Abstract
Our previous work reported cognitive impairments in both young and old mice, particularly in female mice expressing mouse Arg-61 apoE, with a point mutation to mimic the domain interaction feature of human apoE4, as compared to the wildtype mouse (C57BL/6J) apoE. In this study, we further evaluated water maze performance in the female Arg-61 mice at an additional time point and then investigated related hippocampal cyto-architecture in these young female Arg-61 apoE mice vs. the wildtype mice. The results of behavioral performance consistently support our previous report that the young female Arg-61 apoE showed cognitive impairment versus C57BL/6J at the same age. The cyto-architectural results showed that volume of the granular cell layer (GCL) was significantly larger in both 5- and 10-month old Arg-61 apoE mice versus C57BL/6J mice. While the number of newborn calretinin-positive neurons was greater in the sub-granular zone (SGZ) in 5-month old Arg-61 mice, this number dropped significantly in 10-month old Arg-61 mice to a lower level than in age-matched C57BL/6J mice. In addition, the amyloid β species was significantly higher in 5-month old Arg-61 mice versus age-matched C57BL/6J mice. In conclusion, impaired cognitive functions in female Arg-61 apoE mice appear correlated with larger GCL volume and higher calretinin-positive cell number and suggest a compensatory cellular response that may be related to amyloid beta perturbations early in life. Therefore this study suggests a novel cyto-architectural mechanism of apoE4-dependent pathologies and increased susceptibility of APOEε4 subjects to Alzheimer’s disease.
Keywords: Apolipoprotein E, hippocampus, neurogenesis, amyloid β, mice, female
1. INTRODUCTION
Human apolipoprotein E (apoE) ε4 remains the most important genetic risk factor for late onset AD. ApoE4 subjects in various age brackets also have smaller hippocampal volume and this applies to both AD (Lehtovirta et al., 1995; Pievani et al., 2011) and healthy subjects (Burggren et al., 2008; O’Dwyer et al., 2012), and particularly to healthy women (Cohen et al., 2001) as well as women with MCI (Fleisher A, 2005). Furthermore, the APOEε4 gene is associated with enhanced longitudinal hippocampal volume loss (Jak et al., 2007; Moffat et al., 2000) and whole brain atrophy rates are positively correlated with APOEε4 gene-dose in late middle-aged individuals (Chen et al., 2007). Hippocampal volume and shrinkage/atrophy of the hippocampus impacts and predicts memory functions, performance and intelligence in humans (Andreasen et al., 1993; Cohen et al., 2006; Dolek et al., 2012; Grundman et al., 2003; Lye et al., 2006, 2004; Petersen et al., 2000; Stoub et al., 2010). As with smaller hippocampal volume, several studies have reported diminished cognitive functions in apoE4 subjects with or without AD (Caselli, 2009; Dik et al., 2001). Even more interesting is the fact that this AD-independent cognitive impairment may also be independent of old age as cognitive impairments have been reported in young human apoE4 subjects (Acevedo et al., 2010; Deary et al., 2002) and young mice, expressing apoE4 (Liraz et al., 2013). These findings suggest that apoE4 may negatively impact cognitive functions even early in life perhaps due in part to the hippocampal cyto-architecture impairments associated with apoE4.
The Arg-61 apoE mouse, an established model of apoE4 domain interaction, was developed through site directed mutagenesis (Thr61→Arg61), of the endogenous mouse apoE gene that was made to display domain interaction, the main biophysical and pathological feature that differentiates apoE4 from apoE2 and apoE3 (Dong and Weisgraber, 1996; Hatters et al., 2005; Raffaï et al., 2001). We previously reported impaired learning and memory functions in both young and old mice expressing Arg-61 apoE (Adeosun et al., 2014), especially in females. In this study, we carried out hippocampus-dependent learning and memory tests on 5- and 10-month old female Arg-61 and C57BL/6J mice. In addition, we explored changes of hippocampal cyto-architecture in these Arg-61 apoE mice and C57BL/6J controls. We hypothesized that young Arg-61 apoE mice may have smaller hippocampal volume compared to wildtype mice, a factor that may, at least in part, be related to their impaired cognitive functions.
2. MATERIALS AND METHODS
2.1. Mice
Arg-61 apoE mice were backcrossed for more than 10 generations into the C57BL/6J as previously described (Raffaï et al., 2001, Zhong et al., 2009, Zhong et al., 2008). C57BL/6J mice used as controls were obtained from Taconic Labs (Hudson, NY 12534, USA). This study was carried out in strict adherence to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National institute of Health. The protocol (protocol#1155B) was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Mississippi Medical Center. All efforts were made to minimize suffering and the number of animals used.
2.2. Materials.
Rabbit polyclonal calretinin D29k (Cat#214 102) and Aβ 38/40/42; 1:500 (Cat#218 711 Clone 88B12) were purchased from Synaptic Systems (Goettingen, Germany); Mouse anti NeuN (MAB377) from Millipore (Burlington, MA); Rabbit anti-GAPDH antibody (Cat# sc-25778) from SantaCruz Biotechnologies (Dallas, TX). Donkey anti mouse CY3 and donkey anti-rabbit 488 secondary antibodies (for IHC); IRDye 680RD donkey anti-mouse (Cat# 926–68072) and IRDye donkey anti-rabbit (Cat# 926–32213) secondary antibodies from Licor (Lincoln, NE) (for dot blot analysis); normal goat (Cat# S1000) and horse sera (Cat# S2000) from Vista Labs (Burlingame, CA); Triton X-100 (Cat# T8787) and DPX mountant from Sigma-Aldrich (St. Louis, MO).
2.3. Cognitive performance and brain sample collection
The cognitive performance was evaluated by Radial-Arm Water Maze (RAWM), Novel Arm Discrimination (NAD), and Spontaneous Alternation in Y-Maze (SAYM) in young and old mice, as reported previously (Adeosun et al., 2014). At the end of the behavioral experiments, mice were sacrificed at 5 or 10 months-of-age after deep anesthesia with isoflurane and cardiac perfusion with PBS. Brain tissue harvesting and processing for histological staining and protein preparation were done as previously described (Adeosun et al., 2014).
2.4. Nissl staining
Each twelfth section (40 μm thick) obtained through the rostro-caudal extent of the brain was mounted on glass slides and Nissl staining was done according to the procedure described in the ‘Allen Reference Atlas’.
2.5. Immunohistochemistry and unbiased stereology
Immunohistochemical staining was carried out on each twelfth section throughout the rostro-caudal length of the brain as described (Adeosun et al., 2014) with a few modifications. Briefly, sections were incubated overnight with shaking at 4°C with primary antibodies (NeuN 1:500; Calretinin 1:200) diluted with blocking buffer (1X PBS; 0.4% Triton X-100; 2% normal goat serum and 2% normal donkey serum) containing 2.5% each of goat and donkey normal sera. Sections were washed and detected with the appropriate secondary antibodies. The appropriate negative controls (no primary antibody) were carried out along with the stained samples to determine antibody specificity and/or non-specific staining.
Unbiased stereology of the region of interest (ROI) was carried out as previously described (Adeosun et al., 2012). The region of interest included the granular and molecular layers of the dentate gyrus and the hilus.
2.6. Hippocampal volume measurement
Montage images of the whole hemi-brain section were taken at 10X objective magnification using a microscope controlled by the Slidebook 6.0.7 Software (Intelligent Imaging, Denver, CO). Each twelfth section (40 μm) was evaluated, resulting in 6–7 sections containing parts of the hippocampus per animal. The hippocampus, dentate gyrus (DG), granular cell layer (GCL) or CA2–3 of the dentate gyrus were manually traced with a mouse one at a time (Fig. 2a and 2b) and ‘masked’ within each montage image. The surface area of the respective masked region-of-interest was generated in μm2 using the mask statistic menu in the Slidebook program. For each region-of-interest, the estimated volume for each animal was obtained by multiplying the sum of the surface area (in μm2) of all sections measured with the section interval (12 sections × 40 μm = 480μm) (Ojo et al., 2013; Peirce et al., 2003). The averages of these resulting volumes were then compared between C57BL/6J and Arg-61 mice.
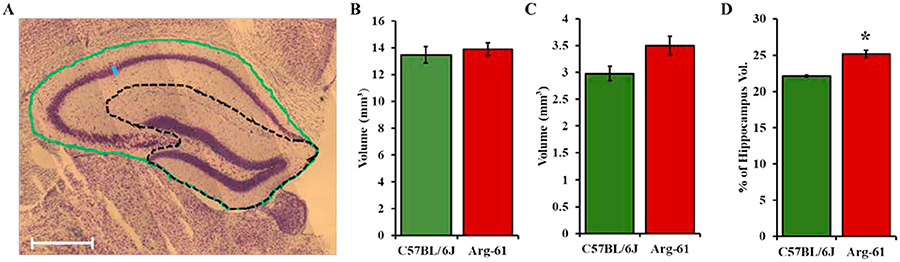
FIGURE 2. Volume of hippocampal regions in 5-month-old, young C57BL/6J and Arg-61 apoE female mice.
(A) Demarcation of some hippocampal regions measured in Nissl-stained section. Green outline represents the surface area of the whole hippocampus (excluding the Fimbria) and the black dotted outline represents the surface area of the dentate gyrus. Montage images were taken at x10 magnification. Scale bar 500μm. Volume of (B) the whole hippocampus or (C) the dentate gyrus (DG) obtained as described in Methods, expressed in mm3. (D) Percentage of the hippocampus occupied by the dentate gyrus is significantly larger in Arg-61 apoE mice than C57BL/6J mice. Data expressed as Mean±SEM. N=4 for both groups. Independent T-test p values *p<0.05.
2.7. NeuN-positive cell density and relative cell number determination
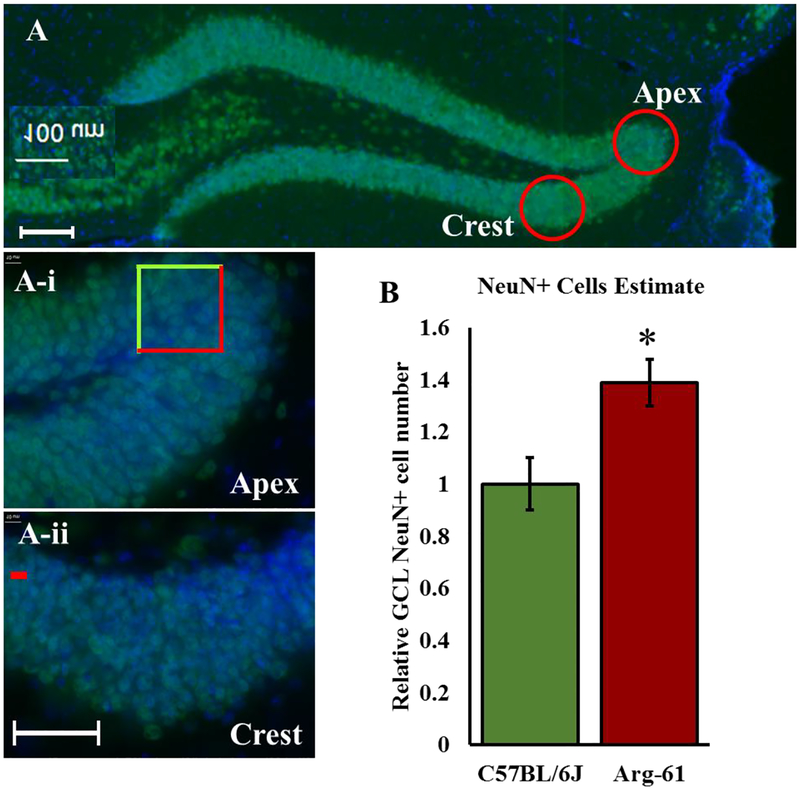
Three sections from the 10-month old mice containing the hippocampus with similar shapes located between bregma −1.455mm and −2.355mm (According to ‘Allen Reference Atlas’) were used to determine the NeuN-positive cell density within the GCL of each mouse. Images were taken at 40X and two 50μm × 50μm squares were positioned within the layer of cells as shown within the ‘apex’ and ‘crest’ (Fig. 6A) of each section. Cells that were in focus and that fell within the square were counted. Cells that crossed the green (top and left) lines were included while those that crossed the red lines (bottom and right) lines were excluded (Fig 6A-i). The mean cell count for each mouse was obtained after which the mean values for all C57BL/6J and Arg-61 mice were compared by Student’s t-test. The relative NeuN cell number within the GCL was obtained by multiplying the cell density obtained for each mouse by the volume measured in each mouse. The number presented was expressed relative to the mean obtained for C57BL/6J mice.
Figure 6. NeuN-positive cell number and size in the granular cell layer of 10-month C57BL/6J and Arg-61 apoE female mice.
(A) Representative image of GCL and the areas (A-i) ‘Apex’ and (A-ii) ‘Crest’ (indicated in A with red circles) within which NeuN cell density was estimated. A sample of one of the two 50μm × 50μm ‘counting frames’ used for the density estimation is shown in A-i. Scale bar is 100μm in A and 50μm in A-i and A-ii. (B) The estimated NeuN+ cell number in the GCL obtained from a product of GCL volume in each animal and the NeuN+ cell density in the GCL expressed relative to C57BL/6J numbers (Independent sample Student’s T-test *p < 0.05). N=3 and 4 respectively for C57BL/6J and Arg-61 apoE mice.
2.8. Cell size determination
The sizes of NeuN+ cells in the GCL were measured by sampling 10 cells (with nuclei) from 4 different positions within each of 3 sections of similar shapes. This made a total of (10×4×3) 120 cells measured per mouse. The respective percentile (10th, 20th, …100th; i.e. from smallest to largest) sizes were obtained for each animal.
2.9. Dot blot
After mice were sacrificed, the hippocampus was dissected and frozen on dry ice. Hippocampal protein was extracted with RIPA buffer supplemented 1:100 with Halt protease inhibitor cocktail. Protein concentrations were determined by BCA assay and protein homogenates were normalized to 2μg/μL with RIPA-Halt. 1.5μL (3μg) of homogenate was spotted on nitrocellulose membrane and allowed to air-dry for 4 hours. Membranes was incubated overnight at 4°C with shaking in a mix of primary antibodies (mouse anti Aβ 38/40/42; 1:500 [Cat# C1 88B12 Synaptic Systems] and Rabbit anti-GAPDH; 1:1000 [Cat# sc, SantaCruz Biotechnologies]). Membranes were washed with PBS-Tween20 and then incubated for 1hr at room temperature with a mix of IRDye 680RD donkey anti-mouse and IRDye donkey anti-rabbit (Cat# 926–68072 and Cat# 926–32213, respectively; Licor) both at 1:10,000 dilution. The membrane was then washed for 15mins three times with PBS-Tween 20 and scanned with the Odyssey scanner. The image was acquired and analyzed using the Licor Image Studio version 5.0. Optical densities of individual Aβ signal in each spot were normalized with the respective GAPDH signal.
2.10. Statistics
Two way ANOVA was used to analyze the effects of age and genotype in 5- and 10-month old C57BL/6J and Arg-61 mice. When data included either only 5- or 10-month old mice, independent sample Student’s t-test was used. Data are presented as mean±SEM and two-sided α-level of statistical significance was set at 0.05.
3. RESULTS
3.1. Cognitive impairment in young female, but not in male, Arg-61 apoE mice
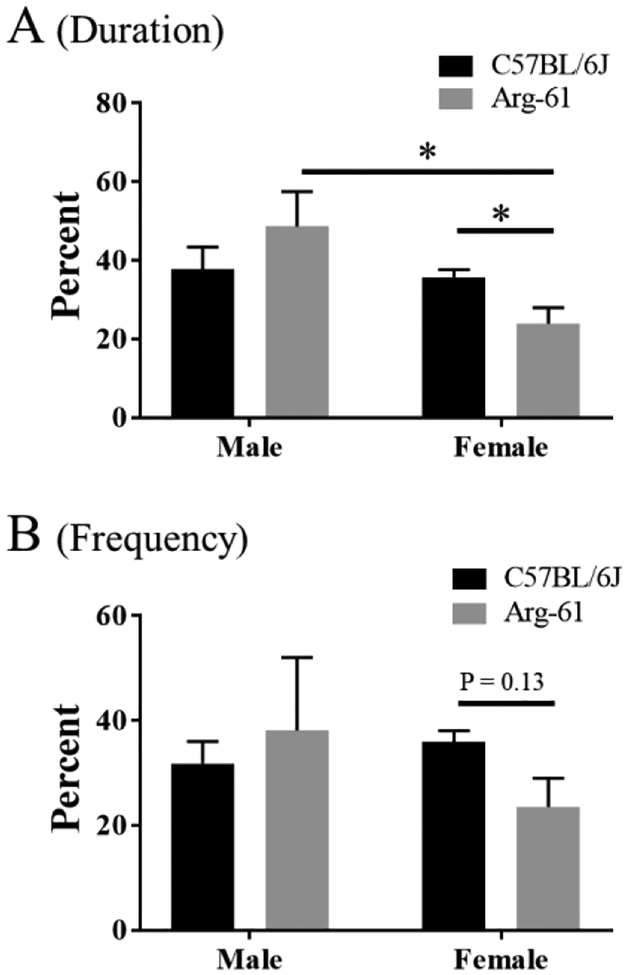
The behavioral performance of the 10-month old Arg-61 mice demonstrated significant cognitive impairments. The analysis showed a significant difference in the latency (time-to-target) during the training trials was specifically impaired among young females (Arg-61 vs. C57BL/6J), but not young males (Adeosun et al., 2014). The percentage time-in-target (duration, Fig. 1A) of young female Arg-61 mice in the 30 seconds probe trial showed spatial memory impairment as they ‘lost’ the preference for the target, while the C57BL/6J mice and the male arg-61 mice retained it (two-way ANOVA, F(1,16) = 3.93, *p < 0.05 for female Arg-61 vs. C57BL/6J; and F(1, 16) = 5.49, *p < 0.05 for female vs. male Arg-61 apoE mice, respective). The percentage entry-into-target (frequency, Fig. 1B) only showed a trend of significance (F(1,16) = 2.43, p = 0.13 for female Arg-61 vs. C57BL/6J mice). These data suggest that, in addition to the previously reported impairments of time-to the target, the time-in the target arm (spatial memory) in old Arg-61 mice (Adeosun et al., 2014), these cognitive impairments are also consistently observed in the young female Arg-61 mice. Taken together the results suggest that domain interaction in apoE protein not only correlates with impairment in older animals but also may actually work independent of age as significant impairment was found also in young female animals. To evaluate if the Arg-61 apoE domain interaction has the similar pathoneurobiological effects with human apoE4 on disruption of hippocampal cyto-architecture, the following results were obtained.
FIGURE 1.
Spatial memory deficits in young female Arg-61 mice. In the 30 seconds probe trial (the platform was removed) in RAWM on day 3, 24 hours after two days training trial, the percent of time-in the target arm (A, duration) and the percent of entry-into the target arm (B, frequency) indicated that spatial memory was impaired in young female Arg-61 mice. Data shown are Mean±SEM. N = 5 males & 5 females in each stains. The data were analyzed by Two Way ANOVA, *p<0.05.
3.2. Larger relative volumes of hippocampal regions in Arg-61 mice
The volume of areas measured included the whole hippocampus (excluding the fimbria), the dentate gyrus (as shown in Fig. 2A) and lastly, CA2–3 (not shown) in Nissl-stained sections of 5-month old female C57BL/6J and Arg-61 apoE mice. There was no significant difference in the whole hippocampus volume between C57BL/6J and Arg-61 mice (13.47±0.62mm3 vs. 13.89±0.47mm3; p=0.614; Fig. 2B). There was also no significant difference in the CA2–3 volume between C57BL/6J and Arg-61 mice in absolute terms (0.86±0.11mm3 vs. 0.95±0.11 mm3; p=0.593), or when expressed relative to body weight (35.8±6.2 vs. 40.2±4.4 mm3g−1; p=0.586), The volume of CA2–3 did not significantly differ between C57BL/6J and Arg-61 mice when expressed as a percentage of the whole hippocampus (6.3±0.6% vs. 6.8±0.6%; p=0.602) (figures not shown). However, there was a trend for a 17.5% marginal increase in dentate gyrus volume in Arg-61 apoE mice (3.50±0.18mm3) versus C57BL/6J mice (2.98±0.14mm3) (p=0.058) (Fig. 2C). Furthermore, there was a significant higher percentage of their hippocampus represented by the dentate gyrus (25±0.5% vs. 22±0.2%; p=0.001) in Arg-61 vs. C57BL/6J mice, respectively (Fig. 2D). Neither the mice body weights (24.2±1.54g vs. 23.4±0.27g; p>0.05) nor the number of sections measured (6.75±0.25 vs. 6.75±0.25; p>0.05) were significantly different between C57BL/6J and Arg-61 apoE mice. While there was no significant difference in the whole hippocampal volume between Arg-61 and C57BL/6J mice and since the dentate gyrus contains the granular cell layer (GCL) made up of the cell bodies of the dentate gyrus neurons, these data suggest that the cellular layer of the hippocampus may be larger in the young Arg-61 apoE mice compared to age-matched C57BL/6J mice.
3.3. More CR+ cells in the hippocampal subgranular zone of Arg-61 mice
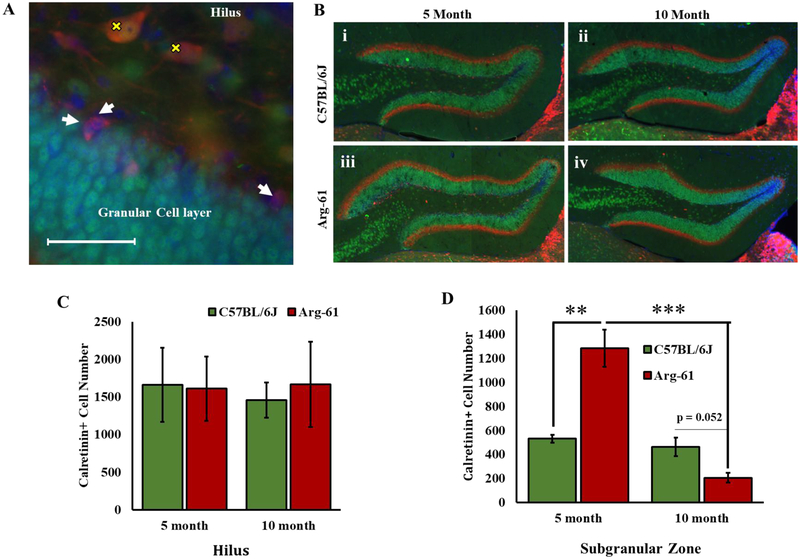
We previously reported an increase in doublecortin-positive (DCX+) cells in the subgranular zone (SGZ) of the hippocampus of young Arg-61 apoE mice vs. C57BL/6J mice, which supports the possibility that more new cells are being added to the GCL. However, as we and others (Heine et al., 2004) have reported, especially in young rodents, it is likely that many of the DCX+ cells die before reaching maturity. To further investigate the basis of the potentially larger GCL volume in Arg-61 apoE mice and a potential age-dependent difference, we analyzed the number of new-born calretinin-positive (CR+) neurons being added to the GCL in the 5- and 10-month old C57BL/6J and Arg-61 female mice. While CR+ neurons were found in several areas of the brain including several layers of the cortex, our focus was on the hippocampus. CR+ neurons were observed more frequently in the ventral than dorsal portions of the hippocampus as reported (Fujise et al., 1997; Fujise and Kosaka, 1999). The majority of the observed CR+ neurons in the ventral levels of the hippocampus are the larger, mossy cells located within the hilar portions of the dentate gyrus (Brandt et al., 2003). The second category of CR+ neurons are smaller than the hilar types and are located within the SGZ at the junction of the granular cell layer and the hilus (Fig. 3A–3B). This category of CR+ neurons consists of post-mitotic newborn neurons which transiently express the protein as they develop into maturity. These cells are more mature than the DCX+ cells that we previously studied in these mice and are therefore more likely to survive to maturity as NeuN-expressing neurons of the GCL (Brandt et al., 2003; Liu et al., 1996).
FIGURE 3. Calretinin-positive, newborn neurons in the hippocampus of 5- and 10-month old C57BL/6J and Arg-61 apoE female mice.
(A) Representative 40x image of the different types of Calretinin-positive (CR+) neurons that were counted. CR+ cells stained with CY3 (red) are widespread in the hippocampus. The cells co-express NeuN stained with 488 (green). The larger CR+ cells are mostly found in the hilus (marked with the yellow x) and they are mossy cells. The smaller CR+ cells which are located between the granular cell layer (GCL) and the hilus (indicated by white arrowheads) are newborn neurons being added to the GCL. Scale bar 50μm. (B) Representative images of CR and NeuN double-staining in the hippocampus; note the higher number of CR+ neurons especially in the young Arg-61 apoE mice (B-iii). (C) Stereology data showing no difference of CR+ cells in the hilus in any of the groups. (D) The number of CR+ cells in sub granular zone were higher in 5 month, but in a trend of lower (p = 0.056) in 10month Arg-61 apoE mice versus age-matched C57BL/6J mice. N=4 for all groups except 10-month Arg-61 mice where N=3. Data was analyzed by 2-way ANOVA followed by Tukey’s multiple comparison post-hoc test. **p<0.01, ***p<0.001.
There was neither a genotype nor an age effect on the number of hilar CR+ cells (Two-way ANOVA, genotype effect, F (1, 11) =0.034; p>0.05; Age effect, F (1, 11) =0.029; p>0.05; genotype X age interaction, F (1, 11) =0.091; p>0.05) (Fig. 3C). However, for the SGZ CR+ cells, there was a significant genotype effect (F (1, 11) =6.651; p=0.026), age effect (F (1, 11) =35.50; p<0.0001) and genotype X age interaction effect (F (1, 11) =27.53; p=0.0003) (Fig. 3C). The stereology estimation suggests a trend that 5-month-old Arg-61 apoE mice have more than twice the number of SGZ CR+ cells compared to age-matched C57BL/6J female mice (1285.7±154.3, CE=0.12 vs. 531.4±32.8, CE=0.062; Tukey’s post-hoc test p>0.05), amounting to a 142% trend increase over the counts in C57BL/6J mice (Fig. 3D). Interestingly, while there was no significant change in SGZ CR+ cell between 5- and 10-months in C57BL/6J mice (531.4±32.8, CE=0.062 vs. 462.9±76.0, CE=0.0164; Tukey’s post-hoc test p>0.05), the high counts of CR+ cells in Arg-61 mice saw a significant 84% drop between 5- and 10-months (1285.7±154.3, CE=0.12 vs. 205.7±39.6, CE=0.16; Tukey’s post-hoc test p<0.0001).
In all groups, we confirmed that the stereological estimation was optimal as coefficients of error (CE= Standard error of mean/Mean) were less than the corresponding coefficients of variation (CV=Standard deviation/Mean) (Volz et al., 2011).
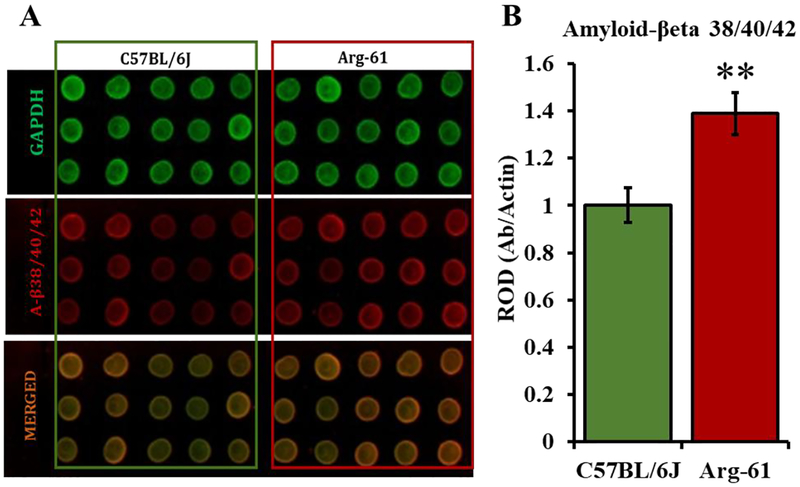
3.4. Domain interaction in mouse Arg-61 apoE stimulates the Aβ formation
Human ApoE4 is known to impact Aβ formation in mice and humans and its domain interaction has been implicated in this process (Jack CR et al., 2015; Ye et al., 2005, p. 4; Zepa et al., 2011). To determine whether the domain interaction in mouse apoE4 may have the similar biological function as human apoE4, we analyzed Aβ level by dot-blot using a monoclonal antibody that detects multiple amyloid beta species (Aβ 38/40/42) in young Arg-61 female mice. As shown in Fig. 4A and 4B, 5-month-old Arg-61 apoE mice have approximately 39% more Aβ 38/40/42 species in their hippocampal homogenates compared to the age-matched C57BL/6J female mice (1.00±0.07 vs. 1.39±0.09 for C57BL/6J and Arg-61 mice respectively; p=0.01). These data suggest that the apoE4 domain interaction in mouse apoE may have the same pathogenic effect as human apoE4 on Aβ production. Aβ reportedly stimulates an ectopic increase in neurogenesis (Chen et al., 2008; Jin et al., 2004; Yu et al., 2009). Although there was no direct evidence showing that ectopic mouse Aβ increases neurogenesis as with human Aβ, the parallel increase of Aβ (Fig. 4) and CR+ cells (Fig. 3D) in 5-month-old Arg-61 female mice suggests such a possibility.
FIGURE 4. Amyloid-β 38/40/42 species immunoreactivity in 5 month, young C57BL/6J and Arg-61 apoE female mice.
(A) Dot blot image of Amyloid-β detected with a monoclonal antibody that recognizes various species (Aβ 38/40/42) detected with IRDye 650 secondary antibody (red; middle) along with GAPDH detected with IRDye 800 secondary antibody (green, top). Three micrograms of hippocampal homogenates were loaded in triplicates for each of C57BL/6J and Arg-61 apoE mice). (B) Optical density of each spot was quantified digitally and simultaneously for both green and red channels. Each dot signal for Aβ 38/40/42 was normalized by the respective GAPDH signal. N=5 for each genotype. Data analyzed by Independent sample, Student T-test. *p<0.01.
3.5. Age-dependent relationship between newborn neurons and granular cell layer (GCL) volume in C57BL/6J and Arg-61 apoE female mice.
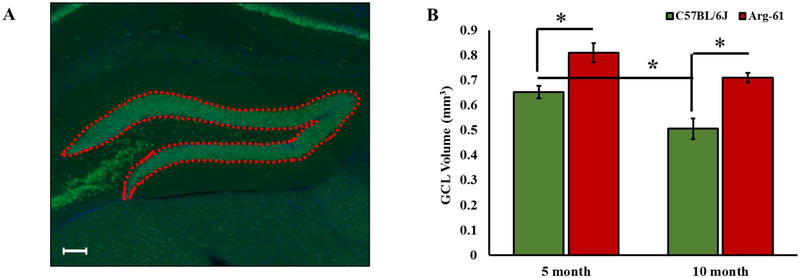
As shown in Fig. 2C, there is a trend for Arg-61 apoE mice to have a larger DG, which constitutes a significantly larger portion of their hippocampus (Fig. 2D) and they have significantly more CR+ newborn neurons present in their GCL (Fig. 3D). We asked whether the granular cell layer (GCL), which is the main portion of the DG that contain cell bodies, is actually larger in Arg-61 mice. The outline of the GCL was traced in NeuN-stained sections to obtain the surface area (Fig. 5A), and as described for the whole hippocampus and other regions we have measured, we obtained the volume of the GCL using each 12th stained sections. 2-way ANOVA shows significant genotype effect (F (1, 11) =27.52; p=0.0003) and age effect (F (1, 11) =12.84; p=0.0043), but no genotype X age interaction (F (1, 11) =0.45; p>0.05) (Fig. 5B). Tukey’s post-hoc analysis shows that, compared to C57BL/6J, Arg-61 apoE mice have larger GCL volume at both 5-months (0.81 0.04mm3 vs. 0.65 0.03mm3 for Arg-61 apoE and C57BL/6J mice, respectively, p=0.0276) and 10-months of age (0.71 0.02mm3 vs. 0.51 0.04mm3 for Arg-61 apoE and C57BL/6J mice respectively; p=0.0091). Interestingly, while there was no 5-month to 10-month age difference in the volume of the GCL in Arg-61 apoE mice (0.81 0.04mm3 vs. 0.71 0.02mm3 for 5- and 10-month-old mice respectively; p>0.05), there was a significant 5-month to 10-month decrease in the volume of the GCL in C57BL/6J mice (0.65 0.03mm3 vs. 0.51 0.02mm3 for 5- and 10-month-old mice respectively; p=0.041) (Fig. 5B).
FIGURE 5. Granular cell layer (GCL) volume in 5-and 10-month old C57BL/6J and Arg-61 apoE female mice.
(A) Demarcation of the granular cell layer of the hippocampal measured in NeuN-stained sections as shown by the red-dotted outline representing the surface area. Montage image was taken at 20x; scale bar = 100μm (B) Volume of the dentate gyrus obtained from the measured surface area (as described in the methods) in 5-month and 10-month Arg-61 apoE mice versus age-matched C57BL/6J mice. N=4 for all bars except 10-month Arg-61 where N=3. Data was analyzed by 2-way ANOVA followed by Tukey’s multiple comparison post-hoc test. *p<0.05.
These data suggest that the larger GCL volume in 5-month-old Arg-61 apoE mice appears to be sustained until later in life in 10-month-old Arg-61 apoE mice.
3.6. Estimated NeuN cell numbers but not cell size are larger in 10-month old Arg-61 apoE female mice.
Although, the addition of newborn neurons to the SGZ constitutes a very small percentage of the total cells in the GCL at any time, it is possible that the larger volume (despite fewer newborn neurons) in 10-month Arg-61 apoE mice may result from matured (NeuN+) neurons being spread more widely apart, that is, demonstrating reduced cell packing density. Therefore, we estimated the density of NeuN+ cells within the GCL using similar sections across all the 10-month-old animals as described in the Methods section 3.6 (Figs. 6A, A-i and A-ii). Independent Student’s t-test showed no significant difference between the NeuN+ neuron densities in the GCL of 10-month-old C57BL/6J and Arg-61 apoE mice (16.7±0.58 vs. 16.5±0.70; p>0.05).
To understand why GCL volume is larger in 10-month-old Arg-61 apoE mice, despite having fewer new neurons added and showing no difference in NeuN+ cell density, we hypothesize that NeuN+ cell size may be larger in Arg-61 apoE mice. We measured 120 random cells within similar levels of the hippocampus in 10 month-old C57BL/6J and Arg-61 apoE mice. There was no significant difference in cell size between C57BL/6J and Arg-61 apoE mice (data not shown).
Using unbiased stereological estimation of doublecortin immune-reactive cell counts in the GCL, we previously concluded that most of the newborn neurons may die before reaching functional maturity in the hippocampus of Arg-61 apoE mice (Adeosun et al., 2014). However, since GCL volume remained larger in Arg-61 apoE mice at 10-months of age (Fig. 5B), despite the significant decrease in SGZ CR+ cell (Fig. 3D), it is likely that the large number of cells added while the animals were young survived to maturity and remained in the hippocampus till the later ages. Therefore, we estimated the total NeuN+ cell numbers in the GCL of the 10- month old mice from the product of the estimated NeuN+ cell density in the GCL and the measured GCL volume. Our data show that NeuN+ neuron number in the GCL of 10-month-old Arg-61 apoE mice are 39% greater than the numbers in age-matched C57BL/6J mice (1.00±0.10 vs 1.39±0.09; p=0.046). The excess newborn neurons added earlier in life may have survived in 10-month-old Arg-61 mice and persisted until at least 10-months of age (Dayer et al., 2003), and may therefore explain the larger NeuN+ cell number and consequently, the greater GCL volume.
4. DISCUSSION
There is major evidence for increased susceptibility to late-onset AD in females and the possibility that females may be more sensitive to the detrimental effects of apoE4. Our results, here and also previously (Adeosun et al., 2014), suggest the detrimental effects of apoE4 initiate at earlier age, and the domain interaction in mouse Arg-61 apoE and human apoE4 is the major pathogenic function of apoE, in terms of Aβ generation (Ewers et al., 2008; Hou et al., 2015, p. 1; Jack CR et al., 2015; Zepa et al., 2011) and cognitive impairment in females (Calafiore et al., 2012; Chen et al., 2008; Jin et al., 2004; Lopez-Toledano et al., 2010; López-Toledano and Shelanski, 2007, 2004; Yu et al., 2009; Adeosun et al., 2014). This cognitive impairment was observed not only in those at 10 months-of-age (comparable to pre-menopause in human), but also in young female mice (comparable to human young adults in the growth period). Interestingly, we observed an increase in volume in the GCL in association with apoE4 domain interaction and it is also worth noting that the hippocampal subregion GCL is an area too small and difficult to delineate using the current resolution of MRI in human studies (Mueller et al., 2010). In combination with the significant 84% decrease of CR+ cells from 5-month to 10-month old Arg-61 apoE mice, we hypothesize a potential compensatory response to neurotoxicity induced by domain interaction in Arg-61 apoE4 in early age and the subsequent depletion of neuro-progenitors late in life.
4.1. Role of Amyloid β species on GCL CR+ newborn neuron number
APOEε4, a late-onset AD (LOAD) related gene, is known to precipitate its effect, at least in part, by affecting amyloid beta formation and/or clearance, or BACE1 expression and/or activity in mice and humans (Ewers et al., 2008, p. 1; Hou et al., 2015, p. 1; Jack CR et al., 2015; Zepa et al., 2011). Interestingly, apoE4 domain interaction has been implicated in this process (Ye et al., 2005, p. 4). Although, some studies have suggested that Aβ reduces neural stem cell proliferation and neurogenesis (Haughey et al., 2002), it has also been demonstrated that amyloid β, especially the oligomeric but not fibrillary form, can stimulate a rather aberrant increase in neurogenesis (Calafiore et al., 2012; Chen et al., 2008; Jin et al., 2004; Lopez-Toledano et al., 2010; López-Toledano and Shelanski, 2007, 2004; Yu et al., 2009). Similar to studies of apoE4 mice with no human APP or PS1 transgene in which (Liraz et al., 2013) higher Aβ levels are reported, larger numbers of slower-maturing adult-born hippocampal neurons are also reported (Li et al., 2009). Our dot-blot method used an antibody that detects many species of Aβ, including the oligomeric forms known to be more relevant to the general AD pathology. Soluble and monomeric forms of Aβ from the SAMP8 mouse model (bearing no human APP transgene) in particular have been shown to facilitate proliferative activity of NSCs (Díaz-Moreno et al., 2013). Thus, the higher levels of Aβ species in the hippocampal homogenates of the Arg-61 apoE mice (Fig. 4A and 3B) may be responsible for the observed increase in production of SGZ CR+ newborn neurons.
4.2. Differential role of CR+ newborn cells to GCL volume at different ages
It is reasonable to explore structural changes in mouse models of AD with an expectation of a lower hippocampal volume since there is much support for hippocampal atrophy in Alzheimer disease (Dolek et al., 2012; Jahn, 2013). This volume reduction is also correlated with a decrease in cognitive performance in AD patients (Grundman et al., 2003; Köhler et al., 1998). The correlation of volume and cognitive performance may be more important in healthy and/or young apoEε4 individuals since smaller hippocampal volume or longitudinal hippocampal volume loss may be an early sign or predisposing factor of the disease (Apostolova LG, 2006; Golomb et al., 1996; Grundman et al., 2002; Wolf et al., 2004) based on the concept of [structural] brain reserve (Stern, 2012; Vuoksimaa et al., 2013).
ApoE4 is associated with lower hippocampal volume and faster hippocampal atrophy in human apoE4 subjects, and based on our previously reported impaired cognitive functions in Arg-61 apoE mice, we hypothesized a decrease in hippocampal volume in Arg-61 apoE mice. However, hippocampal volume as a whole was comparable between Arg-61 apoE and C57BL/6J mice. Conversely, we observed a volume increase in the granular cell layer (Figs. 2D and 5B), which contains the granular cell bodies of the dentate gyrus, including those of newborn neurons. The significant volume increase in the GCL could have resulted from one or more of the following scenarios: (a) an increase in cell number (assuming no decrease in density); (b) an increase in cell size (assuming no increase in cell number) or (c) a decrease in cell density or increase in neuropil (assuming no change in cell number).
It is reasonable to expect larger GCL volume in the young, 5-month-old mice (Figs. 2D and 5B) vs. the10-month-old mice since a lot more newborn CR+ neurons are added to the younger GCL (Fig. 5B). Therefore, we tested the possibility of each of the three hypothetical scenarios above by measuring these parameters in the 10-month-old mice which presented the most interesting phenotype, that is, a sustained larger size of the GCL (Fig. 5B) despite the dramatic reduction in newborn CR+ cell addition to the GCL (Fig. 3D). Our result shows that neither scenario (b) (increased cell size;) nor (c) (decreased cell density) could have impacted this larger GCL volume in 10-month Arg-61 apoE mice (data not shown). On the other hand, our results support scenario (a) (increased cell number; Fig. 6B) as the most plausible explanation for the larger GCL volume in the old mice.
Even more fascinating is the fact that while extant newborn CR+ neuron number could explain the larger GCL volume in 5-month-old Arg-61 apoE mice, the same could not explain the sustained larger GCL size in 10-month-old mice. Apart from the fact that older Arg-61 apoE mice saw a significant drop in newborn neuron generation, even below normal (i.e. vs. 10-month C57BL/6J mice), newborn neuron generation generally decreases with age and there may be too few to impact any significant volume change in the GCL, unlike in much younger mice (Ben Abdallah et al., 2010; Rao et al., 2006). Furthermore, contrary to our earlier hypothesis which was based on increased cleaved caspase-3 expression, most of the excess newborn doublecortin neurons in Arg-61 apoE mice may die before maturing (Adeosun et al., 2014), similar to the reduced survival of increased newborn neurons in presenilin 1 A246E FAD mutant mice (Chevallier et al., 2005). The current data suggest that the newborn neurons may actually survive with NeuN+ and remain part of the GCL for at least 5-months, in the 10-month old Arg-61 apoE mice (Fig. 6B). This is consistent with previous studies which suggest that adult-born hippocampal neurons may survive for at least 5 months after their birth (Dayer et al., 2003). Thus, while newborn CR+ neurons at 10-month of age may not account for the sustained larger GCL volume in 10-month old Arg-61 apoE mice, long term survival of the newborn CR+ neuron at 5 months of age may account for the larger GCL volume at both 5- and 10-month old. These results in Arg-61 mice are in agreement with a recent report of larger volumes of the entorhinal cortex in young adult subjects with apoE4 (DiBattista et al., 2014), although some of the earlier reports reported smaller temporal lobe, entorhinal cortex and/or hippocampus of young apoE4 carriers (Dean et al., 2013; Shaw et al., 2007). The discrepancy of brain region volumes in young subjects may be due to limited MRI data from young subjects and also inconsistent anatomical delineation when using older generation MRI units and analysis methods in human studies (Mueller et al., 2010; DiBattista et al., 2014).
4.3. Pre-emptive Compensation and its detrimental effects
The larger number of newborn CR+ neurons in Arg-61 apoE mice may be a compensatory mechanism as we have previously proposed (Adeosun et al., 2014). This is based on the hypothetical construct of brain reserve capacity (BRC) (Stern, 2012, 2002) which suggests that a larger volume of the hippocampus (furnished by increased new cell addition) or whole brain (Guo et al., 2013; Kim et al., 2008) may be beneficial in protecting against the detrimental effects of AD, or even improve cognitive functions (Vuoksimaa et al., 2013). Although, there are conflicting reports about the beneficial or detrimental role of apoE4 in cognitive functions in young apoE4 human subjects ranging from 24-months of age to adulthood as reviewed by (Tuminello and Han, 2011), the increased hippocampal cell proliferation and volume in young Arg-61 apoE mice is not associated with improved learning and memory performances (Adeosun et al., 2014). A similar, pre-emptive compensatory effect of apoE4 has been observed in young subjects who exhibit higher expression of anti-oxidant enzymes in lymphocyte samples that was considered a consequence of early-life ‘hyper-function’ of antioxidant mechanisms (Badía et al., 2013). Neuronal signaling as demonstrated with CAMKII, ERK1/2 and CREB phosphorylation also follow this age-dependent reversal pattern in 3-month versus 17-month apoE4 mice (Yong et al., 2014). Thus, it is tempting to speculate that Arg-61 apoE mice will eventually see a reduction in their GCL and/or hippocampal volumes as has been reported in much older apoE4 mice of 18- and 24-months of age (Yin et al., 2014, 2011).
5. Conclusion
In conclusion, the current study extended the previous report that apoE domain interaction may play a major role in the cognitive pathogenesis in females, not only in those at 10-months-of-age (comparable to pre-menopause in human), but also in young female mice (comparable to human young adults in the growth period). Furthermore, the results of the current work suggest a novel neurobiological mechanism that enhances perturbations associated with apoE4 domain interaction, including increased levels of Aβ species, leading to the Arg-61 apoE mouse brain that appears to compensate for pathologic changes by increased generation of new cells in the hippocampus granular cell layer (GCL). The addition of newborn neurons to the GCL later in life is possibly hindered as a result of rapid depletion of the neural stem cell pools early in life. Thus, this rescue attempt may contribute to, or set the stage for further AD-related pathologies later in life. In summary, the current work suggests that 1) domain interaction is an AD therapeutic/prophylactic target in apoEε4 subjects; 2) the age-dependent hippocampal cyto-architectural disruption by apoE4 domain interaction may be a brain region- and cell type-specific mechanism of AD progression.
Highlights.
The domain interaction in mouse Arg-61 apoE impairs the cognitive performance in young female mice.
The domain interaction in mouse Arg-61 apoE enhances Aβ generation
Mouse Aβ may stimulate the calretinin positive cells increase in sub-granular zone
The increase of GCL and calretinin cells may be a compensatory response to neurotoxicity induced by domain interaction.
Domain interaction in apoE4 may be an AD therapeutic/prophylactic target in apoEε4 subjects
ACKNOWLEGEMENTS
This study was supported by a NIH/NIAAA/NIA grant (66109610619–01), an Alzheimer’s Association Investigator Initiated Research Grant (133086), a Carraway foundation grant, a training grant from the National Institute for General Medical Science COBRE (P30 GM103328, PI: CS), and a MIND center subcontract to JMW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Acevedo SF, Piper BJ, Craytor MJ, Benice TS, Raber J, 2010. Apolipoprotein E4 and Sex Affect Neurobehavioral Performance in Primary School Children. Pediatr. Res 67, 293–299. doi: 10.1203/PDR.0b013e3181cb8e68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeosun SO, Hou X, Jiao Y, Zheng B, Henry S, Hill R, He Z, Pani A, Kyle P, Ou X, Mosley T, Farley JM, Stockmeier C, Paul I, Bigler S, Brinton RD, Smeyne R, Wang JM, 2012. Allopregnanolone Reinstates Tyrosine Hydroxylase Immunoreactive Neurons and Motor Performance in an MPTP-Lesioned Mouse Model of Parkinson’s Disease. PLoS ONE 7, e50040. doi: 10.1371/journal.pone.0050040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeosun SO, Hou X, Zheng B, Stockmeier C, Ou X, Paul I, Mosley T, Weisgraber K, Wang JM, 2014. Cognitive Deficits and Disruption of Neurogenesis in a Mouse Model of apoE4-Domain Interaction. J. Biol. Chem J Biol Chem, 289, 2946–2959.doi: 10.1074/jbc.M113.497909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, Cristofaro AD, Hsiang H.-L. (Liz), Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW, 2014. Hippocampal Neurogenesis Regulates Forgetting During Adulthood and Infancy. Science 344, 598–602. doi: 10.1126/science.1248903 [DOI] [PubMed] [Google Scholar]
- 5.Andreasen NC, Flaum M, Swayze V 2nd, O’Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT, 1993. Intelligence and brain structure in normal individuals. Am. J. Psychiatry 150, 130–134. [DOI] [PubMed] [Google Scholar]
- 6.Apostolova LG, D. R, 2006. COnversion of mild cognitive impairment to alzheimer disease predicted by hippocampal atrophy maps. Arch. Neurol 63, 693–699. doi: 10.1001/archneur.63.5.693 [DOI] [PubMed] [Google Scholar]
- 7.Badía M-C, Giraldo E, Dasí F, Alonso D, Lainez JM, Lloret A, Viña J, 2013. Reductive stress in young healthy individuals at risk of Alzheimer disease. Free Radic. Biol. Med 63, 274–279. doi: 10.1016/j.freeradbiomed.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 8.Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, Inder TE, Anderson PJ, 2008. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain 131, 2986–2994. doi: 10.1093/brain/awn227 [DOI] [PubMed] [Google Scholar]
- 9.Ben Abdallah NM-B, Slomianka L, Vyssotski AL, Lipp H-P, 2010. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging 31, 151–161. doi: 10.1016/j.neurobiolaging.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Braak E, 1998. Evolution of neuronal changes in the course of Alzheimer’s disease. J. Neural Transm. Suppl 53, 127–140. [DOI] [PubMed] [Google Scholar]
- 11.Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, von der Behrens W, Kempermann G, 2003. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell. Neurosci 24, 603–613. doi: 10.1016/S1044-7431(03)00207-0 [DOI] [PubMed] [Google Scholar]
- 12.Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY, 2008. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. NeuroImage 41, 1177–1183. doi: 10.1016/j.neuroimage.2008.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calafiore M, Copani A, Deng W, 2012. DNA polymerase-? mediates the neurogenic effect of ?- amyloid protein in cultured subventricular zone neurospheres. J. Neurosci. Res 90, 559–567. doi: 10.1002/jnr.22780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caselli RJ, 2009. Age-related Memory Decline and Apolipoprotein E e4. Discov. Med 8, 47–50. [PubMed] [Google Scholar]
- 15.Chen K, Reiman E, Alexander G, Caselli R, Gerkin R, Bandy D, Domb A, Osborne D, Fox N, Crum W, Saunders A, Hardy J, 2007. Correlations Between Apolipoprotein E ε4 Gene Dose and Whole Brain Atrophy Rates. Am. J. Psychiatry 164, 916–921. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Nakajima A, Choi SH, Xiong X, Sisodia SS, Tang Y-P, 2008. Adult neurogenesis is functionally associated with AD-like neurodegeneration. Neurobiol. Dis 29, 316–326. doi: 10.1016/j.nbd.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH, 2005. Perturbed Neurogenesis in the Adult Hippocampus Associated with Presenilin-1 A246E Mutation. Am. J. Pathol 167, 151–159. doi: 10.1016/S0002-9440(10)62962-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen RM, Small C, Lalonde F, Friz J, Sunderland T, 2001. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology 57, 2223–2228. [DOI] [PubMed] [Google Scholar]
- 19.Cohen RM, Szczepanik J, McManus M, Mirza N, Putnam K, Levy J, Sunderland T, 2006. Hippocampal atrophy in the healthy is initially linear and independent of age. Neurobiol. Aging 27, 1385–1394. doi: 10.1016/j.neurobiolaging.2005.07.018 [DOI] [PubMed] [Google Scholar]
- 20.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA, 2003. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol 460, 563–572. doi: 10.1002/cne.10675 [DOI] [PubMed] [Google Scholar]
- 21.Dean DC 3rd, Jerskey BA, Chen K, Protas H, Thiyyagura P, Roontiva A, O’Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Siniard AL, Turk MN, Hua X, Madsen SK, Thompson PM, Fleisher AS, Huentelman MJ, Deoni SCL, Reiman EM, 2013. Brain Differences in Infants at Differential Genetic Risk for Late-Onset Alzheimer Disease: A Cross-sectional Imaging Study. JAMA Neurol doi: 10.1001/jamaneurol.2013.4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ, 2002. Cognitive change and the APOE epsilon 4 allele. Nature 418, 932. doi: 10.1038/418932a [DOI] [PubMed] [Google Scholar]
- 23.Díaz-Moreno M, Hortigüela R, Gonçalves A, García-Carpio I, Manich G, García-Bermúdez E, Moreno-Estellés M, Eguiluz C, Vilaplana J, Pelegrí C, Vilar M, Mira H, 2013. Aβ increases neural stem cell activity in senescence-accelerated SAMP8 mice. Neurobiol. Aging 34, 2623–2638. doi: 10.1016/j.neurobiolaging.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 24.DiBattista AM, Stevens BW, Rebeck GW, Green AE, 2014. Two Alzheimer’s disease risk genes increase entorhinal cortex volume in young adults. Front. Hum. Neurosci 8, 779. doi: 10.3389/fnhum.2014.00779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JW, van Kamp GJ, Deeg DJ, 2001. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology 57, 2217–2222. [DOI] [PubMed] [Google Scholar]
- 26.Dolek N, Saylisoy S, Ozbabalik D, Adapinar B, 2012. Comparison of Hippocampal Volume Measured Using Magnetic Resonance Imaging in Alzheimer’s Disease, Vascular Dementia, Mild Cognitive Impairment and Pseudodementia. J. Int. Med. Res 40, 717–725. doi: 10.1177/147323001204000236 [DOI] [PubMed] [Google Scholar]
- 27.Dong LM, Weisgraber KH, 1996. Human apolipoprotein E4 domain interaction. Arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J. Biol. Chem 271, 19053–19057. [DOI] [PubMed] [Google Scholar]
- 28.Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P, 2007. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS–ADRDA criteria. Lancet Neurol 6, 734–746. doi: 10.1016/S1474-4422(07)70178-3 [DOI] [PubMed] [Google Scholar]
- 29.Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SHR, Piazza P-V, Abrous DN, 2007. Spatial Learning Depends on Both the Addition and Removal of New Hippocampal Neurons. PLoS Biol 5. doi: 10.1371/journal.pbio.0050214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewers M, Zhong Z, Bürger K, Wallin A, Blennow K, Teipel SJ, Shen Y, Hampel H, 2008. Increased CSF-BACE 1 activity is associated with ApoE-ε4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain 131, 1252–1258. doi: 10.1093/brain/awn034 [DOI] [PubMed] [Google Scholar]
- 31.Fleisher A G. M, 2005. SEx, apolipoprotein e ε4 status, and hippocampal volume in mild cognitive impairment. Arch. Neurol 62, 953–957. doi: 10.1001/archneur.62.6.953 [DOI] [PubMed] [Google Scholar]
- 32.Foster JKM, 1999. The Hippocampus and Delayed Recall: Bigger is not Necessarily Better? Memory 7, 715–733. doi: 10.1080/096582199387823 [DOI] [PubMed] [Google Scholar]
- 33.Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN, 1996. Presymptomatic hippocampal atrophy in Alzheimer’s disease A longitudinal MRI study. Brain 119, 2001–2007. doi: 10.1093/brain/119.6.2001 [DOI] [PubMed] [Google Scholar]
- 34.Fujise N, Kosaka T, 1999. Mossy cells in the mouse dentate gyrus: identification in the dorsal hilus and their distribution along the dorsoventral axis. Brain Res 816, 500–511. doi: 10.1016/S0006-8993(98)01202-5 [DOI] [PubMed] [Google Scholar]
- 35.Fujise N, Liu Y, Hori N, Kosaka T, 1997. Distribution of calretinin immunoreactivity in the mouse dentate gyrus: II. Mossy cells, with special reference to their dorsoventral difference in calretinin immunoreactivity. Neuroscience 82, 181–200. doi: 10.1016/S0306-4522(97)00261-3 [DOI] [PubMed] [Google Scholar]
- 36.Geuze E, Vermetten E, Bremner JD, 2005. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol. Psychiatry 10, 160–184. doi: 10.1038/sj.mp.4001579 [DOI] [PubMed] [Google Scholar]
- 37.Golomb J, Kluger A, Leon M.J. de, Ferris SH, Mittelman MP, Cohen J, George AE, 1996. Hippocampal formation size predicts declining memory performance in normal aging. Neurology 47, 810–813. doi: 10.1212/WNL.47.3.810 [DOI] [PubMed] [Google Scholar]
- 38.Grundman M, Jack CR, Petersen RC, Kim HT, Taylor C, Datvian M, Weiner MF, DeCarli C, DeKosky ST, van Dyck C, Darvesh S, Yaffe K, Kaye J, Ferris SH, Thomas RG, Thal LJ, 2003. Hippocampal volume is associated with memory but not nonmemory cognitive performance in patients with mild cognitive impairment. J. Mol. Neurosci 20, 241–248. doi: 10.1385/JMN:20:3:241 [DOI] [PubMed] [Google Scholar]
- 39.Grundman M, Sencakova D, Jack CR Jr, Petersen RC, Kim HT, Schultz A, Weiner MF, DeCarli C, DeKosky ST, van Dyck C, Thomas RG, Thal LJ, Alzheimer’s Disease Cooperative Study, 2002. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J. Mol. Neurosci. MN 19, 23–27. doi: 10.1007/s12031-002-0006-6 [DOI] [PubMed] [Google Scholar]
- 40.Guo L-H, Alexopoulos P, Wagenpfeil S, Kurz A, Perneczky R, 2013. Brain size and the compensation of Alzheimer’s disease symptoms: A longitudinal cohort study. Alzheimers Dement 9, 580–586. doi: 10.1016/j.jalz.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 41.Hatters DM, Budamagunta MS, Voss JC, Weisgraber KH, 2005. Modulation of Apolipoprotein E Structure by Domain Interaction DIFFERENCES IN LIPID-BOUND AND LIPID-FREE FORMS. J. Biol. Chem 280, 34288–34295. doi: 10.1074/jbc.M506044200 [DOI] [PubMed] [Google Scholar]
- 42.Haughey N, Liu D, Nath A, Borchard A, Mattson M, 2002. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid β-peptide. NeuroMolecular Med 1, 125–135. doi: 10.1385/NMM:1:2:125 [DOI] [PubMed] [Google Scholar]
- 43.Heine VM, Maslam S, Joëls M, Lucassen PJ, 2004. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus–pituitary–adrenal axis activation. Neurobiol. Aging 25, 361–375. doi: 10.1016/S0197-4580(03)00090-3 [DOI] [PubMed] [Google Scholar]
- 44.Hou X, Adeosun SO, Zhang Q, Barlow B, Brents M, Zheng B, Wang J, 2015. Differential contributions of ApoE4 and female sex to BACE1 activity and expression mediate Aβ deposition and learning and memory in mouse models of Alzheimer’s disease. Front. Aging Neurosci 207. doi: 10.3389/fnagi.2015.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y, Mucke L, 2012. Alzheimer Mechanisms and Therapeutic Strategies. Cell 148, 1204–1222. doi: 10.1016/j.cell.2012.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jack CR Jr, Wiste HJ, Weigand SD, et al. , 2015. AGe, sex, and apoe ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol 72, 511–519. doi: 10.1001/jamaneurol.2014.4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jahn H, 2013. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci 15, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW, 2007. Differential Cross-Sectional and Longitudinal Impact of APOE Genotype on Hippocampal Volumes in Nondemented Older Adults. Dement. Geriatr. Cogn. Disord 23, 382–389. doi: 10.1159/000101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA, 2004. Increased Hippocampal Neurogenesis in Alzheimer’s Disease. Proc. Natl. Acad. Sci 101, 343–347. doi: 10.1073/pnas.2634794100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KR, Lee KS, Kim EA, Cheong H-K, Oh BH, Hong CH, 2008. The effect of the ApoE genotype on the association between head circumference and cognition. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 16, 819–825. doi: 10.1097/JGP.0b013e3181800551 [DOI] [PubMed] [Google Scholar]
- 51.Köhler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, Foster JK, Moscovitch M, Wincour G, Szalai JP, Bronskill MJ, 1998. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: an MR volumetry study in Alzheimer’s disease. Neuropsychologia 36, 901–914. doi: 10.1016/S0028-3932(98)00017-7 [DOI] [PubMed] [Google Scholar]
- 52.Lehtovirta M, Laakso MP, Soininen H, Helisalmi S, Mannermaa A, Helkala E-L, Partanen K, Ryynänen M, Vainio P, Hartikainen P, Riekkinen PJ Sr, 1995. Volumes of hippocampus, amygdala and frontal lobe in Alzheimer patients with different apolipoprotein E genotypes. Neuroscience 67, 65–72. doi: 10.1016/0306-4522(95)00014-A [DOI] [PubMed] [Google Scholar]
- 53.Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y, 2009. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell 5, 634–645. doi: 10.1016/j.stem.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liraz O, Boehm-Cagan A, Michaelson DM, 2013. ApoE4 induces Aβ42, tau, and neuronal pathology in the hippocampus of young targeted replacement apoE4 mice. Mol. Neurodegener 8, 16. doi: 10.1186/1750-1326-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y, Fujise N, Kosaka T, 1996. Distribution of calretinin immunoreactivity in the mouse dentate gyrus. Exp. Brain Res 108, 389–403. doi: 10.1007/BF00227262 [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Toledano MA, Ali Faghihi M, Patel NS, Wahlestedt C, 2010. Adult neurogenesis: a potential tool for early diagnosis in Alzheimer’s disease? J. Alzheimers Dis. JAD 20, 395–408. doi: 10.3233/JAD-2010-1388 [DOI] [PubMed] [Google Scholar]
- 57.López-Toledano MA, Shelanski ML, 2007. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind). J. Alzheimers Dis. JAD 12, 229–240. [DOI] [PubMed] [Google Scholar]
- 58.López-Toledano MA, Shelanski ML, 2004. Neurogenic Effect of β-Amyloid Peptide in the Development of Neural Stem Cells. J. Neurosci 24, 5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu L, Airey DC, Williams RW, 2001. Complex Trait Analysis of the Hippocampus: Mapping and Biometric Analysis of Two Novel Gene Loci with Specific Effects on Hippocampal Structure in Mice. J. Neurosci 21, 3503–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lye TC, Grayson DA, Creasey H, Piguet O, Bennett HP, Ridley LJ, Kril JJ, Broe GA, 2006. Predicting memory performance in normal ageing using different measures of hippocampal size. Neuroradiology 48, 90–99. doi: 10.1007/s00234-005-0032-5 [DOI] [PubMed] [Google Scholar]
- 61.Lye TC, Piguet O, Grayson DA, Creasey H, Ridley LJ, Bennett HP, Broe GA, 2004. Hippocampal size and memory function in the ninth and tenth decades of life: the Sydney Older Persons Study. J. Neurol. Neurosurg. Psychiatry 75, 548–554. doi: 10.1136/jnnp.2003.010223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM, 2000. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology 55, 134–136. [DOI] [PubMed] [Google Scholar]
- 63.Mori E, Lee K, Yasuda M, Hashimoto M, Kazui H, Hirono N, Matsui M, 2002. Accelerated hippocampal atrophy in Alzheimer’s disease with apolipoprotein E epsilon4 allele. Ann. Neurol 51, 209–214. [DOI] [PubMed] [Google Scholar]
- 64.Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW, 2010. Hippocampal Atrophy Patterns in Mild Cognitive Impairment and Alzheimer’s Disease. Hum. Brain Mapp 31, 1339–1347. doi: 10.1002/hbm.20934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Dwyer L, Lamberton F, Matura S, Tanner C, Scheibe M, Miller J, Rujescu D, Prvulovic D, Hampel H, 2012. Reduced Hippocampal Volume in Healthy Young ApoE4 Carriers: An MRI Study. PLoS ONE 7, e48895. doi: 10.1371/journal.pone.0048895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ojo B, Davies H, Rezaie P, Gabbott P, Colyer F, Kraev I, Stewart MG, 2013. Age-Induced Loss of Mossy Fibre Synapses on CA3 Thorns in the CA3 Stratum Lucidum. Neurosci. J 2013. doi: 10.1155/2013/839535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peirce JL, Chesler EJ, Williams RW, Lu L, 2003. Genetic architecture of the mouse hippocampus: identification of gene loci with selective regional effects. Genes Brain Behav 2, 238–252. doi: 10.1034/j.1601-183X.2003.00030.x [DOI] [PubMed] [Google Scholar]
- 68.Petersen RC, Jack CR Jr, Xu YC, Waring SC, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E, 2000. Memory and MRI-based hippocampal volumes in aging and AD. Neurology 54, 581–587. [DOI] [PubMed] [Google Scholar]
- 69.Peterson BS, V. B, 2000. REgional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284, 1939–1947. doi: 10.1001/jama.284.15.1939 [DOI] [PubMed] [Google Scholar]
- 70.Pievani M, Galluzzi S, Thompson PM, Rasser PE, Bonetti M, Frisoni GB, 2011. APOE4 is associated with greater atrophy of the hippocampal formation in Alzheimer’s disease. NeuroImage 55, 909–919. doi: 10.1016/j.neuroimage.2010.12.081 [DOI] [PubMed] [Google Scholar]
- 71.Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, Saunders AM, Breitner JC, 1997. Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology 48, 985–989. [DOI] [PubMed] [Google Scholar]
- 72.Raffaï RL, Dong L-M, Farese RV, Weisgraber KH, 2001. Introduction of human apolipoprotein E4 “domain interaction” into mouse apolipoprotein E. Proc. Natl. Acad. Sci 98, 11587–11591. doi: 10.1073/pnas.201279298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rao MS, Hattiangady B, Shetty AK, 2006. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell 5, 545–558. doi: 10.1111/j.1474-9726.2006.00243.x [DOI] [PubMed] [Google Scholar]
- 74.Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R, 2007. Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl. Acad. Sci 104, 4642–4646. doi: 10.1073/pnas.0611718104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR, Weiner MW, 2009. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 132, 1067–1077. doi: 10.1093/brain/awp007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, 2007. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol 6, 494–500. doi: 10.1016/S1474-4422(07)70106-0 [DOI] [PubMed] [Google Scholar]
- 77.Stern Y, 2012. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11, 1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stern Y, 2002. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc 8, 448–460. doi: 10.1017/S1355617702813248 [DOI] [PubMed] [Google Scholar]
- 79.Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, deToledo-Morrell L, 2010. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiol. Aging 31, 1089–1098. doi: 10.1016/j.neurobiolaging.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tuminello ER, Han SD, 2011. The Apolipoprotein E Antagonistic Pleiotropy Hypothesis: Review and Recommendations. Int. J. Alzheimers Dis 2011. doi: 10.4061/2011/726197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Petten C, 2004. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia 42, 1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 82.Volz F, Bock HH, Gierthmuehlen M, Zentner J, Haas CA, Freiman TM, 2011. Stereologic estimation of hippocampal GluR2/3- and calretinin-immunoreactive hilar neurons (presumptive mossy cells) in two mouse models of temporal lobe epilepsy. Epilepsia 52, 1579–1589. doi: 10.1111/j.1528-1167.2011.03086.x [DOI] [PubMed] [Google Scholar]
- 83.Vuoksimaa E, Panizzon MS, Chen C-H, Eyler LT, Fennema-Notestine C, Fiecas MJA, Fischl B, Franz CE, Grant MD, Jak AJ, Lyons MJ, Neale MC, Thompson WK, Tsuang MT, Xian H, Dale AM, Kremen WS, 2013. Cognitive reserve moderates the association between hippocampal volume and episodic memory in middle age. Neuropsychologia 51, 1124–1131. doi: 10.1016/j.neuropsychologia.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolf H, Hensel A, Kruggel F, Riedel-Heller SG, Arendt T, Wahlund L-O, Gertz H-J, 2004. Structural correlates of mild cognitive impairment. Neurobiol. Aging 25, 913–924. doi: 10.1016/j.neurobiolaging.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 85.Ye S, Huang Y, Müllendorff K, Dong L, Giedt G, Meng EC, Cohen FE, Kuntz ID, Weisgraber KH, Mahley RW, 2005. Apolipoprotein (apo) E4 Enhances Amyloid B Peptide Production in Cultured Neuronal Cells: ApoE Structure as a Potential Therapeutic Target. Proc. Natl. Acad. Sci. U. S. A 102, 18700–18705. doi: 10.1073/pnas.0508693102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yin J, Turner GH, Coons SW, Maalouf M, Reiman EM, Shi J, 2014. Association of amyloid burden, brain atrophy and memory deficits in aged apolipoprotein ε4 mice. Curr. Alzheimer Res 11, 283–290. [DOI] [PubMed] [Google Scholar]
- 87.Yin J, Turner GH, Lin H, Coons SW, Shi J, 2011. Deficits in spatial learning and memory is associated with hippocampal volume loss in aged apolipoprotein E4 mice. J. Alzheimers Dis. JAD 27, 89–98. doi: 10.3233/JAD-2011-110479 [DOI] [PubMed] [Google Scholar]
- 88.Yong S-M, Lim M-L, Low C-M, Wong B-S, 2014. Reduced neuronal signaling in the ageing apolipoprotein-E4 targeted replacement female mice. Sci. Rep 4, 6580. doi: 10.1038/srep06580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu Y, He J, Zhang Y, Luo H, Zhu S, Yang Y, Zhao T, Wu J, Huang Y, Kong J, Tan Q, Li X-M, 2009. Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus 19, 1247–1253. doi: 10.1002/hipo.20587 [DOI] [PubMed] [Google Scholar]
- 90.Zepa L, Frenkel M, Belinson H, Kariv-Inbal Z, Kayed R, Masliah E, Michaelson DM, 2011. ApoE4-Driven Accumulation of Intraneuronal Oligomerized Aβ42 following Activation of the Amyloid Cascade In Vivo Is Mediated by a Gain of Function. Int. J. Alzheimers Dis 2011. doi: 10.4061/2011/792070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong N, Ramaswamy G, & Weisgraber KH (2009). Apolipoprotein E4 domain interaction induces endoplasmic reticulum stress and impairs astrocyte function. J Biol Chem, 284, 27273–27280. doi/ 10.1074/jbc.M109.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhong N, Scearce-Levie K, Ramaswamy G, & Weisgraber KH (2008). Apolipoprotein E4 domain interaction: synaptic and cognitive deficits in mice. Alzheimers Dement, 4, 179–192. doi/ 10.1016/j.jalz.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]