Abstract
Treprostinil, a prostacyclin analogue, is approved for the treatment of pulmonary arterial hypertension (PAH) in adults. Transition from parenteral to oral treprostinil has been successfully accomplished in adults with PAH but not in children. In this multicenter study, pediatric patients treated with parenteral (Cohort 1) or inhaled (Cohort 2) treprostinil were transitioned to oral treprostinil. Prostacyclin-naïve individuals on background oral PAH therapy received oral treprostinil as add-on therapy (Cohort 3). Successful transition was oral treprostinil dose maintenance through week 24. Patients were monitored for adverse events (AEs), 6-min walk distance (6MWD), PAH symptoms, World Health Organization (WHO) Functional Class (FC), cardiac magnetic resonance imaging (cMRI), cardiopulmonary exercise testing (CPET), and quality of life through 24 weeks. A total of 32 patients were enrolled in the study; 23 (72%) were girls (mean age = 12.2 years). All patients were on background oral PAH therapy. Overall, patients (96.9%) maintained transition to oral treprostinil; one patient (Cohort 1) transitioned to oral treprostinil, then back to parenteral after experiencing syncope and WHO FC change from II to III. Cohorts 1, 2, and 3 received a final mean oral treprostinil dose of 5.6, 3.3, and 4.5 mg t.i.d., respectively. All cohorts had variable changes in 6MWD, cMRI, and CPET. Overall, 12 serious AEs were reported. All patients had drug-related AEs including headache (81%), diarrhea (69%), nausea (66%), vomiting (66%), and flushing (56%). Pediatric patients maintained transition to oral treprostinil with preservation of exercise capacity and WHO FC. Prostanoid-related AEs were most common and similar to those reported in adults.
Keywords: pulmonary arterial hypertension, pediatric, prostacyclin, treprostinil
Pulmonary arterial hypertension (PAH) is a rare but progressive and often fatal disease occurring in both adults and children. Pediatric patients with PAH are currently diagnosed, classified, and treated similarly to adults.1,2 Treatment options have expanded greatly in the past 20 years,3 providing many patients with safer, more convenient therapies. Parenteral prostanoids are recommended for the highest risk patients4 and >4500 patients in the United States are presently using pump-based, parenteral prostacyclin therapy.5 However, intravenous (IV) therapy increases the risk of bloodstream infection and thrombosis;6,7 subcutaneous (SC) treatment frequently causes site pain, which can be severe and require analgesics.8,9 In addition to the parenteral route, inhalation is a common route of administration for prostacyclin therapy in patients with PAH. Although clinically effective, the inhalation system has been reported to be complex and requires frequent dosing. A recent study in stable PAH adult patients receiving IV/SQ treprostinil demonstrated that patients can be safely transitioned to oral treprostinil dosed t.i.d. without a significant decline in exercise capacity or worsening functional class (FC);10 however, to date, data in pediatric patients are lacking.
The management of pediatric PAH remains challenging as treatment decisions continue to depend largely on results from evidence-based adult studies. Treatment efficacy in adults is commonly measured by the change in World Health Organization (WHO) classification or exercise tolerance, usually measured as the change in the 6-min walk distance (6MWD). Unfortunately, the 6MWD can be difficult to reliably assess in young children and may underestimate a child’s exercise limitations.11–13 Cardiopulmonary exercise testing (CPET) with gas exchange is a complementary non-invasive test to measure functional capacity and more accurately reflects functional status, especially in children with a 6MWD >300 m.11,13,14
The primary objective of this study was to assess the safety and tolerability of oral treprostinil in cohorts of pediatric patients with PAH aged 7–17 years: (1) transitioning from IV/SQ treprostinil Always use treprostinil and never Remodulin (trade name); (2) transitioning from inhaled prostacyclin; and (3) adding oral treprostinil de novo to current PAH therapy.
Methods
Nine study centers enrolled participants in this 24-week study. Detailed enrollment criteria, measurements, and transition procedures are presented in the Supplementary Material (available online). Briefly, eligible patients aged 7–17 years and weighed at least 22 kg. Patients had a current diagnosis of PAH (WHO Group 1) and were receiving IV/SQ treprostinil (25 to 125 ng/kg/min), inhaled prostacyclin, or other background PAH therapy (e.g. phosphodiesterase type 5 inhibitor [PDE5-I] or endothelin receptor antagonist [ERA] for at least 90 days) with stable doses for 30 days. The goal was to enroll lower-risk pediatric patients that demonstrated WHO FC I, II, or IIIa symptoms with stable PAH confirmed by prior right heart catheterization (RHC) with the following parameters: mean pulmonary arterial pressure (mPAP) of ≥25 mmHg, pulmonary vascular resistance index (PVRi) of >3 Wood Units*m2, and left ventricular end-diastolic pressure (LVEDP) or pulmonary capillary wedge pressure (PCWP) of ≤15 mmHg. Patients felt to be high risk due to higher doses of PAH therapy or who may be less compliant with three times a day drug dosing were not encouraged to enroll.
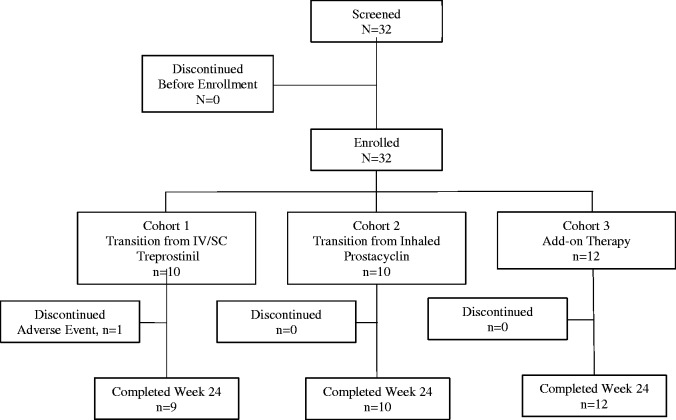
Patients were treated by cohort based on their PAH treatment at the time of enrollment as follows: transitioning from IV/SQ treprostinil (Cohort 1); transitioning from inhaled prostacyclin (Cohort 2); and treated with oral treprostinil as add-on to current PAH therapy in de novo prostacyclin patients (Cohort 3) (Fig. 1). The legal guardians of all 32 patients (all aged <18 years) provided written informed consent to participate and the patients provided written assent after an independent institutional review board approved the study protocol, which conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Fig. 1.
Participant disposition. Of the 32 patients who enrolled, 31 tolerated oral treprostinil maintained therapy through week 24. One participant (Cohort 1) transitioned to oral treprostinil, then back to parenteral after experiencing syncope and WHO FC change from II to III.
The primary endpoint in this study was the safety and tolerability of transitioning patients from IV/SQ treprostinil to oral treprostinil (Cohort 1) or from inhaled prostacyclin (Cohort 2), which were based on the percentage of patients successfully transitioning to oral treprostinil. A successful transition was defined as a subject from Cohort 1 or 2 who received oral treprostinil and was no longer receiving IV/SQ treprostinil or inhaled prostacyclin, respectively, at week 4 and was clinically maintained on oral treprostinil treatment through week 24. A successful initiation of oral treprostinil (Cohort 3) was defined as a patient who was clinically maintained on oral treprostinil through week 24. Secondary endpoints included CPET, symptoms of PAH, FC, 6MWD, Borg dyspnea score, quality of life assessed via the Pediatric Quality of Life Inventory (PedsQL™), plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, cardiac magnetic resonance imagining (cMRI) parameters, and descriptive evaluation of PK (to be published separately).
All patients received oral treprostinil provided as 0.125, 0.25, 1, or 2.5 mg extended-release tablets t.i.d. or q.i.d. at the discretion of the investigator. Dosing occurred approximately every 6–8 h for t.i.d. dosing or approximately every 4–6 h for q.i.d. dosing, with adjustments permitted based on the patient’s lifestyle and schedule. Study individuals were instructed to take their study drug at approximately the same time each day, with food. After baseline assessments were completed, participants in Cohort 1 began the transition from IV/SQ treprostinil in the hospital with a goal of complete transition to oral treprostinil within five days. The initial dose of oral treprostinil for Cohort 1 was calculated from the patient’s dose of IV/SC treprostinil and weight using the formula: oral treprostinil total daily dose (mg) = 0.0072 × treprostinil dose (ng/kg/min) × weight (kg). Individuals in Cohorts 2 and 3 were initiated on 0.125 mg t.i.d. or q.i.d. oral treprostinil with dose escalations possible every 24 h in increments of 0.125 mg t.i.d. or q.i.d. at the discretion of the Investigator during the first four weeks and in increments of either 0.125 mg or 0.25 mg every 24 h thereafter. Oral treprostinil was increased to the maximum tolerated dose, which was assessed by patient side effects. Cross titration occurred for Cohorts 1 and 2, such that doses of IV/SQ treprostinil or inhaled prostacyclin were decreased as patients were fully transitioned to oral treprostinil.
Patients were evaluated by up to seven study visits during the 24-week study at the following time points: screening, baseline, week 0 (Cohort 1 only; inpatient hospital stay days 1–5), week 3, week 6, week 12, and week 24. Patients who terminated the study early were asked to return to the study center for a final evaluation.
Statistical methods
A sample size of approximately 40 pediatric PAH patients aged 7–17 years (25 transition [IV/SQ treprostinil and inhaled] patients and 15 de novo prostacyclin patients) enabled an initial assessment of the safety and tolerability in this participant population for: (1) transition from moderate to high doses of IV/SQ treprostinil to oral treprostinil; (2) transition from inhaled prostacyclin to oral treprostinil; and (3) initiation of oral treprostinil as add-on therapy in de novo prostacyclin patients.
The safety and tolerability of transitioning participants from IV/SQ treprostinil to oral treprostinil (Cohort 1) or from inhaled prostacyclin (Cohort 2) was based on the percentage of individuals successfully transitioning to oral treprostinil. Analysis of secondary endpoints was descriptive in nature. Numeric endpoints for post-baseline assessments were compared to baseline using Wilcoxon signed rank test. P values were calculated for descriptive purposes; no formal hypothesis testing was planned.
Results
A total of 32 participants were screened and enrolled in the study: 10 patients in Cohort 1; 10 patients in Cohort 2; and 12 patients in Cohort 3 (Table 1). All 32 participants received at least one dose of oral treprostinil. Due to enrollment difficulties, the study was halted after 32 patients were enrolled. Thirty-one participants completed the study; 1 patient in Cohort 1 discontinued prematurely due to a serious AE (syncope). This patient was initiated on i.v. treprostinil due to moderate to severe PAH and syncope; after entering the study, the patient experienced syncope again. She was transitioned back to i.v. treprostinil. Participants in Cohorts 1, 2, and 3 received a final mean (±SD) study dose of 5.6 mg (±3.1), 3.3 mg (±0.85), and 4.5 mg (±2.1) t.i.d., respectively, which was substantially higher by weight compared with doses achieved in adult studies of oral treprostinil (Table 2). Only one patient (Cohort 3) required adjustment of background therapy and one patient received q.i.d. dosing (Cohort 3).
Table 1.
Participants’ demographics (n = 32).
| Characteristic | Cohort 1 (transitioning from parenteral, n = 10) | Cohort 2 (transitioning from inhaled, n = 10) | Cohort 3 (as add-on to current PAH therapy, n = 12) | Overall (n = 32) |
|---|---|---|---|---|
| Age at diagnosis (years) (median (range)) | 4.90 (0.2–10.2) | 6.75 (4.0–15.8) | 9.10 (0.0–17.1) | 7.05 (0.0–17.1) |
| Age at enrollment (years) (median (range)) | 10.0 (7–17) | 13.5 (8–17) | 13.5 (8–17) | 12.0 (7–17) |
| Gender: female/male | 9/1 | 7/3 | 7/5 | 23/9 |
| Race (n (%)) | ||||
| White | 7 (70.0) | 9 (90.0) | 10 (83.3) | 26 (81.3) |
| Black or African American | 0 | 0 | 0 | 0 |
| American Indian or Alaska Native | 1 (10.0) | 0 | 0 | 1 (3.1) |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 |
| Asian | 1 (10.0) | 1 (10.0) | 2 (16.7) | 4 (12.5) |
| Unknown | 1 (10.0) | 0 | 0 | 1 (3.1) |
| Current PAH Diagnosis (n (%)) | ||||
| Idiopathic PAH | 8 (80.0) | 8 (80.0) | 8 (66.7) | 24 (75.0) |
| Heritable PAH | 0 | 0 | 1 (8.3) | 1 (3.1) |
| Associated with repaired congenital systemic-to-pulmonary shunts | 1 (10.0) | 2 (20.0) | 2 (16.7) | 5 (15.6) |
| Associated with surgical repair of other congenital heart lesions | 1 (10.0) | 0 | 1 (8.3) | 2 (6.3) |
| Functional Class | ||||
| I | 2 (20.0) | 3 (30.0) | 1 (8.3) | 6 (18.75) |
| II | 8 (80.0) | 7 (70.0) | 9 (75.0) | 24 (75.0) |
| IIIa | 0 | 0 | 2 (16.7) | 2 (6.25) |
ASD, atrial septal defect; PAH, pulmonary arterial hypertension; PDA, patent ductus arteriosus; SD, standard deviation; VSD, ventricular septal defect.
Table 2.
Summary of oral treprostinil dosing and exposure.
| Cohort 1 (transitioning from parenteral, n = 10) | Cohort 2 (transitioning from inhaled, n = 10) | Cohort 3 (as add-on to current PAH therapy, n = 12) | |
|---|---|---|---|
| Initial oral treprostinil dose (mg) | |||
| Mean (SD) | 0.7292 (0.4883) | 0.1250 (0.0000) | 0.1250 (0.0000) |
| Range | 0.313–1.875 | 0.125–0.125 | 0.125–0.125 |
| Oral treprostinil dose when Treprostinil dose is 0.0 ng/kg/min (mg) | |||
| Mean (SD) | 4.7500 (2.8412) | ||
| Range | 1.750–9.625 | ||
| Oral treprostinil dose at time of hospital discharge (mg) | |||
| Mean (SD) | 4.9250 (2.8684) | ||
| Range | 1.750–10.000 | ||
| Oral treprostinil dose when first off inhaled prostacyclin (mg) | |||
| Mean (SD) | 1.2125 (0.6153) | ||
| Range | 0.625–2.750 | ||
| Final oral treprostinil dose (mg) | |||
| Mean (SD) | 5.6125 (3.1410) | 3.3625 (0.8528) | 4.5208 (2.0704) |
| Range | 1.250–10.000 | 1.500–4.500 | 2.375–10.000 |
Oral treprostinil dose was calculated as the sum of all doses given on the designated day divided by the total number of doses given that day.
PAH, pulmonary arterial hypertension; SD, standard deviation.
Efficacy
Overall, CPET was unchanged during transition to oral treprostinil. Although not statistically significant, 6MWD was slightly longer at all time points, resulting in a median numerical increase of 16.0, 19.0, and 23.5 m for Cohorts 1, 2, and 3, respectively, at week 24 (Table 3). Overall, across all cohorts, 20 participants (six each in Cohorts 1 and 2 and eight in Cohort 3) had improvements while 12 participants (four in each cohort) had reductions in 6MWD. Total recovery time following the 6-min walk test (6MWT) was unchanged for all cohorts transitioning to oral treprostinil; median change from baseline varied by no more than 1.5 min at any time point. At week 24, median HR immediately upon stopping the 6MWT was comparable to baseline in all three cohorts. There were no remarkable changes in oxygen saturation (Table 4).
Table 3.
Summary of 6MWT.
| Visit | Statistic | Cohort 1 (transitioning from parenteral, n = 10) | Cohort 2 (transitioning from inhaled, n = 10) | Cohort 3 (as add-on to current PAH therapy, n = 12) |
|---|---|---|---|---|
| Baseline | N | 10 | 10 | 12 |
| Mean (SD) | 468.1 (74.9) | 535.6 (107.0) | 505.0 (84.5) | |
| Median | 440.0 | 537.0 | 516.0 | |
| Range | 402–630 | 302–665 | 374–642 | |
| Week 24 | N | 9 | 10 | 12 |
| Mean (SD) | 472.1 (80.5) | 557.7 (62.0) | 517.8 (91.4) | |
| Median | 465.0 | 563.5 | 528.5 | |
| Range | 360–571 | 473–660 | 336–687 | |
| Change from baseline to week 24 | N | 9 | 10 | 12 |
| Mean (SD) | 2.7 (94.2) | 22.1 (86.5) | 12.8 (49.6) | |
| Median | 16.0 | 19.0 | 23.5 | |
| Range | −196–139 | −137–198 | −87–78 | |
| P value* | 0.7148 | 0.3750 | 0.3921 |
Baseline was the last 6MWT conducted before oral treprostinil dosing.
P values were calculated using Wilcoxon signed-rank test within each cohort.
6MWT, 6-min walk test; PAH, pulmonary arterial hypertension; SD, standard deviation.
Table 4.
Summary of cardiac magnetic resonance imaging parameters (RVEF and LVEF).
| Parameter | Visit | Statistic | Cohort 1 (transitioning from parenteral, n = 10) | Cohort 2 (transitioning from inhaled, n = 10) | Cohort 3 (as add-on to current PAH therapy, n = 12) |
|---|---|---|---|---|---|
| RVEF (%) | Baseline | n | 4 | 9 | 10 |
| Mean (SD) | 50.5 (5.1) | 48.4 (8.6) | 49.1 (4.7) | ||
| Week 24 | n | 4 | 10 | 9 | |
| Mean (SD) | 54.5 (8.1) | 49.0 (4.9) | 48.8 (5.0) | ||
| Change from baseline to week 24 | n | 4 | 9 | 9 | |
| Mean (SD) | 4.0 (7.6) | 0.4 (7.1) | −0.7 (2.7) | ||
| P value* | 0.3707 | 0.8552 | 0.4860 | ||
| LVEF (%) | Baseline | n | 4 | 9 | 10 |
| Mean (SD) | 57.8 (2.2) | 55.7 (5.0) | 59.6 (4.6) | ||
| Week 24 | n | 4 | 10 | 9 | |
| Mean (SD) | 56.8 (2.2) | 57.8 (3.7) | 57.9 (4.3) | ||
| Change from baseline to week 24 | N | 4 | 9 | 9 | |
| Mean (SD) | −1.0 (1.4) | 2.4 (6.0) | −2.4 (3.1) | ||
| P value* | 0.2522 | 0.2581 | 0.0448 |
P values were calculated using paired t-test within each cohort.
LVEF, left ventricular ejection fraction; PAH, pulmonary arterial hypertension RVEF, right ventricular ejection fraction, SD, standard deviation.
There was no effect on Borg dyspnea score and no consistent trends to indicate worsening of PAH symptoms in participants transitioning to oral treprostinil except for a minor increase in fatigue for Cohort 1. At week 24, fatigue grade compared with baseline in Cohort 1 was unchanged for five participants and deteriorated for four participants (two from none to mild; one from mild to moderate; and one from none to severe).
Cardiac MRI was also conducted to evaluate the ventricular size and function with oral treprostinil at baseline and again at week 24. All cMRI studies were performed using a 1.5-T scanner (Siemens Medical Solutions, Erlanger, Germany; Philips Medical Systems, Best, Netherlands). Standard short-axis and horizontal long-axis SSFP cine images were obtained with breath-holding during expiration using a retrospectively gated steady-state free precession sequence. Depending on the size of a patient, the parameters varied as follows: slice thickness = 6–10 mm; TE = 1.1–1.5 ms; TR = 2.8–3.5 ms; and resolution = 1.2–1.4 mm. The endocardial border was manually traced through standard short-axis images with coverage of the ventricles from base to apex as described previously at end-systole and end-diastole using Qmass 7.6 software15 (Medis, Leiden, The Netherlands). There were no consistent trends in either RV or left ventricular ejection fraction (LVEF) to indicate a change from baseline after oral treprostinil treatment, except a slight but significant decrease in LVEF in Group 3 (Table 4). This was also true of cardiac output (data not shown).
Quality of life
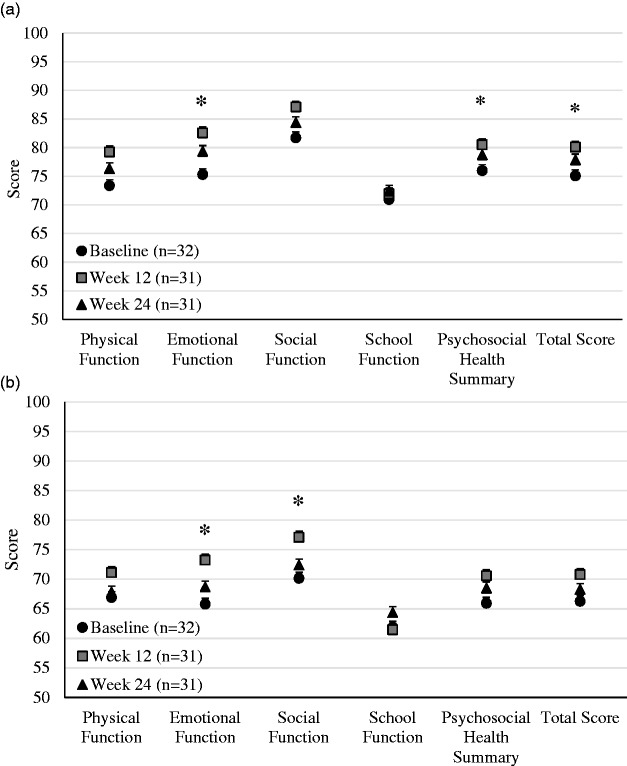
Thirty-two pediatric participants completed baseline quality of life (Pediatric Quality of Life Inventory, PedsQL™) assessments and 31 participants completed the assessments at week 12 and week 24. Social function and school function did not change from baseline to week 24. However, physical function, emotional function, psychosocial health, and total score improved significantly from baseline to week 24 when evaluated across all cohorts (Fig. 2a).
Fig. 2.
Baseline PedsQL™ assessments for (a) pediatric participants and (b) parents of pediatric participants. Asterisks indicate P value < 0.05.
In addition, 32 parents completed baseline quality of life assessments and 31 parents completed week 12 and week 24 assessments. Physical function, school function, psychosocial health, and total score did not change. However, emotional function and social function improved significantly from baseline to week 24 when evaluated across all cohorts (Fig. 2b).
Safety
All participants experienced at least one AE (89, 88, and 146 AEs in Cohorts 1, 2, and 3, respectively) and at least one AE attributable to oral treprostinil (67, 55, and 84 AEs in Cohorts 1, 2, and 3, respectively) (Table 5). Serious treatment-emergent adverse events (TEAEs) were reported for 5 (50.0%) participants in Cohort 1 and 4 (33.3%) participants in Cohort 3. One patient in Cohort 1 discontinued the study prematurely due to an AE of syncope. No TEAEs led to background therapy discontinuation and no deaths were reported. There were no clinically relevant changes in laboratory parameters, vital signs, or ECG parameters over the course of the study in any of the three cohorts.
Table 5.
Overall summary of adverse events.
| Number of participants with at least one: | Cohort 1 (transitioning from parenteral,
n = 10) |
Cohort 2 (transitioning from inhaled,
n = 10) |
Cohort 3 (as add-on to current PAH therapy,
n = 12) |
Overall (n = 32) |
||||
|---|---|---|---|---|---|---|---|---|
| n (%) | AEs (n) | n (%) | AEs (n) | n (%) | AEs (n) | n (%) | AEs (n) | |
| TEAE | 10 (100.0) | 89 | 10 (100.0) | 88 | 12 (100.0) | 146 | 32 (100.0) | 323 |
| Oral treprostinil attributable TEAE | 10 (100.0) | 67 | 10 (100.0) | 55 | 12 (100.0) | 84 | 32 (100.0) | 206 |
| Serious TEAE | 5 (50.0) | 6 | 0 | 0 | 4 (33.3) | 6 | 9 (28.1) | 12 |
| TEAE leading to oral treprostinil discontinuation | 1 (10.0) | 1 | 0 | 0 | 0 | 0 | 1 (3.1) | 1 |
| Background therapy attributable TEAE | 8 (80.0) | 23 | 7 (70.0) | 13 | 9 (75.0) | 51 | 24 (75.0) | 87 |
AE, adverse event; PAH, pulmonary arterial hypertension; TEAE, treatment-emergent adverse event.
For Cohort 1, the most commonly reported TEAEs while transitioning from IV/SQ treprostinil were: vomiting (90% of patients); nausea (70% of patients); diarrhea, flushing, and headache (60% of patients for each); upper abdominal pain (40% of patients); and dizziness (30% of patients). All TEAEs were considered related to oral treprostinil administration.
For Cohort 2, the most commonly reported TEAEs while transitioning from inhaled prostacyclin were: headache (80% of patients); diarrhea (70% of patients); cough, nausea, and vomiting (50% of patients for each); and flushing, upper abdominal pain, and viral upper respiratory tract infection (40% of patients for each). Diarrhea and headache (60% of patients each), nausea and vomiting (50% of patients each), and flushing and upper abdominal pain (40% of patients each) were considered related to oral treprostinil administration.
For Cohort 3, the most commonly reported TEAEs while adding oral treprostinil as a de novo prostacyclin treatment were: headache (100% of patients); diarrhea and nausea (75% of patients for each); flushing (67% of patients); vomiting (58% of patients); pain in jaw (50% of patients); chest pain, pain in extremity, and upper abdominal pain (42% of patients for each); dizziness and dyspnea (33% of patients for each); and fatigue (25% of patients). Headache (100% of patients), nausea (75% of patients), diarrhea, flushing, and vomiting (58% of patients each), pain in jaw (50% of patients), dizziness (33% of patients), and dyspnea, fatigue, and upper abdominal pain (25% of patients each) were considered related to oral treprostinil administration.
Most TEAEs were mild: 55/89 TEAEs, 60/88 TEAEs, and 100/146 TEAEs in Cohorts 1, 2, and 3, respectively. Severe TEAE were experienced by 4 (40%; 7/89 TEAEs), 1 (10%; 2/88 TEAEs), and 3 (25%; 7/146 TEAEs) patients in Cohorts 1, 2, and 3, respectively. Individual severe TEAEs were limited to one or two participants across the cohorts. Moderate TEAE were experienced by 10 (100%; 27/89 TEAEs), 4 (40%; 26/88 TEAEs), and 8 (67%; 39/146 TEAEs) patients in Cohorts 1, 2, and 3, respectively.
A total of 5 (50%) individuals in Cohort 1 experienced six SAEs and 4 (33.3%) participants in Cohort 3 experienced six SAEs. For Cohort 1, SAEs of seizure (two patients) and device-related infection, stoma site infection, and syncope (one participant each) were reported. For Cohort 3, the SAEs were chest pain (two patients), bronchial hyper-reactivity, dyspnea, pneumonia, and tachycardia (one participant each). Of these, one individual in Cohort 1 (syncope) and one participant in Cohort 3 (chest pain and dyspnea) had SAEs that were attributed to oral treprostinil.
Pharmacokinetic parameters of treprostinil were collected and the results are reported elsewhere.
Discussion
Safety and tolerability of oral treprostinil extended-release tablets were similar to adults in this study of pediatric PAH patients aged 7–17 years. Whether transitioning from IV/SQ treprostinil (Cohort 1), inhaled prostacyclin (Cohort 2), or initiating de novo treprostinil therapy orally (Cohort 3), nearly all participants (n = 31; 96.9%) were maintained on oral treprostinil through 24 weeks. All patients but one maintained or improved WHO FC and one participant required background therapy adjustment. All individuals had drug-related AEs. Overall, 12 serious AEs were reported; one patient (Cohort 1) experienced repeat syncope and transitioned back to parenteral treprostinil. There were no significant efficacy results shown in the secondary objective measures. All cohorts had numerical improvements in 6MWD but did not reach statistical significance (20 participants had improvements while 12 (four in each cohort) had reductions in 6MWD). Of note, the 6MWD was normal or near normal at baseline as all cohorts were receiving treatment; therefore, significant improvement was not anticipated. There was a lack of clear improvement in Cohort 3 patients where oral treprostinil was used as an add-on therapy in patients already receiving other oral treatments. No remarkable declines in PAH symptoms or Panama/WHO FC were noted. Additionally, overall quality of life improved over 24 weeks of treatment with oral treprostinil.
The percentage of participants with AEs were high, especially for prostacyclin-related AEs; however, the AE profiles are consistent with the administration of oral treprostinil and the rate of AEs in this study was similar to the AE rate in a transition study in adult patients.10 The majority of the AEs occurred during the first four weeks following the initiation of oral treprostinil. The rate of AEs decreased over time.
The major limitation of this study is the open-label, uncontrolled design and lack of placebo group. Additionally, the sample size was relatively small. Another comparable open-label, uncontrolled study evaluated bosentan, a dual ERA, in a similar pediatric patient population for 12 weeks, showed no significant changes in exercise capacity or WHO FC but did show improvements in hemodynamics.16 Therefore, in lieu of a control group, the duration of treatment and observation in this study was extended to 24 weeks. An additional limitation of the study is the subjective nature of the definition of “successful transition.” The definition of success was similar to previously published transition studies (i.e. that the participant had not deteriorated during the 24 weeks and wished to continue oral treprostinil).16–18 However, this assessment was supplemented by evaluating secondary endpoints such as CPET, symptoms of PAH, FC, 6MWD, Borg dyspnea score, and quality of life. As such, it is important to carefully evaluate patients (e.g. starting dose of IV/SQ therapy and FC) when considering treatment with oral treprostinil. All patients had significant treatment-related side effects, including gastrointestinal (GI) side effects and headache. Some patients had fewer GI side effects if oral treprostinil was taken with increased calories or fiber. Long-term data are needed to evaluate for sustained safety, tolerability, and treatment effect.
In conclusion, the AE profile of oral treprostinil was similar to that reported in adult populations and all children reported GI side effects and/or headaches. The safety results indicated that some pediatric patients with stable PAH, in FC I or II, can be transitioned from parenteral to oral treprostinil and maintained for at least six months. Overall, these data may help inform clinicians seeking a more convenient route of treprostinil administration for their pediatric patients.
Supplemental Material
Supplemental material, Supplemental Material1 for Oral treprostinil in transition or as add-on therapy in pediatric pulmonary arterial hypertension by D. Dunbar Ivy, Jeffrey A. Feinstein, Delphine Yung, Mary P. Mullen, Edward C. Kirkpatrick, Russel Hirsch, Eric D. Austin, Jeffrey Fineman, Uyen Truong, Derek Solum, C.Q. Deng and Rachel K. Hopper in Pulmonary Circulation
Supplemental Material
Supplemental material, Supplemental Material2 for Oral treprostinil in transition or as add-on therapy in pediatric pulmonary arterial hypertension by D. Dunbar Ivy, Jeffrey A. Feinstein, Delphine Yung, Mary P. Mullen, Edward C. Kirkpatrick, Russel Hirsch, Eric D. Austin, Jeffrey Fineman, Uyen Truong, Derek Solum, C.Q. Deng and Rachel K. Hopper in Pulmonary Circulation
Supplemental Material
Supplemental material, Supplemental Material3 for Oral treprostinil in transition or as add-on therapy in pediatric pulmonary arterial hypertension by D. Dunbar Ivy, Jeffrey A. Feinstein, Delphine Yung, Mary P. Mullen, Edward C. Kirkpatrick, Russel Hirsch, Eric D. Austin, Jeffrey Fineman, Uyen Truong, Derek Solum, C.Q. Deng and Rachel K. Hopper in Pulmonary Circulation
Conflict of interest
The University of Colorado contracts with Actelion, Bayer, Lilly, and United Therapeutics for DDI to perform clinical research studies and to be a consultant. DDI receives funding from the NIH and FDA. Vanderbilt University Medical Center contracts with Actelion, Bayer, and United Therapeutics for EDA to perform clinical research studies. EDA receives funding from the NIH and the Cardiovascular Medical Research Foundation. EDA previously performed consultation on clinical trial design (< $10,000) for Acceleron Pharma, Inc.
Funding
This study was funded by United Therapeutics Corporation.
References
- 1.Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D117–126. [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132(21): 2037–2099. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Lau EM, Montani D, et al. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014; 130: 2189–2208. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 5.Chakinala MM, Feldman JP, Rischard F, et al. Transition from parenteral to oral treprostinil in pulmonary arterial hypertension. J Heart Lung Transplant 2017; 36(2): 193–201. [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296–302. [DOI] [PubMed] [Google Scholar]
- 7.Hiremath J, Thanikachalam S, Parikh K, et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant 2010; 29: 137–149. [DOI] [PubMed] [Google Scholar]
- 8.White RJ, Levin Y, Wessman K, et al. Subcutaneous treprostinil is well tolerated with infrequent site changes and analgesics. Pulm Circ 2013; 3: 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathier MA, McDevitt S, Saggar R. Subcutaneous treprostinil in pulmonary arterial hypertension: practical considerations. J Heart Lung Transplant 2010; 29: 1210–1217. [DOI] [PubMed] [Google Scholar]
- 10.White RJ, Chakinala M, Rischard F, et al. Safety and tolerability of transitioning from parenteral treprostinil to oral treprostinil in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2014; 189: A2460. [Google Scholar]
- 11.Ivy DD. Pulmonary arterial hypertension assessment in pediatric cardiology. In: Beghetti M, Barst RJ, Naeije R, et al., eds. Pulmonary Arterial Hypertension Related to Congenital Heart Disease. Atlanta, GA: Elsevier, Urban and Fischer; 2006, pp.93–111.
- 12.Tissot C, Beghetti M. Advances in therapies for pediatric pulmonary arterial hypertension. Expert Rev Respir Med 2009; 3(3): 265–282. [DOI] [PubMed] [Google Scholar]
- 13.Barst RJ, Ertel SI, Beghetti M, et al. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 2011; 37(3): 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adatia I, Haworth SG, Wegner M, et al. Clinical trials in neonates and children: report of the pulmonary hypertension academic research consortium pediatric advisory committee. Pulm Circ 2013; 3(1): 252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James SH, Wald R, Wintersperger BJ, et al. Accuracy of right and left ventricular functional assessment by short-axis vs axial cine steady-state free-precession magnetic resonance imaging: intrapatient correlation with main pulmonary artery and ascending aorta phase-contrast flow measurements. Can Assoc Radiol J 2013; 64(3): 213–219. [DOI] [PubMed] [Google Scholar]
- 16.Barst RJ, Ivy D, Dingemanse J, et al. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther 2003; 73(4): 372–382. [DOI] [PubMed] [Google Scholar]
- 17.Suleman N, Frost AE. Transition from epoprostenol and treprostinil to the oral endothelin receptor antagonist bosentan in patients with pulmonary hypertension. Chest 2004; 126: 808–815. [DOI] [PubMed] [Google Scholar]
- 18.Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest 2006; 130: 1471–1480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Oral treprostinil in transition or as add-on therapy in pediatric pulmonary arterial hypertension by D. Dunbar Ivy, Jeffrey A. Feinstein, Delphine Yung, Mary P. Mullen, Edward C. Kirkpatrick, Russel Hirsch, Eric D. Austin, Jeffrey Fineman, Uyen Truong, Derek Solum, C.Q. Deng and Rachel K. Hopper in Pulmonary Circulation
Supplemental material, Supplemental Material2 for Oral treprostinil in transition or as add-on therapy in pediatric pulmonary arterial hypertension by D. Dunbar Ivy, Jeffrey A. Feinstein, Delphine Yung, Mary P. Mullen, Edward C. Kirkpatrick, Russel Hirsch, Eric D. Austin, Jeffrey Fineman, Uyen Truong, Derek Solum, C.Q. Deng and Rachel K. Hopper in Pulmonary Circulation
Supplemental material, Supplemental Material3 for Oral treprostinil in transition or as add-on therapy in pediatric pulmonary arterial hypertension by D. Dunbar Ivy, Jeffrey A. Feinstein, Delphine Yung, Mary P. Mullen, Edward C. Kirkpatrick, Russel Hirsch, Eric D. Austin, Jeffrey Fineman, Uyen Truong, Derek Solum, C.Q. Deng and Rachel K. Hopper in Pulmonary Circulation