Abstract
Background:
Gastrointestinal therapeutic drugs (GTDs) are extensively prescribed. The aim of this study was to investigate the characteristics of GTD use in a large population: the French general health scheme beneficiaries (87% of the 66 million inhabitants) in 2016.
Methods:
The national health data system was used to identify individual characteristics, diseases and GTD classes reimbursed, together with the costs, using anatomical therapeutic chemical class.
Results:
Among the 57.5 million individuals included, 45% received at least one reimbursement among the 130 million prescriptions reimbursed (90% prescribed by a general practitioner): proton-pump inhibitors (PPI; A02BC: 24%), drugs for functional gastrointestinal disorders (A03: 20%), drugs for constipation (A06: 10%), antidiarrheals, intestinal anti-inflammatory/anti-infective agents (A07: 10%), antiemetics and antinauseants (A04: 7%), other drugs for acid-related disorders (A02X: 6%), other drugs for peptic ulcer and gastro-oesophageal reflux disease (A02BX: 4.5%), antacids (A02A: 1.5%). The overall cost of reimbursed GTDs was €707 million and the mean cost per user was €28. Marked variations were observed according to age, sex, and disease. The rates of at least one reimbursement among infants were A07: 28%, A03: 17%, A02BX: 9%, A02X: 7%, A02BC: 6% and A06: 5%. Women more frequently received a reimbursement than men for each GTD class. Reimbursement rates also varied according to health status (end-stage renal disease A02BC: 66%, pregnancy A03: 53%, A04: 11%), treatments (people with at least six reimbursements for nonsteroidal anti-inflammatory drugs in 2016 A02BC: 62%). Chronic GTD use (>10 reimbursements/year) was observed in 19% of people with at least one A02BC reimbursement, A02BX: 11%, A03: 7%, A04: 2%, A06: 17% and A07: 3%.
Conclusions:
This study demonstrates extensive and chronic use of GTD in France, raising the question of their relevance according to current guidelines. They must be disseminated to general practitioners, who are the main prescribers of these drugs.
Keywords: gastrointestinal drugs, morbidities, nationwide, pharmacoepidemiology, prevalence, treatment duration
Introduction
Apart from the management of long-term diseases such as gastrointestinal cancers, inflammatory bowel disease and viral hepatitis, routine gastroenterology management for gastrointestinal symptoms and functional gastrointestinal disorders generates high health expenditure related to frequent hospitalizations for examinations, emergency and outpatient consultations and acute or chronic treatments. This high expenditure has been evident in various countries for disorders such as gastro-oesophageal reflux disease (GORD), gastritis, peptic ulcers and functional bowel disorders, such as irritable bowel syndrome (IBS), nausea, constipation or diarrhoea.1–5
Statistics quantifying the burden of a disease are useful for public health policy decision making, such as priority settings and resource allocations. The incidence or prevalence of most diagnoses are difficult to estimate and vary according to age, sex, origin, definition or diagnostic criteria, physician diagnosis or self-reporting, symptom severity and frequency and location of study. Available data are mostly derived from representative samples based on small numbers of participants or meta-analyses. The prevalence of GORD, defined by the presence of symptoms at least once a week for 12 months, was estimated to be 13% in a meta-analysis.6 In France, this prevalence was estimated to be 8% in a representative sample in 2003.7 The prevalence of peptic ulcer disease, based on a review of physician diagnosis studies, ranged between 0.12% and 1.50%.8 In a meta-analysis, the prevalence of IBS in adults was estimated as 12% in North America, 21% in South America and 7% in Southeast Asia.9 In France, a recent web-based survey reported a prevalence of 10%.10 Chronic constipation can affect 2–27% of the population, according to either self-reported surveys or questionnaires using the Rome criteria.11 The prevalence of these diagnoses may also vary according to population groups, such as infants or pregnant women.12–14
Another approach to estimating the burden of these diseases and their treatments is to analyse gastrointestinal therapeutic drug (GTD) consumption based on prescriptions or claims databases. The development of nationwide databases such as these increases the potential for observational studies on drug use. In the USA, the sales of prescription GTDs were $25 billion, the 10th leading therapeutic class in terms of sales, with a 10% growth between 2013 and 2014.15,16 Antibiotics, asthma, and gastrointestinal drugs were the outpatient prescriptions most commonly dispensed to infants (0–23 months).17 In the United Kingdom, GTDs were the therapeutic class with the highest growth (1999–2012) in women (22% in 2012) and also increased in men (17% in 2012).18 Overprescription, with respect to guidelines, of certain GTDs [such as proton-pump inhibitors (PPIs)] has also been and can be associated with adverse effects.19–22
The aim of this study, based on 2016 data from the French National Health Data Information System (Système National des Données de Santé, SNDS) comprising drug reimbursement data and patient characteristics, was therefore to analyse the frequency and patterns of use of the various classes of GTD and the related costs according to individual demographic characteristics, disease or health status.
Methods
Data sources
The SNDS is one of the largest claims databases in the world and has been extensively used to guide public health policies in France.23 This database comprehensively collects individual outpatient data (age, sex, date of death…), as well as healthcare prescriptions and procedures reimbursed by French national health insurance, but it does not provide any clinical data concerning the results of physician visits, prescriptions or examinations. Nevertheless, it includes information on the presence of long-term chronic diseases (LTD; Affection de Longue Durée, ALD) eligible for 100% reimbursement of healthcare expenditure, when requested by the patient’s general practitioner and after approval by the health insurance medical consultant. Reimbursed drugs are identified by means of the Anatomical Therapeutic Chemical (ATC) code and therapeutic class, while medical visits are identified by the general nomenclature of professional procedures (Nomenclature Générale des Actes Professionnels). All this information is linked, via the national hospital discharge database (Programme de médicalisation des systèmes d’information, PMSI), to data concerning public and private hospital stays. However, drugs dispensed during a hospital stay are not individually reimbursed and were consequently not included in this study. Hospital diagnoses of the stay and ALD are coded according to the International Classification of Diseases 10th revision (ICD 10).
Population
The French national health insurance general scheme covered about 87% of the 66 million inhabitants of France in 2016. The remaining population was covered by other schemes: the agricultural workers’ health insurance fund (Mutualité Sociale Agricole) and the self-employed health insurance fund (Régime Social des Indépendants), each covering 5% of the population, and the remaining 3% were covered by other schemes. The population of the present study was composed of general health scheme beneficiaries. Beneficiaries of the other funds were not included due to the lack of comprehensive data for LTD status or vital status. As the disease identification algorithms in the SNDS, described below, are based on healthcare use data, general scheme beneficiaries with no reimbursed healthcare consumption in 2014 and 2015 (about 1% of beneficiaries) were excluded from the study.
Outcome variables
Therapeutic classes of GTD were identified by means of ATC codes: A02A: Antacids (A02AB: Aluminium compounds, A02AD: combinations and complexes of aluminium, calcium and magnesium compounds); A02B: drugs for peptic ulcer and GORD (A02BA: H2-receptor antagonists, A02BB: prostaglandins, A02BC: PPI, A02BD: combinations for eradication of Helicobacter pylori); A02BX: other drugs for peptic ulcer and GORD; A02X: other drugs for acid-related disorders (mainly preparations not in the preceding groups); A03: drugs for functional gastrointestinal disorders (A03A: drugs for functional gastrointestinal disorders; A03D: antispasmodics in combination with analgesics; A03F: propulsives); A04: antiemetics and antinauseants; A06: drugs for constipation (A06AA: softeners, emollients; A06AB: contact laxatives; A06AC: bulk-forming laxatives; A06AD: osmotically acting laxatives; A06AG: enemas; A06AH: peripheral opioid receptor antagonists; A06AX: other drugs for constipation); A07: antidiarrheals, intestinal anti-inflammatory/anti-infective agents (A07A: intestinal anti-infectives; A07B: intestinal adsorbents; A07D: antipropulsives; A07E: intestinal anti-inflammatory agents; A07X: other antidiarrhoeals). Other, more specific GTD groups were not studied: A01 (stomatological preparations); A05 (bile and liver therapy); A08 (antiobesity preparations, excluding diet products); and A09 (digestives, including enzymes). A02BA, A02BB and A02BD classes that are prescribed to relatively small numbers of users (25,000 and €3.5 million reimbursed, 55,000 and €0.34 million reimbursed, 98,100 and €4.2 million reimbursed, respectively) were also excluded.
Diseases and health conditions managed in 2015 were identified by algorithms developed by the national health insurance fund for salaried employees (Caisse Nationale d’Assurance Maladie, CNAM) of the general scheme.23–26 Beneficiaries are classified into 60 nonexclusive groups of patients or 13 large categories (Supplementary table). These algorithms were developed based on SNDS data using ICD 10 codes of LTD, hospital diagnoses, drugs that are almost specific for certain diseases and sometimes certain procedures, allowances or diagnosis-related groups. These data were collected over a period of 1–5 years with respect to the year considered for hospital diagnoses and LTDs, which are attributed for renewable 5-year periods (table in Supplementary Material). Algorithms for cardiovascular prevention drugs (antihypertensives or lipid-lowering drugs) or psychotropic drugs are considered on the basis of three annual reimbursements. At least six annual reimbursements were considered for chronic use of analgesics, nonsteroidal anti-inflammatory drugs or corticosteroids. A reference ‘no disease’ group was considered, corresponding to people not presenting with any of the 60 other groups of diseases, treatments, isolated hospitalization or pregnancy. Only certain large categories were described or reported in this study, on the basis of their relevance to the subject.
Analysis
Frequencies of users were calculated for people with at least one GTD or GTD class reimbursement (A02A, A02BC, A02BX, A02X, A03, A04, A06, A07) among all people included and according to age, sex and with or without groups of diseases and health conditions. Median age and interquartile range (IQR) were reported for users of each GTD class. Numbers of people with at least one annual reimbursement for all or one of the GTD classes studied were considered as the denominator to calculate reimbursement frequencies in 2016, also according to age, sex and with or without groups of diseases and health conditions, bearing in mind that some individuals may have been reimbursed for more than one group or for different drugs included in the same drug class.
Expenditures were calculated on the basis of the sums reimbursed to the beneficiary by the national health insurance fund for each drug (100%, 65%, 30% or 15% of the retail price) fixed by the French schemes according to the level of usefulness determined by the French health authority (Haute Autorité de Santé). The total sum reimbursed in 2016 to all national health insurance general scheme beneficiaries was calculated by therapeutic class. The mean expenditure reimbursed per beneficiary with at least one annual reimbursement of each class was also calculated according to individual characteristics and groups of diseases and health conditions.
Specific ethics committee approval was not required for this study. The CNAM, as a health research institute, has permanent access to the SNDS database approved by decree and the French data protection authority (Commission Nationale de l’Informatique et des Libertés). SAS software (version 7.11, SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
Results
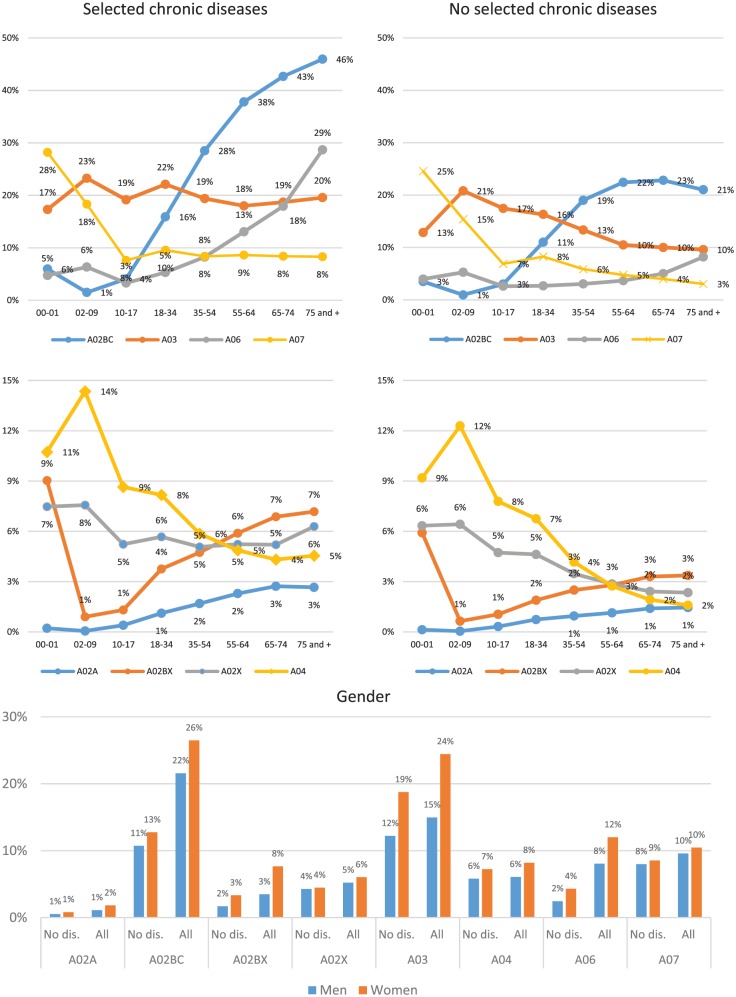
Globally, for the 57.5 million individuals included and the 130 million prescriptions reimbursed, 45% individuals (25.7 million) had at least one selected therapeutic class reimbursement in 2016 and were considered as GTD users. User frequency varied by age according to the GTD classes (Figure 1). Among all users, women had somewhat higher frequencies (by 1 or 2 points) than men for each GTD class, including in the absence of chronic diseases and health conditions (Figure 1). The overall expenditure reimbursed for all GTD was €707 million and the mean expenditure reimbursed by user was €28 euros.
Figure 1.
Frequency of individuals with at least one reimbursement in 2016 for several gastrointestinal therapeutic drug classes by age and sex in the overall population and among those without the selected diseases.
A02A: antacids; A02BC: PPIs; A02BX: other drugs for peptic ulcer and gastro-oesophageal reflux disease; A02X: other drugs for acid-related disorders; A03: drugs for functional gastrointestinal disorders; A04: antiemetics and antinauseants, A06: drugs for constipation, A07: antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents.
PPIs, proton-pump inhibitors.
A02A: antacids
The overall frequency of A02A users [median age: 61 years, interquartile range (IQR): 46–72] was 1.5% with a slow age-related increase: 0.2% (0–1 years) to 2.7% (75 years and older; Figure 1). This increase was less marked in people without the selected chronic diseases and health conditions (0.1% and 1.5%, respectively). Chronic use (>10 reimbursements per year) was identified for 4% of all users with at least one antacid reimbursement in 2016 and 1% of all users without an identified disease. The highest chronic use was observed for people with dementia and neurological disease (10%) or mental illness (8%; Table 1). Among individuals with antacid reimbursement, 9% presented no reimbursement for other GTD classes in the same year, 25% were reimbursed for another class (A02BC 16%), 25% were reimbursed for two other classes and 41% were reimbursed for three other classes (Table 2). Antacids were mainly prescribed by general practitioners (92% of reimbursements), gastroenterologists (5%) or otorhinolaryngologists (1%; Table 3). The global sum reimbursed for A02A drugs was €3 million and the mean reimbursement per A02A user was €3 (Table 4).
Table 1.
Frequency of individuals with low (1–2) or high (>10) numbers of several gastrointestinal therapeutic drug reimbursements in 2016 by sex, age and diseases and other health conditions.
| Gastrointestinal therapeutic drug classes | A02A | A02BC | A02BX | A02X | A03 | A04 | A06 | A07 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of reimbursements | 1–2 | >10 | 1–2 | >10 | 1–2 | >10 | 1–2 | >10 | 1–2 | >10 | 1–2 | >10 | 1–2 | >10 | 1–2 | >10 |
| % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | |
| Total | 71.0 | 4.5 | 54.4 | 19.3 | 51.5 | 10.7 | 94.5 | 0.5 | 62.5 | 7.3 | 91.0 | 1.7 | 50.7 | 16.9 | 84.9 | 2.9 |
| Sex | ||||||||||||||||
| Men | 69.7 | 5.1 | 53.8 | 20.5 | 50.8 | 11.8 | 94.7 | 0.5 | 67.5 | 5.8 | 92.1 | 1.7 | 55.2 | 14.5 | 84.8 | 3.0 |
| Women | 71.7 | 4.2 | 54.9 | 18.4 | 51.9 | 10.1 | 94.3 | 0.5 | 59.8 | 8.1 | 90.3 | 1.7 | 48.1 | 18.3 | 84.9 | 2.8 |
| Age (years) | ||||||||||||||||
| 00–01 | 84.8 | 0.8 | 66.6 | 2.6 | 75.3 | 2.4 | 98.3 | 0.0 | 88.3 | 0.2 | 96.6 | 0.0 | 73.9 | 2.7 | 78.6 | 0.9 |
| 02–09 | 93.4 | 0.4 | 79.1 | 3.9 | 82.2 | 2.1 | 98.7 | 0.0 | 84.8 | 0.2 | 96.6 | 0.0 | 61.4 | 6.1 | 91.4 | 0.1 |
| 10–17 | 92.7 | 0.2 | 89.8 | 1.1 | 80.8 | 0.9 | 98.7 | 0.0 | 75.3 | 0.8 | 96.5 | 0.1 | 68.2 | 4.1 | 95.3 | 0.2 |
| 18–34 | 85.1 | 0.7 | 82.4 | 2.0 | 62.2 | 3.3 | 98.0 | 0.0 | 65.1 | 3.2 | 94.5 | 0.4 | 64.6 | 4.8 | 92.1 | 1.0 |
| 35–54 | 74.6 | 2.8 | 66.3 | 8.5 | 51.4 | 8.7 | 95.1 | 0.3 | 60.3 | 6.4 | 90.4 | 1.8 | 58.5 | 10.2 | 85.2 | 3.1 |
| 55–64 | 67.2 | 5.2 | 50.1 | 19.8 | 45.0 | 13.3 | 91.2 | 0.8 | 51.8 | 12.1 | 81.0 | 5.3 | 55.6 | 14.8 | 77.1 | 6.0 |
| 65–74 | 63.2 | 6.8 | 38.3 | 31.1 | 42.2 | 15.9 | 87.8 | 1.3 | 46.3 | 16.8 | 73.0 | 8.1 | 49.5 | 19.8 | 71.7 | 8.1 |
| 75 and older | 59.2 | 9.0 | 26.5 | 43.5 | 36.9 | 21.3 | 84.4 | 2.0 | 41.2 | 23.6 | 76.4 | 5.9 | 29.7 | 32.3 | 68.6 | 8.9 |
| Diseases and health conditions | ||||||||||||||||
| None | 84.5 | 1.0 | 83.3 | 2.7 | 67.4 | 3.0 | 98.8 | 0.0 | 76.2 | 1.2 | 97.7 | 0.0 | 64.0 | 5.7 | 94.3 | 0.2 |
| Cardiovascular and cerebrovascular disease | 61.5 | 7.7 | 23.2 | 46.6 | 38.3 | 19.1 | 86.2 | 1.6 | 45.3 | 18.7 | 73.5 | 7.2 | 35.0 | 27.3 | 69.0 | 8.9 |
| Cardiovascular prevention (pharmacological)* | 62.6 | 7.1 | 38.9 | 29.8 | 41.4 | 16.6 | 89.4 | 1.2 | 46.6 | 17.3 | 81.3 | 4.8 | 43.2 | 24.3 | 75.9 | 6.3 |
| Diabetes | 60.9 | 7.8 | 28.3 | 41.1 | 37.3 | 19.3 | 84.8 | 1.6 | 45.8 | 17.8 | 76.8 | 6.0 | 39.7 | 23.8 | 65.4 | 10.0 |
| Cancers | 63.5 | 6.7 | 36.3 | 31.8 | 41.5 | 15.2 | 83.3 | 2.0 | 43.9 | 17.6 | 45.7 | 19.2 | 42.4 | 20.7 | 62.7 | 11.5 |
| Mental illness | 61.3 | 8.3 | 35.7 | 33.0 | 39.2 | 18.7 | 86.9 | 1.5 | 45.4 | 19.2 | 79.9 | 4.5 | 30.0 | 32.0 | 71.6 | 8.3 |
| Psychotropic drug treatments** | 60.9 | 7.9 | 33.2 | 35.0 | 39.2 | 18.5 | 87.2 | 1.5 | 42.3 | 20.5 | 77.4 | 6.1 | 35.0 | 29.1 | 71.0 | 8.2 |
| Dementia and neurological disease | 58.8 | 9.9 | 28.5 | 41.1 | 36.0 | 22.2 | 85.0 | 2.1 | 45.3 | 20.8 | 78.8 | 5.1 | 23.3 | 37.4 | 70.2 | 8.7 |
| Chronic respiratory diseases | 61.3 | 7.9 | 33.4 | 34.5 | 40.2 | 18.2 | 90.4 | 0.9 | 52.1 | 14.3 | 83.5 | 3.6 | 40.2 | 23.7 | 76.3 | 5.2 |
| Chronic inflammatory bowel disease | 66.2 | 6.0 | 43.7 | 25.7 | 46.1 | 13.0 | 78.6 | 3.7 | 37.0 | 22.2 | 82.5 | 4.0 | 66.6 | 8.4 | 23.2 | 31.6 |
| HIV or AIDS | 69.6 | 4.8 | 55.3 | 16.7 | 42.2 | 14.4 | 89.3 | 1.1 | 53.6 | 10.4 | 80.7 | 4.6 | 52.2 | 12.8 | 65.8 | 12.5 |
| End-stage renal disease | 67.3 | 6.7 | 13.8 | 54.0 | 35.2 | 20.2 | 81.4 | 2.2 | 45.2 | 17.9 | 73.3 | 6.8 | 38.3 | 21.7 | 54.8 | 15.5 |
| Liver or pancreas diseases | 63.9 | 6.1 | 32.9 | 32.1 | 40.2 | 15.2 | 84.8 | 1.5 | 41.5 | 18.5 | 66.7 | 10.4 | 40.9 | 21.2 | 63.2 | 11.2 |
| LTDs not included elsewhere | 60.7 | 8.4 | 31.2 | 37.8 | 38.2 | 19.5 | 87.1 | 1.6 | 46.4 | 18.6 | 79.3 | 5.4 | 34.1 | 29.3 | 70.4 | 8.8 |
| Pregnancy | 80.6 | 0.6 | 78.3 | 1.6 | 54.7 | 3.7 | 97.8 | 0.0 | 50.8 | 7.0 | 85.8 | 1.2 | 66.2 | 2.9 | 91.3 | 1.1 |
| Nonsteroidal anti-inflammatory drugs° | 73.0 | 3.2 | 39.1 | 9.2 | 46.6 | 12.1 | 92.7 | 0.2 | 50.6 | 8.5 | 89.7 | 0.3 | 51.4 | 12.8 | 82.2 | 1.3 |
| Corticosteroid treatment° | 71.8 | 3.1 | 44.2 | 14.3 | 53.7 | 10.0 | 94.8 | 0.3 | 61.7 | 5.2 | 90.5 | 0.3 | 53.5 | 11.9 | 77.6 | 1.3 |
| Analgesic drug treatment° | 73.5 | 3.2 | 54.6 | 7.6 | 50.9 | 10.1 | 94.6 | 0.1 | 59.8 | 5.3 | 92.1 | 0.1 | 54.9 | 10.6 | 81.9 | 0.7 |
Excluding ischaemic heart disease, cerebrovascular disease, heart failure, peripheral vascular disease, diabetes, end-stage renal disease.
Excluding mental illness.
People with at least six reimbursements per year and without diseases, other chronic treatments or pregnancy during the year.
A02A: antacids; A02BC: PPIs; A02BX: other drugs for peptic ulcer and gastro-oesophageal reflux disease; A02X: other drugs for acid-related disorders; A03: drugs for functional gastrointestinal disorders; A04: antiemetics and antinauseants, A06: drugs for constipation, A07: antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents.
AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; LTD, long-term chronic disease; PPIs, proton-pump inhibitors.
Table 2.
Age and frequency of individuals with at least one gastrointestinal therapeutic drug reimbursed, in combination with another therapeutic class in 2016.
| Gastrointestinal therapeutic drug
classes |
A02A |
A02BC |
A02BX |
A02X |
A03 |
A04 |
A06 |
A07 |
|---|---|---|---|---|---|---|---|---|
| n (million) | 0.9 |
13.9 |
2.6 |
3.3 |
11.5 |
4.1 |
5.9 |
5.8 |
| % | % | % | % | % | % | % | % | |
| Median age (year) | 61 | 65 | 59 | 43 | 47 | 34 | 68 | 42 |
| Interquartile range (Q1–Q3) | 46–72 | 52–76 | 41–72 | 20–64 | 26–66 | 13–58 | 51–81 | 16–62 |
| Gastrointestinal therapeutic drug classes | ||||||||
| Alone | 9.2 | 44.9 | 11.8 | 10.4 | 23.0 | 11.1 | 26.0 | 15.0 |
| With one other class | ||||||||
| A02A | – | 1.0 | 0.2 | 0.1 | 0.4 | 0.1 | 0.3 | 0.1 |
| A02BC | 15.7 | – | 22.1 | 3.8 | 10.9 | 3.4 | 18.0 | 4.7 |
| A02BX | 0.5 | 4.1 | – | 0.4 | 1.0 | 0.3 | 0.7 | 0.5 |
| A02X | 0.4 | 0.9 | 0.6 | – | 3.2 | 2.7 | 0.5 | 2.9 |
| A03 | 5.0 | 9.1 | 4.5 | 11.4 | – | 9.6 | 11.0 | 15.2 |
| A04 | 0.5 | 1.0 | 0.5 | 3.5 | 3.5 | – | 0.7 | 6.0 |
| A06 | 1.8 | 7.6 | 1.7 | 1.0 | 5.6 | 1.0 | – | 1.3 |
| A07 | 0.7 | 2.0 | 1.0 | 5.1 | 7.6 | 8.4 | 1.3 | – |
| All the pairs | 24.7 | 25.9 | 30.6 | 25.4 | 32.4 | 25.6 | 32.7 | 30.8 |
| With two other classes | ||||||||
| A02A and one other class | – | 1.2 | 0.2 | 0.4 | 1.0 | 0.3 | 0.9 | 0.3 |
| A02BC–A03 | 9.6 | – | 1.9 | 4.5 | – | 3.7 | 10.2 | 5.0 |
| A02BC–A06 | 4.1 | – | 1.0 | 0.9 | 5.2 | 1.0 | – | 1.0 |
| A02BC and one other class | 5.0 | 0.0 | 0.7 | 3.6 | 7.4 | 2.7 | 4.5 | 3.0 |
| A02BX–A03 | 0.4 | 1.9 | – | 0.5 | – | 0.4 | 0.6 | 0.5 |
| A02BX and one other class | 0.1 | 1.7 | 0.0 | 0.4 | 0.7 | 0.3 | 0.1 | 0.3 |
| A02X–A03 | 0.6 | 1.0 | 0.1 | – | – | 5.1 | 0.8 | 4.4 |
| A02X–A07 | 0.2 | 0.5 | 0.0 | – | 2.2 | 2.1 | 0.3 | |
| A02X and one other class | 0.2 | 0.4 | 0.0 | 0.0 | 2.2 | 0.2 | 0.1 | 1.8 |
| A03–A04 | 0.7 | 1.1 | 0.1 | 6.5 | – | – | 1.2 | 10.3 |
| A03–A06 | 2.3 | 4.3 | 0.3 | 1.5 | – | 1.7 | – | 1.7 |
| A03–A07 | 1.0 | 2.1 | 0.2 | 7.9 | – | 14.4 | 1.7 | – |
| A04–A06 | 0.1 | 0.3 | 0.0 | 0.2 | 0.6 | – | – | 0.4 |
| A04–A07 | 0.2 | 0.4 | 0.1 | 2.7 | 5.2 | – | 0.4 | – |
| A06–A07 | 0.1 | 0.4 | 0.0 | 0.5 | 0.8 | 0.5 | – | – |
| All the triplets | 25.3 | 15.7 | 25.4 | 29.7 | 26.0 | 32.7 | 21.5 | 28.9 |
| With three or more other classes | 40.8 | 13.4 | 32.4 | 34.5 | 18.6 | 30.6 | 19.8 | 25.4 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Other therapeutic classes | ||||||||
| Vitamin K antagonists (B01AA) | 2.4 | 3.2 | 2.4 | 2.1 | 1.6 | 1.2 | 4.5 | 1.7 |
| Heparin (B01AB) | 5.2 | 6.5 | 5.4 | 4.3 | 4.7 | 4.2 | 7.9 | 3.6 |
| Platelet-aggregation inhibitors (B01AC) | 13.0 | 16.3 | 12.2 | 8.3 | 7.2 | 5.2 | 17.4 | 7.0 |
| Thrombin inhibitors (B01AE) | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.1 | 0.4 | 0.2 |
| Direct-factor-Xa inhibitors (B01AF) | 1.7 | 2.1 | 1.6 | 1.2 | 1.0 | 0.8 | 2.6 | 1.0 |
| Other antithrombotics (B01AX) | 0.7 | 0.7 | 0.6 | 0.5 | 0.5 | 0.4 | 0.7 | 0.4 |
| At least one platelet-aggregation inhibitor or antithrombotic | 20.2 | 25.1 | 19.5 | 14.1 | 13.1 | 10.1 | 28.0 | 11.8 |
| Nonsteroidal anti-inflammatory drugs | 53.3 | 61.5 | 49.3 | 49.3 | 51.0 | 51.5 | 43.7 | 48.7 |
| Corticosteroids | 33.6 | 32.2 | 32.8 | 32.5 | 30.7 | 35.1 | 29.7 | 34.1 |
A02A: antacids; A02BC: PPIs; A02BX: other drugs for peptic ulcer and gastro-oesophageal reflux disease; A02X: other drugs for acid-related disorders; A03: drugs for functional gastrointestinal disorders; A04: antiemetics and antinauseants, A06: drugs for constipation, A07: antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents.
PPIs, proton-pump inhibitors.
Table 3.
Prescriber specialty according to the gastrointestinal therapeutic drug classes reimbursed in 2016.
| Gastrointestinal therapeutic drug
classes |
A02A |
A02BC |
A02BX |
A02X |
A03 |
A04 |
A06 |
A07 |
All |
|---|---|---|---|---|---|---|---|---|---|
| Prescriptions (n million) | 1.6 | 61.5 | 5.5 | 4.1 | 24.0 | 5.8 | 18.0 | 9.1 | 129.6 |
| % | % | % | % | % | % | % | % | % | |
| General practitioner | 91.5 | 90.7 | 90.9 | 93.9 | 90.0 | 88.1 | 83.2 | 90.1 | 89.5 |
| Gastroenterologist | 4.5 | 1.5 | 1.3 | 1.0 | 2.6 | 1.0 | 9.9 | 3.4 | 3.0 |
| Paediatrician | 0.3 | 0.5 | 0.7 | 2.2 | 1.2 | 2.4 | 1.3 | 3.0 | 1.1 |
| Gynaecologist and obstetrician | 0.6 | 0.3 | 1.1 | 0.3 | 2.4 | 0.5 | 0.6 | 0.2 | 0.8 |
| Rheumatologist | 0.2 | 0.4 | 2.5 | 0.1 | 0.1 | 0.1 | 0.2 | 0.6 | 0.7 |
| Cardiologist | 0.2 | 0.3 | 0.4 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.6 |
| Oncologist | 0.1 | 1.2 | 0.2 | 0.5 | 0.3 | 4.4 | 0.2 | 0.5 | 0.4 |
| Otolaryngologist | 1.1 | 1.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 |
| Psychiatrist | 0.2 | 0.3 | 0.3 | 0.2 | 0.2 | 0.1 | 0.7 | 0.2 | 0.3 |
| Lung specialist | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.5 | 0.1 | 0.1 | 0.2 |
| Nephrologist | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 |
| Endocrinologist | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 |
| Dermatologist | 0.1 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Geriatrician | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 |
| Other specialist | 1.0 | 2.7 | 1.7 | 1.2 | 2.5 | 2.4 | 3.0 | 1.4 | 2.5 |
A02A: antacids; A02BC: PPIs; A02BX: other drugs for peptic ulcer and gastro-oesophageal reflux disease; A02X: other drugs for acid-related disorders; A03: drugs for functional gastrointestinal disorders; A04: antiemetics and antinauseants, A06: drugs for constipation, A07: antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents.
PPIs, proton-pump inhibitors.
Table 4.
Total reimbursements (euros) in 2016 for several gastrointestinal therapeutic drug classes and mean cost per individual with at least one reimbursement, by sex, age, diseases or other health conditions.
| Gastrointestinal therapeutic drug classes | Mean per individual |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A02A | A02BC | A02BX | A02X | A03 | A04 | A06 | A07 | All | All | |
|
n individuals with at least one
reimbursement for the class (million) |
0.86 | 13.9 | 2.6 | 3.3 | 11.5 | 4.1 | 5.9 | 5.8 | 25.7 | |
| Total reimbursement (€ million) | 2.6 | 422.7 | 11.3 | 8.1 | 47.4 | 59.1 | 71.7 | 76.0 | 706.9 | |
| All | 3 | 30 | 4 | 2 | 4 | 14 | 12 | 13 | 28 | 706.9 |
| Sex | ||||||||||
| Men | 3 | 32 | 5 | 3 | 4 | 15 | 12 | 14 | 28 | 296.7 |
| Women | 3 | 29 | 4 | 2 | 4 | 14 | 12 | 12 | 27 | 410.3 |
| Age (year) | ||||||||||
| 00–01 | 1 | 33 | 4 | 2 | 1 | 1 | 2 | 5 | 9 | 5.9 |
| 02–09 | 1 | 28 | 3 | 2 | 1 | 1 | 4 | 3 | 5 | 10.7 |
| 10–17 | 1 | 9 | 1 | 2 | 1 | 2 | 4 | 4 | 5 | 7.5 |
| 18–34 | 2 | 10 | 3 | 2 | 3 | 3 | 6 | 9 | 11 | 47.6 |
| 35–54 | 2 | 19 | 3 | 2 | 4 | 15 | 10 | 18 | 22 | 145.8 |
| 55–64 | 3 | 32 | 5 | 3 | 6 | 45 | 13 | 23 | 38 | 139.8 |
| 65–74 | 4 | 43 | 6 | 4 | 8 | 69 | 15 | 25 | 49 | 162.4 |
| 75 and older | 5 | 55 | 7 | 5 | 11 | 38 | 18 | 23 | 57 | 187.2 |
| Diseases and other health conditions | ||||||||||
| None | 1 | 10 | 2 | 2 | 1 | 1 | 4 | 3 | 6 | 61.5 |
| Cardiovascular and cerebrovascular disease | 5 | 62 | 8 | 5 | 11 | 54 | 18 | 25 | 68 | 182.1 |
| Cardiovascular prevention (pharmacological)* | 3 | 40 | 5 | 3 | 7 | 36 | 15 | 22 | 43 | 197.0 |
| Diabetes | 6 | 56 | 9 | 5 | 11 | 45 | 18 | 22 | 61 | 124.8 |
| Cancers | 6 | 49 | 9 | 7 | 14 | 176 | 18 | 27 | 85 | 148.2 |
| Mental illness | 6 | 50 | 9 | 4 | 12 | 23 | 25 | 21 | 57 | 73.9 |
| Psychotropic drug treatments** | 4 | 48 | 6 | 4 | 9 | 42 | 18 | 22 | 56 | 203.6 |
| Dementia and neurological disease | 7 | 57 | 10 | 5 | 15 | 26 | 27 | 23 | 62 | 53.2 |
| Chronic respiratory diseases | 5 | 50 | 7 | 3 | 8 | 27 | 17 | 16 | 52 | 106.2 |
| Chronic inflammatory bowel disease | 8 | 45 | 9 | 10 | 22 | 27 | 14 | 357 | 276 | 49.3 |
| HIV or AIDS | 7 | 34 | 12 | 5 | 11 | 33 | 16 | 24 | 43 | 2.8 |
| End-stage renal disease | 10 | 83 | 19 | 9 | 19 | 37 | 25 | 28 | 98 | 6.2 |
| Liver or pancreas diseases | 6 | 52 | 9 | 6 | 13 | 84 | 20 | 38 | 75 | 26.3 |
| LTDs nonincluded elsewhere | 7 | 57 | 10 | 5 | 14 | 34 | 23 | 32 | 65 | 60.7 |
| Pregnancy | 3 | 13 | 5 | 2 | 6 | 6 | 5 | 11 | 15 | 12.8 |
| Nonsteroidal anti-inflammatory drugs° | 2 | 23 | 3 | 2 | 3 | 2 | 7 | 4 | 22 | 4.2 |
| Corticosteroid treatment° | 2 | 27 | 3 | 2 | 2 | 2 | 7 | 7 | 20 | 0.4 |
| Analgesic drug treatment° | 2 | 19 | 3 | 2 | 2 | 1 | 6 | 4 | 14 | 15.9 |
A02A: antacids; A02BC: PPIs; A02BX: other drugs for peptic ulcer and gastro-oesophageal reflux disease; A02X: other drugs for acid-related disorders; A03: drugs for functional gastrointestinal disorders; A04: antiemetics and antinauseants, A06: drugs for constipation, A07: antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents.
Excluding: ischaemic heart disease, cerebrovascular disease, heart failure, peripheral vascular disease, diabetes, end-stage renal disease.
Excluding: mental illness.
People with at least six reimbursements per year and without diseases, other chronic treatments or pregnancy during the year.
AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; LTD, long-term chronic disease; PPIs, proton-pump inhibitors.
A02BC: proton-pump inhibitors (PPIs)
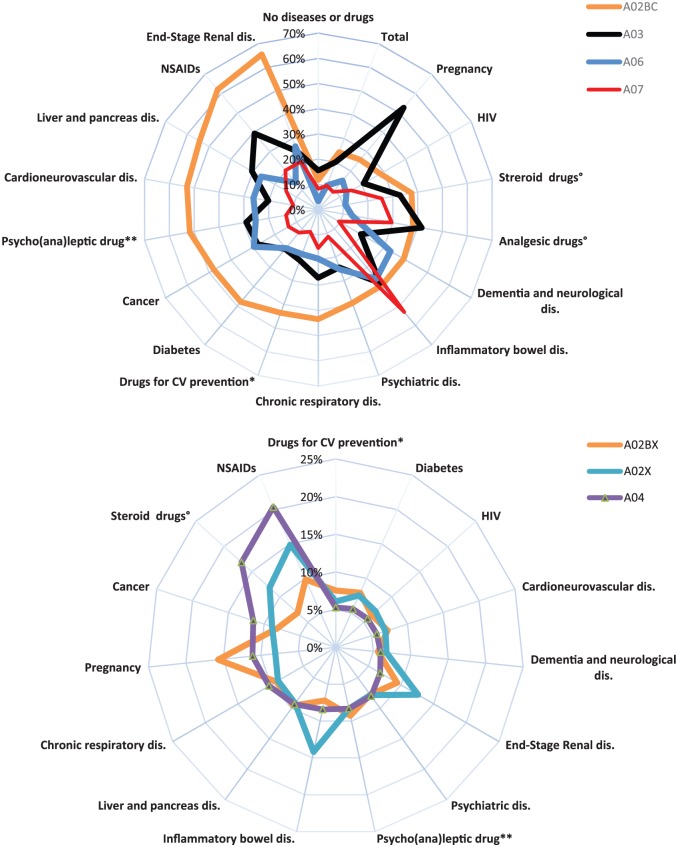
The overall frequency of A02BC users with or without a chronic disease (median age: 65 years, IQR: 52–76) was 24%, and 12% for those without a chronic disease. A peak of 6% was observed for infants (0–1 years) followed by a decrease and then dramatic increase from the 10–17-year age group (4%) to the group 75 years and older (46%; Figure 1). The frequency of A02BC users was lower in the absence of chronic disease (4%, 3% and 21%, respectively). Higher user frequencies were observed for several diseases, such as end-stage renal disease (ESRD; 66%), people with at least six reimbursements of a nonsteroidal anti-inflammatory drug (62%) or corticosteroids (38%), liver or pancreatic disease (54%), and cardiovascular diseases (53%; Figure 2). Chronic use (>10 reimbursements per year) was observed for 19% of users (0–1 year: 3%, 75 years and older: 44%; Table 1) and 3% of those without chronic diseases. Chronic use was more frequent for several diseases, such as ESRD (54%), cardiovascular disease (47%), dementia or other neurological diseases (41%) and diabetes (41%). The frequency of chronic use was 9% among people with at least six reimbursements of a nonsteroidal anti-inflammatory drug and 14% among people reimbursed for corticosteroids. No drug from other GTD classes was reimbursed during the same year in 45% of individuals with one A02BC reimbursement, while one drug from another class was reimbursed in 26% (9% A03), two drugs from other classes were reimbursed in 16% and three or more drugs from other classes were reimbursed in 13% (Table 2). In 2016, 25% of people with A02BC reimbursement received at least one reimbursement for platelet-aggregation inhibitors or antithrombotic agents. General practitioners prescribed 91% of all reimbursed drugs for peptic ulcer and GORD (Table 3). The total sum reimbursed was €423 million and the mean reimbursement per A02BC user was €30 (Table 4).
Figure 2.
Frequency of individuals with at least one reimbursement in 2016 for gastrointestinal therapeutic drugs, depending on diseases and other health conditions.
A02A: antacids; A02BC: PPIs; A02BX: other drugs for peptic ulcer and gastro-oesophageal reflux disease; A02X: other drugs for acid-related disorders; A03: drugs for functional gastrointestinal disorders; A04: antiemetics and antinauseants, A06: drugs for constipation, A07: antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents.
*Excluding ischaemic heart disease, cerebrovascular disease, heart failure, peripheral vascular disease, diabetes, end-stage renal disease.
**Excluding mental illness.
°People with at least six reimbursements per year and with no other diseases, other chronic treatments or pregnancy during the year.
CV, cardiovascular; HIV, human immunodeficiency virus; NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton-pump inhibitors.
A02BX: other drugs for peptic ulcer and gastro-oesophageal reflux disease
The overall frequency of A02BX users (median age: 59 years, IQR: 41–72) was 4.5%, and 1.9% for those without a chronic disease. A peak was also observed for infants (0–1 year, 9%) followed by a decrease and then a slow increase from 10 to 17 years (1%) to the group 75 years and older (7.2%) (Figure 1). Lower frequencies of use were observed in the absence of chronic disease: 6%, 1% and 3%, respectively. Higher frequencies were mainly observed during pregnancy (16%), in people with at least six reimbursements for anti-inflammatory drugs (10%) and those with ESRD (9%; Figure 2). Chronic use was observed for 11% of all users (0–1 year: 2%; 75 years and older: 21%; Table 1) and 3% of those without a chronic disease. A high rate of chronic use was also observed for patients with ESRD (20%) and dementia or other neurological diseases (22%). Chronic use was observed for 12% of people with at least six reimbursements for a nonsteroidal anti-inflammatory drug and 10% for those reimbursed for corticosteroids. No drug from other GTD classes was reimbursed during the same year for 12% of individuals with one A02BX reimbursement, while one drug from another class was reimbursed in 31% of patients (A02BC: 22%), two drugs from other classes were reimbursed in 25% and three or more drugs from other classes were reimbursed in 32% (Table 2). In 2016, 20% of people with a reimbursement for A02BX received at least one reimbursement for platelet-aggregation inhibitors or antithrombotic agents. General practitioners prescribed 91% of all reimbursed drugs for peptic ulcer and GORD (Table 3). The total sum reimbursed was €2.6 million and the mean reimbursement per A02BX user was €4 (Table 4).
A02X: other drugs for acid-related disorders
The overall frequency of A02X users (median age: 43 years, IQR: 20–64) was 5.7%, and 4.4% for those without a chronic disease. The highest frequency (7%) was found for infants (0–1 year), followed by a plateau around 5% (Figure 1). Higher frequencies were observed for patients with at least six reimbursements for analgesics (15%), nonsteroidal anti-inflammatory drugs (12%) or corticosteroids (12%), chronic inflammatory bowel disease (14%) and ESRD (13%; Figure 2). Chronic use was identified for 1% of users (75 years and older: 2%; Table 1). No drug from other GTD classes was reimbursed during the same year for 10% of individuals with one A02X reimbursement, one drug from another class was reimbursed in 25% (A03: 11%), two drugs from other classes were reimbursed in 30% and three or more drugs from other classes were reimbursed in 35% (Table 2). General practitioners prescribed 94% of all reimbursed drugs of this class and paediatricians prescribed 2% (Table 3). The total sum reimbursed was €8 million and the mean reimbursement per A02X user was €2 (Table 4).
A03: drugs for functional gastrointestinal disorders
The overall frequency of A03 users (median age: 47 years, IQR: 26–66) was 20%, and 16% for those without a chronic disease. Overall frequency by age remained stable around 20% by age group (Figure 1). The highest frequencies were observed in pregnant women (53%), in patients with chronic inflammatory bowel disease (38%), and patients taking analgesics (42%), nonsteroidal anti-inflammatory drugs (39%) and corticosteroids (33%; Figure 2). Chronic use was identified for 7% of users (75 years and older: 24%; Table 1), and was associated with several chronic diseases, with a frequency of about 20% among people with chronic inflammatory bowel disease, dementia or other neurological diseases and psychotropic drug treatment. No drug from other GTD classes was reimbursed during the same year for 23% of individuals with one A03 reimbursement, one drug from another class was reimbursed in 32% (A02BC: 11%), two drugs from other classes were reimbursed in 26% and three or more drugs from other classes were reimbursed in 19% (Table 2). General practitioners prescribed 90% of all reimbursed drugs of this class, gastroenterologists prescribed 3%, gynaecologists prescribed 2% and paediatricians prescribed 1% (Table 3). The total sum reimbursed was €47 million and the mean reimbursement per patient was €4 and more specifically, €22 for patients with chronic inflammatory bowel disease (Table 4).
A04: antiemetics and antinauseants
The overall frequency of A04 users (median age: 34 years, IQR: 13–58) was 7.2%, and 6.5% for those without a chronic disease. More users of these drugs were observed before the age of 18 years (2–9 years: 14%), followed by a regular decrease (5% after the age of 55 years; Figure 1). The highest frequencies were observed for people with analgesic drug treatments (21%), corticosteroid treatments (17%), pregnancy (11%) and cancer (12%; Figure 2). Chronic use was observed for 2% of all users (65–74 years 8%; Table 1), with higher rates among patients with cancer (19%) and liver or pancreatic disease (10%). No drug from other GTD classes was reimbursed during the same year for 11% of individuals with one A04 reimbursement, one drug from another class was reimbursed in 26% (A03: 10%, A07: 8%), two drugs from other classes were reimbursed in 33% and three or more drugs from other classes were reimbursed in 31% (Table 2). General practitioners prescribed 88% of all reimbursed drugs of this class, oncologists prescribed 4%, and paediatricians prescribed 2% (Table 3). The total sum reimbursed was €59 million and the mean reimbursement per patient was €14, with variations as high as €176 for cancer patients (Table 4).
A06: drugs for constipation
The overall frequency of A06 users (median age: 68 years, IQR: 51–81) was 10.2% and 3.4% for those without a chronic disease. The frequency of A06 drug use was 5% for infants (0–1 years), followed by a progressive increase from 5% (18–34) to 29% (75 years and older; Figure 1). The highest reimbursement frequencies were observed for patients with chronic inflammatory bowel disease (36%), dementia and neurological disease (33%), cancer (30%), and ESRD (27%; Figure 2). Chronic use was observed for 17% of users (0–1 year: 3%, 75 years and older: 32%; Table 1) and for 6% of users with no chronic disease. High reimbursement rates were also observed for people with dementia and neurological disease (37%) and mental illness 32%. No drug from other GTD classes was reimbursed during the same year in 26% of individuals with one A06 drug reimbursement, one drug from another class was reimbursed in 33% (A02BC: 18%), two drugs from other classes were reimbursed in 22% and three or more drugs from other classes were reimbursed in 20% (Table 2). General practitioners prescribed 83% of all reimbursed drugs of this class, 83% were prescribed by a general practitioner, gastroenterologists prescribed 10%, and paediatricians prescribed 1% (Table 3). The total sum reimbursed was €72 million and the mean reimbursement per patient was €12 (Table 4).
A07: antidiarrheals, intestinal anti-inflammatory/anti-infective agents
The overall frequency of A07 users (median age: 42 years, IQR: 16–62) was 10.1% and 8.3% for those without a chronic disease. A peak frequency of at least one reimbursement (28%) was observed for infants (0–1 years) followed by a decrease (10–17 years: 8%) and a plateau at 8% (Figure 1). The highest reimbursement frequencies were observed for patients with chronic inflammatory bowel disease (53%), analgesic drug treatment (30%), and corticosteroid treatment (26%; Figure 2). Chronic use was observed for 3% of users (0–1 year: 1%, 75 years and older: 9%; Table 1) and 0.2% of those without a chronic disease. Chronic use was also observed for people with chronic inflammatory bowel disease (32%), ESRD (16%) and cancer (12%). No drug from other GTD classes was reimbursed during the same year in 15% of individuals with one A07 drug reimbursement, one drug from another class was reimbursed in 31% (15% A03), two drugs from other classes were reimbursed in 29% and three or more drugs from other classes were reimbursed in 25% (Table 2). General practitioners prescribed 90% of all reimbursed drugs of this class, gastroenterologists prescribed 3% and paediatricians prescribed 1% (Table 3). The total sum reimbursed was €76 million and the mean reimbursement per patient was €13 (Table 4).
Discussion
The key results of this national observational study based on the main gastrointestinal drug classes reimbursed in 2016 indicate very frequent use, reported at least once by almost half of the French population, that is, 25.7 million people, corresponding to 130 million prescriptions/pharmacy dispensaries per year. The drugs most commonly reimbursed were PPIs, reimbursed at least once to 24% of the population, that is, 13.8 million people, and at least 10 times a year to 2.6 million people. Other commonly used drugs were those used to treat functional gastrointestinal disorders (20% of the population) and constipation (10%), antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents (10%), antiemetics and antinauseants (7%) and other drugs for acid-related disorders (6%).
This study also demonstrates extensive and chronic use of gastrointestinal drugs in the youngest and oldest age groups, among specific groups such as pregnant women or children aged 0–1 year, but also among people with specific diseases (ESRD, chronic inflammatory bowel disease, mental illness) or taking other chronic treatments (psychotropic drugs, nonsteroidal anti-inflammatory drugs). Overuse was clearly suspected for some GTD classes, such as PPIs, and combinations of different GTD class were frequently observed. GTD drugs were mostly prescribed by general practitioners. The large sums reimbursed (a total of €707 million) can mainly be attributed to PPI due to their extensive use.
Frequency of use of the various GTD classes according to age reflect the known prevalence of gastrointestinal diseases and classical indications. GORD, gastritis and dyspepsia were the main indications for the prescription of antacids. Only limited population-based data are available describing the frequency of antacid prescription or reimbursement. A web survey in Denmark found a 23% use of antacids/alginate among adults (16% without PPI),27 which is much higher than in our study (A02A: 1.5%, A02BX: 4.5%) and could be explained by the fact that antacids are frequently sold over the counter in France and are consequently not reimbursed and not identified in our database.
A similar French study confirmed our 24% prevalence of PPI (A02BC) use (at least one reimbursement), and 12% of users without PPI reimbursement during the previous year.28 A Danish prescription registry study reported a prevalence of 7.4% in 2014 (20% for people 80 years and older),19 which is much lower than our results of 24% for all ages and 46% after the age of 75 years. Chronic use was also observed for 19% of users in the present study. The prevalence of GORD, defined by symptoms present at least once a week for 12 months, was estimated to be 13% in a meta-analysis and 8% in France.6,7 This prevalence is lower than the prevalence of reimbursement for PPI. The prevalence of peptic ulcer disease based on a review of physician diagnosis studies ranged between 0.12% and 1.50%.8 The 6% peak in infants (0–1 years) is probably due to GORD treatment. However, the widespread use of PPI in such settings, involving the treatment of nonacid-related symptoms and the safety of this treatment remain a subject of debate. PPI are also used to systematically prevent gastrointestinal complications in patients treated with nonsteroidal anti-inflammatory agents or antiplatelet agents. In this study, two thirds of patients with at least six reimbursements for nonsteroidal anti-inflammatory drugs had at least one reimbursement for PPI during the same year. Many studies have been published concerning prescription or long-term use of PPI that does not comply with guidelines. For example, prescription of a PPI in patients with ESRD may be questionable in two thirds of patients in light of recent data showing an increased risk of renal impairment with PPI treatment,29–32 despite the prevention of aspirin-related adverse effects in patients with multiple comorbidities.
Few data are available concerning the nationwide use of other therapeutic classes. The prevalence of adult IBS was estimated at 12% in North America and 10% in France,9,10 where 20% of people received at least one reimbursement for drugs for functional gastrointestinal disorders and 10% received at least one reimbursement for antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents. According to a national claims database in Korea, 6% of the population sought medical care for IBS; antispasmodics were prescribed to 75% of patients, and a combination of drugs was also frequently prescribed, as in the present study.33
Chronic constipation can affect 2–27% of the population.11 In the present study, 10% of people had received at least one reimbursement for drugs for constipation and 17% of them were chronic users. The association between constipation and neurological disease or mental illness may be secondary to decreased mobility and the use of treatments that can impair intestinal motility.34 Nevertheless, these drugs have various indications. No reimbursement data concerning the use of antiemetics and antinauseants were found in the literature, but the 15% rate of antiemetic or antinauseant prescriptions between the ages of 2 and 9 years in our study does not comply with current guidelines concerning the use of these drugs in gastroenteritis in this age group.35 The use of both laxative and antidiarrhoeal GTD classes in IBS patients can be explained by constipation after postoperative bowel stenosis in some cases and insufficient control of diarrhoea in other patients.
User and prescriber characteristics
Two age groups presented with relatively high frequency of reimbursements: children and older adults. Children less than 10 years of age are frequent users of many GTD classes: A02BC, A02X, A03, A04, and A07, which is likely to reflect acute disease, as they present low rates of chronic use. In the United States, 0.7% of the 0–19-year age group had a PPI prescription (0.7% 6–11 years). H2 antagonists were somewhat more frequent in the youngest age group: 2.1% in the 0–23-month age group.36 Older adults present much higher rates of PPI use and drugs for constipation and these treatments may contribute to polypharmacy in the oldest patients. These patients are also frequently chronic users.
Pregnant women more frequently suffer from gastrointestinal disorders, GORD, nausea, vomiting, constipation and diarrhoea, and high rates of drug use were observed in this study (A02BC: 26%, A04: 11%, A03: 53%, A06: 15%, A07: 9%). However, very low rates of drug use were reported in Norway: A02B: 2%, A03: 5%, A07: 1%.37 French pregnant women seem particularly exposed to drugs with one of the highest average number of specialties prescribed in developed countries, as noted in a meta-analysis in 2011.38
General practitioners prescribed approximately 90% of all GTD expenditure, regardless of the drug class. Gastroenterologists accounted for more than 3% of prescriptions for three classes, antacids, drugs for functional gastrointestinal diseases, and drugs for diarrhoea and about 10% of total expenditure for drugs for constipation. None of the other categories of prescribers exceeded 2% of total expenditure for any drug classes except for paediatricians for the treatment of acid-related disorders, vomiting and diarrhoea and obstetricians–gynaecologists for the treatment of functional gastrointestinal disorders.
Expenditure
The €707 million reimbursed for these main GTD classes accounted for 3.6% of the total expenditure reimbursed for ambulatory drugs and 1.2% of all reimbursements for ambulatory healthcare.24 The main GTD class contributing to this expenditure was PPI, accounting for €420 million. Although these expenditures cannot be compared with those observed in other countries due to a lack of international data, they are in line with data from the US, where abdominal pain, diarrhoea, nausea, constipation and heartburn are the leading gastrointestinal symptoms and diagnoses in ambulatory settings.5 The US expenditure also does not include GTD drugs dispensed over the counter without reimbursement. The IMS Institute for Healthcare reports $25 billion for GTD products sales for 2014 (3% of global sales for prescription medications).16 Nevertheless, comparisons of expenditure or reimbursement between countries are limited due to the variability of drug pricing, reimbursement rates, and availability of generic drugs.17 Rates of reimbursements or prescription per individual could be useful for comparison of various countries, although many aspects may differ across countries: characteristics, diagnoses, criteria, practices, guidelines, health use and system, insurance coverage, etc.
Strengths and limitations
The main strengths of this study are the use of the SNDS population database comprising almost 87% of the French population. However, some over-the-counter GTDs are not reimbursed, leading to underestimation of our high rates. It is also possible that not all prescriptions result in dispensing by a pharmacy. It is also likely that not all reimbursed drugs are used, and some may be stockpiled at home. Other limitations are related to disease information, which is based on algorithms and not directly coded by the clinician for each visit. For example, diseases that did not require hospitalization, LTD, or a specific treatment cannot be identified, essentially corresponding to GTD used for symptomatic complaints.
Conclusion
This study highlights the value of national drug reimbursement databases to monitor drug prescription in the general population. It demonstrates extensive and sometimes chronic use of GTD and suggests that this large-scale use does not comply with current guidelines, raising the question of the relevance of this drug use. This work supports the need to disseminate guidelines published by learned societies to general practitioners, who are the main prescribers of these drugs.
Supplemental Material
Supplemental material, Burden_of_drug_use_for_TAIG_supplementary_table for Burden of drug use for gastrointestinal symptoms and functional gastrointestinal disorders in France: a national study using reimbursement data for 57 million inhabitants by Philippe Tuppin, , Sébastien Rivière, David Deutsch, Christelle Gastaldi-Menager and Jean-Marc Sabaté in Therapeutic Advances in Gastroenterology
Acknowledgments
PT is the guarantor of the article. PT and JMS designed the study. SR and DD contributed to the data analysis and all authors contributed to the interpretation of the data. PT, CGM and JMS drafted the manuscript and all authors participated in the interpretation of the data and revision of the content of the manuscript. The final version of the manuscript was revised and approved by all authors.
Footnotes
Funding: Self-funded by CNAM.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Philippe Tuppin  https://orcid.org/0000-0001-5698-9215
https://orcid.org/0000-0001-5698-9215
Contributor Information
Philippe Tuppin, Caisse Nationale d’Assurance Maladie (CNAM), Direction de la Stratégie des Études et des Statistiques, 26–50, avenue du Professeur André Lemierre, 75986 Paris Cedex 20, France.
Sébastien Rivière, Caisse Nationale d’Assurance Maladie (CNAM), Paris, France.
David Deutsch, Service de Gastroentérologie Hôpital Avicenne, AP-HP, Bobigny, France.
Christelle Gastaldi-Menager, Caisse Nationale d’Assurance Maladie (CNAM), Paris, France.
Jean-Marc Sabaté, Service de Gastroentérologie Hôpital Avicenne, AP-HP, Bobigny, France INSERM U-987, Physiopathologie et Pharmacologie Clinique de la Douleur, Hôpital Ambroise Paré, Boulogne-Billancourt, France.
References
- 1. Miwa H, Takeshima T, Iwasaki K, et al. Medical cost, incidence rate, and treatment status of gastroesophageal reflux disease in Japan: analysis of claims data. J Med Econ 2016; 19: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 2. Buono JL, Mathur K, Averitt AJ, et al. Economic burden of irritable bowel syndrome with diarrhea: retrospective analysis of a U.S. commercially insured population. J Manag Care Spec Pharm 2017; 23: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dapoigny M, Bellanger J, Bonaz B, et al. Irritable bowel syndrome in France: a common, debilitating and costly disorder. Eur J Gastroenterol Hepatol 2004; 16: 995–1001. [DOI] [PubMed] [Google Scholar]
- 4. Choung RS, Branda ME, Chitkara D, et al. Longitudinal direct medical costs associated with constipation in women. Aliment Pharmacol Ther 2011; 33: 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015; 149: 1731–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut 2018; 67: 430–440. [DOI] [PubMed] [Google Scholar]
- 7. Bretagne JF, Honnorat C, Richard-Molard B, et al. Comparative study of characteristics and disease management between subjects with frequent and occasional gastro-oesophageal reflux symptoms. Aliment Pharmacol Ther 2006; 23: 607–616. [DOI] [PubMed] [Google Scholar]
- 8. Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 2009; 29: 938–946. [DOI] [PubMed] [Google Scholar]
- 9. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–721.e4. [DOI] [PubMed] [Google Scholar]
- 10. Schnabel L, Buscail C, Sabate JM, et al. Association between ultra-processed food consumption and functional gastrointestinal disorders: results from the French NutriNet-Santé cohort. Am J Gastroenterol 2018; 113: 1217–1228. [DOI] [PubMed] [Google Scholar]
- 11. Sanchez MI, Bercik P. Epidemiology and burden of chronic constipation. Can J Gastroentero. 2011; 25(Suppl B): 11B–15B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferreira-Maia AP, Matijasevich A, Wang YP. Epidemiology of functional gastrointestinal disorders in infants and toddlers: a systematic review. World J Gastroenterol 2016. 28; 22: 6547–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haas DM, Marsh DJ, Dang DT, et al. Prescription and other medication use in pregnancy. Obstet Gynecol 2018; 131: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demailly R, Escolano S, Quantin C, et al. Prescription drug use during pregnancy in France: a study from the national health insurance permanent sample. Pharmacoepidemiol Drug Saf 2017; 26: 1126–1134. [DOI] [PubMed] [Google Scholar]
- 15. Lindsley CW. 2014 prescription medications in the United States: tremendous growth, specialty/orphan drug expansion, and dispensed prescriptions continue to increase. ACS Chem Neurosci 2015; 6: 811–812. [DOI] [PubMed] [Google Scholar]
- 16. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA 2016; 316: 858–871. [DOI] [PubMed] [Google Scholar]
- 17. Chai G, Governale L, McMahon AW, et al. Trends of outpatient prescription drug utilization in US children, 2002-2010. Pediatrics 2012; 130: 23–31. [DOI] [PubMed] [Google Scholar]
- 18. Zhang F, Mamtani R, Scott FI, et al. Increasing use of prescription drugs in the United Kingdom. Pharmacoepidemiol Drug Saf 2016; 25: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pottegård A, Broe A, Hallas J, et al. Use of proton-pump inhibitors among adults: a Danish nationwide drug utilization study. Therap Adv Gastroenterol 2016; 9: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Othman F, Card TR, Crooks CJ. Proton pump inhibitor prescribing patterns in the UK: a primary care database study. Pharmacoepidemiol Drug Saf 2016; 25: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 21. Wallerstedt SM, Fastbom J, Linke J, et al. Long-term use of proton pump inhibitors and prevalence of disease- and drug-related reasons for gastroprotection-a cross-sectional population-based study. Pharmacoepidemiol Drug Saf 2017; 26: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haastrup PF, Thompson W, Søndergaard J, et al. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol 2018; 123: 114–121. [DOI] [PubMed] [Google Scholar]
- 23. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the Système National d’Information Interrégimes de l’Assurance Maladie (SNIIRAM) to the Système National des Données de Santé (SNDS) in France. Rev Epidemiol. Sante Publique 2017; 65: S149–S167. [DOI] [PubMed] [Google Scholar]
- 24. Caisse nationale d’assurance maladie. Améliorer la qualité du système de santé et maîtriser les dépenses. Rapport au ministre chargé de la Sécurité sociale et au Parlement sur l’évolution des charges et des produits de l’Assurance Maladie. CNAM; 2016, pp. 171–185. www.ameli.fr
- 25. Quantin C, CNAM. Etude des algorithmes de définition des pathologies dans le Sniiram. CNAM, 2015, p. 272. www.ameli.fr [Google Scholar]
- 26. Constantinou P, Tuppin P, Fagot-Campagna A, et al. Two morbidity indices developed in a nationwide population permitted performant outcome-specific severity adjustment. J Clin Epidemio 2018; 103: 60–70. [DOI] [PubMed] [Google Scholar]
- 27. Lødrup A, Reimer C, Bytzer P. Use of antacids, alginates and proton pump inhibitors: a survey of the general Danish population using an internet panel. Scand J Gastroentero. 2014; 49: 1044–1050. [DOI] [PubMed] [Google Scholar]
- 28. Lassale M, Le Tri T, Bardou M, et al. Etude d’utilisation des inhibiteurs de la pompe à protons en France en 2015. Rev Epidemiol. Sante Publique 2018; 66S: S221. [Google Scholar]
- 29. Safe M, Chan WH, Leach ST, et al. Widespread use of gastric acid inhibitors in infants: are they needed? Are they safe? World J Gastrointest Pharmacol Ther 2016; 7: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 2017; 152: 706–715. [DOI] [PubMed] [Google Scholar]
- 31. Mares-García E, Palazón-Bru A, Martínez-Martín Á, et al. Non-guideline-recommended prescribing of proton pump inhibitors in the general population Curr Med Res Opin 2017; 33: 1725–1729. [DOI] [PubMed] [Google Scholar]
- 32. Wijarnpreecha K, Thongprayoon C, Chesdachai S, et al. Associations of proton-pump inhibitors and H2 receptor antagonists with chronic kidney disease: a meta-analysis. Dig Dis Sci 2017; 62: 2821–2827. [DOI] [PubMed] [Google Scholar]
- 33. Jung HK, Kim YH, Park JY, et al. Estimating the burden of irritable bowel syndrome: analysis of a nationwide Korean database. J Neurogastroenterol Motil 2014; 20: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Camilleri M, Ford AC, Mawe GM, et al. Chronic constipation. Nat Rev Dis Primers 2017; 3: 17095. [DOI] [PubMed] [Google Scholar]
- 35. Guarino A, Lo Vecchio A, Dias JA, et al. Universal recommendations for the management of acute diarrhea in non-malnourished children. J Pediatr Gastroenterol Nutr 2018; 67: 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hales CM, Kit BK, Gu Q, et al. Trends in prescription medication use among children and adolescents-United States, 1999-2014. JAMA 2018; 319: 2009–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Engeland A, Bjørge T, Klungsøyr K, et al. Trends in prescription drug use during pregnancy and postpartum in Norway, 2005 to 2015. Pharmacoepidemiol Drug Saf 2018; 27: 995–1004. [DOI] [PubMed] [Google Scholar]
- 38. Daw JR, Hanley GE, Greyson DL, et al. Prescription drug use during pregnancy in developed countries: a systematic review. Pharmacoepidemiol Drug Saf 2011; 20: 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Burden_of_drug_use_for_TAIG_supplementary_table for Burden of drug use for gastrointestinal symptoms and functional gastrointestinal disorders in France: a national study using reimbursement data for 57 million inhabitants by Philippe Tuppin, , Sébastien Rivière, David Deutsch, Christelle Gastaldi-Menager and Jean-Marc Sabaté in Therapeutic Advances in Gastroenterology