Abstract
Background:
The low-expressive short (S) allele of a functional polymorphism (5-HTTLPR) within the serotonin (5-hydroxytriptamine; 5-HT) transporter gene (SLC6A4) has been associated with a reduced functioning of the brain 5-HT system relative to the long (L) allele. As a consequence, the S-allele is found to predispose individuals to a higher risk of sleep quality reduction and clinical insomnia.
Aims:
The present study investigated whether subchronic pre-sleep tryptophan administration could compensate for this predisposition by improving sleep in 5-HTTLPR S-allele carriers.
Methods:
In a double-blind placebo-controlled crossover design a sample of homozygous 5-HTTLPR S-allele (n = 47) and L-allele (n = 51) carriers were assessed for subjective (sleep diary) and objective (actigraphy) sleep during a treatment protocol consisting of 1 week of placebo (1000 mg/day) and 1 week of tryptophan administration (1000 mg/day).
Results:
The results support the sleep-promoting effects of tryptophan. Tryptophan improved objective sleep efficiency and objective wake after sleep onset irrespective of allelic variation. There was a marginally significant improvement of subjective sleep quality in the 5-HTTLPR S-allele group but not in the L-allele group following tryptophan relative to placebo intake. In contrast, a significantly poorer sleep quality in the S-allele as opposed to the L-allele group in the placebo condition was not observed in the tryptophan condition.
Conclusions:
Tryptophan augmentation promises to be a valuable treatment strategy for sleep impairments related to genetic deficiencies in 5-HT functioning.
Keywords: Sleep, insomnia, 5-HTTLPR, serotonin, tryptophan
Introduction
Sleep disturbances are highly prevalent in the general population. Consensus has been reached that around 30% of the adult population reports some form of sleep complaint (Ancoli-Israel and Roth, 1999; Leger et al., 2008) whereas approximately 6–8% meets the diagnostic criteria for clinical insomnia (Ohayon, 1997; Uhlig et al., 2014). This is of high clinical relevance as inadequate sleep contributes to a diversity of medical conditions (Cappuccio et al., 2010; Palagini et al., 2013; Schwartz et al., 1999) and is one of the primary risk factors for major depression (Baglioni et al., 2011). These findings emphasize the need to identify factors involved in the aetiology of sleep disturbances and develop adequate clinical interventions.
Genetic factors considerably contribute to the aetiology of sleep disturbances as demonstrated in heritability (Bastien and Morin, 2000; Beaulieu-Bonneau et al., 2007; Dauvilliers et al., 2005; Lind et al., 2015; Wing et al., 2012) and genome-wide association studies (Hammerschlag et al., 2017; Jansen et al., 2019; Lane et al., 2017). As a specific candidate gene, accumulating evidence suggests that a functional polymorphism (serotonin transporter gene-linked polymorphic region; 5-HTTLPR) within the promoter region of the serotonin (5-hydroxytriptamine; 5-HT) transporter gene (SLC6A4) modulates the risk for sleep impairment. This polymorphism regulates the transcriptional efficiency of the 5-HT transporter via an allelic variation: the short (S) allele is associated with reduced expression and functioning of the 5-HT transporter relative to the long (L) allele (Heils et al., 1996), although complex mechanisms may mediate a more diverse influence on 5-HT neurotransmission (Murphy and Lesch, 2008). As a consequence of this deficient 5-HT functioning, the 5-HTTLPR S-allele is found to predispose to risk of stress-related (Brummett et al., 2007; Huang et al., 2014) and depression-related (Polito et al., 2015) sleep disturbance as well as clinical insomnia (Deuschle et al., 2010; Huang et al., 2014), even though these findings have not always been consistently replicated (Barclay et al., 2011; van Dalfsen and Markus, 2015, 2019). In line with the involvement of 5-HT in sleep regulation (Ursin, 2002), these findings suggest that genetic influences on 5-HT neurotransmission considerably contribute to the aetiology of sleep impairment.
The observation that the 5-HTTLPR S-allele promotes sleep disturbance encourages the search for pharmacological interventions aimed to compensate for deficient 5-HT functioning. Brain 5-HT is synthesized in serotonergic neurons from the essential amino acid tryptophan, where it is first metabolized into the immediate 5-HT precursor 5-hydroxytryptophan (5-HTP) by the enzyme tryptophan hydroxylase. Since this enzyme is not fully saturated, it provides a rate-limiting step that controls further 5-HT synthesis depending on tryptophan availability (Fernstrom, 2012; Wurtman et al., 1980). In this context, the synthetic pathway of 5-HT is very sensitive to pharmacological increments in tryptophan availability, which are found to rapidly increase brain 5-HT levels (Eccleston et al., 1970; Fernstrom, 1983) even at low pharmaceutical dosage (Fernstrom and Wurtman, 1971). Specific effects of tryptophan on sleep regulation have been repeatedly demonstrated in both healthy subjects and those with insomnia including a reduced sleep onset latency, increased total sleep time and decreased wake after sleep onset (for review, see Hartmann and Greenwald, 1984; Schneider-Helmert and Spinweber, 1986; Silber and Schmitt, 2010). Taken together, these findings indicate that tryptophan augmentation holds promise as a pathway to compensate for the 5-HT-related sleep disturbances associated with the 5-HTTLPR S-allele. Although one previous investigation supports particular sleep-promoting effects in this allelic variant (van Dalfsen and Markus, 2015), studies including evening tryptophan administration, more detailed sleep assessment and within-subjects comparisons are desirable to further establish this pharmacogenetic effect.
The present study investigated whether subchronic pre-sleep tryptophan administration improves sleep depending on allelic variation in 5-HTTLPR. In a double-blind placebo-controlled crossover design a well-screened sample of homozygous S-allele and L-allele carriers were monitored for subjective (sleep diary) and objective (actigraphy) sleep during a treatment protocol consisting of 1 week of pre-sleep placebo (1000 mg/day) and 1 week of pre-sleep tryptophan administration (1000 mg/day). It is expected that tryptophan improves objectively and subjectively measured sleep, particularly in the low-expressive 5-HTTLPR S-allele.
Methods
Participants
Participants were obtained from a recently created 5-HTTLPR database (n = 804) and were carefully selected based on the following inclusion and exclusion criteria. Inclusion criteria encompassed: homozygous S’/S’ (S/S, S/Lg, Lg/Lg) or L’/L’ (La/La) 5-HTTLPR genotype. Exclusion criteria comprised: diabetes, pregnancy or breast feeding, bladder problems (physical irritation, bladder cancer), gastrointestinal diagnosis (diminished amount of gastric acid, diminished absorption in the upper bowel), psychiatric diagnosis, sleep disorder diagnosis, heavy snoring and shift work (contemporary or during the past month) as well as the use of sleep medication, antidepressants, antipsychotics or recreational drugs (during or within 1 month prior to study participation). The final sample (n = 98), consisting of S’/S’ (n = 47) and L’/L’ (n = 51) 5-HTTLPR genotypes, included 25 (25.5%) male and 73 (74.5%) female participants aged between 20 and 30 years of age (M = 22.21, SD = 2.00). The procedures followed were approved by the local ethics committee of Maastricht University (Ethical Review Committee Psychology and Neuroscience; ERCPN) and are in line with the guidelines laid down in the Declaration of Helsinki 1975, revised in 2013 (World Medical Association, 2013). Written informed consent was obtained from all subjects, and participants received financial compensation for their contribution to the study.
Genotyping
Sterile swabs (Omni Swabs; Whatman, Maidstone, United Kingdom) were used to acquire buccal cell samples for 5-HTTLPR determination. Isolation of genomic DNA was performed using QIamp DNA Mini Kits (Qiagen, Leusden, The Netherlands). Polymerase chain reaction protocol was followed for the subsequent 5-HTTLPR genotyping (Glatz et al., 2003). More recent findings revealed that fragments in the 5-HTTLPR L-allele affect the transcription of the 5-HT transporter including a high-expressive La and a low-expressive Lg variant (Nakamura et al., 2000). Consistent with previous research (Zalsman et al., 2006), tri-allelic variants were therefore reclassified into a functionally relevant bi-allelic model including S’/S’ (S/S, S/Lg, Lg/Lg), S’/L’ (S/La, Lg/La) and L’/L’ (La/La) respectively. Hardy–Weinberg equilibrium (HWE) was determined for the initial database and revealed that genotype frequencies of S’/S’ (n = 190), S’/L’ (n = 405) and L’/L’ (n = 209) did not differ from HWE (χ2 = 0.052, p = 0.820).
Procedure
The influence of tryptophan on objective and subjective sleep was assessed using a double-blind placebo-controlled crossover design. Homozygous S-allele and L-allele carriers completed a within-subjects treatment protocol consisting of 1 week of placebo and 1 week of tryptophan administration (1000 mg/day; administered 20 minutes before bedtime). Treatment order was randomized across participants and counterbalanced within 5-HTTLPR genotype groups. Sleep assessment comprised a daily sleep diary and actigraphy measurements during the night. Prior to the start of the study, participants completed general questionnaires and received verbal instructions regarding the treatment administration, sleep diary and actigraphy device. Participants were further asked to attain similar bedtimes and rising times throughout the experimental period.
Treatment
During the treatment protocol, participants self-administered two capsules every evening exactly 20 minutes prior to bedtime (i.e. lights out). Depending on the treatment week, capsules either contained tryptophan (500 mg per capsule, 1000 mg in total) or placebo (microcrystalline cellulose) but were identical in appearance. This administration interval allows for a substantial amount of tryptophan uptake as peak plasma levels are generally obtained in 1 to 2 hours after administration (Yuwiler et al., 1981). In order to assess treatment compliance participants were asked to write down the exact time of administration or indicate that they did not administer the respective capsules.
Sleep diary
The morning diary of the consensus sleep diary (CSD) was used for subjective sleep assessment (Carney et al., 2012). This daily questionnaire has been developed by leading sleep experts and provides a standardized sleep diary to improve the comparability of sleep studies (Carney et al., 2012). Apart from general bedtimes and rising times, this sleep diary included subjective reports of sleep onset latency, amount of nocturnal awakenings, wake after sleep onset, sleep quality (ranging from 1 very poor to 5 very good) and refreshed sleep (ranging from 1 not rested at all to 5 very well rested). Recent comparisons between insomnia patients and healthy controls support the validity and clinical utility of the CSD (Maich et al., 2016).
Actigraphy
Activity-based sleep–wake monitoring (i.e. actigraphy) was used for objective sleep assessment. Methodological research indicates that actigraphy is a valid and reliable method for objective sleep assessment that is particularly sensitive to detecting sleep alterations related to pharmacological interventions (for review, see Sadeh, 2011). Sleep data were recorded using the GT3X+ activity monitor (ActiGraph, Pensacola, Florida, USA). This device includes a tri-axial solid-state accelerometer to measure activity. The actigraph was worn on the wrist of the non-dominant hand according to common conventions. Data were recorded at a sampling rate of 30 Hz with a low- and high-pass frequency filter of 2.5 Hz and 0.25 Hz respectively. Recorded sleep data were processed using ActiLife software (ActiLife 6.0, ActiGraph, Pensacola, Florida, USA).
Bedtimes and rising times reported in the sleep diary were entered to indicate the sleep period for each respective night. Data for each night were visually inspected to determine whether the actigraph was worn during the night and corresponded with reported sleep times. Sleep–wake scoring of the data was performed in 1-minute epochs using a commonly used algorithm validated for the age of the present sample (Sadeh et al., 1994). The scored data were subsequently used to calculate the sleep parameters of interest including sleep onset latency, sleep efficiency, time in bed, wake after sleep onset, nocturnal awakenings and average awakening length. Polysomnographic comparisons revealed that a GT3X+ actigraphy device combined with the algorithm used in the present study produces a sensitivity, specificity and accuracy of 90%, 46% and 84% respectively (Slater et al., 2015).
Missing data
Data concerning the sleep diary and actigraphy variables were indicated as missing for a respective night if treatment compliance could not be ensured (placebo: M = 0.22, SD = 0.83; tryptophan: M = 0.22, SD = 0.78), either because the time of capsule administration was missing or the participant reported that the treatment was not administered. Data on actigraphy variables were further excluded for a respective night if the device was either not worn (placebo: M = 0.34, SD = 0.61; tryptophan: M = 0.31, SD = 0.58) or became detached during the night (placebo: M =0.03, SD = 0.17; tryptophan: M = 0.01, SD = 0.10), the sleep period could not be determined because the sleep diary bedtime or rising time was missing (placebo: M = 0.35, SD = 0.92; tryptophan: M = 0.34, SD = 0.93) or when the reported sleep period time was not corresponding with actigraphy data based on visual inspection (placebo: M = 0.59, SD = 1.02; tryptophan: M = 0.53, SD = 0.84). On average 12.64 (SD = 2.78) and 11.43 (SD = 2.94) of the 14 nights were included per participant for the sleep diary (placebo: M = 6.30, SD = 1.53; tryptophan: M = 6.35, SD = 1.43) and actigraphy data (placebo: M = 5.63, SD = 1.74; tryptophan: M = 5.80, SD = 1.49) respectively. This indicates that compliance in the present study was generally high.
Statistical analyses
Statistical analyses were conducted in SPSS (SPSS 24.0 statistics for Windows, IBM, Armonk, New York). Linear mixed model analyses including a random intercept (indicating repeated measurements for each participant) were performed to investigate whether the influence of treatment (tryptophan versus placebo) on subjective (sleep diary) and objective (actigraphy) sleep parameters varied as a function of 5-HTTLPR (S’/S’ versus L’/L’). Sleep variables of interest were retrieved from the subjective sleep diary reports (i.e. sleep onset latency, nocturnal awakenings, wake after sleep onset, total sleep time, sleep quality, refreshed sleep) and objective actigraphy recordings (i.e. sleep onset latency, sleep efficiency, total sleep time, wake after sleep onset, nocturnal awakenings). General influences of treatment were first explored in a linear mixed model that included treatment as a single predictor for sleep variables. The differential influence of treatment depending on 5-HTTLPR was subsequently investigated in a linear mixed model that included treatment, 5-HTTLPR and an interaction term of the two predictors. Simple main effects of treatment within 5-HTTLPR genotype groups and of 5-HTTLPR within treatment conditions were used to interpret significant interactions.
Results
Sample characteristics
Table 1 presents the sample characteristics in relation to allelic variation in 5-HTTLPR. As demonstrated, no significant mean differences were observed between the two genotype groups regarding relevant variables.
Table 1.
Sample characteristics M (SD).
| S’/S’ | L’/L’ | t-test | ||
|---|---|---|---|---|
| t(96) | p | |||
| n | 47 | 51 | ||
| Men | 11 | 14 | ||
| Women | 36 | 37 | ||
| Age | 22.53 (2.12) | 21.92 (1.86) | 1.514 | 0.133 |
| PSQI | 4.47 (1.97) | 4.10 (2.42) | 0.820 | 0.414 |
| ISI | 5.04 (3.05) | 4.55 (3.88) | 0.703 | 0.484 |
| CES-D | 7.43 (7.39) | 7.00 (7.00) | 0.291 | 0.771 |
CES-D: Center for Epidemiological Studies Depression Scale; ISI: Insomnia Severity Index; M: mean; n: number of participants; p: p-value; PSQI: Pittsburgh Sleep Quality Index; SD: standard deviation; t: t-statistic.
Subjective sleep
Linear mixed model analyses including treatment (tryptophan versus placebo) as a single predictor revealed no significant main effects on any of the subjective sleep variables (Table 2).
Table 2.
Estimated marginal means M (SE) of sleep variables within treatment conditions.
| Placebo |
Tryptophan |
|||||
|---|---|---|---|---|---|---|
| S’/S’ | L’/L’ | Total | S’/S’ | L’/L’ | Total | |
| Sleep diary | ||||||
| Sleep onset latency (min) | 20.11 (2.53) | 17.05 (2.46) | 18.53 (1.76) | 18.78 (2.53) | 15.48 (2.46) | 17.08 (1.76) |
| Nocturnal awakenings | 1.61 (0.13) | 1.36 (0.13) | 1.48 (0.09) | 1.49 (0.13) | 1.54 (0.13) | 1.51 (0.09) |
| Wake after sleep onset (min) | 11.93 (1.69) | 8.83 (1.63) | 10.33 (1.18) | 12.77 (1.69) | 10.44 (1.64) | 11.57 (1.18) |
| Total sleep time (h) | 7.67 (0.11) | 7.58 (0.10) | 7.62 (0.07) | 7.58 (0.11) | 7.66 (0.10) | 7.62 (0.07) |
| Sleep quality | 3.60 (0.07)a,b | 3.90 (0.07)b | 3.75 (0.05) | 3.71 (0.07)a | 3.81 (0.07) | 3.76 (0.05) |
| Refreshed sleep | 3.26 (0.09) | 3.43 (0.88) | 3.35 (0.06) | 3.28 (0.09) | 3.37 (0.88) | 3.24 (0.06) |
| Actigraphy | ||||||
| Sleep onset latency (min) | 6.64 (0.72) | 6.06 (0.70) | 6.34 (0.50) | 6.96 (0.71) | 6.03 (0.69) | 6.48 (0.50) |
| Sleep efficiency (%) | 84.42 (0.75) | 84.97 (0.73) | 84.70 (0.52)c | 85.26 (0.74) | 86.08 (0.73) | 85.68 (0.52)c |
| Total sleep time (h) | 6.78 (0.12) | 6.71 (0.11) | 6.74 (0.08) | 6.76 (0.12) | 6.86 (0.11) | 6.81 (0.08) |
| Wake after sleep onset (min) | 68.06 (3.60) | 65.53 (3.50) | 66.76 (2.50)c | 63.13 (3.57) | 60.09 (3.49) | 61.57 (2.49)c |
| Nocturnal awakenings | 24.34 (0.92) | 23.54 (0.89) | 23.93 (0.64) | 23.69 (0.91) | 23.37 (0.89) | 23.52 (0.64) |
M: mean; SE: standard error.
Main effect of treatment in S’/S’ group (p < 0.10).
Main effect of 5-HTTLPR in placebo condition (p < 0.01).
Main effect of treatment (p < 0.001).
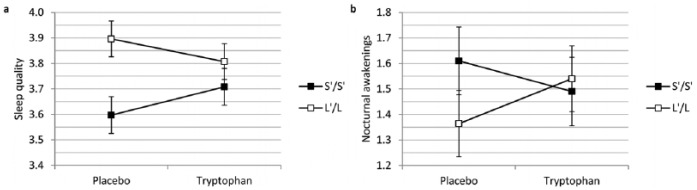
As illustrated in Figure 1, linear mixed model analyses including treatment (tryptophan versus placebo), 5-HTTLPR (S’/S’ versus L’/L’) and an interaction term as predictors for subjective sleep variables revealed a significant interaction between treatment and 5-HTTLPR on subjective sleep quality (b = 0.200, t(1208.72) = 2.490, p = 0.013) and subjective amount of nocturnal awakenings (b = −0.296, t(1216.69) = −2.045, p = 0.041). Simple main effects of treatment within 5-HTTLPR genotype groups revealed a marginally significant improvement of subjective sleep quality in the S’/S’ (b = 0.111, t(1208.23) = 1.929, p = 0.054) but not in the L’/L’ group (b = −0.89, t(1209.24) = −1.587, p = 0.113) following tryptophan compared to placebo intake. Moreover, a significantly poorer sleep quality in the S’/S’ as opposed to the L’/L’ group in the placebo condition (b = −0.300, t(133.92) = −2.985, p = 0.003) was not observed in the tryptophan condition (b = −0.099, t(133.65) = −0.990, p = 0.324). Simple effects within 5-HTTLPR and treatment groups did not reveal further significant main effects except for a marginally significant increase in the number of nocturnal awakenings in the L’/L’ group following tryptophan relative to placebo intake (b = 0.175, t(1216,80) = 1.740, p = 0.082). No significant interactions were observed for the remainder of subjective sleep variables (Table 2).
Figure 1.
Interaction between 5-HTTLPR and treatment for (a) subjective sleep quality (p < 0.05) and (b) subjective number of nocturnal awakenings (p < 0.05).
Objective sleep
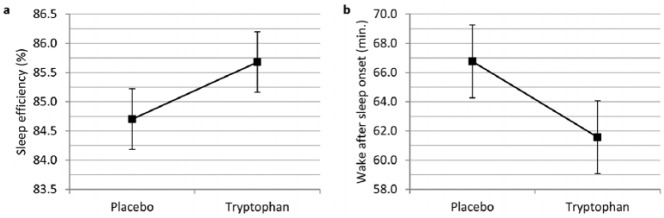
Linear mixed model analyses with treatment (tryptophan versus placebo) as a single predictor for objective sleep variables revealed a significant increase in sleep efficiency (b = 0.977, t(1027.10) = 3.337, p < 0.001) and a reduction in objective wake after sleep onset (b = −5.188, t(1027.83) = −3.502, p < 0.001) following tryptophan relative to placebo intake (Figure 2). No significant main effects of treatment were observed for the remainder of the objective sleep variables (Table 2).
Figure 2.
Main effect of treatment on (a) objective sleep efficiency (p < 0.001) and (b) objective wake after sleep onset (p < 0.001).
Linear mixed model analyses with treatment (tryptophan versus placebo, 5-HTTLPR (S’/S’ versus L’/L’) and an interaction term as predictors for objective sleep variables revealed no significant interactions (Table 2). This indicates that 5-HTTLPR does not moderate the effect of tryptophan on objectively measured sleep.
Discussion
The results of the present study support the sleep-promoting effects of tryptophan and indicate that some influences depend on allelic variation in 5-HTTLPR. While tryptophan improved objective sleep efficiency and objective wake after sleep onset irrespective of allelic variation, 5-HTTLPR was found to moderate the influence of tryptophan on subjective sleep quality and subjective number of nocturnal awakenings, as measured by the respective items of the sleep diary. A marginally significant improvement of sleep quality in the S-allele but not in the L-allele group illustrates the value of tryptophan as a compensatory treatment for the presumed elevated susceptibility for sleep impairment in this allelic variant. This is further supported by the observation that a significantly lower subjective sleep quality in the S-allele relative to the L-allele in the placebo condition diminished following tryptophan treatment.
The significantly lower sleep quality in the 5-HTTLPR S-allele during placebo administration corresponds with previous work revealing an increased susceptibility for sleep quality reductions (Brummett et al., 2007) and clinical insomnia (Deuschle et al., 2010; Huang et al., 2014) in this allelic variant. While 5-HTTLPR-dependent differences were observed for subjective sleep quality, no genetic influences were found for objective measurements. A low correspondence between subjective sleep quality and actigraphy has been commonly reported (Sadeh, 2011), and it has been suggested that the two assessment methods capture different sleep dimensions (Aili et al., 2017). This parallels the discrepancy in subjective and objective sleep commonly observed in clinical insomnia (Riemann et al., 2012). The distinction between subjective sleep perception and objective sleep measures might be particularly relevant to the field of 5-HTTLPR as previous research mainly included subjective sleep reports (Barclay et al., 2011; Brummett et al., 2007; Huang et al., 2014; Polito et al., 2015; van Dalfsen and Markus, 2015, 2019). Hence, this could suggest that 5-HTTLPR particularly modulates subjective aspects of sleep. Nonetheless, given the enduring validity issues with actigraphy (Sadeh, 2011), and in the absence of polysomnographic studies, it cannot be excluded that objective sleep alterations account for 5-HTTLPR-dependent differences in subjective sleep quality.
The present study revealed that tryptophan improves subjective sleep quality in S-allele but not in L-allele carriers. This finding is in accordance with previous work demonstrating that tryptophan administration is associated with significantly better sleep quality in stress vulnerable individuals carrying two copies of the S-allele (van Dalfsen and Markus, 2015). However, in the present study the effect of tryptophan in the S-allele group was relatively small and only approached statistical significance. Although this may intrinsically relate to the effect of tryptophan, it is likely that this at least in part reflects a ceiling effect related to the high sleep quality of the studied sample. Hence, this profoundly limits the room for improvement on the already restricted response options of the subjective sleep quality item. Subjective results further revealed a marginally significant increase in the reported number of nocturnal awakenings in the L-allele group. Taken together, the direction of 5-HTTLPR-dependent treatment effects provides some indications that tryptophan may improve subjective sleep in the S-allele group, whereas it might impair subjective sleep in the L-allele group.
In line with a large body of research supporting the sleep-promoting effects of tryptophan (Hartmann and Greenwald, 1984; Schneider-Helmert and Spinweber, 1986; Silber and Schmitt, 2010), actigraphic findings revealed that tryptophan improves sleep efficiency and reduces wake after sleep onset irrespective of allelic 5-HTTLPR variation. Comparing these objective effects with the influence of tryptophan on subjective measures, there appears to be a marked difference among sleep assessment procedures. Hence, while tryptophan improved objective sleep irrespective of allelic variation, it exclusively increased subjective sleep quality in S-allele relative to L-allele carriers. This might suggest that tryptophan specifically improves subjective sleep perception in the 5-HTTLPR S-allele, whereas it does not result in an actual improvement in objectively measured sleep. This could result from a reduction in the negative attentional bias associated with this allelic variant (Pergamin-Hight et al., 2012) and, hence, the involvement of the serotonergic system in the inhibition of such maladaptive cognitive processes (Robinson et al., 2013). Alternatively, tryptophan might selectively improve sleep architecture in the 5-HTTLPR S-allele resulting in an objective sleep improvement that cannot be detected by actigraphic measures.
The precise mechanism underlying the general sleep-promoting effects of tryptophan remains largely unknown; however, there is specific evidence that the biosynthetic pathway of 5-HT is involved in sleep regulation. This is mainly derived from animal research revealing that pharmacological blocking of the enzyme tryptophan hydroxylase with para-chlorophenylalanine (pCPA) results in profound sleep impairments (Borbely et al., 1981; Jouvet, 1972; Koella et al., 1968; Ursin, 1972) that can be reversed by 5-HTP (Koella et al., 1968; Pujol et al., 1971) and tryptophan (Borbely et al., 1981) administration. Since this rate-limiting enzyme is also thought to mediate the effects of tryptophan on 5-HT synthesis, these findings provide indirect support for the sleep-promoting effects of tryptophan augmentation. It remains uncertain, however, how general increments in brain 5-HT synthesis exert sleep-inducing effects. This relates to the complex involvement of 5-HT in sleep–wake regulation, as its activity promotes both sleep and wakefulness depending on current behavioural state, brain region and 5-HT receptor type as well as its interrelationships with other neurotransmitter and hormonal systems (for review, see Ursin, 2002).
The diminished expression of the 5-HT transporter in the 5-HTTLPR S-allele produces a variety of neurochemical changes in the brain 5-HT system. Expanding on the influence of tryptophan on 5-HT-mediated sleep regulation, such alterations might explain why sleep-improving effects may particularly occur in this allelic variant. As comprehensively reviewed (Murphy and Lesch, 2008), research in humans and mice with comparable levels of 5-HT transporter expression as homozygous S-allele carriers (i.e. SLC6A4+/–) indicates that a diminished transcriptional activity of the 5-HT transporter decreases the amount of 5-HT transporter binding sites. This reduces 5-HT reuptake resulting in elevated extracellular 5-HT concentrations and prolonged 5-HT clearance. This augmented 5-HT signalling is accompanied by a reduced basal 5-HT firing rate in the dorsal raphe as well as a downregulation of 5-HT1A, 5-HT1B and 5-HT3 receptors. Since the 5-HT1A receptor is thought to facilitate sleep (for review, see Ursin, 2002), a decreased sensitivity might theoretically contribute to the sleep quality reductions associated with the 5-HTTLPR S-allele. Research in mice lacking 5-HT transporter expression (i.e. SLC6A4−/–) further suggests that 5-HT synthesis and turnover might increase to compensate for the reduced recycling of 5-HT (for review, see Murphy and Lesch, 2008). Since an elevation in tryptophan availability increases 5-HT synthesis and release (Fernstrom, 2012; Wurtman et al., 1980), such mechanisms provide a potential pathway as to how tryptophan might compensate for the deficient 5-HT functioning and related sleep disturbances in the 5-HTTLPR S-allele. Regardless of the precise molecular mechanism of action, research on a variety of outcome measures supports the assumption that the 5-HTTLPR S-allele is generally more susceptible to manipulations of tryptophan availability (for review, see Gibson, 2018).
The following limitations should be considered for the present study. The first limitation relates to the relatively high sleep quality of the present sample. This is an important issue as this may considerably reduce potential treatment effects, and future research in clinical populations is therefore encouraged. The second limitation pertains to the objective sleep assessment. Although 1 week of actigraphy sleep assessment is found to provide a reliable estimate of sleep parameters (Aili et al., 2017) the accuracy of detecting nocturnal awakenings during sleep periods (i.e. specificity) is relatively limited, and polysomnographic studies are needed to detect more valid objective influences. Further research involving different doses of tryptophan is also desirable, as higher doses of tryptophan administration might produce more profound effects on sleep regulation.
In conclusion, current findings support the sleep-promoting effects of tryptophan augmentation. The present study elaborates on more general findings revealing that tryptophan may particularly improve subjective sleep quality in genetic variants associated with deficient 5-HT functioning. Given the involvement of the 5-HTTLPR S-allele in the aetiology of reduction in sleep quality and clinical insomnia, tryptophan supplementation holds promise as a valuable treatment strategy to compensate for this predisposition, although research in clinical populations is desirable.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jens H van Dalfsen  https://orcid.org/0000-0002-4388-8447
https://orcid.org/0000-0002-4388-8447
References
- Aili K, Astrom-Paulsson S, Stoetzer U, et al. (2017) Reliability of actigraphy and subjective sleep measurements in adults: The design of sleep assessments. J Clin Sleep Med 13: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Roth T. (1999) Characteristics of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey. I. Sleep 22: S347–S353. [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, et al. (2011) Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 135: 10–19. [DOI] [PubMed] [Google Scholar]
- Barclay NL, Eley TC, Mill J, et al. (2011) Sleep quality and diurnal preference in a sample of young adults: Associations with 5HTTLPR, PER3, and CLOCK 3111. Am J Med Genet B Neuropsychiatr Genet 156B: 681–690. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Morin CM. (2000) Familial incidence of insomnia. J Sleep Res 9: 49–54. [DOI] [PubMed] [Google Scholar]
- Beaulieu-Bonneau S, LeBlanc M, Merette C, et al. (2007) Family history of insomnia in a population-based sample. Sleep 30: 1739–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, Neuhaus HU, Tobler I. (1981) Effect of p-chlorophenylalanine and tryptophan on sleep, EEG and motor activity in the rat. Behav Brain Res 2: 1–22. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Krystal AD, Ashley-Koch A, et al. (2007) Sleep quality varies as a function of 5-HTTLPR genotype and stress. Behav Brain Res 69: 621–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, et al. (2010) Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 33: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, et al. (2012) The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 35: 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Morin C, Cervena K, et al. (2005) Family studies in insomnia. J Psychosom Res 58: 271–278. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Schredl M, Schilling C, et al. (2010) Association between a serotonin transporter length polymorphism and primary insomnia. Sleep 33: 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston D, Ashcroft GW, Crawford TB. (1970) Effect of tryptophan administration on 5HIAA in cerebrospinal fluid in man. J Neurol Neurosurg Psychiatry 33: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom JD. (1983) Role of precursor availability in control of monoamine biosynthesis in brain. Physiol Rev 63: 484–546. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD. (2012) Effects and side effects associated with the non-nutritional use of tryptophan by humans. J Nutr 142: 2236s–2244s. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Wurtman RJ. (1971) Brain serotonin content: Physiological dependence on plasma tryptophan levels. Science 173: 149–152. [DOI] [PubMed] [Google Scholar]
- Gibson EL. (2018) Tryptophan supplementation and serotonin function: Genetic variations in behavioural effects. Proc Nutr Soc 77: 174–188. [DOI] [PubMed] [Google Scholar]
- Glatz K, Mossner R, Heils A, et al. (2003) Glucocorticoid-regulated human serotonin transporter (5-HTT) expression is modulated by the 5-HTT gene-promotor-linked polymorphic region. J Neurochem 86: 1072–1078. [DOI] [PubMed] [Google Scholar]
- Hammerschlag AR, Stringer S, de Leeuw CA, et al. (2017) Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat Genet 49: 1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E, Greenwald D. (1984) Tryptophan and human sleep: An analysis of 43 studies. In: Schlossberger HG, Kochen W, Linzen B, et al. (eds) Progress in Tryptophan and Serotonin Research. Berlin: Walter de Gruyter, pp. 297–304. [Google Scholar]
- Heils A, Teufel A, Petri S, et al. (1996) Allelic variation of human serotonin transporter gene expression. J Neurochem 66: 2621–2624. [DOI] [PubMed] [Google Scholar]
- Huang C, Li J, Lu L, et al. (2014) Interaction between serotonin transporter gene-linked polymorphic region (5-HTTLPR) and job-related stress in insomnia: A cross-sectional study in Sichuan, China. Sleep Med 15: 1269–1275. [DOI] [PubMed] [Google Scholar]
- Jansen PR, Watanabe K, Stringer S, et al. (2019) Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet 51: 394–403. [DOI] [PubMed] [Google Scholar]
- Jouvet M. (1972) The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb Physiol 64: 166–307. [DOI] [PubMed] [Google Scholar]
- Koella WP, Feldstein A, Czicman JS. (1968) The effect of para-chlorophenylalanine on the sleep of cats. Electroencephalogr Clin Neurophysiol 25: 481–490. [DOI] [PubMed] [Google Scholar]
- Lane JM, Liang J, Vlasac I, et al. (2017) Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat Genet 49: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger D, Poursain B, Neubauer D, et al. (2008) An international survey of sleeping problems in the general population. Curr Med Res Opin 24: 307–317. [DOI] [PubMed] [Google Scholar]
- Lind MJ, Aggen SH, Kirkpatrick RM, et al. (2015) A longitudinal twin study of insomnia symptoms in adults. Sleep 38: 1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maich KHG, Lachowski AM, Carney CE. (2016) Psychometric properties of the consensus sleep diary in those with insomnia disorder. Behav Sleep Med 16: 1–18. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. (2008) Targeting the murine serotonin transporter: Insights into human neurobiology. Nat Rev Neurosci 9: 85–96. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, et al. (2000) The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry 5: 32–38. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. (1997) Prevalence of DSM-IV diagnostic criteria of insomnia: Distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res 31: 333–346. [DOI] [PubMed] [Google Scholar]
- Palagini L, Bruno RM, Gemignani A, et al. (2013) Sleep loss and hypertension: A systematic review. Curr Pharm Des 19: 2409–2419. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Bakermans-Kranenburg MJ, van Ijzendoorn MH, et al. (2012) Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: A meta-analysis. Biol Psychiatry 71: 373–379. [DOI] [PubMed] [Google Scholar]
- Polito L, Davin A, Vaccaro R, et al. (2015) Serotonin transporter polymorphism modifies the association between depressive symptoms and sleep onset latency complaint in elderly people: Results from the ‘InveCe.Ab’ study. J Sleep Res 24: 215–222. [DOI] [PubMed] [Google Scholar]
- Pujol JF, Buguet A, Froment JL, et al. (1971) The central metabolism of serotonin in the cat during insomnia. A neurophysiological and biochemical study after administration of P-chlorophenylalanine or destruction of the Raphe system. Brain Res 29: 195–212. [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Nissen C, et al. (2012) REM sleep instability: A new pathway for insomnia? Pharmacopsychiatry 45: 167–176. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Allen PS, et al. (2013) The role of serotonin in the neurocircuitry of negative affective bias: Serotonergic modulation of the dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit. Neuroimage 78: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A. (2011) The role and validity of actigraphy in sleep medicine: An update. Sleep Med Rev 15: 259–267. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. (1994) Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep 17: 201–207. [DOI] [PubMed] [Google Scholar]
- Schneider-Helmert D, Spinweber CL. (1986) Evaluation of L-tryptophan for treatment of insomnia: A review. Psychopharmacology 89: 1–7. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Anderson WM, Cole SR, et al. (1999) Insomnia and heart disease: A review of epidemiologic studies. J Psychosom Res 47: 313–333. [DOI] [PubMed] [Google Scholar]
- Silber BY, Schmitt JA. (2010) Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci Biobehav Rev 34: 387–407. [DOI] [PubMed] [Google Scholar]
- Slater JA, Botsis T, Walsh J, et al. (2015) Assessing sleep using hip and wrist actigraphy. Sleep Biol Rhythms 13: 172–180. [Google Scholar]
- Uhlig BL, Sand T, Odegard SS, et al. (2014) Prevalence and associated factors of DSM-V insomnia in Norway: The Nord-Trondelag Health Study (HUNT 3). Sleep Med 15: 708–713. [DOI] [PubMed] [Google Scholar]
- Ursin R. (1972) Differential effect of para-chlorophenylalanine on the two slow wave sleep stages in the cat. Acta Physiol Scand 86: 278–285. [DOI] [PubMed] [Google Scholar]
- Ursin R. (2002) Serotonin and sleep. Sleep Med Rev 6: 55–69. [DOI] [PubMed] [Google Scholar]
- van Dalfsen JH, Markus CR. (2015) Interaction between 5-HTTLPR genotype and cognitive stress vulnerability on sleep quality: Effects of sub-chronic tryptophan administration. Int J Neuropsychopharmacol 18: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dalfsen JH, Markus CR. (2019) The serotonin transporter 5-HTTLPR polymorphism and the risk for insomnia: A non-replication. Sleep Med 53: 195–196. [DOI] [PubMed] [Google Scholar]
- Wing YK, Zhang J, Lam SP, et al. (2012) Familial aggregation and heritability of insomnia in a community-based study. Sleep Med 13: 985–990. [DOI] [PubMed] [Google Scholar]
- World Medical Association (2013) World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Hefti F, Melamed E. (1980) Precursor control of neurotransmitter synthesis. Pharmacol Rev 32: 315–335. [PubMed] [Google Scholar]
- Yuwiler A, Brammer GL, Morley JE, et al. (1981) Short-term and repetitive administration of oral tryptophan in normal men. Effects on blood tryptophan, serotonin, and kynurenine concentrations. Arch Gen Psychiatry 38: 619–626. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Huang YY, Oquendo MA, et al. (2006) Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry 163: 1588–1593. [DOI] [PubMed] [Google Scholar]