Abstract
Cell surface carbohydrates, termed “glycans,” are ubiquitous posttranslational effectors that can tune cancer progression. Often aberrantly displayed or found at atypical levels on cancer cells, glycans can impact essentially all progressive steps, from malignant transformation to metastases formation. Glycans are structural entities that can directly bind promalignant glycan-binding proteins and help elicit optimal receptor–ligand activity of growth factor receptors, integrins, integrin ligands, lectins, and other type-1 transmembrane proteins. Because glycans play an integral role in a cancer cell’s malignant activity and are frequently uniquely expressed, preclinical studies on the suitability of glycans as anticancer therapeutic targets and their promise as biomarkers of disease progression continue to intensify. While sialylation and fucosylation have predominated the focus of cancer-associated glycan modifications, the emergence of blood group I antigens (or I-branched glycans) as key cell surface moieties capable of modulating cancer virulence has reenergized investigations into the role of the glycome in malignant progression. I-branched glycans catalyzed principally by the I-branching enzyme GCNT2 are now indicated in several malignancies. In this Perspective, the putative role of GCNT2/I-branching in cancer progression is discussed, including exciting insights on how I-branches can potentially antagonize the cancer-promoting activity of β-galactose–binding galectins.
Keywords: GCNT2, poly-N-acetylglucosamine, cancer-associated glycans, galectins, I-branching
While a cancer cell’s ability to proliferate, survive, generate a vascular bed, adapt to metabolic stress, evade the immune system, and metastasize are widely considered hallmarks of cancer (1), the dysregulated assembly and structure of glycans on cancer cells is still reluctantly acknowledged (2). However, altered cancer cell glycosylation can regulate numerous malignancy-associated pathways, including cell proliferation, death, migration/invasion, angiogenesis, metastasis, and immune evasion (3–5). Glycans represent the unifying “structural” thread through these functional activities, critical to the development and progression of cancer. By controlling cellular protein stability, membrane dynamics, subcellular trafficking, homo/heterophilic interactions, and extrinsic/intrinsic lectin-binding activities, cancer-associated glycans are uniquely poised to impact all virulent pathways. In this Perspective, established cancer-associated glycans and their roles in cancer will be contextualized to an emerging cancer glycomic feature characterized by blood group I-antigen (or I-branched glycans) and I-branching enzymatic activity of β1,6 N-acetylglucosaminyltransferase 2, GCNT2. Beyond highlighting how GCNT2/I-branched glycans regulate cancer cell activities, their putative role in modulating the functional activities of protumorigenic galactose-binding galectins will also be introduced.
Established Cancer-Associated Glycome Features
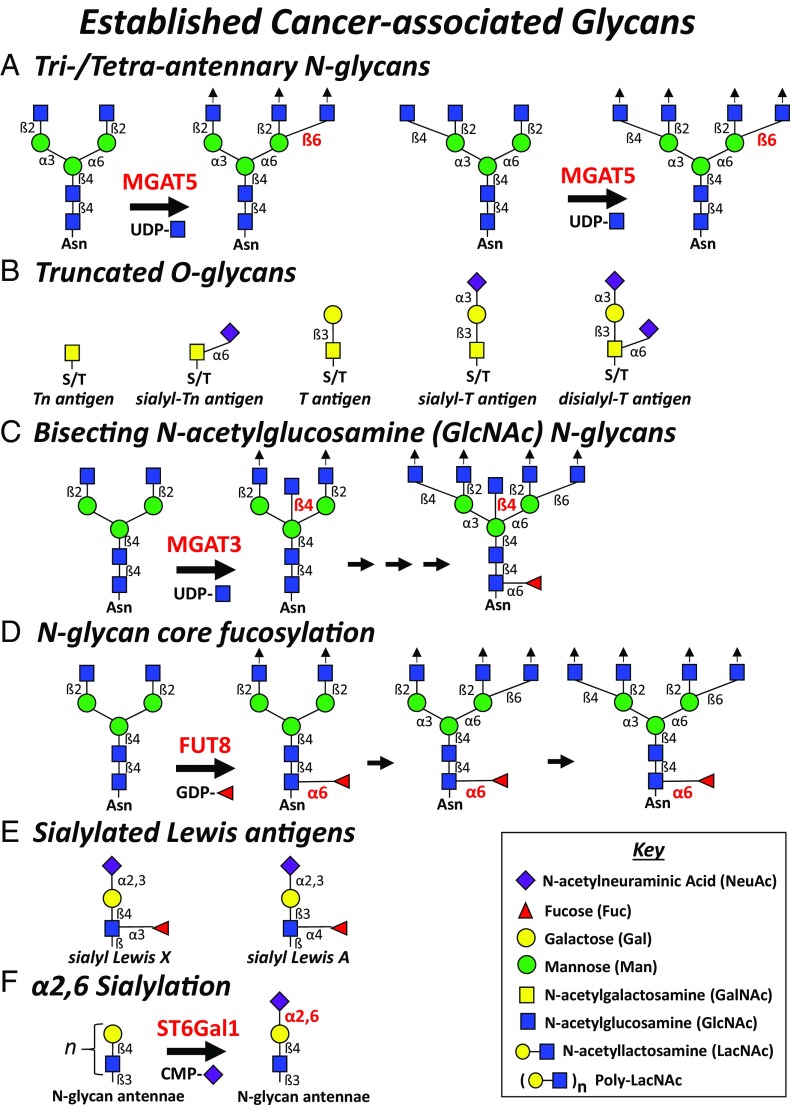
Glycans as markers of malignancy were first described at least 6 decades ago (6–8). The cancer glycomics field has slowly progressed from methods using a dearth of anticarbohydrate antibodies or plant lectins that detect blood group/oncofetal antigens and glycan peculiarities on cancer cells to more precise modalities using matrix-assisted laser/desorption ionization time-of-flight mass spectrometry on asparagine (N)-linked glycans released from cancer cell surfaces (9). Together, these technologies have provided several key discoveries on cancer cell glycan phenotypes characterized by bulky tri/tetra-antennary N-glycans, truncated serine/threonine (O)-linked glycans, bisecting N-acetylglucosamine (GlcNAc) N-glycans, N-glycan core fucosylation, sialylated Lewis antigens, and α2,6 sialylation. These cancer-associated glycans have been shown to subtly tune a cancer cell’s ability to proliferate, survive, invade, evade the immune system, and form distant metastases (10). Expression of cancer-associated glycans is conspicuously contingent on a distinct cell type with lineage-specific gene-expression patterns uniquely leveraged upon cellular transformation and malignant progression. These common cancer glycome features and their impact on distinct cancer subtypes are briefly described as follows.
Tri/Tetra-Antennary N-Glycans.
One of the most impactful posttranslational modifications on Golgi-derived membrane and secreted proteins is N-glycosylation. Notably, the enzymatic activity of α-mannosyl-β1,6 N-acetylglucosaminyltransferase-V (GnT-V; MGAT5) generates a bulky tri/tetra-antennary N-glycan species that can modify a protein’s half-life, stability, membrane dynamics, extracellular-binding partners, and functional activity (Fig. 1A). Not surprisingly, the heightened expression and utilization of MGAT5 by cancer cells have long been recognized key features in the synthesis of cancer-associated glycans. There is a preponderance of experimental evidence showing that elevations in MGAT5 and resultant large tri/tetra-antennary N-glycans can affect cancer cell virulence: MGAT5 expression promotes homo-/heterotypic adhesion and migratory activity, tumorigenicity, and metastasis in mouse models of breast and lung cancer (11–13). Specific MGAT5 N-glycan–dependent alterations on gastric cancer cells cause destabilization and aberrant membrane localization of E-cadherin and of adherens-junctions that impair homotypic cell–cell aggregation (14). Enforced MGAT5 overexpression in fibrosarcoma cells compromises N-cadherin clustering and signaling activity and increases cell motility via phosphorylation of catenins (15) and reduces α5β1 clustering to enhance migration and invasion (16). Interestingly, elevations in MGAT5 and tetra-antennary N-glycan levels correspond better with the fibronectin integrin receptor-mediated adhesion and motility of a metastatic melanoma cell line compared with the matching localized melanoma cell line variant (17). What is increasingly associated with MGAT5-modified N-glycans is that resultant tri/tetra-antennae often contain N-acetyllactosamine (LacNAc) moieties that bind galectins, form organized lattices, and accentuate promalignant activity of growth factor receptor tyrosine kinases (RTK) and integrins (3, 18, 19).
Fig. 1.
Established cancer-associated glycans. The following cell surface carbohydrates on N- or O-glycans and their enzymatic regulators (in red) and respective nucleotide-sugar donor play key roles in cancer progression: (A) tri/tetra-antennary N-glycans (MGAT5), (B) truncated O-glycans, (C) bisecting GlcNAc N-glycans (MGAT3), (D) N-glycan core fucosylation (FUT8), (E) sialylated Lewis antigens, and (F) α2,6 sialylation (ST6Gal1).
Truncated O-Glycans.
O-glycosylations, another major Golgi-derived protein glycosylation modification, are represented by a series of 8 diverse core structures. O-glycan biosynthesis is initiated by addition of N-acetylgalactosamine (GalNAc) by one of 20 polypeptide N-acetylgalactosaminyltransferase family members to form a simple Tn antigen moiety. In cancer, enzymatic extension of Tn antigen with N-acetylneuraminic acid (NeuAc) or galactose (Gal) to generate sialo-Tn or core 1 O-glycans (T antigen) (Fig. 1B) or with β1,6 GlcNAc to build core 2 O-glycans is often dysregulated and associated with numerous malignancies (20, 21). The action of core 1 β1,3 galactosyltransferase 1 (C1GalT1) with the core 1 synthase chaperone Cosmic; α-GalNAc-α2,6 sialyltransferases-1, -2, -3 and -4 (ST6GalNAc1-4); α2,3 sialyltransferase 1 (ST3Gal-1); or core 2 β1,6 N-acetylglucosaminyltransferases 1 and 2 (GCNT1 or 3) are synthetically positioned to compete for these budding Tn and core 1 O-glycan acceptors, which are often aberrantly expressed and commonly related to cancer progression and poor prognosis (20, 21). These enzymes function sequentially and often in competition for the same glycan acceptor to produce structurally diverse O-glycan species. For example, elevations in ST6GalNAc enzymes or expression of mutant nonfunctional Cosmic increase levels of sialo-Tn, whereas reductions in ST6GalNAc enzymes heighten core 2 O-glycan levels. Whether overexpressed or down-regulated depending on the cancer subtype, these O-glycan–modifying enzymes can function as critical biosynthetic regulators of siglec- or galectin-binding O-glycosylations. Cancer cells harness their dysregulated glycoenzyme signatures to preferentially yield truncated O-glycans, sialo-Tn, or sialo-core 1 or extended core 2 O-glycans, translating to siglec- or galectin-dependent malignant behaviors, respectively (21).
Cancer-associated truncated O-glycans have been directly linked with breast (22), ovarian (23), gastric (24, 25), colorectal (26), and pancreatic (27) malignancies and have been shown to impact several oncogenic features, including cell adhesion, migration and invasion, and immunoregulation (28). Furthermore, cancer-associated truncated O-glycans (or lack thereof) are also integral in modifying the binding activities of galectin (Gal)-1 and Gal-3 and of tumor-associated macrophage siglec-15 that, upon binding, render an intrinsic malignant activity or a TGF-β–dependent protumor immune microenvironment, respectively (29–32). That is, reductions in truncating O-glycan–modifying ST6GalNAc1-4 can elevate Gal-1 binding extended poly-LacNAc core 2 O-glycans, while elevations in these enzymes can increase Gal-3 binding core 1 O-glycans to help confer growth, adhesive and metastatic seeding activities (29–31).
Bisecting GlcNAc N-Glycans.
As noted above, aberrancies in complex N-glycan processing are a hallmark glycosylation phenotype in cancer cells. Hybrid or biantennary complex N-glycans can be bisected with GlcNAc by β-mannosyl-β1,4 N-acetylglucosaminyltransferase-III (GnT-III; MGAT3) (Fig. 1C). While this GlcNAc addition is not typically elongated, it can theoretically impart molecular rigidity or a “spacer” moiety that affects how N-glycosylation impacts a protein’s function. So, depending on cancer cell type, this N-glycan maturation step can either compromise or promote malignant activities. Lung metastatic activity of murine melanomas is lowered by enforcing MGAT3 expression (33); cancer cell growth factor receptor signaling is attenuated (34–36); and absence of MGAT3 in murine mammary tumors increases tumor growth, migration, and metastasis, whereas overexpression of MGAT3 inhibits early mammary tumor development and tumor cell migration (36). Bisecting GlcNAcs have also been shown to alter cancer cell E-cadherin and integrin receptor stability and function (17, 37–39) and boost Notch receptor activity related to ovarian cancer progression (40).
N-Glycan Core Fucosylation.
Cell surface α1,3/4 fucosylation is best known for generating sialylated Lewis antigens, critical for cancer cell binding to endothelial (E)-selectin, vascular adhesion, and seeding in distant tissues (41, 42). However, more recent data suggest that α1,6 fucosylation of the most proximal GlcNAc in the N-glycan chitobiose core by α1,6 fucosyltransferase 8 (FUT8) (Fig. 1D) is a key structure regulating the function of cancer cell membrane receptors. When FUT8 gene expression and resultant α1,6 fucosyl moieties are elevated, breast cancer cells exhibit an enhanced ability to signal through TGF-β receptor pathway and undergo malignancy-associated epithelial to mesenchymal transition and related metastatic activities (43). Similarly, core N-glycan α1,6 fucosylation on lung cancer cells enhances EGFR-dependent signaling activity and regulates E-cadherin–dependent nuclear translocation of β-catenin (44, 45) and, when silenced on melanoma cell adhesion molecules, suppresses invasion and tumor dissemination (46).
Sialylated Lewis Antigens.
Sialylated Lewis antigens, α2,3 sialyl Lewis A (sLeA) and α2,3 sialyl Lewis X (sLeX), are commonly elevated on aggressive cancer cells and linked to metastatic potential (10, 42, 47) (Fig. 1E). The most widely recognized function of sLeX/A on cancer cells is its ability to bind vascular endothelial (E)- and platelet (P)-selectins and promote vascular endothelial cell adhesion to help deliver circulating cancer cells to distant tissues. Cancer cell–selectin binding interactions characteristically yield tethering and rolling events on the luminal aspect of postcapillary venules that precede firm adherence and tissue entry, analogous to the leukocyte homing paradigm (48). While most cancer cells are enzymatically equipped to generate terminal α2,3 sialyl LacNAc moieties by ST3Gal3, ST3Gal4, and ST3Gal6 at the termini of their N-glycans, core 2 O-glycans, and neolacto glycosphingolipids, selectin-binding proficiency is consummated by the action of α1,3/4 fucosyltransferases (FUT3–7, 9–11) to synthesize sLeX or sLeA antigens (3, 41, 47, 49–56). Whereas FUT3 and, to a minor extent, FUT5 exhibit α1,4 fucosyltransferase activity for sLeA synthesis, FUT3, FUT5–7, and FUT9 predominantly provide the α1,3 fucosyltransferase activity necessary for synthesizing sLeX and related selectin-binding activities (57–61). Uniformly, decades of experimental and correlative analyses indicate that a high level of sLeX and sLeA antigens inversely correlates with the survival of patients with most if not all types of malignancies. Cancer of the colon (62–68), breast (69–72), prostate (41, 51, 52, 56, 73, 74), multiple myeloma (75), and pancreas (76–78) commonly leverage their elevated sLeX/A moieties to mount shear-resistant, vascular E/P-selectin–mediated adhesion and enhance metastatic potential.
α2,6 Sialylation.
N-glycan antennae terminated with α2,6 NeuAc moieties (Fig. 1F), principally governed by the action of β-galactosyl-α2,6 sialyltransferase (ST6Gal-1), are becoming one of the more critical glycomic features correlated with malignant and metastatic progression (79). In colon (80, 81), mammary (82), ovarian (83–88), liver (89–91), and pancreatic (84–86, 88, 92) cancers, α2,6 sialylation can enhance several malignancy-associated activities. Cancer cell α2,6 sialylation can elicit its functional activity on N-glycosylated membrane proteins via a binding moiety (e.g., ligand for siglec-2/CD22) or by imparting optimal stability, membrane organization, or homo/heterophilic interactive capacity. When β1 integrins on cancer cells display ST6Gal-1–synthesized α2,6 sialylated moieties, adhesive and migratory activities and related focal adhesion kinase activities are accentuated (80, 82, 89–91). Protection from chemotherapeutics, including EGFR-targeted therapy, and Fas-mediated death, promotion of survival pathways, and evasion of hypoxic stress are also boosted in cancer cells via ST6Gal-1–dependent sialylation (83–87, 92). Beyond these malignancy-associated traits, tumor-initiating cell activity and expression of stem cell markers have been correlated positively with ST6Gal-1 expression (88).
Emergence of I-Branched Glycans and β1,6 I-Branching Enzyme GCNT2 as Modulators of Cancer Progression
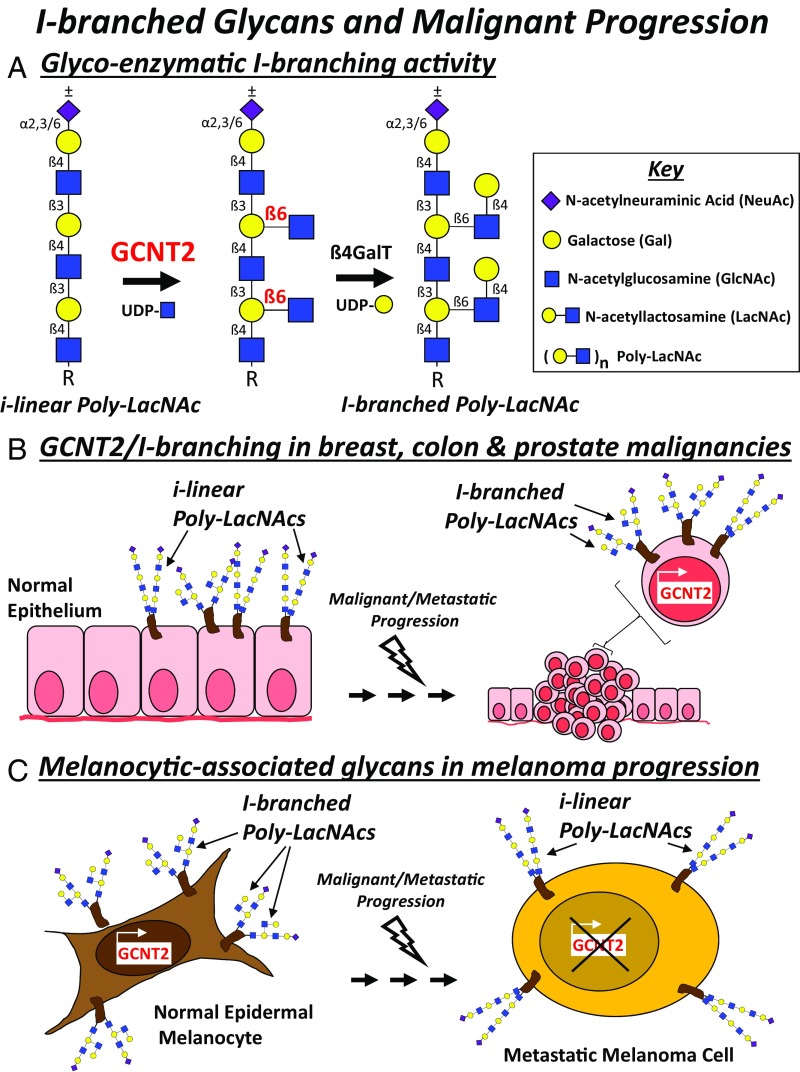
Sialylation, fucosylation, number/length of N-glycan antennae, and O-glycan complexity have predominated the focus of the cancer glycomics research field. However, more recent studies on the identity of cancer-associated glycans have revealed a critical new role for blood group I-antigen (I-branches) in cancer progression. Synthesis of I-branched glycans, Galß1,4GlcNAc moieties linked in a β1,6 conformation to internal galactose residues on fetal i-antigen [linear poly-LacNAc; (Galβ1,4-GlcNAcβ1,3)n], is chiefly initiated by the developmental I-branching GCNT2 (Fig. 2A) (93). A linear poly-LacNAc synthesized by the repeating action of β3GnTs and β4GalTs (94) provides its internal galactose residues as an acceptor for the β1,6 GlcNAc transferring action of GCNT2 and subsequent ubiquitous β1,4Gal capping activity of β4GalTs (9, 95). GCNT2 exists as isoforms A, B, and C (also referred to as variants 1, 2, and 3) and governs the conversion of linear poly-LacNAcs commonly expressed on fetal and cord blood cells to I-branched glycans normally found on adult erythrocytes, mucosal epithelia, and cells of the eye and olfactory bulb (93, 94, 96–101). Ineffective I-branch conversion has been linked to loss of GCNT2 expression and early-onset congenital cataracts (102). In cancer, GCNT2/I-branched glycans have been correlated both positively (103–105) and negatively (9, 106) with cancer progression, regulating malignancy-associated adhesive, migratory, signaling, growth, and metastatic activities as follows.
Fig. 2.
I-branched glycans and malignant progression. (A) I-branching activity of GCNT2 and subsequent β1,4 galactosyltransferase (β4GalT) activity on i-linear poly-LacNAc is depicted. The current models of GCNT2-regulated I-branched glycans driving the malignant or metastatic progression of breast, colon, and prostate cancer (B) or, alternatively, slowing the progression of malignant melanomas (C) are illustrated.
Breast Cancer.
The seminal investigation on the role of GCNT2 in breast cancer reveals a strong relationship between functional expression of GCNT2 and breast cancer metastasis (103). Expression array and immunohistochemical datasets show strong GCNT2 expression on metastatic breast cancer cell lines, high-grade breast tumors and tumors of a basal-like histotype, and breast cancer metastases (103), implicating GCNT2 expression with breast cancer progression. Functionally, studies using GCNT2-enforced or -silenced breast cancer cell lines provide strong evidence that high GCNT2 levels elicit greater cell migratory, invasive, and metastatic activities, including a promoting role in TGF-β–induced epithelial to mesenchymal transition and in AKT and ERK survival/proliferation signaling pathways. Whether and how GCNT2-synthesized I-branched glycans impact distinct breast cancer growth factor receptors, integrins, and other membrane proteins, notably cell adhesion molecules, involved in malignancy-associated extracellular or intracellular signaling pathways in breast cancer, however, are still unknown. How GCNT2 gene and isoform splicing are regulated in breast cancer cells is also undefined.
Colon Cancer.
Using a comparative real-time qPCR for glycogene approach on primary colorectal cancer and normal colonic mucosal tissue specimens, data show that GCNT2 gene expression is severely depressed in the cancer tissues compared with normal mucosa (106). In fact, all 3 GCNT2 gene variants are suppressed in colorectal cancer tissues. Subsequent experiments on the epigenetic factors putatively regulating GCNT2 expression reveal that these 3 GCNT2 gene variants, which are controlled by unique promoter regions, appear to be hypermethylated in GCNT2 depressed cell lines, suggesting that GCNT2 expression is suppressed by methylation (106). However, in lymph node metastases specimens, closer scrutiny and associative analysis of the individual GCNT2 variant methylation status shows that GCNT2 variant 2 promoter is, in fact, hypomethylated compared with normal colonic mucosa (106). So, while GCNT2 gene levels are depressed from normal mucosa to malignant transition, hypomethylation of GCNT2 variant 2 in lymph node metastases suggests that epigenetic regulation of GCNT2 may help predict metastatic potential. Whether elevated levels of the I-branched glycan correspond with GCNT2 hypomethylation to influence malignancy-associated pathways in colorectal cancer disease progression is still unknown. However, an interesting follow-up study on the role of GCNT2 on the malignant activity of colorectal cancer shows that GCNT2 and its I-branched glycan product, indeed, accelerate epithelial-to-mesenchymal transition among other malignant traits and are regulated negatively by the expression of microRNA, miR-199a/b-5p (105).
Prostate Cancer.
Similar to GCNT2’s association with the malignant and metastatic activity of breast and colon cancer, respectively, GCNT2 expression in primary prostatic cancer tissue localizes at invasive protrusions and directly correlates with a higher risk of prostate-specific antigen recurrence after radical prostatectomy (104). Furthermore, experiments using GCNT2-overexpressing or -silenced prostate cancer cell lines show that high GCNT2/I-branching levels encourage higher invasive and migratory activity, partially through α5β1 integrin and related signaling activity. However, I-branching in these cell line models does not appear to directly affect α5β1 heterodimerization or fibronectin-binding affinity, suggesting that global cell surface I-branching can alter membrane protein function via indirect glycocalyx mechanisms (104). Of note, GCNT2/I-branching in DU145 prostate cancer cells appears to largely occur on glycolipids and partially on O-glycans (104), demonstrating the ability of GCNT2 to act on poly-LacNAcs present on N- and O-glycans as well as on glycolipids.
Malignant Melanoma.
The most recent study on the role of I-branched glycans and cancer reveals a striking role for GCNT2/I-branching in melanoma progression (9). While prior reports in breast, colon, and prostate cancer indicate an oncogenic role for GCNT2/I-branching, new data suggest that, in contrast, GCNT2/I-branching acts as a putative tumor suppressor, inhibiting several malignancy-associated activities in melanoma cells and xenografts (9). N-glycan antennae on normal epidermal melanocytes almost uniformly display I-branches, whereas primary melanomas variably express I-branched glycans and metastatic melanomas mostly lack I-branches concomitant with depressed GCNT2 expression (9). Data-mining analysis and immunohistochemical analysis of GCNT2 in clinical primary and metastatic melanoma specimens establish a strong inverse relationship between GCNT2 expression and melanoma metastases, suggesting that GCNT2 expression (or loss thereof) could help serve as a biomarker and predict clinical outcome. While the regulation of GCNT2 expression in normal and malignant melanocytes has not yet been addressed, biochemical data show that GCNT2 catalyzes global I-branch synthesis to N-glycans on several classes of membrane proteins expressed by melanoma cells (9). The presence of GCNT2-synthesized I-branches on growth factor RTKs and α/β integrin chains, such as insulin-like growth factor 1 receptor (IGF1R) and α4-, β1-, and β3-chains, can inhibit IGF1 and extracellular matrix-binding activities and attenuate related downstream signaling and prosurvival factors in melanoma cells (9).
Mechanistically, the I-branches on normal and malignant melanocytes do not appear to contain sialylated or fucosylated moieties, indicating that the effects of I-branching are likely not through ancillary sialo-fucosylations, but rather as bulky capping moieties causing either direct or indirect steric interference of receptor–ligand interactions. Further studies are needed to explore: (i) how GCNT2 expression is regulated in melanomas, (ii) whether GCNT2 expression can predict which patients with thick primary melanomas will (or will not) metastasize, and (iii) how I-branches antagonize RTK/integrin function in melanoma cells. To help cement GCNT2’s negative regulatory role in melanoma progression in vivo, additional murine studies could be performed using an inducible melanoma mouse model (107) in a wild-type or GCNT2 null background.
Implications for I-Branched Glycans Controlling Galectin-Binding Activities
In that I-branched glycans—Galß1,4GlcNAc moieties linked in a β1,6 conformation to internal galactose residues on linear poly-LacNAcs—can potentially serve as β-galactoside-binding determinants for galectins and that galectins possess key immunoregulatory and protumorigenic functions, GCNT2/I-branching activity could function as a critical regulator of cancer progression. Because β3GnT extension activity is necessary for linear poly-LacNAc synthesis, β3GnT(s) and GCNT2 could theoretically compete to dually regulate the synthesis of linear vs. I-branched poly-LacNAc. GCNT2/I-branching activity, however, appears to serve as an end-stage glycosylation event. GCNT2 and B3GNT2, when coexpressed, have a cooperative relationship, in which I-branched poly-LacNAcs are synthesized from linear poly-LacNAcs and the level of I-branched poly-LacNAcs directly correlates with GCNT2 expression (9, 94, 95). Such end-stage glycosylation events, akin to α2,6 sialylation and α1,3 fucosylation, often have profound effects on galectin-binding activities (21, 108–110). Indeed, data in studies on GCNT2/I-branching in melanoma progression (9) reveal a potential role for GCNT2/I-branching activity as a native inhibitor of Gal-3 binding activity. As opposed to Gal-1, Gal-3 binds linear poly-LacNAcs on melanoma cells more avidly than to GCNT2-synthesized I-branched glycans, which is consistent with Gal-3’s preference for linear poly-LacNAcs on glycan microarrays (111, 112). Additional observations from our laboratory demonstrate that GCNT2/I-branching activity also blunts Gal-9 ligand activities in numerous melanoma cell lines. Because melanoma progression is directly related to Gal-3 expression in melanoma cells (113), melanoma-intrinsic GCNT2 action could theoretically offset functional activities triggered by Gal-3 binding. That is, in melanoma patients with moderate- to late-stage disease where GCNT2 expression is progressively lost (9), renewing GCNT2/I-branching activity could potentially antagonize Gal-3–dependent malignant activities and slow melanoma progression. This therapeutic notion is dependent, however, on the future discovery and engagement of regulatory factors controlling GCNT2 expression.
Coincident with evidence of GCNT2/I-branching antagonizing melanoma galectin ligand activity, intensive glycomic interrogation of human B cell subsets depicts GCNT2 as a major factor controlling Gal-9 binding activity (95). In contrast to robust binding on naïve and memory B cells, Gal-9 binding to germinal center B cells is markedly less due, in part, to up-regulation of GCNT2/I-branching activity. Gal-9, in the absence of I-branched glycans, imposes a regulatory activity via CD45 binding and downstream suppression of B cell receptor signaling and cell activation (95). Notably, our laboratory also finds that elevated GCNT2/I-branching activity in human B cell lines associates with depressed Gal-3 binding, suggesting that GCNT2 indeed elicits its galectin inhibitory effects across normal and malignant settings.
Collectively, the putative tumor-intrinsic and immunological consequences of GCNT2/I-branching on Gal-3 and Gal-9 function provide opportunities for anticancer therapeutic targeting of GCNT2. Whether tuning galectin-dependent immunoregulation of antitumor immune cells or malignancy-associated activities intrinsic to cancer cells, GCNT2/I-branching could provide an attractive therapeutic target to the burgeoning field of cancer immunotherapy.
Conclusions
The field of glycobiology is now penetrating the interests of most cancer researchers, because nearly all malignancy-associated pathways are impacted by glycan modification. As the methods for detection, isolation, and characterization of glycans and their enzymatic regulators continue to improve, cancer glycomics is poised to provide exciting new insights on the pathogenesis of cancer. Studies on cancer cell surface sialylation, fucosylation, N-/O-glycan maturity and now i-linear/I-branched poly-LacNAcs dominate much of our current interests due to their importance in uncovering regulators of membrane protein folding, clustering, organization, recycling, lectin-/ligand-binding, and signaling. Moreover, growth factor RTKs, such as EGFR, IGF1R, and VEGFR2, along with other Ig superfamily members and integrins, are some of the most vulnerable biosynthetic targets due to their abundance of glycans (9, 11, 13, 16, 19, 30, 45, 73, 84, 114, 115). EGFR contains N-glycans that comprise ∼30% of its molecular mass (114, 115), and α/β-chains of IGF1R and all α/β-integrin chains are heavily N-glycosylated (9), implicating RTKs and integrins governing growth/survival activity as prime candidates for modulation by a cancer glyco-phenotype. Where GCNT2/I-branching plays a role in cancer progression, these protein candidates can also potentially be influenced by Gal-3– or -9–binding activities that are known regulators of membrane protein dynamics and function.
Importantly, through the pioneering efforts to reengage the host immune system to fight cancer, cancer-associated glycans can also be targeted in these revitalized anticancer therapeutic strategies. Development of vaccines, anticarbohydrate-drug conjugate Abs, and chimeric antigen receptor T cells against cancer-specific glycans are rapidly evolving as promising cancer immunotherapeutic approaches (116–120). As reviewed here, there are several cancer-associated glycan features that can be leveraged to design rational drug or immune system targets. To maximize antitumor activities and overcome mechanisms of cancer neoantigen evolution, multiple cancer glycome features/glyco-enzymatic regulators should be targeted as more standard treatment paradigm. The emergence of I-branched glycans and enzymatic regulator GCNT2 now provides additional opportunities to target glycome peculiarities of cancer and elicit anticancer activity. Whether boosting or blunting GCNT2/I-branching activity (depending on GCNT2’s effects on a given cancer subtype) (Fig. 2 B and C), anticancer glycan therapeutics is now armed with new glycome target. Furthermore, monitoring GCNT2/I-branch expression through facile immunohistochemical methods can potentially be used to predict metastatic potential and help guide long-term treatment decisions. There are still unexplored aspects of GCNT2 action, such as gene and enzymatic regulation and the impact of I-branching on galectin-binding sensitivities, that need to be explored to ensure the most appropriate mode of intervention with minimal side effects.
Acknowledgments
I thank Drs. Jenna Geddes Sweeney, Nicholas Giovannone, Aristotelis Antonopoulos, and Stuart Haslam for their early insights and groundbreaking data on the structure and function of GCNT2/I-branching in cancer and immunity. This Perspective was supported by the NIH/National Cancer Institute Alliance of Glycobiologists for Cancer Research: Biological Tumor Glycomics Laboratory U01 CA225644 (to C.J.D.) and a Mizutani Foundation for Glycoscience Research grant (to C.J.D.). The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Vajaria B. N., Patel P. S., Glycosylation: A hallmark of cancer? Glycoconj. J. 34, 147–156 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Pinho S. S., Reis C. A., Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Munkley J., Elliott D. J., Hallmarks of glycosylation in cancer. Oncotarget 7, 35478–35489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuster M. M., Esko J. D., The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 5, 526–542 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Ladenson R. P., Schwartz S. O., Ivy A. C., Incidence of the blood groups and the secretor factor in patients with pernicious anemia and stomach carcinoma. Am. J. Med. Sci. 217, 194–197 (1949). [DOI] [PubMed] [Google Scholar]
- 7.Hakomori S. I., Murakami W. T., Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc. Natl. Acad. Sci. U.S.A. 59, 254–261 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feizi T., Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature 314, 53–57 (1985). [DOI] [PubMed] [Google Scholar]
- 9.Sweeney J. G., et al. , Loss of GCNT2/I-branched glycans enhances melanoma growth and survival. Nat. Commun. 9, 3368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glavey S. V., et al. , The cancer glycome: Carbohydrates as mediators of metastasis. Blood Rev. 29, 269–279 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Demetriou M., Nabi I. R., Coppolino M., Dedhar S., Dennis J. W., Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V. J. Cell Biol. 130, 383–392 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seberger P. J., Chaney W. G., Control of metastasis by Asn-linked, β1-6 branched oligosaccharides in mouse mammary cancer cells. Glycobiology 9, 235–241 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Granovsky M., et al. , Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6, 306–312 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Pinho S. S., et al. , E-cadherin and adherens-junctions stability in gastric carcinoma: Functional implications of glycosyltransferases involving N-glycan branching biosynthesis, N-acetylglucosaminyltransferases III and V. Biochim. Biophys. Acta 1830, 2690–2700 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Guo H. B., Lee I., Kamar M., Pierce M., N-acetylglucosaminyltransferase V expression levels regulate cadherin-associated homotypic cell-cell adhesion and intracellular signaling pathways. J. Biol. Chem. 278, 52412–52424 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Guo B., Lee I., Kamar M., Akiyama S. K., Pierce M., Aberrant N-glycosylation of β1 integrin causes reduced α5β1 integrin clustering and stimulates cell migration. Cancer Res. 62, 6827–6845 (2002). [PubMed] [Google Scholar]
- 17.Pocheć E., et al. , Expression of integrins α3β1 and α5β1 and GlcNAc β1,6 glycan branching influences metastatic melanoma cell migration on fibronectin. Eur. J. Cell Biol. 92, 355–362 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Di Lella S., et al. , When galectins recognize glycans: From biochemistry to physiology and back again. Biochemistry 50, 7842–7857 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croci D. O., et al. , Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 156, 744–758 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Kudelka M. R., Ju T., Heimburg-Molinaro J., Cummings R. D., Simple sugars to complex disease—Mucin-type O-glycans in cancer. Adv. Cancer Res. 126, 53–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimitroff C. J., Galectin-binding O-glycosylations as regulators of malignancy. Cancer Res. 75, 3195–3202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leivonen M., Nordling S., Lundin J., von Boguslawski K., Haglund C., STn and prognosis in breast cancer. Oncology 61, 299–305 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H., Terao T., Kawashima Y., Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J. Clin. Oncol. 10, 95–101 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Victorzon M., Nordling S., Nilsson O., Roberts P. J., Haglund C., Sialyl Tn antigen is an independent predictor of outcome in patients with gastric cancer. Int. J. Cancer 65, 295–300 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Pinho S., et al. , Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 249, 157–170 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Itzkowitz S. H., et al. , Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer 66, 1960–1966 (1990). [DOI] [PubMed] [Google Scholar]
- 27.Kim G. E., et al. , Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 123, 1052–1060 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Radhakrishnan P., et al. , Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl. Acad. Sci. U.S.A. 111, E4066–E4075 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reticker-Flynn N. E., Bhatia S. N., Aberrant glycosylation promotes lung cancer metastasis through adhesion to galectins in the metastatic niche. Cancer Discov. 5, 168–181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yazawa E. M., et al. , Melanoma cell galectin-1 ligands functionally correlate with malignant potential. J. Invest. Dermatol. 135, 1849–1862 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murugaesu N., et al. , An in vivo functional screen identifies ST6GalNAc2 sialyltransferase as a breast cancer metastasis suppressor. Cancer Discov. 4, 304–317 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Takamiya R., Ohtsubo K., Takamatsu S., Taniguchi N., Angata T., The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 23, 178–187 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura M., Nishikawa A., Ihara Y., Taniguchi S., Taniguchi N., Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc. Natl. Acad. Sci. U.S.A. 92, 8754–8758 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebbaa A., et al. , Gene transfection-mediated overexpression of β1,4-N-acetylglucosamine bisecting oligosaccharides in glioma cell line U373 MG inhibits epidermal growth factor receptor function. J. Biol. Chem. 272, 9275–9279 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Ihara Y., Sakamoto Y., Mihara M., Shimizu K., Taniguchi N., Overexpression of N-acetylglucosaminyltransferase III disrupts the tyrosine phosphorylation of Trk with resultant signaling dysfunction in PC12 cells treated with nerve growth factor. J. Biol. Chem. 272, 9629–9634 (1997). [DOI] [PubMed] [Google Scholar]
- 36.Song Y., Aglipay J. A., Bernstein J. D., Goswami S., Stanley P., The bisecting GlcNAc on N-glycans inhibits growth factor signaling and retards mammary tumor progression. Cancer Res. 70, 3361–3371 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura M., Ihara Y., Matsuzawa Y., Taniguchi N., Aberrant glycosylation of E-cadherin enhances cell-cell binding to suppress metastasis. J. Biol. Chem. 271, 13811–13815 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Kitada T., et al. , The addition of bisecting N-acetylglucosamine residues to E-cadherin down-regulates the tyrosine phosphorylation of beta-catenin. J. Biol. Chem. 276, 475–480 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Isaji T., et al. , Introduction of bisecting GlcNAc into integrin alpha5beta1 reduces ligand binding and down-regulates cell adhesion and cell migration. J. Biol. Chem. 279, 19747–19754 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Allam H., et al. , The glycosyltransferase GnT-III activates Notch signaling and drives stem cell expansion to promote the growth and invasion of ovarian cancer. J. Biol. Chem. 292, 16351–16359 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barthel S. R., et al. , Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc. Natl. Acad. Sci. U.S.A. 106, 19491–19496 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barthel S. R., Gavino J. D., Descheny L., Dimitroff C. J., Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin. Ther. Targets 11, 1473–1491 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu C. F., Wu M. Y., Lin Y. C., Kannagi R., Yang R. B., FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 19, 111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu P., et al. , E-cadherin core fucosylation regulates nuclear beta-catenin accumulation in lung cancer cells. Glycoconj. J. 25, 843–850 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Liu Y. C., et al. , Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc. Natl. Acad. Sci. U.S.A. 108, 11332–11337 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agrawal P., et al. , A systems biology approach identifies FUT8 as a driver of melanoma metastasis. Cancer Cell 31, 804–819.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natoni A., Macauley M. S., O’Dwyer M. E., Targeting selectins and their ligands in cancer. Front. Oncol. 6, 93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biancone L., Araki M., Araki K., Vassalli P., Stamenkovic I., Redirection of tumor metastasis by expression of E-selectin in vivo. J. Exp. Med. 183, 581–587 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim Y. J., Borsig L., Varki N. M., Varki A., P-selectin deficiency attenuates tumor growth and metastasis. Proc. Natl. Acad. Sci. U.S.A. 95, 9325–9330 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varki A., Kannagi R., Toole B., Stanley P., “Glycosylation changes in cancer” in Essentials of Glycobiology, Varki A., et al., Eds. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, ed. 3, 2017). [Google Scholar]
- 51.Dimitroff C. J., et al. , Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res. 65, 5750–5760 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimitroff C. J., Lechpammer M., Long-Woodward D., Kutok J. L., Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer Res. 64, 5261–5269 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Mondal N., et al. , ST3Gal-4 is the primary sialyltransferase regulating the synthesis of E-, P-, and L-selectin ligands on human myeloid leukocytes. Blood 125, 687–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mollicone R., et al. , Activity, splice variants, conserved peptide motifs, and phylogeny of two new alpha1,3-fucosyltransferase families (FUT10 and FUT11). J. Biol. Chem. 284, 4723–4738 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Mondal N., et al. , Glycosphingolipids on human myeloid cells stabilize E-selectin-dependent rolling in the multistep leukocyte adhesion cascade. Arterioscler. Thromb. Vasc. Biol. 36, 718–727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barthel S. R., et al. , Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology 18, 806–817 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buffone A. Jr, et al. , Silencing α1,3-fucosyltransferases in human leukocytes reveals a role for FUT9 enzyme during E-selectin-mediated cell adhesion. J. Biol. Chem. 288, 1620–1633 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carvalho A. S., et al. , Differential expression of alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 42, 80–89 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Sackstein R., The first step in adoptive cell immunotherapeutics: Assuring cell delivery via glycoengineering. Front. Immunol. 9, 3084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mondal N., Buffone A. Jr, Neelamegham S., Distinct glycosyltransferases synthesize E-selectin ligands in human vs. mouse leukocytes. Cell Adhes. Migr. 7, 288–292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mondal N., et al. , Distinct human α(1,3)-fucosyltransferases drive Lewis-X/sialyl Lewis-X assembly in human cells. J. Biol. Chem. 293, 7300–7314 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burdick M. M., Chu J. T., Godar S., Sackstein R., HCELL is the major E- and L-selectin ligand expressed on LS174T colon carcinoma cells. J. Biol. Chem. 281, 13899–13905 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Hanley W. D., Burdick M. M., Konstantopoulos K., Sackstein R., CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res. 65, 5812–5817 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Burdick M. M., McCaffery J. M., Kim Y. S., Bochner B. S., Konstantopoulos K., Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am. J. Physiol. Cell Physiol. 284, C977–C987 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Yoshihama N., et al. , A novel function of CD82/KAI1 in sialyl Lewis antigen-mediated adhesion of cancer cells: Evidence for an anti-metastasis effect by down-regulation of sialyl Lewis antigens. PLoS One 10, e0124743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khatib A. M., Fallavollita L., Wancewicz E. V., Monia B. P., Brodt P., Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res. 62, 5393–5398 (2002). [PubMed] [Google Scholar]
- 67.Köhler S., Ullrich S., Richter U., Schumacher U., E-/P-selectins and colon carcinoma metastasis: First in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. Br. J. Cancer 102, 602–609 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimitroff C. J., et al. , Cell surface n-acetylneuraminic acid alpha2,3-galactoside-dependent intercellular adhesion of human colon cancer cells. Biochem. Biophys. Res. Commun. 256, 631–636 (1999). [DOI] [PubMed] [Google Scholar]
- 69.Carrascal M. A., et al. , A functional glycoproteomics approach identifies CD13 as a novel E-selectin ligand in breast cancer. Biochim. Biophys. Acta Gen. Subj. 1862, 2069–2080 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Carrascal M. A., et al. , Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation, and ERK1/2 and p38 MAPK activation. Mol. Oncol. 12, 579–593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shirure V. S., Reynolds N. M., Burdick M. M., Mac-2 binding protein is a novel E-selectin ligand expressed by breast cancer cells. PLoS One 7, e44529 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Julien S., et al. , Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 71, 7683–7693 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barthel S. R., et al. , Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res. 73, 942–952 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gakhar G., et al. , Circulating tumor cells from prostate cancer patients interact with E-selectin under physiologic blood flow. PLoS One 8, e85143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glavey S. V., et al. , The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood 124, 1765–1776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pérez-Garay M., et al. , α2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. Int. J. Biochem. Cell Biol. 45, 1748–1757 (2013). [DOI] [PubMed] [Google Scholar]
- 77.Remmers N., et al. , Aberrant expression of mucin core proteins and O-linked glycans associated with progression of pancreatic cancer. Clin. Cancer Res. 19, 1981–1993 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakamori S., et al. , Molecular mechanism involved in increased expression of sialyl Lewis antigens in ductal carcinoma of the pancreas. J. Exp. Clin. Cancer Res. 18, 425–432 (1999). [PubMed] [Google Scholar]
- 79.Lu J., Gu J., Significance of beta-galactoside alpha2,6 sialyltranferase 1 in cancers. Molecules 20, 7509–7527 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swindall A. F., Bellis S. L., Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J. Biol. Chem. 286, 22982–22990 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seales E. C., et al. , Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 65, 4645–4652 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Hedlund M., Ng E., Varki A., Varki N. M., Alpha 2-6-Linked sialic acids on N-glycans modulate carcinoma differentiation in vivo. Cancer Res. 68, 388–394 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Britain C. M., Dorsett K. A., Bellis S. L., The glycosyltransferase ST6Gal-I protects tumor cells against serum growth factor withdrawal by enhancing survival signaling and proliferative potential. J. Biol. Chem. 292, 4663–4673 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Britain C. M., Holdbrooks A. T., Anderson J. C., Willey C. D., Bellis S. L., Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J. Ovarian Res. 11, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holdbrooks A. T., Britain C. M., Bellis S. L., ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. J. Biol. Chem. 293, 1610–1622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones R. B., Dorsett K. A., Hjelmeland A. B., Bellis S. L., The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1α signaling. J. Biol. Chem. 293, 5659–5667 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schultz M. J., et al. , ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J. Ovarian Res. 6, 25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schultz M. J., et al. , The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell Transcription factors and confers a cancer stem cell phenotype. Cancer Res. 76, 3978–3988 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang S., et al. , α2,6-linked sialic acids on N-glycans modulate the adhesion of hepatocarcinoma cells to lymph nodes. Tumour Biol. 36, 885–892 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Yu S., et al. , Caveolin-1 up-regulates integrin α2,6-sialylation to promote integrin α5β1-dependent hepatocarcinoma cell adhesion. FEBS Lett. 587, 782–787 (2013). [DOI] [PubMed] [Google Scholar]
- 91.Yu S., et al. , Caveolin-1 up-regulates ST6Gal-I to promote the adhesive capability of mouse hepatocarcinoma cells to fibronectin via FAK-mediated adhesion signaling. Biochem. Biophys. Res. Commun. 427, 506–512 (2012). [DOI] [PubMed] [Google Scholar]
- 92.Chakraborty A., et al. , ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage. J. Biol. Chem. 293, 984–994 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bierhuizen M. F., Mattei M. G., Fukuda M., Expression of the developmental I antigen by a cloned human cDNA encoding a member of a beta-1,6-N-acetylglucosaminyltransferase gene family. Genes Dev. 7, 468–478 (1993). [DOI] [PubMed] [Google Scholar]
- 94.Henion T. R., Schwarting G. A., N-linked polylactosamine glycan synthesis is regulated by co-expression of β3GnT2 and GCNT2. J. Cell. Physiol. 229, 471–478 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Giovannone N., et al. , Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans. Nat. Commun. 9, 3287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wiener A. S., Unger L. J., Cohen L., Feldman J., Type-specific cold auto-antibodies as a cause of acquired hemolytic anemia and hemolytic transfusion reactions: Biologic test with bovine red cells. Ann. Intern. Med. 44, 221–240 (1956). [DOI] [PubMed] [Google Scholar]
- 97.Pruzanski W., Shumak K. H., Biologic activity of cold-reacting autoantibodies (second of two parts). N. Engl. J. Med. 297, 583–589 (1977). [DOI] [PubMed] [Google Scholar]
- 98.Pruzanski W., Shumak K. H., Biologic activity of cold-reacting autoantibodies (first of two parts). N. Engl. J. Med. 297, 538–542 (1977). [DOI] [PubMed] [Google Scholar]
- 99.Fukuda M., Fukuda M. N., Hakomori S., Developmental change and genetic defect in the carbohydrate structure of band 3 glycoprotein of human erythrocyte membrane. J. Biol. Chem. 254, 3700–3703 (1979). [PubMed] [Google Scholar]
- 100.Yu L. C., Twu Y. C., Chang C. Y., Lin M., Molecular basis of the adult i phenotype and the gene responsible for the expression of the human blood group I antigen. Blood 98, 3840–3845 (2001). [DOI] [PubMed] [Google Scholar]
- 101.Magnet A. D., Fukuda M., Expression of the large I antigen forming beta-1,6-N-acetylglucosaminyltransferase in various tissues of adult mice. Glycobiology 7, 285–295 (1997). [DOI] [PubMed] [Google Scholar]
- 102.Yu L. C., et al. , The molecular genetics of the human I locus and molecular background explain the partial association of the adult i phenotype with congenital cataracts. Blood 101, 2081–2088 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Zhang H., et al. , Engagement of I-branching beta-1, 6-N-acetylglucosaminyltransferase 2 in breast cancer metastasis and TGF-beta signaling. Cancer Res. 71, 4846–4856 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mikami J., et al. , I-branching N-acetylglucosaminyltransferase regulates prostate cancer invasiveness by enhancing α5β1 integrin signaling. Cancer Sci. 107, 359–368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chao C. C., et al. , Downregulation of miR-199a/b-5p is associated with GCNT2 induction upon epithelial-mesenchymal transition in colon cancer. FEBS Lett. 591, 1902–1917 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Nakamura K., et al. , Aberrant methylation of GCNT2 is tightly related to lymph node metastasis of primary CRC. Anticancer Res. 35, 1411–1421 (2015). [PubMed] [Google Scholar]
- 107.Dankort D., et al. , Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 41, 544–552 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toscano M. A., et al. , Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 8, 825–834 (2007). [DOI] [PubMed] [Google Scholar]
- 109.Stowell S. R., et al. , Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 283, 10109–10123 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leppänen A., Stowell S., Blixt O., Cummings R. D., Dimeric galectin-1 binds with high affinity to alpha2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J. Biol. Chem. 280, 5549–5562 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Noll A. J., et al. , Galectins are human milk glycan receptors. Glycobiology 26, 655–669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song X., et al. , Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem. Biol. 16, 36–47 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Braeuer R. R., et al. , Galectin-3 contributes to melanoma growth and metastasis via regulation of NFAT1 and autotaxin. Cancer Res. 72, 5757–5766 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taylor E. S., Pol-Fachin L., Lins R. D., Lower S. K., Conformational stability of the epidermal growth factor (EGF) receptor as influenced by glycosylation, dimerization and EGF hormone binding. Proteins 85, 561–570 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaszuba K., et al. , N-Glycosylation as determinant of epidermal growth factor receptor conformation in membranes. Proc. Natl. Acad. Sci. U.S.A. 112, 4334–4339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dube D. H., Bertozzi C. R., Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 4, 477–488 (2005). [DOI] [PubMed] [Google Scholar]
- 117.Posey A. D. Jr, et al. , Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44, 1444–1454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eavarone D. A., et al. , Humanized anti-sialyl-Tn antibodies for the treatment of ovarian carcinoma. PLoS One 13, e0201314 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Starbuck K., et al. , Treatment of ovarian cancer by targeting the tumor stem cell-associated carbohydrate antigen, sialyl-Thomsen-nouveau. Oncotarget 9, 23289–23305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Prendergast J. M., et al. , Novel anti-sialyl-Tn monoclonal antibodies and antibody-drug conjugates demonstrate tumor specificity and anti-tumor activity. MAbs 9, 615–627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]