Abstract
Postharvest fungal disease is one of the significant factors that limits the storage period and marketing life of peaches, and even result in serious economic losses worldwide. Biological control using microbial antagonists has been explored as an alternative approach for the management of postharvest disease of fruits. However, there is little information available regarding to the identification the fungal pathogen species that cause the postharvest peach diseases and the potential and mechanisms of using the Bacillus subtilis JK-14 to control postharvest peach diseases. In the present study, a total of six fungal isolates were isolated from peach fruits, and the isolates of Alternaria tenuis and Botrytis cinerea exhibited the highest pathogenicity and virulence on the host of mature peaches. In the culture plates, the strain of B. subtilis JK-14 showed the significant antagonistic activity against the growth of A. tenuis and B. cinerea with the inhibitory rates of 81.32% and 83.45% at 5 days after incubation, respectively. Peach fruits treated with different formulations of B. subtilis JK-14 significantly reduced the mean disease incidences and lesion diameters of A. tenuis and B. cinerea. The greatest mean percent reduction of the disease incidences (81.99% and 71.34%) and lesion diameters (82.80% and 73.57%) of A. tenuis and B. cinerea were obtained at the concentration of 1 × 107 CFU mL−1 (colony forming unit, CFU). Treatment with the strain of B. subtilis JK-14 effectively enhanced the activity of the antioxidant enzymes-superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) in A. tenuis and B. cinerea inoculated peach fruits. As such, the average activities of SOD, POD and CAT were increased by 36.56%, 17.63% and 20.35%, respectively, compared to the sterile water treatment. Our results indicate that the isolates of A. tenuis and B. cinerea are the main pathogens that cause the postharvest peach diseases, and the strain of B. subtilis JK-14 can be considered as an environmentally-safe biological control agent for the management of postharvest fruits diseases. We propose the possible mechanisms of the strain of B. subtilis JK-14 in controlling of postharvest peach diseases.
Keywords: Bacillus spp., peach fruits, postharvest diseases, antagonistic activity, antioxidative defense system
1. Introduction
Postharvest losses refer to the losses that occur along the food supply chain due to pathogens infection, handling, storage, transportation and processing, thereby resulting in the reduction in quality, quantity and market value of agricultural commodities [1,2]. Food and Agriculture Organization reported that global average loss due to the food postharvest losses in North America, Europe and Oceania was about 29%, compared to an average of about 38% in industrialized Asia, Africa, Latin America and South East Asia [3]. Among all the factors for reducing the losses on food supply, postharvest diseases of fruits present a major factor that causes the postharvest losses and limits the duration of storage [4,5]. In addition, postharvest diseases are often the major concern in influencing consumer prices, requirements and mode of transportation [6]. China is the largest producer of peaches with a production of 13.5 million metric tons (MMT), and exporter to North Korea, Russia, Singapore, USA, Philippines and Malaysia, with a very different climate (from tropical, continental or oceanic climatic climates), but the postharvest diseases of peach fruits have been considered one of the most severe factors that results in the loss of production [7]. Additionally, the diseases caused by fungal pathogens in harvested fresh fruits are considered as one of the most serious losses of production at the postharvest and consumption levels [8,9,10]. Some research showed that the main worldwide postharvest diseases caused by fungi in peach fruits are brown rot caused by Monilinia fructicola or M. laxa, Rhizopus; rot caused by R. stolonifer; grey mold caused by Botrytis cinerea [11], and other economically important fungal diseases such as those of stone fruits caused by Penicillium spp., Cladosporium spp., Alternaria spp. and Aspergillus spp. [12,13,14]. However, little is known about the species of main fungal pathogens that cause the postharvest disease of peaches in China.
A number of strategies have been adapted to manage of postharvest diseases worldwide [15,16]. Chemical control (synthetic fungicides) is known to be highly effective and widely applied method in orchard after harvesting [17,18]. However, some fungicides have toxicological risks, such as dangerous to human health and causing environmental pollution, even in some cases their use is prohibited by law in postharvest phase [19,20,21]. Particularly, the increased level of fungicide use in fruit orchards has led to the growing public concern over the health and environmental hazards associated with fungicides [22]. Therefore, development of alternative safe and natural methods in controlling postharvest diseases have become urgent in recent years worldwide [23,24]. In particular, there has been extensive research to reduce synthetic fungicide usage based on microbial antagonists to biologically control postharvest pathogens in the past years with higher control efficiency [10,15,23,25]. In recent years, the bacterium Bacillus spp. has been widely studied as a potential biological agent against various plant diseases, increases plant systemic resistance and improves rhizosphere microbial community structure [26,27,28,29]. It is common in nature and nontoxic and harmless to humans and other animals, and nonpathogenic to plants [30]. However, there is little information on the bio-control activity of the bacterial antagonist B. subtilis JK-14 and its mechanisms involved in the postharvest disease management of peaches.
Therefore, the objectives of the present study were to (i) isolate and identify the main species of fungal pathogens causing postharvest disease on peaches, (ii) explore the antifungal potential and controlling efficiency of B. subtilis JK-14 against the main postharvest fungal infection, and (iii) determine the possible mechanisms involved in the strain of B. subtilis JK-14 in controlling postharvest fruit diseases on peaches.
2. Results
2.1. Isolation and Identification of Postharvest Fungal Pathogens
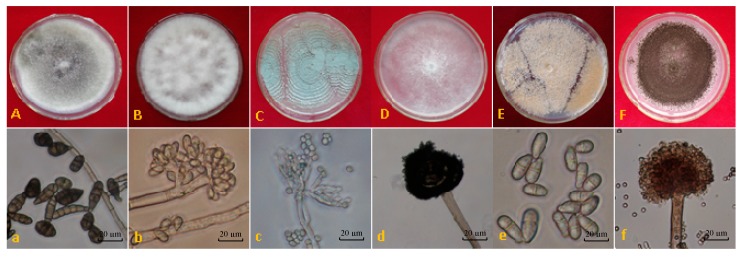
In the present study, a total of six fungal isolates were isolated from the mature peach (Prunus persica L.) fruits during the storage period. They were identified as Alternaria tenuis (Figure 1A,a), Botrytis cinerea (Figure 1B,b), Penicillium digitatum (Figure 1C,c), Rhizopus nigricans (Figure 1D,d), Trichothecium roseum (Figure 1E,e), and Aspergillus niger (Figure 1F,f), respectively, according to the characteristics of colony (Figure 1A–F) and conidia (Figure 1a–f) under the microscope observation. The isolates of A. tenuis, B. cinerea, P. digitatum, T. roseum, A. niger, and R. nigricans were all grown better on potato dextrose agar (PDA) medium (pH = 6.0) at 25 °C as the ecophysiological conditions.
Figure 1.
Characteristics of colony (A–F) and conidia (a–f) of the pathogenic isolates of peaches grew on potato dextrose agar (PDA) for 5 days. Where (A) and (a) the colony and conidia of Alternaria tenuis; (B) and (b) Botrytis cinerea; (C) and (c) Penicillium digitatum; (D) and (d) Rhizopus nigricans; (E) and (e) Trichothecium roseum; (F) and (f) Aspergillus niger.
2.2. Determination of the Pathogenicity of the Isolates
The difference in disease incidences of infection caused by the six fungal isolates on the mature fruits was highly significant between intact and wounded fruits. The non-inoculated control fruits, including intact and wounded did not develop decay symptoms. In contrast, all the wounded fruits developed rot and decay, regardless of the isolates used. Particularly, the isolates of A. tenuis, B. cinerea and R. nigricans presented the highest disease incidences after inoculation onto the wounded fruits, and the disease incidences were all 100%. In addition, the highest disease incidences were observed after inoculation with the isolates of A. tenuis and B. cinerea on the intact fruits, and the disease incidences were 100% and 92.33%, respectively. Whereas the isolate of T. roseum was unable to infect the intact fruits under the same experimental conditions (Table 1).
Table 1.
The pathogenicity of six fungal isolates after inoculation onto the postharvest peach fruits.
| Isolates | Disease Incidences (%) | |
|---|---|---|
| Wound Inoculation | Intact Inoculation | |
| Alternaria tenuis | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| Botrytis cinerea | 100.00 ± 0.00 a | 92.33 ± 3.16 c |
| Penicillium digitatum | 95.46 ± 4.21 b | 56.67 ± 3.02 f |
| Trichothecium roseum | 34.54 ± 2.56 g | 0.00 ± 0.00 i |
| Rhizopus nigricans | 100.00 ± 0.00 a | 83.33 ± 2.89 d |
| Aspergillus niger | 67.63 ± 2.33 e | 17.34 ± 1.54 h |
| Control | 0.00 ± 0.00 i | 0.00 ± 0.00 i |
Data are means ± standard error of replicates and those in a column followed by different letters are significantly different at p < 0.05, based on Duncan’s new multiple range test using multi-way ANOVA (n = 18). The disease incidences (%) were determined at 5 days after inoculation with the six isolates. Control represents the fruits inoculation with sterile water but not with the isolates.
2.3. Inhibitory Effect of Bacillus subtilis JK-14 against Alternaria tenuis and Botrytis cinerea
Our results showed that the strain of B. subtilis JK-14 presented the significant antagonistic activity on the pathogens of A. tenuis and B. cinerea compared to the control. In the culture plates (PDA), the colony growth of A. tenuis and B. cinerea were significantly inhibited at 5 days after inoculated with the antagonistic strain of B. subtilis JK-14. The inhibitory rates of A. tenuis and B. cinerea were 81.32% and 83.45% at 5 days after inoculation with the strain of B. subtilis JK-14, respectively (Table 2).
Table 2.
Antagonistic activity of Bacillus subtilis JK-14 against Alternaria tenuis and Botrytis cinerea.
| Treatments | Inhibitory Rates (%) | |
|---|---|---|
| Alternaria tenuis | Botrytis cinerea | |
| Bacillus subtilis JK-14 | 81.32 ± 2.11 b | 83.45 ± 1.54 a |
| Control | - | - |
Data are means ± standard error of replicates and those in a column followed by different letters are significantly different at p < 0.05, based on Duncan’s new multiple range test using multi-way ANOVA (n = 12). The inhibitory rates (%) were determined at 5 days after inoculation with pathogens of Alternaria tenuis and Botrytis cinerea. Control represents the media inoculation with Alternaria tenuis or Botrytis cinerea but not with Bacillus subtilis JK-14.
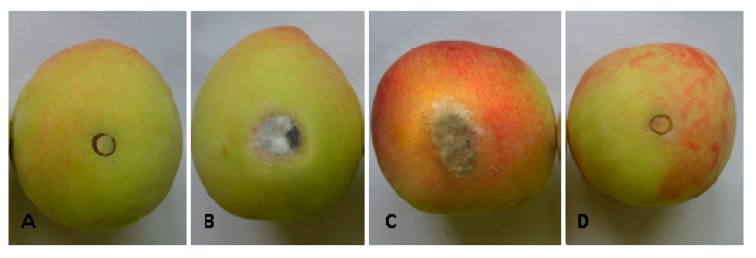
To further confirm the antagonistic activity of B. subtilis JK-14 in controlling the A. tenuis and B. cinerea decay on fresh peaches fruits. We used the bacterial cell suspension (BCS) of B. subtilis JK-14 treatment and found that it was effective in inhibiting the fresh fruits decay caused by the pathogens of A. tenuis and B. cinerea, compared to the control. The disease incidences and lesion diameters on the peach fruits treated with the BCS of B. subtilis JK-14 at the tested concentration of 1 × 108 CFU mL−1 were significantly reduced compared to those on the control fruits. The disease incidences and lesion diameters were 14.8% and 3.0 mm for A. tenuis, and 14.1% and 3.2 mm for B. cinerea after 5-day incubation, whereas the average disease incidences and lesion diameters of A. tenuis and B. cinerea decay in the control fruits were 93.7% and 12.6 mm, respectively (Table 3). In addition, there was no significant symptoms and mycelium around the inoculation site of fresh fruits after inoculated with the pathogen of A. tenuis and the antagonist of B. subtilis JK-14 (Figure 2A), and the pathogen of B. cinerea and B. subtilis JK-14 together (Figure 2D) in treatment group, whereas the fruits in the control group were decayed significantly and a large number of hyphae grown around the wound sites, regardless of the pathogens of A. tenuis (Figure 2B) and B. cinerea (Figure 2C) that was inoculated alone in this experiment without B. subtilis JK-14.
Table 3.
Effect of Bacillus subtilis JK-14 on disease incidences and lesion diameters of fresh peach fruits after inoculation with Alternaria tenuis and Botrytis cinerea.
| Treatments | Alternaria tenuis | Botrytis cinerea | ||
|---|---|---|---|---|
| Disease Incidences (%) | Lesion Diameters (mm) | Disease Incidences (%) | Lesion Diameters (mm) | |
| Bacillus subtilis JK-14 | 14.8 ± 3.40 b | 3.0 ± 0.06 d | 14.1 ± 3.40 b | 3.2 ± 0.16 d |
| Control | 94.8 ± 1.29 a | 12.0 ± 0.12 c | 92.6 ± 2.57 a | 13.1 ± 0.17 bc |
Data are means ± standard error of replicates and those in a column followed by different letters are significantly different at p < 0.05, based on Duncan’s new multiple range test using multi-way ANOVA (n = 18). The disease incidences (%) and lesion diameters (mm) were determined at 5 days after inoculation with the pathogens. Control represents the peach fruits inoculation with Alternaria tenuis or Botrytis cinerea but not with Bacillus subtilis JK-14.
Figure 2.
Inhibitory activities of Bacillus subtilis JK-14 in controlling Alternaria tenuis and Botrytis cinerea on fresh peaches. (A) Bacillus subtilis JK-14 and Alternaria tenuis inoculation; (B) Alternaria tenuis inoculation; (C) Botrytis cinerea inoculation; (D) Bacillus subtilis JK-14 and Botrytis cinerea inoculation.
2.4. Effect of Bacillus subtilis JK-14 in Controlling Alternaria tenuis and Botrytis cinerea Decay on Peaches
The disease incidences and lesion diameters of postharvest decay of peaches treated with the different formulations of B. subtilis JK-14 at all tested concentrations (1 × 105–1 × 109 CFU mL−1) were significantly reduced compared to those of the control fruits for the decay caused by the pathogens of A. tenuis (Table 4) and B. cinerea (Table 5). Among all the different concentrations of the formulations, the disease incidences and lesion diameters of postharvest decay of peaches were significantly reduced by the application of fermentation liquid bacterial cells (FLBC) and BCS of B. subtilis JK-14 at 1 × 107 CFU mL−1. The disease incidences and lesion diameters were 18.52% and 3.79 mm for A. tenuis and 17.78% and 3.74 mm for B. cinerea at 5 days after inoculated with the FLBC formulations of B. subtilis JK-14 at 1 × 107 CFU mL−1, 14.82% and 3.06 mm for A. tenuis and 14.07% and 3.19 mm for B. cinerea after inoculated with the BCS. In contrast, the disease incidences and lesion diameters of A. tenuis decay in the control fruits were 92.59% and 11.95 mm, respectively (Table 4), and 92.59% and 13.11 mm, respectively, of B. cinerea decay in the control fruits (Table 5).
Table 4.
Effect of different formulations of Bacillus subtilis JK-14 on disease incidences and lesion diameters of mature peach fruits after inoculation with Alternaria tenuis.
| Concentrations (CFU mL−1) | Disease Incidences (%) | Lesion Diameters (mm) | ||
|---|---|---|---|---|
| FLBC | BCS | FLBC | BCS | |
| 1 × 109 | 47.41 ± 1.28 c | 45.18 ± 3.40 cd | 6.13 ± 0.13 c | 5.69 ± 0.08 d |
| 1 × 108 | 38.52 ± 3.39 d | 36.30 ± 3.40 de | 5.61 ± 0.06 d | 5.16 ± 0.16 e |
| 1 × 107 | 18.52 ± 2.56 f | 14.82 ± 3.40 f | 3.79 ± 0.08 fg | 3.06 ± 0.06 g |
| 1 × 106 | 34.07 ± 3.40 e | 31.11 ± 2.22 e | 4.95 ± 0.06 e | 4.11 ± 0.09 f |
| 1 × 105 | 65.92 ± 2.60 b | 61.48 ± 3.39 b | 7.36 ± 0.14 b | 6.72 ± 0.19 bc |
| Control | 92.59 ± 1.28 a | 11.95 ± 0.09 a | ||
Data are means ± standard error of replicates and those in a column followed by different letters are significantly different at p < 0.05, based on Duncan’s new multiple range test using multi-way ANOVA (n = 18). The disease incidences (%) and lesion diameters (mm) were determined at 5 days after inoculation with the pathogen. Control represents the peach fruits inoculation with Alternaria tenuis but not with Bacillus subtilis JK-14. FLBC represents the fermentation liquid with bacterial cells; BCS represents the bacterial cells suspension. CFU represents colony forming unit.
Table 5.
Effect of different formulations of Bacillus subtilis JK-14 on disease incidences and lesion diameters of mature peach fruits after inoculation with Botrytis cinerea.
| Concentrations (CFU mL−1) | Disease Incidences (%) | Lesion Diameters (mm) | ||
|---|---|---|---|---|
| FLBC | BCS | FLBC | BCS | |
| 1 × 109 | 44.44 ± 2.23 c | 43.70 ± 3.40 c | 6.03 ± 0.19 c | 5.74 ± 0.22 c |
| 1 × 108 | 39.26 ± 1.28 d | 36.30 ± 1.28 e | 5.37 ± 0.25 d | 5.13 ± 0.15 d |
| 1 × 107 | 17.78 ± 2.22 h | 14.07 ± 3.40 i | 3.74 ± 0.16 f | 3.19 ± 0.16 g |
| 1 × 106 | 28.15 ± 1.28 f | 24.44 ± 2.23 g | 4.80 ± 0.17 e | 4.22 ± 0.16 e |
| 1 × 105 | 62.22 ± 2.22 b | 60.00 ± 2.22 b | 7.15 ± 0.08 b | 6.66 ± 0.34 b |
| Control | 92.59 ± 2.57 a | 13.11 ± 0.17 a | ||
Data are means ± standard error of replicates and those in a column followed by different letters are significantly different at p < 0.05, based on Duncan’s new multiple range test using multi-way ANOVA (n = 18). The disease incidences (%) and lesion diameters (mm) were determined at 5 days after inoculation with the plant pathogen. Control represents the peach fruits inoculation with Botrytis cinerea but not with Bacillus subtilis JK-14. FLBC represents the fermentation liquid with bacterial cells; BCS represents the bacterial cells suspension. CFU represents colony forming unit.
In addition, the controlling effect of different formulations of the strain of B. subtilis JK-14 was different between the formulations of FLBC and BCS at all the tested concentrations. The average disease incidences and lesion diameters of A. tenuis and B. cinerea decay of the fruits treated with the BCS formulations were lower than the formulation of FLBC. Therefore, the BCS formulations of the strain of B. subtilis JK-14 exhibited the highest controlling effect on the A. tenuis and B. cinerea decay compared to the control (Table 4 and Table 5).
2.5. Effect of Bacillus subtilis JK-14 on the Symptoms of Fruits Decay after Inoculation with the Pathogens on Peaches
Overall, the different concentrations of BCS formulation of B. subtilis JK-14 (1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 CFU mL−1) had the different inhibitory and controlling effects on the A. tenuis (Figure 3A) and B. cinerea (Figure 3B) decay of the fruits. The BCS formulation of B. subtilis JK-14 at the concentration of 1 × 107 CFU mL−1 had a stronger and more significant inhibitory and controlling effect (Figure 3). At the concentrations of 1 × 105, 1 × 108 and 1 × 109 CFU mL−1, the fruits were decayed significantly and a large number of hyphae grown on the surface. At the concentration of 1 × 106 CFU mL−1, the fruits became decay and a fewer number of hyphae grew on the surface. However, there was no significant symptoms and mycelium around the inoculation site at the concentration of 1 × 107 CFU mL−1. In contrast, the fruits were exhibited significant decay and a large number of hyphae grew around the wound site in the untreated control fruits.
Figure 3.
Effect of Bacillus subtilis JK-14 on the symptoms of peach fruits decay at 5 days after inoculation with the pathogens of (A) Alternaria tenuis and (B) Botrytis cinerea.
2.6. Effect of Bacillus subtilis JK-14 on the Activities of Defense-Related Enzymes of Peaches
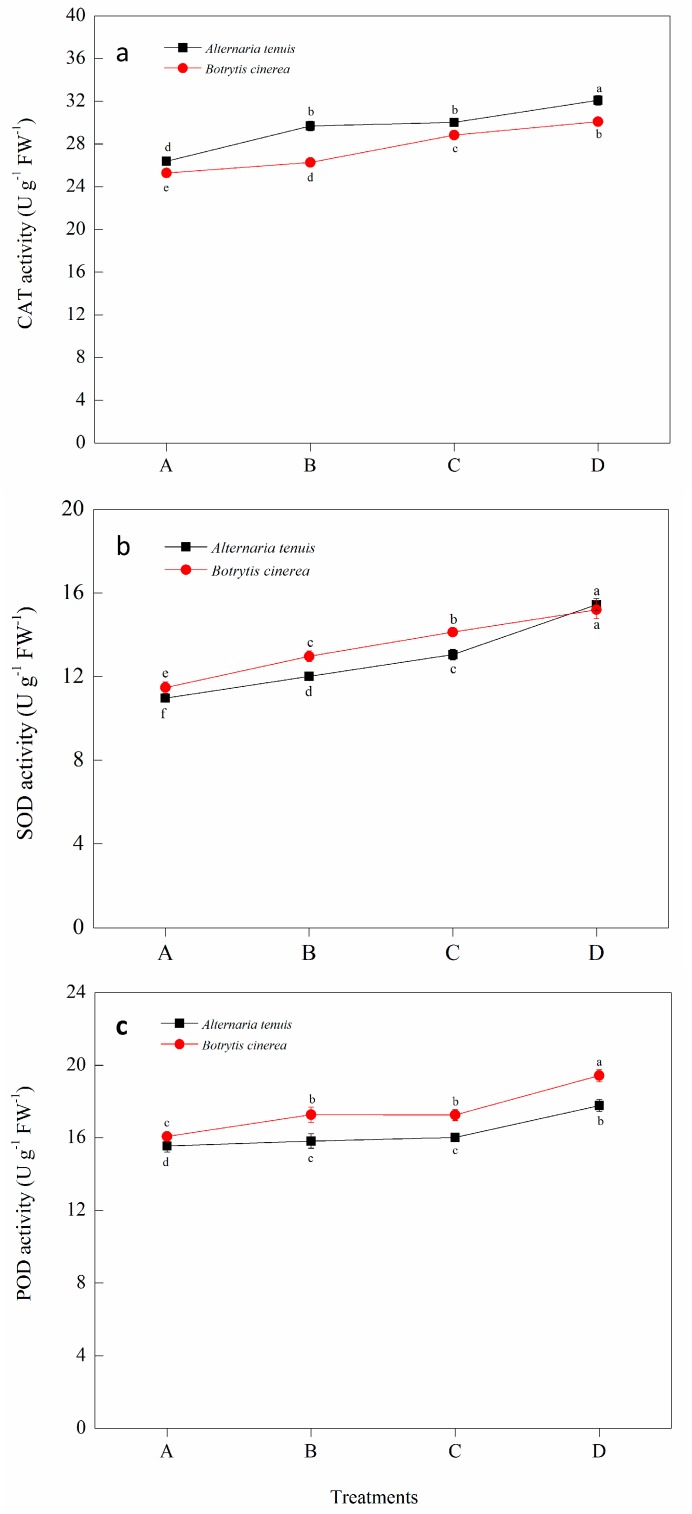
The effects of B. subtilis JK-14 on the activities of defense-related enzymes of peaches fruits were determined after inoculation with the pathogens of A. tenuis and B. cinerea onto the fruits. Our results found that the pathogens (A. tenuis and B. cinerea) or the B. subtilis JK-14 alone induced and increased the defense-related enzymes activities in peach fruits, including the activities of CAT, SOD and POD (Figure 4) in comparison to the sterile water treatments. Moreover, the activities of CAT, SOD and POD were significantly increased after being treated with the strain of B. subtilis JK-14, and the pathogens of A. tenuis or B. cinerea onto fruits, compared to the sterile water, B. subtilis JK-14, and the pathogens-inoculated fruits alone. Compared to the sterile water (Treatment A), the activity of CAT (Figure 4a), SOD (Figure 4b) and POD (Figure 4c) were significantly increased by 21.67%, 40.69% and 14.32%, respectively, on the 4th day after inoculation with the strain of B. subtilis JK-14 and the pathogen of A. tenuis (Treatment D) and 19.04%, 32.43% and 20.93%, respectively, after the inoculation with B. subtilis JK-14 and the pathogen of B. cinerea (Treatment D). In addition, the B. subtilis JK-14 and A. tenuis (Treatment D) treatment increased the activity of CAT (Figure 4a), SOD (Figure 4b) and POD (Figure 4c) by 8.09%, 28.55% and 12.39% on the 4th day, respectively, and 14.58%, 17.19% and 12.53%, respectively, after treatment with B. subtilis JK-14 and B. cinerea (Treatment D) in comparison to the pathogens inoculation (Treatment B). Moreover, the activity of POD in seedlings treated with B. subtilis JK-14 alone (Treatment C) did not differ from fruits treated with the pathogens treatments (Treatment B) (Figure 4c), whereas the activities of SOD differed significantly between the two treatments (Treatment B and C) after inoculation with A. tenuis or B. cinerea (Figure 4b).
Figure 4.
Effect of Bacillus subtilis JK-14 on the activity of (a) CAT, (b) SOD and (c) POD of peach fruits after inoculation with the pathogens of Alternaria tenuis and Botrytis cinerea. A—sterile water, B—pathogens inoculation, C—the strain of Bacillus subtilis JK-14 inoculation, D—Bacillus subtilis JK-14 and pathogens inoculation. Each value is the mean of two experiments. Line bars represent the standard errors of the means. Data in columns with the different letters are significantly different according to Duncan’s multiple range test at p < 0.05 (n = 18).
3. Discussion and Conclusions
Peach is one of the most ancient and world-popular fruits due to its high marketing value with favorable taste and abundant phytonutrients [31]. However, postharvest fungal diseases limit the storage period and marketing life of peaches, and result in serious economic losses worldwide. Recently, application of bio-control agents for the management of postharvest fruit decay has been explored as an alternative method instead of synthetic fungicides worldwide [15]. Bacillus spp. has been considered as the bio-control agent in controlling number of plant diseases with a high efficacy [32,33]. However, there is little information available regarding the identification the fungal pathogen species that cause the peach postharvest diseases, and explore the potential and mechanisms of Bacillus subtilis JK-14 in controlling postharvest peach diseases. Our present study showed that a total of six fungal isolates were isolated from the mature peaches, and in particular the species of Alternaria tenuis and Botrytis cinerea have been identified as the main pathogens for causing the host of mature peach decay. Interestingly, the strain of B. subtilis JK-14 has been found and exhibited a potent activity in inhibiting the growth of A. tenuis and B. cinerea, and controlling peaches fruits fungal disease in the present study. The possible mechanisms for the strain of B. subtilis JK-14 in inhibiting and controlling postharvest peaches fungal disease were due to the direct effect by inhibiting the pathogens infection, and the indirect effect by activating the host defense response to pathogens infection. To the best of our knowledge, the present study is the first to discover the role of the antagonistic B. subtilis JK-14 in controlling peach fungal disease that are caused by the pathogens of A. tenuis and B. cinerea. In view of the high control efficacy in comparison to the control, the strain of B. subtilis JK-14 can be considered as an environmentally-safe biological control agent instead of chemical fungicides for the management of postharvest disease.
Some previous studies found and identified numerous postharvest pathogens which can cause the decay of stone fruits and belong to the genera of Monilinia, Rhizopus, Penicillium, Alternaria, Botrytis, Cladosporium, Colletotrichum and Stigmina [34], Trichothecium [35] and Aspergillus [36]. Interestingly, six fungal isolates were isolated from the mature peach fruits in the present study, including A. tenuis, B. cinerea, P. digitatum, T. roseum, R. nigricans and A. niger. Our results confirm for the first time that these species are pathogenic to peach fruit and cause decay on wounded peach fruits. However, we have discovered that the isolate of T. roseum was not pathogenic to the intact peach fruits. The reason may due to the lack of wounds that prevent the T. roseum invasion. A similar study demonstrated that the wounds can provide the pathways for the pathogens invasion [25]. In addition, some previous studies revealed that the gray mold decay, blue mold decay and Rhizopus decay caused by the fungi of B. cinerea, P. expansum and R. stolonifer were the most economically significant and destructive postharvest diseases of peaches [5,8,37,38]. However, our results found that the isolates of A. tenuis and B. cinerea presented the highest pathogenicity and virulence on the host of mature peaches, and also considered as the main pathogens that cause the postharvest disease of peach fruits. The average disease incidences of A. tenuis and B. cinerea were 100% and 96.17% after inoculation onto the wounded and intact fruits, respectively. The difference from the previous studies may due to the relationship between the pathogenicity of microbial isolates and the ripening index of peach fruits at harvest [39,40].
In view of the need for reducing environmental pollution due to fungicide over-use in controlling plant diseases in previous years, recently, biological control has emerged as an effective strategy to combat major postharvest decay of fruits [25,41]. It is well-known that B. subtilis is an effective antagonistic bacterium and has been applied in controlling plant fungal diseases such as root diseases [42], foliar diseases [43] and postharvest diseases [15]. A significant advancement from the present study is the finding that B. subtilis JK-14 provided a significant inhibitory effect on the peach fruits pathogens of A. tenuis and B. cinerea, and also different formulations of B. subtilis JK-14 exhibited significant controlling effect on the peach fruits decay after inoculation with pathogens of A. tenuis and B. cinerea. Our findings suggest that the strain of B. subtilis JK-14 can be considered as a bio-control agent in the effort of developing alternative approaches to control postharvest diseases of fruits.
A previous study showed that Bacillus sp. C06 suppressed the disease incidences of the postharvest disease brown rot by 92% and decreased the lesion diameters by 88% compared to the pathogen-only, and Bacillus sp.T03-c reduced disease incidences and lesion diameters by 40% and 62%, respectively [44]. Similarly, Xu et al. reported that the treatment with Pichia caribbica significantly reduced the disease incidences and lesion diameters of Rhizopus decay of peaches compared with the control fruits in a dose dependent manner [7]. However, our results revealed that the greatest mean percent reduction of disease incidences and lesion diameters of peach postharvest fungal disease by 82.40% and 72.46% after the application of B. subtilis JK-14 at 1 × 107 CFU mL−1 among all the different concentrations from 1 × 105 to 1 × 109 CFU mL−1. Such differences may be related to the effect of the species of pathogens and different conditions of ripening index of peach fruits (pH value) at harvest on the inhibitory effect of B. subtilis JK-14 [39].
To further understand the mechanisms of B. subtilis JK-14 in controlling postharvest diseases of peaches, we explored the effects of B. subtilis JK-14 on the activities of defense-related enzymes after inoculation with the pathogens in the present study, and found that the treatment of peach fruits with B. subtilis JK-14 effectively enhanced the activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) after inoculation with the pathogen of A. tenuis or B. cinerea. Our results indicate that the enhanced activities of defense-related enzymes may play a significant role in the resistance of peaches to the pathogens infection and the induced activity of defense-related enzymes to be part of the mechanism of B. subtilis JK-14 in controlling postharvest diseases of peach fruits. Some previous studies revealed that one of the important mechanisms for the genus Bacillus in controlling plant diseases is by increasing and activating the plant systemic resistance [45,46,47]. In addition, the enhanced activities of antioxidant enzymes (SOD, POD, CAT and ascorbate peroxidase, APX) and their coordinated action have been reported to be a part of the mechanism implicated in the alleviation of lipid peroxidation and delay of senescence in peach fruits [48]. Similarly, Xu et al. [7] have demonstrated that peach fruits inoculated with P. caribbica exhibited higher level of POD, CAT and phenylalanine aminolase (PAL) activities than the untreated fruits during the storage period.
In summary, a total of six isolates were isolated from the peach fruits, and the isolates of A. tenuis and B. cinerea were considered as the main pathogens with the highest pathogenicity and virulence on the host of mature peaches. The strain of B. subtilis JK-14 exhibits a high efficacy in controlling postharvest decay of peaches, and may be considered as an environmentally-safe biological control agent for the management of postharvest decay diseases. The possible mechanisms of B. subtilis JK-14 for the management of peach postharvest disease were due to (i) the direct effect by inhibiting the postharvest fungal pathogens growth and infection, and (ii) the indirect effect by activating the defense-related enzymes to enhance the resistance of peaches response to the postharvest fungal pathogens infection during the storage period.
4. Materials and Methods
Experiments were carried out at the Gansu Provincial Biocontrol Engineering Laboratory of Crop Diseases and Pests. The peach (Prunus persica L., cultivar Baifeng) fruits were collected from the stone fruit orchards in Gansu, China. Gansu is located in the northwest of China, at the longitude of 103.8264470 E and latitude of 36.0595610 N, with a dry and strong continental temperate monsoon climate. The average temperature, precipitation and relative humidity of the air were about 8 °C, 300 mm and 30% in 2011–2012.
4.1. Fungal Pathogens Isolation and Identification
During 2011–2012, the mature peach (cultivar Baifeng) fruits were collected from the stone fruit orchards in Gansu, China. The ripening index of peach fruits at harvest: pH 3.75–3.98, organic acid 2.28–2.64 mg g−1, ethylene production 16.23–21.46 µL kg−1 h−1, soluble solids content 12.24–13.16%, total sugar 90.85–110.90 mg g−1, pectic substances 9.2–13.8 mg g−1. Thereafter, fruits were moist-incubated by placing in plastic containers with lids, lined with moist paper towels to maintain high relative humidity, and incubated at room temperature (20 °C) for 1–2 weeks to promote the pathogens growth and development. Small fruits sections (2 cm) were surface sterilized with 2% sodium hypochlorite (NaClO) for 3 min, and followed by 3 min rinses in sterile water. Fruits were then cut lengthwise along the lesion (1 cm) and placed individually onto PDA for 5 days at 25 °C. The spores and mycelium were transferred with a sterile needle from the colony to fresh Petri dishes containing PDA medium at Day 5. These cultures were grown for 5 days in an incubator at 25 °C, and then identified according to the colony and spores characteristics. Finally, all isolates were maintained and stored in 20% glycerol at −80 °C until use.
4.2. Spore Suspensions of Fungal Pathogen Preparation
The identified pathogens of peaches were cultured on PDA medium for 5 days, and then suspended in 5 mL of sterile water containing 0.05% (v/v) Tween-80. Thereafter, the spore suspensions were filtered through 0.22 mm Millipore membranes to remove any adhering mycelia. The concentration of the spore suspension was determined using a hemacytometer, and then, the final concentration was adjusted to 1 × 106 CFU mL−1 [49].
4.3. Fruit Preparation
For inoculum production, the experiments were conducted with the peach (Prunus persica L.) fruit cultivars Baifeng. The fresh fruits (pH = 3.53–3.64) were collected one week before commercial harvest during the 2012 production season, and the mature fruits (pH = 3.75–3.98) were collected and harvested at the mature stage, and sorted based on the size and the absence of physical injuries or disease infection. Before treatments, fruits were disinfected on the surface with 2% (v/v) NaClO for 3 min, and then rinsed with sterile water and air-dried for approximately 30 min at room temperature (20 °C) prior to use [50] and inoculation.
4.4. Pathogenicity of the Isolates on Peach Fruits
All the isolates in the present study were tested for pathogenicity on the mature peach fruits. Two groups of treatments were designed in this experiment, (i) one group of the sterile fruits were wounded once to a depth of 3 mm with a sterilized needle in the equatorial zone (wounded fruits) and (ii) another group of the sterile fruits were non-wounded with a sterilized needle (intact fruits). A 5-mm-diameter plug from a 5-day-old mycelial culture of isolates was inoculated onto intact and wounded peach fruits. Additionally, a 5-mm-diameter PDA plug was used as the untreated control treatment. Thereafter, all the treatments fruits were moist-incubated by placing in plastic containers with lids, lined with moist paper towels to maintain high relative humidity and incubated at room temperature (20 °C). Pathogenicity was determined as the ability to cause the typical decay symptom, and the number of fruit infected. The parameter of disease incidences was measured at 5 days after inoculation. Each experiment had three replications and each replication had three fruits, and all the experiments were repeated twice. The highest pathogenic pathogens were used to determine the antagonistic activity of Bacillus subtilis JK-14 in later experiments.
4.5. Formulations of Bacillus Subtilis JK-14 Preparation
The strain of B. subtilis JK-14 used in the present study was obtained from the College of Plant Protection, Gansu Agricultural University, isolated from the surface of peach fruits from an orchard in Gansu, China, and tested for its antifungal potential against the highest pathogenicity of the isolates on mature peach fruits. The active colony was then prepared by culturing on nutrient agar (NA, pH = 7.0) in Petri dishes for 3 days at 28 °C. A culture of B. subtilis JK-14 was obtained by transferring a colony from the activated culture plate into a 150 mL flask containing 30 mL liquid broth (peptone 0.3 g, yeast extract 0.3 g, NaCl 0.05 g) and shaking in an orbital shaker (200 rpm min−1) at 28 °C for 48 h. A formulation of fermentation liquid with bacterial cells (FLBC) was made by incubating the bacterial culture under the same conditions, and then dissolved with the sterile water to prepare the final concentrations of FLBC from 1 × 105 to 1 × 109 CFU mL−1. A formulation of bacterial cell suspension (BCS) was prepared by centrifuging the fermentation liquid at 12, 000 rpm min−1 at 4 °C for 20 min, and filtered by 0.22 µm biofilter to collect the bacterial sediment. Thereafter, the bacterial sediment was washed with an equal volume of saline (0.85% NaCl), and then dissolved with the sterile water to prepare the final concentrations of BCS from 1 × 105 to 1 × 109 CFU mL−1. The two formulations were stored at 4 °C for later use.
4.6. In Vitro and in Vivo Antagonistic Activity Determination
In vitro experiments, the antagonistic activity of B. subtilis JK-14 against the main pathogens, were conducted following dual culture plate technique [51]. The inhibitory effects of B. subtilis JK-14 on the isolates with the highest pathogenicity were done by examining the growth rates inhibition using the paper–disc method on PDA [52,53]. Each experiment had six replications and was repeated twice.
For the confirmation of the antagonistic activity of B. subtilis JK-14 (BCS formulation) in controlling A. tenuis and B. cinerea decay in fresh peach wounds, the fruits experiments were conducted to determine the controlling effects in vivo. A uniform wound (3 mm diameter and 3 mm deep) was made at the equator of each peach fruit using sterilized needle. An aliquot (30 µL) of B. subtilis JK-14 at 1 × 108 CFU mL−1 was pipetted into each wound site, and 30 µL of sterile water in place of the B. subtilis JK-14 was used as the control. Two hours later, 15 µL spores suspension of A. tenuis and B. cinerea (1 × 106 CFU mL−1) were inoculated into each wound, respectively. After air drying, the peaches were stored in enclosed plastic containers to maintain a high relative humidity (RH 85%) at 20 °C. Disease incidences and lesion diameters, and the symptoms of the treated peach fruits were measured and observed at 5 days after inoculation. All treatments were carried out with three replicates and three fruits for each treatment, and the experiment was conducted twice.
4.7. Efficacy of Bacillus subtilis JK-14 in Controlling of Peach Postharvest Disease
For the fruits inoculation, peach fruit samples were treated as described above to determine the antagonistic activity of B. subtilis JK-14 formulations (FLBC and BCS) in inhibiting A. tenuis and B. cinerea decay in mature peach wounds in vivo. An aliquot (30 µL) of different formulations of B. subtilis JK-14 at 1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 CFU mL−1 was pipetted into each wound site, and 30 µL of sterile water in place of the B. subtilis JK-14 formulations was used as the control. Two hours later, 15 µL spores suspension of A. tenuis and B. cinerea (1 × 106 CFU mL−1) were inoculated into each wound, respectively. After air drying, the incubation condition of treated peaches as described above. Disease incidences and lesion diameters, and the symptoms of the treated mature peach fruits were measured and observed at 5 days after inoculation. All treatments were carried out with three replicates and three fruits for each treatment, and the experiment was conducted twice.
4.8. Effects of Bacillus subtilis JK-14 on the Activities of Defense-Related Enzymes of Peaches
Peach fruit samples were treated as described above to test the efficacy of B. subtilis JK-14 in inhibiting A. tenuis and B. cinerea decay in mature peach wounds. The wounds were then treated with 30 µL of BCS of B. subtilis JK-14 at 1 × 107 CFU mL−1, and 30 µL of sterile water in place of the BCS formulation of B. subtilis JK-14 was used as the control. Two hours later, 15 µL spores suspension of the highest pathogenicity of the isolates of A. tenuis and B. cinerea (1 × 106 CFU mL−1) were inoculated into each wound. The treatments of sterile water and B. subtilis JK-14 alone were considered as the controls. The peach fruits were stored in enclosed plastic containers to maintain a high relative humidity (RH 85%) and incubated at 20 °C after air drying. In order to measure the activities of defense-related enzymes of peaches after the treatment of B. subtilis JK-14, the tissue surrounding each wound of fruit was collected at Day 4 after treatment. Three replicates consistent of three fruits were sampled in both inoculated group and control group, and the experiments were conducted twice.
4.9. Determination and Analysis of Defense-Related Enzyme Activities of Peaches
The extraction procedures of the enzyme extract from the collected samples were conducted following the method of Xu et al. [7]. The tissue surrounding each wound of fruits (2 g) were collected and homogenized with 4 mL of ice-cold sodium phosphate buffer (50 mM, pH 7.8) containing 1.33 mM EDTA and 1% PVP. Thereafter, the homogenates were then centrifuged at 12,000× g for 15 min at 4 °C, and the supernatants were collected and used as enzyme extract to assay the activity of POD, SOD and CAT of peaches after extraction using the spectrophotometer (AOE (UV1900), Shanghai, China).
POD activity was assayed following the method of Meng et al., with some minor modifications [54]. The reaction mixture containing 0.2 mL of the enzyme extract and 2.2 mL of 0.3% guaiacol was incubated for 5 min at 30 °C, and then the reaction was initiated immediately by adding 0.6 mL of 0.3% H2O2. The activity of POD was determined by measuring absorbance at 470 nm, and expressed as U per g fresh weight (U g−1 FW−1).
SOD activity was measured following the method of Giannopolitis and Ries, and determined by assaying the ability to inhibit the photochemical reduction of nitroblue tetrazolium chloride (NBT) [55]. The reaction mixture (1.5 mL) contained 50 mM phosphate buffer (pH 7.8), 0.1 µM EDTA, 13 mM methionine, 75 µM NBT, 2 µM riboflavin and 50 µL enzyme extracts. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the NBT photo reduction rate, and the results were expressed as U g−1 FW−1.
CAT activity was measured according to the method described by Wang et al. [56], with some modifications. The reaction mixtures contained 1.4 mL buffered substrate (50 mM sodium phosphate, pH 7.8, and 30 mM H2O2) and 100 µL of enzyme extracts. The decomposition of H2O2 was measured by the decline in absorbance at 240 nm. One unit of the CAT activity was defined as the amount of the H2O2 decomposing, and the activity was expressed as U g−1 FW−1.
4.10. Statistical Analysis
Data presented in the present paper were pooled across two independent repeated experiments. All statistical analyses were performed with SPSS version 16.0 (SPSS Inc., Chicago, IL, USA, 2007). Data were analyzed by multi-way ANOVA. Duncan’s multiple range test were computed using standard error and T values of adjusted degrees of freedom. Differences at p < 0.05 were considered significant.
Acknowledgments
We appreciate the critical proof reading by Jing-Jiang Zhou of JJ Scientific Consultant Ltd, UK and Rothamsted Research, UK.
Author Contributions
Experiment design, S.Z., Q.Z. and B.X.; Methodology, S.Z. and B.X.; Data analysis, S.Z. and J.L.; Writing-original draft preparation, S.Z.; Writing-review and editing, S.Z., Q.Z. and B.X; Funding acquisition, S.Z.; Project administration, S.Z. and B.X.
Funding
This work was funded by Special Funds for Discipline Construction [GAU-XKJS-2018-147]; Scientific Research Start-up Funds for Openly-recruited Doctors [2017RCZX-07]; Research Program Sponsored by Gansu Provincial Key Laboratory of Aridland Crop Science, Gansu Agricultural University [GSCS-2017-1]; Special Funds for Discipline Construction [GAU-XKJS-2018-145]; National Natural Science Foundation of China [31860526]; Gansu Provincial Science Fund for Distinguished Young Scholars [18JR3RA161]; Gansu Provincial system of Modern fruit industry [GARS-SG-2]; International Scientific and Technological Cooperation of Gansu Province [1604WKCA010] and Hall of Gansu Province Farming Herd Biology Technology [GNSW-2013-19].
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
The isolates of Alternaria tenuis and Botrytis cinerea were discovered and identified as the main pathogens that cause the postharvest peach diseases. Bacillus subtilis JK-14 exhibits a high efficacy in controlling postharvest disease of peaches, and the possible mechanisms are due to the direct effect by inhibiting the fungal growth. There is an indirect effect by activating the antioxidative defense system in peaches to enhance the resistance of response to the pathogens infection.
References
- 1.Kader A.A. Increasing food availability by reducing postharvest losses of fresh produce. Int. Postharvest Symp. Acta Hort. 2005;682:2169–2178. doi: 10.17660/ActaHortic.2005.682.296. [DOI] [Google Scholar]
- 2.Parfitt J., Barthel M., Macnaughton S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. 2010;365:3065–3081. doi: 10.1098/rstb.2010.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Agriculture Organization of the United Nations . Global Food Losses and Food Waste: Extent, Causes and Prevention. FAO; Rome, Italy: 2011. [Google Scholar]
- 4.Liu H.X., Jiang W.B., Bi Y., Luo Y.B. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005;35:263–269. doi: 10.1016/j.postharvbio.2004.08.006. [DOI] [Google Scholar]
- 5.Karabulut O.A., Baykal N. Integrated control of postharvest diseases of peaches with a yeast antagonist, hot water and modified atmosphere packaging. Crop Prot. 2004;23:431–435. doi: 10.1016/j.cropro.2003.09.012. [DOI] [Google Scholar]
- 6.Gatto M.A., Ippolito A., Linsalata V., Cascarano N.A., Nigro F., Vanadia S., Di Venere D. Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol. Technol. 2011;61:72–82. doi: 10.1016/j.postharvbio.2011.02.005. [DOI] [Google Scholar]
- 7.Xu B.T., Zhang H.Y., Chen K.P., Xu Q., Yao Y., Gao H. Biocontrol of postharvest Rhizopus decay of peaches with Pichia caribbica. Curr. Microbiol. 2013;67:255–261. doi: 10.1007/s00284-013-0359-9. [DOI] [PubMed] [Google Scholar]
- 8.Karabulut O.A., Cohen L., Wiess B., Daus A., Lurie S., Droby S. Control of brown rot and blue mold of peach and nectarine by short hot water brushing and yeast antagonists. Postharvest Biol. Technol. 2002;24:103–111. doi: 10.1016/S0925-5214(01)00132-6. [DOI] [Google Scholar]
- 9.Vitoratos A., Dimitrios B., Karkanis A., Efthimiadou A. Antifungal activity of plant essential oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Hort. Agrobot. 2013;41:86–92. doi: 10.15835/nbha4118931. [DOI] [Google Scholar]
- 10.Spadaro D., Droby S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Tech. 2016;47:39–49. doi: 10.1016/j.tifs.2015.11.003. [DOI] [Google Scholar]
- 11.Arrebola E., Sivakumar D., Bacigalupo R., Korsten L. Combined application of antagonist Bacillus amyloliquefaciens and essential oils for the control of peach postharvest diseases. Crop Prot. 2010;29:369–377. doi: 10.1016/j.cropro.2009.08.001. [DOI] [Google Scholar]
- 12.Mari M., Bautista-Banos S., Sivakumar D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016;122:70–81. doi: 10.1016/j.postharvbio.2016.04.014. [DOI] [Google Scholar]
- 13.Usall J., Casals C., Sisquella M., Palou L., de Cal A. Alternative technologies to control postharvest diseases of stone fruits. Stewart Postharvest Rev. 2015;11:1–6. [Google Scholar]
- 14.Usall J., Torres R., Vias I., Abadias M., Teixidó N. Poscosecha de pera, manzana y melocotón. Mundiprensa; Madrid, Spain: 2013. Principales enfermedades de postcosecha y su control; pp. 247–280. [Google Scholar]
- 15.Sharma R., Singh D., Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control. 2009;50:205–221. doi: 10.1016/j.biocontrol.2009.05.001. [DOI] [Google Scholar]
- 16.Wisniewski M., Droby S., Norelli J., Liu J., Schena L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016;122:3–10. doi: 10.1016/j.postharvbio.2016.05.012. [DOI] [Google Scholar]
- 17.Holmes G.J., Eckert J.W. Sensitivity of Penicillium digitatum and P. italicum to postharvest citrus fungicides in California. Phytopathology. 1999;89:716–721. doi: 10.1094/PHYTO.1999.89.9.716. [DOI] [PubMed] [Google Scholar]
- 18.Sansone G., Rezza I., Calvente V., Benuzzi D., Tosetti M.I.S. Control of Botrytis cinerea strains resistant to iprodione in apple with rhodotorulic acid and yeasts. Postharvest Biol. Technol. 2005;35:245–251. doi: 10.1016/j.postharvbio.2004.09.005. [DOI] [Google Scholar]
- 19.Adaskaveg J.E., Michailides T.J. Cancellation of postharvest use of Rovral 50WP on stone fruit crops and other label changes of the fungicide. Cent. Vall. Postharvest Newslett. 1996;5:9–10. [Google Scholar]
- 20.He D., Zheng X.D., Yin Y.M., Sun P., Zhang H.Y. Yeast application for controlling apple postharvest diseases associated with Penicillium expansum. Bot. Bull Acad. Sinica. 2003;44:211–216. [Google Scholar]
- 21.Hong C.X., Michailides T.J., Holtz B.A. Effects of wounding, inoculum density and biological control agents on postharvest brown rot of stone fruits. Plant Dis. 1998;82:1210–1216. doi: 10.1094/PDIS.1998.82.11.1210. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Reyes J.G., Spadaro D., Prelle A., Garibaldi A., Gullino M.L. Efficacy of plant essential oils on postharvest control of rots caused by fungi on different stone fruits in vivo. J. Food Prot. 2013;76:631–639. doi: 10.4315/0362-028X.JFP-12-342. [DOI] [PubMed] [Google Scholar]
- 23.Droby S., Wisniewski M., Macarisin D., Wilson C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm. Postharvest Biol. Technol. 2009;52:137–145. doi: 10.1016/j.postharvbio.2008.11.009. [DOI] [Google Scholar]
- 24.Romanazzi G., Lichter A., Mlikota Gabler F., Smilanick J.L. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012;63:141–147. doi: 10.1016/j.postharvbio.2011.06.013. [DOI] [Google Scholar]
- 25.Janisiewicz W.J., Korsten L. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002;40:411–441. doi: 10.1146/annurev.phyto.40.120401.130158. [DOI] [PubMed] [Google Scholar]
- 26.Cavaglieri L., Orlando J., Rodriguez M.I., Chulze S., Etcheverry M. Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res. Microbiol. 2005;156:748–754. doi: 10.1016/j.resmic.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 27.De Jensen C.D., Percich J.A., Graham P.H. Integrated management strategies of bean root rot with Bacillus subtilis and Rhizobium in Minnesota. Field Crop Res. 2002;74:107–115. doi: 10.1016/S0378-4290(01)00200-3. [DOI] [Google Scholar]
- 28.Sharma N., Sharma S. Control of foliar diseases of mustard by Bacillus from reclaimed soil. Microbiol. Res. 2008;163:408–413. doi: 10.1016/j.micres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Scherm H., Ngugi H.K., Savelle A.T., Edwards J.R. Biological control of infection of blueberry flowers caused by Monilinia vaccinii-corymbosi. Biol. Control. 2004;29:199–206. doi: 10.1016/S1049-9644(03)00154-3. [DOI] [Google Scholar]
- 30.Acea M.J., Moore C.R., Alexander M. Survival and growth of bacteria introduced into soil. Soil Biol. Biochem. 1988;20:509–515. doi: 10.1016/0038-0717(88)90066-1. [DOI] [Google Scholar]
- 31.Lurie S., Crisosto C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005;37:195–208. doi: 10.1016/j.postharvbio.2005.04.012. [DOI] [Google Scholar]
- 32.Vreeland R.H., Rosenzweig W.D., Powers D.W. Isolation of a 250 million-year-old halo tolerant bacterium from a primary salt crystal. Nature. 2000;407:897–900. doi: 10.1038/35038060. [DOI] [PubMed] [Google Scholar]
- 33.Lee S.C., Yoo J.S., Kim S.H., Chung S.Y., Hwang C.W., Joo C.H., Choi Y.L. Production and characterization of lipopeptide biosurfactant from Bacillus subtilis A8-8. J. Microbiol. Biotechnol. 2006;16:716–723. [Google Scholar]
- 34.Snowdon A.L. Colour Atlas of Postharvest-Harvest Diseases and Disorders of Fruit and Vegetables, Volume 1: General Introduction and Fruits. Manson Publishing Ltd.; London, UK: 2010. [Google Scholar]
- 35.Hong C.X., Michailides T.J. Prune, plum, and nectarine as hosts of Trichothecium roseum in California orchards. Plant Dis. 1997;81:112. doi: 10.1094/PDIS.1997.81.1.112D. [DOI] [PubMed] [Google Scholar]
- 36.Mclaughlin R.J., Wilson C.L., Droby S., Benarie R., Chalutz E. Biological control of postharvest diseases of grape, peach and apple with the yeasts kloeckera-apiculata and candida-guilliermondii. Plant Dis. 1992;76:470–473. doi: 10.1094/PD-76-0470. [DOI] [Google Scholar]
- 37.De Cal A., Sandín-Espana P., Martinez F., Egüen B., Chien-Ming C., Lee M.H., Melgarejo P., Prusky D. Role of gluconic acid and pH modulation in virulence of Monilinia fructicola on peach fruit. Postharvest Biol. Technol. 2013;86:418–423. doi: 10.1016/j.postharvbio.2013.07.012. [DOI] [Google Scholar]
- 38.Gell I., De Cal A., Torres R., Usall J., Melgarejo P. Relationship between the incidence of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: Factors affecting latent infection. Eur. J. Plant Pathol. 2008;121:487–498. doi: 10.1007/s10658-008-9268-3. [DOI] [Google Scholar]
- 39.Fan Q., Tian S.P. Postharvest biological control of Rhizopus rot of nectarine fruits by Pichia membranefaciens. Plant Dis. 2000;84:1212–1216. doi: 10.1094/PDIS.2000.84.11.1212. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H.Y., Wang L., Dong Y., Jiang S. Postharvest biological control of gray mold decay of strawberry with Rhodotorula glutinis. Biol. Control. 2007;40:287–292. doi: 10.1016/j.biocontrol.2006.10.008. [DOI] [Google Scholar]
- 41.Zhang H.Y., Yang Q.Y., Ge L.L., Zhang G.C., Zhang X.L., Zhang X.Y. Chitin enhances biocontrol of Rhodotorula mucilaginosa to postharvest decay of peaches. Int. J. Biol. Macromol. 2016;88:465–475. doi: 10.1016/j.ijbiomac.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Noble R., Coventry E. Suppression of soil-borne plant diseases with composts: A review. Biocontrol Sci. Technol. 2005;15:3–20. doi: 10.1080/09583150400015904. [DOI] [Google Scholar]
- 43.Jacobsen A.M., Hallingsørensen B., Ingerslev F., Hansen S.H. Simultaneous extraction of tetracycline, macrolide and sulfonamide antibiotics from agricultural soils using pressurised liquid extraction, followed by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2004;1038:157–170. doi: 10.1016/j.chroma.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 44.Zhou T., Schneider K.E., Li X.Z. Development of biocontrol agents from food microbial isolates for controlling post-harvest peach brown rot caused by Monilinia fructicola. Int. J. Food Microbiol. 2008;126:180–185. doi: 10.1016/j.ijfoodmicro.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Abdallah B., Stedel R.A., Garagounis C., Nefzi C., Jabnoun-Khiareddine A., Papadopoulou H., Daami-Remadi K.K. Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Prot. 2017;99:45–58. doi: 10.1016/j.cropro.2017.05.008. [DOI] [Google Scholar]
- 46.Fu L., Penton C.R., Ruan Y. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017;104:39–48. doi: 10.1016/j.soilbio.2016.10.008. [DOI] [Google Scholar]
- 47.Wu B., Wang X., Yang L. Effects of Bacillus amyloliquefaciens ZM9 on bacterial wilt and rhizosphere microbial communities of tobacco. Appl. Soil Ecol. 2016;103:1–12. doi: 10.1016/j.apsoil.2016.03.002. [DOI] [Google Scholar]
- 48.Zheng X.L., Tian S.P., Meng X.H., Li B.Q. Physiological and biochemical responses in peach fruit to oxalic acid treatment during storage at room temperature. Food Chem. 2007;45:281–284. doi: 10.1016/j.foodchem.2006.11.015. [DOI] [Google Scholar]
- 49.Zhang S.W., Gan Y.T., Xu B.L., Xue Y.Y. The parasitic and lethal effects of Trichoderma longibrachiatum against Heterodera avenae. Biol. Control. 2014;72:1–8. doi: 10.1016/j.biocontrol.2014.01.009. [DOI] [Google Scholar]
- 50.Liu J., Tian S.P., Meng X.H., Xu Y. Control effects of chitosan on postharvest diseases and physiological response of tomato fruit. Postharvest Biol. Tech. 2007;44:300–306. doi: 10.1016/j.postharvbio.2006.12.019. [DOI] [Google Scholar]
- 51.Bell D.K., Wells H.D., Markham C.R. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology. 1982;72:379–382. doi: 10.1094/Phyto-72-379. [DOI] [Google Scholar]
- 52.Liu B., Zhou J. Effect study on isolation and screening of antagonistic actinomycetes in soil. Mod. Agric. Technol. 2013;17:225–228. [Google Scholar]
- 53.Dasari V., Nikku M.Y., Donthireddy S.R.R. Screening of antagonistic marine actinomycetes: Optimization of process parameters for the production of novel antibiotic by Amycolatopsis alba var. nov. DVR D4. J. Antimicrob. Chemother. 2011;3:92–98. [Google Scholar]
- 54.Meng X.H., Li B.Q., Liu J., Tian S.P. Physiological responses and quality attributes of table grape fruit to chitosan postharvest spray and postharvest coating during storage. Food Chem. 2008;106:501–508. doi: 10.1016/j.foodchem.2007.06.012. [DOI] [Google Scholar]
- 55.Giannopolitis C.N., Ries S.K. Superoxide dismutases: I. occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y.S., Tian S.P., Xu Y., Qin G.Z., Yao H.J. Changes in the activities of protein and antioxidant enzymes in peach fruit inoculated with Cryptococcus laurentii or Penicillium expansum at 0 or 20 °C. Postharvest Biol. Tech. 2004;34:21–28. doi: 10.1016/j.postharvbio.2004.04.003. [DOI] [Google Scholar]