Abstract
Objective:
To determine the extent of dasatinib uptake and effect on Src kinase activity in tumor, normal adjacent tissue, and blood in newly diagnosed endometrial cancer patients.
Methods:
Dasatinib was dosed at 100 or 200 mg PO BID at 32 and 8 hours preoperatively. Blood and tissue were collected pre-treatment and at surgery to assess active (pY419) and total Src protein (pharmacodynamics [PD]) and pharmacokinetics (PK). Plasma PK and PD were also analyzed at 2, 4 and 8 hours following the second dose.
Results:
Ten patients completed the study, 5 at each dose level (DL). Average (median, standard deviation, range) 2 h plasma concentration of drug was 119 (121, 80, 226) and 236 (162, 248, 633) ng/mL, for the 100 and 200 mg DL, respectively. Average ratio of 8 h normal and tumor tissue to plasma concentration overall was 3.6 (2.3, 3.4, 9.6) and 8.3 (3.2, 11.9, 38.7), respectively. Dasatinib concentration in tumor was higher than in plasma for both DL. Four patients displayed significant reductions in pTyr419Src at ≥1 time points in blood, and four patients satisfied the PD activity criteria in tissue, with reductions in pTyr419Src of ≥60%.
Conclusions:
This is the first study to show PK and PD effects of dasatinib in tumor tissue, allowing evaluation of tissue PD markers as a function of tumor dasatinib concentration. Dasatinib tissue concentrations at 8h after dosing were associated with modulation of pTyr419Src, total Src protein, and pTyr419Src/Src ratio. All patients had reduction in at least one Src parameter in either tissue or blood.
Keywords: dasatinib, endometrial cancer, src inhibition, pharmacodynamics
INTRODUCTION
The Src family of kinases (SFKs) includes nine highly related, membrane-associated non-receptor tyrosine kinases that are involved in intracellular signaling pathways that regulate cell proliferation, motility and invasion, angiogenesis, and survival.1,2 High levels of the prototypical member of this family, c-Src proto-oncoprotein (Src), and Src activity are correlated with advanced malignancy and poor prognosis of multiple tumor types.2 Src is an attractive therapeutic target because of its involvement in multiple cellular processes and its activation in numerous cancers as measured by auto-phosphorylation of Src on Tyr 419 in the kinase domain.3 However, despite a central role in tumorigenesis and the successful therapeutic application of SFK inhibitors in certain hematologic malignancies,3 these inhibitors have been less efficacious in solid tumors for unclear reasons.4,5
Endometrial cancer provided an optimal setting to conduct a study of dastatinib in solid tumor tissue as it lends itself well to the surgical window paradigm. Endometrial cancer is the most common of the gynecologic malignancies, affecting 63,230 women in the US in 2018 and 319,605 women worldwide.6,7 Primary treatment of localized endometrial cancer is surgical, providing an opportunity for tissue sampling for biomarker, pharmacokinetic (PK), and pharmacodynamic (PD) studies. Evaluation of effects on the adjacent normal endometrium and endometrial stroma are also facilitated as the second biopsy includes the entire uterine corpus.
Elevated protein levels and catalytic activity of c-Src have been detected in endometrial cancer.5 Src activates signaling cascades, including the MAPK and AKT pathways, in an estrogen/estrogen receptor (ER)-dependent manner and potentiates tamoxifen-agonist action in tamoxifen-resistant endometrial adenocarcinoma cells.8 Src also functions in ER-independent signaling pathways,9 suggesting that Src-mediated ER-dependent and independent pathways could contribute to the development, progression, and tamoxifen-resistance of endometrial cancer. In breast cancer, c-Src functions as a signal transducer between cell surface receptors such as the epidermal growth factor receptor and the ER.10 In fact, c-Src directly phosphorylates the ER to affect its mitogenic signals. Additionally, phosphorylation responses to estradiol in breast cancer cells are inhibited by pharmacological inhibitors of c-Src.10
Dasatinib is a potent, oral inhibitor of SFK family members, with a preference for Src kinase.11 We designed a window-of-opportunity trial to study the effect of dasatinib in endometrial cancer. The primary endpoint was to determine drug- and dose-specific changes in levels of Src activity (pTyr419Src) in tumor and blood. We hypothesized that changes in levels of and/or activation status of Src family of kinases in blood would be correlated with changes in levels of and/or activation status of SFK protein in endometrial tumor tissue induced by two different doses of dasatinib. Drug levels in both blood and tissue were also assessed.
PATIENTS AND METHODS
Eligibility Criteria
Patients with newly diagnosed, endometrioid adenocarcinoma of the endometrium that was to be treated surgically with hysterectomy/bilateral salpingo-oophorectomy were eligible. Patients were ≥ 18 years old, had ECOG performance status ≤ 1, and were amenable to a second (research) biopsy of the endometrium. Laboratory criteria included: absolute neutrophil count (ANC) ≥ 1500/mcL, platelet count ≥ 100,000/mcL, hemoglobin ≥ 9.0g/dl, PT < 1.5 times upper limit of normal (ULN), PTT < 1.5 x ULN, creatinine ≤ 1.5 times ULN, bilirubin ≤ 1.5 times ULN, alanine transaminase and aspartate transaminase ≤ 2.5 times ULN. Patients with a pleural or pericardial effusion or history of significant bleeding disorder were excluded. Patients with a cardiac history including uncontrolled angina, congestive heart failure, or myocardial infarction within 6 months, clinically significant ventricular arrhythmias, congenital long QT Syndrome or a prolonged QTc interval (QTc>480 msec) on pre-entry EKG were excluded. Patients taking potent CYP3A4 inhibitors were ineligible unless they discontinued these agents ≥ 7 days prior to study entry. All patients gave written informed consent before study entry (NCT01482728).
Study Design and Drug Administration
A baseline research endometrial biopsy was performed using standard pipelle aspiration at the pre-operative office visit, within 4 weeks of planned surgery. Dasatinib (Sprycel® provided by Bristol-Myers Squibb Company), was administered 32 and 8 hours prior to surgery at either 100 mg (Dose Level [DL] 1) or 200 mg (DL 2). Doses were chosen based on attaining drug concentrations in blood 8 hours after last dose at concentrations known to statistically significantly inhibit Src autophosphorylation.12,13 A sub-therapeutic (100 mg) dose was chosen to begin the study with the hypothesis that we would demonstrate Src inhibition at a sub-therapeutic dose level. A second higher (therapeutic) dose (200 mg) was added to ensure that a response was seen and to potentially demonstrate a dose response.
Adverse events (AEs) related to dasatinib were collected from study entry through six weeks following definitive surgery using the NCI Common Toxicity Criteria for Adverse Events (NCI CTCAE) version 4. Any toxicity ≥ grade 2 thought to be secondary to dasatinib would necessitate stopping accrual to the trial for review. Study accrual would be halted for review and potential modification if any grade ≥ 3 intra-operative bleeding or any grade ≥ 2 post-operative bleeding events occurred in the first 5 subjects in each DL.
Sample collection
Blood sampling was performed at baseline concomitant with baseline fresh biopsy, and at 2, 4 and 8 h ± 1 h after the second dasatinib dose. Peripheral blood mononuclear cells (PBMC) and plasma from EDTA tubes were separated, and aliquots were immediately frozen at −135 0C until analysis. Paired fresh normal adjacent and tumor tissue samples were collected from the hysterectomy specimen.
PK Analysis
Concentrations of dasatinib were quantitated with a validated LC-MS/MS assay as previously described.14 Tissue samples were ground to powder in a liquid nitrogen-cooled mortar and pestle. This powder was weighted out and diluted in control plasma at a ratio of 10–20 (plasma to tissue powder, v/g). Ten to 20 μL of tissue homogenate were added to 200 μL volume QC samples which resulted in an average accuracy of 87% at a precision of 7.4% supporting analysis of plasma-diluted tissue homogenate samples with our plasma assay.
PD Evaluations
Frozen PBMC pellets were sonicated in ice-cold lysis buffer containing protease and phosphatase inhibitors (50 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1% deoxycholate, 5 μM PMSF, 100 nM NaF, 300 nM NaVO4, and HALT™ Protease Inhibitor Cocktail I [Calbiochem #539134]) and centrifuged to remove cell debris. Supernatant protein concentrations were determined using the Invitrogen EZQ® Protein Quantitation Kit, and 30–60 μg protein was separated by electrophoresis and transferred to Immobilon-FL-PDVF membrane using standard procedures. Membranes were probed using primary Src protein-specific monoclonal antibody (mAb) 2–17 (a gift of the NCI mAb collection), pY419Src activation-specific mAb (Invitrogen #4466OG), and anti-Ran mAb (BD Transduction Laboratories #610340) as a loading control. Membranes were incubated with IRDye800CW and IRDye 680 secondary antibodies to generate 2-color images of total Src protein and activated Src, respectively, and scanned using a LiCor Odyssey Infrared Imaging system. Densitometry analysis of the superimposable images was analyzed with LiCor Image Studio v2.1 software. Values from 3–4 replicate experiments/sample were obtained for pY419Src, total Src protein, and activated fraction (pY419Src/Src).
Powdered frozen tissue samples were lysed in Tissue Protein Extraction Reagent buffer (T-PER, Pierce #78510) containing 5 μM PMSF, 100 nM NaF, 300 nM NaVO4, and Halt™ Protease Inhibitor Cocktail. Thirty-sixty μg protein were analyzed by gel electrophoresis and immunoblotting, as described for the PBMC samples. Values from hysterectomy tumor tissue were compared to those of normal tissue adjacent to tumor.
Tumor ER expression and estradiol levels
All tumor tissue underwent immunohistochemistry (IHC) for ER using estrogen receptor (ER) IHC (Ventana clone SP1, pre-diluted) and interpreted by a single gynecologic pathologist (AMM). Tumor cell staining was scored based on optical assessment of nuclear intensity (0=negative, 1+=weak, 2+=moderate, 3+=strong) and extent (% positive cells, 1–100%). Background endometrial glands and stroma served as the internal control. ER score was based on the hysterectomy specimen if there was deficient control reactivity or insufficient lesional tissue for assessment. The H score was calculated by multiplying the staining intensity score by the percentage of positive cells.15,16 Blood samples were subjected to clinical 17β-estradiol assay.
Statistical Analysis and Interim Assessments
This study was designed as a proof-of-concept trial to evaluate reduction of activated Src levels in tissue after treatment with dasatinib. Target sample size was 10 evaluable patients per dose level. Significant reduction of Src activity (pTyr419Src) was defined as a reduction in Src activity compared to baseline that satisfied two criteria: 1) reduction post treatment was at least 50%; and, 2) reduction was 1.95 times greater than the baseline standard deviation (SD). The criteria were calculated from the variance of the difference of two normally distributed variables using the t-distribution to define the cut-off values. Variability was measured on log-transformed values. If the PD effect criteria were satisfied, then the effect was considered to be a statistically significant response at the 1-sided 0.10 level. Significant inhibition by dasatinib within a dose level would be declared if eight of ten evaluable patients had significant inhibition in either the blood or tumor measures. This yielded 90% confidence that the true significant inhibition rate was at least 55%. This was based upon the one-sided lower limit of an exact binomial confidence interval. A pre-planned evaluation of PD response after assessment of the first five evaluable patients was done for each DL. If 2 or fewer PD responses were observed in the first 5 evaluable patients then the criteria for significant inhibition could not be satisfied and accrual to the DL would stop.
Scatterplots were generated as visual displays of potential pairwise associations and Pearson product moment correlation coefficients were used to assess the strength of the pairwise associations. Pairwise comparisons included assessment between normal endometrium and adjacent tumor for the PD and PK outcome measures, as well as assessment between ER H score and estradiol levels for the reduction of activated Src levels in tumor tissue or circulating PBMC.
RESULTS
Clinical Summary
Thirteen patients were consented. Two patients were screen failures: one study biopsy demonstrated blood only, and one patient had ineligible pathology. Eleven patients started treatment. One patient took one dose of dasatinib and declined further study treatment due to grade 2 headache. Ten patients completed the study, 5 at each DL (Table 1).
Table 1.
Patient Demographics
| Characteristic | Number |
|---|---|
| Evaluable patients | 10 |
| Median age, years (range) | 56, (45–70) |
| Median Body Mass Index range, kg/m2 (range) | 37, (21–49) |
| Performance Status 0 | 10 |
| Ethnicity White | 10 |
| Histology, endometrioid | 10 |
| Stage, Grade | |
| Stage 1A, Grade 1 | 6 |
| Stage 1B, Grade 1 | 2 |
| Stage 1A Grade 2 | 1 |
| Stage 2, Grade 1 | 1 |
Dasatinib was well-tolerated with no grade 3 or 4 drug-related adverse events (Table 2). No surgeries were delayed or canceled due to dasatinib-related toxicity. Four patients had grade 1 and 1 patient had grade 2 prolonged QTc, all resolved in one day (grade 1) and one week (grade 2). The patient with grade 2 thrombocytopenia (non-protocol blood draw) was attributed to underlying disease and not to dasatnib. All other grade 2 AEs listed in Table 2 were expected for pre-operative bowel preparation, anesthesia, and surgery.
Table 2.
All adverse events by dose and grade*
|
Toxicity Category |
Adverse Event |
Dose Level 1 N=5 |
Dose Level 2 N=6 |
||
|---|---|---|---|---|---|
| G1 | G2 | G1 | G2 | ||
| Blood and lymphatic | Anemia | 2 | 1 | 1 | - |
| GI disorders | Nausea | - | - | 3 | - |
| Injury/procedural | Bruising | - | 1 | - | - |
| Hemorrhage | 1 | - | - | - | |
| Investigations | Creatinine increased | - | - | 1 | - |
| QTc prolonged | 2 | - | 2 | 1 | |
| Platelets decreased | - | - | 2 | 1 | |
| Metabolism | Hyperglycemia | 1 | 1 | 2 | - |
| Hyperkalemia | - | - | 1 | - | |
| Hypocalcemia | 2 | 1 | 1 | - | |
| Nervous system | Dizziness | - | - | 1 | - |
| Headache | 3 | - | 1 | 1 | |
| Psychiatric | Anxiety | 1 | - | 1 | - |
There were no grade 3 or 4 drug related toxicities
Pharmacokinetics:
All ten patients provided samples for pharmacokinetic analysis. Plasma, normal tissue, and tumor dasatinib concentrations are displayed in Figure 1. The average (median, standard deviation, range) 2 h plasma concentration was 119 (121, 80, 226) and 236 (162, 248, 633) ng/mL, for DL1 and 2, respectively. The average ratio of 8 h normal and tumor tissue concentration to plasma concentration overall was 3.6 (2.3, 3.4, 9.6) and 8.3 (3.2, 11.9, 38.7), respectively; the average ratio of tumor to normal tissue was 2.0 (1.8, 0.9, 3.1). Dasatinib concentration in operative tumor samples was higher than the plasma concentration in both DLs. Concentrations in tumor were higher than in paired normal tissue in both DLs (Wilcoxon rank sum p=0.014).
Figure 1.

Patient dasatinib concentrations over time after 100 mg (○) or 200 mg (Δ) of dasatinib PO, in plasma (2–8 h), 8–9 h normal tissue (N) or tumor (T), and the tumor/normal tissue ratio (T/N, secondary Y-axis).
Pharmacodynamics:
Src activity (pTyr419Src), total Src protein, and relative Src activity (pTyr419Src/Src) were measured in tumor tissue and blood (Figure 2, panels A-F). Four of ten patients (4, 5, 7, 11), two in each DL, satisfied the PD activity criteria, exhibiting reductions of tumor tissue pTyr419Src of 60% or greater (Figure 2A, denoted by asterisks). Similarly, changes were observed in PBMCs (Figure 2B); four patients (4, 5, 6, 8) displayed significant reductions in pTyr419Src at one or more time points (denoted by asterisks). Only patients 4 and 5, both DL1, met the pTyr419Src PD response criteria in both tumor tissue and blood. Table 3 summarizes these results, demonstrating that the pTyr419Src PD response to dasatinib was variable, and that a majority of patients (6/10) met the PD response criteria in either tumor tissue or blood. All ten patients had a dasatinib PD effect (Fig. 2A and B). Figures 2 G and H show representative immunoblots from Patient 5 tumor tissue and PBMC, respectively.
Figure 2.
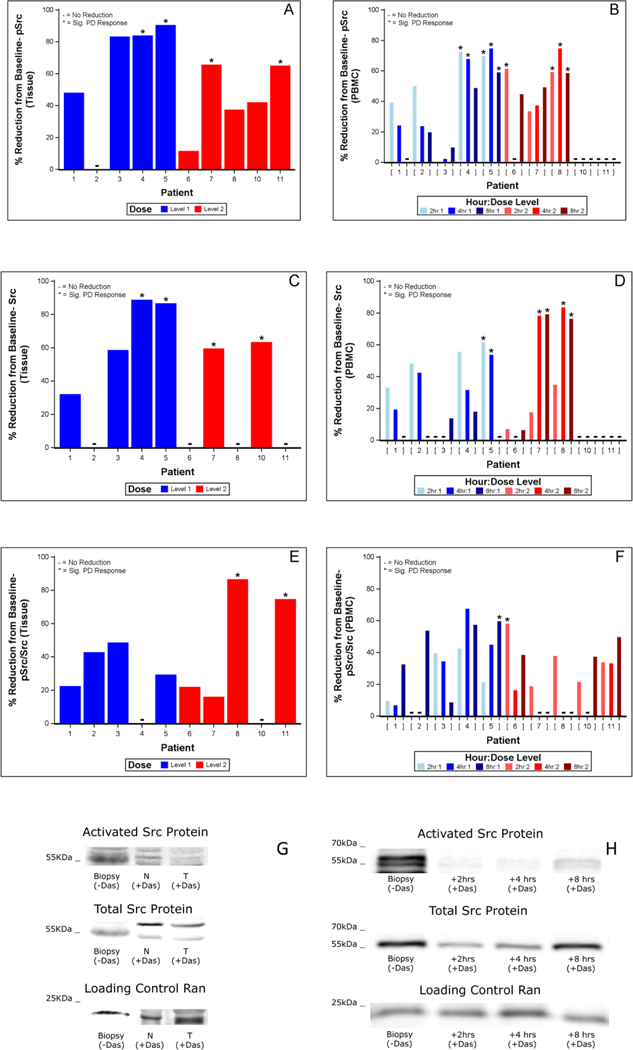
Reduction of Src activity and protein levels following dasatinib treatment.
Figure 2A. Percent reduction from baseline biopsy of pTyr419Src activity (pSrc) in tumor (T) and adjacent normal tissue (N) following 8 hr dasatinib treatment. Values were derived from 3–4 replicates per sample measured in densitometry units (DU) from immunoblots of pSrc relative to Ran loading control. Dose Level 1: blue; Dose Level 2: red. * denotes PD-level response values; +: no reduction. Results are from individual patients, who are identified by unique patient number.
Figure 2B. Percent reduction from baseline of PBMC pSrc. Blood samples from time of biopsy (baseline) as well as 2, 4, and 8 hr after dasatinib treatment were analyzed per patient as in Fig. 2A.
Figure 2C. Percent reduction of total Src protein levels following dasatinib treatment in N and T tissue relative to Src protein levels from baseline biopsy.
Figure 2D. Percent reduction of total Src protein levels following dasatinib treatmen in PBMC relative to Src protein levels from baseline.
Figure 2E. Percent reduction of pSrc/Src following dasatinib treatment in N and T tissue relative to pSrc/Src from baseline biopsy.
Figure 2F. Percent reduction of pSrc/Src following dasatinib treatment. in PBMC relative to pSrc/Src from baseline.
Figure 2G. Representative immunoblot of patient 5 tissue showing pSrc, Src protein and Ran levels in T and N tissue following dasatinib treatment relative to pre-dasatinib biopsy.
Figure 2H. Representative immunoblot of patient 5 PBMC showing pSrc, Src protein and Ran levels in biopsy and 2, 4 and 8 hr following dasatinib treatment.
Table 3.
Pharmacodynamic response as measured by pSrc
| Measure | Type | Dose Level | Dose Level | Total |
|---|---|---|---|---|
| # with significant PD response / total | ||||
| pSrc | Tissue | 2/5 | 2/5 | 4/10 |
| PBMC any time | 2/5 | 2/5 | 4/10 | |
| Tissue or PBMC | 2/5 | 4/5 | 6/10 | |
| Tissue & PBMC | 2/5 | 0/5 | 2/10 | |
| Src | Tissue | 2/5 | 2/5 | 4/10 |
| PBMC any time | 1/5 | 2/5 | 3/10 | |
| Tissue or PBMC | 2/5 | 3/5 | 5/10 | |
| Tissue & PBMC | 1/5 | 1/5 | 2/10 | |
| pSrc/Src | Tissue | 0/5 | 2/5 | 2/10 |
| PBMC any time | 1/5 | 1/5 | 2/10 | |
| Tissue or PBMC | 1/5 | 3/5 | 4/10 | |
| Tissue & PBMC | 0/5 | 0/5 | 0/10 | |
| # with any reduction / total | ||||
| pSrc | Tissue | 4/5 | 5/5 | 9/10 |
| PBMC any time | 5/5 | 3/5 | 8/10 | |
| Tissue or PBMC | 5/5 | 5/5 | 10/10 | |
| Tissue & PBMC | 4/5 | 3/5 | 7/10 | |
| Src | Tissue | 4/5 | 2/5 | 6/10 |
| PBMC any time | 5/5 | 3/5 | 8/10 | |
| Tissue or PBMC | 5/5 | 4/5 | 9/10 | |
| Tissue & PBMC | 4/5 | 1/5 | 5/10 | |
| pSrc/Src | Tissue | 4/5 | 4/5 | 8/10 |
| PBMC any time | 5/5 | 5/5 | 10/10 | |
| Tissue or PBMC | 5/5 | 5/5 | 10/10 | |
| Tissue & PBMC | 4/5 | 4/5 | 8/10 | |
PBMC=peripheral blood mononuclear cells
PD=pharmacodynamic
Similarly, Src protein levels were evaluated. Figures 2C–D and Table 3 show that 4 of 10 patients (4, 5, 7, 10) had a reduction in tumor tissue Src protein, and 3 (5, 7, 10) had a reduction in PBMC Src. Finally, the ratio of pTyr419Src/Src as an assessment of relative Src activity was evaluated (Figure 2E–F). Two patients, both on DL2 (8, 11), exhibited a significant reduction of 75–80% in tumor tissue pTyr419Src/Src ratio. Figure 2F shows that two other patients (5, 6) exhibited reductions in PMBC pTyr419Src/Src that met the PD effect criteria. Together these data show that 9/10 patients responded to dasatinib by one of three measures, pTyr419Src, Src protein, and pTyr419Src/Src, although only about half of these responses met our study criteria for significance. Importantly, pTyr419Src, Src, pTyr419Src/Src levels and dasatinib concentration in the normal adjacent tissue were positively correlated with those measured in the tumor (Figure 3). However, no clear exposure-response relationships between the PD measures of pTyr419Src, Src protein, and pTyr419Src/Src and the PK measures of dasatinib concentrations in the respective samples of tumor tissue, normal adjacent tissue or blood were found (normal comparison defined as normal adjacent tissue; data not shown).
Figure 3.

Correlation of dasatinib levels in normal endometrium with levels in adjacent tumor of each patient in the study. Blue (Dose level 1); Red (Dose level 2).
There was no correlation between ER H score or serum estradiol levels and active Src (pTyr419Src), Src protein or relative Src activity (pTyr419Src/Src) noted (data not shown).
DISCUSSION
The window-of-opportunity design allows evaluation of biochemical, molecular, and other changes in blood, tumor, and normal tissue, and is most readily applied to cancers where surgical intervention is planned. We applied this approach to study the effect of dasatinib on Src levels and activity in endometrioid endometrial cancer. Our results demonstrate for the first time that dasatinib entered solid tumor tissue and modulated Src activity, Src protein, and relative Src activity. Dasatinib concentrations were greater in tumor than in adjacent normal tissue. All patients had reduction in at least one Src parameter in either tumor tissue or circulating PBMCs, indicating even limited dasatinib exposure is pharmacodynamically functional in the absence of toxicity.
A previous study in endometrial cancer demonstrated that Src protein levels were 10–15 fold increased in adenocarcinomas versus benign endometrium.17 The relative activity of Src in benign tissue was shown to be greater than in the carcinomas; however, the total Src activity was estimated to be 2–4 fold greater in carcinomas versus benign endometrium. Thus, this study suggests that blocking Src activity may be of value in the treatment of advanced or recurrent endometrial cancer, perhaps in combination with chemotherapy, hormonal treatment, or targeted therapy.
One goal of our study was to determine whether intra-tumoral, active Src (pTyr419Src) was inhibited by dasatinib. We demonstrated that 40% of patients met at least one of the study criteria for pTyr419Src inhibition, independent of dosage, and another 50% exhibited some level of inhibition of pTyr419Src in tumor tissue. Although significant inhibition of active Src was shown, the question arose as to why more patients did not meet the protocol defined PD criteria for a response. For example, 9 of 10 patients responded to dasatinib with reductions in pSrc ranging from 10–90% in tumor tissue, while only 4 of those 10 qualified for a PD response. Of the five that did not qualify, four samples were inhibited ≥40%. A similar pattern was seen when post-treatment pSrc levels in PBMC samples were examined and when Src protein or pSrc/Src levels were measured in tumor tissue and PBMC. However, the PD response required not only a 50% reduction but also that the variability of the measured reduction be 1.95 times greater than the baseline standard deviation. Therefore, samples failed the PD designation not only because inhibition did not reach 50%, but also because the variability in observed measures was too great when compared to the baseline variability.
Several possibilities may have contributed to the observed inter-patient variability in the baseline values of pTyr419Src, Src, and relative Src activity. First, the variability may be due to the quality of the tissue samples, especially the pre-treatment biopsy (baseline), where the greatest data variability was encountered. The blind nature of the pipelle sample likely resulted in a mixture of normal and malignant endometrium as well as endometrial stroma; micro-dissection might have improved reproducibility, but sample size then would have become technically limiting. Second, patient and tumor heterogeneity could be associated with variability in measured outcomes. Third, the immunoblot assay employed has multiple steps, each with a small but unavoidable variability. Alternative methods also have limitations, and may lack the ability to measure Src specifically. Because of our focus on Src in this study, immunoblotting remained the method of choice.
While we expected to observe inhibition of active Src (pTyr419Src) following dasatinib treatment, we did not anticipate reduction in Src protein, as dasatinib-induced loss of Src protein has not been reported. Tissue samples satisfying the PD criteria for dasatinib-induced Src protein reduction were observed at the same frequency as seen for reduction in activated pTyr419Src (4/10 for each) and 3/10 vs. 4/10 for PBMC. Similar frequencies of reduction between Src protein and pTyr419Src were observed in samples exhibiting any response to dasatinib (6/10 vs 9/10 for tissue; 8/10 vs 8/10 for PBMC). The reasons for the observed reduction in Src protein are not clear. Degradation of highly activated Src (high levels of pTyr419) has been shown to occur more rapidly than that of steady-state (low levels of pTyr419) Src in experimental laboratory cell systems.18 Thus, inhibition of highly activated Src should be correlated with maintenance of or increases in Src protein, not accelerated loss, as we observed. From a clinical perspective, reducing levels of an oncoportein as well as inhibiting its activity provides double assurance of neutralizing its pro-oncogenic effects. We propose, therefore, that the clinical efficacy of dasatinib for solid tumors may improve if patient biopsies were screened for high levels of total and/or active Src. Future studies are needed to address this question.
Our finding that the 2 h dasatinib plasma concentrations were approximately twice those previously reported in a solid tumor phase I trial, with a Cmax of 56 (CV% 118) ng/mL at 1.5 h after a 100 mg dose, and exposure reported in the CML population, with a Cmax of 55 (CV% 56) ng/mL after 100 mg, was unexpected.12,19 Dasatinib exposure is known to be variable between patients, with coefficients of variation up to 100% for both AUC and Cmax. This difference could be due to chance given the small cohort sized studied in this trial.12,20 In contrast, the finding of tissue concentrations in this study are novel, and the ratio > 1 of tumor to normal endometrium is hypothesis generating. There are only two preclinical reports addressing tissue concentration. The first reports mouse brain concentrations, which is known to be shielded from dasatinib exposure by BCRP/Pgp mediated efflux,21 while the second reports rat [14C]-dasatinib-derived radioactivity to be 2–3 times higher in ovaries than in plasma.22 The latter closely agrees with tissue to plasma ratios observed in the current report, and is in line with the volume of distribution at steady-state in mice, rats, dogs and monkey after intravenous administration, which varies between 3.5 and 6.3 L/kg.13 With these relatively low numbers of samples, no clear concentration-effect relationship could be discerned, also likely in part due to inherent imprecision in the activity readouts and intra-tumor heterogeneity of samples analyzed for effect and concentration, respectively.
In January 2016, the NCI convened a Clinical Trials Planning Meeting in endometrial cancer. Based on insights gained at this meeting, several window of opportunity trials investigating targeted therapy for endometrial cancer were proposed. In completing the current study, we have demonstrated the feasibility of performing such window of opportunity studies in women with endometrial cancer which permitted the collection and study of fresh tissue (rather than formalin fixed), the quantification of drug concentrations and biologic endpoints in tumor tissue, and the evaluation of their associations. Further treatment trials with dasatinib are needed to assess the clinical efficacy of dasatinib in this population of women. As examples, NRG Oncology conducted a study administering dasatinib as monotherapy in recurrent gynecologic clear cell cancers (NCT02059265) and MD Anderson Cancer Center investigators are studying dasatinib in combination with paclitaxel and carboplatin in recurrent endometrial disease (NCT01440998). We await the results of these studies to understand the role of dasatinib in the treatment of women with endometrial cancer and the need for further translational work in these women.
Statement of translational relevance:
Window of opportunity trials represent a relatively novel trial mechanism designed to ask translational questions about drug mechanism without intention of clinical benefit. In the case of endometrial cancer, the penultimate “biopsy” is the surgical specimen, allowing study of the tumor tissue as well as the adjacent normal endometrium, the endometrial stroma (the microenvironment) and the myometrium.
Prior surgical window studies in endometrial cancer have demonstrated the feasibility and acceptability of this trial format. These studies allow investigators to answer complex scientific questions regarding drug mechanism of action in the tumor, as well as pharmacokinetic and pharmacodynamic questions. In the current study we demonstrated for the first time that dasatnib was measurable and concentrated in tumor tissue 8 hours after dosing. Additionally, we demonstrated drug activity in tumor and normal tissue. These findings will allow further study of dasatinib in solid tumor, as well as inform future trials.
Acknowledgments
Pharmacokinetic studies were funded by Bristol-Myers Squibb Company as a Laboratory Service Agreement to University of Pittsburgh, Pittsburgh PA. This part of the project utilized the UPCI Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by award P30-CA47904.
Dr. Duska receives research funding to her department (OB/GYN) for clinical trial research.
Jan Beumer reports receiving research funding from BMS to his institute.
Dr. Fracasso reports that she became an employee of Bristol-Myers Squibb Company (BMS) as of May 1, 2014, and as such, she has stock with the company. Prior to her employment with BMS, she was a Professor of Medicine and Obstetrics and Gynecology at the University of Virginia where she is now affiliated as a Visiting Professor of Medicine and Obstetrics and Gynecology. This clinical study was done while she was a Professor at the University of Virginia and no activities in this work have any relationship to her work at BMS.
Funding:
This study was supported by the UVA Cancer Center through the Women’s Oncology Program, NCI Cancer Center Support Grant, P30 CA44579.
Drug was supplied by Bristol-Myers Squibb Company.
This work was presented in part at the Annual Meeting on Women’s Cancer. Society of Gynecologic Oncologists. Tampa, Florida. March 22–24, 2014.
Footnotes
Compliance with Ethical Standards:
Ethical approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Financial Conflict of Interest Report:
Linda R. Duska, Gina R. Petroni, Heather Lothamer, William Faust, Susan M. Christner, Anne M. Mills, and Sarah J. Parsons have no financial conflicts of interest.
REFERENCES
- 1.Frame MC: Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602:114–30, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Biscardi JS, Maa MC, Tice DA, et al. : c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274:8335–43, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Keating GM: Dasatinib: A Review in Chronic Myeloid Leukaemia and Ph+ Acute Lymphoblastic Leukaemia. Drugs 77:85–96, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Irby RB, Yeatman TJ: Role of Src expression and activation in human cancer. Oncogene 19:5636–42, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Ishizawar R, Parsons SJ: c-Src and cooperating partners in human cancer. Cancer Cell 6:209–14, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA Cancer J Clin 68:7–30, 2018 [DOI] [PubMed] [Google Scholar]
- 7.http://globocan.iarc.fr/Pages/fact_sheets_population.aspx, Accessed May 5, 2018
- 8.Shah YM, Rowan BG: The Src kinase pathway promotes tamoxifen agonist action in Ishikawa endometrial cells through phosphorylation-dependent stabilization of estrogen receptor (alpha) promoter interaction and elevated steroid receptor coactivator 1 activity. Mol Endocrinol 19:732–48, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Feng W, Webb P, Nguyen P, et al. : Potentiation of estrogen receptor activation function 1 (AF-1) by Src/JNK through a serine 118-independent pathway. Mol Endocrinol 15:32–45, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Shupnik MA: Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene 23:7979–89, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Lombardo LJ, Lee FY, Chen P, et al. : Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 47:6658–61, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Demetri GD, Lo Russo P, MacPherson IR, et al. : Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res 15:6232–40, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Kamath AV, Wang J, Lee FY, et al. : Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): a potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother Pharmacol 61:365–76, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Twardowski PW, Beumer JH, Chen CS, et al. : A phase II trial of dasatinib in patients with metastatic castration-resistant prostate cancer treated previously with chemotherapy. Anticancer Drugs 24:743–53, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang A, Pettigrew NM, Watson PH: Immunohistochemical assay for oestrogen receptors in paraffin wax sections of breast carcinoma using a new monoclonal antibody. J Pathol 180:223–7, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Vereide AB, Kaino T, Sager G, et al. : Effect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-alpha and ER-beta) in human endometrial hyperplasia. Gynecol Oncol 101:214–23, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Desouki MM, Rowan BG: SRC kinase and mitogen-activated protein kinases in the progression from normal to malignant endometrium. Clin Cancer Res 10:546–55, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Laszlo GS, Cooper JA: Restriction of Src activity by Cullin-5. Curr Biol 19:157–62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Roy A, Hochhaus A, et al. : Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of a Phase III study. Clin Pharmacol 5:85–97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai G, Pfister M, Blackwood-Chirchir A, et al. : Importance of characterizing determinants of variability in exposure: application to dasatinib in subjects with chronic myeloid leukemia. J Clin Pharmacol 48:1254–69, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Agarwal S, Mittapalli RK, Zellmer DM, et al. : Active efflux of Dasatinib from the brain limits efficacy against murine glioblastoma: broad implications for the clinical use of molecularly targeted agents. Mol Cancer Ther 11:2183–92, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He K, Lago MW, Iyer RA, et al. : Lacteal secretion, fetal and maternal tissue distribution of dasatinib in rats. Drug Metab Dispos 36:2564–70, 2008 [DOI] [PubMed] [Google Scholar]


