Abstract
Background
Ibrutinib is a Bruton’s tyrosine-kinase (BTK) inhibitor that is approved as a second-line treatment in chronic lymphocytic leukemia (CLL). While recent trials have demonstrated impressive results for ibrutinib, there remains a paucity of real-world data on its use in the clinical setting.
Methods
In this single-center study carried out at Brighton and Sussex University Hospitals, we retrospectively compared outcomes in 38 patients with relapsed CLL who received ibrutinib versus those who received conventional first- and second-line therapies.
Results
Our results demonstrate improved progression-free survival (PFS, p=0.022) with ibrutinib versus conventional second-line therapies and survival comparable to conventional first-line therapies. However, there was a high frequency (81.6%) of adverse events associated with ibrutinib therapy, including 2 cases of death secondary to sepsis and a further 7 cases of discontinuation of treatment due to adverse events. We also identify del13q14.3 as an adverse predictor of response to ibrutinib with respect to both overall survival (p=0.014) and PFS (p=0.008), suggesting that these patients may be better suited to receiving the BCL2 inhibitor venetoclax.
Conclusion
Whilst there is robust evidence for improved outcomes with ibrutinib, we find that survival in patients with del13q14.3 is reduced and that the rate of adverse events and discontinuation in clinical practice is higher than anticipated from clinical trials.
Keywords: CLL, chronic lymphocytic leukaemia, relapsed, real-world, ibrutinib, adverse events
Introduction
At present, conventional first-line treatment for chronic lymphocytic leukemia (CLL) in the UK is intermittent chemotherapy or chemo-immunotherapy delivered intravenously in the hospital setting; and until recently, the treatment for the relapsed disease was much the same.
The landmark success of imatinib, a targeted oral chemotherapy agent producing impressive and durable remissions in chronic myeloid leukemia,1–3 heralded research into the development of similar agents in CLL. Increased understanding of the central role of Bruton’s tyrosine kinase (BTK) in B-cell function led to the development of ibrutinib,4,5 a small molecule producing targeted inhibition of BTK, which has been shown to effectively impair B-cell receptor signaling and cellular proliferation.6,7
In clinical trials, ibrutinib has since been shown to be highly efficacious in relapsed or refractory CLL, with progression-free survival (PFS) rates reported at 96% at 30 months in the RESONATE trial.8 Consequently, ibrutinib became the first oral chemotherapeutic agent to be approved in the UK by NICE for the treatment of relapsed CLL or CLL with high-risk cytogenetics. Furthermore, with increasing evidence of the efficacy of ibrutinib as a first-line treatment and in combination with other agents,9–11 its use in clinical practice may soon become more widespread. Clinical trials are ongoing to evaluate its efficacy in this context.
There is also increasing research into prognostic factors influencing response to ibrutinib treatment. When compared with alternative chemotherapy agents, the evidence from clinical trials suggests that ibrutinib improves the response rate in patients with a 17p deletion or TP53 mutation,8,12,13 making it a valuable option now approved as first-line treatment in patients with this high-risk cytogenetic abnormality. However, the significance of other genetic abnormalities in predicting response to treatment is yet to be established.
Most notably, despite 3 years of routine use in the UK, data reflecting real-world experience and long-term follow-up with ibrutinib in the clinical setting are lacking. Of particular interest to patients, there remains only limited information available outside of the context of clinical trials, on the adverse event profile of the drug and the likelihood of a disruption in or discontinuation of therapy. Research suggests that disruption to continuous therapy, as is often required when managing adverse effects of treatment, is more frequent than was anticipated from the trial data and is likely to have a detrimental impact on the rate of disease progression.14,15
Consequently, in this single-center retrospective study, we report on the clinical experience of ibrutinib therapy, focusing on the outcomes from ibrutinib as compared to conventional first- and second-line treatment as well as the adverse event profile and rates of transformation to high-grade lymphoma. We also identify prognostic markers, which, if validated in other studies, may be used to direct individualized patient management with respect to ibrutinib therapy.
Methods
This research was conducted at the Royal Sussex County Hospital, Brighton, and Sussex University Hospital NHS Trust, a provider of secondary level care for patients with hematological disorders. The study conformed to the NHS Health Research Authority criteria for not requiring Research Ethics Committee approval (http://www.hra-decisiontools.org.uk/ethics/index.html). It was approved by the Individual Research Project Review Committee of Brighton and Sussex Medical School and deemed not to require patient consent as all patient data was anonymized. It was conducted with complete confidentiality of patient information and with full compliance with the Declaration of Helsinki.
One hundred and twenty patients were identified via pharmacy chemotherapy records to be included in the study. Electronic patient records were utilized for retrospective data collection of patient outcomes and adverse events. TP53 mutational status, as well as fluorescence in situ hybridization (FISH) data for cytogenetic abnormalities, was obtained where available. Very few patients were screened for mutation of IGH; therefore, this data was not included in any analyses.
Primary outcome measures were overall survival (OS) and PFS. Time to disease progression was defined as the time to first evidence of progressive disease, identified in most cases according to the criteria published by the International Workshop on CLL,16 or if these data were not available, the time to starting subsequent therapy. Since interval bone marrow assessment and regular comprehensive imaging is not routine clinical practice, the accurate reporting of remission status was not possible and consequently is not included in the analysis. The Kaplan–Meier survival method and log-rank tests were used for OS and PFS analyses. Statistical analysis of survival data was performed in RStudio Version 1.1.383.
Results
In our patient population, 38 patients received ibrutinib therapy between 2013 and 2017, of which 25 were male (65.8%). All patients had a documented reason for starting treatment with ibrutinib. Reasons for starting treatment included progressive nodal disease on CT, splenomegaly, lymphocyte doubling time less than 6 months, B-symptoms, progressive cytopaenias or extensive bone marrow infiltration. All patients receiving ibrutinib had received at least one course of chemotherapy prior to starting ibrutinib, median 2 (1–5). Median blood results prior to starting therapy were hemoglobin 109 (68–133), platelets 107 (22–221) and lymphocytes 63.6 (0.6–260). Eighteen of 38 patients had an LDH above laboratory reference range prior to starting ibrutinib therapy, median 453 (199–1175). Median length of follow-up for ibrutinib was 23 months (4–56).
The conventional treatment cohorts comprise of 82 patients receiving first-line therapy between 2008 and 2017 and 25 patients who went on to receive second-line therapy between 2011 and 2016. Conventional treatment groups typically received two or more agents in combination, most commonly rituximab with fludarabine/cyclophosphamide, bendamustine or chlorambucil, selected according to the patient profile. Other agents included idelalesib in 2 patients and alemtuzumab in 1 patient.
The patient demographics across all groups were comparable with respect to age and gender (Table 1). The median time from diagnosis to initiating treatment was 22 (0–293), 50.5 months (7–205) and 81 months (13–310) in first-line treatment, second-line treatment and ibrutinib cohort, respectively.
Table 1.
Patient demographics and outcomes from treatment
| Conventional first-line therapy | Conventional second-line therapy | Ibrutinib | |
|---|---|---|---|
| Number of patients | 82 | 25 | 38 |
| Male gender (%) | 48/82 (58.5%) | 16/25 (64.0%) | 25/38 (65.8%) |
| Median age at diagnosis (range) | 69 (38–91) | 70 (42–83) | 64 (47–80) |
| Median age at time of treatment (range) | 72.5 (40–94) | 75 (44–87) | 72 (53–87) |
| Median number of previous therapies (range) | 0 (0–0) | 1 (1–1) | 2 (1–5) |
| Median months from diagnosis to starting treatment (range) | 22 (0–293) | 50.5 (7–205) | 81 (13–310) |
| Median number of genetic aberrations (range) | 0 (0–3) | 1 (0–3) | 1 (0–7) |
| Median OS | 2,333 days | 1,097 days | Not reached |
| OS at 1 year (95% CI) | 85.1% (77–93) | 96.0% (89–100) | 100% (100–100) |
| OS at 3 years (95% CI) | 76.2% (67–87) | 50.8% (32–81) | 71.5% (48–100) |
| Median PFS | 1,462 days | 593 days | Not reached |
| PFS at 1 year (95% CI) | 77.1% (68–87) | 70.8% (55–92) | 88.9% (79–100) |
| PFS at 3 years (95% CI) | 57.4% (47–71) | 31.8% (16–65) | 69.8% (56–88) |
Abbreviations: OS, overall survival; PFS, progression-free survival.
FISH data or TP53 mutational status were available for 58/82 patients in the first-line therapies cohort, 17/25 patients in the conventional second-line therapies cohort and 29/38 patients in the ibrutinib cohort. 17p deletions or TP53 mutations were reported in and 6/58 (10.3%), 3/17 (17.6%) and 9/29 patients (31.0%), respectively. Del13q14.3 was the most frequently occurring genetic aberration, with frequencies of 18/58 (31.0%), 5/17 (29.4%) and 9/29 (31.0%) in each group. The types and frequency of mutations detected within the different treatment groups are shown in Table 2.
Table 2.
Frequency of genetic aberrations by treatment cohort
| Frequency in conventional first-line therapy group | Frequency in conventional second-line therapy group | Frequency in ibrutinib group | |
|---|---|---|---|
| Del17p or TP53 mutation | 6/58 (10.3%) | 3/17 (17.6%) | 9/29 (31.0%) |
| Del13q14.3 | 18/58 (31.0%) | 5/17 (29.4%) | 9/29 (31.0%) |
| Trisomy 12 | 16/58 (27.6%) | 6/17 (35.3%) | 4/29 (13.8%) |
| Del ATM locus | 6/58 (10.3%) | 1/17 (5.9%) | 5/29 (17.2%) |
| Del IGH locus | 7/58 (12.1%) | 3/17 (17.6%) | 1/29 (3.4%) |
| Other | 3/58 (5.2%) | 3/17 (17.6%) | 3/29 (10.3%) |
| Normal | 16/58 (27.6%) | 1/17 (5.9%) | 4/29 (13.7%) |
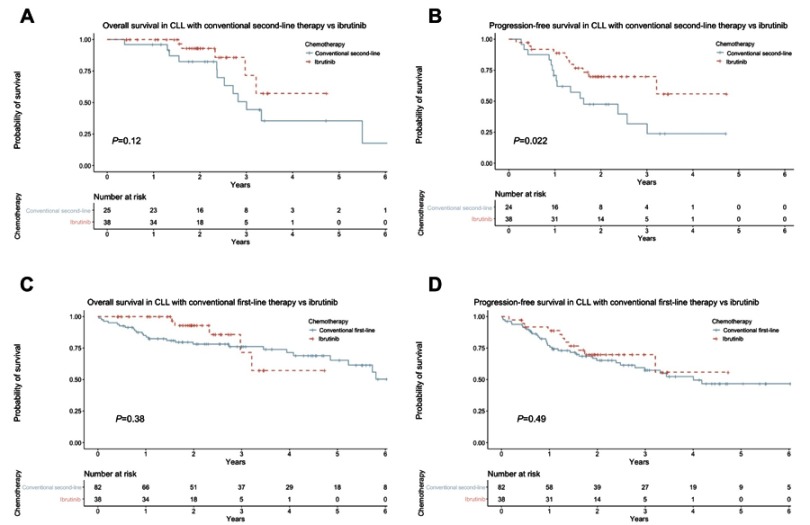
In this patient population, comparing ibrutinib with conventional second-line therapies revealed a marked trend towards improved OS with ibrutinib therapy, with median OS not reached versus median of 1,097 days (p=0.12) for patients on conventional second-line therapies (Figure 1A). PFS was significantly improved with ibrutinib therapy, median not reached versus median of 593 days for conventional therapies (p=0.022) (Figure 1B). Correspondingly, at 1 year after starting treatment, 88.9% of patients receiving ibrutinib were alive and free from progressive disease, compared to 70.8% in patients receiving conventional second-line therapies.
Figure 1.
(A) Overall survival with ibrutinib versus conventional second-line therapy; (B) progression-free survival with ibrutinib versus conventional second-line therapy; (C) overall survival with ibrutinib versus conventional first-line therapy; (D) progression-free survival with ibrutinib versus conventional first-line therapy.
In light of the improved outcomes for patients on ibrutinib when compared with conventional second-line therapies, we sought to compare outcomes also with first-line therapies. We find that ibrutinib treatment yields comparable PFS and OS to first-line treatments, with median OS of 2,333 days with first-line therapy, versus median not reached with ibrutinib (Figure 1C). PFS with first-line therapy was 1,462 days, versus median not reached with ibrutinib (Figure 1D).
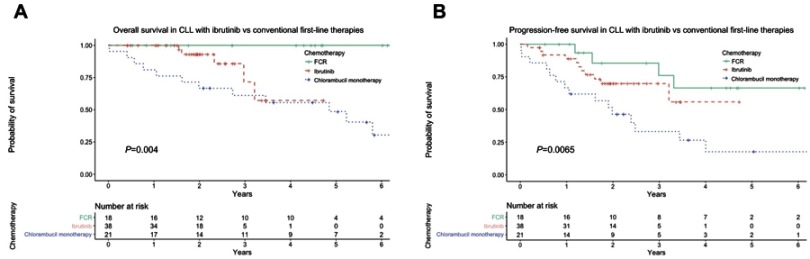
Notably, when patients receiving first-line therapy are stratified according to the type of chemotherapy received, there is a significant difference in both OS (p=0.002) and PFS (p=0.007) between ibrutinib, FCR and chlorambucil monotherapy, with patients receiving FCR having the best outcomes (Figure 2).
Figure 2.
(A) Overall survival with ibrutinib versus FCR and chlorambucil monotherapy, (B) progression-free survival with ibrutinib versus FCR and chlorambucil monotherapy. FCR, fludarabine, cyclophosphamide, rituximab.
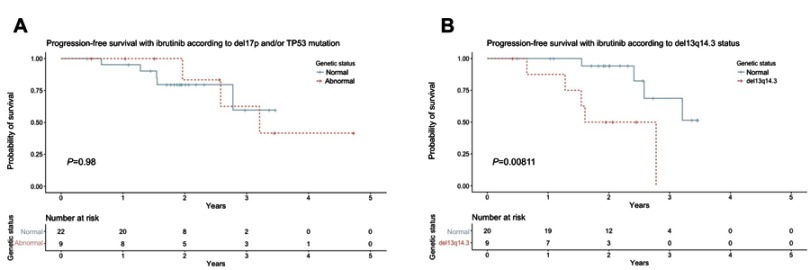
In the ibrutinib group, patient factors including age >70, gender, number of prior lines of therapy and number of cytogenetic mutations were not found to correlate with the outcome from treatment. Similarly, when considered together, 17p deletion and/or TP53 mutation status did not impact significantly on either OS or PFS with ibrutinib (Figure 3A). Interestingly, del13q14.3 was found to correlate with reduced OS (p=0.014) and reduced PFS (p=0.008) (Figure 3B).
Figure 3.
(A) Progression-free survival with ibrutinib according to del17p and/or TP53 mutation status; (B) progression-free survival with ibrutinib according to del13q14.3 status.
Adverse events were common in the ibrutinib arm and affected 31 out of 38 patients (81.6%) (Table 3). Those with the highest incidence were bruising or bleeding problems (15 patients) and gastrointestinal disturbance (14 patients). Recurrent or severe infections and neutropenia were also reported with a relatively high frequency, affecting 7/38 and 6/38 patients, respectively. Amongst these were two cases of severe sepsis that resulted in death.
Table 3.
Frequency of adverse events with ibrutinib therapy
| Adverse event | Frequency |
|---|---|
| GI disturbance (dyspepsia, nausea, diarrhoea) | 14 |
| Bleeding/bruising | 14 |
| Recurrent infections/sepsis | 7 |
| Neutropenia | 6 |
| Rash | 5 |
| Cardiac rhythm abnormalities | 3 |
| Arthralgia | 3 |
| Headache | 3 |
| Leg swelling | 2 |
| Brittle nails | 2 |
| Leg cramps | 2 |
| Blurred vision | 2 |
| Pneumonitis | 1 |
| Malaise | 1 |
| Oral ulceration | 1 |
| Pancolitis | 1 |
| Uveitis | 1 |
| EBV reactivation | 1 |
Abbreviations: GI, gastrointestinal; EBV, Epstein–Barr virus.
Overall, ibrutinib was stopped for a minimum of 1 week in 21/38 patients due to an adverse event or in order to prophylactically minimize bleeding risk for an invasive procedure. Therapy was discontinued completely in 9/38 patients due to serious adverse events, most notably, cardiac rhythm abnormalities in 3 cases. Seven of these 9 patients were still alive at the time of follow-up and 4 of these had developed progressive disease.
From our cohort of 38 patients receiving ibrutinib, 4 discontinued ibrutinib therapy due to the development of Richter’s transformation, with 2 cases of DLBCL and 2 Hodgkin’s lymphoma. Other reasons for cessation of therapy were progressive CLL in one case and development of advanced metastatic pancreatic adenocarcinoma in one case.
Discussion
The approval of ibrutinib therapy in 2014 has prompted a dramatic shift in the clinical management of patients with relapsed or high-risk CLL, away from further courses of intravenous chemotherapy toward oral chemotherapy, allowing patients to manage their treatment at home. This is an appealing prospect for both patients and doctors, but critically, the success of this novel treatment in practice is dependent on its ability to produce favorable clinical outcomes with an associated acceptable level of toxicity.
The results of this study support the data on survival outcomes from clinical trials, demonstrating improved PFS with ibrutinib therapy as compared to conventional second-line therapies, with an estimated PFS at 1 year of 88.9%. The patients included in our study were similar in terms of age, gender and cytogenetic profile to those included in the RESONATE trial, which reported 84% PFS at 1 year.8 However, consideration should also be given to studies of ibrutinib use in clinical practice, which have reported lower PFS rates.15,17 For example, the Polish Adult Leukaemia Group (PALG) study of 165 patients enrolled in a compassionate use program for ibrutinib reported an estimated PFS of 79.7% at 1 year.18 PALG authors suggest that the lower PFS rate may be due to poorer performance status in their study population.
The data presented here are also suggestive of improved OS when compared with conventional second-line therapies, with an estimated 1-year OS with ibrutinib of 100%, although the difference was not statistically significant. The reason for this may be due to the heterogeneity of the type of conventional second-line therapies included in this study, with certain regimes having better OS than others. Interestingly, in a recent retrospective study indirectly comparing the use of bendamustine and rituximab versus ibrutinib as second-line treatment, there was no significant difference in OS.19 Whereas, when directly compared with ofatumumab, ibrutinib is associated with significantly improved OS.8 The 1-year OS rate with ibrutinib measured in the ofatumumab trial and others is lower than reported here.8,20 The reason for this discrepancy is unclear, but may reflect that the patients included in this study had comparatively lower exposure to prior therapies than in other trials, or perhaps represents a difference in baseline characteristics that is unmeasured in this study.
There is emerging evidence from clinical trials that ibrutinib monotherapy is effective as a first-line treatment in patients aged ≥65 and has favorable outcomes when compared with conventional chemotherapy or chemoimmunotherapy combinations.9,11 The use of ibrutinib monotherapy as a first-line treatment in a more general context is still to be established. When compared retrospectively with conventional first-line therapies, these data suggest that BTK inhibitor therapy produces second remissions that are similar in their durability to first remissions. When stratified according to the type of first-line therapy, however, the results of our study demonstrate a significant difference in both OS and PFS between ibrutinib, FCR and chlorambucil monotherapy, with FCR producing superior outcomes, and chlorambucil producing comparatively poor outcomes.
These results are in keeping with the report from the RESONATE-2 trial, which showed improved OS and PFS with ibrutinib as compared to chlorambucil monotherapy as first-line management in older patients.9 The data from prior clinical trials of FCR demonstrate the efficacy of this combination of agents as a first-line treatment, particularly in those with a favorable cytogenetic profile, with many patients achieving a long-term remission.21,22 These data suggest that at present, for younger patients with low-risk characteristics, and who are able to tolerate FCR chemotherapy, this is the optimal approach to initial management.
It is necessary to consider; however, that in this retrospective study, the ibrutinib group received their treatment more recently than the conventional first-line therapy population. This is acknowledged as a potential confounder, which would tend to bias toward favoring ibrutinib therapy, for example, due to improvements in general supportive care over time. Additionally, patients receiving ibrutinib, chlorambucil monotherapy or FCR were not matched for baseline characteristics, since the chemotherapy agent was selected according to patient profile, taking in to account age and comorbidities, and in clinical practice, the toxicity associated with the FCR regime often precludes many patients from being eligible to receive it. Therefore, large-scale randomized clinical trials will be essential in resolving unanswered questions with regard to ibrutinib as first-line treatment.
Of note, there is unpublished trial data to suggest that perhaps a move away from first-line FCR as standard in younger patients with cytogenetically favorable profiles may be on the horizon. Results of the ECOG-ACRIN E1912 trial, presented at the American Society of Haematology meeting, are the first to demonstrate the potential for ibrutinib in combination with rituximab as a first-line agent in younger patients. In their study of patients aged ≤70, at a median follow-up of 33.4 months, ibrutinib plus rituximab produced improved OS, PFS and reduced toxicity when compared with standard FCR chemotherapy.23 Additionally, the results of the phase III FLAIR trial in the UK are eagerly anticipated, particularly as this will address the outstanding question of the efficacy of ibrutinib monotherapy as first-line in younger patients. At present, the use of ibrutinib in this context remains guarded.
The impact of high-risk cytogenetic abnormalities such as 17p deletion is an area of particular clinical interest, since these patients typically respond poorly to conventional chemotherapy.24 Early clinical trial data demonstrated encouraging responses to treatment in this patient cohort.8,12,14 In accordance with these results, the data presented here suggest that there is no clinically significant difference in either OS or PFS in patients with 17p deletion or TP53 mutation treated with ibrutinib.
However, these results must be interpreted with caution, considering the modest sample size in this study. Indeed, it is worthwhile noting that the data from longer-term follow up of patients in clinical trials suggest that responses are less durable in this patient cohort receiving ibrutinib as first-line therapy, with median PFS reduced to 26 months compared to median not reached over a median follow-up period of 5 years.25 Additionally, the data presented by the Swedish Group demonstrated a significantly reduced OS and PFS over a relatively short follow-up period of 10.2 months.17
Interestingly, in our cohort of patients receiving ibrutinib, an adverse effect was seen with del13q14.3, leading to a reduction in both OS and PFS. This cytogenetic abnormality is conventionally considered to be a favorable prognostic marker for disease progression,24 although its influence on outcomes from ibrutinib treatment is not known. Possible explanations for this finding were considered, in particular, due to the prevalence of del13q14.3 in this study population, and hence its frequent occurrence in combination with other cytogenetic abnormalities, that the detrimental effect of this abnormality may reflect the effect of a more complex karyotype. However, other cytogenetic abnormalities and total number of abnormalities did not appear to affect response to ibrutinib. Validation of these findings in larger studies with greater power to detect more subtle impact on the outcome for rare events will be required.
Considering these data alongside that from clinical trials, there is substantial evidence that ibrutinib improves disease control and survival in the setting of relapsed and high-risk CLL. The direction of future research is therefore pointing toward increasing application, with potential for use as a first-line therapy or in combination with other agents, with initial clinical trials in this area showing promising results.10
Nevertheless, as with all cytotoxic agents, off-target effects limit its utility in practice. In the RESONATE trial, overall rate of adverse events that were grade 3 or higher was 57% in patients receiving ibrutinib.8 Subsequent studies of ibrutinib use in practice have reported similar rates of clinically significant adverse events.15 However, there are many reasons why in practice, the experienced rates of adverse events are likely to be higher than those reported in clinical trials and retrospective research.
Despite some evidence that over time ibrutinib therapy is associated with improved immune function,26 the data from clinical trials demonstrate that the risk of infection remains a significant risk, particularly within the first year of starting treatment.27 Indeed, in this cohort of 38 patients, there were 7 cases of clinically significant infection and 2 cases of death due to severe sepsis. Additionally, there have been multiple reports of opportunistic infections occurring on ibrutinib therapy, including invasive aspergillosis and Cryptococcus.28,29 There is currently no clear guidance on antimicrobial prophylaxis or monitoring of patients on ibrutinib therapy, and consequently, severe infections continue to pose a significant risk.
Of additional concern is the frequency of bleeding problems, which has resulted in serious adverse events and, in some cases, fatality.15 Despite the exclusion of patients deemed to have a high bleeding risk from clinical trials, such as those taking warfarin or a strong CYP3A4/5 inhibitor, the reported frequency of bleeding problems remained significant at 44%, with 1% of patients experiencing a major hemorrhage,8 and similar results were reported in the Swedish experience.17 Pertinently, a secondary analysis of trial data found that the incidence of major bleeding events in patients concomitantly receiving other types of anticoagulation or antiplatelet agents was still elevated at 3%, suggesting that these alternative agents are also likely to increase risk of bleeding.30
It has therefore been proposed that bleeding risk may be managed by avoiding co-medicating with anticoagulants and antiplatelet agents and temporarily holding treatment for procedures with a risk of bleeding.31 However, in clinical practice, it is often challenging to balance up the relative risks of bleeding and thrombotic events, and until further research is conducted in this area, guidance on best practice is lacking.
Furthermore, the increased rates of cardiac arrhythmias with ibrutinib use create an unfortunate paradox in anticoagulant management in the context of ibrutinib-induced bleeding risk.32 In many cases, the complexity of this medical scenario ultimately results in discontinuation of ibrutinib therapy,33 as was seen in 3 patients in this study. If ibrutinib is continued, an anticoagulant with lower bleeding risk may be introduced in order to reduce the risk of a secondary thrombotic event, although as described above, objective evidence for the safety of this approach is lacking.
The consequence of any serious adverse events in most cases is an initial temporary pause in treatment. In some cases, this may lead to a permanent discontinuation or the possibility of a trial of reintroduction of therapy at a reduced dose. We find that temporary pauses in treatment of at least 7 days occur in the majority of patients, which is in agreement with the findings of the UK CLL group,15 indicating that the rate of pauses in treatment with use in clinical practice is likely to be greater than that reported in trials.14 We also found that 9/38 patients discontinued treatment permanently due to reasons other than disease progression, a rate which is again higher than that reported in the pooled data from 4 clinical trials of ibrutinib, which was described in 45/308 patients.34 It is likely that this discrepancy between real-world use and clinical trial data is due to the careful selection of trial participants, with exclusion of patients with comorbidities that may impact on the ability to comply with treatment.
It is not surprising that permanent cessation of therapy results in both development of progressive disease and reduced survival,35 but perhaps unexpectedly, treatments breaks of a relatively short duration have also been shown to have a significant impact on outcomes from treatment.15,36 On the other hand, there is evidence from the UK CLL group and PALG that a dose reduction of ibrutinib does not affect OS,15,18 which supports the use of this strategy where possible in managing adverse events in clinical practice.
Of additional interest in this study is the relatively high rate of Richter’s transformation of CLL to lymphoma, at 10.5%. The early clinical trials reported very low levels of disease transformation with ibrutinib, with two cases reported in the RESONATE trial of 195 patients and zero in the RESONATE-2 trial, where the authors report that the only case of high-grade Richter’s transformation to DLBCL occurred in the group of patients receiving chlorambucil monotherapy.8,9 However, the rates reported in clinical use appear to be higher, with 9/95 reported in the Swedish group and 18/315 biopsy-proven cases reported in the UK CLL group studies.15,17 These results suggest that rates of transformation are likely to be higher with routine clinical use than anticipated from clinical trials.
Conclusion
Ibrutinib is a promising novel therapy for CLL, with evidence for improved PFS and OS when compared with conventional second-line therapies and comparable outcomes to first-line therapies. The improvement in PFS as compared to chlorambucil as a first-line agent raises the possibility of ibrutinib as a first-line agent in patients deemed unfit for FCR. Responses to ibrutinib therapy amongst patients with 17p deletions and TP53 mutations were similar to nonmutated patients, although we find that del13q14.3 negatively impacts survival. Given the fact that del13q14.3 is associated with increased expression of BCL2 due to loss of miR15a/16a, it is tempting to speculate that these patients might respond better to the BCL2 inhibitor venetoclax than to a BTK inhibitor such as ibrutinib.
Our real-world data also reveal a number of serious adverse events resulting in permanent cessation of therapy in 7 patients. Of further concern, 2 patients died of sepsis and 4 patients developed Richter’s transformation. This research highlights the need for further clinical trials to investigate the use of ibrutinib as a first-line agent in selected patient cohorts. Additionally, larger scale studies will be required to identify and validate prognostic markers in order to effectively predict and monitor response to treatment. Finally, research into mechanisms of ibrutinib toxicity and the development of guidance on managing the risk to patients will be crucial in reducing the frequency and severity of adverse events.
Declarations of interest
This paper was presented at the Annual Scientific Meeting of the British Society for Haematology Conference 2018 as a poster presentation with interim findings. The poster’s abstract was published in “Abstracts” in the British Journal of Haematology: https://onlinelibrary.wiley.com/doi/full/10.1111/bjh.15226
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–652. doi: 10.1056/NEJMoa011573 [DOI] [PubMed] [Google Scholar]
- 2.Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99(6):1928 LP–1937. doi: 10.1182/blood.V99.6.1928 [DOI] [PubMed] [Google Scholar]
- 3.Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–927. doi: 10.1056/NEJMoa1609324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saouaf SJ, Mahajan S, Rowley RB, et al. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc Natl Acad Sci U S A. 1994;91(20):9524–9528. doi: 10.1073/pnas.91.20.9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brorson K, Brunswick M, Ezhevsky S, et al. xid affects events leading to B cell cycle entry. J Immunol. 1997;159(1):135–143. [PubMed] [Google Scholar]
- 6.Ponader S, Chen -S-S, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng S, Ma J, Guo A, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28(3):649–657. doi: 10.1038/leu.2013.358 [DOI] [PubMed] [Google Scholar]
- 8.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi: 10.1056/NEJMoa1509388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. doi: 10.1016/S1470-2045(15)00465-9 [DOI] [PubMed] [Google Scholar]
- 11.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528. doi: 10.1056/NEJMoa1812836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farooqui MZH, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2):169–176. doi: 10.1016/S1470-2045(14)71182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. doi: 10.1016/S1470-2045(16)30212-1 [DOI] [PubMed] [Google Scholar]
- 14.Barr PM, Brown JR, Hillmen P, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood. 2017;129(19):2612. doi: 10.1182/BLOOD-2016-12-737346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563–1572. doi: 10.3324/haematol.2016.147900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winqvist M, Asklid A, Andersson P, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica. 2016;101(12):1573–1580. doi: 10.3324/haematol.2016.144576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iskierka-Jażdżewska E, Hus M, Giannopoulos K, et al. Efficacy and toxicity of compassionate ibrutinib use in relapsed/refractory chronic lymphocytic leukemia in Poland: analysis of the Polish Adult Leukemia Group (PALG). Leuk Lymphoma. 2017;58(10):2485–2488. doi: 10.1080/10428194.2017.1292353 [DOI] [PubMed] [Google Scholar]
- 19.Cuneo A, Follows G, Rigolin GM, et al. Efficacy of bendamustine and rituximab as first salvage treatment in chronic lymphocytic leukemia and indirect comparison with ibrutinib: a GIMEMA, ERIC and UK CLL FORUM study. Haematologica. 2018;103(7):1209LP–1217. doi: 10.3324/haematol.2018.189837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet (London, England). 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5 [DOI] [PubMed] [Google Scholar]
- 22.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215. doi: 10.1182/blood-2015-06-651125 [DOI] [PubMed] [Google Scholar]
- 23.Shanafelt TD, Wang V, Kay NE, et al. A Randomized phase III study of ibrutinib (PCI-32765)-based therapy vs. standard Fludarabine, Cyclophosphamide, and Rituximab (FCR) chemoimmunotherapy in untreated younger patients with Chronic Lymphocytic Leukemia (CLL): A trial of the ECOG-ACRIN Cancer Research Group (E1912). Blood. 2018;132(Suppl 1):LBA-4 LP-LBA-4. doi: 10.1182/blood-2018-120779 [DOI] [Google Scholar]
- 24.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. doi: 10.1056/NEJM200012283432602 [DOI] [PubMed] [Google Scholar]
- 25.O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018. doi: 10.1182/blood-2017-10-810044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052–3064. doi: 10.1172/JCI89756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32(1):83–91. doi: 10.1038/leu.2017.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghez D, Calleja A, Protin C, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018. doi: 10.1182/blood-2017-11-818286 [DOI] [PubMed] [Google Scholar]
- 29.Baron M, Zini JM, Challan Belval T, et al. Fungal infections in patients treated with ibrutinib: two unusual cases of invasive aspergillosis and cryptococcal meningoencephalitis. Leuk Lymphoma. 2017;58(12):2981–2982. doi: 10.1080/10428194.2017.1320710 [DOI] [PubMed] [Google Scholar]
- 30.Jones JA, Hillmen P, Coutre S, et al. Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single-agent ibrutinib. Br J Haematol. 2017;178(2):286–291. doi: 10.1111/bjh.14660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown JR. How I treat CLL patients with ibrutinib. Blood. 2018;131(4):379–386. doi: 10.1182/blood-2017-08-764712 [DOI] [PubMed] [Google Scholar]
- 32.Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138–140. doi: 10.1182/blood-2016-05-712828 [DOI] [PubMed] [Google Scholar]
- 33.Thompson PA, Lévy V, Tam CS, et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175(3):462–466. doi: 10.1111/bjh.14324 [DOI] [PubMed] [Google Scholar]
- 34.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80. doi: 10.1001/jamaoncol.2014.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067. doi: 10.1182/blood-2014-09-603670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–2506. doi: 10.1182/blood-2014-10-606038 [DOI] [PMC free article] [PubMed] [Google Scholar]