Abstract
Cyanobacteria have been shown to produce a number of bioactive compounds, including toxins. Some bioactive compounds obtained from a marine cyanobacterium Moorea producens (formerly Lyngbya majuscula) have been recognized as drug leads; one of these compounds is aplysiatoxin. We have isolated various aplysiatoxin derivatives from a M. producens sample obtained from the Okinawan coastal area. The frozen sample was extracted with organic solvents. The ethyl acetate layer was obtained from the crude extracts via liquid–liquid partitioning, then separated by HPLC using a reversed-phase column. Finally, 1.1 mg of the compound was isolated. The chemical structure of the isolated compound was elucidated with spectroscopic methods, using HR-MS and 1D and 2D NMR techniques, and was revealed to be oscillatoxin I, a new member of the aplysiatoxin family. Oscillatoxin I showed cytotoxicity against the L1210 mouse lymphoma cell line and diatom growth-inhibition activity against the marine diatom Nitzschia amabilis.
Keywords: aplysiatoxin, cyanobacteria, Moorea producens, cytotoxicity, biosynthesis
1. Introduction
The marine cyanobacterium Lyngbya majuscula is known to produce aplysiatoxins [1,2] and lyngbyatoxins [3,4], which cause severe contact dermatitis [1,3]. Contact dermatitis due to L. majuscula has been reported in tropical and subtropical waters, especially in the Pacific region [5,6]. Aplysiatoxin and lyngbyatoxin have also caused food poisoning via the ingestion of the red alga Gracilaria coronopifolia [7] and the green turtle Chelonia mydas [8], respectively. Recently, blooms of L. majuscula have been increasing worldwide, threatening human health and eco-systems; this increase is presumably due to climate change [9]. In Japan, several blooms of L. majuscula have also been recorded. During the summer of 2010, a mass occurrence of Moorea producens (formerly L. majuscula) was observed in the Okinawa Prefecture and lasted for almost one month. A cyanobacterial sample collected at this site during the 2010 mass occurrence was used in this study. In a recent study, we identified 15 aplysiatoxin derivatives in this Okinawan cyanobacteria sample [10]. Aplysiatoxins have been shown to act as protein kinase C (PKC) activators and potent tumor promoting compounds [11,12,13,14,15]; their toxicity results from their high PKC activation potency [11,12,13,14,15]. The compound bryostatin-1, which is obtained from the marine bryozoan Bugula neritina [16], is also a potent PKC activator [17]. However, bryostatin-1 has low tumor-promoting activity and potent anti-cancer activity [17,18]. Therefore, bryostatin-1 has been recognized as a lead compound against various cancers [18]. The regulation of PKC activity has been shown to be a valuable therapeutic strategy for producing anti-cancer drugs [19,20]. For example, simplified analogues of aplysiatoxin have been shown to exhibit anti-cancer activity [21,22]. Thus, aplysiatoxins are attractive compounds due to their unique activities. So far, many aplysiatoxins have been isolated and reported from marine cyanobacteria [23,24,25,26,27,28,29]. We have also continued to isolate aplysiatoxin-related compounds from the Okinawan M. producens sample. In this paper, the isolation, structural elucidation, and bioactivities of a new aplysiatoxin derivative, oscillatoxin I (1, Figure 1), are discussed.
Figure 1.
Structures of oscillatoxins obtained from the Okinawan Moorea producens.
2. Results
The frozen sample of the marine cyanobacterium M. producens was extracted using MeOH. The obtained methanol extract was subjected to successive separation steps, and finally purified by HPLC using an octadecyl C18 column to yield compound 1 (1.1 mg).
2.1. Structural Elucidation of Compound 1
Compound 1 was isolated as a colorless solid ([α]D17 +19 (c 0.01, MeOH)). The 1H NMR spectrum (Figure S1) showed that compound 1 was an aplysiatoxin-related compound. The molecular formula of compound 1 was deduced to be C32H43BrO8 ([M−H]− 635.2174 and 637.2173, calcd. 635.2220 and 637.2200) (Figure S2), suggesting that compound 1 contained a bromophenol side chain similar to aplysiatoxin. The UV maxima at 228 nm (ε 15,880) and 275 nm (ε 8,190) suggested that compound 1 contained not only a bromophenol chromophore, but also a conjugated system. The HSQC spectra (Figure S3) of compound 1 showed seven methyls (two singlets, four doublets, and a methoxy), four methylenes, three methines bonded to methyls in the aliphatic region, four oxygenated methines, two olefinic methines, and three aromatic protons in the bromophenol side chain and nine quaternary (one aliphatic, two olefinic, three aromatic in the bromophenol, two esters, and one ketone) carbons (Table 1). The carbon signal at δC 197.4 and an HMBC (Figure S4) correlation from Me-26 (δH 1.08) to the ketone suggested that compound 1 was related to 17-bromo-30-methyloscillatoxin D (2) [10,23]. Analogously to 17-bromo-30-methyloscillatoxin D (2), connectivity was observed between the H-4 (Me-26) and H2-5 protons in the 1H–1H COSY spectrum (Figure 2, Figure S5), although the methine singlet of H-2 in 17-bromo-30-methyloscillatoxin D was not observed. HMBC correlations were observed between Me-26 and C-3, and between Me-24 and Me-25 and a quaternary carbon (C-7). The carbon chemical shifts of C-2 at δC 130.0 and C-7 at δC 164.0 suggested that these quaternary carbons were olefinic carbons in a conjugated system. These observations revealed that compound 1 contained a cyclohexenone structure. A partial structure from H-8 to H-12 was assigned by analysis the 1H–1H COSY spectrum. The proton chemical shifts of H-8 and H-9 suggested that these peaks corresponded to olefinic protons; these peaks were shifted downfield from δH 5.78 and δH 5.52 in 17-bromo-30-methyloscillatoxin D (2) [10] to δH 6.19 and δH 6.07 in compound 1, respectively. In addition to this, HMBC correlations between H-8 and C-2/C-7 were observed. This indicated that H-8 and H-9 also belonged to the conjugated system. The hydroxy proton observed at δH 3.50 was coupled to H-11, apparently indicating that the hydroxy group was located on C-11. The position of the hydroxy group and the unsaturation number (11) indicated the absence of a six-membered ether ring in compound 1. The methoxy (Me-32) proton at δH 3.22 was confirmed to be bonded to C-15 via the HMBC spectrum. The existence of a γ-lactone was deduced from the unsaturation number and the proton chemical shift of H-30 at δH 4.84, which was close to that of the corresponding peak in 17-bromo-30-methyloscillatoxin D (δH 4.81) [10]. The HMBC correlation between H-29 at δH 5.54 and C-1 (δC 166.6) confirmed the existence of an ester linkage between C-1 and C-29. The proton coupling constants of 4.8 Hz for H-4/H-5b and 13.7 Hz for H-4/H-5a rationalized the equatorial orientation of Me-26 on C-4. The large proton coupling constant (16 Hz) indicated an E configuration of Δ8. The proton coupling constants of H-11 (11.3 and 1.2 Hz) were similar to those of H-11 in aplysiatoxins that have six-membered ether rings, which suggested that the conformations of H-10-H-11 and H-11-H-12 in compound 1 were likely anti and gauche, respectively, like in aplysiatoxin and its derivatives. The structure of compound 1 is shown in Figure 1. Previously, we reported compounds 3 and 4 as oscillatoxin E and F, respectively [10]. However, the same nomenclature (oscillatoxin E and F) was applied to different aplysiatoxin derivatives by Tang et al. at almost the same time [29]. To avoid confusion, we have renamed compounds 3 and 4 oscillatoxin G and H, respectively. Furthermore, compound 1 was designated as oscillatoxin I.
Table 1.
NMR data for oscillatoxin I in acetone-d6 (600 MHz for 1H and 150 MHz for 13C).
| No. | δH Multiplicity (J in Hz) |
δC | No. | δH Multiplicity (J in Hz) |
δC |
|---|---|---|---|---|---|
| 1 | - | 166.6, C | 17 | - | 111.1, C |
| 2 | - | 130.0, C | 18 | 7.38 d (8.6) | 133.4, CH |
| 3 | - | 197.4, C | 19 | 6.73 dd (3.1, 8.6) | 116.3, CH |
| 4 | 2.66 m | 37.1, CH | 20 | - | 157.6, C |
| 5a | 1.75 dd (13.7, 13.7) | 45.3, CH2 | 21 | 6.97 d (3.0) | 114.2, CH |
| 5b | 1.86 dd (4.8, 13.4) | - | 22 | 0.94 d (6.6) | 13.8, CH3 |
| 6 | - | 35.8, C | 23 | 1.05 d (6.9) | 17.4, CH3 |
| 7 | - | 164.0, C | 24 | 1.21 s | 28.3, CH3 |
| 8 | 6.19 dd (0.8, 16.0) | 125.4, CH | 25 | 1.31 s | 24.7, CH3 |
| 9 | 6.07 dd (8.6, 16.0) | 124.2, CH | 26 | 1.08 d (6.6) | 13.9, CH3 |
| 10 | 2.49 m | 41.4, CH | 27 | - | 174.1, C |
| 11 | 3.25 dd (1.2, 11.3) | 77.6, CH | 28a | 2.66 dd (1.1, 18.1) | 36.3, CH2 |
| 12 | 1.61 m | 24.7, CH | 28b | 3.07 dd (6.0, 18.1) | - |
| 13a | 1.59 m | 35.4, CH2 | 29 | 5.54 m | 72.4, CH |
| 13b | 1.59 m | - | 30 | 4.84 m | 78.7, CH |
| 14a | 1.67 m | 34.1, CH2 | 31 | 1.43 d (6.6) | 13.9, CH3 |
| 14b | 1.67 m | - | 32 | 3.22 s | 56.4, CH3 |
| 15 | 4.47 dd (4.5, 7.5) | 82.2, CH | 11-OH | 3.50 d (5.7) | - |
| 16 | - | 143.0, C | 20-OH | 8.55 s | - |
Figure 2.
NMR interpretation of oscillatoxin I. Bold line: COSY correlation; Arrow: HMBC correlations.
2.2. Biological Activity of Oscillatoxin I (1)
Oscillatoxin I (1) showed 100% inhibition activities in both cytotoxicity and diatom growth inhibition tests at a concentration of 10 µg/mL. The IC50 values of oscillatoxin I (1) in the cytotoxicity test and diatom growth inhibition test were 4.6 µg/mL and 1.2 µg/mL, respectively.
3. Discussion
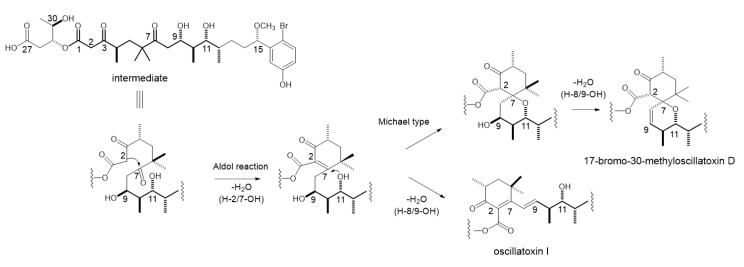
Oscillatoxin I (1) is an aplysiatoxin analog that lacks a six-membered ether ring in the molecule. The characteristic bicyclo ring systems of aplysiatoxin and its known derivatives have been proposed to be biosynthesized from a common intermediate [10]. The formation of the ring system of 17-bromo-30-methylosicllatoxin D (2) is preceded by an intramolecular aldol reaction between C-2 and C-7 and the dehydration of H-2/7-OH to generate a conjugated ketone, followed by an intramolecular Michael-type addition of 11-OH to C-7 (Figure 3). The presence of a cyclohexenone ring in oscillatoxin I (1) suggests that the biosynthesis of oscillatoxin I branches from a biosynthetic intermediate of 17-bromo-30-methyloscillatoxin D (2). The biosynthesis of oscillatoxin I is presumed to occur as follows: the aldol reaction of C-2/C-7 followed by the dehydration of H-2/7-OH and H-8/9-OH leads to the biosynthesis of oscillatoxin I. The E configuration of Δ8 prevents the Michael-type addition of 11-OH to C-7. Therefore, the isolation of oscillatoxin I (1) reinforced our proposal regarding the biosynthesis of aplysiatoxin derivatives [10].
Figure 3.
Proposed biosynthetic pathway of 17-bromo-30-methyloscillatoxin D and oscillatoxin I.
Osillatoxin I (1) showed moderate toxicity in the cytotoxicity test (IC50; 4.6 µg/mL) and diatom growth inhibition test (IC50; 1.2 µg/mL). We also studied the toxicities of aplysiatoxin and its fourteen derivatives, and found they had mild activities in these bioactivity tests [10]. Oscillatoxin I showed one of the most potent toxicities among aplysiatoxin and its analogues in the cytotoxicity and diatom growth inhibition tests.
4. Materials and Methods
4.1. General Procedure
HPLC was performed using a Hitachi Chromaster HPLC System (Hitachi High-Tech Science Co., Tokyo, Japan). HR-ESI-MS spectral data were collected using a Bruker micrOTOF QII (Bruker Co., Bremen, Germany) mass spectrometer. NMR spectra were recorded in acetone-d6 using a Bruker AVANCE III 600 spectrometer. Optical rotations were measured using a JASCO P-2100 (JASCO Co., Tokyo, Japan) using a 10 mm length cell. UV spectra were measured using a JASCO V-550 UV-spectrometer (JASCO Co., Tokyo, Japan). Bioassay results were recorded on a Model 550 microplate reader (Bio-Rad, Hercules, CA, USA).
4.2. Marine Cyanobacterium M. producens
Samples of the marine cyanobacterium M. producens were collected from Kuba Beach, Nakagusuku, Okinawa, Japan in July of 2010. After freeze-drying, the samples were stored at −30 °C until the experiments were performed. Identification of the sample was accomplished via morphological observation under a microscope by Dr. Masayuki Fukuoka of Tokyo University of Marine Science and Technology. Moorea producens was a dominant cyanobacteria species in the sample. The sample also contained some unidentified diatoms.
4.3. Isolation of Oscillatoxin I
A frozen sample of the cyanobacterium M. producens (dry weight: 0.87 kg) was soaked for several days in ethanol at room temperature. After filtering the ethanol extract, it was extracted five times with methanol and once with acetone. The extracts were then combined and concentrated in vacuo to yield a residue (37.8 g), which was partitioned first between methanol/water (4:1, v/v) and hexane. The solvent of the 80% MeOH-soluble layer was then removed, and the remaining sample was partitioned using distilled water and ethyl acetate (EtOAc). The EtOAc layer was evaporated to dryness. The distilled-water layer was then dissolved with 1-butanol (BuOH) and separated into two extracts. Since the EtOAc layer of the extracts from the cyanobacterium M. producens showed the most potent bioactivity, this layer was separated using an open glass column (PEGASIL ODS, Senshu Co., Tokyo, Japan) measuring 20 × 120 mm with stepwise elution in 50%, 70%, 90%, and 100% methanol. The 70% methanol eluate was then purified via HPLC using a reversed-phase column (Cosmosil 5C18-AR-II, 10 × 250 mm, Nakalai Tesque Inc., Kyoto, Japan). Finally, oscillatoxin I (1, 1.1 mg) was isolated.
4.4. Biological Tests of Oscillatoxin I
Cytotoxicity assays against mouse L1210 leukemia cells were carried out for the isolated compounds. The growth inhibition activity of the compounds against the marine diatom Nitzschia amabilis were also evaluated. Both types of bioactive assays were performed using the XTT colorimetric reaction method as previously reported [30,31].
Acknowledgments
The authors gratefully acknowledge Naomasa Oshiro, Setsuko Iwanaga and Daijiro Kamiya of Okinawa Prefectural Institute of Health and Environment for the cyanobacteria sample collection. The authors would like to thank Mana Horiuchi of Tokyo University of Marine Science and Technology for a part of biological assay.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/11/6/366/s1, Figure S1: ESI-HRMS spectrum of oscillatoxin G in positive ion mode. Figure S2: 1H NMR spectrum of oscillatoxin I in acetone- d6. Figure S3: 13C NMR spectrum of oscillatoxin I in acetone- d6. Figure S4: 1H–1H COSY NMR spectrum of oscillatoxin I in acetone- d6. Figure S5: 1H–13C HSQC spectrum of oscillatoxin I in acetone- d6. Figure S6: 1H–13C HMBC spectrum of oscillatoxin I in acetone- d6.
Author Contributions
Isolation: K.I., S.S. Chemical analyses: H.N., S.S., K.H., H.U., M.S. Biological tests: M.K. All the authors participated in the design of experiments and the writing and proofreading of the manuscript.
Funding
This study was supported partly by Japan Society for the Promotion of Science (JSPS) Grant in-Aid for Scientific Research Grant Number 16K01911 and 19K06220 (Hiroshi Nagai) and 15K01798 (Masayuki Satake).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Key Contribution
A new aplysiatoxin derivative, oscillatoxin I, was isolated from the marine cyanobacterium Moorea producens. Oscillatoxin I showed cytotoxicity and diatom growth-inhibition activity.
References
- 1.Mynderse J.S., Moore R.E., Kashiwagi M., Norton T.R. Antileukemia activity in the Osillatoriaceae: isolation of debromoaplysiatoxin from Lyngbya. Science. 1977;196:538–540. doi: 10.1126/science.403608. [DOI] [PubMed] [Google Scholar]
- 2.Moore R.E., Blackman A.J., Cheuk C.E., Mynderse J.S., Matsumoto G.K., Clardy J., Woodard R.W., Craig J.C. Absolute stereochemistries of the aplysiatoxins and oscillatoxin A. J. Org. Chem. 1984;49:2484–2489. doi: 10.1021/jo00187a035. [DOI] [Google Scholar]
- 3.Cardellina J.H., II, Marner F.J., Moore R.E. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193–195. doi: 10.1126/science.107586. [DOI] [PubMed] [Google Scholar]
- 4.Aimi N., Odaka H., Sakai S.I., Fujiki H., Suganuma M., Moore R.E., Patterson G.M.L. Lyngbyatoxins B and C, two new irritants from Lyngbya majuscula. J. Nat. Prod. 1990;53:1593–1596. doi: 10.1021/np50072a035. [DOI] [PubMed] [Google Scholar]
- 5.Osborne N.J., Webb P.M., Shaw G.R. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ. Int. 2001;27:381–392. doi: 10.1016/S0160-4120(01)00098-8. [DOI] [PubMed] [Google Scholar]
- 6.Kimberly A.W., Marquart L., Norton S.A. Lyngbya dermatitis (toxic seaweed dermatitis) Int. J. Dermatol. 2012;51:59–62. doi: 10.1111/j.1365-4632.2011.05042.x. [DOI] [PubMed] [Google Scholar]
- 7.Nagai H., Yasumoto T., Hokama Y. Aplysiatoxin and debromoaplysiatoxin as the causative agents of a red alga Gracilaria coronopifolia poisoning in Hawaii. Toxicon. 1996;34:753–761. doi: 10.1016/0041-0101(96)00014-1. [DOI] [PubMed] [Google Scholar]
- 8.Yasumoto T. Fish poisoning due to toxins of microalgal origins in the Pacific. Toxicon. 1998;36:1515–1518. doi: 10.1016/S0041-0101(98)00142-1. [DOI] [PubMed] [Google Scholar]
- 9.Paerl H.W., Paul V.J. Climate change: links to global expansion of harmful cyanobacteria. Water Res. 2012;46:1349–1363. doi: 10.1016/j.watres.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Nagai H., Watanabe M., Sato S., Kawaguchi M., Xiao Y.Y., Hayashi K., Watanabe R., Uchida H., Satake M. New aplysiatoxin derivatives from the Okinawan cyanobacterium Moorea producens. Tetrahedron. 2019;75:2486–2494. doi: 10.1016/j.tet.2019.03.020. [DOI] [Google Scholar]
- 11.Fujiki H., Suganuma M., Nakayasu M., Hoshino H., Moore R.E., Sugimura T. The third class of new tumor promoters, polyacetates (debromoaplysiatoxin and aplysiatoxin), can differentiate biological actions relevant to tumor promoters. Gann. 1982;73:495–497. [PubMed] [Google Scholar]
- 12.Fujiki H., Tanaka Y., Miyake R., Kikkawa U., Nishizuka Y., Sugimura T. Activation of calcium-activated, phospholipid-dependent protein kinase (protein kinase C) by new classes of tumor promoters: teleocidin and debromoaplysiatoxin. Biochem. Biophys. Res. Commun. 1984;120:339–343. doi: 10.1016/0006-291X(84)91259-2. [DOI] [PubMed] [Google Scholar]
- 13.Suganuma M., Fujiki H., Tahira T., Cheuk C., Moore R.E., Sugimura T. Estimation of tumor promoting activity and structure-function relationships of aplysiatoxins. Carcinogenesis. 1984;5:315–318. doi: 10.1093/carcin/5.3.315. [DOI] [PubMed] [Google Scholar]
- 14.Arcoleo J.P., Weinstein I.B. Activation of protein kinase C by tumor promoting phorbol esters, teleocidin and aplysiatoxin in the absence of added calcium. Carcinogenesis. 1985;6:213–217. doi: 10.1093/carcin/6.2.213. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura H., Kishi Y., Pajares M.A., Rando R.R. Structural basis of protein kinase C activation by tumor promoters. Proc. Natl. Acad. Sci. USA. 1989;86:9672–9676. doi: 10.1073/pnas.86.24.9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettit G.R., Herald C.L., Doubek D.L., Herald D.L., Arnold E., Clardy J. Isolation and structure of bryostatin 1. J. Am. Chem. Soc. 1982;104:6846–6848. doi: 10.1021/ja00388a092. [DOI] [Google Scholar]
- 17.Hennings H., Blumberg P.M., Pettit G.R., Herald C.L., Shores R., Yuspa S.H. Bryostatin 1, an activator of protein kinase C, inhibits tumor promotion by phorbol esters in SENCAR mouse skin. Carcinogenesis. 1987;8:1343–1346. doi: 10.1093/carcin/8.9.1343. [DOI] [PubMed] [Google Scholar]
- 18.Hale K.J., Hummersone M.G., Manaviazar S., Frigerio M. The chemistry and biology of the bryostatin antitumour macrolides. Nat. Prod. Rep. 2002;19:413–453. doi: 10.1039/b009211h. [DOI] [PubMed] [Google Scholar]
- 19.Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol. Therapeut. 1993;59:257–280. doi: 10.1016/0163-7258(93)90070-T. [DOI] [PubMed] [Google Scholar]
- 20.Antal C.E., Hudson A.M., Kang E., Zanca C., Wirth C., Stephenson N.L., Trotter E.W., Gallegos L.L., Miller C.J., Furnary F.B., et al. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell. 2015;160:489–502. doi: 10.1016/j.cell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa Y., Yanagita R.C., Hamada N., Murakami A., Takahashi H., Saito N., Nagai H., Irie K. A simple analogue of tumor-promoting aplysiatoxin is an antineoplastic agent rather than a tumor promoter: development of a synthetically accessible protein kinase C activator with bryostatin-like activity. J. Am. Chem. Soc. 2009;131:7573–7579. doi: 10.1021/ja808447r. [DOI] [PubMed] [Google Scholar]
- 22.Irie K., Yanagita R.C. Synthesis and biological activities of simplified analogs of the natural PKC ligands, bryostatin-1 and aplysiatoxin. Chem. Rec. 2014;14:251–267. doi: 10.1002/tcr.201300036. [DOI] [PubMed] [Google Scholar]
- 23.Entzeroth M., Blackman A.J., Mynderse J.S., Moore R.E. Structures and stereochemistries of oscillatoxin B, 31-noroscillatoxin B, oscillatoxin D, and 30-methyloscillatoxin D. J. Org. Chem. 1985;50:1255–1259. doi: 10.1021/jo00208a019. [DOI] [Google Scholar]
- 24.Nagai H., Yasumoto T., Hokama Y. Manauealides, Some of the causative agents of a red alga Gracilaria coronopifolia poisoning in Hawaii. J. Nat. Prod. 1997;60:925–928. doi: 10.1021/np970193c. [DOI] [PubMed] [Google Scholar]
- 25.Chlipara G.E., Tri P.H., Hung N.V., Krunic A., Shim S.H., Soejarto D.D., Orjala J. Nhatrangins A and B, aplysiatoxin-related metabolites from the marine cyanobacterium Lyngbya majuscula from Vietnam. J. Nat. Prod. 2010;73:784–787. doi: 10.1021/np100002q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta D.K., Kaur P., Leong S.T., Tan L.T., Prinsep M.R., Chu J.J.H. Anti-Chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium Trichodesmium erythraeum. Mar. Drugs. 2014;12:115–127. doi: 10.3390/md12010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han B.N., Liang T.T., Keen L.J., Fan T.T., Zhang X.D., Xu L., Zhao Q., Wang S.P., Lin H.W. Two marine cyanobacterial aplysiatoxin polyketides, neo-debromoaplysiatoxin A and B, with K+ channel inhibition activity. Org. Lett. 2018;20:578–581. doi: 10.1021/acs.orglett.7b03672. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y.H., Liang T.T., Fan T.T., Keen L.J., Zhang X.D., Xu L., Zhao Q., Zeng R., Han B.N. Neo-debromoaplysiatoxin C, with new structural rearrangement, derived from debromoaplysiatoxin. Nat. Prod. Res. 2019 doi: 10.1080/14786419.2019.1577840. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y.H., Wu J., Fan T.T., Zhang H.H., Gong X.X., Cao Z.Y., Zhang J., Lin H.W., Han B.N. Chemical and biological study of aplysiatoxin derivatives showing inhibition of potassium channel Kv1.5. RSC Adv. 2019;9:7594–7600. doi: 10.1039/C9RA00965E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawabata T., Lindsay D.J., Kitamura M., Konishi S., Nishikawa J., Nishida S., Kamio M., Nagai H. Evaluation of the bioactivities of water-soluble extracts from twelve deep-sea jellyfish species. Fish. Sci. 2013;79:487–494. doi: 10.1007/s12562-013-0612-y. [DOI] [Google Scholar]
- 31.Jiang W., Akagi T., Suzuki H., Takimoto A., Nagai H. A new diatom growth inhibition assay using the XTT colorimetric method. Comp. Biochem. Physiol. Part C. 2016;185:13–19. doi: 10.1016/j.cbpc.2016.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.