Key Points
Question
Is high lifespan cognitive reserve (CR) indicator associated with a reduction in dementia risk, and how strong is this association in the presence of high brain pathologies?
Findings
In this cohort study including 1602 dementia-free older adults, high lifespan CR was associated with a decreased risk of dementia. This association was present in people with high Alzheimer disease and vascular pathologies.
Meaning
Accumulative educational and mentally stimulating activities enhancing CR throughout life might be a feasible strategy to prevent dementia, even for people with high brain pathologies.
This cohort study examines the association of lifespan cognitive reserve with dementia risk.
Abstract
Importance
Evidence on the association of lifespan cognitive reserve (CR) with dementia is limited, and the strength of this association in the presence of brain pathologies is unknown.
Objective
To examine the association of lifespan CR with dementia risk, taking brain pathologies into account.
Design, Setting, and Participants
This study used data from 2022 participants in the Rush Memory and Aging Project, an ongoing community-based cohort study with annual follow-up from 1997 to 2018 (mean follow-up, 6 years; maximum follow-up, 20 years). After excluding 420 individuals who had prevalent dementia, missing data on CR, or dropped out, 1602 dementia-free adults were identified at baseline and evaluated to detect incident dementia. During follow-up, 611 died and underwent autopsies. Data were analyzed from May to September 2018.
Exposures
Information on CR factors (education; early-life, midlife, and late-life cognitive activities; and social activities in late life) was obtained at baseline. Based on these factors, lifespan CR scores were captured using a latent variable from a structural equation model and was divided into tertiles (lowest, middle, and highest).
Main Outcomes and Measures
Dementia was diagnosed following international criteria. Neuropathologic evaluations for Alzheimer disease and other brain pathologies were performed in autopsied participants. The association of lifespan CR with dementia or brain pathologies was estimated using Cox regression models or logistic regression.
Results
Of the 1602 included participants, 1216 (75.9%) were women, and the mean (SD) age was 79.6 (7.5) years. During follow-up, 386 participants developed dementia (24.1%), including 357 participants with Alzheimer disease–related dementia (22.3%). The multiadjusted hazards ratios (HRs) of dementia were 0.77 (95% CI, 0.59-0.99) for participants in the middle CR score tertile and 0.61 (95% CI, 0.47-0.81) for those in the highest CR score tertile compared with those in the lowest CR score tertile. In autopsied participants, CR was not associated with most brain pathologies, and the association of CR with dementia remained significant after additional adjustment for brain pathologies (HR, 0.60; 95% CI, 0.42-0.86). The highest CR score tertile was associated with a reduction in dementia risk, even among participants with high Alzheimer disease pathology (HR, 0.57; 95% CI, 0.37-0.87) and any gross infarcts (HR, 0.34; 95% CI, 0.18-0.62).
Conclusions and Relevance
High lifespan CR is associated with a reduction in dementia risk, even in the presence of high brain pathologies. Our findings highlight the importance of lifespan CR accumulation in dementia prevention.
Introduction
The cognitive reserve (CR) hypothesis has been proposed as a compensatory mechanism to cope with age-related brain damage and to account for interindividual variability in the ability to maintain cognitive function in the presence of brain pathologies.1 Education, occupation attainment, and social and cognitive activities have been considered as proxy measures of CR.2,3,4 However, emerging evidence has suggested that CR is an active construct that develops from continued life experiences.5 One reserve-enhancing factor during a certain period alone could not fully explain the accumulation of cognitive activities over the life course.6 So far, evidence on whether and to what extent lifespan CR accumulation may reduce dementia risk is still limited.6
According to CR theory, Stern et al7 have suggested that 3 components are required for CR-related research: a measure of CR, clinical or cognitive performance outcomes, and the status of the brain (reflecting brain pathologies). However, as in vivo measures of neuronal pathology are not widely available, few studies presenting the association of the proxy of CR with dementia have taken brain pathologies into account. Several studies have shown that CR might be directly associated with neuropathology and resist the accumulation of brain pathologies.8,9,10 However, other studies have indicated that CR might bypass classic brain pathologies11 and represent other pathways, such as enhancing brain network efficiency to compensate for dementia pathology.12,13 Therefore, the role of brain pathologies in the association of CR with cognitive outcomes remains unclear.
We previously reported that more frequent cognitive activities from early to late life and social activities in late life were associated with slower cognitive decline.14,15,16 In the present study, we aim to verify the hypothesis that high lifespan CR accumulation is associated with a reduction in clinical dementia risk and to estimate the strength of this association in the presence of brain pathologies using data from a long-term community-based cohort study in which people donated their brains for autopsy.
Methods
Study Design, Setting, and Participants
The Rush Memory and Aging Project17 is an ongoing prospective cohort study that investigates risk factors for common chronic neurodegenerative conditions in older adults. At the time of enrollment and thereafter, all participants underwent a comprehensive clinical assessment, including medical history, neurological examination, and detailed cognitive function testing.17
Beginning in 1997 through 2018, a total of 2022 participants were enrolled. The participants were annually followed-up with, for a maximum of 20 years. In this study, among 2022 participants, a total of 420 were excluded, including 112 with prevalent dementia, 101 with missing data on CR-enhancing factors at baseline, 31 not eligible for their first follow-up because of mental disorder, 136 who dropped out before the first follow-up evaluation, and 40 who died. Thus, 1602 participants were available for the current study. During the follow-up period, 747 participants died, of whom 611 (81.8%) underwent autopsy (eFigure 1 in the Supplement).
The study was approved by the Institutional Review Board of Rush University Medical Center and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all participants, and Uniform Anatomic Gift Act documentation was obtained for all participants who underwent autopsy. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Assessment of Lifespan CR
Data on stimulating mental and social activities over the life course and social network collected at baseline were considered to construct an indicator of CR. These factors included education; early-life, midlife, and late-life cognitive activities; social activity in late life; and social network in late life.
For education, years of education was calculated based on the number of years of regular school reported at baseline.18 Among 1602 participants, the mean (range) years of education was 14.76 (0-29) years.
For early-life, midlife, and late-life cognitive activities, participants completed a 37-item cognitive activity questionnaire at baseline.14,16 The activities included reading books, visiting a library, and writing letters during childhood (aged 6 to 12 years), young adulthood (aged approximately 18 years), middle age (aged approximately 40 years), and late life (at the study enrollment). Frequencies of participation in each activity at different periods of life were rated from 1 (once a year or less) to 5 (every day or about every day).14 In the questionnaire, there were 7 late-life (ie, at baseline) activities and 21 early-life activities, including 11 regarding childhood, 10 regarding young adulthood, and 9 regarding midlife cognitive activity. Item scores were averaged to yield separate early-life, midlife, and late-life activity measures.19
For social activity in late life, frequency of activity was assessed using a 6-item scale, including (1) going to restaurants, sporting events, or teletract (off-track betting); (2) going on day or overnight trips; (3) doing unpaid community or volunteer work; (4) visiting relatives’ or friends’ houses; (5) participating in groups, such as at a senior center; and (6) attending church or religions services. Participants were asked to rate how often they participated in each activity based on a 5-point scale from 1 (once a year or less) to 5 (every day or about every day). Item scores were summed and averaged to obtain a composite measure of social activity.20,21
For social network in late life, participants were asked about the number of children they have and meet monthly. They were also asked about the number of relatives (besides spouse and children) and other close friends to whom they feel close and with whom they felt at ease and could talk to about private matters and could call on for help as well as how many of these people they see monthly. Social network size was the number of these individuals (children, family, and friends) seen at least once per month.22
Assessment of Dementia, Alzheimer Disease–Related Dementia, and Mild Cognitive Impairment
Clinical diagnoses of dementia and Alzheimer disease (AD)–related dementia were based on criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association.23 The diagnosis of mild cognitive impairment (MCI) referred to persons with cognitive impairment diagnosed by the neuropsychologist but without a clinical diagnosis of dementia by the examining clinician.24,25
Assessment of Brain Pathologies
Postmortem neuropathologic evaluations were conducted on the 611 autopsied brains (median [interquartile range] postmortem interval, 6.90 [3.53-10.27] hours). Global AD pathology burden, β-amyloid plaques, and tangles were quantified26 and also categorized as being at low or high levels. Chronic infarcts, including gross infarcts and microinfarcts14; cerebral vascular disease pathology, including atherosclerosis, arteriolosclerosis, and cerebral amyloid angiopathy27; Lewy bodies14; and typical hippocampal sclerosis28 were assessed as present or absent.
Other Variable Assessments
A broad range of potential confounders and proxies for confounders were considered, including age, sex, smoking, alcohol consumption, physical activity, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), Mini-Mental State Examination (MMSE) score, heart disease, hypertension, diabetes, and apolipoprotein E (APOE) ε4 status. They are described in detail in eMethods 1 in the Supplement.
Statistical Analysis
The characteristics of the study population by dementia or survival status were compared using t tests and nonparametric tests (χ2 and Wilcoxon rank sum tests). To construct the lifespan CR accumulation based on stimulating mental and social activities during life course, structural equation modeling (SEM) was performed to derive a best-fitting measurement model using full information maximum likelihood estimation (eMethods 2 in the Supplement). The lifespan CR score was operationalized as both a continuous and a categorical variable (based on the tertile distribution; reference, lowest tertile).
Cox regression models were used to estimate the hazard ratios (HRs) and 95% CIs for dementia incidence associated with the CR score. Follow-up time was calculated as the time from study entry to dementia diagnosis, death, or the last examination. The basic models were adjusted for age and sex. The multiadjusted models were further adjusted for smoking, alcohol consumption, physical activity, BMI, MMSE score, heart disease, hypertension, cerebrovascular disease, diabetes, APOE ɛ4, death, and brain pathologies. To test a dose-dependent association of CR with dementia risk, the categorized CR variable (1 for lowest tertile, 2 for middle tertile, and 3 for highest tertile) as a continuous variable was used in Cox regression models. Multiadjusted multinomial logistic regression was used to estimate odds ratios (ORs) with 95% CIs of the association of CR with the burden of AD and other brain pathologies. Incidence rates and 95% CIs of dementia per 1000 person-years were calculated for CR categories by different levels of brain pathologies, adjusting for the confounders.
In sensitivity analysis, multiple imputation by chained equation was used to impute data for 166 participants with missing values. Further, we excluded 420 individuals with MCI at baseline. Considering death as a competing risk for dementia, a competing risks model (the Fine-Gray model) was used to estimate HR for dementia associated with CR score. The level of statistical significance was set at a P value less than .05, and all P values were 2-tailed. All analyses were performed with Stata/SE version 15.0 (StataCorp).
Results
Characteristics of the Study Population
Of the 1602 participants, 1216 (75.9%) were women, and the mean (SD) age was 79.6 (7.5) years. During follow-up (mean follow-up, 6 years; maximum follow-up, 20 years; total follow-up time, 9721.7 person-years), 386 participants developed dementia (24.1%), including 357 participants with AD-related dementia (22.3%). Table 1 presents the baseline characteristics of the study population by incident dementia.
Table 1. Baseline Characteristics of the Study Population by Dementia Status.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Dementia-Free (n = 1216) | Incident Dementia (n = 386) | ||
| Age, mean (SD), y | 78.5 (7.6) | 83.0 (6.0) | <.001 |
| Female | 924 (76.0) | 292 (75.7) | .89 |
| Life course cognitive reserve factors | |||
| Education, mean (SD), y | 14.8 (3.3) | 14.5 (3.1) | .08 |
| Early-life cognitive activity, mean (SD) | 3.1 (0.6) | 3.0 (0.6) | .11 |
| Midlife cognitive activity, mean (SD) | 3.3 (0.6) | 3.2 (0.7) | .04 |
| Late-life cognitive activity, mean (SD) | 3.2 (0.7) | 3.1 (0.8) | .003 |
| Social activity in late life, mean (SD) | 2.7 (0.6) | 2.5 (0.6) | <.001 |
| Social network, median (IQR) | 6.0 (3.0-10.0) | 5.0 (3.0-9.0) | .03 |
| Smoking status | |||
| Never | 696 (57.2) | 245 (63.5) | .08 |
| Ever | 483 (39.7) | 133 (34.5) | |
| Current | 37 (3.1) | 8 (2.0) | |
| Alcohol consumptiona | |||
| Never/occasional | 731 (60.8) | 261 (67.6) | .03 |
| Light/moderate | 340 (27.6) | 89 (23.1) | |
| Heavy | 144 (11.7) | 36 (9.3) | |
| Physical activity, median (IQR) | 2.4 (0.8-4.5) | 2.5 (1.0-4.7) | .98 |
| BMI, mean (SD)b | 27.7 (5.6) | 26.5 (4.5) | <.001 |
| MMSE score, median (IQR)a | 29.0 (28.0-30.0) | 28.0 (26.0-29.0) | <.001 |
| Heart diseasea | 109 (9.0) | 36 (9.3) | .83 |
| Hypertension | 826 (67.9) | 242 (62.7) | .06 |
| Cerebrovascular diseasec | 96 (8.7) | 41 (11.3) | .14 |
| Type 2 diabetes | 187 (15.4) | 39 (10.1) | .01 |
| Any APOE ɛ4d | 224 (19.5) | 121 (32.0) | <.001 |
| Death during follow-up | 458 (37.7) | 289 (74.9) | <.001 |
Abbreviations: APOE ɛ4, apolipoprotein ɛ4 allele; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; MMSE, Mini-Mental State Examination.
Data missing for 1 participant.
Data missing for 30 participants.
Data missing for 139 participants.
Data missing for 72 participants.
Extracting CR Score
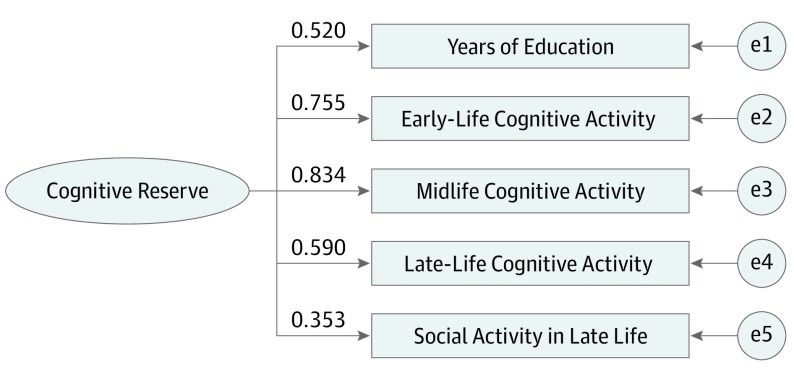
The final CR indicator was generated based on the best-fitting SEM with the 5 CR-enhancing factors (education; early-life, midlife, and late-life cognitive activities; and social activities in late life), as presented in Figure 1. The model fit was satisfactory, and the correlation coefficients among the 5 factors varied from 0.17 to 0.64 (eTable 1 in the Supplement). A unique value for the CR score was obtained for each participant, and the score ranged from −8.00 to 5.81.
Figure 1. Standardized Estimates From the Structural Equation Model With 5 Observable Factors of a Latent Reserve Construct.
The values indicate the loadings of the 5 factors to cognitive reserve. e1, e2, e3, e4, and e5 indicate the measurement error for each cognitive reserve factor. Structural equation modeling fit statistics: χ25 = 41.919; P < .001; comparative fit index = 0.981; standardized root mean squared residual = 0.026; root mean squared error of approximation = 0.068.
Association of CR With Dementia Risk in All Participants
In both basic and multiadjusted Cox regression models, the CR score (as a continuous variable) was associated with a reduction in risk of dementia. After adjusting for age, sex, smoking, alcohol consumption, physical activity, BMI, MMSE score, heart disease, hypertension, cerebrovascular disease, diabetes, APOE ɛ4, and death, compared with those in the lowest CR score tertile, the HRs of dementia were 0.77 (95% CI, 0.59-0.99) for those in the middle CR score tertile and 0.61 (95% CI, 0.47-0.81) for those in the highest CR score tertile, and the HRs of AD-related dementia were 0.77 (95% CI, 0.59-1.00) for those in the middle CR score tertile and 0.61 (95% CI, 0.46-0.81) for those in the highest CR score tertile. The association of the CR score with dementia and AD-related dementia risk was dose dependent (Table 2).
Table 2. Association of Levels of Cognitive Reserve With Dementia and Alzheimer Disease (AD)–Related Dementia Among All Participants.
| Cognitive Reserve | No. of Participants | Dementia | AD-Related Dementia | ||||
|---|---|---|---|---|---|---|---|
| No. | HR (95% CI)a | Multiadjusted HR (95% CI)b | No. | HR (95% CI)a | Multiadjusted HR (95% CI)b | ||
| Continuous | 1364c | 349 | 0.89 (0.85-0.93) | 0.93 (0.88-0.97) | 320 | 0.89 (0.85-0.93) | 0.92 (0.88-0.97) |
| Categorical tertile | |||||||
| Lowest | 442 | 138 | 1 [Reference] | 1 [Reference] | 126 | 1 [Reference] | 1 [Reference] |
| Middle | 453 | 108 | 0.71 (0.55-0.92) | 0.77 (0.59-0.99) | 99 | 0.71 (0.55-0.93) | 0.77 (0.59-1.00) |
| Highest | 469 | 103 | 0.55 (0.42-0.71) | 0.61 (0.47-0.81) | 95 | 0.55 (0.42-0.72) | 0.61 (0.46-0.81) |
| P for trend | NA | NA | <.001 | <.001 | NA | <.001 | <.001 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Adjusted for age and sex.
Adjusted for age, sex, smoking, alcohol consumption, physical activity, body mass index, Mini-Mental State Examination score, heart disease, hypertension, cerebrovascular disease, diabetes, apolipoprotein E ɛ4, and death.
A total of 238 participants had missing data (alcohol consumption, 1; body mass index, 30; Mini-Mental State Examination, 1; heart disease, 1; cerebrovascular disease, 139; and apolipoprotein E ɛ4, 72).
Kaplan-Meier survival analysis showed that median (interquartile range) dementia onset time was 12.30 (8.78-15.82) years in participants in the lowest CR score tertile, 14.98 (11.14-18.82) years in participants in the middle CR score tertile, and more than 20 years in participants with highest CR. Participants in the highest CR score tertile had later dementia onset by more than 7 years compared with those in the lowest CR score tertile (eFigure 2 in the Supplement).
CR and Dementia Risk in Autopsied Participants
Of the 747 people who died, 611 (81.8%; 440 [72.0%] women; mean [SD] age, 83.0 [5.8] years) underwent autopsy, of whom 241 were diagnosed as having incident dementia. Baseline and neuropathological characteristics of the autopsied participants by dementia status are shown in eTable 2 in the Supplement.
In the post mortem data analysis, multiadjusted multinomial logistic regression analyses showed that compared with the lowest CR score tertile, the middle and highest CR score tertiles at baseline were not associated with the burden of AD pathology and other brain pathologies, except for gross infarcts (OR, 0.49; 95% CI, 0.31-0.78) (eTable 3 in the Supplement). There was no statistically significant association of MCI with global AD pathology (OR, 0.78; 95% CI, 0.37-1.67; P = .53) or gross infarcts (OR, 1.04; 95% CI, 0.38-2.82; P = .94) among those in the highest CR score tertile.
The multiadjusted Cox regression models showed that the highest CR score tertile was significantly associated with a reduction in risk of dementia (HR, 0.60; 95% CI, 0.42-0.86) and AD-related dementia (HR, 0.60; 95% CI, 0.41-0.87) compared with the lowest CR score tertile after additional adjustment for global AD pathology and other brain pathologies. The associations of CR score tertile with risk of dementia and AD-related dementia were dose dependent (eTable 4 in the Supplement).
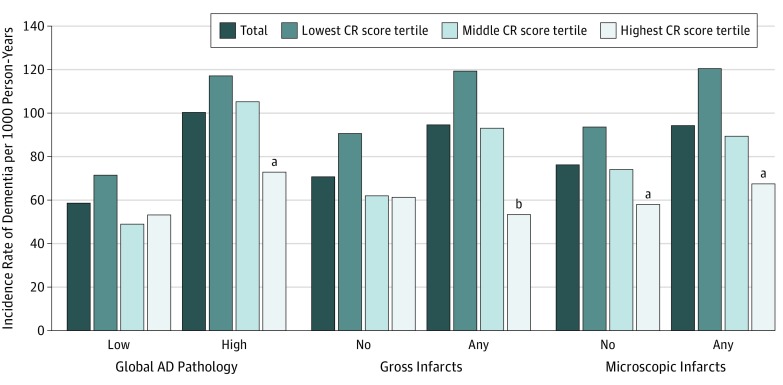
Compared with those with high brain pathologies but in the lowest CR score tertile, the incident rates of dementia were about 38% to 55% lower in people both in the highest CR score tertile and with high brain pathologies (including global AD pathology, gross infarcts, and microscopic infarcts) (Figure 2; eTable 5 in the Supplement). In stratified analysis by level of brain pathology, the association of high CR score tertile with a reduction in dementia risk remained significant in participants with high AD pathology (HR, 0.57; 95% CI, 0.37-0.87) and any gross infarcts (HR, 0.34; 95% CI, 0.18-0.62) (Table 3).
Figure 2. Incidence Rates of Dementia per 1000 Person-Years by Cognitive Reserve (CR) Tertile and Brain Pathology.
Incidence rates were adjusted for age, sex, smoking, alcohol consumption, physical activity, body mass index, heart disease, hypertension, cerebrovascular disease, diabetes, and apolipoprotein E ɛ4. AD indicates Alzheimer disease.
aP < .05 compared with lowest CR score tertile.
bP < .01 compared with lowest CR score tertile.
Table 3. Association of Cognitive Reserve (CR) With Dementia and Alzheimer Disease (AD)–Related Dementia by Presence of Brain Pathologyc.
| Brain Pathology | CR Tertile | No. of Participants | Dementia | AD-Related Dementia | ||||
|---|---|---|---|---|---|---|---|---|
| No. | HR (95% CI)a | Multiadjusted HR (95% CI)b | No. | HR (95% CI)a | Multiadjusted HR (95% CI)b | |||
| Global AD pathology burden | ||||||||
| Low | Lowest | 94 | 32 | 1 [Reference] | 1 [Reference] | 30 | 1 [Reference] | 1 [Reference] |
| Middle | 91 | 21 | 0.58 (0.33-1.02) | 0.66 (0.36-1.23) | 17 | 0.53 (0.30-0.92) | 0.58 (0.30-1.12) | |
| Highest | 102 | 29 | 0.55 (0.32-0.94) | 0.63 (0.35-1.13) | 26 | 0.51 (0.28-0.93) | 0.60 (0.33-1.11) | |
| High | Lowest | 104 | 63 | 1 [Reference] | 1 [Reference] | 58 | 1 [Reference] | 1 [Reference] |
| Middle | 95 | 45 | 0.87 (0.59-1.27) | 0.94 (0.63-1.39) | 43 | 0.88 (0.59-1.30) | 0.97 (0.64-1.47) | |
| Highest | 83 | 38 | 0.63 (0.42-0.95) | 0.57 (0.37-0.87) | 37 | 0.64 (0.43-0.98) | 0.58 (0.37-0.90) | |
| Gross infarcts | ||||||||
| No | Lowest | 113 | 47 | 1 [Reference] | 1 [Reference] | 44 | 1 [Reference] | 1 [Reference] |
| Middle | 112 | 33 | 0.63 (0.39-0.96) | 0.64 (0.40-1.03) | 32 | 0.63 (0.42-0.97) | 0.65 (0.40-1.05) | |
| Highest | 138 | 48 | 0.61 (0.42-0.95) | 0.61 (0.40-0.93) | 46 | 0.62 (0.39-0.98) | 0.60 (0.39-0.93) | |
| Any | Lowest | 85 | 48 | 1 [Reference] | 1 [Reference] | 44 | 1 [Reference] | 1 [Reference] |
| Middle | 74 | 33 | 0.83 (0.53-1.31) | 0.78 (0.49-1.23) | 28 | 0.80 (0.49-1.29) | 0.74 (0.46-1.22) | |
| Highest | 47 | 19 | 0.41 (0.23-1.31) | 0.34 (0.18-0.62) | 17 | 0.39 (0.22-0.72) | 0.32 (0.17-0.61) | |
Abbreviation: HR, hazard ratio.
Adjusted for age and sex.
Adjusted for age, sex, smoking, alcohol consumption, physical activity, body mass index, heart disease, hypertension, cerebrovascular disease, diabetes, and apolipoprotein E ɛ4.
A total of 43 participants had missing data (body mass index, 11; cerebrovascular disease, 26; and apolipoprotein E ɛ4, 6).
Supplementary Analyses
The results were not altered much compared with those from initial analyses when we repeated the following analyses by (1) multiple imputation for missing values (eTable 6 in the Supplement), (2) excluding 420 individuals with MCI at baseline (eTable 7 in the Supplement), (3) using competing risks models in all participants (eTable 8 in the Supplement), (4) removing education from the SEM (eTable 9 in the Supplement), (5) removing late-life cognitive and social activities from the SEM (eTable 10 in the Supplement), and (6) assessing the association of individual factors included in CR with dementia risk (eTable 11 in the Supplement). Finally, we found that sex did not significantly affect the association of CR with dementia risk in all participants (HR, 1.05; 95% CI, 0.78-1.43; P = .74) or in autopsied participants (HR, 1.01; 95% CI 0.68-1.49; P = .97).
Discussion
In this community-based prospective study of dementia-free older adults, we found that (1) high lifespan CR indicator accumulated through education, early-life cognitive activities, midlife cognitive activities, late-life cognitive activities, and social activities in late life was associated with a reduction in risk of dementia in a dose-dependent manner; (2) CR was not associated with most brain pathologies, and the association of CR with dementia remained significant after additional adjustment for brain pathologies; and (3) high CR could be associated with a reduction in dementia risk even in the presence of high AD burden and vascular pathologies. Neuropathological and neuroimaging studies have suggested that many people may tolerate considerable AD-related neuropathology without expressing the clinical syndrome.1 Indeed, about 25% of cognitively healthy older adults have increased levels of β-amyloid plaques in the brain.4 The concept of CR refers to the capacity to be resilient to age-related brain changes and the disease-related pathology in the brain without developing clinical dementia29 through enhanced brain network efficiency, capacity, or flexibility.13 Although a number of CR-related factors, including higher education attainment,2,30 complex occupation status,29 and rich cognitive and social activities,3,31,32 have been individually associated with a reduction in dementia risk, the association of each individual component with dementia could also be because of many alternative paths instead of a direct relation to the hypothesized CR. For example, lower education that is associated with dementia risk may also contribute to the deleterious effects of low socioeconomic status or cardiovascular disorders.33
In recent years, the use of CR indices has been suggested to evaluate the CR based on cumulative reserve factors,34,35 and the specific weight of each proxy indicator has been controversial. In the present study, to extract the CR score, we used SEM based on lifespan (ie, through early life, midlife, and late life) cognitive-enhancing activities and social activities in late life, and the weight of each CR factor was generated from SEM according to its contribution to the score, which was not equally weighted. We found that lifespan CR indicators in the middle and highest tertiles were associated with an approximately 23% to 39% reduction in risk of dementia. Furthermore, the association of CR with dementia was dose dependent, suggesting that accumulative educational and mentally stimulating activities throughout life are of great significance, given that there is currently no effective treatment for dementia.
So far, few studies presenting the association of the proxy of CR with dementia have taken brain pathologies into account. A 1999 study8 found that lower education was associated with the occurrence of cerebral infarcts. However, many other studies have failed to find a direct association of CR factors (such as education,11 cognitive activity,16 or cognitive lifestyle score36) with common dementia neuropathology. In the present study, we found that high lifespan CR indicator was not associated with most brain pathologies, except for gross infarcts, and baseline MCI status did not modify the association of brain pathology with CR. Further, high CR indicator was associated with a reduction in the risk of dementia independently of AD, vascular, and other brain pathologies. In addition, high lifespan CR indicator may be associated with a reduction in the risk of dementia even in the presence of high AD and vascular pathologies. These results were consistent with other studies11 and the CR theory7 that CR could reduce dementia risk and compensate for or cope with dementia pathology through other pathways rather than avoiding pathology directly.
Strengths and Limitations
This study has high rates of clinical evaluation and autopsy, which might minimize selective bias. Furthermore, the use of latent factors could capture the comprehensive effect of multiple CR factors across the lifespan. Nonetheless, some limitations need to be pointed out. First, the generalizability of the findings is limited because the study participants were volunteers. Second, as the brains were obtained at the end of the study, the causal inference of the neuropathologic basis in the association of CR with dementia must be further explored carefully. Third, CR-related factors were assessed by retrospective self-report, which could be subject to measurement error. However, use of a SEM-based latent variable approach allowed for the correction of unreliability in these factors. Fourth, as brain changes might occur nearly 15 years before clinical diagnosis of dementia, reverse causality between dementia and exposure studied at baseline could have occurred. We excluded those with incident dementia during the first follow-up, and the observed association remained significant. Fifth, nonresponse bias might have occurred owing to missing data. However, we repeated the analysis by multiple imputation for missing values, and the main results were not altered much.
Conclusions
This study provides evidence that high lifespan CR indicator, encompassing education, early-life, midlife, and late-life cognitive activities, and social activities in late life, is associated with a reduction in dementia risk, even in people with high AD and vascular pathologies. Our findings suggest that accumulative educational and mentally stimulating activities enhancing CR throughout life might be a feasible strategy to prevent dementia, even in people with high AD or vascular pathologies. Further large population-based longitudinal studies are warranted to establish the strategies of engagement in CR-related activities for the prevention of dementia.
eMethods 1. Covariate details.
eMethods 2. SEM computation details.
eTable 1. Pearson correlation coefficient matrix of observed contributors to cognitive reserve indicator included in the calculation of a latent variable.
eTable 2. Baseline and neuropathological characteristics of the 611 autopsied participants by dementia.
eTable 3. Association of brain pathologies with middle and highest cognitive reserve at baseline using multinomial logistic regression.
eTable 4. Hazard ratios (HRs) with 95% CIs of dementia and Alzheimer disease–related dementia in relation to cognitive reserve among participants with autopsies.
eTable 5. Incidence rates (per 1000 person-years) of dementia and Alzheimer disease–related dementia by different levels of cognitive reserve and brain pathologies.
eTable 6. Hazard ratios (HRs) with 95% CIs of the association of levels of cognitive reserve with dementia and Alzheimer disease–related dementia with multiple imputation for missing data.
eTable 7. Hazard ratios (HRs) and 95% CIs of the association of cognitive reserve with dementia and Alzheimer disease–related dementia after excluding MCI.
eTable 8. Hazard ratios (HRs) and 95% CIs of the association of cognitive reserve with dementia and Alzheimer disease–related dementia among all participants: results from competing risk models.
eTable 9. Hazard ratios (HRs) and 95% CIs of the association of cognitive reserve with dementia and Alzheimer disease–related dementia among all participants.
eTable 10. Hazard ratios (HRs) and 95% CIs of the association of cognitive reserve with dementia and Alzheimer disease–related dementia among all participants.
eTable 11. Hazard ratios (HRs) and 95% CIs of the associations of individual cognitive reserve indicators with dementia and Alzheimer disease–related dementia among all participants.
eFigure 1. Participant flow chart.
eFigure 2. Kaplan-Meier survival curves of dementia by cognitive reserve.
References
- 1.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006-1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M, Acosta D, Ferri CP, et al. Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. Lancet. 2012;380(9836):50-58. doi: 10.1016/S0140-6736(12)60399-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee ATC, Richards M, Chan WC, Chiu HFK, Lee RSY, Lam LCW. Association of daily intellectual activities with lower risk of incident dementia among older Chinese adults. JAMA Psychiatry. 2018;75(7):697-703. doi: 10.1001/jamapsychiatry.2018.0657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA. Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathol. 2014;127(1):137-150. doi: 10.1007/s00401-013-1226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58(4):617-622. doi: 10.1002/ana.20637 [DOI] [PubMed] [Google Scholar]
- 6.Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17(4):593-601. doi: 10.1017/S1355617710001748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. ; Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup . Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance [published online September 14, 2018]. Alzheimers Dement.30222945 [Google Scholar]
- 8.Del Ser T, Hachinski V, Merskey H, Munoz DG. An autopsy-verified study of the effect of education on degenerative dementia. Brain. 1999;122(pt 12):2309-2319. doi: 10.1093/brain/122.12.2309 [DOI] [PubMed] [Google Scholar]
- 9.Sumowski JF, Rocca MA, Leavitt VM, et al. Searching for the neural basis of reserve against memory decline: intellectual enrichment linked to larger hippocampal volume in multiple sclerosis. Eur J Neurol. 2016;23(1):39-44. doi: 10.1111/ene.12662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology. 2018;90(15):695-703. doi: 10.1212/WNL.0000000000005303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brayne C, Ince PG, Keage HA, et al. ; EClipSE Collaborative Members . Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133(pt 8):2210-2216. doi: 10.1093/brain/awq185 [DOI] [PubMed] [Google Scholar]
- 12.Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging. 2007;28(5):784-798. doi: 10.1016/j.neurobiolaging.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17(10):502-509. doi: 10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81(4):314-321. doi: 10.1212/WNL.0b013e31829c5e8a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James BD, Wilson RS, Barnes LL, Bennett DA. Late-life social activity and cognitive decline in old age. J Int Neuropsychol Soc. 2011;17(6):998-1005. doi: 10.1017/S1355617711000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920. doi: 10.1212/01.wnl.0000271087.67782.cb [DOI] [PubMed] [Google Scholar]
- 17.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646-663. doi: 10.2174/156720512801322663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RS, Yu L, Lamar M, Schneider JA, Boyle PA, Bennett DA. Education and cognitive reserve in old age. Neurology. 2019;92(10):e1041-e1050. doi: 10.1212/WNL.0000000000007036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marquine MJ, Segawa E, Wilson RS, Bennett DA, Barnes LL. Association between cognitive activity and cognitive function in older Hispanics. J Int Neuropsychol Soc. 2012;18(6):1041-1051. doi: 10.1017/S135561771200080X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James BD, Boyle PA, Buchman AS, Bennett DA. Relation of late-life social activity with incident disability among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66(4):467-473. doi: 10.1093/gerona/glq231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchman AS, Boyle PA, Wilson RS, Fleischman DA, Leurgans S, Bennett DA. Association between late-life social activity and motor decline in older adults. Arch Intern Med. 2009;169(12):1139-1146. doi: 10.1001/archinternmed.2009.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322-2326. doi: 10.1212/01.WNL.0000147473.04043.B3 [DOI] [PubMed] [Google Scholar]
- 23.Fleischman DA, Leurgans S, Arfanakis K, et al. Gray-matter macrostructure in cognitively healthy older persons: associations with age and cognition. Brain Struct Funct. 2014;219(6):2029-2049. doi: 10.1007/s00429-013-0622-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133-1142. doi: 10.1212/WNL.56.9.1133 [DOI] [PubMed] [Google Scholar]
- 25.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200-208. doi: 10.1002/ana.21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378-384. doi: 10.1001/archneur.61.3.378 [DOI] [PubMed] [Google Scholar]
- 27.Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018;91(6):e517-e525. doi: 10.1212/WNL.0000000000005951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crystal HA, Schneider JA, Bennett DA, Leurgans S, Levine SR. Associations of cerebrovascular and Alzheimer’s disease pathology with brain atrophy. Curr Alzheimer Res. 2014;11(4):309-316. doi: 10.2174/1567205011666140302194358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement. 2008;4(5):324-331. doi: 10.1016/j.jalz.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271(13):1004-1010. doi: 10.1001/jama.1994.03510370056032 [DOI] [PubMed] [Google Scholar]
- 31.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508-2516. doi: 10.1056/NEJMoa022252 [DOI] [PubMed] [Google Scholar]
- 32.Sajeev G, Weuve J, Jackson JW, et al. Late-life cognitive activity and dementia: a systematic review and bias analysis. Epidemiology. 2016;27(5):732-742. doi: 10.1097/EDE.0000000000000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166-2178. doi: 10.1161/CIRCULATIONAHA.117.029652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavrencic LM, Richardson C, Harrison SL, et al. Is there a link between cognitive reserve and cognitive function in the oldest-old? J Gerontol A Biol Sci Med Sci. 2018;73(4):499-505. doi: 10.1093/gerona/glx140 [DOI] [PubMed] [Google Scholar]
- 35.Wang HX, MacDonald SW, Dekhtyar S, Fratiglioni L. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: a community-based cohort study. PLoS Med. 2017;14(3):e1002251. doi: 10.1371/journal.pmed.1002251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenzuela MJ, Matthews FE, Brayne C, et al. ; Medical Research Council Cognitive Function and Ageing Study . Multiple biological pathways link cognitive lifestyle to protection from dementia. Biol Psychiatry. 2012;71(9):783-791. doi: 10.1016/j.biopsych.2011.07.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Covariate details.
eMethods 2. SEM computation details.
eTable 1. Pearson correlation coefficient matrix of observed contributors to cognitive reserve indicator included in the calculation of a latent variable.
eTable 2. Baseline and neuropathological characteristics of the 611 autopsied participants by dementia.
eTable 3. Association of brain pathologies with middle and highest cognitive reserve at baseline using multinomial logistic regression.
eTable 4. Hazard ratios (HRs) with 95% CIs of dementia and Alzheimer disease–related dementia in relation to cognitive reserve among participants with autopsies.
eTable 5. Incidence rates (per 1000 person-years) of dementia and Alzheimer disease–related dementia by different levels of cognitive reserve and brain pathologies.
eTable 6. Hazard ratios (HRs) with 95% CIs of the association of levels of cognitive reserve with dementia and Alzheimer disease–related dementia with multiple imputation for missing data.
eTable 7. Hazard ratios (HRs) and 95% CIs of the association of cognitive reserve with dementia and Alzheimer disease–related dementia after excluding MCI.
eTable 8. Hazard ratios (HRs) and 95% CIs of the association of cognitive reserve with dementia and Alzheimer disease–related dementia among all participants: results from competing risk models.
eTable 9. Hazard ratios (HRs) and 95% CIs of the association of cognitive reserve with dementia and Alzheimer disease–related dementia among all participants.
eTable 10. Hazard ratios (HRs) and 95% CIs of the association of cognitive reserve with dementia and Alzheimer disease–related dementia among all participants.
eTable 11. Hazard ratios (HRs) and 95% CIs of the associations of individual cognitive reserve indicators with dementia and Alzheimer disease–related dementia among all participants.
eFigure 1. Participant flow chart.
eFigure 2. Kaplan-Meier survival curves of dementia by cognitive reserve.