Abstract
Tetrodotoxin (TTX) is a potent alkaloid typically from tropical ecosystems, but in the last decade its presence has been more pronounced in the temperate waters of the Atlantic. In its last scientific opinion, the European Food Safety Authority (EFSA) stressed the need for data regarding TTX prevalence in European waters. To address EFSA’s concerns, benthic organisms such as mollusks, crustaceans, echinoderms and fish with different feeding habits were collected along the Portuguese continental coast, islands (São Miguel, Azores, and Madeira) and the northwestern Moroccan coast. A total of 165 samples were analyzed by ultra high performance liquid chromatography high resolution mass spectrometry (UHPLC-HRMS) and ultra high performance chromatography mass spectrometry (UHPLC-MS/MS). Geographical tendencies were detected as follows, by descending order: S. Miguel Island (Azores), Moroccan coast, Madeira Island and Portuguese continental coast. The toxin amounts detected were significant, above the Dutch limit value established in 2017, showing the importance and the need for continuity of these studies to gain more knowledge about the prevalence of these toxins, unraveling new vectors, in order to better assess human health risk. This work represents a general overview of new TTX bearers (7) most of them in gastropods (Patella depressa, Nucella lapillus, Onchidella celtica and Aplysia depilans), followed by echinoderms (Echinus esculentus and Ophidiaster ophidianus) and puffer fish Sphoeroides marmoratus.
Keywords: tetrodotoxin, new vectors, North Atlantic Waters
1. Introduction
Tetrodotoxin (TTX) is an extremely potent alkaloid typical from warm ecosystems that over recent years became more frequent in temperate waters [1,2,3]. Named after the Tetraodontidae puffer fish family where it was first isolated, TTX was later identified also in different taxa not close related (from bacteria; marine invertebrates; terrestrial and marine vertebrates) [4,5]. The ubiquity of TTX is due to its exogenous origin, bacteria from the different phyla (Proteobacteria, Firmicutes, Bacterioides and Actinobacteria) associated with dinoflagellate blooms of Prorocentrum have been pointed to as potential producers [6,7,8].
Chemically, TTX is a crystalline weakly basic heterocyclic molecule with six hydroxyl groups, a pyridine ring with additional fused ring systems and a guanidinium group, positively charged at a physiological pH, with a molecular formula of C11H17O8N3 (Table 1) [1]. This powerful alkaloid exhibits its action by binding selectively and extracellularly to receptor-site 1 of voltage-gated sodium channels (Navs), once it mimics the sodium hydrated cation, occluding the outer pore. Thus, avoiding the access of monovalent cations to the pore, the generation and propagation of the action potential is blocked, inhibiting the communication between electrically excitable cells in muscle and nerve tissues, causing paralysis which results in death by cardio-respiratory failure [9,10,11]. About 30 analogues have been described to date, whose toxicity varies with structure implying their greater or lesser affinity for their molecular target [12]. The rise of water temperature together with anthropological intervention aided the migration and establishment of this neurotoxin typically from tropical ecosystems, the Pacific and Indian oceans, into European waters, where it has become more prevalent in the last decade [13,14,15,16,17]. Regarding consumer protection in the EU, regulations 853/2004/EC and 854/2004/EC were published in 2004, preventing fish species reported as TTX-bearers (Tetraodontidae, Canthigasteridae, Molidae and Diodontidae) from market placement [18,19]; the Netherlands being the pioneer in including the scrutinizing of this toxin in its monitoring program, establishing 44 µg TTX equivalents/kg of shellfish as a safe concentration [20]. Still, more data are needed in order to better protect consumer health. In EFSA’s latest opinion it’s stated that more occurrence data on TTX and its derivatives in edible parts of gastropods and bivalves are necessary in order to complement a reliable exposure assessment, since it was not possible to include gastropods in the risk characterization due to insufficient studies [21]. The primary aim of this work was to screen the presence of TTX in different benthic marine species along the Portuguese continental and archipelagos and western Moroccan coasts, thus unraveling its prevalence and geographical trends. We hope to bridge and to contribute to EFSA’s recommendations, towards the development of effective and robust monitoring programs.
Table 1.
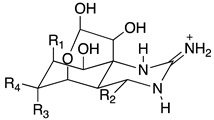
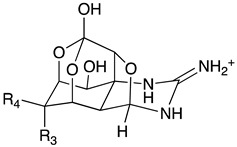
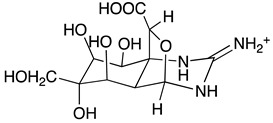
Tetrodotoxin and analogues molecular structure.
| Structure | Analogue | R1 | R2 | R3 | R4 | Molecular Formula | Exact Mass |
|---|---|---|---|---|---|---|---|
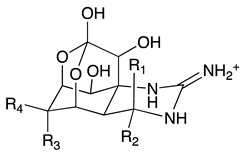
|
TTX | H | OH | OH | CH2OH | C11H17N3O8 | 320.1088 |
| 4-epiTTX | OH | H | OH | CH2OH | C11H17N3O8 | 320.1088 | |
| 6-epiTTX | H | OH | CH2OH | OH | C11H17N3O8 | 320.1088 | |
| 11-deoxyTTX | H | OH | OH | CH3 | C11H17N3O7 | 304.1139 | |
| 11-norTTX-6(R/S)-ol | H | OH | H | OH | C10H15N3O7 | 290.0983 | |
| 6,11-dideoxyTTX | H | OH | H | CH3 | C11H17N3O6 | 288.1190 | |
| 11-oxoTTX | H | OH | OH | CHO | C11H17N3O9 | 336.1038 | |
|
5-deoxyTTX | H | OH | OH | CH2OH | C11H17N3O7 | 304.1139 |
| 5,11-dideoxyTTX | H | OH | H | CH3 | C11H17N3O6 | 288.1190 | |
| 5,6,11-trideoxyTTX | H | OH | H | CH3 | C11H17N3O5 | 272.1214 | |
|
4,9-anhydroTTX | --- | --- | OH | CH2OH | C11H15N3O7 | 302.0983 |
| 6-epi-4,9-anhydroTTX | --- | --- | CH2OH | OH | C11H15N3O7 | 302.0983 | |
|
Tetrodonic acid | --- | --- | --- | --- | C11H17N3O8 | 320.1088 |
2. Results and Discussion
A total of 165 samples collected from 25 different sampling points (Figure 1) were screened using the techniques of ultra high performance chromatography mass spectrometry (UHPLC-MS/MS) and ultra high performance liquid chromatography high resolution mass spectrometry (UHPLC-HRMS) [8,22]. These two techniques are complementary when performing a complete and undoubted identification of TTX analogues when standards are not available. UHPLC-MS/MS in product ion scan (PIS) mode allowed to check the mass fragmentation pattern of this family of compounds and HRMS was used to obtain the exact mass of each compound. Finally, the positive samples were quantified by UHPLC-MS/MS in multiple reaction monitoring (MRM) mode using a TTX standard. About 30 species of edible, with commercial interest, and non-edible benthic organisms were collected in the intertidal zone and by Self-Contained Underwater Breathing Apparatus (SCUBA) diving, with the aim of searching new vectors and determining the prevalence of TTXs in the food web: bivalves (mussels), gastropods (limpets, sea-snails, sea-slugs), echinoderms (starfish, sea-urchins, sea-cucumbers), crustaceans (barnacles) and fish (puffer). Sampling points and collected species are described in detail in the Materials and Methods section.
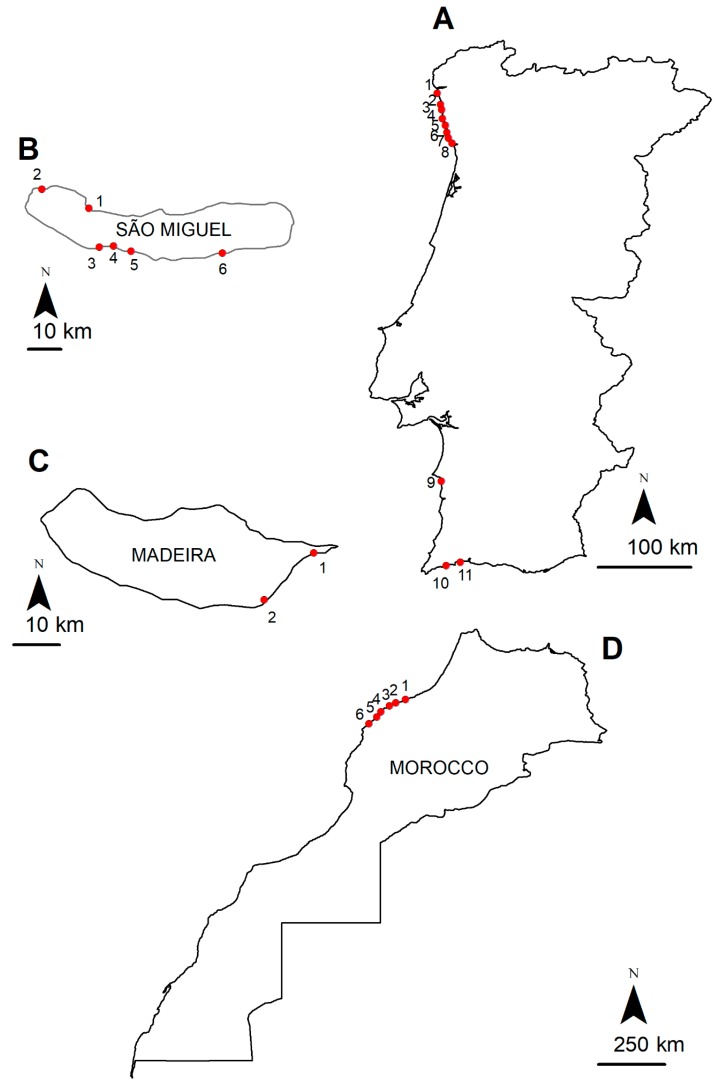
Figure 1.
Location of the sampling points: (A) Portuguese continental coast: 1, Esposende; 2, Póvoa do Varzim; 3, Angeiras; 4, Memória; 5, Pedra de Jacó; 6, Pêlo Negro; 7, Valadares; 8, Ovar; 9, Porto Côvo; 10, Camilo; 11, Luz. (B) Madeira Island coast: 1, Caniçal and 2, Reis Magos. (C) São Miguel Island coast, Azores archipelago: 1, Cruzeiro; 2, Mosteiros; 3, Étar de Ponta Delgada; 4, São Roque; 5, Lagoa; 6, Caloura. (D) Northwestern Moroccan coast: 1, Casablanca Corniche; 2, El Jadida Haras; 3, El Jadida Sâada; 4, Sidi Bouzid; 5, Mrizika; 6, Oualidia.
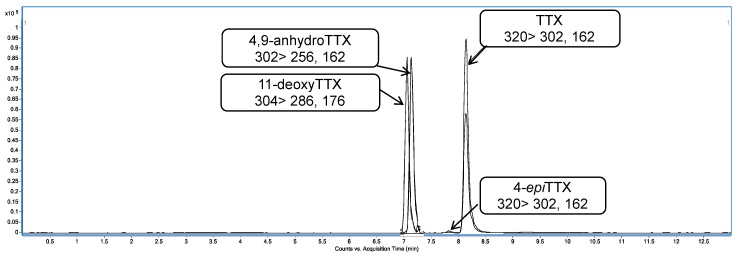
In the present study, a variety of samples from different animals were analyzed using the UHPLC-MS/MS method after the extraction procedure. Initially, samples were analyzed in MRM mode for 18 TTX analogues described in the literature [1] and some compounds of this family were detected in several samples. This preliminary analysis showed many positive samples for TTX plus analogues. One of the great challenges of the emerging toxins is the lack of reference material, as far as TTX is concerned, therefore only the analogues present in the standard (TTX, 4,9-anhydroTTX, 4-epiTTX and 11-deoxyTTX) could be confirmed (Figure 2). However, all positive extracts presented several TTX analogues. To confirm these positive results, their mass fragmentation pattern was studied [8,23,24]. The fragmentation pathway of TTX standard shows two losses of water and m/z 256, m/z 178 and m/z 162 as product ions (Figure 3) [8,24,25].
Figure 2.
Multiple reaction monitoring (MRM) chromatogram obtained in positive mode of tetrodotoxin (TTX) standard.
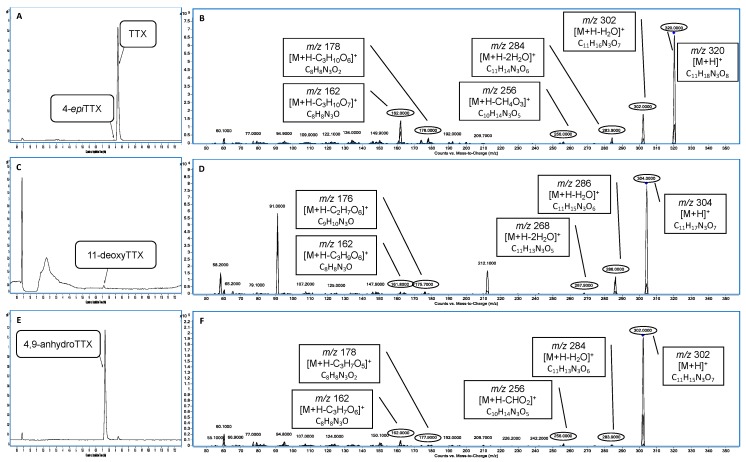
Figure 3.
Chromatogram and mass spectrum of TTX standard. (A) Chromatogram of TTX and 4-epiTTX obtained by PIS. (B) Mass fragmentation pattern of TTX and 4-epiTTX. (C) Chromatogram of 11-deoxyTTX obtained by PIS. (D) Mass fragmentation pattern of 11-deoxyTTX. (E) Chromatogram of 4,9-anhydroTTX obtained by PIS. (F) Mass fragmentation pattern of 4,9-anhydroTTX.
In this way, TTXs analogues were confirmed in several samples. As shown in Table 2, a loss of water and m/z 178 (or 176) and m/z 162 are the product ions of TTX analogues detected in positive samples.
Table 2.
TTXs analogues present in positive samples analyzed by MS/MS.
| Suspicious TTXs | Multiple Reaction Monitoring (MRM) | Product Ions Scan (PIS) | |||
|---|---|---|---|---|---|
| Precursor Ion | Product Ions | [M+H]+ | [M+H-H2O]+ | Other Product Ions | |
| oxoTTX | 336 | 318/162 | 336 | 318 | 300/176/162 |
| TTX and epimers | 320 | 302/162 | 320 | 302 | 284/256/178/162 |
| deoxyTTX | 304 | 286/162 | 304 | 286 | 268/176/162 |
| anhydroTTX | 302 | 256/162 | 302 | 284 | 256/178/162 |
| norTTX | 290 | 272/162 | 290 | 272 | 176/162 |
| dideoxyTTX | 288 | 270/224 | 288 | 270 | 224/178/162 |
| trideoxyTTX | 272 | 254/162 | 272 | 254 | 236/178/162 |
| anhydrotrideoxyTTX | 254 | 236/162 | 254 | 236 | 178/162 |
At the same time, the suspicious positive samples were also analyzed using High Resolution Mass Spectrometry (HRMS) to confirm and identify TTX analogues. The mass fragmentation pathway of TTX analogues has been reported in many LC-HRMS studies [8,26]. In the current study the exact mass of TTX analogues was determined by HRMS using the formula predictor software to characterize and predict the molecular formula of these unknown compounds. In this way, elemental composition, theoretical value m/z, experimental m/z and mass errors in mDa for TTX analogues were obtained after MS1 spectra (Table 3, Table 4 and Table 5).
Table 3.
Sample information from continental Portugal. TTXs analogues in positive samples analyzed by high resolution mass spectrometry (HRMS) and quantified by MRM mode.
| Sampling Date | Sampling Site | Sample | Species | Trophic Level [Ref] | Edible | Code | Suspicious TTXs | RT (min) | Elemental Composition | Theoretical m/z | Experimental m/z | Error (mDa) | TTX eq (µg/Kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| September 2011 | Porto Côvo | Limpet | Patella depressa | Grazer [27] | Yes | 225 | 11-oxoTTX | 7.2 | C11H17N3O9 | 336.1038 | 336.1000 | −3.8 | <LOQ |
| Gastropod | Phorcus lineatus | Grazer [28] | Yes | 226 | trideoxyTTX | 7.7 | C11H17N3O5 | 272.1241 | 272.1232 | −0.9 | <LOQ | ||
| October 2011 | Pedra de Jacó | Sea-urchin | Echinus esculentus | Grazer [29] | Yes | 229_2 | 11-oxoTTX | 7.2 | C11H17N3O9 | 336.1038 | 336.1020 | −1.8 | <LOQ |
| Ovar | Gastropod | Charonia lampas | 3rd Level Predator [30] | Yes | 232_2 | trideoxyTTX | 7.7 | C11H17N3O5 | 272.1241 | 272.1230 | −1.1 | <LOQ | |
| 232_3 | trideoxyTTX | 7.7 | C11H17N3O5 | 272.1241 | 272.1251 | 1.0 | <LOQ | ||||||
| November 2011 | Memória | Gastropod | Nucella lapillus | 1st Level Predator [27] | Yes | 236 | trideoxyTTX | 7.7 | C11H17N3O5 | 272.1241 | 272.1239 | −0.2 | <LOQ |
| Phorcus lineatus | Grazer [28] | Yes | 239 | 11-oxoTTX | 7.2 | C11H17N3O9 | 336.1038 | 336.1000 | −3.8 | <LOQ | |||
| Gibbula umbilicalis | Grazer [28] | Yes | 240 | trideoxyTTX | 7.7 | C11H17N3O5 | 272.1241 | 272.1238 | −1.1 | <LOQ |
Table 4.
Sample information São Miguel, Azores archipelago. TTXs analogues in positive samples analyzed by HRMS and quantified by MRM mode.
| Sampling Date | Sampling Site | Sample | Species | Trophic Level [Ref] | Edible | Code | Standard TTX | Suspicious TTXs | RT (min) | Elemental Composition | Theoretical m/z | Experimental m/z | Error (mDa) | TTX eq (µg/Kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| June 2013 | Lagoa | Starfish | Ophidiaster ophidianus | Detritivorous [32] | No | 412 | norTTX | 3.1 | C10H15N3O7 | 290.0983 | 290.2489 | 150.6 | 30,902 | |
| norTTX | 4.6 | C10H15N3O7 | 290.0983 | 290.2303 | 132.0 | 699 | ||||||||
| June 2013 | Ilhéu de São Roque | Starfish | Ophidiaster ophidianus | Detritivorous [32] | No | 424 | norTTX | 3.1 | C10H15N3O7 | 290.0983 | 290.2475 | 149.2 | 42,604 | |
| norTTX | 4.6 | C10H15N3O7 | 290.0983 | 290.2298 | 131.5 | 1638 | ||||||||
| June 2013 | Caloura | Starfish | Ophidiaster ophidianus | Detritivorous [32] | No | 435 | norTTX | 3.1 | C10H15N3O7 | 290.0983 | 290.2478 | 149.9 | 20,853 | |
| June 2013 | Caloura | Fish (Gonads) | Sphoeroides marmoratus | 3rd Level Predator [33] | Yes | 438 G | TTX | 8.1 | C11H17N3O8 | 320.1088 | 320.1088 | 0.0 | 352,886 | |
| 4-epiTTX | 7.8 | C11H17N3O8 | 320.1088 | 320.1082 | −0.6 | 3157 | ||||||||
| deoxyTTX | 7.7 | C11H17N3O7 | 304.1139 | 304.1129 | −1.0 | 446 | ||||||||
| 11-deoxyTTX | 7.0 | C11H17N3O7 | 304.1139 | 304.1126 | −1.3 | 157,156 | ||||||||
| deoxyTTX | 6.5 | C11H17N3O7 | 304.1139 | 304.1124 | −1.5 | 108,375 | ||||||||
| 4,9-anhydroTTX | 7.1 | C11H15N3O7 | 302.0983 | 302.1005 | 2.2 | 91 | ||||||||
| dideoxyTTX | 6.4 | C11H17N3O6 | 288.1190 | 288.1157 | −3.3 | 22,232 | ||||||||
| trideoxyTTX | 5.0 | C11H17N3O5 | 272.1241 | 272.1198 | −4.3 | 12,708 | ||||||||
| trideoxyTTX | 4.8 | C11H17N3O5 | 272.1241 | 272.1228 | −1.3 | 37,367 | ||||||||
| trideoxyTTX | 4.6 | C11H17N3O5 | 272.1241 | 272.1227 | −1.4 | 1849 | ||||||||
| anhydrotrideoxyTTX | 4.7 | C11H15N3O4 | 254.1135 | 254.1122 | −1.3 | 364 | ||||||||
| June 2013 | Caloura | Fish (Liver) | Sphoeroides marmoratus | 3rd Level Predator [33] | Yes | 438 F | TTX | 8.1 | C11H17N3O8 | 320.1088 | 320.1098 | 1.0 | 41 | |
| 4-epiTTX | 7.8 | C11H17N3O8 | 320.1088 | nd | 6 | |||||||||
| 11-deoxyTTX | 7.0 | C11H17N3O7 | 304.1139 | nd | <LOQ | |||||||||
| deoxyTTX | 6.9 | C11H17N3O7 | 304.1139 | nd | 4 | |||||||||
| deoxyTTX | 6.5 | C11H17N3O7 | 304.1139 | 304.1153 | 1.4 | 136 | ||||||||
| 4,9-anhydroTTX | 7.1 | C11H15N3O7 | 302.0983 | nd | <LOQ | |||||||||
| dideoxyTTX | 6.4 | C11H17N3O6 | 288.1190 | nd | 283 | |||||||||
| trideoxyTTX | 5.0 | C11H17N3O5 | 272.1241 | nd | 31 | |||||||||
| trideoxyTTX | 4.8 | C11H17N3O5 | 272.1241 | 272.1263 | 2.2 | 250 | ||||||||
| trideoxyTTX | 4.6 | C11H17N3O5 | 272.1241 | nd | 14 | |||||||||
| June 2013 | Caloura | Fish (Muscle) | Sphoeroides marmoratus | 3rd Level Predator [33] | Yes | 438 M | TTX | 8.1 | C11H17N3O8 | 320.1088 | 320.1088 | 0.0 | 9918 | |
| 4-epiTTX | 7.8 | C11H17N3O8 | 320.1088 | 320.1067 | −2.1 | 118 | ||||||||
| deoxyTTX | 7.7 | C11H17N3O7 | 304.1139 | 304.1164 | 2.5 | 29 | ||||||||
| 11-deoxyTTX | 7.0 | C11H17N3O7 | 304.1139 | 304.1172 | 3.3 | 48 | ||||||||
| deoxyTTX | 6.9 | C11H17N3O7 | 304.1139 | 304.1123 | −1.6 | 65 | ||||||||
| deoxyTTX | 6.5 | C11H17N3O7 | 304.1139 | 304.1100 | −3.9 | 2085 | ||||||||
| 4,9-anhydroTTX | 7.1 | C11H15N3O7 | 302.0983 | 302.0958 | −2.5 | 45 | ||||||||
| dideoxyTTX | 6.4 | C11H17N3O6 | 288.1190 | 288.1145 | −4.5 | 2953 | ||||||||
| trideoxyTTX | 5.0 | C11H17N3O5 | 272.1241 | nd | 122 | |||||||||
| trideoxyTTX | 4.8 | C11H17N3O5 | 272.1241 | 272.1217 | −2.4 | 507 | ||||||||
| trideoxyTTX | 4.6 | C11H17N3O5 | 272.1241 | nd | 26 | |||||||||
| anhydrotrideoxyTTX | 4.7 | C11H15N3O4 | 254.1135 | 254.1129 | −0.6 | 5 |
nd—not detected.
Table 5.
Sample information from the Moroccan coast. TTXs analogues in positive samples analyzed by HRMS and quantified by MRM mode.
| Sampling Date | Sampling Site | Sample | Species | Trophic Level [Ref] | Edible | Code | Standard TTX | Suspicious TTXs | RT (min) | Elemental Composition | Theoretical m/z | Experimental m/z | Error (mDa) | TTX eq (µg/Kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| July 2013 | Mrizika | Gastropod | Onchidella celtica | Grazer [43] | No | 474 | TTX | 8.1 | C11H17N3O8 | 320.1088 | 320.1082 | −0.6 | 12 | |
| 4-epiTTX | 7.8 | C11H17N3O8 | 320.1088 | 320.1071 | 1.7 | 8 | ||||||||
| TTX epimer | 7.5 | C11H17N3O8 | 320.1088 | nd | 4 | |||||||||
| July 2013 | Oualidia | Gastropod | Aplysia depilans | Grazer [44] | No | 476 | TTX | 8.1 | C11H17N3O8 | 320.1088 | nd | <LOQ | ||
| 4-epiTTX | 7.8 | C11H17N3O8 | 320.1088 | nd | <LOQ |
nd—no detected.
Once the presence of TTX analogues was verified, the toxin amount was quantified using LC-MS/MS in MRM mode (Table 3, Table 4 and Table 5). In order to calculate the concentrations of TTXs, the calibration curve was done with TTX standard, assuming that related analogues would give a similar response of TTX. The quantification was done using MRM acquisition in positive mode. A seven-point calibration curve with range between 1.5 ng/mL–100 ng/mL was used (R2 = 0.999). The limit of detection (LOD) was 1.64 µg/Kg and the limit of quantification (LOQ) 5.46 µg/Kg.
2.1. Portuguese Continental Coast
In a total of 62 analyzed samples collected between September 2011 and January 2012 at the 11 sampling points (Figure 1), 8 positive results were detected, all below the LOQ (Table 3).
Regarding positive hits for TTXs, despite not quantifiable, we count three first reports of this group of toxins, two in gastropods, P. depressa and N. lapillus, for the analogs 11-oxoTTX and trideoxyTTX, respectively and one in echinoderms, E. esculentus positive for 11-oxoTTX. In our former work, the analogue trideoxyTTX was identified only in one sample of the species C. lampas [16]; in the present study, this analogue was the most common along the Portuguese coast, being present in five of seven positive organisms, all gastropods: P. lineatus, C. lampas, N. lapillus, G. umbilicalis.
The analogue m/z 336 was detected in P. depressa (225), E. esculentus (229_2) and P. lineatus (239). The fragmentation pattern of this molecule shows m/z 318, corresponding to [M+H−H2O]+, m/z 176 and m/z 162 [31]. After HRMS (Table 3) the analogue 11-oxoTTX can be proposed as m/z 336 with a small error (−3.8 and −1.8 mDa).
P. lineatus (226), C. lampas (232_2 and 232_3), N. lapillus (236) and G. umbilicalis (240) presented a TTX analogue with m/z 272 and a fragmentation pathway with loss of water, m/z 254, and the product ions m/z 178 and m/z 162 [24]. The TTX analogue with an elemental composition of C11H17N3O5 is suggested (error of 0.9–1.1 mDa) after HRMS analysis (Table 3).
Finally, the samples were quantified by MRM using TTX standard, assuming that the TTX analogues have a similar response. In this case, the quantification was not possible since the amount present in the samples was below the LOQ.
2.2. Madeira Island (Madeira Archipelago)
From a total of 23 samples harvested in two sampling points (Figure 1) during the summer of 2012, we reported zero positive hits for TTX and its derivatives.
2.3. São Miguel Island (Azores Archipelago)
São Miguel island, Azores, was screened during June 2013. From the 39 harvested samples along 6 sampling points (Figure 1), we detected 4 positive hits (Table 4).
Regarding toxin uptake, we found in São Miguel, Azores, a distinct toxin profile concerning positive taxa and detected toxins, also all detected values were above the current limit established in the Netherlands [20]. Starting with echinoderms, all positive samples were in the red velvet starfish, O. ophidianus, for an unknown TTX analogue since the molecule that follows TTX mass fragmentation pattern, further studies are needed. The sample of S. marmoratus was analyzed per tissue (muscle, gonads and liver), since the specimen belongs to the Tetraodontidae family, and there was sufficient extractable biomass. Discussing uptake by tissue, the values detected curiously increased in the following order: liver, muscle, gonads. Surprisingly, the liver had half the values detected in the muscle and ten times less than in the gonads, which deviates from the typical accumulation pattern for this family of fish [34,35]. However, a study performed in the United States of America, between 2002 and 2004, showed the same uptake pattern, for the saxitoxin group, in the species Sphoeroides nephelus, detecting concentrations in the muscle 5 to 20 fold higher in comparison to the liver [36]. Concerning toxic profile, all tissues were positive for TTX, 4-epiTTX, monodeoxyTTX, 4,9-anhydroTTX, dideoxyTTX, trideoxyTTX and anhydrotrideoxyTTX, being the later derivative not detected in the liver tissue.
In comparison with other works regarding TTX concentrations in starfish [37,38], though they are a distinct ecotype and species, the present measured concentrations are higher. Though chronologically distant from the Japanese studies, in the present work, the considerable detected amounts could be explained by the rise of water temperature. In a simplistic approach, it has been shown that seawater temperature warming correlates with the global warming phenomenon; it was also proved that TTX production has a positive relationship with water temperature [39]. Other studies concerning other emergent toxin, ciguatera, predict that global warming is expected to be the catalyst for the increment of the incidence and prevalence of poisoning cases [40,41]. From our first report in 2012 on this neurotoxin group in Portugal, Angeiras was the northernmost point of TTX in Europe [16]. The latest reports corroborate this trend, since the last work points to its presence in England [42]. It is noteworthy, that this is the first report of TTXs in both species: O. ophidianus and S. marmoratus.
Three samples of O. ophidianus presented two TTX analogues with m/z 290 and different retention times (3.1 min and 4.6 min). The fragmentation mass pattern of all of them showed a loss of water and m/z 176 and m/z 162 product ions, as TTX. When these samples were analyzed using HRMS, a high error was observed compared to the 11-norTTX theoretical mass. Therefore, it can be concluded that these two TTX analogues have other elemental compositions different from 11-norTTX. Being aware of the error, and since it is in the same species, it could be due to matrix composition and the feeding habits of this particular species [32]. Further studies are needed.
The female fish, S. marmoratus, was divided in three different samples, gonads (438 G), liver (438 F), and muscle (438 M). Twelve analogues were detected in these samples, m/z 320 (TTX and 4-epiTTX), m/z 304 (11-deoxyTTX and other three deoxyTTX analogues), m/z 302 (4,9-anhydroTTX), m/z 288, m/z 272 (three analogues) and m/z 254. The fragmentation pathway of these peaks is similar to TTX, with a loss of water, m/z 178 (or 176) and m/z 162 product ions. Next, these samples were studied using HRMS (Table 4). As it was expected, TTX and 4-epiTTX (m/z 320 ions), 4,9-anhydroTTX (m/z 302) were confirmed with small errors (<2.5 mDa). Two m/z 304 analogues with retention times of 6.5 and 7.7 min were identified as deoxyTTX (error of 1-3.9 mDa). Another analogue, m/z 288 was recognized as dideoxyTTX with a retention time 6.4 min and an error of −3.3 mDa. Compound m/z 272 was detected in three peaks, retention times of 4.6, 4.8 min and 5 min. These three molecules could be associated with an elemental composition of C11H17N3O5, and errors 1.4, 1.3 and 4.3 mDa, respectively. Finally, TTX analogue m/z 254 was identified in S. marmoratus samples with an error of 0.6–1.3 mDa and elemental composition of C11H15N3O4.
2.4. Moroccan Coast
Thirty-eight samples were harvested along the northwestern Moroccan Coast for TTXs, during July 2013 at the 6 sampling points (Figure 1), with two positive hits for TTXs (Table 5).
Though the values detected were all below the reference value implemented in the Netherlands [20], all positives were in gastropods and first report of TTX in these two species, A. depilans and O. celtica, as TTX vectors. O. celtica had quantifiable amounts of TTX, 4-epiTTX and other TTX epimer, and A. depilans was positive below the limit of quantification for TTX and 4-epiTTX.
TTX and 4-epiTTX (m/z 320) were identified in O. celtica (474) and A. depilans (476) samples (Table 5). Besides, in sample 474 another molecule with m/z 320 and different retention time was detected. In this case, the fragmentation pattern was studied and the loss of water and m/z 178 and m/z 162 as product ions were obtained. This TTX analogue was analyzed by HRMS but due to the small quantity it could not be confirmed. As shown in Table 5, TTX and 4-epiTTX were confirmed by HRMS (error −0.6 mDa and 1.7 mDa). The low quantity of 476 sample prevents the confirmation of these compounds by HRMS.
2.5. Statistical Analysis
The TTX content per sample is a continuous variable with a large number of samples showing a value of 0 and potentially, overdispersion. Without overdispersion, the best models would be a zero-inflated or not zero-inflated model with Poisson distribution of model error. With overdispersion, we would need to change the error distribution of the model to quasipoisson or negative binomial. Then, in order to decide which statistical model to use, we first tested for overdispersion of our data.
Overdispersion was tested by fitting a generalized function overdispersion test, from the AER R package. This function was applied to a model with Poisson error distribution. In this model, the TTX content was the dependent variable, and the sampling site a factor with 4 levels (São Miguel island, Madeira, continental Portugal and Morocco). TTX content was rounded to appear as a discrete variable (needed for a model with Poisson error distribution). The overdispersion test results were non-significant (z = 1.22, p = 0.11), so it was possible to keep the Poisson model instead of using another one accounting for overdispersion. The goodness of fit of the model error to a Poisson distribution was also tested, with non-significant results (p = 1). Then, it was also not necessary to account for zero-inflation in our data.
An analysis of deviance applied to the previous model showed that ‘sampling site’ had a significant effect (χ2 = 882507, p < 0.001). The model coefficients are shown in Table 6, indicating that samples from São Miguel Island had the highest content of TTX, followed by Morocco, whereas Madeira and continental Portugal had the same coefficient value. Due to the large error of the estimate, these latter two coefficients were not significantly different from 0.
Table 6.
Coefficients of the Poisson model with ‘sampling site’ as factor.
| Sampling Sites | Coefficents ± SE | z | p |
|---|---|---|---|
| São Miguel | 8.96 ± 0.001 | 5000.3 | <0.001 |
| Madeira | −19.26 ± 21.38 | −0.9 | 0.37 |
| Morocco | −10.67 ± 0.38 | −28.2 | <0.001 |
| Continental Portugal | −19.26 ± 13.30 | −1.4 | 0.15 |
3. Conclusions
In summary, the primary aim of this work was to search for new vectors for TTXs off the Portuguese coast, islands and the northwestern coast of Morocco using UHPLC-HRMS and UHPLC-MS/MS techniques. We report for the first time seven new TTX bearers, 57% of them in gastropods (P. depressa, N. lapillus, O. celtica and A. depilans), 29% in echinoderms (E. esculentus and O. ophidianus) and 14% in fish (S. marmoratus). All the measurable concentrations ranged from 5 to 352,886 TTX eq µg/Kg of SM. Also, an unknown TTX analogue was detected only in the velvet starfish O. ophidianus, feeding habits can be responsible, but further studies are needed for confirmation.
Regarding geographical tendencies, S. Miguel Island in the Azores was the location with greatest propensity to find these biotoxin groups, followed by the Moroccan Coast; whereas Madeira and continental Portugal had the same coefficient value (≈0). Despite Madeira Island and Morocco having the same latitude, and the sampling being performed one year apart, the tendency values are quite different, which could be due to less anthropogenical interference in Madeira.
We hope this work represents a step forward in the emergent toxin phenomenon and in particularly in the tetrodotoxin theme in line with EFSA’s recommendations, stressing the importance of these works for better understanding this emergent subject and protection of public health.
4. Materials and Methods
4.1. Sampled Points and Selected Species
TTX plus analogs were screened in 30 different species of benthic organisms, harvested from intertidal areas during low tide and in SCUBA diving expeditions. The areas surveyed comprised: the Portuguese continental coast (11 sampling points), Madeira and São Miguel Islands (8 sampling points) and the northwestern Moroccan coast (6 sampling points) (Table 7). The Portuguese continental coast was screened between September 2011 and January 2012, Madeira Island in September 2012, S. Miguel Island, Azores, and the northwestern coast of Morocco in June and July 2013 respectively. Different species of edible and non-edible organisms were screened to search for new potential vectors for TTXs: bivalves (Mytilus galloprovincialis, Mytilus spp.), sea-urchins (Echinus esculentus, Arbacia lixula, Paracentrotus lividus, Sphaerechinus granularis, Diadema africanum), sea-cucumber (Holothuria (Platyperona) sanctori), starfish (Asterias rubens, Astropecten aranciacus, Marthasterias glacialis, Echinaster sepositus, Ophidiaster ophidianus), crustaceans (Pollicipes pollicipes), gastropods (Phorcus lineatus, Gibbula umbilicalis, Stramonita haemastoma, Charonia lampas, Aplysia depilans, Nucella lapillus, Patella depressa, Patella gomesii, Patella aspera, Patella sp., Umbraculum umbraculum, Patella ordinaria, Cerithium vulgatum, Onchidella celtica), and fish (Sphoeroides marmoratus). The samples of P. ordinaria and P. aspera were purchased in local markets in Madeira, caught off the northern coast of the island (32°51′17.02″ N; 17°01′54.02″ W ). The organisms were transported to the laboratory in refrigerated containers. The samples that were not immediately processed were carefully stored at −20 °C.
Table 7.
Sampling sites and respective geographical coordinates, surveyed during September 2011 till January 2012, September 2012 and June and July 2013.
| Date | Location | Sampling Site | Geographic Coordinates |
|---|---|---|---|
| September 2011 | Continental Portugal | Luz | 37°04′37″ N; 8°44′51″ W |
| Camilo | 37°05′14″ N; 8°40′06″ W | ||
| Porto Côvo | 37°53′33″ N; 8°47′38″ W | ||
| Pelo Negro | 41°19′22″ N; 8°71′69″ W | ||
| October 2011 | Continental Portugal | Pedra de Jacó | 41°17′14″ N; 8°70′78″ W |
| Ovar | 40°52′58″ N; 8°40′31″ W | ||
| Esposende | 41°29′5″ N; 8°46′45″ W | ||
| November 2011 | Continental Portugal | Memória | 41°13′50″ N; 8°43′18″ W |
| December 2011 | Continental Portugal | Esposende | 41°29′5″ N; 8°46′45″ W |
| Valadares | 41°5′29″ N; 8°39′27″ W | ||
| January 2012 | Continental Portugal | Póvoa do Varzim | 41°22′41″ N; 8°46′7″ W |
| Angeiras | 41°15′50″ N; 8°43′37″ W | ||
| September 2012 | Madeira Island | Reis Magos | 32°39′16″ N; 16°49′05″ W |
| Caniçal | 32°44′20″ N; 16°44′17″ W | ||
| June 2013 | São Miguel Island | Cruzeiro | 37° 50′31″ N; 25° 41′33″ W |
| Étar de Ponta Delgada | 37°44′19″ N; 25°39′38″ W | ||
| São Roque | 37°45′15″ N; 25°38′31″ W | ||
| Mosteiros | 37°53′25″ N; 25°49′14″ W | ||
| Lagoa | 37°44′42″ N; 25°19′47″ W | ||
| Caloura | 37°42′49″ N; 25°29′54″ W | ||
| July 2013 | Morocco Coast | Casablanca corniche | 33°36′01″ N; 7°39′57″ W |
| El Jadida Haras | 33°14′42″ N; 8°28′37″ W | ||
| El Jadida Sâada | 33°14′42″ N; 8°32′26″ W | ||
| Sidi Bouzid | 33°13′57″ N; 8°33′20″ W | ||
| Mrizika | 32°57′21″ N; 8°46′53″ W | ||
| Oualidia | 32°43′55″ N; 9°02′57″ W |
4.2. Reagents
Pure TTX was purchased from Laboratorio CIFGA S.A. (Lugo, Spain). Ampoules contained 0.5 mL of solution with 25.9 ± 1.3 µg TTX/g and 2.99 ± 0.16 µg 4,9-anhydroTTX/g.
Acetonitrile, methanol and acetic acid were supplied by Panreac (Barcelona, Spain). All solvents employed in this work were HPLC or analytical grade and the water was distilled and passed through a water purification system (Milli-Q, Millipore, Mollet des Vallès, Spain). Formic acid was purchased from Merck (Darmstadt, Germany). Ammonium formate was from Fluka (Sigma-Aldrich, Madrid, Spain).
4.3. Sample Extraction
Samples were extracted on Silva et al. (2012) extraction protocol with appropriate amendments to the type of sample [16,23,45,46,47]. Animals were dissected and homogenized with a blender (A320R1, 700 W, Moulinex, Lisbon, Portugal) in pooled groups in order to obtain 1 g of tissue, with the exception of M. glacialis, A. rubens, E. esculentus, C. lampas, O. ophidianus, U. umbraculum, P. lividus, S. granularis, D. africanum, A. depilans, H. sanctori and S. marmoratus (here we divided the extraction into: gonads, muscle and liver). These species where treated individually, since each organism had enough extractable biomass. Samples were extracted in in 3 mL of acetic acid (1%)/methanol with the help of a vortex mixer for 5 min (Top Mix 1118, Fisher Bioblock Scientific) and ultrasonic bath, (5 min, 100 Hz) (RK100H, Bandelin SONOREX). A double extraction was performed, extracts were centrifuged at 4495× g for 15 min at 4°C (Centrifugal-Legend RT, Sorvall, Kendro Laboratory Products, Asheville, NC, USA), supernatants were combined and adjusted to a final volume of 7 mL. Then 1 mL of the extract was cleaned through a C18 solid-phase extraction (SPE), using cartridges (500 mg/3 mL volume from Supelco, Bellefonte, PA, USA) previously conditioned with 6 mL of methanol, followed by 6 mL of water (milliQ). The sample was eluted with 10 mL of 100% methanol and diluted with the same solvent to a final volume of 12 mL. Finally, each sample was concentrated by drying and re-suspending in 1 mL of methanol, and 500 µL were filtered through 0.22 µm filters (UltraFree-MC centrifugal devices, Millipore, Spain) before UHPLC-MS/MS analysis.
4.4. UHPLC Conditions
Chromatographic separation was carried out using a 1290 Infinity ultra-high-performance liquid chromatography system coupled to a 6460 Triple Quadrupole mass spectrometer (Agilent Technologies, Waldbronn, Germany). The toxins were separated using a column AQUITY UPLC BEH Amide (2.1 × 100 mm, 1.7 µm, Waters) at 35°C. Mobile phase A was 100% water with 10 mM formic acid and 10 mM ammonium formate. Mobile phase B was acetonitrile-water (95:5), containing 5 mM formic acid and 2 mM ammonium formate. The gradient program with a flow rate of 0.4 mL/min was started with 100% B and then a linear gradient to 65% B in 7 min. After an isocratic hold time linear of 2 min at 65% B and return to the starting conditions of 100% B in 0.5 min. Finally, 100% B was kept for 3.5 min before the next injection. The samples in the autosampler were cooled to 4°C and injection volume was 5 µL.
4.5. MS Methods
4.5.1. Source Conditions
MS/MS detection was performed using an Agilent G6460C Triple Quadrupole mass spectrometer with an Agilent Jet Stream Electrospray source (Agilent Technologies, Waldbronn, Germany). Source conditions were optimized to achieve the best sensitivity for all compounds drying gas temperature of 250°C and drying gas flow 5 L/min, nebulizer gas pressure 55 psi (Nitrocraft NCLC/MS from Air Liquid), sheath gas temperature 400°C and sheath gas flow 12 L/min. The capillary voltage was set to 3000 V in positive mode with a nozzle voltage of 0 V. The fragmentor was 152 and the cell accelerator voltage was 2 for each TTX analogue in this method.
HRMS was acquired by IT-TOF mass spectrometer instrument from Shimadzu (Kyoto, Japan). The mass spectrometer was operated with the following conditions: a heat block and curve desolvation line temperature, 200°C; nebulizing gas flow, 1.5 L/min; detector voltage, 1.65 kV; and drying gas pressure, 105 kPa. The mass range was calibrated prior to data acquisition employing a direct infusion of IT-TOF standard sample.
The nitrogen generator was a Nitrocraft NCLC/MC from Air Liquid (Meicende, Spain).
4.5.2. UHPLC-MS/MS Analysis: MRM Mode
The Agilent G6460C Triple Quadrupole mass spectrometer was operated in MRM in positive mode and the collision energy (CE) was optimized using MassHunter Optimizer software (Table 8). Two product ions were analyzed per compound, one for quantification and another for confirmation.
Table 8.
Main characteristics of UHPLC-MS/MS method in positive mode for TTX and detection of its analogues. The optimized transitions for each toxin are indicated in the table together with CE, fragmentor voltages, cell accelerator voltage (CAV) and polarity. Fragmentor voltage was 160 and CAV was 2 for each toxin.
| Compound | Precursor Ion | Product Ion | CE |
|---|---|---|---|
| 11-oxo-TTX | 336 | 318 | 24 |
| 178 | 40 | ||
| TTX 4-epiTTX TTX epimers Tetrodonic acid |
320 | ||
| 302 | 24 | ||
| 161.9 | 36 | ||
| 11-deoxyTTX deoxyTTX |
304 | 286 | 24 |
| 176 | 40 | ||
| 4,9-anhydro-TTX anhydroTTX |
302 | 256 | 28 |
| 161.9 | 40 | ||
| 11-norTTX-6(R/S)-ol | 290 | 272 | 24 |
| 162 | 40 | ||
| dideoxyTTX | 288 | 270 | 24 |
| 224 | 40 | ||
| hydroxytrideoxyTTX | 288 | 270 | 24 |
| 162 | 40 | ||
| trideoxyTTX | 272 | 254 | 24 |
| 162 | 40 | ||
| hydroxyanhydrotrideoxyTTX | 270 | 252 | 24 |
| 162 | 40 | ||
| anhydrotrideoxyTTX | 254 | 236 | 24 |
| 162 | 40 |
4.5.3. UHPLC-MS/MS Analysis: PIS Mode
To confirm and identify TTX analogues, the fragmentation pathway for each molecule was used. The Agilent G6460C Triple Quadrupole mass spectrometer was operated in PIS in positive mode for each TTX analogue with a scan range from 50 to 350 m/z, scan time 120 msec and collision energy from 20 to 50 eV. The characteristic ions were always formed [M+H-H2O], m/z 162 and m/z 178/176 and sometimes also [M+H-2H2O].
4.5.4. UHPLC-HRMS
HRMS was acquired using a UHPLC-MS-IT-TOF mass spectrometer instrument from Shimadzu (Kyoto, Japan) with a resolving power of 12,000 to identify TTX analogues. The mass spectra were acquired in full scan MS mode in positive mode with a mass range of 50–150 and 150–500 Da. The molecules were analysed using an ion accumulation time of 10 msec, with an event time of 100 msec and 1 repetition.
Acknowledgments
We acknowledge the project EMERTOX (grant 734748), funded by H2020-MSCA-RISE 2016 and UID/Multi/04423/2019.
Author Contributions
M.S. and V.V. conceived the idea, M.S. performed the sampling, M.S. and I.R. performed the sample analyses and wrote the paper. A.B. contributed to the experimental design and statistical analyses. M.H. collaborated in sample collection. A.A. contributed with experimental design. V.V. and L.M.B. contributed to funding and to materials and analyses tools. V.V., B.S., A.I.N., and M.K. collaborated in the sample collection and provided sampling and laboratory facilities. All authors participated in proof reading of the manuscript.
Funding
This research was partially funded by the Portuguese Foundation of Science and Technology (FCT) project UID/Multi/04423/2013 and by the projects ALERTOXNET (EAPA_317/2016), funded by the Interreg Atlantic program. The research leading to these results has received funding from the following FEDER cofunded-grants. From Conselleria de Cultura, Educacion e Ordenación Universitaria, Xunta de Galicia, 2017 GRC GI-1682 (ED431C 2017/01). From CDTI and Technological Funds, supported by Ministerio de Economía, Industria y Competitividad, AGL2014-58210-R, AGL2016-78728-R (AEI/FEDER, UE), ISCIII/PI16/01830 and RTC-2016-5507-2, ITC-20161072. From European Union POCTEP 0161-Nanoeaters -1-E-1, Interreg Agritox EAPA-998-2018. Additional funding was provided by National Funds through FCT—Fundação para a Ciência e a Tecnologia, under the projects UID/BIA/00329/2013, 2015 - 2018 and UID/BIA/00329/2019.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
Detection of TTXs in non-traditional vectors; first report of new vectors, addressing EFSA’s concerns and recommendations for this group of biotoxins in Portuguese territory and Moroccan waters.
References
- 1.Bane V., Lehane M., Dikshit M., O’Riordan A., Furey A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins. 2014;6:693–755. doi: 10.3390/toxins6020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botana L.M., Alfonso A., Rodriguez I., Botana A.M., Louzao M.C., Vieytes M.R. How safe is safe for marine toxins monitoring? Toxins. 2016;8:208. doi: 10.3390/toxins8070208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva M., Pratheepa V.K., Botana L.M., Vasconcelos V. Emergent toxins in North Atlantic temperate waters: A challenge for monitoring programs and legislation. Toxins. 2015;7:859–885. doi: 10.3390/toxins7030859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tahara Y., Hirata Y. Studies on the puffer fish toxin. J. Pharm. Soc. Jpn. 1909;29:587–625. [Google Scholar]
- 5.Miyazawa K., Noguchi T. Distribution and origin of tetrodotoxin. J. Toxicol. Toxin Rev. 2001;20:11–33. doi: 10.1081/TXR-100103081. [DOI] [Google Scholar]
- 6.Pratheepa V., Vasconcelos V. Microbial diversity associated with tetrodotoxin production in marine organisms. Environ. Toxicol. Pharmacol. 2013;36:1046–1054. doi: 10.1016/j.etap.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Magarlamov T.Y., Melnikova D.I., Chernyshev A.V. Tetrodotoxin-Producing Bacteria: Detection, Distribution and Migration of the Toxin in Aquatic Systems. Toxins. 2017;9:166. doi: 10.3390/toxins9050166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez I., Alfonso A., Alonso E., Rubiolo J., Roel M., Vlamis A., Katikou P., Jackson S., Margassery L., Dobson A., et al. The association of bacterial C 9-based TTX-like compounds with Prorocentrum minimum opens new uncertainties about shellfish seafood safety. Sci. Rep. 2017;7:40880. doi: 10.1038/srep40880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary G., Yotsu-Yamashita M., Shang L., Yasumoto T., Dudley S.C., Jr. Interactions of the C-11 hydroxyl of tetrodotoxin with the sodium channel outer vestibule. Biophys. J. 2003;84:287–294. doi: 10.1016/S0006-3495(03)74849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheib H., McLay I., Guex N., Clare J., Blaney F., Dale T., Tate S., Robertson G. Modeling the pore structure of voltage-gated sodium channels in closed, open, and fast-inactivated conformation reveals details of site 1 toxin and local anesthetic binding. J. Mol. Model. 2006;12:813–822. doi: 10.1007/s00894-005-0066-y. [DOI] [PubMed] [Google Scholar]
- 11.Cestele S., Catterall W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/S0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 12.Yotsu-Yamashita M., Sugimoto A., Takai A., Yasumoto T. Effects of specific modifications of several hydroxyls of tetrodotoxin on its affinity to rat brain membrane. J. Pharmacol. Exp. Ther. 1999;289:1688–1696. [PubMed] [Google Scholar]
- 13.Bentur Y., Ashkar J., Lurie Y., Levy Y., Azzam Z.S., Litmanovich M., Golik M., Gurevych B., Golani D., Eisenman A. Lessepsian migration and tetrodotoxin poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon. 2008;52:964–968. doi: 10.1016/j.toxicon.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Katikou P., Georgantelis D., Sinouris N., Petsi A., Fotaras T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece) Toxicon. 2009;54:50–55. doi: 10.1016/j.toxicon.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez P., Alfonso A., Vale C., Alfonso C., Vale P., Tellez A., Botana L.M. First Toxicity Report of Tetrodotoxin and 5,6,11-TrideoxyTTX in the Trumpet Shell Charonia lampas lampas in Europe. Anal. Chem. 2008;80:5622–5629. doi: 10.1021/ac800769e. [DOI] [PubMed] [Google Scholar]
- 16.Silva M., Azevedo J., Rodriguez P., Alfonso A., Botana L.M., Vasconcelos V. New Gastropod Vectors and Tetrodotoxin Potential Expansion in Temperate Waters of the Atlantic Ocean. Marine Drugs. 2012;10:712–726. doi: 10.3390/md10040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner A.D., Higgins C., Higman W., Hungerford J. Potential threats posed by tetrodotoxins in UK waters: Examination of detection methodology used in their control. Mar. Drugs. 2015;13:7357–7376. doi: 10.3390/md13127070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for Food of Animal Origin OJ L 139, 30.4.2004. [(accessed on 14 December 2010)]; Available online: http://eur-lex.europa.eu.
- 19.Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Rules for the Organisation of Official Controls on Products of Animal Origin Intended for Human Consumption. OJ L 139, 30.4.2004 (18 June 2015) [(accessed on 14 December 2010)];:206–320. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32007R1246.
- 20.Gerssen A., Bovee T., Klijnstra M., Poelman M., Portier L., Hoogenboom R. First report on the occurrence of tetrodotoxins in bivalve mollusks in the Netherlands. Toxins. 2018;10:450. doi: 10.3390/toxins10110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knutsen H.K., Alexander J., Barregard L., Bignami M., Bruschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Edler L., Grasl-Kraupp B., et al. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017;15:4752. doi: 10.2903/j.efsa.2017.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlamis A., Katikou P., Rodriguez I., Rey V., Alfonso A., Papazachariou A., Zacharaki T., Botana A.M., Botana L.M. First detection of tetrodotoxin in Greek Shellfish by UPLC–MS/MS potentially linked to the presence of the Dinoflagellate Prorocentrum minimum. Toxins. 2015;7:1779–1807. doi: 10.3390/toxins7051779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoji Y., Yotsu-Yamashita M., Miyazawa T., Yasumoto T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001;290:10–17. doi: 10.1006/abio.2000.4953. [DOI] [PubMed] [Google Scholar]
- 24.Yotsu-Yamashita M., Abe Y., Kudo Y., Ritson-Williams R., Paul V.J., Konoki K., Cho Y., Adachi M., Imazu T., Nishikawa T., et al. First Identification of 5,11-Dideoxytetrodotoxin in Marine Animals, and Characterization of Major Fragment Ions of Tetrodotoxin and Its Analogs by High Resolution ESI-MS/MS. Mar. Drugs. 2013;11:2799–2813. doi: 10.3390/md11082799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez P., Alfonso A., Otero P., Katikou P., Georgantelis D., Botana L.M. Liquid chromatography–mass spectrometry method to detect Tetrodotoxin and Its analogues in the puffer fish Lagocephalus sceleratus (Gmelin, 1789) from European waters. Food Chem. 2012;132:1103–1111. doi: 10.1016/j.foodchem.2011.11.081. [DOI] [Google Scholar]
- 26.Bane V., Brosnan B., Barnes P., Lehane M., Furey A. High-resolution mass spectrometry analysis of tetrodotoxin (TTX) and its analogues in puffer fish and shellfish. Food Addit. Contam. Part A Chem. Anal. Control Expos. Risk Assess. 2016;33:1468–1489. doi: 10.1080/19440049.2016.1218070. [DOI] [PubMed] [Google Scholar]
- 27.Knox G.A. Hard Shores. In: Kennish M.J., editor. The Ecology of Seashores. CRC Press; Boca Raton, FL, USA: 2001. pp. 20–86. [Google Scholar]
- 28.Crothers J.H. Common topshells: An introduction to the biology of Osilinus lineatus with notes on other species in the genus. Field Stud. 2001;10:115–160. [Google Scholar]
- 29.Forster G.R. The ecology of Echinus esculentus L. Quantitative distribution and rate of feeding. J. Mar. Biol. Assoc. UK. 1959;38:361–367. doi: 10.1017/S0025315400006147. [DOI] [Google Scholar]
- 30.Lin S.J., Hwang D.F. Possible source of tetrodotoxin in the starfish Astropecten scoparius. Toxicon. 2001;39:573–579. doi: 10.1016/S0041-0101(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 31.Yotsu-Yamashita M., Mebs D. Occurrence of 11-oxotetrodotoxin in the red-spotted newt, Notophthalmus viridescens, and further studies on the levels of tetrodotoxin and its analogues in the newt’s efts. Toxicon. 2003;41:893–897. doi: 10.1016/S0041-0101(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson J.C. Feeding activity in echinaster and its induction with dissolved nutrients. Biol. Bull. 1969;136:374–384. doi: 10.2307/1539682. [DOI] [Google Scholar]
- 33.Harmelin-Vivien M.L., Harmelin J.G., Almeida A.J. Structure of fish assemblages on coastal rocky shores of the Azores. Boletim do Museu Municipal do Funchal. 2001;6:127–138. [Google Scholar]
- 34.Noguchi T., Ebesu J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol. Toxin Rev. 2001;20:1–10. doi: 10.1081/TXR-100103080. [DOI] [Google Scholar]
- 35.Noguchi T., Arakawa O. Tetrodotoxin—Distribution and Accumulation in Aquatic Organisms, and Cases of Human Intoxication. Mar. Drugs. 2008;6:220–242. doi: 10.3390/md20080011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landsberg J.H., Hall S., Johannessen J.N., White K.D., Conrad S.M., Abbott J.P., Flewelling L.J., Richardson R.W., Dickey R.W., Jester E.L.E., et al. Saxitoxin Puffer Fish Poisoning in the United States, with the First Report of Pyrodinium bahamense as the Putative Toxin Source. Environ. Health Perspect. 2006;114:1502–1507. doi: 10.1289/ehp.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruyama J., Noguchi T., Narita H., Nara M., Jeon J.K., Otsuka M., Hashimoto K. Occurrence of Tetrodotoxin in a Starfish, Astropecten scoparius. Agric. Biol. Chem. 1985;10:3069–3070. [Google Scholar]
- 38.Miyazawa K., Noguchi T., Maruyama J., Jeon J.K., Otsuka M., Hashimoto K. Occurrence of tetrodotoxin in the starfishes Astropecten polyacanthus and A. scoparius in the Seto Inland Sea. Mar. Biol. 1985;90:61–64. doi: 10.1007/BF00428215. [DOI] [Google Scholar]
- 39.Sugita H., Iwata J., Miyajima C., Kubo T., Noguchi T., Hashimoto K., Deguchi Y. Changes in microflora of a puffer fish Fugu niphobles, with different water temperatures. Mar. Biol. 1989;101:299–304. doi: 10.1007/BF00428125. [DOI] [Google Scholar]
- 40.Llewellyn L.E. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the south Pacific: Reassessing the link between ciguatera and climate change. Toxicon. 2010;56:691–697. doi: 10.1016/j.toxicon.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Ashton M., Tosteson T., Tosteson C. The effect of elevated temperature on the toxicity of the laboratory cultured dinoflagellate Ostreopsis lenticularis (Dinophyceae) Revista de Biología Tropical. 2003;51:1–6. [PubMed] [Google Scholar]
- 42.Turner A.D., Fenwick D., Powell A., Dhanji-Rapkova M., Ford C., Hatfield R.G., Santos A., Martinez-Urtza J., Bean B.P., Baker-Austin C., et al. New Invasive Nemertean Species (Cephalothrix simula) in England with High Levels of Tetrodotoxin and a Microbiome Linked to Toxin Metabolism. Mar. Drugs. 2018;16:452. doi: 10.3390/md16110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dayrat B. Review of the current knowledge of the systematics of onchidiidae (mollusca: Gastropoda: Pulmonata) with a checklist of nominal species. Zootaxa. 2009;2068:1–26. [Google Scholar]
- 44.Carefoot T.H. Aplysia: Its biology and ecology. Oceanogr. Mar. Biol. Annu. Rev. 1987;25:167–284. [Google Scholar]
- 45.Ito K., Okabe S., Asakawa M., Bessho K., Taniyama S., Shida Y., Ohtsuka S. Detection of tetrodotoxin (TTX) from two copepods infecting the grass puffer Takifugu niphobles: TTX attracting the parasites? Toxicon. 2006;48:620–626. doi: 10.1016/j.toxicon.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Jen H.C., Lin S.J., Tsai Y.H., Chen C.H., Lin Z.C., Hwang D.F. Tetrodotoxin poisoning evidenced by solid-phase extraction combining with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2008;871:95–100. doi: 10.1016/j.jchromb.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 47.Tsai Y.H., Ho P.H., Hwang C.C., Hwang P.A., Cheng C.A., Hwang D.F. Tetrodotoxin in several species of xanthid crabs in southern Taiwan. Food Chem. 2006;95:205–212. doi: 10.1016/j.foodchem.2004.12.032. [DOI] [Google Scholar]