Joe A., Guo M., Korneli C., Yang H., Wang P., Yu M., Cerny R.L., Staiger D., et al. Structure function analysis of an ADP-ribosyltransferase type III effector and its RNA-binding target in plant immunity. J. Biol. Chem. 2011;286:43272–43281. doi: 10.1074/jbc.M111.290122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang A.E., Schmidt G., Schlosser A., Hey T.D., Larrinua I.M., Sheets J.J., Mannherz H.G., Aktories K. Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science. 2010;327:1139–1142. doi: 10.1126/science.1184557. [DOI] [PubMed] [Google Scholar]
- 46.Lin Y., Wang P., Yang H., Xu Y. Crystallization and preliminary crystallographic analysis of the ADP-ribosyltransferase HopU1. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010;66:932–934. doi: 10.1107/S1744309110022463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Von Elsner L., Hagemann S., Just I., Rohrbeck A. C3 exoenzyme impairs cell proliferation and apoptosis by altering the activity of transcription factors. Naunyn Schmiedebergs Arch. Pharmacol. 2016;389:1021–1031. doi: 10.1007/s00210-016-1270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derossi D., Joliot A.H., Chassaing G., Prochiantz A. The third helix of the antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 49.Winton M.J., Dubreuil C.I., Lasko D., Leclerc N., McKerracher L. Characterization of new cell permeable C3-like proteins that inactivate Rho and stimulate neurite outgrowth on inhibitory substrates. J. Biol. Chem. 2002;277:32820–32829. doi: 10.1074/jbc.M201195200. [DOI] [PubMed] [Google Scholar]
- 50.Fahrer J., Kuban J., Heine K., Rupps G., Kaiser E., Felder E., Benz R., Barth H. Selective and specific internalization of clostridial C3 ADP-ribosyltransferases into macrophages and monocytes. Cell. Microbiol. 2010;12:233–247. doi: 10.1111/j.1462-5822.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 51.Rohrbeck A., Schroder A., Hagemann S., Pich A., Holtje M., Ahnert-Hilger G., Just I. Vimentin mediates uptake of C3 exoenzyme. PLoS ONE. 2014;9:e101071. doi: 10.1371/journal.pone.0101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rohrbeck A., Just I. Cell entry of C3 exoenzyme from Clostridium botulinum. Curr. Top. Microbiol. Immunol. 2017;406:97–118. doi: 10.1007/82_2016_44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohrbeck A., Holtje M., Adolf A., Oms E., Hagemann S., Ahnert-Hilger G., Just I. The Rho ADP-ribosylating C3 exoenzyme binds cells via an Arg-Gly-Asp motif. J. Biol. Chem. 2017;292:17668–17680. doi: 10.1074/jbc.M117.798231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohishi I., Yanagimoto A. Visualizations of binding and internalization of two nonlinked protein components of botulinum C2 toxin in tissue culture cells. Infect. Immun. 1992;60:4648–4655. doi: 10.1128/iai.60.11.4648-4655.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barth H., Preiss J.C., Hofmann F., Aktories K. Characterization of the catalytic site of the ADP-ribosyltransferase Clostridium botulinum C2 toxin by site-directed mutagenesis. J. Biol. Chem. 1998;273:29506–29511. doi: 10.1074/jbc.273.45.29506. [DOI] [PubMed] [Google Scholar]
- 56.Stiles B.G., Blocker D., Hale M.L., Guetthoff M.A., Barth H. Clostridium botulinum C2 toxin: Binding studies with fluorescence-activated cytometry. Toxicon. 2002;40:1135–1140. doi: 10.1016/S0041-0101(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 57.Barth H., Roebling R., Fritz M., Aktories K. The binary Clostridium botulinum C2 toxin as a protein delivery system: Identification of the minimal protein region necessary for interaction of toxin components. J. Biol. Chem. 2002;277:5074–5081. doi: 10.1074/jbc.M109167200. [DOI] [PubMed] [Google Scholar]
- 58.Funfhaus A., Poppinga L., Genersch E. Identification and characterization of two novel toxins expressed by the lethal honey bee pathogen Paenibacillus larvae, the causative agent of American foulbrood. Environ. Microbiol. 2013;15:2951–2965. doi: 10.1111/1462-2920.12229. [DOI] [PubMed] [Google Scholar]
- 59.Vogelsgesang M., Aktories K. Exchange of glutamine-217 to glutamate of Clostridium limosum exoenzyme C3 turns the asparagine-specific ADP-ribosyltransferase into an arginine-modifying enzyme. Biochemistry. 2006;45:1017–1025. doi: 10.1021/bi052253g. [DOI] [PubMed] [Google Scholar]
- 60.Pautsch A., Vogelsgesang M., Trankle J., Herrmann C., Aktories K. Crystal structure of the C3bot-RalA complex reveals a novel type of action of a bacterial exoenzyme. EMBO J. 2005;24:3670–3680. doi: 10.1038/sj.emboj.7600813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ammam F., Marvaud J.C., Lambert T. Distribution of the vanG-like gene cluster in Clostridium difficile clinical isolates. Can. J. Microbiol. 2012;58:547–551. doi: 10.1139/w2012-002. [DOI] [PubMed] [Google Scholar]
- 62.Han S., Craig J.A., Putnam C.D., Carozzi N.B., Tainer J.A. Evolution and mechanism from structures of an ADP-ribosylating toxin and NAD complex. Nat. Struct. Biol. 1999;6:932–936. doi: 10.1038/13300. [DOI] [PubMed] [Google Scholar]