Abstract
Bee venom contains a number of pharmacologically active components, including enzymes and polypeptides such as phospholipase A2 (PLA2) and melittin, which have been shown to exhibit therapeutic benefits, mainly via attenuation of inflammation, neurotoxicity, and nociception. The individual components of bee venom may manifest distinct biological actions and therapeutic potential. In this study, the potential mechanisms of action of PLA2 and melittin, among different compounds purified from honey bee venom, were evaluated against Parkinson’s disease (PD). Notably, bee venom PLA2 (bvPLA2), but not melittin, exhibited neuroprotective activity against PD in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. MPTP-induced behavioral deficits were also abolished after bvPLA2 treatment, depending on the PLA2 content. Further, bvPLA2 administration activated regulatory T cells (Tregs) while inhibiting inflammatory T helper (Th) 1 and Th17 cells in the MPTP mouse model of PD. These results indicate that bvPLA2, but not melittin, protected against MPTP and alleviated inflammation in PD. Thus, bvPLA2 is a promising and effective therapeutic agent in Parkinson’s disease.
Keywords: bee venom, phospholipase A2, melittin, Parkinson’s disease, neuroprotection, inflammation, regulatory T cells
1. Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disorder characterized by the progressive loss of dopaminergic (DA) neurons in the substantia nigra [1]. Generally, most cases of PD are of unclear etiology and occur sporadically. Evidence suggests that inflammation is one of the key contributing factors to the pathogenesis of PD [2]. The enhanced inflammatory response in PD has been detected in the post-mortem brain tissue of PD patients, which demonstrated increased T cell infiltration [3]. Numerous studies have expanded our understanding of the potential role of inflammation in PD pathogenesis. Specifically, recent studies support the role of regulatory T cells (Tregs) in inhibiting T cell inflammatory factors associated with immunosuppressive activity in the brain, which is the potential target in neurodegenerative disease.
Bee venom (BV), delivered by bee sting, consists of a complex mixture of polypeptides, enzymes, lipids, and bioactive amines [4]. BV has been widely associated with significant therapeutic effects under a variety of conditions, including for inflammatory [5] and neurodegenerative diseases [6]. Based on the molecular target, each component of bee venom may have different pharmacological activities. Hence, it is essential to understand the biological function of each component for bee-venom-based drug discovery.
Previously, we reported that bee venom phospholipase A2 (bvPLA2) protected the brain against neurodegenerative disorders such as Parkinson’s disease [7] and Alzheimer’s disease [8]. Even though the mechanism of action is still unclear, it appears that bvPLA2 may have a neuroprotective anti-inflammatory effect, mainly by inducing the activation of regulatory T cells (Tregs). Moreover, there is mounting evidence to support the pharmacological aspects of melittin, one of the main components of honey BV [9]. Indeed, some components of BV, including bvPLA2 and melittin, are currently under investigation as promising therapeutic tools. Unfortunately, there are many challenges and issues involved in BV-based drug discovery. Specifically, a clear functional characterization of each BV component needs to be evaluated.
In the present study, we purified bioactive components of BV, such as bvPLA2 and melittin, from crude BV by obtaining different concentrations of the components. We investigated the neuroprotective effects of bvPLA2 or melittin against Parkinson’s disease in mouse models by administering purified bvPLA2 and melittin extracts. We also analyzed the anti-inflammatory effects of purified extracts, based on the activation of microglia and Tregs in the mice.
2. Results
2.1. Purification PLA2 and Melittin from Crude Bee Venom
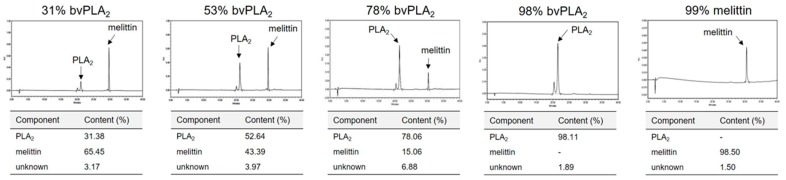
In order to determine the therapeutic potential of bvPLA2 and melittin, we isolated and purified them from the active component of BV. As our primary goal was to identify and compare the therapeutic effects of bvPLA2 and melittin, we obtained multiple extracts with diverse compositions of phospholipase A2 (PLA2) and melittin. The different products were quantified using high-performance liquid chromatography (HPLC) (Figure 1). Based on the corresponding HPLC profiles, the appropriate conditions for separation and purification were established. Furthermore, a satisfactory yield of bvPLA2 and melittin was obtained after removing the endotoxins and environmental pollutants such as heavy metals. Thus, the purified extracts appeared to be appropriate for the assessment of the therapeutic potential of bvPLA2 and melittin.
Figure 1.
Purification and manufacture of bee venom extracts containing PLA2 and melittin. Bioactive compounds from bee venom were purified according to their PLA2 and melittin content using high-performance liquid chromatography (HPLC) (upper panel). The components of the purified extracts derived from bee venom are displayed (lower panel).
2.2. BvPLA2 with Enriched PLA2 Levels Protects against Neurotoxicity in Parkinson’s Disease in MPTP-Induced Mice
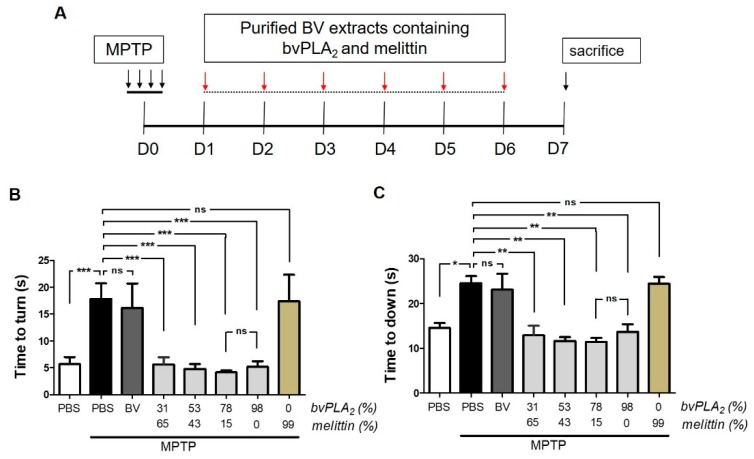
First, we carried out a pole test to examine the efficacy of bvPLA2 or melittin against motor deficits induced by the neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mice were treated with each purified extract for six consecutive days, beginning 1 day after MPTP treatment (Figure 2A). As expected, MPTP-injected mice required a longer timeframe to orient downwards (Figure 2B) and a longer period to descend the pole (Figure 2C) when compared with the controls. By contrast, the administration of bvPLA2 with enriched PLA2 extracts (78% and 98%) effectively suppressed any motor deficits in MPTP-challenged mice. No significant difference was found between the two groups of mice injected with these enriched PLA2 agents. By contrast, no significant improvement in motor function was observed in PD mice treated with melittin. Even the MPTP-challenged mice injected with highly purified melittin (99%) showed no significant difference in motor function.
Figure 2.
Beneficial effects of bvPLA2 enriched with PLA2 against motor deficits in the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-challenged mice. (A) Experimental timeline: the bee venom (BV) extracts containing different levels of PLA2 and melittin were administered to MPTP-exposed mice for 6 consecutive days, starting on day 1 after MPTP injection. (B,C) The motor deficits induced by MPTP were examined on Day 6 after MPTP. Time to orient downward and descend the pole was measured with the pole test. The data are expressed as the means ± standard error of the mean (SEM). n = 3–4 per group; *p < 0.05, ns, not significant.
2.3. BvPLA2 with Enriched PLA2 Rescues Loss of Dopaminergic Neurons in MPTP-Treated Mice
We further investigated whether bvPLA2 inhibited the loss of dopaminergic neurons in Parkinson’s disease in a PLA2-dependent manner. MPTP exposure induced dramatic cellular loss of dopaminergic neurons in the substantia nigra of mice, based on immunohistochemical assays for tyrosine hydroxylase (Figure 3). However, purified bvPLA2 protected against MPTP-induced neuronal loss, and the protection was positively correlated with higher PLA2 content. Approximately 78% of the purified bvPLA2 was adequate to prevent neuronal loss in Parkinson’s disease. Similar to the effects on motor function, melittin appeared to induce no significant changes in the loss of dopaminergic neurons, as demonstrated by their residual population. All these observations suggest that bvPLA2-based intervention is an efficient strategy to inhibit neurodegeneration and to rescue neurobehavioral function in Parkinson’s disease.
Figure 3.
Neuroprotective effect of standard bvPLA2 with enriched PLA2 on dopaminergic neurons in MPTP-injected mice. On Day 7 post-MPTP, immunohistochemical analysis of tyrosine hydroxylase (TH) was performed in MPTP mice injected with bee venom extracts containing PLA2 and melittin. (A) Representative images of sections containing TH-positive neurons, with high magnifications of dotted squares in substantia nigra pars compacta (SNpc). Phosphate buffered saline (PBS)-injected group was assigned to control. Upper panels are low magnification images (10×). Lower panels are high magnification images of the boxed areas in corresponding upper panels. (B) Unbiased stereological estimation for TH-positive neurons in substantia nigra. MPTP + bvPLA2 group: MPTP-exposed mice were treated with the enriched bvPLA2 (78% of PLA2 and 15% of melittin). Data are expressed as the means ± standard error of the mean (SEM). n = 3–4 per group; *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant. Scale bar: 100 μm.
2.4. BvPLA2 with Enriched PLA2 Levels Induces Differentiation of Regulatory T Cells in MPTP-Challenged Mice
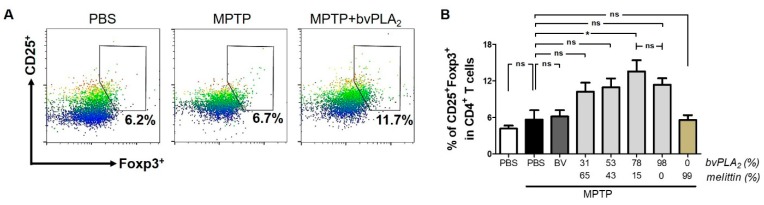
A growing body of evidence has shown that Tregs control the privileged immune status of mouse brains in neurodegenerative disease [10]. Interestingly, a Treg dysfunction was observed in PD patients [11] and in an animal model of PD [12]. Thus, we investigated whether the neuroprotective action of bvPLA2 was related to Tregs in Parkinson’s disease. We found no significant difference in CD4+CD25+Foxp3+ Treg cell populations between control and MPTP mice (Figure 4). However, bvPLA2 administration induced Treg cell differentiation in mice, depending on the PLA2 content. By contrast, melittin treatment did not alter the proportion of Tregs in mice when compared with control mice. These findings suggest that bvPLA2, and not melittin, stimulated the differentiation of Treg cells and their immunosuppressive and anti-inflammatory activity.
Figure 4.
Induction of regulatory T cells in MPTP-treated mice following administration of bvPLA2. Seven days after MPTP injection, CD4+CD25+Foxp3+ regulatory T cells from splenocytes of each group mice were analyzed by flow cytometry. Bee venom agents with different levels of PLA2 and melittin were administered to MPTP-challenged mice. Representative plots (A) and quantification (B) of CD25+Foxp3+ cells in CD4+ T cells are shown. MPTP + bvPLA2 group: MPTP-exposed mice were treated with enriched bvPLA2 (78% of PLA2 and 15% of melittin). Data are expressed as the means ± standard error of the mean (SEM). n = 3–4 per group; *p < 0.05, ns, not significant.
2.5. BvPLA2 with Enriched PLA2 Suppresses the Differentiation of CD4+ Effector T Cells in PD
A number of studies have suggested the importance of maintaining the balance between T cell subsets for immune homeostasis [13]. Thus, we examined whether mouse models of PD displayed an imbalance between CD4+ T cell subtypes. Clearly, MPTP-treated mice exhibited increased numbers of Th1 and Th17 cells, based on the secretion of interferon-γ (IFN-γ) by Th1 cells and interleukin-17A (IL-17A) by Th17 cells (Figure 5). However, bvPLA2 treatment reduced the cellular populations of CD4+ T cells with Th1 and Th17 phenotypes in MPTP-challenged mice. This inhibitory effect of bvPLA2 appeared to show a positive correlation with PLA2 content. Conversely, no such inhibitory effects against Th1 or Th17 differentiation were observed in mice treated with melittin in a dose-dependent manner.
Figure 5.
Inhibitory effect of bvPLA2 on the differentiation of CD4+ effector T cells in MPTP-treated mice. IFN-γ- (A) and IL-17A-positive (B) splenic CD4+ T cells were analyzed using flow cytometry on Day 7 post-MPTP to evaluate the number of Th1 and Th17 cells, respectively. Bee venom extracts containing different levels of PLA2 and melittin were administered to MPTP-treated mice. Data are expressed as the means ± standard error of the mean (SEM). n = 3–4 per group; *p < 0.05, **p < 0.01, ns, not significant.
3. Discussion
In this study, we isolated the bioactive components of PLA2 and melittin from crude bee venom. In order to analyze their therapeutic potential, we purified different formulations of PLA2 and melittin, exhibiting different purity. We investigated the effect of purified extracts of PLA2 and melittin in a mouse model of PD. Interestingly, bvPLA2, but not melittin, extracts containing enriched PLA2 levels improved motor function and provided substantial protection against MPTP-induced neurodegeneration. Furthermore, we observed an increase in regulatory T cells and a decrease in the inflammatory Th1 and Th17 cells in PD mice, depending on the PLA2 concentration.
Indeed, bee venom therapy (BVT) has been widely used to treat several conditions, including rheumatoid arthritis and skin diseases [14]. The most widely studied venoms showing therapeutic effects are those derived from the European honey bee, Apis mellifera. These honey BVs contain a variety of biologically active components, including enzymes and peptides [15]. Among these active components, PLA2 has been extensively studied for its therapeutic effects in a variety of diseases. Specifically, evidence suggests a therapeutic role of bvPLA2 in neurodegenerative diseases [16]. Even though the mechanism of action remains unclear, there is a possible association between PLA2 and inflammatory response in the pathogenesis of neurodegenerative disease. Accumulated evidence indicates that enhanced inflammatory response and damage is associated with the development and progression of PD [17]. In particular, Tregs have been of great interest for the treatment of PD due to their anti-inflammatory properties, attenuation of microglial activation, and enhanced neuronal survival in the neurodegeneration of PD [12]. In this study, we observed a clear induction of CD4+CD25+Foxp3+ regulatory T cells, depending on bvPLA2. Moreover, PLA2 isolated and purified from BV displayed a distinct inhibitory effect on inflammatory Th1 and Th17 phenotypes in a mouse model of PD. Hence, it is possible that increased Tregs induced by bvPLA2 suppress inflammation in PD with a concomitant decrease in Th1 and Th17 cell populations. Notably, substantial evidence implicates CD4+CD25+Foxp3+ regulatory T cell dysfunction in the pathogenesis of PD, which suggests that it may be a major contributing factor in the progression of neurodegeneration [10,12]. However, further studies are needed to provide a deeper understanding of the mechanisms underlying the therapeutic effects of bvPLA2 in PD.
Melittin, one of the main active components of BV, is a peptide with diverse therapeutic activities, including anti-microbial, anti-tumor, and anti-inflammatory effects [18]. However, recent studies reveal distinct biological actions of melittin in a strict dose-dependent manner. Small doses of melittin induce beneficial anti-inflammatory effects [9], while in high doses, melittin elicits pain and exacerbates the inflammatory response [19]. Numerous studies have reported distinct mechanisms of melittin under different conditions [18]. In this study, we found a limited therapeutic effect of melittin against PD. Interestingly, in this study, we observed that a purified BV extract, containing 78% of PLA2 and 15% of melittin, displayed a strong neuroprotective effect and improved motor function in PD. It is possible that the action of bvPLA2 is increased by melittin [20]. Further studies are necessary to demonstrate the relationship between bvPLA2 and melittin in their therapeutic activity against neurodegenerative disease.
4. Conclusions
Bioactive components of BV such as PLA2 and melittin are currently available in the market. Despite their widespread availability and use, their formulations are difficult to obtain. In this study, we isolated and produced BV extracts containing different levels of bvPLA2 and melittin. Based on further biological experiments, we observed a strong neuroprotective effect, improved motor function, and inhibition of inflammatory T cell phenotypes in a mouse model of PD in a strictly dose-dependent manner with bvPLA2, but not melittin. Overall, bvPLA2 may be a potential candidate for treating Parkinson’s disease and other diseases associated with neuroinflammation.
5. Materials and Methods
5.1. Preparation and Manufacturing of bvPLA2 and Melittin
The standardized BV phospholipase A2 was prepared by Inist St Co. Ltd. (Eumseong-gun, South Korea). Briefly, crude BV was purchased from Bee Venom Lab LLC (Tbilisi, GA, USA) and applied to a polytetrafluoroethylene (PTFF) membrane filter (pore size 0.45 μm; Sigma-Aldrich, St. Louis, MO, USA). In order to reduce the volume, the mixtures were concentrated by Ultracel-10 kDa membrane with Pellicon 3 devices (Merck Millipore, Billerica, MA, USA). The separation was carried out using reversed-phase high-performance liquid chromatography (RP-HPLC) on a C18 column (Sigma-Aldrich, St. Louis, MO, USA). The area of the detected peak was measured to determine the recovery of bvPLA2 and melittin. Commercial standard bvPLA2 (Sigma-Aldrich, St. Louis, MO, USA) was used as PLA2 with high purity (98% of PLA2). All these procedures were performed in an aseptic good manufacturing practice (GMP) facility. For quality management, a purity test was performed to confirm the absence of detectable heavy metals, endotoxins, or microbes.
5.2. Animals
All animal experiments were carried out in accordance with the approved animal protocols and guidelines established by the Kyung Hee Univesity [KHUASP(SE)17-149]. The experiments were conducted on 7 to 8 weeks old make C57BL/6J mice (20–22 g; Japan SLC, Hamatsu, Japan). Mice were maintained in a pathogen-free facility on a 12 h light/dark cycle with food and water provided ad libitum.
5.3. MPTP-Induced Mouse Model
MPTP (20 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) was intraperitoneally (i.p.) administered to mice four times a day at 2 h intervals, as previously described [21]. The physical condition of the mice was monitored.
5.4. BvPLA2 Treatment
MPTP-treated mice were exposed to either bvPLA2 or phosphate buffered saline (PBS) for a consecutive 6 days, beginning 1 day after the last MPTP injection. Purified standard bvPLA2 formulations and commercial grade bvPLA2 (Sigma-Aldrich, St. Louis, MO, USA) formulations were dissolved in PBS and administered as a single daily subcutaneous injection at a concentration of 0.5 mg/kg.
5.5. Immunohistochemistry
Immunohistochemical analysis was performed, as previously described [7]. Mouse brain tissues were prepared for histology in 4% paraformaldehyde overnight, washed in PBS, and then immersed in 30% sucrose until they sank. The brains were then sliced into 30 μm coronal sections using a sliding microtome. Next, the sections were processed for 30 min in 1% H2O2 in PBS and then blocked with PBS buffer containing 1% BSA and 0.1% Triton X-100 during 1 h. After an overnight incubation with tyrosine hydroxylase (TH) antibody (Wako Pure Chemic Industries, Osaka, Japan) at 4 °C, the tissues were incubated with biotinylated goat anti-rabbit immunoglobulin G (IgG) secondary antibody (Vector Laboratories, Burlingame, CA, USA). Following another wash, the brains were subjected to incubation in avidin–biotin complex using ABC kit (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA, USA). The chromogen reactions were then performed with diaminobenzidine (DAB) (Vector Laboratories, Burlingame, CA, USA).
5.6. Unbiased Stereological Estimation
An unbiased stereological estimation of the number of TH-positive cells was performed using an optical fractionator, as previously described with minor modifications [22]. Briefly, the sections extending from the rostral tip of the substantia nigra pars compacta (SNpc) to the caudal end of the substantia nigra pars reticulate (SNR) were selected and counted using Olympus CAST-Grid system (Olympus). The total number of cells was calculated using the optical fractionator.
5.7. Flow Cytometry
To determine the number of regulatory T cells in the splenocytes, flow cytometry was performed, as previously described [7]. The splenocytes were washed with PBS and stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4 (eBioscience, San Diego, CA, USA) and phycoerythrin (PE)-conjugated anti-mouse CD25 (eBioscience, San Diego, CA, USA). Next, the cells were fixed and stained with Alexa Fluor 647 anti-mouse Foxp3 (BD Biosciences, San Jose, CA, USA) overnight at 4 °C in the dark. After washing, the cells were analyzed. In order to measure Th1 and Th17 cells in the splenocytes, FITC-conjugated anti-mouse CD4 (eBioscience, San Diego, CA, USA), PE-conjugated anti-mouse IFN-γ (eBioscience, San Diego, CA, USA), and PerCP/Cyanine5.5-conjugated anti-mouse IL-17A (eBioscience, San Diego, CA, USA) were stained according to the supplier’s instructions.
5.8. Behavioral Test
The pole test was performed to measure motor coordination and balance, as previously described [23]. In brief, the mice were placed on the top of a gauze-banded wooden pole (diameter 1 cm; height 50 cm). The time at which mice successfully oriented themselves downward and climbed down was measured using a cut-off limit of 30 s. The average of three trials was used. Trials were excluded if the mouse dropped or jumped off.
5.9. Statistical Analysis
Statistical analysis was carried out using GraphPad Prism software (v5.0; GraphPad Software, La Jolla, CA, USA). Conditions were compared using a one-way analysis of variance (ANOVA) followed by Newman–Keuls test for multiple comparisons. A paired t-test was used to compare two groups. Data were expressed as the mean ± standard error of the mean (SEM). p < 0.05 was considered significant.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation (NRF) of South Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2017R1A2B3009574), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C0978).
Author Contributions
Conceptualization: H.B. and K.H.K.; investigation: K.H.K., M.K., J.L., and H.N.J.; resources: S.H.K.; supervision: H.B.; writing—original draft: K.H.K.; writing—review and editing: H.B. and K.H.K.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This study analyzes the therapeutic effects of phospholipase A2 compared with melittin isolated from honey bee venom, against Parkinson’s disease. The bee venom extracts containing high phospholipase A2 exhibit strong neuroprotective effects.
References
- 1.Bertram L., Tanzi R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Investig. 2005;115:1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan J., Fu Q., Cheng L., Zhai M., Wu W., Huang L., Du G. Inflammatory response in Parkinson’s disease (review) Mol. Med. Rep. 2014;10:2223–2233. doi: 10.3892/mmr.2014.2563. [DOI] [PubMed] [Google Scholar]
- 3.Brochard V., Combadiere B., Prigent A., Laouar Y., Perrin A., Beray-Berthat V., Bonduelle O., Alvarez-Fischer D., Callebert J., Launay J.M., et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis E.A., Cao J., Hsu Y.H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.Y., Kang S.S., Kim J.H., Bae C.S., Choi S.H. Inhibitory effect of whole bee venom in adjuvant-induced arthritis. In Vivo. 2005;19:801–805. [PubMed] [Google Scholar]
- 6.Silva J., Monge-Fuentes V., Gomes F., Lopes K., dos Anjos L., Campos G., Arenas C., Biolchi A., Goncalves J., Galante P., et al. Pharmacological alternatives for the treatment of neurodegenerative disorders: Wasp and bee venoms and their components as new neuroactive tools. Toxins. 2015;7:3179–3209. doi: 10.3390/toxins7083179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung E.S., Lee G., Lee C., Ye M., Chung H.S., Kim H., Bae S.J., Hwang D.S., Bae H. Bee venom phospholipase A2, a novel Foxp3+ regulatory T cell inducer, protects dopaminergic neurons by modulating neuroinflammatory responses in a mouse model of parkinson’s disease. J. Immunol. 2015;195:4853–4860. doi: 10.4049/jimmunol.1500386. [DOI] [PubMed] [Google Scholar]
- 8.Ye M., Chung H.S., Lee C., Yoon M.S., Yu A.R., Kim J.S., Hwang D.S., Shim I., Bae H. Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J. Neuroinflamm. 2016;13:10. doi: 10.1186/s12974-016-0476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghuraman H., Chattopadhyay A. Melittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- 10.He F., Balling R. The role of regulatory T cells in neurodegenerative diseases. Wiley interdisciplinary reviews. Syst. Biol. Med. 2013;5:153–180. doi: 10.1002/wsbm.1187. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y., Qi B., Xu W., Ma B., Li L., Chen Q., Qian W., Liu X., Qu H. Clinical correlation of peripheral CD4+cell subsets, their imbalance and Parkinson’s disease. Mol. Med. Rep. 2015;12:6105–6111. doi: 10.3892/mmr.2015.4136. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds A.D., Stone D.K., Hutter J.A., Benner E.J., Mosley R.L., Gendelman H.E. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson′s disease. J. Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J., Yamane H., Paul W.E. Differentiation of effector CD4 T cell populations. Ann. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awad K., Abushouk A.I., AbdelKarim A.H., Mohammed M., Negida A., Shalash A.S. Bee venom for the treatment of Parkinson′s disease: How far is it possible? Pharmacother. Biomed. Pharmacother. 2017;91:295–302. doi: 10.1016/j.biopha.2017.04.065. [DOI] [PubMed] [Google Scholar]
- 15.Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 16.De Souza J.M., Goncalves B.D.C., Gomez M.V., Vieira L.B., Ribeiro F.M. Animal toxins as therapeutic tools to treat neurodegenerative diseases. Front. Pharmacol. 2018;9:145. doi: 10.3389/fphar.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stojkovska I., Wagner B.M., Morrison B.E. Parkinson’s disease and enhanced inflammatory response. Exp. Biol. Med. 2015;240:1387–1395. doi: 10.1177/1535370215576313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee G., Bae H. Anti-inflammatory applications of melittin, a major component of bee venom: Detailed mechanism of action and adverse effects. Molecules. 2016;21:616. doi: 10.3390/molecules21050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., Guan S.M., Sun W., Fu H. Melittin, the major pain-producing substance of bee venom. Neurosci. Bull. 2016;32:265–272. doi: 10.1007/s12264-016-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue H.Y., Fujita T., Kumamoto E. Phospholipase A2 activation by melittin enhances spontaneous glutamatergic excitatory transmission in rat substantia gelatinosa neurons. Neuroscience. 2005;135:485–495. doi: 10.1016/j.neuroscience.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 21.Jackson-Lewis V., Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 22.West M.J. New stereological methods for counting neurons. Neurobiol. Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-O. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa N., Hirose Y., Ohara S., Ono T., Watanabe Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res. Commun. Chem. Pathol. Pharmacol. 1985;50:435–441. [PubMed] [Google Scholar]