Abstract
Background:
A reduced willingness to perform effort based on the magnitude and probability of potential rewards has been associated with diminished dopamine function and may be relevant to attention-deficit/hyperactivity disorder (ADHD). Here, we investigated the influence of ADHD status and methylphenidate on effort-based decisions. We hypothesized that ADHD participants would make fewer high-effort selections than non-ADHD subjects, and that methylphenidate would increase the number of high-effort selections. Furthermore, we hypothesized there would be associations among ADHD severity and methylphenidate-related changes in effort-based and attentional performance across all participants.
Methods and participants:
ADHD (n = 23) and non-ADHD (n = 23) adults completed the Effort Expenditure for Rewards Task in which participants select between low-effort and high-effort options to receive monetary rewards at varying levels of reward magnitude and probability. A test of attentional performance was also completed.
Results:
Overall, participants made more high-effort selections as potential reward magnitude and probability increased. ADHD participants did not make fewer high-effort selections than non-ADHD participants, but ADHD participants showed greater methylphenidate-related increases in high-effort selections. ADHD participants had worse attentional performance than non-ADHD participants. ADHD severity was associated with methylphenidate-related changes in high-effort selections, but not changes in attentional performance.
Conclusions:
These results indicate that methylphenidate increases the willingness to perform effort in individuals with ADHD, possibly due to disorder-related motivational deficits. This provides support for theories of insufficient effort allocation among individuals with ADHD.
Keywords: Attention-deficit/hyperactivity disorder, effort, methylphenidate, attention
1.0. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset neuropsychiatric disorder marked by symptoms of inattention, hyperactivity, and impulsivity that interfere with school, work, and social responsibilities (APA, 2013). About half of childhood cases of ADHD are thought to persist into adulthood (Biederman et al., 2000) and ADHD is estimated to affect 2.5% of the U.S. adult population (Simon et al., 2009). ADHD is well known to cause problems with cognitive performance (Hervey et al., 2004; Boonstra et al., 2005). In addition, several theoretical models posit that attention deficits are associated with altered reinforcement sensitivity and reduced motivation (Nigg, 2005; Tripp and Wickens, 2009; Luman et al., 2010) based on research indicating that ADHD subjects prefer smaller, immediate over larger, delayed rewards and their cognitive performance improves when provided with larger reinforcements for performance (Marx et al., 2013; Modesto-Lowe et al., 2013). Many of these theoretical models suggest altered reinforcement sensitivity and motivation are related to dysregulated or diminished dopamine function (Nigg, 2005; Tripp and Wickens, 2009; Luman et al., 2010).
ADHD symptoms may arise from hypo-active dopamine (DA) and other catecholamine systems (Faraone and Biederman, 1998; Faraone et al., 2015). Indeed, adults with ADHD have reduced markers of DA function in the reward pathway (Volkow et al., 2009), and reduced DA receptors in adults with ADHD have been shown to correlate with less trait motivation (Volkow et al., 2011). DA is an important regulator of effort and energy expenditure, which are important components of motivation (Salamone et al., 2016). Effort expenditure is costly, and effort-based decision making weighs the value or preference of a reinforcer, as well as the likelihood of receiving it, against the amount of effort needed to obtain it (Salamone et al., 2016). Preclinical and clinical studies have shown that increasing or decreasing DA transmission via drugs, DA depletions, or genetic manipulations can enhance or diminish (respectively) behavioral responding for a reinforcer, especially when a large amount of effort is required to obtain it (Caul and Brindle, 2001; Floresco et al., 2008; Venugopalan et al., 2011; Wardle et al., 2011; Beierholm et al., 2013; de Jong et al., 2015). Importantly, the hedonic value of the reinforcer does not appear to be affected, e.g., rodents with dopamine depletions/antagonists still prefer highly-palatable foods to standard chow and maintain low-effort responding for palatable food and its consumption (Salamone et al., 2016). The willingness to exert effort to obtain reinforcers is an important feature of healthy decision making. DA transmission may enable the decision to engage in effortful activity and alterations in DA-ergic activity may result in individual differences in effortful decision making. This decision making may be impaired in psychiatric disorders marked by dysfunction in the DA system, such as ADHD.
It has been theorized that deficits in cognitive performance and motivation reported among individuals with ADHD may be related to insufficient effort allocation (Sergeant, 2005), however, little empirical research has been conducted. One study using a verbal learning test reported ADHD children use less effortful learning strategies, which could account for poor memory performance (Egeland et al., 2010). Another study used a handheld dynamometer to test the willingness to perform physical effort among children with and without ADHD (Winter et al., 2016). Participants chose between a high effort-high reward option and a low effort-low reward alternative. Individuals with ADHD did not choose fewer high effort-high reward options, although they did fail to recruit sufficient effort to successfully perform the high effort task more often than controls (Winter et al., 2016). However, participants were not asked to refrain from using medication to treat ADHD on the study day, and stimulant medication may have mitigated any performance differences (Winter et al., 2016).
A common stimulant medication for ADHD is methylphenidate (MPH), which increases extracellular DA levels in the brain (Volkow et al., 2002). MPH modestly improves cognitive performance deficits, such as working memory, reaction time variability, and vigilance (Pievsky and McGrath, 2018). MPH may indirectly improve cognitive performance by enhancing the saliency of a cognitive challenge, thus making it more interesting (Volkow et al., 2004). MPH has also been shown to affect performance on progressive ratio tasks, which require an increasing number of lever presses to obtain a reinforcer, thus testing the willingness to exert effort. DA depletion decreases persistence on progressive ratio tasks in preclinical studies (Salamone et al., 2006), and, conversely, MPH has been shown to increase progressive ratio persistence in children with ADHD (Wilkison et al., 1995; Chelonis et al., 2011). Altogether, this suggests that diminished DA function produces deficits in motivation and effort allocation reported in ADHD, and DA-ergic medications such as MPH may improve these specific deficits.
In the present study, medication-free male and female adults with and without ADHD were administered a single dose of MPH (40 mg) or placebo in counter-balanced order on two separate study days. They were administered a measure of willingness to exert effort known as the Effort Expenditure for Rewards Task (EEfRT). This task presents a choice between a low-effort button-pressing task for a small monetary reward and a high-effort button-pressing task for a larger monetary reward. The probability of receiving a reward upon successful task completion, and the magnitude of the high-effort reward, vary across trials so that the selection of high-effort tasks is informed by the expected value (probability × magnitude) of the reward (Treadway et al., 2009). The design of this task is based on preclinical work in animal models revealing DA modulation of effort expenditure (Salamone et al., 2016). In healthy adults, drugs that enhance DA transmission increase effort expenditure in the EEfRT (Wardle et al., 2011; Treadway et al., 2012b). In the present study, participants were also administered a measure of attentional performance and reaction time to explore whether differences in effort expenditure were associated with cognitive deficits. This measure, the Attention Network Test (ANT), assesses the efficiency with which reflexive and voluntary attention is oriented to special cues (Fan et al., 2002). We hypothesized that, in the placebo condition, participants with ADHD would select fewer high-effort selections than non-ADHD controls, and that MPH would increase the number of high-effort selections from the placebo condition. Furthermore, we hypothesized that there would be associations among ADHD severity and changes in performance from placebo to MPH in EEfRT and ANT measures across all participants.
2.0. Methods
2.1. Participants.
Participants were recruited from the Durham, North Carolina (n = 14 ADHD, 9 non-ADHD) and Little Rock, Arkansas (n = 14 ADHD, 9 non-ADHD) communities via social media, flyers, and word-of-mouth. Participants completed a phone interview and in-person screening session to determine eligibility. Eligible participants were between the ages of 18–45 years. To be eligible, ADHD participants had to have T-scores ≥ 65 for inattentive and/or hyperactive-impulsive symptoms on the Conners’ Adult ADHD Rating Scale (CAARS) (Conners et al., 1998), and were evaluated to meet criteria for a primary diagnosis of ADHD based on the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (CAADID) (Epstein et al., 2001). Non-ADHD participants had to have T-scores < 55 for inattentive, hyperactive-impulsive, and total symptoms.
Participants were excluded if they reported serious health problems (e.g., uncontrolled cardiovascular disease) or neurological problems (e.g., seizure disorder or traumatic brain injury), met criteria for a psychiatric disorder other than ADHD (except for symptoms of depression or anxiety co-morbid with ADHD) based on the MINI International Neuropsychiatric Interview (Sheehan et al., 2009), reported drug or alcohol dependence in the past 12 months (other than tobacco), reported daily use of medication for ADHD in the past 6 months, had hypertension (i.e., blood pressure > 140/90 mmHg), or had contraindications for MPH (e.g., motor tics). Participants were also excluded if they tested positive for drugs (iCup, Alere Toxicology Services Portsmouth, VA), alcohol (Alco-Sensor III, Intoximeters Inc St. Louis, MO), or pregnancy (QuickVue+, Quidel Corporation San Diego, CA).
Seventy-nine individuals were consented and screened to participate in the study, and 28 participants were ineligible because they did not meet ADHD/non-ADHD criteria (n = 11), had hypertension (n = 6), had a positive drug screen (n = 4) had another Axis I diagnosis (n = 3), withdrew before the study day (n = 4). Of the 51 participants that met eligibility criteria and began the study, 46 participants completed all aspects of the study and were included in the data analysis. Participants provided written informed consent and this protocol was approved by Duke University’s and University of Arkansas for Medical Sciences’ Institutional Review Boards.
2.2. Effort Expenditure for Rewards Task (EEfRT) (Treadway et al., 2009):
In each trial of the EEfRT, participants choose between two task options to earn money. Both task options consist of repeated manual button presses within a short amount of time, and completed button presses are represented onscreen by the height of a vertical bar. The low effort option requires 30 button presses with the dominant index finger within 7 sec. The high effort option requires 100 button presses with the nondominant little finger within 21 sec. Participants were monitored during the task to ensure they used the correct finger.
In low-effort trials, participants could receive $1.00 if they completed the task on time. In high-effort trials, participants could receive a variable amount between $1.24 and $4.30 (i.e., reward magnitude). Across trials, the likelihood of receiving money upon successful completion of the task was either 12%, 50%, or 88% (i.e., reward probability). The probability level applied to both the low and high effort tasks. At the start of each trial, participants were shown the reward magnitude for both task options and the probability level. They had 5 sec to make a choice or else they would be randomly assigned to a task. Then, they completed the button press task and received feedback informing them if the task was completed successfully or not, and whether they received money for that trial. Participants were told a single trial that resulted in money reward would be selected at random at the end of the EEfRT, and the participant would be given this amount as bonus pay. See Figure 1.
Figure 1.
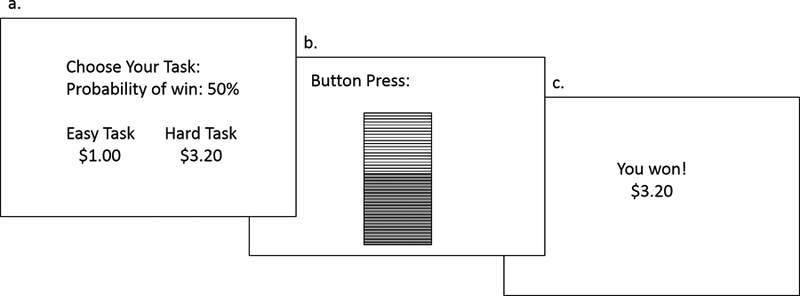
Representation of a single trial of the Effort Expenditure for Rewards Task (EEfRT). a) Participants are shown information about the reward magnitude of the high-effort task and the probability of receiving the reward for that trial. b) Participants made button presses to complete the chosen task for 7 sec (low-effort task) or 21 sec (high-effort task). c) Participants received feedback indicating whether and how much reward was received for that trial.
Low-effort trials lasted approximately 15 sec, and high-effort trials lasted approximately 30 sec. Participants were told they had 20 min to play as many trials as possible. They were informed of the trade-off between choosing too many high-effort tasks and missing out on playing large-magnitude, large-probability trials later in the game to discourage the exclusive selection of either the low-effort or high-effort task. This also helped ensure that decisions were based on the expected value of the reward, and not based on a strategy to always select high-effort or low-effort trials. Trials were presented in the same randomized order to all participants. The primary dependent variable was the percent high-effort selections by reward probability and magnitude (divided into 4 bins with an equal number of trials for analysis). Other performance metrics consisted of total number of trials completed and the ratio of high-effort trials completed/selected.
2.3. Attention Network Test (ANT) (Fan et al., 2002).
The ANT combines a cued reaction-time test and flanker test to measure the efficiency and accuracy of three cognitive networks: alerting, orienting, and conflict (Posner and Petersen, 1990). On each trial, a row of five horizontal black lines, with arrowheads pointing left or right, is shown onscreen above or below a center fixation cross. The target is the center arrow. The target is flanked by arrows pointing in the same direction (congruent condition), or in the opposite direction (incongruent condition) or by lines (neutral condition). Participants indicate the direction of the target arrow using the arrow keys. The arrows are preceded by four types of cues (no cue, center cue, double, spatial cue), which either indicate the arrows will appear soon and/or predict the location of the arrows above or below the fixation cross. The primary dependent variables were the alerting, orienting, and executive function scores. The alerting score is the difference in reaction time between the temporally informative cue condition and the temporally uninformative cue condition. The orienting score is the difference in reaction time between the spatially informative cue condition and the spatially uninformative cue condition. The conflict score is the difference in reaction time between the congruent flanker condition and the incongruent flanker condition. Other performance metrics consisted of overall percent accuracy and average reaction time.
2.4. Procedure.
After consenting and eligibility evaluation, the participants were scheduled for two study visits. These study visits were scheduled within two weeks of each other, but were at least 48 hours apart. For each participant, both study visits occurred either in the afternoon or the morning. Participants were instructed to skip the meal prior to the study visit (i.e., either breakfast or lunch). Participants were administered either immediate-release methylphenidate (MPH: 40 mg) or a matching placebo (PLA) under double-blind conditions. Drugs were ordered and compounded through a pharmacy, and the placebo consisted of lactose. The prescription and medical oversight was provided by a study physician. After administration, participants were given two cereal bars, a fruit cup, and 8 oz of water and rested for 1 hour to allow for drug absorption. The study visit lasted for a total of 3 hours, and the EEfRT and ANT were completed approximately 2.5 hours after drug administration. The Positive and Negative Affect Scale (PANAS) (Watson et al., 1988) was administered prior to drug administration and at the end of the study visit. At the end of the visit, participants also rated to what extent they felt a drug effect on a scale from 1 (not at all) to 10 (extremely). Compensation for participation was provided at the end of the study and participants were told they could earn up to $5 in bonus pay for each task, depending on their task performance.
2.5. Data Analysis.
Participant demographics were analyzed using independent-samples t-tests and Chi-Square tests. Age was included as a covariate in subsequent analyses due to differences between groups. The drug effects questionnaire, PANAS positive and negative scales, performance metrics in the EEfRT and ANT, and ANT dependent variables were analyzed using separate 2 (Group) × 2 (Drug) repeated-measures ANCOVAs. Percent high-effort selections in the EEfRT were analyzed using a 2 (Group) × 2 (Drug) × 4 (Reward Magnitude) × 3 (Reward Probability) repeated-measures ANCOVA. Follow-up comparisons were univariate ANCOVAs. Since participants could complete a variable number of trials during the 20 min of the EEfRT, only data from the first 50 trials were used for consistency (Treadway et al., 2012a). Associations between CAARS scores and the EEfRT and ANT dependent variables were performed using partial correlations (controlling for age). Data were analyzed using SPSS v24 (Chicago: SPSS Inc).
An initial exploration of the EEfRT and ANT performance metrics revealed no differences by site (UAMS vs Duke); thus, site was not included as a covariate in the analyses.
3.0. Results.
3.1. Participants.
A total of 23 ADHD (12 men) and 23 non-ADHD (11 men) participants were included in the analysis. Participant demographics are shown in Table 1. Groups did not differ in sex ratio or years of education. The ADHD group was older (t(44) = 2.3, p = .027) and had fewer Black individuals (Chi-square(2) = 8.3, p = .016). As expected, the ADHD group had greater CAARS T-scores for Inattentive Symptoms (t(44) = 20.5, p < .001), Hyperactivity Symptoms (t(44) = 11.9, p < .001), and DSM ADHD score (t(44) = 18.3, p < .001).
Table 1.
Participant demographics for ADHD and non-ADHD groups, mean ± standard deviation.
| ADHD (n=23) | non-ADHD (n=23) | p-value | |
|---|---|---|---|
| Sex (M/F) | 12/11 | 11/12 | p > .7 |
| Age (years) | 32.9 ± 8.4 | 27.8 ± 6.5 | p = .027 |
| Years of education | 16.0 ± 3.1 | 15.8 ± 2.8 | p > .8 |
| Race (White/Black/other) |
18/0/5 | 12/7/4 | p = .016 |
| CAARS DSM Inattentive T-score | 82.5 ± 8.6 | 41.1 ± 4.5 | p < .001 |
| CAARS DSM Hyperactivity T-score | 71.3 ± 11.7 | 39.5± 5.3 | p < .001 |
| CAARS DSM ADHD T-score | 81.7 ± 9.9 | 39.2 ± 5.1 | p < .001 |
On the PANAS, positive mood scores decreased from (M ± SD) 26.6 ± 6.8 to 21.2 ± 5.8 after PLA, and increased from 26.4 ± 6.6 to 27.5 ± 7.1 after MPH across both groups (Drug × Time interaction effect: F(1,42) = 5.8, p = .020). Negative mood scores decreased from 12.2 ± 2.2 to 11.0 ± 1.4 after PLA, and increased from 11.9 ± 1.9 to 12.3 ± 2.2 after MPH across both groups (Drug × Time interaction effect: F(1,42) = 6.6, p = .014). Across both drug conditions, negative mood scores decreased from 13.1 ± 2.4 to 11.7 ± 2.0 in the ADHD group and increased from 11.0 ± 2.4 to 11.6 ± 2.0 in the non-ADHD group (Group × Time interaction effect: F(1,42) = 8.8, p = .005). There were no other significant effects of group, drug, or time on PANAS scores. PANAS data from a non-ADHD participant was missing and the participant was excluded from the data analysis.
The participants reported feeling a greater drug effect after MPH (M ± SD: 5.0 ± 3.0) compared to PLA (1.8 ± 1.5) (F(1,42) = 7.0, p = .012). There were no other significant effects of group or group × drug interactions in drug effects scores.
3.2. EEfRT.
The performance metric analyses revealed no differences between groups or drug conditions in the number of trials completed (M ± SD: 63.4 ± 8.2) or the ratio of high-effort trials completed/selected (0.87 ± 0.22), indicating no differences between groups, drug conditions, or group × drug interactions in the ability to perform the task.
Across all participants, high-effort selections in the EEfRT increased as the reward magnitude increased (Magnitude effect: F(3,41) = 3.3, p = .029) and increased as the reward probability increased (Probability effect: F(2,42) = 9.7, p < .001). See Figure 2.
Figure 2.
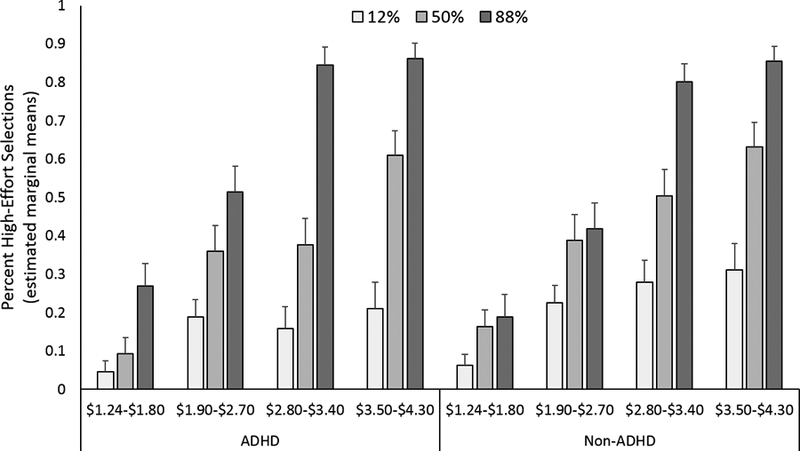
The percent high-effort selections made across reward magnitude (x-axis) and reward probability (different colored bars) in ADHD (on the left) and non-ADHD (on the right) groups. Across all participants and drug conditions, high-effort selections increased as reward magnitude and probability increased (p’s < .05). Shown are the estimated marginal means (including age as a covariate of no interest). Error bars are S.E.M.
Between groups, MPH had a greater effect on high-effort selections among the ADHD group than the non-ADHD group (Group × Drug interaction effect: F(1,43) = 13.6, p = .001). This was due to a greater increase in the average number of high-effort selections from PLA to the MPH condition among ADHD (mean difference ± SD: 0.10 ± .10) compared to non-ADHD (−0.01 ± .10) (F(1,43) = 13.6, p = .001). See Figure 3.
Figure 3.
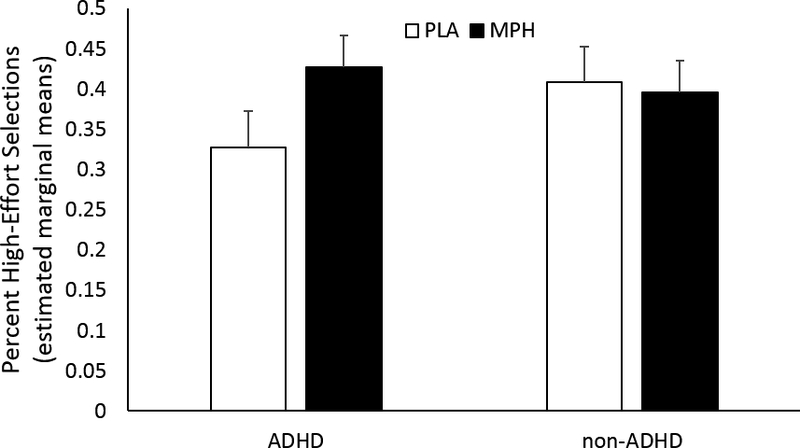
The percent high-effort selections among ADHD and non-ADHD groups after placebo (PLA, white bars) and methylphenidate (MPH, black bars). MPH had a greater effect in the ADHD group than the non-ADHD group (Group × Drug interaction effect, p = .001). Shown are the estimated marginal means (including age as a covariate of no interest). Error bars are S.E.M.
Across both drug conditions, reward probability had a greater effect on high-effort selections among the ADHD group than the non-ADHD group (Group × Probability interaction effect: F(2,42) = 3.4, p = .044). This was due to a greater increase in high-effort selections from the 50% to the 88% probability conditions among ADHD (mean difference ± SD: 0.26 ± 0.16) compared to non-ADHD (0.14 ± 0.16) (F(1,43) = 6.4, p = .015). There were no other significant main or interaction effects.
3.3. ANT.
The performance metric analyses revealed no differences between groups or drug conditions in overall percent accuracy (M ± SD: 0.96 ± 0.05) and average reaction time (619.1 ± 111.3 msec), indicating no differences between groups or drug conditions in the ability to perform the task.
Across all participants, the alerting score was smaller after MPH (M ± SD: 34.5 ± 18.0) compared to PLA (42.2 ± 21.6) (Drug effect: F(1,43) = 4.3, p = .045). Between groups, the alerting score was larger (i.e., indicating worse performance) among the ADHD group (46.7 ± 22.8) than the non-ADHD group (30.0 ± 22.8) (Group effect: F(1,43) = 5.8, p = .020). See Figure 4. There was no significant group by drug interaction effect, nor were there any significant effects for orienting scores or conflict scores.
Figure 4.
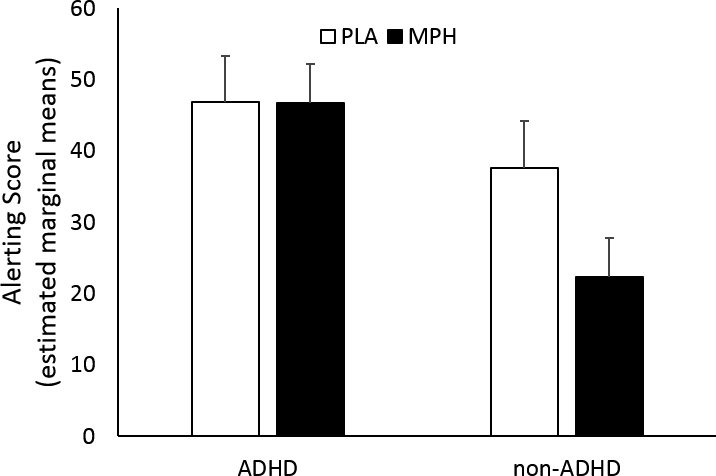
The alerting score from the Attention Network Test (ANT) among ADHD and non-ADHD groups after placebo (PLA, white bars) and methylphenidate (MPH, black bars). Across both groups, the alerting score was smaller after MPH, and between groups, the alerting score was larger among the ADHD group than the non-ADHD group (p’s < .05). Shown are the estimated marginal means (including age as a covariate of no interest). Error bars are S.E.M.
3.4. Correlations across variables.
Partial correlations (controlling for age) were conducted to test whether the CAARS DSM ADHD T-score was associated with changes in performance (MPH minus PLA) for the EEfRT and ANT dependent variables (EEfRT percent high-effort selections; ANT alerting, orienting, and conflict scores). The ADHD T-score only correlated with the difference in EEfRT percent high-effort selections (averaged across all reward magnitudes and probabilities) (r = .484, p = .001). In other words, as ADHD symptoms became more severe, MPH produced a larger increase in the percent of high-effort selections. There were no significant correlations between EEfRT and ANT dependent variables.
4.0. Discussion
The goal of this study was to investigate the effects of MPH on effort-based decision making in medication-free adults with and without ADHD. Participants selected between low-effort/low-reward and high-effort/high-reward alternatives, with the probability of both rewards and the magnitude of the high-effort alternative varying across trials. We report that MPH had a greater effect on increasing high-effort selections among the ADHD group, although the ADHD group did not make fewer high-effort selections than the non-ADHD group during the PLA condition as hypothesized. Across both MPH and PLA conditions, the ADHD group was more sensitive to changes in high-effort reward probability than the non-ADHD group. In contrast, attentional performance on a separate measure of cognition was worse among the ADHD group than the non-ADHD group. MPH improved attentional performance across both groups, although this effect appeared to have been driven primarily by changes in the non-ADHD group. ADHD severity correlated with MPH-related increases in effort-based decision making, but not in attentional performance. To our knowledge, this is the first study to relate ADHD severity to the effects of MPH on effort-based decision making, and the results suggest that MPH increases the willingness to perform effort in individuals with ADHD. This change in motivation may have indirect effects on other cognitive functions and goal-directed behavior.
Many previous studies on motivation and decision-making in ADHD have focused on delay of gratification or delay discounting (i.e., an aversion to a delay to reward receipt). Individuals with ADHD have been shown to prefer smaller-sooner over larger-later rewards more than non-ADHD peers (Jackson and MacKillop, 2016; Patros et al., 2016). Delay discounting and other types of risky decision making associated with ADHD are considered forms of impulsivity (Malloy-Diniz et al., 2007; Drechsler et al., 2008; Matthies et al., 2012). A diminished willingness to perform effort could also be construed as an impulsive behavior, in that low-effort/small-rewards are preferred to high-effort/large-rewards. It is possible that effort discounting is modulated by the same underlying mechanism as delay discounting, and MPH has been shown to reduce experiential delay discounting in children with ADHD (Shiels et al., 2009). However, a meta-analysis in healthy adults found that delay, probability, and effort discounting were unrelated and all were generally uncorrelated with DA D2 receptor availability (Castrellon et al., 2019). Furthermore, evidence from behavioral economic and neuroimaging research conducted with healthy subjects indicates that physical effort is dissociable from delay- and risk-related decision making (Prevost et al., 2010; Burke et al., 2013; Klein-Flugge et al., 2015). In support of this, adolescents with ADHD were shown to have steeper experiential delay discounting than non-ADHD peers, but groups did not differ in effort discounting (Mies et al., 2018). Potentially, constructs that are dissociable in healthy subjects may be less separable in individuals with psychiatric disorders, as psychopathologies differ in how DA function and its downstream effects are disrupted, and more research is needed to understand the relationship between effort and delay discounting among individuals with motivational deficits (Castrellon et al., 2019).
Previous research using the EEfRT has shown that participants with major depressive disorder were less likely to make high-effort selections (Treadway et al., 2012a; Yang et al., 2014) and were less sensitive to reward magnitude and probability than non-depressed controls (Treadway et al., 2012a). Similarly, participants with schizophrenia were less sensitive to reward magnitude and probability, although they were not less likely to make high-effort selections than healthy controls (Barch et al., 2014; Treadway et al., 2015). These two psychiatric disorders have also been linked to DA dysfunction (Davis et al., 1991; Nestler and Carlezon, 2006; Pierce and Kumaresan, 2006; Volkow et al., 2009). However, in the present study ADHD participants were more sensitive to reward probability than non-ADHD participants. While this is somewhat inconsistent with previous studies (Luman et al., 2009), it does support theories of altered reinforcement sensitivity in ADHD (Luman et al., 2010). However, this raises the question of how DA dysfunction could give rise to either a heightened or diminished sensitivity to the expected value of rewards. The answer may lie in how large-scale brain networks are affected by different psychopathologies (Castellanos and Proal, 2012).
The EEfRT is a measure of willingness to perform physical energy, but attentional and executive function deficits reported in ADHD have been related to a reduced willingness to perform cognitive effort (APA, 2013; Hsu et al., 2017). The willingness to exert physical versus cognitive effort may or may not be separable dimensions (Verguts et al., 2015; Westbrook and Braver, 2015). Cognitive effort does not have the metabolic cost of physical effort, and little is known about the core mechanisms underlying the decision to engage in cognitive effort (Westbrook and Braver, 2015). In addition, different neural systems may subserve cognitive (e.g., prefrontal-parietal network) versus physical effort (e.g., motor cortices) (Schmidt et al., 2012). Yet, like physical effort, cognitive effort (e.g., adaptive working memory allocation) is modulated by DA (Westbrook and Braver, 2016) and there is evidence that physical and cognitive effort share a common motivational hub in the striatum, putatively modulated by DA (Schmidt et al., 2012). Furthermore, a study using simulated data from a neurocomputational model of reinforcement learning predicted that cognitive and physical effort relies on similar circuitry and performance would be correlated (Verguts et al., 2015). There are cognitive effort tasks, one in particular parallels the structure of the EEfRT (Lopez-Gamundi and Wardle, 2018), which could be used in conjunction with physical effort tasks to test this prediction.
In the current study, we report that the ADHD group had worse alerting scores on the ANT than the non-ADHD group, but groups had similar conflict and orienting scores. This is similar to two previous studies in children with and without ADHD (Mullane et al., 2011; Samyn et al., 2017). Most studies report differences in conflict scores (reviewed in Casagrande et al., 2012), although there is a great deal of heterogeneity in ANT performance across studies (reviewed in Casagrande et al., 2012; Samyn et al., 2017). Indeed, heterogeneity has been noted across measures and cognitive domains (Mostert et al., 2015), making it difficult to isolate a particular deficit for therapeutic intervention. While the ANT is not a measure of motivation or willingness to perform cognitive effort, we had hypothesized that improved motivation/willingness to perform effort would translate into improved attentional performance, which does not appear to be the case. Alternatively, the ANT might not be the best measure of the effects of MPH on attentional deficits and future studies should include other tasks (e.g., Continuous Performance Test) that may be better suited for this purpose (Losier et al., 1996).
The strengths of this study include a placebo-controlled, counterbalanced design and the use of an effort-based measure shown to have good test-retest reliability (Reddy et al., 2015). However, participants reported a greater drug effect after receiving MPH than PLA, suggesting discrimination of the stimulant medication. Another method of blinding the participants would be to provide a list of alternative medications (e.g., caffeine) they may receive during the study visit, with MPH and PLA being two possibilities. Another potential limitation is that many children with ADHD have abnormal motor skills (Kaiser et al., 2015), which could have made it more difficult for the ADHD group to perform the EEfRT. However, the performance metrics indicated that the ADHD group completed the same ratio of high-effort completions to selections and completed a similar number of trials compared to non-ADHD group, so this confound appears to be minimal. Although a better way of eliminating this confound would be to measure finger tapping speed, to eliminate potential motor function differences between groups (Barch et al., 2014). Other limitations include the potential for variation across individuals in the salience of the EEfRT task and the monetary reward, the sample size may have been underpowered to detect group differences in EEfRT performance in the placebo condition, and the lack of group-matching based on race.
In summary, this study suggests that increasing ADHD severity relates to a larger effect of MPH on increasing the willingness to perform physical effort. The results of this study may have clinical implications. Children with ADHD tend to have academic problems related to their symptoms (Faraone et al., 1993), and adults with ADHD self-report lower levels of trait achievement (Volkow et al., 2011). MPH improves lab-based cognitive performance (Coghill et al., 2014; Pievsky and McGrath, 2018), as well as academic achievement in children and adolescents with ADHD (Evans et al., 2001; Hechtman et al., 2004; Kortekaas-Rijlaarsdam et al., 2019). The effects of MPH on cognitive performance may be directly or indirectly modulated by motivation level and the willingness to exert effort. Little research has been conducted on effort-based decision making in ADHD, but such research could help shed light on the relationship between DA function and motivation.
Highlights:
MPH increased effort-related behavior in individuals with ADHD
Attentional performance was worse in individuals with ADHD
ADHD severity is associated with MPH-related changes in effort behavior
Acknowledgements:
This work was supported by the Brain and Behavior Research Foundation (Grant #23703, PI: Addicott). The authors would like to thank Michael T. Treadway for providing the EEfRT.
Footnotes
Trial registration: Clinicaltrials.gov Identifier, NCT02630017.
Conflict of Interest: All authors declare that they have no conflict of interest pertaining to this manuscript.
Compliance with ethical standards: This protocol followed all applicable regulations for protection of human subjects as outlined by the United States Department of Health & Human Services, including the Food and Drug Administration; and was approved by our local Institutional Review Board.
References
- APA (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC, American Psychiatric Association. [Google Scholar]
- Barch DM, Treadway MT, Schoen N (2014). Effort, Anhedonia, and Function in Schizophrenia: Reduced Effort Allocation Predicts Amotivation and Functional Impairment. J Abnorm Psychol 123: 387–397. DOI: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierholm U, Guitart-Masip M, Economides M, Chowdhury R, Duzel E, Dolan R, Dayan P (2013). Dopamine Modulates Reward-Related Vigor. Neuropsychopharmacol 38: 1495–1503. DOI: 10.1038/npp.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV (2000). Age-dependent decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. Am J Psychiat 157: 816–818. DOI: DOI 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Boonstra AM, Oosterlaan J, Sergeant JA, Buitelaar JK (2005). Executive functioning in adult ADHD: a meta-analytic review. Psychol Med 35: 1097–1108. DOI: 10.1017/S003329170500499x. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Brunger C, Kahnt T, Park SQ, Tobler PN (2013). Neural Integration of Risk and Effort Costs by the Frontal Pole: Only upon Request. J Neurosci 33: 1706-+ DOI: 10.1523/Jneurosci.3662-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande M, Martella D, Ruggiero MC, Maccari L, Paloscia C, Rosa C, Pasini A (2012). Assessing Attentional Systems in Children with Attention Deficit Hyperactivity Disorder. Arch Clin Neuropsych 27: 30–44. DOI: 10.1093/arclin/acr085. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E (2012). Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci 16: 17–26. DOI: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrellon JJ, Searnan KL, Crawford JL, Young JS, Smith CT, Dang LC, Hsu M, Cowan RL, Zald DH, Samanez-Larkin GR (2019). Individual Differences in Dopamine Are Associated with Reward Discounting in Clinical Groups But Not in Healthy Adults. J Neurosci 39: 321–332. DOI: 10.1523/Jneurosci.1984-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caul WF, Brindle NA (2001). Schedule-dependent effects of haloperidol and amphetamine: multiple-schedule task shows within-subject effects. Pharmacol Biochem Be 68: 53–63. DOI: Doi 10.1016/S0091-3057(00)00431-7. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Johnson TA, Ferguson SA, Berry KJ, Kubacak B, Edwards MC, Paule MG (2011). Effect of Methylphenidate on Motivation in Children With Attention-Deficit/Hyperactivity Disorder. Exp Clin Psychopharm 19: 145–153. DOI: 10.1037/a0022794. [DOI] [PubMed] [Google Scholar]
- Coghill DR, Seth S, Pedroso S, Usala T, Currie J, Gagliano A (2014). Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol Psychiatry 76: 603–615. DOI: 10.1016/j.biopsych.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow E (1998). The Conners adult ADHD rating scale (CAARS). Toronto, Multi-Health Systems, Inc. [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M (1991). Dopamine in Schizophrenia - a Review and Reconceptualization. Am J Psychiat 148: 1474–1486. [DOI] [PubMed] [Google Scholar]
- de Jong JW, Roelofs TJM, Mol FMU, Hillen AEJ, Meijboom KE, Luijendijk MCM, van der Eerden HAM, Garner KM, Vanderschuren LJMJ, Adan RAH (2015). Reducing Ventral Tegmental Dopamine D2 Receptor Expression Selectively Boosts Incentive Motivation. Neuropsychopharmacol 40: 2085–2095. DOI: 10.1038/npp.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler R, Rizzo P, Steinhausen HC (2008). Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. J Neural Transm (Vienna) 115: 201–209. DOI: 10.1007/s00702-007-0814-5. [DOI] [PubMed] [Google Scholar]
- Egeland J, Johansen SN, Ueland T (2010). Do Low-Effort Learning Strategies Mediate Impaired Memory in ADHD? J Learn Disabil-Us 43: 430–440. DOI: 10.1177/0022219409355473. [DOI] [PubMed] [Google Scholar]
- Epstein J, Johnson DE, Conners CK (2001). Conners’ adult ADHD diagnostic interview for DSM-IV (CAADID). New York, MHS. [Google Scholar]
- Evans SW, Pelham WE, Smith BH, Bukstein O, Gnagy EM, Greiner AR, Altenderfer L, Baron-Myak C (2001). Dose-response effects of methylphenidate on ecologically valid measures of academic performance and classroom behavior in adolescents with ADHD. Exp Clin Psychopharm 9: 163–175. DOI: 10.1037//1064-1297.9.2.163. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002). Testing the efficiency and independence of attentional networks. J Cognitive Neurosci 14: 340–347. DOI: Doi 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJS, Tannock R, Franke B (2015). Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 1 DOI: UNSP 15020 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J (1998). Neurobiology of attention-deficit hyperactivity disorder. Biol Psychiat 44: 951–958. DOI: Doi 10.1016/S0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Lehman BK, Spencer T, Norman D, Seidman LJ, Kraus I, Perrin J, Chen WJ, Tsuang MT (1993). Intellectual-Performance and School Failure in Children with Attention-Deficit Hyperactivity Disorder and in Their Siblings. J Abnorm Psychol 102: 616–623. DOI: Doi 10.1037//0021-843x.102.4.616. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MTL, Ghods-Sharifi S (2008). Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacol 33: 1966–1979. DOI: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Hechtman L, Abikoff H, Klein RG, Weiss G, Respitz C, Kouri J, Blum C, Greenfield B, Etcovitch J, Fleiss K, Pollack S (2004). Academic achievement and emotional status of children with ADHD treated with long-term methylphenidate psychosocial and multimodal treatment. J Am Acad Child Psy 43: 812–819. DOI: 10.1097/01.chi.0000128796.84202.eb. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF (2004). Neuropsychology of adults with attention-deficit/hyperactivity disorder: A meta-analytic review. Neuropsychology 18: 485–503. DOI: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Hsu CF, Eastwood JD, Toplak ME (2017). Differences in Perceived Mental Effort Required and Discomfort during a Working Memory Task between Individuals At-risk And Not At-risk for ADHD. Frontiers in Psychology 8 DOI: ARTN 407 10.3389/fpsyg.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JN, MacKillop J (2016). Attention-Deficit/Hyperactivity Disorder and Monetary Delay Discounting: A Meta-Analysis of Case-Control Studies. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 316–325. DOI: 10.1016/j.bpsc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser ML, Schoemaker MM, Albaret JM, Geuze RH (2015). What is the evidence of impaired motor skills and motor control among children with attention deficit hyperactivity disorder (ADHD)? Systematic review of the literature. Res Dev Disabil 36: 338–357. DOI: 10.1016/j.ridd.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Klein-Flugge MC, Kennerley SW, Saraiva AC, Penny WD, Bestmann S (2015). Behavioral Modeling of Human Choices Reveals Dissociable Effects of Physical Effort and Temporal Delay on Reward Devaluation. Plos Comput Biol 11 DOI: UNSP e1004116 10.1371/journal.pcbi.1004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekaas-Rijlaarsdam AF, Luman M, Sonuga-Barke E, Oosterlaan J (2019). Does methylphenidate improve academic performance? A systematic review and meta-analysis. European Child & Adolescent Psychiatry 28: 155–164. DOI: 10.1007/s00787-018-1106-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Gamundi P, Wardle MC (2018). The cognitive effort expenditure for rewards task (C-EEfRT): A novel measure of willingness to expend cognitive effort. Psychol Assess 30: 1237–1248. DOI: 10.1037/pas0000563. [DOI] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM (1996). Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: A meta-analytic review. Journal of Child Psychology and Psychiatry and Allied Disciplines 37: 971–987. DOI: DOI 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A (2010). Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neuroscience and biobehavioral reviews 34: 744–754. DOI: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Luman M, van Meel CS, Oosterlaan J, Sergeant JA, Geurts HM (2009). Does reward frequency or magnitude drive reinforcement-learning in attention-deficit/hyperactivity disorder? Psychiat Res 168: 222–229. DOI: 10.1016/j.psychres.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A (2007). Impulsive behavior in adults with attention deficit/hyperactivity disorder: Characterization of attentional, motor and cognitive impulsiveness. J Int Neuropsych Soc 13: 693–698. DOI: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Marx I, Hopcke C, Berger C, Wandschneider R, Herpertz SC (2013). The Impact of Financial Reward Contingencies on Cognitive Function Profiles in Adult ADHD. Plos One 8 DOI: ARTN e67002 10.1371/journal.pone.0067002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies S, Philipsen A, Svaldi J (2012). Risky decision making in adults with ADHD. J Behav Ther Exp Psy 43: 938–946. DOI: 10.1016/j.jbtep.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Mies GW, Ma I, de Water E, Buitelaar JK, Scheres A (2018). Waiting and working for rewards: Attention-Deficit/Hyperactivity Disorder is associated with steeper delay discounting linked to amygdala activation, but not with steeper effort discounting. Cortex 106: 164–173. DOI: 10.1016/j.cortex.2018.05.018. [DOI] [PubMed] [Google Scholar]
- Modesto-Lowe V, Chaplin M, Soovajian V, Meyer A (2013). Are Motivation Deficits Underestimated in Patients With ADHD? A Review of the Literature. Postgrad Med 125: 47–52. DOI: 10.3810/pgm.2013.07.2677. [DOI] [PubMed] [Google Scholar]
- Mostert JC, Onnink AMH, Klein M, Dammers J, Harneit A, Schulten T, van Hulzen KJE, Kan CC, Slaats-Willemse D, Buitelaar JK, Franke B, Hoogman M (2015). Cognitive heterogeneity in adult attention deficit/hyperactivity disorder: A systematic analysis of neuropsychological measurements. Eur Neuropsychopharm 25: 2062–2074. DOI: 10.1016/j.euroneuro.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane JC, Corkum PV, Klein RM, McLaughlin EN, Lawrence MA (2011). Alerting, Orienting, and Executive Attention in Children With ADHD. Journal of Attention Disorders 15: 310–320. DOI: 10.1177/1087054710366384. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA (2006). The mesolimbic dopamine reward circuit in depression. Biol Psychiat 59: 1151–1159. DOI: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nigg JT (2005). Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: The state of the field and salient challenges for the coming decade. Biol Psychiat 57: 1424–1435. DOI: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, Hudec KL (2016). Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clin Psychol Rev 43: 162–174. DOI: 10.1016/j.cpr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V (2006). The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and biobehavioral reviews 30: 215–238. DOI: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pievsky MA, McGrath RE (2018). Neurocognitive effects of methylphenidate in adults with attention-deficit/hyperactivity disorder: A meta-analysis. Neuroscience and biobehavioral reviews 90: 447–455. DOI: 10.1016/j.neubiorev.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE (1990). The Attention System of the Human Brain. Annual Review of Neuroscience 13: 25–42. DOI: DOI 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Prevost C, Pessiglione M, Metereau E, Clery-Melin ML, Dreher JC (2010). Separate Valuation Subsystems for Delay and Effort Decision Costs. J Neurosci 30: 14080–14090. DOI: 10.1523/Jneurosci.2752-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Barch DM, Buchanan RW, Dunayevich E, Gold JM, Lyons N, Marder SR, Treadway MT, Wynn JK, Young JW, Green MF (2015). Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 1-Psychometric Characteristics of 5 Paradigms. Schizophrenia Bull 41: 1045–1054. DOI: 10.1093/schbul/sbv089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM (2006). Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: Implications for understanding anergia and psychomotor slowing in depression. Current Psychiatry Reviews 2: 1–14. [Google Scholar]
- Salamone JD, Yohn SE, Lopez-Cruz L, Miguel NS, Correa M (2016). Activational and effort-related aspects of motivation: neural mechanisms and implications for psychopathology. Brain 139: 1325–1347. DOI: 10.1093/brain/aww050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samyn V, Roeyers H, Bijttebier P, Wiersema JR (2017). Attentional Networks in Boys With ADHD or Autism Spectrum Disorder and the Relationship With Effortful Control. Journal of Attention Disorders 21: 228–239. DOI: 10.1177/1087054712473183. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Lebreton M, Clery-Melin ML, Daunizeau J, Pessiglione M (2012). Neural Mechanisms Underlying Motivation of Mental Versus Physical Effort. Plos Biol 10 DOI: ARTN e1001266 10.1371/journal.pbio.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant JA (2005). Modeling attention-deficit/hyperactivity disorder: A critical appraisal of the cognitive-energetic model. Biol Psychiat 57: 1248–1255. DOI: 10.1016/j.bps.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Janavas J, Harnett-Sheehan K, Sheehan M, Gray C (2009). MINI International Neuropsychiatric Interview (English Version 6.0.0). Tampa, FL, University of South Florida College of Medicine. [Google Scholar]
- Shiels K, Hawk LW, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE, Waxmonsky JG, Gangloff BP (2009). Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol 17: 291–301. DOI: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V, Czobor P, Balint S, Meszaros A, Bitter I (2009). Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Brit J Psychiat 194: 204–211. DOI: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH (2012a). Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. J Abnorm Psychol 121: 553–558. DOI: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH (2012b). Dopaminergic Mechanisms of Individual Differences in Human Effort-Based Decision-Making. J Neurosci 32: 6170–6176. DOI: 10.1523/Jneurosci.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH (2009). Worth the ‘EEfRT’? The Effort Expenditure for Rewards Task as an Objective Measure of Motivation and Anhedonia. Plos One 4 DOI: ARTN e6598 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Peterman JS, Zald DH, Park S (2015). Impaired effort allocation in patients with schizophrenia. Schizophr Res 161: 382–385. DOI: 10.1016/j.schres.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Wickens JR (2009). Neurobiology of ADHD. Neuropharmacology 57: 579–589. DOI: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Venugopalan VV, Casey KF, O’Hara C, O’Loughlin J, Benkelfat C, Fellows LK, Leyton M (2011). Acute Phenylalanine/Tyrosine Depletion Reduces Motivation to Smoke Cigarettes Across Stages of Addiction. Neuropsychopharmacol 36: 2469–2476. DOI: 10.1038/npp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verguts T, Vassena E, Silvetti M (2015). Adaptive effort investment in cognitive and physical tasks: a neurocomputational model. Front Behav Neurosci 9 DOI: ARTN 57 10.3389/fnbeh.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, Ding YS, Gatley SJ, Gifford A, Zhu W, Swanson JM (2002). Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: Therapeutic implications. Synapse 43: 181–187. DOI: DOI 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, Gatley SJ, Pappas N, Wong C, Vaska P, Zhu W, Swanson JM (2004). Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiat 161: 1173–1180. DOI: DOI 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma YM, Pradhan K, Wong C, Swanson JM (2009). Evaluating Dopamine Reward Pathway in ADHD Clinical Implications. Jama-J Am Med Assoc 302: 1084–1091. DOI: DOI 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, Fowler JS, Goldstein RZ, Klein N, Logan J, Wong C, Swanson JM (2011). Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatr 16: 1147–1154. DOI: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H (2011). Amping Up Effort: Effects of d-Amphetamine on Human Effort-Based Decision-Making. J Neurosci 31: 16597–16602. DOI: 10.1523/Jneurosci.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988). Development and Validation of Brief Measures of Positive and Negative Affect - the Panas Scales. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Westbrook A, Braver TS (2015). Cognitive effort: A neuroeconomic approach. Cogn Affect Behav Ne 15: 395–415. DOI: 10.3758/s13415-015-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Braver TS (2016). Dopamine Does Double Duty in Motivating Cognitive Effort. Neuron 89: 695–710. DOI: 10.1016/j.neuron.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkison PC, Kircher JC, Mcmahon WM, Sloane HN (1995). Effects of Methylphenidate on Reward Strength in Boys with Attention-Deficit Hyperactivity Disorder. J Am Acad Child Psy 34: 897–901. DOI: Doi 10.1097/00004583-199507000-00013. [DOI] [PubMed] [Google Scholar]
- Winter Y, Ben-Pazi H, Pollak Y (2016). Effort Allocation in Children With ADHD: Abnormal Decision-Making or Poor Execution? J Atten Disord. DOI: 10.1177/1087054716654569. [DOI] [PubMed] [Google Scholar]
- Yang XH, Huang J, Zhu CY, Wang YF, Cheung EFC, Chan RCK, Xie GR (2014). Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiat Res 220: 874–882. DOI: 10.1016/j.psychres.2014.08.056. [DOI] [PubMed] [Google Scholar]


