Abstract
The co-occurrence of various cyanobacterial toxins can potentially induce toxic effects different than those observed for single cyanotoxins, as interaction phenomena cannot be discarded. Moreover, mixtures are a more probable exposure scenario. However, toxicological information on the topic is still scarce. Taking into account the important role of mutagenicity and genotoxicity in the risk evaluation framework, the objective of this study was to assess the mutagenic and genotoxic potential of mixtures of two of the most relevant cyanotoxins, Microcystin-LR (MC-LR) and Cylindrospermopsin (CYN), using the battery of in vitro tests recommended by the European Food Safety Authority (EFSA) for food contaminants. Mixtures of 1:10 CYN/MC-LR (CYN concentration in the range 0.04–2.5 µg/mL) were used to perform the bacterial reverse-mutation assay (Ames test) in Salmonella typhimurium, the mammalian cell micronucleus (MN) test and the mouse lymphoma thymidine-kinase assay (MLA) on L5178YTk± cells, while Caco-2 cells were used for the standard and enzyme-modified comet assays. The exposure periods ranged between 4 and 72 h depending on the assay. The genotoxicity of the mixture was observed only in the MN test with S9 metabolic fraction, similar to the results previously reported for CYN individually. These results indicate that cyanobacterial mixtures require a specific (geno)toxicity evaluation as their effects cannot be extrapolated from those of the individual cyanotoxins.
Keywords: genotoxicity, mutagenicity, Cylindrospermopsin, Microcystin-LR, mixture
1. Introduction
Nowadays, a proliferation of cyanobacterial species can be seen globally because of water eutrophication and climate change, leading to an increasing occurrence of cyanotoxins [1,2,3]. Cyanotoxins are toxic secondary metabolites produced by various species of cyanobacteria, which involved an ample variety of compounds with different structural and physicochemical properties [4]. Humans may be exposed to cyanotoxins via different routes, but oral exposure by means of contaminated water and foods (fish, crops, vegetables and food supplements) is by far the most important [5,6]. Microcystins (MCs) and cylindrospermopsins (CYN) are among the most frequently investigated cyanotoxins due to their toxicity and extensive distribution.
MCs are cyclic heptapeptides and 246 variants were identified so far [7], with Microcystin-LR (MC-LR) as the reference congener. The liver is the main target organ in MC-LR toxicity because of its uptake into hepatocytes by the organic anion transport system [8]. MC-LR inhibits the protein serine/threonine phosphatases by covalent binding, especially PP1 and PP2. Thus, the proteins are hyperphosphorylated leading to the modification of cytoskeleton and disruption of actin filaments [9]. In addition, MCs induce oxidative stress [1,10], disrupt different enzymatic activities [11,12] and induce apoptosis [13]. MC-LR was classified as possible human carcinogen (Group 2B) by the International Agency of Research on Cancer (IARC) [14]. It can produce genotoxic effects in vitro and in vivo [15], although the mechanisms involved are not yet completely understood [16].
Cylindrospermopsins are guanidine alkaloid hepatotoxins with five known analogues [17]. Cylindrospermopsin (CYN) has zwitterionic characteristics, thus being highly water soluble and chemically stable at high temperatures and a wide range of pH [18,19]. For these reasons, humans can be more likely exposed to CYN than to other cyanotoxins as up to 90% of total CYN is presented in surrounding waters. Although the liver and kidney are target organs of CYN, other organs such us lungs, heart, thymus, stomach, spleen, intestinal tract, skin, nervous, immune, vascular and lymphatic systems could also be damaged [1,20,21,22].
The absorption mechanism of CYN is not totally elucidated, but it was shown that paracellular transport is involved in the intestinal uptake [1,23]. The main mechanisms of CYN toxicity is the irreversible inhibition of protein synthesis [24,25] and glutathione (GSH) depletion [26] related to the oxidative stress induced by CYN [27,28,29]. Moreover, the bioactivation of CYN by cytochrome P-450 plays an important role in its mechanism of toxicity [30]. CYN was shown to induce DNA fragmentation and DNA strands breaks [31,32,33,34,35,36,37,38]. However, it was not yet classified by its carcinogenic potential by the IARC.
Both cyanotoxins have been extensively studied individually, but there are very few studies that evaluate their combined effects, as indicated by the European Food Safety Authority (EFSA) [5]. The simultaneous occurrence of MCs and CYN was reported repeatedly [39,40]. They have different chemical structures and mechanisms of action, thus interaction phenomena such as synergism, antagonism or toxicity potentiation must be considered. Moreover, a risk assessment can be greatly influenced when diverging from individual toxin exposure to a multi-toxin exposure scenario. Gutiérrez-Praena et al. [41] found an antagonistic effect of CYN and MC-LR when investigating the cytotoxicity of binary mixtures in comparison to the individual toxins in HepG2 cells. Hercog et al. [42] observed a genotoxic potential of CYN/MC-LR mixtures comparable to that of CYN alone when using the micronucleus (MN) and comet assays in the same experimental model.
The exploration of the genotoxic potential of CYN/MC-LR applicable to food and feed safety assessment is of great current interest. EFSA has indicated the need for further data on the toxicity of cyanotoxins mixtures [5] following recommended genotoxicity testing strategies [43].
Thus, the purpose of this research was to assess the mutagenic and genotoxic potential of the CYN/MC-LR mixtures trough a complete battery of different in vitro tests. This battery included: (1) The bacterial reverse-mutation assay in five strains of Salmonella typhimurium (Ames test, OECD 471 [44]) which detects gene mutations in the absence and presence of the microsomal fraction S9; (2) the Micronucleus test (MN, OECD 487 [45]) on L5178Y Tk+/− cells that detects clastogenic and aneugenic chromosome aberrations in the absence and presence of the microsomal fraction S9; (3) the standard and enzyme modified comet assays with restriction enzymes (Endonuclease III (Endo III) and Formamide pyrimidine glycosylase (FPG)) that detect DNA strand breaks and oxidative DNA damage in Caco-2 cells; (4) the mouse lymphoma thymidine-kinase assay (MLA, OECD 490 [46]) on L5178Y Tk+/− cells to detect gene mutations in the timidine kinase (Tk) locus in the absence and presence of the microsomal fraction S9. The microsomal fraction S9 was used to assess if CYN/MC-LR genotoxicity is due to metabolic bioactivation of these toxins or due to the parent compounds.
2. Results
2.1. Ames Test
No signals of toxicity and/or test solutions instability were observed during the test performance. CYN/MC-LR mixtures did not induce changes in any of the S. typhimurium strains without S9 fraction (Table 1). On the contrary, a significant increase in the number of revertants per plate was observed with TA97A, TA102 and TA135 strains. However, a MI higher than 2 was not obtained in any of the assayed experimental conditions. Solvent controls (MetOH 2% and DMSO) did not induce statistical significant changes versus the negative controls.
Table 1.
Effect of CYN-MC-LR mixtures on the Ames test in three independent experiments by triplicate. Data are given as mean ± SD revertants/plate. * p < 0.05. ** p < 0.01 in comparison to negative control.
| Concentration (µg/mL) | TA97A | TA98 | TA100 | TA102 | TA1535 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −S9 | MI | +S9 | MI | −S9 | MI | +S9 | MI | −S9 | MI | +S9 | MI | −S9 | MI | +S9 | MI | −S9 | MI | +S9 | MI | ||
| Pure CYN-MC-LR mixture | Negative controls | 231 ± 42 | - | 244 ± 5 | - | 21 ± 2 | - | 24 ± 9 | - | 117 ± 25 | - | 135 ± 14 | - | 215 ± 12 | - | 292 ± 11 | - | 293 ± 23 | - | 273 ± 33 | - |
| 0.125–1.25 | 297 ± 37 | 1.4 | 319 ± 51 | 1.3 | 19 ± 2 | 0.9 | 18 ± 8 | 0.8 | 136 ± 40 | 1.2 | 153 ± 21 | 1.1 | 230 ± 36 | 1.1 | 440 ± 29 ** | 1.5 | 327 ± 25 | 1.1 | 376 ± 54 * | 1.3 | |
| 0.25–2.5 | 165 ± 28 | 0.8 | 334 ± 49 ** | 1.4 | 20 ± 1 | 1.0 | 17 ± 7 | 0.7 | 144 ± 12 | 1.2 | 166 ± 24 | 1.2 | 217 ± 29 | 1.0 | 380 ± 33 ** | 1.3 | 311 ± 10 | 1.1 | 411 ± 54 ** | 1.4 | |
| 0.5–5 | 213 ± 15 | 1.0 | 290 ± 58 | 1.2 | 26 ± 9 | 1.3 | 20 ± 10 | 0.9 | 154 ± 13 | 1.3 | 143 ± 19 | 1.1 | 251 ± 17 | 1.2 | 296 ± 18 | 10. | 309 ± 42 | 1.1 | 336 ± 18 * | 1.1 | |
| 1–10 | 168 ± 10 | 0.8 | 234 ± 43 | 1.0 | 21 ± 2 | 1.0 | 19 ± 9 | 1.0 | 146 ± 18 | 1.2 | 130 ± 10 | 1.0 | 134 ± 12 | 0.6 | 383 ± 44 ** | 1.3 | 250 ± 43 | 0.9 | 464 ± 44 ** | 1.6 | |
| 2–20 | 205 ± 31 | 1.0 | 295 ± 25 | 1.2 | 19 ± 5 | 0.9 | 25 ± 8 | 1.0 | 104 ± 31 | 0.9 | 143 ± 19 | 1.1 | 151 ± 1 | 0.7 | 397 ± 32 ** | 1.4 | 276 ± 15 | 0.9 | 476 ± 52 ** | 1.6 | |
| Positive controls | 613 ± 66 ** | 2.9 | 527 ± 19 ** | 2.2 | 883 ± 55 ** | 42.0 | 960 ± 53 ** | 40.9 | 816 ± 11 ** | 7.0 | 583 ± 39 ** | 4.3 | 950 ± 118 ** | 4.4 | 671 ± 22 ** | 2.3 | 833 ± 25 ** | 2.8 | 659 ± 39 ** | 2.2 | |
| MeOH 2% | 176 ± 25 | 0.8 | 316 ± 32 | 1.3 | 17 ± 5 | 0.8 | 25 ± 13 | 1.1 | 92 ± 13 | 0.8 | 87 ± 29 | 0.6 | 192 ± 8 | 0.8 | 280 ± 12 | 0.6 | 313 ± 9 | 1.1 | 233 ± 35 | 0.9 | |
| DMSO | 209 ± 66 | 1.3 | 184 ± 38 | 0.8 | 25 ± 2 | 1.2 | 30 ± 6 | 1.3 | 115 ± 5 | 1.0 | 113 ± 17 | 0.8 | 250 ± 65 | 1.2 | 231 ± 35 | 0.8 | 342 ± 63 | 1.2 | 298 ± 16 | 1.1 | |
Negative control: Milli Q water. Control solvent: MeOH 2% and DMSO. Positive controls without S9 for TA97A: 9-aminoacridine (50 µg/plate), TA98: 2-nitrofluorene (0.1 µg/plate), TA100 and TA1535: NaN3 (1.5 µg/plate) and TA102: mytomicin C (2.5 µg/plate). Positive control for all strains with S9: 2-aminofluorene (20 µg/plate).
2.2. Micronucleus Test
In the absence of S9 fraction, CYN/MC-LR mixtures did not increase the number of binucleated cells with MN in any of the concentration assayed (Table 2). However, a significant reduction of the cytokinesis-block proliferation index (CBPI) was observed at the highest concentration (1.35 µg/mL CYN + 13.5 µg/mL MC-LR). Positive controls for clastogens (MMC) and aneugens (colchicine) showed a significant increase in the frequency of binucleated cells with micronuclei (BNMN) (p < 0.01).
Table 2.
Percentage of binucleated cells with micronuclei (BNMN) and cytokinesis-block proliferation index (CBPI) in cultured mouse lymphoma cells L5178YTk+/− exposed to CYN+MC-LR mixture (n = 3). The genotoxicity assay was performed in the absence and presence of the metabolic fraction S9. The values are expressed as mean ± SD. ** p < 0.01, *** p < 0.001 in comparison to negative control group values.
| Experimental Group | Absence of S9 | Presence of S9 | ||||||
|---|---|---|---|---|---|---|---|---|
| Exposure Time (h) | Concentrations (µg/mL) | BNMN (%) ± SD | CBPI ± SD | Exposure Time (h) | Concentrations (µg/mL) | BNMN (%) ± SD | CBPI ± SD | |
| Negative control | 24 | - | 2.3 ± 0.5 | 1.9 ± 0.1 | 4 | - | 2.5 ± 1.0 | 1.8 ± 0.1 |
| Positive control | 24 | Mitomycin C 0.0625 | 10.5 ± 4.1 *** | 1.5 ± 0.1 *** | 4 | Cyclophosfamide 8 | 8.3 ± 1.9 ** | 1.8 ± 0.1 |
| Colchicine 0.0125 | 9.6 ± 1.7 *** | 1.8 ± 0.0 | ||||||
| CYN+MC-LR | 24 | 0.084–0.84 | 1.8 ± 1.5 | 1.9 ± 0.0 | 4 | 0.125–1.25 | 4.8 ± 2.6 | 1.8 ± 0.1 |
| 24 | 0.168–1.68 | 2.3 ± 1.0 | 1.9 ± 0.0 | 4 | 0.250–2.5 | 4.0 ± 1.4 | 1.8 ± 0.1 | |
| 24 | 0.336–3.36 | 2.5 ± 0.6 | 1.8 ± 0.0 | 4 | 0.5–5 | 5.8 ± 1.5 | 1.8 ± 0.1 | |
| 24 | 0.672–6.72 | 1.3 ± 0.5 | 1.7 ± 0.1 | 4 | 1–10 | 8.8 ± 4.2 ** | 1.8 ± 0.1 | |
| 24 | 1.35–13.5 | 0.8 ± 1.0 | 1.3 ± 0.3 *** | 4 | 2–20 | 4.8 ± 0.5 | 1.8 ± 0.1 | |
Clastogen and aneugen positive controls: mitomicyn C (0.0625 µg/mL) and colchicine (0.0125 µg/mL), respectively.
In the presence of S9 fraction, CYN/MC-LR induced an increase of BNMN (%) when compared to the negative control, but only at 1 µg/mL CYN + 10 µg/mL MC-LR this change was statistically significant (p < 0.01).
2.3. Mouse Lymphoma Thymidine-Kinase Assay (MLA)
Results of the MLA are shown in Table 3, Table 4 and Table 5. None of the evaluated CYN/MC-LR mixture concentrations induced a mutagenic response in the absence or presence of S9 fraction, neither after a short treatment (4 h) nor a long treatment (24 h). Concurrent vehicle control did not show changes in comparison to negative control (data not shown).
Table 3.
Toxicity and mutagenicity of CYN/MC-LR in L5178YTk+/− cells after 4 h without S9 fraction by the mouse lymphoma thymidine-kinase assay (MLA) (n = 2). a: Total mutant frequency divided into small/large (S/L) colony mutant frequencies. The induced mutant frequency (IMF) was determined according to the formula IMF = MF-SMF, where MF is the test culture mutant frequency and SMF is the spontaneous mutant frequency. *** p < 0.001.
| Concentration (µg/mL) | Relative Total Growth | Percent Plating Efficiency | Mutant Frequency (× 10−6) | MF (S/L) a | IMF (MF-SMF) (× 10−6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | |
| 0 | 100 | 100 | 91 | 124 | 107 | 152 | 51/56 | 33/41 | - | - |
| 0.04 CYN-0.4 MC | 77 | 90 | 98 | 98 | 126 | 143 | 95/48 | 86/57 | 56 | 70 |
| 0.08 CYN-0.8 MC | 98 | 100 | 93 | 70 | 202 | 157 | 111/91 | 102/55 | 95 | 83 |
| 0.16 CYN-1.6 MC | 82 | 86 | 102 | 82 | 71 | 162 | 44/27 | 100/62 | −14.4 | 89 |
| 0.33 CYN-3.3 MC | 64 | 72 | 98 | 91 | 165 | 150 | 84/81 | 80/70 | 58 | 76 |
| 0.67 CYN-6.7 MC | 57 | 58 | 95 | 88 | 174 | 144 | 106/68 | 60/84 | 67 | 71 |
| MMS (10 µg/mL) | 46 | 70 | 69 | 82 | 728 *** | 738 *** | 407/321 | 424/314 | 621 | 664 |
Positive controls: methylmethanesulfonate, MMS 10 μg/mL without S9 fraction and cyclophosphamide, CP 3 μg/mL with S9 fraction.
Table 4.
Toxicity and mutagenicity of CYN/MC-LR in L5178YTk+/− cells after 4 h with S9 fraction by the mouse lymphoma thymidine-kinase assay (MLA) (n = 2). a: Total mutant frequency divided into small/large (S/L) colony mutant frequencies. The induced mutant frequency (IMF) was determined according to the formula IMF = MF-SMF, where MF is the test culture mutant frequency and SMF is the spontaneous mutant frequency. *** p < 0.001.
| Concentration (µg/mL) | Relative Total Growth | Percent Plating Efficiency | Mutant Frequency (× 10−6) | MF (S/L) a | IMF (MF-SMF) (× 10−6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | |
| 0 | 100 | 100 | 93 | 102 | 155 | 146 | 96/59 | 82/64 | - | - |
| 0.04 CYN-0.4 MC | 96 | 84 | 82 | 84 | 94 | 100 | 43/51 | 42/58 | −61 | −46 |
| 0.08 CYN-0.8 MC | 82 | 72 | 91 | 91 | 95 | 95 | 50/45 | 50/45 | −60 | −51 |
| 0.16 CYN-1.6 MC | 58 | 51 | 95 | 100 | 98 | 95 | 48/50 | 49/47 | −57 | −51 |
| 0.33 CYN-3.3 MC | 58 | 56 | 102 | 100 | 98 | 105 | 56/43 | 60/45 | −57 | −41 |
| 0.67 CYN-6.7 MC | 26 | 31 | 118 | 113 | 120 | 132 | 62/58 | 77/55 | −35 | −14 |
| 1.35 CYN-13.5 MC | 16 | 16 | 130 | 116 | 70 | 91 | 29/41 | 38/53 | −85 | −55 |
| CP (3 µg/mL) | 99 | 81 | 65 | 73 | 480 *** | 433 *** | 228/252 | 213/220 | 325 | 286 |
Positive controls: methylmethanesulfonate, MMS 10 μg/mL without S9 fraction and cyclophosphamide, CP 3 μg/mL with S9 fraction.
Table 5.
Toxicity and mutagenicity of CYN/MC-LR in L5178YTk+/− cells after 24 h without S9 fraction by the mouse lymphoma thymidine-kinase assay (MLA) (n = 2). a: Total mutant frequency divided into small/large (S/L) colony mutant frequencies. The induced mutant frequency (IMF) was determined according to the formula IMF = MF-SMF, where MF is the test culture mutant frequency and SMF is the spontaneous mutant frequency. *** p < 0.001.
| Concentration (µg/mL) | Relative Total Growth | Percent Plating Efficiency | Mutant Frequency (× 10−6) | MF (S/L) a | IMF (MF-SMF) (× 10−6) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | |
| 0 | 100 | 100 | 113 | 124 | 170 | 170 | 106/72 | 87/92 | - | - |
| 0.04 CYN-0.4 MC | 103 | 115 | 90 | 87 | 107 | 78.9 | 62/45 | 48/30 | −71 | −100 |
| 0.08 CYN-0.8 MC | 91 | 102 | 102 | 93 | 121 | 124 | 50/71 | 72/52 | −57 | −55 |
| 0.16 CYN-1.6 MC | 79 | 96 | 76 | 108 | 143 | 100 | 81/66 | 56/44 | −35 | −79 |
| 0.33 CYN-3.3 MC | 71 | 74 | 116 | 104 | 115 | 168 | 64/51 | 109/59 | −63 | −12 |
| 0.67 CYN-6.7 MC | 39 | 39 | 127 | 104 | 113 | 195 | 74/39 | 77/118 | −66 | 16 |
| MMS (10 µg/mL) | 52 | 66 | 35 | 34 | 778 *** | 897 *** | 370/408 | 459/438 | 599 | 718 |
Positive controls: methylmethanesulfonate, MMS 10 μg/mL without S9 fraction and cyclophosphamide, CP 3 μg/mL with S9 fraction.
2.4. Standard and Enzyme-Modified Comet Assays
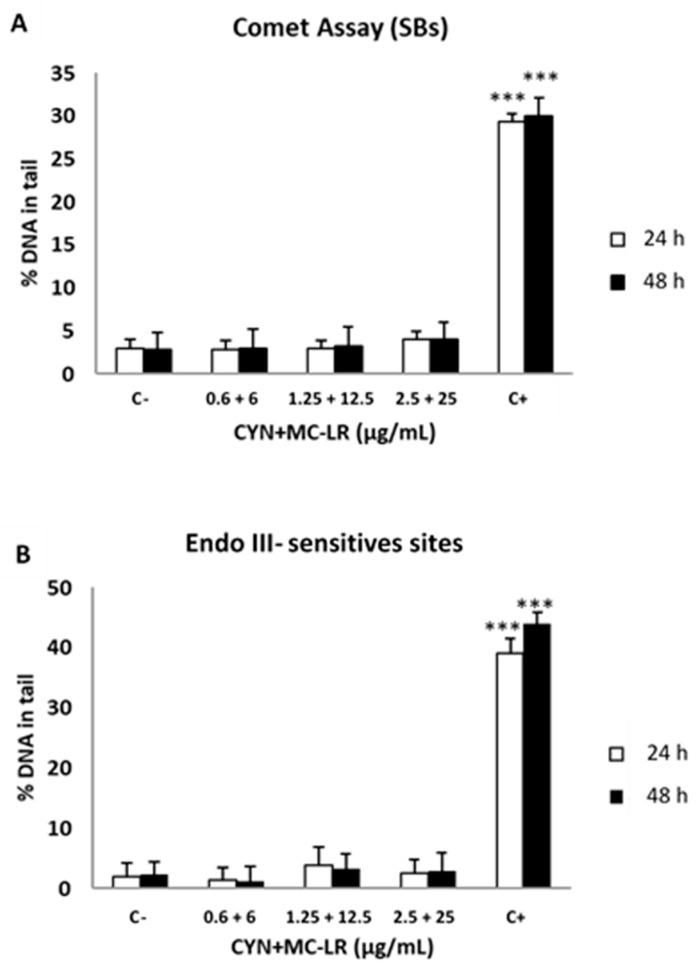
Caco-2 cells exposure to CYN/MC-LR mixtures did not result in DNA strand breaks in the standard comet assay after 24 and 48 h (Figure 1a). In addition, an oxidative damage induced genotoxicity was not observed as the experiments performed with Endo III and FPG enzymes did not show a significant increase of % DNA in tail (Figure 1b,c). Results for the solvent control were similar to the negative control (data not shown) and only positive controls showed a significant (p < 0.001) genotoxicity.
Figure 1.
DNA damage in Caco-2 cells after exposure to CYN+MC-LR mixtures for 24 and 48 h. Results expressed as the formation of strand breaks (a) and oxidative DNA damage as Endo III-sensitive sites (b) and FPG-sensitive sites (c) (n = 3). The level of DNA strand-breaks (SBs), oxidized pyrimidines and oxidized purines are expressed as % DNA in tail. All values are expressed as mean ± SD. Negative control (C-): culture medium. Positive controls (C+): 100 μM H2O2 for the standard comet assay and Endo III-sensitive sites, and 2 μM of Ro 19-8022 photosensitizer with light irradiation for FPG-sensitive sites. *** p < 0.001.
3. Discussion
The data on the genotoxicity of a chemical is of key importance as it drives the type of human risk assessment to be performed. While a genotoxic chemical and health-based guidance value is usually set, for an unavoidable chemical, that is, a genotoxic carcinogen, the Margin of Exposure approach is usually applied [47]. For the generation and evaluation of data on genotoxic potential, the EFSA [43] recommends a step-wise approach for the generation and evaluation of data on genotoxic potential that begins with a basic battery of in vitro tests, including a bacterial reverse mutation assay and an in vitro MN assay. Moreover, further in vitro assays should be conducted in case of inconclusive, conflicting or equivocal results. The need for using several assays is justified as it is considered that there is no single mutagenicity test which can detect all kinds of potential human mutagens with 100% accuracy or prediction. This was shown to be true as mutagenesis itself is multifactorial [48].
Moreover, the genotoxicity evaluation of chemical mixtures is of great current interest and the EFSA has recently published a statement on the topic [49]. Thus, the Scientific Committee advocates for chemically fully defined mixtures, a component based approach, i.e., assessing all components individually using all suitable information including read across and quantitative structure–activity relationship (QSAR) considerations about their genotoxic potential, following the Scientific Committee guidance already mentioned [43]. In the present case, there are available data on CYN genotoxicity following EFSA recommendations [38], while MC-LR, was classified by the IARC in group 2B [14]. Moreover, the two single toxicity studies dealing with CYN/MC-LR mixtures have shown an antagonistic effect regarding cytotoxicity [41] and genotoxicity [42] in HepG2 cells. In addition, the genotoxicity of CYN/MC-LR mixtures has not been previously evaluated following a complete battery of in vitro tests, and a potential antagonic result for the mixture could affect the risk evaluation.
The first assay included in the basic battery was the Ames test. The mixture did not show a mutagenic response at the conditions tested, similar to previous results obtained for CYN [35]. In both cases, TA102 was one of the most responsive strains although the mutagenic indexes (MI) was always lower than 2. As CYN concentrations were similar in both studies, the results obtained suggest that MC-LR does not contribute to the genotoxicity of the mixture. This agrees with Sieroslawska [50] who found no effects in the Ames microplate format mutagenicity assay for pure MC-LR, pure CYN and neither for a mixture CYN/MC-LR/Anatoxin-a (1 µg/mL each).
A MN test is included in the basic battery to cover potential structural and numerical chromosome aberrations in addition to the Ames test. Chromosomal abnormalities, such as increased chromosomal breakage or chromosomal loss, are associated with enhanced risk of carcinogenesis and progression of neoplastic transformation [51]. In the case of the CYN/MC-LR mixture, an increase of MN was only observed with S9 fraction, similar to CYN in an individual exposure [38]. Moreover, single CYN showed this enhancement from lower concentrations (0.25 µg/mL) whereas the mixture showed this effect at 1 µg/mL CYN (+10 µg/mL MC-LR). This finding suggests that MC-LR ameliorates in this case the CYN response. However, in the scientific literature, there are contradictory data on the genotoxic potential of MC-LR by the MN assay. Thus, Abramsson-Zetterberg et al. [52] did not observe changes in vitro (in human lymphocytes, up to 2.0 mg extract of freeze-dried cyanobacteria per ml cell culture) and in vivo (in mice up to 55 µg/kg bw pure MC-LR by i.p. administration). On the contrary, Dias et al. [15] found that MC-LR treatment (5 and 20 μM) caused a significant induction in the MN frequency in kidney- (Vero-E6) and liver-derived (HepG2) cell lines and, interestingly, a similar positive effect was observed in mouse reticulocytes (37.5 μg MCLR/kg, i.p. route). Huang et al. [53] found that MC-LR induced a 1.6-fold increase in MN frequency in a human–hamster hybrid AL cell line after 30 days of exposure to 0.1 μg/mL (but no changes after 1 and 3 days of exposure). Regarding cyanobacterial mixtures, there is a single study that explored the MN induction of a CYN/MC-LR mixture and found that 0.5 µg/mL CYN + 1 µg/mL MC-LR induced a significant increase of MN in HepG2 cells [42].
Additional in vitro methods were applied (MLA and Comet assay), following the recommendations of [43], because the results obtained with the Ames test and the MN assay did not allow confirmation of the genotoxicity (or absence of genotoxicity) of the mixture.
The MLA results did not provide new evidence as no changes were observed at any of the conditions tested. Puerto et al. [38] also did not find a mutagenic response when single CYN exposure was evaluated. Zhan et al. [54] performed the TK gene mutation assay in the TK6 human lymphoblastoid cell line for MC-LR and found TK mutation in a concentration-dependent manner. The MLA is the most extensively used of the different in vitro mammalian gene-mutation assays [55]. Both MN assay and MLA are performed in the same experimental model, the L5178YTk+/− cells, recommended in the Organization for Economic Co-operation and Development OECD guidelines. It seems that MN assay is more sensitive, or that the potential mutagenicity of the evaluated cyanotoxins is related mostly with chromosomal aberrations and to a lesser extent, with gene (point) mutations. However, the MLA detects intragenic events, mainly point mutations, and also loss of heterozygosity. This can result from the entire Tk gene loss, leading to karyotypically visible deletions and rearrangements of the Tk+/− bearing chromosome [56]. These features make the MLA especially useful to evaluate the ability of chemicals to induce a broad variety of mutational events [57].
Similarly, the Comet assay also did not evidence DNA damage induced by the CYN/MC-LR mixture in any of the procedures performed, that is, the standard assay and the modified version to detect oxidative DNA-damage. CYN alone showed the same response in similar conditions: Experimental model, concentrations and times of exposure [38]. Other authors, however, have observed genotoxic effects for CYN in the Comet assay both in vitro [31,33,34] and in vivo [58,59]. MC-LR single exposure was also reported to induce DNA strand breaks by the comet assay in vitro [15,60,61,62] and in vivo [15]. There is a single study [42] that showed DNA strand breaks induction by cyanobacterial mixtures CYN/MC-LR in HepG2 cells after 24 h exposure, but to lesser degree than CYN. Once more, it seems that MC-LR ameliorates the genotoxicity induced by CYN.
Overall, it is difficult to derive any statement about the (geno)toxicity of CYN/MC-LR mixtures because the available studies in the scientific literature for the individual toxins mostly use different model systems and exposure concentrations. This is the first time that a thorough investigation using 4 different mutagenicity and genotoxicity assays has been performed for cyanobacterial mixtures and the results indicate that the mixture does not show a higher genotoxicity compared to CYN. However, taking into account that MC-LR was classified in the group 2B by the IARC due to its tumour promotion mechanism [14], caution is required when trying to elucidate its role in the mixture toxicity.
As Zouaoui et al. [63] highlighted, the type of interactions among toxins could be related with the different chemical structures and properties, and the competition or not, for the same cell receptor. It is, therefore, required to explore the cyanotoxins mechanisms of action when they are alone or in mixtures. In this case, the investigated cyanotoxins showed different toxicity mechanisms but also share others, such as the oxidative stress induction. Thus, Gutiérrez-Praena et al. [41] suggested that the depletion of GSH could be related with the antagonistic response as it could decrease the uptake ratio of CYN. Other authors such as Hercog et al. [42] pointed out to their different kinetics as MC-LR and CYN are detoxified and toxified, respectively, after [30,64] and also to the compromise of DNA repair mechanisms induced by MC-LR [65]. In any case, further studies would be required to fully understand the mechanisms involved in the toxicity of mixtures. Moreover, despite using the battery proposed by EFSA [43], considering the results obtained (positive effects only in one of the four tests performed) and the limitations of in vitro genotoxicity tests to predict the in vivo situation suggested by Nesslany [66], the further step would be to assess in vivo the genotoxicity of cyanobacterial mixtures.
4. Conclusions
The in vitro mutagenicity and genotoxicity showed by CYN/MC-LR mixtures do not differ substantially from that observed for CYN tested individually. This effect was evident only when S9 fraction was used, indicating the relevance of CYN on the mixture toxicity at the conditions tested. The increased knowledge of cyanotoxins mixture genotoxic potential would contribute to perform more realistic risk evaluations.
5. Materials and Methods
5.1. Chemicals and Reagents
Cylindrospermopsin (95% purity) and Microcystin-LR (99% purity) standards were provided by Alexis Corporation (Lausen, Switzerland). Chemicals for different assays were supplied by Gibco (Biomol, Sevilla, Spain), Sigma -Aldrich (Madrid, Spain), C-Viral S.L. (Sevilla, Spain) and Moltox (Trinova, Biochem, Germany).
5.2. Cells and Culture Conditions
Five Salmonella typhimurium histidine-auxotrophic strains TA97A, TA98, TA100, TA102 and TA1535 were used for the Ames test. L5178Y Tk+/− mouse lymphoma cells used for the MN test and MLA were originally provided by Dr. Oliver Gillardeux (Safoni-Synthélabo, Paris, France). Caco-2 cell line, used for standard and enzyme-modified comet assays, come from a human colon adenocarcinoma (ATCC© HTB-37). L5178Y Tk+/− cells and Caco-2 cells were maintained in an incubator with 5% CO2 and 95% relative humidity at 37 °C.
5.3. Test Solutions
Stock solution of CYN (1000 µg/mL) and MC-LR (4000 µg/mL) were prepared in milliQ sterile water and water: MeOH, respectively and stored at less than 4 °C. The exposure concentration solutions were prepared by dilution in sterile MilliQ water (Ames test), RPMI 1640 medium (MN and MLA assays) or MEM medium (standard and enzyme-modified comet assays). Test concentrations were selected individually for every test as they need to fulfil toxicity criteria in each of the experimental models used. The selected concentrations of MC-LR were 10 times higher than that of CYN since MC-LR is normally more abundant in nature [1,2,67].
5.4. Bacterial Reverse Mutation Test (Ames Test)
The Ames test was performed following the OECD Guideline 471 [44] and Maron et al. [68] with minor modifications as follows. Five Salmonella typhimurium histidine-auxotrophic strains (TA97, TA98, TA100, TA102 and TA1535) obtained from TRINOVA BIOCHEM GmbH (Germany) were cultured following the provider instructions. The mutagenic activity of CYN/MC-LR mixtures was assessed in the absence and presence of the external metabolic activation system from rat livers (S9 fraction). Each experiment was conducted with five growing concentrations of CYN/MC-LR mixtures (0.125–2 µg/mL CYN and 1.25–20 µg/mL MC-LR) selected according to the results obtained by Puerto et al. [38] when CYN mutagenicity was assessed by the Ames test. Also, a negative control (distilled sterile water), solvent controls (MeOH and DMSO) and a positive control for each strain in accordance with the presence or absence of S9 fraction were included. Nine-aminoacridine (50 µg/plate) was the positive control for TA97A without S9 fraction; 2-Nitrofluorene (2-NF) (0.1 µg/plate) for TA98; sodium azide (NaN3) (1 µg/plate) for TA100 and TA1535; and mitomycin C (MMC) (2.5 µg/plate) for TA102. The positive control in the presence of S9 fraction was 2-aminofluorene (2-AF) (20 µg/plate) for all strains. At least 3 independent experiments were performed using triplicate plates for each test concentration. Results are expressed as revertant colonies and mutagenic indexes (MI).
5.5. Micronucleus Test (MN)
The MN test was carried out following the OECD guideline 487 [45]. L5178Y Tk+/− cells were seeded at a density of 2.0 × 105 cell/mL and exposed to five different concentrations of CYN/MC-LR mixture (0.084–1.35 µg/mL CYN and 0.84–13.5 µg/mL MC-LR in the absence of S9 fraction for 24 h, and 0.125–2 µg/mL CYN and 1.25–20 µg/mL MC-LR for 4 h in the presence of S9 fraction). These concentrations were selected taking into account previous results obtained in cytotoxicity assays and carried out according to the OECD Guideline 487 [45]. The RPMI medium was used as negative control; MeOH as vehicle control; and 0.0625 µg/mL MMC and 0.0125 colchicine (without S9 fraction) and 8 µg/mL cyclophosphamide (CP) (with S9 fraction) as positive controls. Cells were exposed to CYN/MC-LR mixtures (4 or 24 h, with and without S9 mix, respectively), then exposed to cythochalasin B (Cyt-B) (6 µg/mL) for 20 h to block cytokinesis and obtain binucleated cells. Afterward, cells were exposed to a hypotonic treatment with KCl and fixed. Subsequently, cells were dripped on slides and stained with Giemsa 10%. Quantification of binucleated cells with micronuclei (BNMN) and cytokinesis-block proliferation index (CBPI) were carried out following the OECD 487 guideline [45] by analysing at least 2000 binucleated cells/concentration.
5.6. Mouse Lymphoma Thymidine-Kinase Assay (MLA)
The MLA assay was performed in agreement to OECD Guideline 490 [46] and Maisanaba et al. [69]. Each experiment includes a negative control (fresh media), a solvent control (MeOH), a positive control (methylmetanosulfonate, MMS 10 µg/mL in absence of S9 fraction and cyclophosphamide, CP 3 µg/mL in presence of S9 fraction), five concentrations of CYN/MC-LR mixture in the absence of S9 fraction for 4 and 24 h assays (0.04–0.67 µg/mL CYN and 0.4–6.7 µg/mL MC-LR) and six concentrations in the presence of S9 fraction for 4 h assay (0.04–1.35 µg/mL CYN and 0.4–13.5 µg/mL MC-LR). These concentrations were selected in accordance with previous tests performed to define the cytotoxicity of CYN/MC-LR mixtures by the relative total growth (RTG) after 4 and 24 h of treatment without S9 fraction. According to the ICH Expert Working Group [70], the highest concentration chosen for the mutagenicity test must be higher than 10–20% of RTG. RTG values were employed to determine the acceptability of the toxicity at each concentration. Cells were seeded at 104 cells/mL in 96-well plates (two replicates per experimental group) to assess the viability and mutagenicity. The mutation analysis cells were exposed to 4 µg/mL trifluorothymidine (TFT), and both the viability plates and the mutagenicity plates were incubated at 37 °C and 5% CO2 for 12 days. Afterwards, viable colonies and TFT mutation colonies were counted. Thiazolyl blue tetrazolium (MTT) (2.5 mg/mL) was added to wells to facilitate the counting of mutant colonies, and the plates were incubated for 4 h. According to Honma et al. [71], the size of the colonies were described as small (less than 1/3 of well diameter) or large (higher than 1/3 of well diameter) colonies. Moreover, the induced mutant frequency (IMF) was also analyzed.
5.7. Standard and Enzyme-Modified Comet Assay
The standard comet assay was carried out to evaluate genotoxicity, and a modified version of this assay with endonuclease III (Endo III) and formamidopyrimidine (FPG), which recognise oxidized pyrimidines and purines, was performed to determine oxidative DNA damage, respectively.
The standard and enzyme-modified comet assays were carried out to assess the genotoxicity of CYN/MC-LR mixtures, as previously described by Collins et al. [72] and Llana-Ruiz-Cabello et al. [73]. Caco-2 cell line was selected as cyanotoxins are food contaminants and it is a commonly used enterocytic model in toxicological studies [74,75,76,77]. Cells were seeded at 3.5 × 105 cells/mL into 24-well tissue culture plates and treated with increasing concentrations of CYN/MC-LR mixtures (0.6–2.5 µg/mL CYN and 6–25 µg/mL MC-LR) for 24 h and 48 h, according to the value obtained in the most sensitive cytotoxicity endpoint assayed [76]. Cells were treated with a negative control (medium) and a positive control (H2O2 100 μM) for standard comet assay and Endo III sensitives sites and Ro 19-8022 (2 µM) for FPG-sensitive sites. After exposure time, cells were washed, trypsinized and re-suspended in phosphate buffer saline (PBS) at 2.5 × 105 cell/mL. Cells suspensions were mixed with 1% (w/v) low-melting-agarose in PBS and placed on agarose precoated glass slides. Afterwards, lysis, incubation with Endo III and FPG (in the case of modified comet assay), denaturing, electrophoresis, neutralization, washing, fixation, dying, staining with SYBR Gold and quantification of nuclei were performed.
Olympus BX61 (fluorescence microscope) with the comet assay IV software (Perceptive Instruments, UK) available at the Microscopy Service of the University of Seville (CITIUS) was used to score the cells. The results were expressed as mean % DNA in tail respect to the negative control group. The % DNA in tail represents the amount of DNA breakage. Both types of comet assays (standard and modified) were performed in at least three independent experiments and using a triplicate/experiment.
5.8. Statistical Analysis
The statistical analysis was performed with Graph-Pad InStat software (Graph-Pad Software Inc., La Jolla, CA, USA). The non-parametric Wilcoxon matched-pairs signed-rank test was employed to compare the exposed samples with the negative control. Differences were considered significant at * p < 0.05, ** p < 0.01 and *** p < 0.001, respectively.
Acknowledgments
Spanish Ministerio de Economía y Competitividad for the project AGL2015-64558-R, MINECO/FEDER, UE, and for the grant FPI (BES-2016-078773) awarded to Leticia Díez-Quijada Jiménez.
Author Contributions
Conceptualization, A.M.C. and Á.J.; methodology, L.D.-Q., M.P. and A.I.P.; software, L.D.-Q., M.P. and A.I.P.; formal analysis, L.D.-Q., M.P., A.I.P., Á.J. and A.M.C.; investigation, L.D.-Q., M.P. and A.I.P.; resources, A.M.C. and Á.J.; writing—original draft preparation, L.D.-Q., Á.J. and A.M.C.; writing—review and editing, A.M.C. and Á.J.; supervision, A.M.C. and Á.J.; project administration, A.M.C. and Á.J.; funding acquisition, A.M.C. and Á.J.
Funding
This research was funded by the SPANISH MINISTERIO DE ECONOMÍA Y COMPETITIVIDAD (AGL2015-64558-R, MINECO/FEDER, UE); by the FPI grant number BES-2016-078773 awarded to Leticia Díez-Quijada Jiménez.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
A genotoxic and mutagenic assessment of cyanotoxin binary mixtures of CYN and MC-LR was performed by a battery of in vitro tests. Results showed a similar response to CYN individually. Thus, evaluation of mixtures is required as interactions can occur.
References
- 1.Buratti F.M., Manganelli M., Vichi S., Stefanelli M., Scardala S., Testai E., Funari E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017;91:1049–1130. doi: 10.1007/s00204-016-1913-6. [DOI] [PubMed] [Google Scholar]
- 2.Diez-Quijada L., Puerto M., Gutierrez-Praena D., Llana-Ruiz-Cabello M., Jos A., Camean A.M. Microcystin-RR: Occurrence, content in water and food and toxicological studies. A review. Environ. Res. 2019;168:467–489. doi: 10.1016/j.envres.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Diez-Quijada L., Prieto A.I., Guzman-Guillen R., Jos A., Camean A.M. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019;125:106–132. doi: 10.1016/j.fct.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Codd G.A., Meriluoto J., Metcalf J.S. Introduction: Cyanobacteria, cyanotoxins, their human impact, and risk management. Handb. Cyanobact. Monit. Cyanotoxin Anal. 2016:1–8. doi: 10.1002/9781119068761.ch1. [DOI] [Google Scholar]
- 5.Testai E., Buratti F.M., Funari E., Manganelli M., Vichi S., Arnich N., Biré R., Fessard V., Sialehaamoa A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016;13:1–309. doi: 10.2903/sp.efsa.2016.EN-998. [DOI] [Google Scholar]
- 6.Roy-Lachapelle A., Solliec M., Bouchard M.F., Sauvé S. Detection of Cyanotoxins in Algae Dietary Supplements. Toxins. 2017;9:76. doi: 10.3390/toxins9030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spoof L., Catherine A. Appendix 3: Tables of microcystins and nodularins. Handb. Cyanobact. Monit. Cyanotoxin Anal. 2016:526–537. doi: 10.1002/9781119068761.app3/pdf. [DOI] [Google Scholar]
- 8.Fischer W.J., Altheimer S., Cattori V., Meier P.J., Dietrich D.R., Hagenbuch B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005;203:257–263. doi: 10.1016/j.taap.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.MacKintosh C., Beattie K.A., Klumpp S., Cohen P., Codd G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264:187–192. doi: 10.1016/0014-5793(90)80245-E. [DOI] [PubMed] [Google Scholar]
- 10.Prieto A.I., Jos A., Pichardo S., Moreno I., Cameán A.M. Protective role of vitamin E on the microcystin-induced oxidative stress in tilapia fish (Oreochromis niloticus) Environ. Toxicol. Chem. 2008;27:1152–1159. doi: 10.1897/07-496.1. [DOI] [PubMed] [Google Scholar]
- 11.Moreno I., Mate A., Repetto G., Vázquez C., Cameán A.M. Influence of microcystin-LR on the activity of membrane enzymes in rat intestinal mucosa. J. Physiol. Biochem. 2003;59:293–299. doi: 10.1007/BF03179887. [DOI] [PubMed] [Google Scholar]
- 12.Atencio L., Moreno I., Prieto A.I., Moyano R., Molina A.M., Camean A.M. Acute effects of microcystins MC-LR and MC-RR on acid and alkaline phosphatase activities and pathological changes in intraperitoneally exposed tilapia fish (Oreochromis sp.) Toxicol. Pathol. 2008;36:449–458. doi: 10.1177/0192623308315356. [DOI] [PubMed] [Google Scholar]
- 13.Valério E., Vasconcelos V., Campos A. New insights on the mode of action of microcystins in animal cells-a review. Mini Rev. Med. Chem. 2016;16:1032–1041. doi: 10.2174/1389557516666160219130553. [DOI] [PubMed] [Google Scholar]
- 14.Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. [(accessed on 10 April 2019)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK326544/pdf/Bookshelf_NBK326544.pdf.
- 15.Dias E., Louro H., Pinto M., Santos T., Antunes S., Pereira P., Silva M.J. Genotoxicity of microcystin-LR in in vitro and in vivo experimental models. BioMed. Res. Int. 2014 doi: 10.1155/2014/949521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Žegura B. An overview of the mechanisms of microcystin-LR genotoxicity and potential carcinogenicity. Mini Rev. Med. Chem. 2016;16:1042–1062. doi: 10.2174/1389557516666160308141549. [DOI] [PubMed] [Google Scholar]
- 17.Kokociński M., Cameán A.M., Carmeli S., Guzmán-Guillén R., Jos Á., Mankiewicz-Boczek J., Metcalf J.S., Moreno I.M., Prieto A.I., Sukenik A. Cylindrospermopsin and congeners. Handb. Cyanobact. Monit. Cyanotoxin Anal. 2017:127–137. doi: 10.1002/9781119068761.ch12. [DOI] [Google Scholar]
- 18.Chiswell R.K., Shaw G.R., Eaglesham G., Smith M.J., Norris R.L., Seawright A.A., Moore M.R. Stability of cylindrospermopsin, the toxin from the cyanobacterium, Cylindrospermopsis raciborskii: Effect of pH, temperature, and sunlight on decomposition. Environ. Toxicol. Int. J. 1999;14:155–161. doi: 10.1002/(SICI)1522-7278(199902)14:1<155::AID-TOX20>3.0.CO;2-Z. [DOI] [Google Scholar]
- 19.Falconer I.R., Humpage A.R. Cyanobacterial (blue-green algal) toxins in water supplies: Cylindrospermopsins. Environ. Toxicol. 2006;21:299–304. doi: 10.1002/tox.20194. [DOI] [PubMed] [Google Scholar]
- 20.Guzmán-Guillén R., Puerto M., Gutiérrez-Praena D., Prieto A., Pichardo S., Jos Á., Campos A., Vasconcelos V., Cameán A. Potential use of chemoprotectants against the toxic effects of cyanotoxins: A review. Toxins. 2017;9:175. doi: 10.3390/toxins9060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinojosa M., Gutiérrez-Praena D., Prieto A., Guzmán-Guillén R., Jos A., Cameán A. Neurotoxicity induced by microcystins and cylindrospermopsin: A review. Sci. Total Environ. 2019;668:547–565. doi: 10.1016/j.scitotenv.2019.02.426. [DOI] [PubMed] [Google Scholar]
- 22.Poniedziałek B., Rzymski P., Kokociński M. Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environ. Toxicol. Pharmacol. 2012;34:651–660. doi: 10.1016/j.etap.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Pichardo S., Devesa V., Puerto M., Vélez D., Cameán A.M. Intestinal transport of Cylindrospermopsin using the Caco-2 cell line. Toxicol. In Vitro. 2017;38:142–149. doi: 10.1016/j.tiv.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Terao K., Ohmori S., Igarashi K., Ohtani I., Watanabe M., Harada K., Ito E., Watanabe M. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezakia natans. Toxicon. 1994;32:833–843. doi: 10.1016/0041-0101(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 25.Froscio S.M., Humpage A.R., Burcham P.C., Falconer I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. Int. J. 2003;18:243–251. doi: 10.1002/tox.10121. [DOI] [PubMed] [Google Scholar]
- 26.Runnegar M.T., Kong S.-M., Zhong Y.-Z., Lu S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 1995;49:219–225. doi: 10.1016/S0006-2952(94)00466-8. [DOI] [PubMed] [Google Scholar]
- 27.Gutiérrez-Praena D., Pichardo S., Jos Á., Cameán A.M. Toxicity and glutathione implication in the effects observed by exposure of the liver fish cell line PLHC-1 to pure cylindrospermopsin. Ecotoxicol. Environ. Saf. 2011;74:1567–1572. doi: 10.1016/j.ecoenv.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Puerto M., Jos A., Pichardo S., Gutiérrez-Praena D., Cameán A.M. Acute effects of pure cylindrospermopsin on the activity and transcription of antioxidant enzymes in tilapia (Oreochromis niloticus) exposed by gavage. Ecotoxicology. 2011;20:1852–1860. doi: 10.1007/s10646-011-0723-0. [DOI] [PubMed] [Google Scholar]
- 29.Poniedziałek B., Rzymski P., Karczewski J. The role of the enzymatic antioxidant system in cylindrospermopsin-induced toxicity in human lymphocytes. Toxicol. In Vitro. 2015;29:926–932. doi: 10.1016/j.tiv.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Norris R., Seawright A., Shaw G., Senogles P., Eaglesham G., Smith M., Chiswell R., Moore M. Hepatic xenobiotic metabolism of cylindrospermopsin in vivo in the mouse. Toxicon. 2002;40:471–476. doi: 10.1016/S0041-0101(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 31.Humpage A.R., Fontaine F., Froscio S., Burcham P., Falconer I.R. Cylindrospermopsin genotoxicity and cytotoxicity: Role of cytochrome P-450 and oxidative stress. J. Toxicol. Environ. Health A. 2005;68:739–753. doi: 10.1080/15287390590925465. [DOI] [PubMed] [Google Scholar]
- 32.Humpage A.R., Fenech M., Thomas P., Falconer I.R. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat. Res. 2000;472:155–161. doi: 10.1016/S1383-5718(00)00144-3. [DOI] [PubMed] [Google Scholar]
- 33.Štraser A., Filipič M., Žegura B. Genotoxic effects of the cyanobacterial hepatotoxin cylindrospermopsin in the HepG2 cell line. Arch. Toxicol. 2011;85:1617–1626. doi: 10.1007/s00204-011-0716-z. [DOI] [PubMed] [Google Scholar]
- 34.Žegura B., Gajski G., Štraser A., Garaj-Vrhovac V. Cylindrospermopsin induced DNA damage and alteration in the expression of genes involved in the response to DNA damage, apoptosis and oxidative stress. Toxicon. 2011;58:471–479. doi: 10.1016/j.toxicon.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Štraser A., Metka F., Matjaž N., Bojana Ž. Double strand breaks and cell-cycle arrest induced by the cyanobacterial toxin cylindrospermopsin in HepG2 cells. Mar. Drugs. 2013;11:3077–3090. doi: 10.3390/md11083077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sieroslawska A., Rymuszka A. Cylindrospermopsin induces oxidative stress and genotoxic effects in the fish CLC cell line. J. Appl. Toxicol. 2015;35:426–433. doi: 10.1002/jat.3040. [DOI] [PubMed] [Google Scholar]
- 37.Pichardo S., Cameán A.M., Jos Á.M. In vitro toxicological assessment of cylindrospermopsin: A review. Toxins. 2017;9:402. doi: 10.3390/toxins9120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puerto M., Prieto A.I., Maisanaba S., Gutierrez-Praena D., Mellado-Garcia P., Jos A., Camean A.M. Mutagenic and genotoxic potential of pure Cylindrospermopsin by a battery of in vitro tests. Food Chem. Toxicol. 2018;121:413–422. doi: 10.1016/j.fct.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Bittencourt-Oliveira M., Carmo D., Piccin-Santos V., Moura A.N., Aragão-Tavares N.K., Cordeiro-Araújo M.K. Cyanobacteria, microcystins and cylindrospermopsin in public drinking supply reservoirs of Brazil. An. Acad. Bras. Cienc. 2014;86:297–310. doi: 10.1590/0001-3765201302512. [DOI] [PubMed] [Google Scholar]
- 40.Jančula D., Straková L., Sadílek J., Maršálek B., Babica P. Survey of cyanobacterial toxins in Czech water reservoirs—the first observation of neurotoxic saxitoxins. Environ. Sci. Pollut. Res. Int. 2014;21:8006–8015. doi: 10.1007/s11356-014-2699-9. [DOI] [PubMed] [Google Scholar]
- 41.Gutiérrez-Praena D., Guzmán-Guillén R., Pichardo S., Moreno F.J., Vasconcelos V., Jos Á., Cameán A.M. Cytotoxic and morphological effects of microcystin-LR, cylindrospermopsin, and their combinations on the human hepatic cell line HepG2. Environ. Toxicol. 2018;34:240–251. doi: 10.1002/tox.22679. [DOI] [PubMed] [Google Scholar]
- 42.Hercog K., Maisanaba S., Filipič M., Jos Á., Cameán A.M., Žegura B. Genotoxic potential of the binary mixture of cyanotoxins microcystin-LR and cylindrospermopsin. Chemosphere. 2017;189:319–329. doi: 10.1016/j.chemosphere.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 43.EFSA S.C. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA J. 2011;9 doi: 10.2903/j.efsa.2011.2379. [DOI] [Google Scholar]
- 44.OECD Guidelines for the Testing of Chemicals, Bacterial Reverse Mutation Test. [(accessed on 1 April 2019)]; Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948418.pdf.
- 45.OECD Guidelines for the Testing of Chemicals, In Vitro Mammalian Cell Micronucleus Test. [(accessed on 1 April 2019)]; Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-tg487-2014-508.pdf.
- 46.OECD Guidelines for the Testing of Chemicals, In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene. [(accessed on 1 April 2019)]; Available online: https://www.oecd-ilibrary.org/docserver/9789264264908-en.pdf?expires=1559275406&id=id&accname=guest&checksum=323552A68EC3041C6EA6F9E8A7ACF632.
- 47.EFSA Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005;3:282. doi: 10.2903/j.efsa.2005.282. [DOI] [Google Scholar]
- 48.Kamath G.H., Rao K. Genotoxicity guidelines recommended by International Conference of Harmonization (ICH) Methods Mol. Biol. 2013;1044:431–458. doi: 10.1007/978-1-62703-529-3_24. [DOI] [PubMed] [Google Scholar]
- 49.EFSA S.C., More S., Bampidis V., Benford D., Boesten J., Bragard C., Halldorsson T., Hernandez-Jerez A., Hougaard-Bennekou S., Koutsoumanis K. Genotoxicity assessment of chemical mixtures. EFSA J. 2019;17:5519. doi: 10.2903/j.efsa.2019.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sieroslawska A. Assessment of the mutagenic potential of cyanobacterial extracts and pure cyanotoxins. Toxicon. 2013;74:76–82. doi: 10.1016/j.toxicon.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Guerin M.R. Energy Sources of Polycyclic Aromatic Hydrocarbons. [(accessed on 1 April 2019)]; Available online: https://www.osti.gov/servlets/purl/7303055.
- 52.Abramsson-Zetterberg L., Sundh U.B., Mattsson R. Cyanobacterial extracts and microcystin-LR are inactive in the micronucleus assay in vivo and in vitro. Mutat. Res. 2010;699:5–10. doi: 10.1016/j.mrgentox.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Huang P., Xu A. Genotoxic effects of microcystin-LR in mammalian cells; Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering; Beijing, China. 11–16 June 2009. [Google Scholar]
- 54.Zhan L., Sakamoto H., Sakuraba M., Wu D.-S., Zhang L.-S., Suzuki T., Hayashi M., Honma M. Genotoxicity of microcystin-LR in human lymphoblastoid TK6 cells. Mutat. Res. 2004;557:1–6. doi: 10.1016/j.mrgentox.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Moore M.M., Honma M., Clements J., Bolcsfoldi G., Cifone M., Delongchamp R., Fellows M., Gollapudi B., Jenkinson P., Kirby P. Mouse lymphoma thymidine kinase gene mutation assay: International Workshop on Genotoxicity Tests Workgroup report—Plymouth, UK 2002. Mutat. Res. 2003;540:127–140. doi: 10.1016/j.mrgentox.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Chen T., Harrington-Brock K., Moore M.M. Mutant frequencies and loss of heterozygosity induced by N-ethyl-N-nitrosourea in the thymidine kinase gene of L5178Y/Tk+/−-3.7.2C mouse lymphoma cells. Mutagenesis. 2002;17:105–109. doi: 10.1093/mutage/17.2.105. [DOI] [PubMed] [Google Scholar]
- 57.Demir E., Kaya B., Soriano C., Creus A., Marcos R. Genotoxic analysis of four lipid-peroxidation products in the mouse lymphoma assay. Mutat. Res. 2011;726:98–103. doi: 10.1016/j.mrgentox.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Shen X., Lam P., Shaw G., Wickramasinghe W. Genotoxicity investigation of a cyanobacterial toxin, cylindrospermopsin. Toxicon. 2002;40:1499–1501. doi: 10.1016/S0041-0101(02)00151-4. [DOI] [PubMed] [Google Scholar]
- 59.Bazin E., Huet S., Jarry G., Hégarat L.L., Munday J.S., Humpage A.R., Fessard V. Cytotoxic and genotoxic effects of cylindrospermopsin in mice treated by gavage or intraperitoneal injection. Environ. Toxicol. 2012;27:277–284. doi: 10.1002/tox.20640. [DOI] [PubMed] [Google Scholar]
- 60.Žegura B., Sedmak B., Filipič M. Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon. 2003;41:41–48. doi: 10.1016/S0041-0101(02)00207-6. [DOI] [PubMed] [Google Scholar]
- 61.Lankoff A., Krzowski Ł., Głąb J., Banasik A., Lisowska H., Kuszewski T., Góźdź S., Wójcik A. DNA damage and repair in human peripheral blood lymphocytes following treatment with microcystin-LR. Mutat. Res. 2004;559:131–142. doi: 10.1016/j.mrgentox.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Žegura B., Gajski G., Štraser A., Garaj-Vrhovac V., Filipič M. Microcystin-LR induced DNA damage in human peripheral blood lymphocytes. Mutat. Res. 2011;726:116–122. doi: 10.1016/j.mrgentox.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Zouaoui N., Mallebrera B., Berrada H., Abid-Essefi S., Bacha H., Ruiz M.-J. Cytotoxic effects induced by patulin, sterigmatocystin and beauvericin on CHO–K1 cells. Food Chem. Toxicol. 2016;89:92–103. doi: 10.1016/j.fct.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Pflugmacher S., Wiegand C., Oberemm A., Beattie K.A., Krause E., Codd G.A., Steinberg C.E. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Biophys. Acta. 1998;1425:527–533. doi: 10.1016/S0304-4165(98)00107-X. [DOI] [PubMed] [Google Scholar]
- 65.Lankoff A., Bialczyk J., Dziga D., Carmichael W., Gradzka I., Lisowska H., Kuszewski T., Gozdz S., Piorun I., Wojcik A. The repair of gamma-radiation-induced DNA damage is inhibited by microcystin-LR, the PP1 and PP2A phosphatase inhibitor. Mutagenesis. 2006;21:83–90. doi: 10.1093/mutage/gel002. [DOI] [PubMed] [Google Scholar]
- 66.Nesslany F. The current limitations of in vitro genotoxicity testing and their relevance to the in vivo situation. Food Chem. Toxicol. 2017;106:609–615. doi: 10.1016/j.fct.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 67.De La Cruz A.A., Hiskia A., Kaloudis T., Chernoff N., Hill D., Antoniou M.G., He X., Loftin K., O’Shea K., Zhao C., et al. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process. Impacts. 2013;15:1979. doi: 10.1039/c3em00353a. [DOI] [PubMed] [Google Scholar]
- 68.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 69.Maisanaba S., Prieto A.I., Puerto M., Gutiérrez-Praena D., Demir E., Marcos R., Cameán A.M. In vitro genotoxicity testing of carvacrol and thymol using the micronucleus and mouse lymphoma assays. Mutat. Res. 2015;784:37–44. doi: 10.1016/j.mrgentox.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 70.International Conferences on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use. [(accessed on 1 April 2019)]; Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step4/S2R1_Step4.pdf.
- 71.Honma M., Hayashi M., Shimada H., Tanaka N., Wakuri S., Awogi T., Yamamoto K.I., Kodani N.-U., Nishi Y., Nakadate M. Evaluation of the mouse lymphoma Tk assay (microwell method) as an alternative to the in vitro chromosomal aberration test. Mutagenesis. 1999;14:5–22. doi: 10.1093/mutage/14.1.5. [DOI] [PubMed] [Google Scholar]
- 72.Collins A.R., Azqueta A. Methods Cell Biology. Volume 112. Elsevier; Amsterdam, The Netherlands: 2012. Chapter 4: Single-cell gel electrophoresis combined with lesion-specific enzymes to measure oxidative damage to DNA; pp. 69–92. [Google Scholar]
- 73.LLana-Ruiz-Cabello M., Maisanaba S., Puerto M., Prieto A.I., Pichardo S., Jos Á., Cameán A.M. Evaluation of the mutagenicity and genotoxic potential of carvacrol and thymol using the Ames Salmonella test and alkaline, Endo III-and FPG-modified comet assays with the human cell line Caco-2. Food Chem. Toxicol. 2014;72:122–128. doi: 10.1016/j.fct.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Sambuy Y., De Angelis I., Ranaldi G., Scarino M., Stammati A., Zucco F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 75.Puerto M., Pichardo S., Jos Á., Cameán A.M. Comparison of the toxicity induced by microcystin-RR and microcystin-YR in differentiated and undifferentiated Caco-2 cells. Toxicon. 2009;54:161–169. doi: 10.1016/j.toxicon.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 76.Puerto M., Pichardo S., Jos Á., Cameán A.M. Microcystin-LR induces toxic effects in differentiated and undifferentiated Caco-2 cells. Arch. Toxicol. 2010;84:405–410. doi: 10.1007/s00204-010-0513-0. [DOI] [PubMed] [Google Scholar]
- 77.Gutiérrez-Praena D., Pichardo S., Jos Á., Moreno F.J., Cameán A.M. Biochemical and pathological toxic effects induced by the cyanotoxin Cylindrospermopsin on the human cell line Caco-2. Water Res. 2012;46:1566–1575. doi: 10.1016/j.watres.2011.12.044. [DOI] [PubMed] [Google Scholar]