Abstract
Background:
Results of our recently published phase III randomized clinical trial of ultrasound-enhanced thrombolysis (sonothrombolysis) using an operator-independent, high frequency ultrasound device revealed heterogeneity of patient recruitment among centers.
Methods:
We performed a post hoc analysis after excluding subjects that were recruited at centers reporting a decline in the balance of randomization between sonothrombolysis and concurrent endovascular trials.
Results:
From a total of 676 participants randomized in the CLOTBUST-ER trial we identified 52 patients from 7 centers with perceived equipoise shift in favor of endovascular treatment. Post hoc sensitivity analysis in the intention-to-treat population adjusted for age, National Institutes of Health Scale score at baseline, time from stroke onset to tPA bolus and baseline serum glucose showed a significant (p < 0.01) interaction of perceived endovascular equipoise shift on the association between sonothrombolysis and 3 month functional outcome [adjusted common odds ratio (cOR) in centers with perceived endovascular equipoise shift: 0.22, 95% CI 0.06–0.75; p = 0.02; adjusted cOR for centers without endovascular equipoise shift: 1.20, 95% CI 0.89–1.62; p = 0.24)]. After excluding centers with perceived endovascular equipoise shift, patients randomized to sonothrombolysis had higher odds of 3 month functional independence (mRS scores 0–2) compared with patients treated with tPA only (adjusted OR: 1.53; 95% CI 1.01–2.31; p = 0.04).
Conclusion:
Our experience in CLOTBUST-ER indicates that increasing implementation of endovascular therapies across major academic stroke centers raises significant challenges for clinical trials aiming to test noninterventional or adjuvant reperfusion strategies.
Keywords: endovascular, equipoise shift, intracranial hemorrhage, mechanical thrombectomy, outcome, recanalization, sonothrombolysis, stroke, ultrasound-enhanced thrombolysis
Introduction
Preliminary evidence has indicated that the addition of high-frequency, pulsed-wave ultrasound to tissue plasminogen activator (tPA) may increase the odds of recanalization and favorable functional outcomes in patients with acute ischemic stroke (AIS) with proximal intracranial occlusions.1,2 Nevertheless, the largest to date phase III randomized-controlled clinical trial (RCT) evaluating the safety and efficacy of ultrasound-enhanced thrombolysis (sonothrombolysis) using an operator-independent high-frequency ultrasound device compared with intravenous thrombolysis (IVT) alone reported that delivery of sonothrombolysis was feasible and safe, but failed to offer additional clinical benefit in AIS patients with baseline moderate-to-severe stroke.3
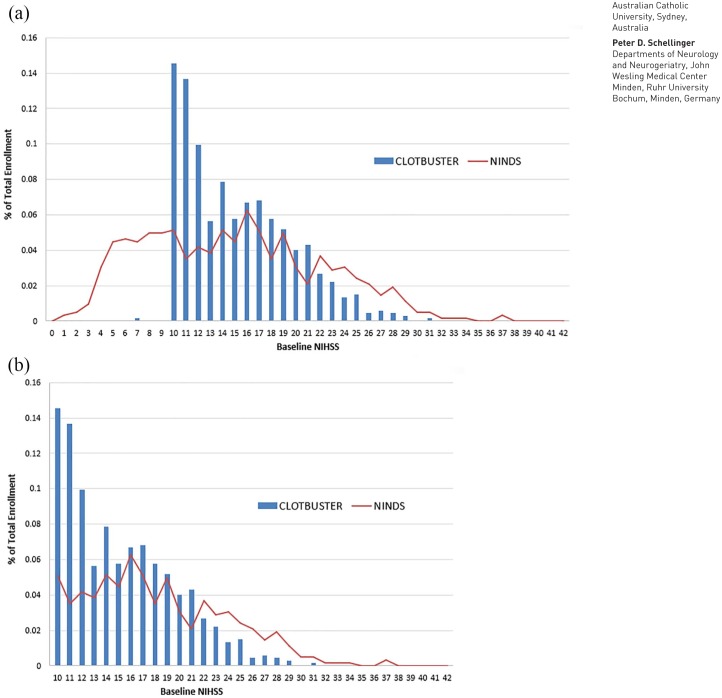
Review of the results of this RCT revealed heterogeneity of patient recruitment among centers, a finding that potentially reflected practice drift at sites that were also participating in concurrent mechanical thrombectomy trials, reflecting loss of equipoise in favor of open-label mechanical thrombectomy or preferential endovascular trial recruitment among patients with large vessel occlusions (LVOs). This concern has also been corroborated by the substantial representation (28%) of patients with baseline National Institutes of Health Scale (NIHSS) scores of 10 or 11 enrolled into the trial (Figure 1), given the lower positive predictive value of a cut-off of 10 points in NIHSS score compared with the cut-off of 12 points in detecting LVO in AIS patients.4
Figure 1.
Overview of the distribution of the (a) National Institutes of Health Stroke Scale (NIHSS) scores of all patients with acute ischemic stroke and (b) patients with acute ischemic stroke and NIHSS scores 10 points or greater randomized in the CLOTBUST-ER trial (blue bars). Indirect comparison with NIHSS scores of patients with acute ischemic stroke randomized in the National Institute for Neurological Disorders rt-PA Stroke Trial (red line).
In view of these considerations, we performed an additional post hoc analysis in order to explore how practice drift in favor of endovascular thrombectomy might have affected the findings of the sonothrombolysis trial.
Methods
Trial design and study population
The Combined Lysis of Thrombus using Ultrasound and Systemic tPA for Emergent Revascularization (CLOTBUST-ER) was a multinational, double-blind, sham-controlled RCT.5 Detailed descriptions of the methods and results of the CLOTBUST-ER trial are available in relevant publications.3,5 In brief, AIS patients aged 18–80 years with baseline NIHSS scores of ⩾10 points who were eligible for intravenous tPA treatment within a 4.5 h treatment window worldwide and within a 3 h treatment window in North America were randomized 1:1 to active ultrasound + tPA (intervention group) or to sham ultrasound + tPA (control group) using web-based central randomization.3,5 The trial was approved by the institutional review board at each site or national ethics committee.3 Written informed consent was obtained from the patient or a legal representative before enrolment.3
Investigational procedure
All eligible subjects received full-dose intravenous tPA (0.9 mg/kg; 90 mg maximum; 10% bolus followed by 90% intravenous infusion over 60 min) and activation of the headframe within 30 min of tPA bolus to achieve maximum overlap between exposure to the device and tPA infusion.3,5 All subjects regardless of device activation time were required to wear the headframe for a total of 120 min. Devices were programmed based on a randomization code that maintained blinding of treating physicians, patients, and the sponsor to treatment group assignment (active or sham).3,5
Primary and secondary outcomes
The primary efficacy outcome was assessed using modified Rankin scale (mRS) scores at 90 ± 10 days from randomization, ascertained by trained and certified personnel blinded to treatment assignment.3,5 The primary analysis included a cumulative ordinal logistic regression (shift analysis of mRS scores in the direction of functional improvement) for those subjects enrolled within 3 h of stroke symptom onset according to the US Food and Drug Administration regulatory requirements (‘US’ primary outcome).3,5 This analysis was repeated for all patients who were enrolled within 4.5 h (‘Global’ primary outcome).3,5
Other secondary efficacy endpoints included the differences in NIHSS scores between the two groups at 2 h, 24 h, day 7, and day 90, the differences in mRS scores at day 7 and the difference in rates of dichotomous 90 day mRS 0–1 and mRS 0–2 between the two groups.3,5 We also further assessed the rates of dramatic clinical recovery at 2 h, clinical recovery at 24 h, and clinical recovery at day 90 (defined as a reduction of 10 or more points in NIHSS compared with pretreatment, or a total NIHSS score of 3 or less), neurological improvement at 24 h (defined as a reduction of 5 or more points on NIHSS compared with the pretreatment score), and neurologic worsening at 24 h (defined as an increase of 4 or more points on NIHSS compared with the pretreatment score).3,5
Symptomatic intracranial hemorrhage (sICH) per study protocol was defined as neurological deterioration (⩾4 points worsening on the NIHSS compared with the best prior examination) within 24 h after tPA bolus with documented parenchymal hemorrhage type 2 or remote parenchymal hemorrhage type 2.3,5 All prespecified adverse events were reported by the blinded clinical investigators of the participating centers, while reviewed and adjudicated by a blinded independent adjudication panel within the Data and Safety Monitoring Board (DSMB) committee.3,5
Endovascular equipoise shift definition
A number of CLOTBUST-ER centers were concurrently participating in ongoing endovascular thrombectomy trials. Following the presentation of the results of MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) in October 2014,6 a slowing of recruitment rates during the first months of 2015 was detected. This led to repeated Steering Committee discussions regarding potential conflicts between CLOTBUST-ER and ongoing mechanical thrombectomy trials. The Global and National Principal Investigators contacted centers with perceived conflicts due to competing endovascular reperfusion therapies during conduct of CLOTBUST-ER to verify and address these concerns as well as to discuss their continuation in CLOTBUST-ER. As result of these discussions, one center had to stop enrollment.
In order to explore the potential impact of shifting treatment practice at centers participating in endovascular trials on CLOTBUST-ER, an additional post hoc analysis was performed after excluding those subjects that were recruited at centers meeting following criteria: (1) centers with 24/7 available endovascular services; and (2) decline in equal randomization rates between sonothrombolysis and endovascular trials or (3) decline in preference to randomize patients with LVO to CLOTBUST-ER (opting instead to treat them with interventional treatment as standard of care). Investigators from these centers have openly stated during the Steering Committee meetings that they were consistently selecting AIS patients with LVO presence on baseline computed tomography angiography (CTA) for enrollment in endovascular trials that were competing with CLOBUST-ER (ESCAPE, REVASCAT, SWIFT PRIME), while they preferred to enroll only patients with no vessel occlusion (lacunar strokes) or distal vessel occlusion on CTA in the CLOBUST-ER trial. These centers were subsequently considered to be prone to patient selection bias due to the expressed treatment preference in favor of mechanical thrombectomy (MT) and were excluded from the present analyses. The centers that were involved in RCTs of MT and decided to equally randomize patients in MT and sonothrombolysis RCTs were not excluded from the present analyses. No further clarifications (e.g. by self-report questionnaires or telephone interviews) were made.
The Steering Committee was blinded to the 3 month functional outcomes of all patients during the process of identifying centers that met these criteria. Interaction testing using proportional odds and binary logistic regression was performed between the dichotomous variable ‘perceived endovascular equipoise shift’ and ‘treatment assignment’.
Statistical analysis
All reported analyses were performed in the intention-to-treat population. The primary endpoint analysis was performed in subjects who received tPA within 3 h of symptom onset, using the proportional odds logistic regression (polr command in R) over the 90 day mRS distribution after collapsing grades 5 and 6.3,5 For all secondary efficacy and safety outcomes we performed unadjusted and adjusted analyses for confounders (baseline NIHSS, age, baseline serum glucose, time to tPA bolus) that were chosen by the steering committee prior to unblinding of the data. Prespecified secondary outcomes were tested in the unadjusted analyses with Fisher’s two-sided test of proportion and confidence intervals (CIs) were provided. Interaction testing using proportional odds and binary logistic regression was performed between the dichotomous variable ‘perceived endovascular equipoise shift’ and ‘treatment assignment’. The threshold of statistical significance for interaction testing was set at p < 0.1
Results
A total of 676 participants were randomized in the CLOTBUST-ER trial (335 to the intervention group and 341 to the control group) at a total of 76 medical centers between August 2013 and April 2015. CLOTBUST-ER was stopped early for futility at the second interim analysis by the DSMB according to prespecified stopping rules. Subjects who were enrolled in the study at the time of the futility determination were followed until 90 days post-tPA administration by the site investigators. A significant (Pearson chi-squared: 106.379; df = 75; p = 0.01) variation in the reported rates of favorable functional outcomes (mRS scores of 0–1) was documented across participating centers after analyzing both treatment groups combined. Moreover, this variation persisted in the reported rates of 3 month functional independence (Pearson chi-squared: 92.181; df = 75; mRS scores of 0–2; p = 0.09) and in the distribution of reported 3 month mRS scores (Pearson chi-squared: 491.188; df = 450; p = 0.09).
A total of 52 patients (7.7%) were enrolled at 7 centers with perceived endovascular equipoise shift. Post hoc sensitivity analysis in the intention-to-treat population showed a significant (p < 0.01) interaction of perceived endovascular equipoise shift on the effect of sonothrombolysis on 3 month functional outcome compared with standard tPA treatment [adjusted common odds ratio (cOR) for Global outcome in centers with perceived endovascular equipoise shift: 0.22, 95% CI 0.06–0.75; p = 0.02; adjusted cOR for Global outcome in centers without endovascular equipoise shift: 1.20, 95% CI 0.89–1.62; p = 0.24); Figure 2].
Figure 2.
Subgroup analysis of the primary global outcomes according to potential endovascular equipoise shift.
The forest plot shows the effect size in the primary global outcome variable (common odds ratio for improvement on the modified Rankin scale at 90 days of patients treated with intravenous thrombolysis within 4.5 h from stroke onset) analyzed according to ordinal logistic regression after collapsing mRS scores 5 and 6 and adjusting for age, National Institutes of Health Stroke Scale (NIHSS) score at baseline; time from stroke onset to tPA (tissue plasminogen activator) bolus and baseline serum glucose according to potential endovascular equipoise shift. The thresholds for age and NIHSS score (range, 0–42, with higher scores indicating more severe neurologic deficits) were chosen at the median. The threshold for time from stroke onset to tissue plasminogen activator bolus was prespecified.
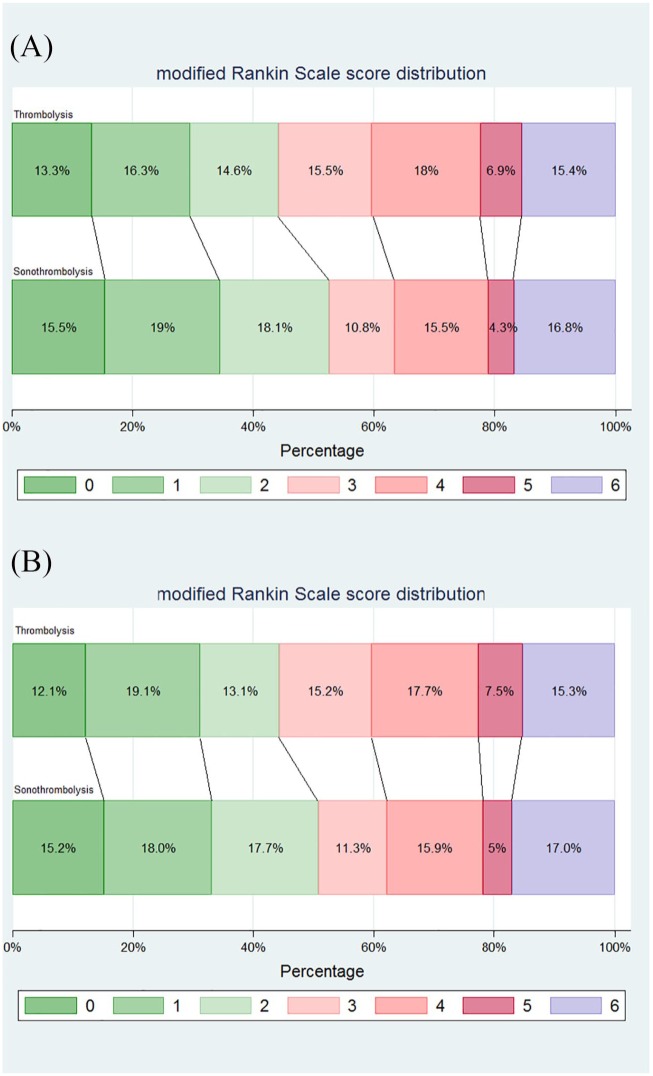
After excluding patients from centers with perceived endovascular equipoise shift, the two groups of the remaining study population (310 in the intervention group and 314 in the control group) did not differ in any of the baseline characteristics (Table 1). The distribution of the mRS scores of patients randomized within 3 and 4.5 h are shown in Figure 3A and B, respectively. Sonothrombolysis was not associated with higher likelihood of functional improvement compared with IVT [adjusted cOR for Global outcome: 1.20 (95% CI 0.89–1.62); p = 0.24]. Patients randomized to sonothrombolysis had higher odds of 3 month functional independence (mRS scores 0–2) compared with patients treated with tPA only (adjusted OR: 1.53; 95% CI 1.01–2.31; p = 0.04 and adjusted OR: 1.47; 95% CI 1.02–2.13; p = 0.04 for patients randomized within 3 and 4.5 h, respectively). No difference between the two groups was evident on the probability of favorable functional outcome at 3 months (adjusted OR: 1.17, 95% CI 0.80–1.72, p = 0.41). The sensitivity analyses between the two groups in terms of other secondary efficacy outcomes are presented in Table 2.
Table 1.
Baseline characteristics of the study population after removing centers with perceived endovascular equipoise shift.
| Variables | Intervention (n = 310) | Control (n = 314) | p |
|---|---|---|---|
| Mean age ± SD, years | 67.1 ± 10.3 | 67.0 ± 10.6 | 0.86 |
| Male sex, n (%) | 175 (56.4) | 187 (59.5) | 0.47 |
| Median NIHSS score (IQR), points | 15 (11–18) | 14 (11–18) | 0.81 |
| Hypertension, n (%) | 178 (57.4) | 194 (61.8) | 0.29 |
| Diabetes mellitus, n (%) | 68 (21.9) | 75 (23.9) | 0.57 |
| Atrial fibrillation, n (%) | 56 (18.1) | 53 (16.9) | 0.75 |
| Prestroke modified Rankin scale score 0–1, n (%) | 309 (99.7) | 312 (99.4) | >0.99 |
| Mean systolic blood pressure before tPA bolus ± SD, mmHga | 150.0 ± 20.2 | 150.4 ± 20.1 | 0.81 |
| Mean diastolic blood pressure before tPA bolus ± SD, mmHgb | 81.4 ± 13.4 | 81.8 ± 13.0 | 0.71 |
| Mean serum glucose before tPA bolus ± SD, mg/dl | 139.4 ± 50.5 | 138.0 ± 53.5 | 0.74 |
| Median time from symptom onset to tPA bolus (IQR), min | 117.5 (95.0–161.5) | 128.0 (97.2–165.8) | 0.12 |
| Time from symptom onset to tPA bolus within 3 h, n (%) | 255 (82.3) | 262 (83.4) | 0.74 |
| Median time from symptom onset to headframe activation (IQR), min | 136 (118–182) | 150 (116–188) | 0.38 |
Intervention: sonothrombolysis.
Control: intravenous thrombolysis.
Missing data in 7 and 13 patients in the intervention and control arms respectively.
Missing data in 6 and 13 patients in the intervention and control arms respectively.
IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; tPA, tissue plasminogen activator.
Figure 3.
Modified Rankin scale scores at 90 days in the intention-to-treat population that was treated with intravenous thrombolysis within 3 h (‘US’ primary outcome) after removing centers with perceived endovascular equipoise shift (A). Modified Rankin scale scores at 90 days in the intention-to-treat population that was treated with intravenous thrombolysis within 4.5 h (‘Global’ primary outcome) after removing centers with perceived endovascular equipoise shift (B).
Shown is the distribution of scores on the modified Rankin scale. Scores range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability (patient is able to look after own affairs without assistance, but is unable to carry out all previous activities), 3 moderate disability (patient requires some help, but is able to walk unassisted), 4 moderately severe disability (patient is unable to attend to bodily needs without assistance and unable to walk unassisted), 5 severe disability (patient requires constant nursing care and attention), and 6 death.
Table 2.
Primary and secondary efficacy outcomes in the intention-to-treat population after removing centers with perceived endovascular equipoise shift.
| Variables | Intervention (n = 310) |
Control (n = 314) |
Unadjusted OR (95% CI) |
p | Adjusteda
OR (95% CI) |
p |
|---|---|---|---|---|---|---|
| Primary outcome: ordinal analysis of mRS score at 90 days (median, IQR) | ||||||
| USb | 2.0 (1.0–4.0) | 3.0 (1.0–4.0) | 1.22 (0.88–1.68) | 0.22 | 1.20 (0.87–1.68) | 0.27 |
| Globalc | 2.0 (1.0–4.0) | 3.0 (1.0–4.0) | 1.16 (0.86–1.54) | 0.33 | 1.20 (0.89–1.62) | 0.24 |
| Secondary outcomes | ||||||
| mRS score at 7 days or discharged US | 3.0 (1.0–4.0) | 4.0 (1.0–5.0) | 1.18 (0.86–1.63) | 0.30 | 1.20 (0.86–1.67) | 0.27 |
| mRS score at 7 days or discharged Global | 3.0 (1.0–4.0) | 4.0 (1.0–5.0) | 1.12 (0.84–1.50) | 0.43 | 1.22 (0.90–1.64) | 0.20 |
| mRS score at 90 days 0–1; USb, n (%) | 80 (34.5%) | 69 (29.6%) | 1.25 (0.85–1.85) | 0.27 | 1.30 (0.85–2.00) | 0.22 |
| mRS score at 90 days 0–1; Globalc, n (%) | 94 (33.2%) | 88 (31.2%) | 1.10 (0.77–1.56) | 0.65 | 1.17 (0.80–1.72) | 0.41 |
| mRS score at 90 days 0–2; USb, n (%) | 122 (52.6%) | 103 (44.2%) | 1.40 (0.97–2.02) | 0.08 | 1.53 (1.01–2.31) | 0.04 |
| mRS score at 90 days 0–2; Globalc, n (%) | 144 (50.9%) | 125 (44.3%) | 1.30 (0.93–1.81) | 0.13 | 1.47 (1.02–2.13) | 0.04 |
| Independent functional outcome at 90 dayse; USb, n (%) | 92 (39.7%) | 82 (35.2%) | 1.21 (0.83–1.76) | 0.34 | 1.25 (0.84–1.86) | 0.27 |
| Independent functional outcome at 90 dayse; Globalc, n (%) | 109 (38.5%) | 102 (36.2%) | 1.10 (0.79–1.55) | 0.60 | 1.18 (0.83–1.69) | 0.36 |
| Dramatic clinical recovery at 2 hf; US, n (%) | 54 (22.0%) | 52 (20.5%) | 1.10 (0.71–1.69) | 0.74 | 1.12 (0.72–1.74) | 0.63 |
| Dramatic clinical recovery at 2 hf; Global, n (%) | 56 (18.8%) | 57 (18.7%) | 1.00 (0.67–1.51) | 1.00 | 1.05 (0.68–1.61) | 0.84 |
| Clinical recovery at 24 hg; US, n (%) | 78 (32.6%) | 90 (36.1%) | 0.86 (0.59–1.24) | 0.45 | 0.85 (0.58–1.25) | 0.42 |
| Clinical recovery at 24 hg; Global, n (%) | 95 (32.8%) | 102 (34.3%) | 0.93 (0.66–1.31) | 0.73 | 0.96 (0.68–1.37) | 0.84 |
| Neurological improvement at 24 hh; US, n (%) | 141 (59.0%) | 139 (55.8%) | 1.14 (0.79–1.63) | 0.52 | 1.16 (0.80–1.69) | 0.43 |
| Neurological improvement at 24 hh; Global, n (%) | 169 (58.3%) | 163 (54.9%) | 1.15 (0.83–1.59) | 0.45 | 1.20 (0.86–1.69) | 0.29 |
| Neurological deterioration at 24 hi; US, n (%) | 20 (8.4%) | 17 (6.8%) | 1.25 (0.63–2.44) | 0.61 | 1.17 (0.58–2.37) | 0.66 |
| Neurological deterioration at 24 hi; Global, n (%) | 26 (9.0%) | 19 (6.4%) | 1.44 (0.78–2.67) | 0.28 | 1.29 (0.69–2.44) | 0.43 |
| NIHSSj at day 7 US, median (IQR) | 5 (1–10.25) | 6 (1–13) | 0.25 | |||
| NIHSSj at day 7 Global, median (IQR) | 5 (1–11) | 6 (1–12.75) | 0.24 | |||
| NIHSSk at day 90 US, median (IQR) | 2 (0–5) | 2 (0–5) | 0.37 | |||
| NIHSSk at day 90 Global, median (IQR) | 2 (0–6) | 2 (0–6) | 0.26 | |||
| Duration of hospital stay until discharge; US, days, median (IQR)l | 7 (5–10.25) | 7 (5.0–11.00) | 0.77 | |||
| Duration of hospital stay until discharge; Global, days, median (IQR)m | 7 (5–12) | 7 (4.75–11.00) | 0.92 | |||
Intervention: sonothrombolysis.
Control: intravenous thrombolysis.
ORs were adjusted for age, NIHSS score at baseline; time from stroke onset to tPA bolus, and baseline serum glucose.
Patients treated with intravenous thrombolysis within 3 h from symptom onset; there were 23 and 28 patients with missing data in the intervention and control arms, respectively.
Patients treated with intravenous thrombolysis within 4.5 h from symptom onset; there were 27 and 32 patients with missing data in the intervention and control arms, respectively.
There were 26 subjects in the intervention arm 20 subjects in the control arm missing day 7 or discharge mRS in the US cohort. There were 31 subjects in the intervention arm and 25 in the control arm missing day 7 or discharge mRS for the Global cohort.
Independent functional outcome adjusting for pretreatment NIHSS assessed at 90 ± 10 days post-treatment includes a mRS score of 0–1 for subjects with pretreatment NIHSS 10–14, or a mRS score of 0–2 for subjects with pretreatment NIHSS > 14.
Dramatic clinical recovery assessed at 120 ± 15 mins after headframe activation includes a reduction of 10 or more points on NIHSS compared with pretreatment, or a total NIHSS score of 3 or less. There were 31 and 33 patients with missing data in the intervention and control arms, respectively, for the US outcome of dramatic clinical recovery. There were 36 and 39 patients with missing data in the intervention and control arms, respectively, for the Global outcome of dramatic clinical recovery.
Clinical recovery assessed at 24 ± 2 h after headframe activation includes a reduction of 10 or more points on NIHSS compared with pretreatment, or a total NIHSS score of 3 or less. There were 37 and 37 patients with missing data in the intervention and control arms, respectively, for the US outcome of clinical recovery. There were 44 and 45 patients with missing data in the intervention and control arms, respectively, for the Global outcome of dramatic clinical recovery.
Neurological improvement assessed at 24 ± 2 h after headframe activation requires a reduction of 5 or more points on NIHSS compared with the pretreatment score. There were 37 and 37 patients with missing data in the intervention and control arms, respectively, for the US outcome of neurological improvement at 24 h. There were 44 and 45 patients with missing data in the intervention and control arms, respectively, for the Global outcome of neurological improvement at 24 h.
Neurological worsening assessed at 24 ± 2 h after headframe activation requires an increase of 4 or more points on NIHSS compared with the pretreatment score. There were 37 and 37 patients with missing data in the intervention and control arms, respectively, for the US outcome of neurological worsening at 24 h. There were 44 and 45 patients with missing data in the intervention and control arms, respectively, for the Global outcome of neurological worsening at 24 h.
There were 31 subjects in the intervention arm 24 subjects in the control arm missing day 7 NIHSS in the US cohort. There were 39 subjects in the intervention arm and 32 in the control arm missing day 7 NIHSS for the Global cohort.
There were 92 subjects in the intervention arm 102 subjects in the control arm missing day 90 NIHSS in the US cohort. There were 111 subjects in the intervention arm and 118 in the control arm missing day 90 NIHSS for the Global cohort.
There were 39 and 29 patients with missing data in the intervention and control arms respectively.
There were 47 and 38 patients with missing data in the intervention and control arms, respectively.
Common ORs were computed using ordinal logistic regression analyses after collapsing mRS scores 5 and 6.
CI, confidence interval; IQR, interquartile range; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; SD, standard deviation; tPA, tissue plasminogen activator.
The sensitivity analyses of safety outcomes between the two groups are presented in Table 3. The two groups did not differ in any of the safety outcomes including symptomatic (OR: 1.01, 95% CI 0.35–2.92, p > 0.99) and asymptomatic intracranial hemorrhage, cerebral edema, brain herniation, and death. The two groups also did not differ in any of the serious adverse events with the exception of nausea (12.6% versus 7.3%; p = 0.03) and atrial fibrillation (9.3% versus 4.5% without exclusion of patients with atrial fibrillation at baseline assessment, p = 0.02; 7.7% versus 3.8% after exclusion of patients with atrial fibrillation at baseline assessment, p = 0.04), which were more common in the intervention group.
Table 3.
Safety variables and serious adverse events within 90 days after randomization after removing centers with perceived endovascular equipoise shift.
| Variables | Intervention (n = 310) |
Control (n = 314) |
OR (95% CI) | p |
|---|---|---|---|---|
| Symptomatic intracranial hemorrhage at 24 ha, n (%) | 7 (2.3%) | 7 (2.2%) | 1.01 (0.35–2.92) | >0.99 |
| Symptomatic intracranial hemorrhage at 36 hb, n (%) | 8 (2.6%) | 8 (2.5%) | 1.01 (0.37–2.73) | >0.99 |
| Asymptomatic intracranial hemorrhage at 24 h, n (%) | 35 (11.3%) | 22 (7.0%) | 1.69 (0.97–2.95) | 0.07 |
| Death, n (%) | 48 (17.0%) | 43 (15.3%) | 1.14 (0.72–1.78) | 0.65 |
| Death due to serious adverse event, n (%) | 35 (11.3%) | 34 (10.8%) | 1.05 (0.63–1.73) | 0.90 |
| Serious adverse events, n (%) | 84 (27.1%) | 79 (25.2%) | 1.11 (0.77–1.58) | 0.59 |
| Cerebral edema, n (%) | 16 (5.2%) | 9 (2.9%) | 1.84 (0.80–4.24) | 0.16 |
| Brain herniation, n (%) | 11 (3.5%) | 4 (1.3%) | 2.85 (0.90–9.05) | 0.07 |
| Midline shift, n (%) | 8 (2.6%) | 9 (2.9%) | 0.90 (0.34–2.36) | >0.99 |
| Study discontinuation due to adverse events, n (%) | 19 (6.1%) | 25 (8.0%) | 0.75 (0.41–1.40) | 0.44 |
| Most common adverse event (headache), n (%) | 59 (19.0%) | 51 (16.2%) | 1.21 (0.80–1.83) | 0.40 |
| Second most common adverse event, (pyrexia), n (%) | 31 (10.0%) | 38 (12.1%) | 0.81 (0.49–1.33) | 0.44 |
| Third most common adverse event (nausea), n (%) | 39 (12.6%) | 23 (7.3%) | 1.82 (1.06–3.13) | 0.03 |
| Fourth most common adverse event (constipation), n (%) | 28 (9.0%) | 31 (9.9%) | 0.91 (0.53–1.55) | 0.78 |
| Fifth most common adverse event (pneumonia/aspiration pneumonia), n (%) | 33 (10.6%) | 25 (8.0%) | 1.38 (0.80–2.38) | 0.30 |
| Atrial fibrillation as adverse event, n (%) | 29 (9.3%) | 14 (4.5%) | 2.21 (1.14–4.27) | 0.02 |
| Atrial fibrillation as adverse event after exclusion of patients with atrial fibrillation at baseline, n (%) | 24 (7.7%) | 12 (3.8%) | 2.11 (1.04–4.30) | 0.04 |
Intervention: sonothrombolysis.
Control: intravenous thrombolysis.
sICH is defined as neurological deterioration (⩾4 points worsening on the NIHSS compared with the best prior examination) within 24 h after rt-PA bolus with documented parenchymal hemorrhage type 2 or type 2 remote (PH2/PH2r) where PH2 is defined as ICH volume at least one-third of the infarct volume, or death due to hemorrhage within 24 h after rt-PA bolus.
sICH is defined as neurological deterioration (⩾4 points worsening on the NIHSS compared with the best prior examination) within 36 h after rt-PA bolus with documented parenchymal hemorrhage type 2 or type 2 remote (PH2/PH2r) where PH2 is defined as ICH volume at least one-third of the infarct volume, or death due to hemorrhage within 36 h after rt-PA bolus.
CI, confidence interval; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; sICH, symptomatic intracranial hemorrhage; rt-PA, recombinant tissue plasminogen activator.
Discussion
In the present post hoc analysis of the CLOTBUST-ER trial we explored the potential interaction of shift in treatment practice in favor of endovascular thrombectomy and the effect of sonothrombolysis on 3 month functional outcome in a randomized controlled trial. The findings indicate a significant interaction between the effect of sonothrombolysis on outcome and center experience with endovascular treatment. The presence of equipoise shift in favor of endovascular treatment is further portrayed by the fact that in the CLOTBUST-ER trial the median baseline NIHSS score in the interventional group was 15 (11–18), whereas more than one-quarter (28%) of included patients presenting with baseline NIHSS scores of 10 or 11 points (Figure 1).3 This varies considerably from the patient population with severe stroke (NIHSS⩾10) included in the previous phase II RCT of sonothrombolysis (CLOTBUST) that reported a median NIHSS of 17 (14–21) for the intervention group with only 14% of the study population with severe stroke presenting with baseline NIHSS scores of 10 or 11 points.7
Enhancing current reperfusion treatments remains an important global priority for acute stroke treatment. This applies equally in healthcare systems with developed endovascular treatment networks, where a substantial proportion of patients commence IVT prior to prolonged inter-hospital transfer to endovascular centers, and in the many healthcare systems where endovascular treatment is unavailable or very poorly available.8,9 Endovascular treatment implementation requires reorganization of the stroke infrastructure, including a network referral to comprehensive stroke centers from secondary and primary stroke care centers.10 It is known that AIS patients with direct admission to a comprehensive stroke center with endovascular capacities have better 90 day functional outcomes compared with those referred from a primary stroke care center.11 Given that inter-hospital transfer is a critical and as yet unavoidable component in the treatment of patients with LVO transferred for mechanical thrombectomy, with the time from arrival to primary stroke care center to arrival in the comprehensive stroke center exceeding 120 min even in high-volume primary stroke centers,12 it becomes apparent that ancillary methods to facilitate vessel reperfusion need to be developed for use in settings where MT is still totally unavailable and for use during patient transfer to the comprehensive stroke center.
Endovascular treatment has become the standard of care for patients with LVO. Since endovascular treatment facilities are concentrated in the same large academic centers that recruit a high proportion of patients in clinical trials, it has become extremely challenging for clinical trials of alternative pharmacological or nonpharmacological reperfusion therapies to initiate and sustain patient recruitment.13 Although there is still room for improvement to enhance the effectiveness of endovascular treatments and to expand their application to a larger subset of stroke patients,14 all ancillary therapies for acute LVO treatment have the extremely difficult task of proving their additive effect on top of the huge effect size of endovascular treatment.15
Our experience in CLOTBUST-ER indicates that the increasing implementation of endovascular therapies across major academic stroke centers raises significant challenges for clinical trials aiming to test noninterventional or adjuvant reperfusion strategies if undertaken at the same centers. After taking into account that the positive results of recent thrombectomy trials have positioned CTA as standard of care in AIS patients with LVO,16 we have redesigned the operator-independent ultrasound device used in CLOTBUST-ER to take advantage of CTA-located LVO to increase the insonation of the occlusion by only insonating the suboccipital, the right transtemporal, or the left transtemporal window in accordance with the occlusion location identified on the CTA. This new device will be tested in the recently launched TRUST trial (ClinicalTrials.gov identifier: NCT03519737), in which all patients with LVO who meet standard tPA criteria and are being transferred from primary to comprehensive stroke centers (‘drip-and-ship’) will be randomized to ultrasound or no ultrasound with the primary endpoint being complete recanalization at receiving hospitals on digital subtraction angiography prior to thrombectomy.17 The results of TRUST trial will provide definitive answers regarding the efficacy of sonothrombolysis for improving tPA-induced reperfusion rates in AIS patients with LVO.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RM has been supported by the National Program of Sustainability II (MEYS CR; project number LQ1605) and by FNUSA-ICRC (project number CZ.1.05/1.1.00/02.0123; OP VaVpI); AHK has been supported by a Research Experience Fellowship from the European Academy of Neurology.
Conflict of interest statement: The authors declare that there is no conflict of interests.
ORCID iD: Andrei V. Alexandrov  https://orcid.org/0000-0001-8871-1023
https://orcid.org/0000-0001-8871-1023
Contributor Information
Andrei V. Alexandrov, Department of Neurology, University of Tennessee Health Science Center, 855 Monroe Avenue, Suite 415, Memphis, TN 38163, USA.
Georgios Tsivgoulis, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN, USA; Second Department of Neurology, ‘Attikon’ University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Martin Köhrmann, Department of Neurology, Universitaetsklinikum Erlangen, Erlangen, Germany; Department of Neurology, University Duisburg-Essen, Essen, Germany.
Aristeidis H. Katsanos, Second Department of Neurology, ‘Attikon’ University Hospital, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece Department of Neurology, St Josef Hospital, Ruhr University Bochum, Bochum, Germany.
Lauri Soinne, Department of Neurology, Helsinki University Hospital and Clinical Neurosciences, Neurology, University of Helsinki Helsinki, Finland.
Andrew D. Barreto, Department of Neurology, University of Texas Health Science Center at Houston, Houston, TX, USA
Travis Rothlisberger, Cerevast Therapeutics, Inc., Redmond, WA, USA.
Vijay K. Sharma, Yong Loo Lin School of Medicine, National University of Singapore and Division of Neurology, Department of Medicine, National University Hospital, Singapore, Singapore
Robert Mikulik, International Clinical Research Centre and Department of Neurology, St. Anne’s University Hospital in Brno and Medical Faculty, Masaryk University, Brno, Czech Republic.
Keith W. Muir, Institute of Neuroscience and Psychology, University of Glasgow, Queen Elizabeth University Hospital, Glasgow, UK
Christopher R. Levi, Department of Neurology, John Hunter Hospital, University of Newcastle, Newcastle, Australia
Carlos A. Molina, Stroke Unit, Department of Neurology, Vall d’Hebron University Hospital, Vall d’Hebron Research Institute, Autonomous University of Barcelona, Barcelona, Spain
Maher Saqqur, Department of Medicine (Neurology), University of Alberta, Edmonton, Alberta, Canada; Neuroscience Institute, Hamad Medical Corporation, Doha, Qatar.
Dimitris Mavridis, Department of Primary Education, School of Education, University of Ioannina, Ioannina, Greece.
Theodora Psaltopoulou, Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Athens, Greece.
Milan R. Vosko, Department of Neurology 2, Med Campus III, Kepler University Hospital, Linz, Austria
Jochen B. Fiebach, Center for Stroke Research Berlin, Charité-University Medicine Berlin, Berlin, Germany
Pitchaiah Mandava, Stroke Outcomes Laboratory, Department of Neurology, Baylor College of Medicine, Houston, TX, USA; Michael E. DeBakey VA Medical Center Stroke Program and Center for Translational Research on Inflammatory Diseases, Houston, TX, USA.
Thomas A. Kent, Stroke Outcomes Laboratory, Department of Neurology, Baylor College of Medicine, Houston, TX, USA Michael E. DeBakey VA Medical Center Stroke Program and Center for Translational Research on Inflammatory Diseases, Houston, TX, USA.
Anne W. Alexandrov, Department of Neurology, University of Tennessee Health Science Center, Memphis, TN, USA Australian Catholic University, Sydney, Australia.
Peter D. Schellinger, Departments of Neurology and Neurogeriatry, John Wesling Medical Center Minden, Ruhr University Bochum, Minden, Germany
References
- 1. Tsivgoulis G, Eggers J, Ribo M, et al. Safety and efficacy of ultrasound-enhanced thrombolysis: a comprehensive review and meta-analysis of randomized and nonrandomized studies. Stroke 2010; 41: 280–287. [DOI] [PubMed] [Google Scholar]
- 2. Ricci S, Dinia L, Del Sette M, et al. Sonothrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2012; 10: CD008348. [DOI] [PubMed] [Google Scholar]
- 3. Alexandrov AV, Köhrmann M, Soinne L, et al. Efficacy and safety of sonothrombolysis for acute ischemic stroke: a multi-centre, double-blind, phase 3, randomised controlled trial. Lancet Neurol 2019; 18: 338–347. [DOI] [PubMed] [Google Scholar]
- 4. Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke 2005; 36: 2121–2125. [DOI] [PubMed] [Google Scholar]
- 5. Schellinger PD, Alexandrov AV, Barreto AD, et al. ; CLOTBUST-ER Investigators. Combined lysis of thrombus with ultrasound and systemic tissue plasminogen activator for emergent revascularization in acute ischemic stroke (CLOTBUST-ER): design and methodology of a multinational phase 3 trial. Int J Stroke 2015; 10: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 6. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 7. Barlinn K, Tsivgoulis G, Barreto AD, et al. Outcomes following sonothrombolysis in severe acute ischemic stroke: subgroup analysis of the CLOTBUST trial. Int J Stroke 2014; 9: 1006–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsivgoulis G, Safouris A, Katsanos AH, et al. Mechanical thrombectomy for emergent large vessel occlusion: a critical appraisal of recent randomized controlled clinical trials. Brain Behav 2016; 6: e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischer U, Aguiar de, Sousa D, Norrving B, et al. Status and perspectives of acute stroke care in Europe. Stroke 2018; 49: 2281–2282. [DOI] [PubMed] [Google Scholar]
- 10. Daubail B, Ricolfi F, Thouant P, et al. Impact of mechanical thrombectomy on the organization of the management of acute ischemic stroke. Eur Neurol 2016; 75: 41–47. [DOI] [PubMed] [Google Scholar]
- 11. Ismail M, Armoiry X, Tau N, et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J Neurointerv Surg 2019; 11: 14–19. [DOI] [PubMed] [Google Scholar]
- 12. Ng FC, Low E, Andrew E, et al. Deconstruction of interhospital transfer workflow in large vessel occlusion: real-world data in the thrombectomy era. Stroke 2017; 48: 1976–1979. [DOI] [PubMed] [Google Scholar]
- 13. Savitz SI, Baron JC, Yenari MA, et al. Reconsidering neuroprotection in the reperfusion era. Stroke 2017; 48: 3413–3419. [DOI] [PubMed] [Google Scholar]
- 14. Linfante I, Cipolla MJ. Improving reperfusion therapies in the era of mechanical thrombectomy. Transl Stroke Res 2016; 7: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsivgoulis G, Katsanos AH, Alexandrov AV. Reperfusion therapies of acute ischemic stroke: potentials and failures. Front Neurol 2014; 5: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 17. Aureva transcranial ultrasound device with tPA in patients with acute ischemic stroke (TRUST), https://clinicaltrials.gov/ct2/show/NCT03519737 (accessed 2 January 2019).