Abstract
D2 dopamine receptors (D2Rs) represent an important class of receptors in the pharmacological development of novel therapeutic drugs for the treatment of schizophrenia. Recent research into D2R signaling suggests that receptor properties are dependent on interaction with a coh01t of dopamine receptor interacting proteins (DRIPs) within a macromolecular structure termed the signalplex. One component of this signalplex is neuronal calcium sensor I (NCS-1) a protein found to regulate the phosphorylation, trafficking, and signaling profile of the D2R in neurons. It has also been found that NCS-1 can contribute to the pathology of schizophrenia and may play a role in the efficacy of antipsychotic drugs medication in the brain. In this review we discuss how the selective targeting of a DRIP, such as NCS-1, can be utilized as a novel strategy of drug design for the creation of new therapeutics for a disease such as schizophrenia.
Using a fluorescence polarization assay we explore how the ability to detect changes in D2R/NCS-1 interaction can be exploited as an effective screening tool in the isolation and development of lead compounds for antipsychotic drugs development This line of work explores a novel direction in targeting D2Rs via their signalplex components and supports the notion that receptor interacting proteins represent an emerging new class of molecular targets for pharmacological drug development.
Keywords: Drug development, schizophrenia, drug design, calcium signaling, therapeutics, dopamine receptor interacting protein, neuronal calcium, antipsychotic, GPCR, D2R/NCS-1 interaction
INTRODUCTION
The dopamine receptor (DR) is a functionally diverse class of the G protein coupled receptors (GPCRs) found in a wide array of structures within the nervous system. It has been implicated in the regulation of a myriad of neuronal functions from motor and memory processes to reward and attention [1–3]. Using pharmacological techniques, in situ hybridization and gene cloning, five major DR isoforms have been discovered [4–6]. Dl-like (Dl and D5) receptors couple to stimulatory (Gs) G-protein groups whereas D2-like (D2–D4) receptors couple to inhibitory (Gi) G-proteins [7]. In addition, D2-like receptors, have been linked to Phosphatidylinositol (PI) signaling, Calcium-Calmodulin Kinase II (CaMKII) and Calcineurin activation as well as to the regulation of Nitric Oxide (NO) in cells [8]. As with most others GPCRs, DRs can also regulate a diversity of ligand and voltage-gated ion channels, pumps and transporters thereby providing a key modulatory switch in neuronal firing activity and ionic balance [1, 9].
The classical view of GPCR function suggests essentially a bimodal state where downstream signaling is mediated almost exclusively by the components of the G protein pathway [5]. In recent years, however, findings on the discovery of an abundance of receptor-protein interactions suggests an alternative model in which the receptor is directly bound to components of the signaling milieu in a structure termed the signalplex or the receptor complex [10–12]. To date, the D2R subtype is the primary prototype DR complex in the brain [10]. Findings based on yeast two-hybrid screen, immunoprecipitation and pulldown strategies have defined well over 20 dopamine receptor interacting proteins (DRIPs) that represent at least some of the components of the D2R signalplex (Table 1). Shtdies show that DRIPs regulate key aspects of receptor signaling behavior such as ligand binding, phosphorylation, trafficking and coupling to other membrane proteins [10]. Some DRIPs, such as Neuronal Calcium Sensor I (NCS-1) and PAR 4, appear to play an important role in dopamine related diseases (Table 1).
Table 1.
Signalplex Proteins of the Dopamine D2 Receptor In the Brain, Proteins are Grouped Based on their Function at the D2R Site. Regions of Proteins Interaction within the D2R arc Indicated. Changes in Protein/mRNA Levels for DRIPs in Post-Mortem Tissue from Schizophrenic Patients of Primates following Antipsychotic Treatment Relative to Controls (↑: Upregulatlon, ↓: Downregulation in Expression Level)
| Protein | Interaction Site | Schizophrenia (mRNA/protein) | Antipsychotic: human/monkey (mRNA/protein) | Reference |
|---|---|---|---|---|
| Receptors: | -- | -- | ||
| Dopamine, D2L/S Dopamine, D3/D1 |
-- -- |
↑ mRNA | ↑ RNA (haloperidol, clozapine) | [39] |
| Glutamate, AMPA (GluR1/2) | -- | -- | [40] | |
| Cannabinoid, CB1 | -- | -- | [41] | |
| Adenosine, A2A | -- | -- | [42] | |
| Somatostatin, SSTR5 | -- | -- | [43] | |
| Glutamale, NMDA (NR20) | 3rd cytoplasmic loop | -- | [44] | |
| Ion Channels: | ||||
| CLIC-6 | C-terminal tail | -- | [37] | |
| Kir3 | -- | -- | [45] | |
| Scaffolding, trafficking and anchoring: | ||||
| 4.1 (N/B/G/R) | 3rd cytoplasmic loop | -- | [46] | |
| Arresfin (2/3) | 2nd and 3rd cytoplasmic loop | -- | [47] | |
| Dynamin-2 | -- | -- | [48] | |
| Filamin-A | 3rd cytoplasmic loop | -- | [49] | |
| GASP | -- | -- | [50] | |
| GIPC | C-terminal tail | -- | [51] | |
| H-FABP | 3rd cytoplasmic loop | -- | [52] | |
| Calnexin | -- | -- | [53] | |
| Signaling: | ||||
| Calmodulin | 3rd cytoplasmic loop | -- | [54] | |
| NCS-1 | C-terminal tail | ↑ mRNA, protein | [26] | |
| Par-4 | 3rd cytoplasmic loop | -- | [55] | |
| Gα i/z/o | 3rd cytoplasmic loop | ↓ protein | [6] | |
| GRK2 | -- | -- | [26] | |
| RGS19 | -- | -- | [56] | |
| Spinophilin | 3rd cytoplasmic loop | ↑ mRNA | ↓ protein (haloperidol) | [57] |
| Synaptic: | ||||
| CAPS | 2nd cytoplasmic loop | -- | [46] | |
| NSF | 3rd cytoplasmic loop | -- | [40] | |
| DAT | 3rd cytoplasmic loop | -- | [58] | |
It is important to note that DRIP interactions appear to be receptor subtype-specific suggesting that individual DRIPs can be targeted for the regulation of select DRs in the brain. In this scenario, the pharmacological targeting of DRIPs represents a promising new tool for selectively regulating DRs as an alternative (or in conjunction with) the common ligand binding method, which continues to maintain notable limitations on selectivity for various receptor subtypes [5]. Moreover, since DRIPs play an important role in the regulation of various functions of the DR (e.g. its phosphorylation, membrane localization, etc.) it is especially appealing to consider that the targeting of DRIPs in drug development can aim to mediate select features of the DR beyond a broad mode of receptor antagonism/agonism at the cell surface. This pivotal direction in drug design promises to contribute to the efficacy of treatments for disorders such as Parkinson’s disease, addiction, and schizophrenia [13, 14]. In this review we explore the possibility of exploiting the D2R/NCS-l interaction in the development of novel antipsychotic drugs for the treatment of schizophrenia.
D2 DOPAMINE RECEPTORS IN THE TREATMENT OF SCHIZOPHRENIA
Schizophrenia is one of the most devastating disorders of humankind. The cardinal symptom of schizophrenia is characterized by inappropriate associations, which are manifested by thought or speech disorganization. Psychotic symptoms include hallucinations (typically auditory) and delusions, which frequently involve persecution and/or megalomania [15]. Psychotic symptoms and severe thought disorganization are often grouped under the term positive symptoms. Deficit symptoms are also commonly referred to as negative symptoms and are manifest as flat affect, lack of volition (apathy), poverty of speech, lack of pleasure (anhedonia), as well as social withdrawal [16].
Dysfunction within the dopamine neurotransmission system has been widely implicated in the pathogenesis of schizophrenia. The most effective known treatments for schizophrenia are those that principally antagonize the D2 receptor (D2R) subtype [17, 18]. The view that imbalances in dopamine-mediated signaling may underlie at least some of the deficits seen in schizophrenia was formulated ~30 years ago as the dopaminergic hypothesis of schizophrenia [19]. This hypothesis posits that schizophrenia arises from dysregulation of dopamine neurotransmission in mesolimbic and mesocortical pathways of the brain. The dopamine hypothesis of schizophrenia is strongly supported by the pharmacologic actions of antipsychotic drugs, which specifically interact with and block mesolimbic and mesocortical dopamine receptors [17, 19]. The role of D2Rs in the etiology of schizophrenia has been further substantiated in a transgenic mouse model [20]. Overexpression of D2Rs in the striatum of transgenic mice leads to alterations in prefrontal cortex functioning. This includes selective cognitive impairments in working memory [20], one of the hallmark endophenotypes characteristics of schizophrenia.
Antipsychotic drugs (typicals and atypicals) that are currently available are particularly effective in treating the positive symptoms of schizophrenia. Both typical (i.e. chlorpromazine, haloperidol) and atypical (i.e., olanzapine, aripiprazole, clozapine) antipsychotics can greatly reduce the severity of hallucinations, delusions, and destructive behavior in psychotic patients. However, typical antipsy-chotics can cause discomforting side effects such as unwanted sedation, tremors, movement disorders such as tardive dyskinesia, and in extreme cases, life-threatening neuroleptic malignant syndrome. The newer generation of atypical antipsychotics also have serious side effects. These include agranulocytosis (clozapine), cataracts (quetiapine), weight gain and increased risk of Type-2 diabetes (olanzapine, aripiprazole). Long term treatment with atypical antipsychotics may also lead to extrapyramidal side effects including tardive dyskinesia. Because schizophrenia and other forms of psychosis are such a pressing mental health problem, there is a critical need for the development of alternative drug therapies which can alleviate symptoms of the disease without the deleterious side effects associated with currently available antipsychotic drugs.
For the past two decades, the dopamine neurotransmission system has been a major focus in the search for new and better antipsychotic drugs [15]. There is considerable evidence that dysfunction of the prefrontal cortical dopaminergic system represents an important component in the manifestation of schizophrenia [21, 22]. Paradoxically, there is no direct genetic or biochemical evidence linking defects in DR genes with the etiology of schizophrenia [23, 24], even though the D2R represents the major cellular target of antipsychotic drugs. It is now generally accepted that the cascade of intracellular events set off by D2R activation is mediated by multi-protein signaling complexes (signalplexes) which are composed of the receptor and a cohort of structural, regulatory and signaling proteins formed through protein-protein interactions [10, 25]. It is possible that the dysfunctional dopaminergic signaling that occurs in schizophrenia may result from alterations in expression or function of DRIPs as well as possible aberrations in the interaction properties between DRIPs and DRs. DRIPs therefore represent a promising new class of potential molecular targets for the development of novel antipsychotic drugs [22].
TARGETING D2R/NCS-1 INTERACTIONS FOR DRUG DEVELOPMENT
NCS-1 was identified as a DRIP in a yeast two-hybrid screen [26]. NCS-1 is a member of the EF-hand family of calcium binding proteins and is the mammalian ortholog of Drosophila frequenin. Interaction between the D2R and NCS-1 regulates desensitization of activated D2Rs and causes a significant increase in the half-life of the D2R at the plasma membrane following ligand stimulation [26]. The D2R/NCS-l interaction is thus likely to have important implications for how DR density (and ultimately function) may be regulated within the brain. Recent studies have demonstrated elevated levels of NCS-1 in the dorsolateral prefrontal cortex of schizophrenic patients [27, 28], suggesting that upregulation of NCS-1 could functionally augment D2R signaling in the prefrontal cortex [29]. Overexpression of NCS-1 may therefore contribute to the cellular adaptations within affected neural networks that occur in schizophrenia. Because the D2R/NCS-1 interaction plays such a key role in modulating D2R-mediated signaling, this interaction represents a novel (but plausible) target for advancing drug development.
We have developed a high throughput screening (HTS) format, utilizing fluorescence anisotropy (FA), to identify small molecules that disrupt the D2R/NCS-1 interaction. A number of important studies have begun to demonstrate the efficacy of the FA assay for the study of targeting protein-protein interactions in cellular systems. Here, by developing a novel assay that can provide a quantitative measure of changes in D2R/NCS-1 interaction, it is now possible to systematically screen for compounds capable of blocking (or altering) interactions between D2Rs and NCS-1 under various biochemical conditions. We predict that such compounds may serve as lead compounds for the development of novel antipsychotic drugs via their specific targeting of the D2R/NCS-1 interaction in cells.
FA offers numerous advantages over more conventional methods that study protein-protein binding. For example, no hazardous radioactive waste is generated and sub-micro-molar to nanomolar quantities of proteins are required to verify an interaction [30]. Additionally, no separation of components are required and FA assays provide for an environment in which binding kinetics are easily measured. FA is a very sensitive and a widely used high throughput technique utilized in the study of protein-protein interactions [31]. In this article, the term anisotropy refers to anisotropy of fluorescence polarization (FA) and is used interchangeably with the term fluorescence polarization (FP). Although the two quantities are subtly different, for detailed biophysical analysis the use of anisotropy is more straightforward[35].
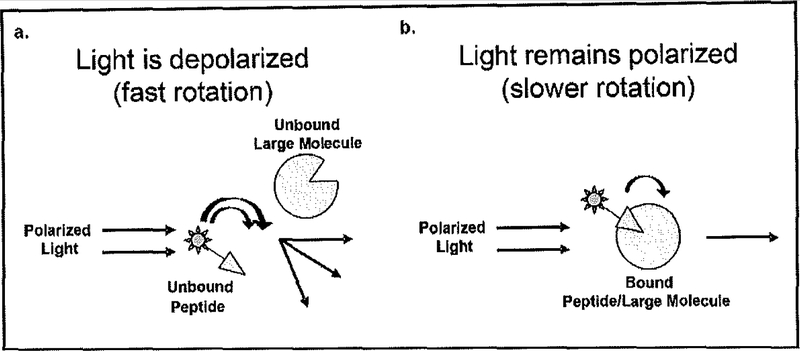
In its common use the FA/FP technique can be highly effective in the detection of molecular interactions between small fluorescently tagged molecules and larger (non-tagged) molecules. A number of published articles and chapters can be found elsewhere explaining in greater detail the FA/FP assay. In summary, the assay exploits the ability to detect changes in polarized light in the plane of excitement when a small fluorescently tagged molecule interacts with a larger (untagged) molecule as shown in Fig. (1). When vertical polarized light excites a fluorophore, light is emitted in the same vertical polarized plane, assuming that the fluorophore remains motionless during the excited state. However, molecules in the presence of liquid, display properties of dynamic movement, and are thus always in motion [32]. Therefore, to determine the degree of movement by the fluorophore, the intensity of the emitted light can be monitored in vertical and horizontal planes. If the fluorophore molecule is small, rotation and tumbling (Brownian movement) are faster and the emitted light is depolarized in relation to the excitation plane. If the fluorophore molecule is very large, little movement occurs during excitation and the emitted light remains highly polarized. Thus, the difference in movement between a small molecular fluorophore and a large molecular fluorophore can be distinguished by collecting and comparing emission intensities from the different planes. The smaller fluorophore will exhibit a larger degree of motion and have a decrease in emitted polarized light in the plane of excitation as shown in Fig. (1a) while the larger fluorophore will have slower motion and increased polarized light in the plane of excitation as shown in Fig. (1b) [33, 34]. Under these experimental conditions, we aim to be able to isolate novel small molecule compounds, with putative antipsychotic properties, as based on their ability to interact with and disrupt the NCS-1/D2R interaction.
Fig. (1).
Representation of fluorescence polarization (FP) assay. a) Diagram of when the fluorescence tagged peptide does not interact with a larger molecule and has a fast rotation, all polarized light is not emitted on the same plane as excitation polarized light due to fast rotation. b) Diagram of when fluorescence tagged peptide interacts with a larger molecule and has a slower rotation, polarized light is emitted on the same plane as excitation polarized light due to slower rotation.
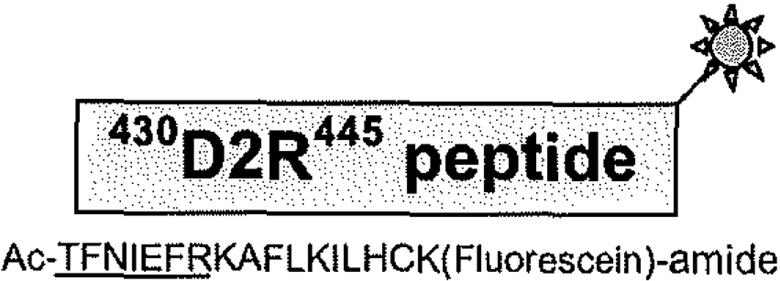
The interaction between the D2R and NCS-1 was initially detected in a yeast-two hybrid screen and then confirmed in cell free as well as cell based interaction assays and in brain [26]. Deletion mapping localized the NCS-l binding site to residues 428–436 within the C-terminal tail of the D2R, and the D2R binding site to the N-terminal 71 residues of NCS-1 [26]. Knowledge of these protein interaction domains was utilized to develop an FA-based D2R/NCS-1 interaction assay. As depicted in Fig. (2), a small 17 amino acid-long fluorescein-labeled peptide encompassing the NCS-1 binding site on the D2R serves as a tracer, while full-length wild-type NCS-l functions as the larger binding molecule.
Fig. (2).
Schematic representation of the fluorescein-labeled D2R-peptide tracer. The peptide spans residues 430–445 within the C-terminal tail of the D2R. Fluorescein tag is represented by the green sunburst. Fluorescein labeling of the peptide was performed during synthesis. Sequence of the D2R-peptide is shown below. The NCS-1 binding site is underlined.
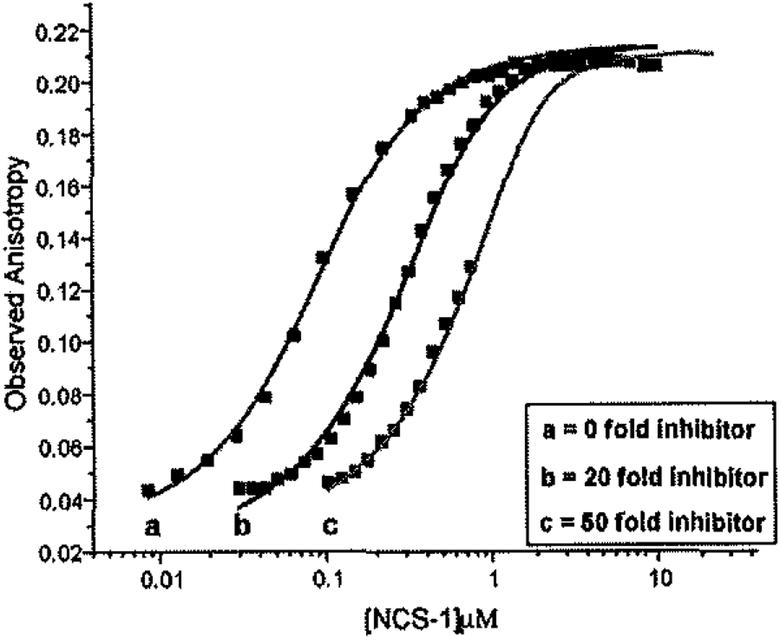
Preliminary experiments used FA to analyze the interaction between the labeled D2R-peptide tracer and wild-type (non-myristoylated) NCS-1. This interaction was observed to occur with a Kd between 100 and 300 nM. The specificity of the labeled D2R-peptide/NCS-l interaction was determined using a competition binding assay. FA was measured at saturating concentrations of NCS-1 and at concentrations of unlabeled D2R-peptide present at 0, 20, and 50 fold excess of the labeled D2R-peptide as shown in Fig. (3). Increasing the ratio of unlabeled to labeled peptide caused a shift of the curves to the right, an indication that the unlabeled peptide competes with the labeled peptide for a specific binding site on NCS-1. Best fit of the data to a global fit model allowed us to calculate a Kd of 148 nM for the unlabeled D2R-peptide/NCS-l interaction with a Z factor> 0.88 (Z-factor is a measure of the quality or power of an (HTS) assay). A Z-factor of 0.5–1.0 is considered extremely robust [36]. These preliminaty studies highlight the possibility of using FA as a tool to screen for compounds capable of specifically blocking the association between the D2R and NCS-1 using a high throughput format.
Fig. (3).
Competition binding cmve using unlabeled D2R-peptide as an inhibitor against the labeled D2R-peptide/NCS-I complex interaction. Anisotropy is given as a function of NCS-1 concentration. Unlabelled D2R-peptide concentrations were 0 (a), 20 (b), and 50 (c) fold greater than the labeled D2R-peptide (32.4 nM). Trcndlines were derived from a global fit of the data (R2 = 0.996). Error bars are small enough to be completely obscured by sample points.
AN FA-BASED SCREEN FOR INHIBITORS OF THE D2R/NCS-1 INTERACTION
We have used the FA-based assay to screen a natural products library (composed of 480 compounds) in order to identify small molecule inhibitiors of the D2R/NCS-l interaction. In this screen, we identified 12 compounds which potentially disrupt the D2R/NCS-1 interaction. In the FA-based assay, the fluorescien-labeled peptide (D2R-FL) sequence corresponds to the C-terminal tail of the D2R, which spans the NCS-1 binding site, and is a 17 amino acid residue peptide (TFNIEFRKAFLKILHCK-fluorescein) synthesized with a C-terminal lysine for fluorophore attachment D2R-FL was synthesized (New England Peptide, Gardner, MA) and used as a tracer in FA experiments. An unlabeled D2R-peptide containing an acylated C-terminus instead of fluorescein was also synthesized (Proteomics Core Facility, Penn State College of Medicine, Hershey, PA,) for use as a competitive inhibitor of the D2R-FL/NCS-1 interaction. The full length NCS-l protein expression construct (NCS-l TEV) was engineered to contain a tobacco etch virus (TEV) protease cleavable N-terminal His6-tag from the pET-28-TEV vector (gift of Stephen Burley, Rockefeller University). NCS-1 TEV was expressed in E. coli BL21 (DE3) cells (Novagen) grown in ZYP-5052 auto-induction media and purified as previously described [59].
To perform the screen, the cuvette-based FA assay was miniaturized to conform to an HTS format. Assay components were aliquoted into a black 384-well polystyrene plate by using an automated Eppendorf- epMotion 5070 (Hauppauge, NY) loading instrument Test compounds from the natural products libraty (Timtec- 480 Natural Product Library, Newark, DE) were diluted to 25 uM into each test well from a 96-well storage plate by the Eppendorf automated loading instrument. Fluorescence anisotropy (FA) readings were immediately performed using a Flexstation 3 fluorimeter, (Sunnyvale, CA) at 30° C. Samples were excited at 485 nm and the emission spectrum was monitored at 538 nm with a cutoff filter at 530 nm.
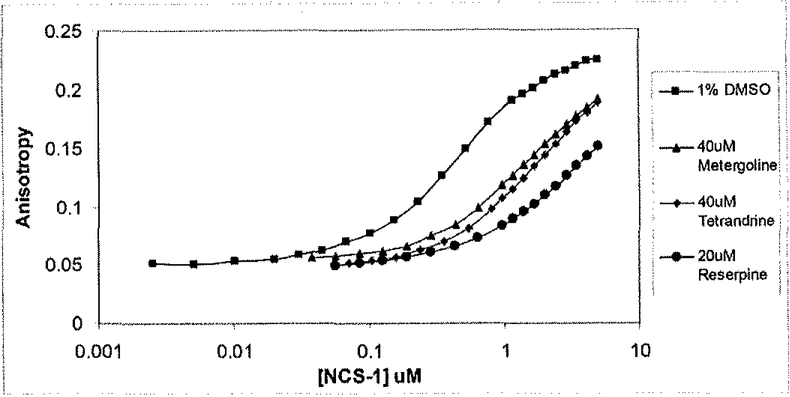
The entire 480 small molecule library of natural compounds was evaluated for inhibitors of the D2R-FL/NCS-l interaction. Compounds whose anisotropies ranged between 0.087 and 0.06 were defined as hits (i.e. blocked the D2R/NCS-l interaction) in the primary screen. From this screen, 12 primary hits were subsequently confirmed in a secondary 384-well plate based assay. Of the 12 compounds confirmed as positive hits in the HTS format, three known psychoactive compounds (metergoline, tetrandrine, and reserpine) were identified as putative inhibitors of the D2R/NCS-l interaction in a tertiary cuvette-based FA assay (Fig. 4).
Fig. (4).
Natural products library screen. Of the 480 compounds screened, 14 produced anisotropy values between 0.087 and 0.06 (an anisotropy range determined to be a hit), and 12 were confirmed in a secondary 384-well plate based FA assay. Three were subsequently confirmed using the cuvette-based FA assay, as shown.
The FA assay presented here represents a powerful new method for detecting and validating protein-protein interactions in a high throughput manner. The identification of novel inhibitors of the D2R/NCS-l interaction would represent an important advance as both a research tool for evaluating the function of the D2R/NCS-I complex in cells, and possibly for the development of novel antipsychotics for the treatment of disorders such as schizophrenia.
FUTURE DIRECTIONS IN DOPAMINE RECEPTOR TARGETING FOR ANTIPSYCHOTIC DRUG DEVELOPMENT
The development of the FA assay presents an important first screening tool for the high throughput testing of candidate blockers of protein-protein interaction at the D2RNCS-l inte1face. The C-terminal tail region of the D2R represents an important site of receptor regulation in addition to its role in the binding ofNCS-1 in neurons [37].
Biochemical mapping studies reveal a number of specific receptor residues within each of the 3 cytoplasmic loop domains as well as the C-terminal tail of the D2R that are critical for receptor-protein interactions (Table 1). D2R interaction with individual DRIPs appears modifiable via the targeting of such interaction interfaces using methods such as site-directed mutagenesis, the expression of competing/blocking peptides, and/or the delivery of small molecule therapeutics into cells [25]. The later two approaches represent more appealing therapeutic strategies due to high efficacy and low toxicity profiles as well as the ability to circumvent the use of viral vectors for the delivery of mutant proteins into patients.
With the emergence of peptide mimetics and peptide-based therapeutics as promising strategies in therapeutic drug design for human brain diseases [38], it is interesting to consider that basic research on the properties of receptor complexes such as the D2R signalplex are central to the advancement of the peptide based drug platform and is vital for continuing to expand the repertoire of molecular targets available for drug development. This article highlights the targeting of D2R/NCS-l interaction as an especially promising new strategy for antipsychotic drug development however it is without doubt that future research on methods and tools for the facilitation as well as the disruption of protein interactions within various components the receptor signalplex will be of value in advancing drug design and available therapies for disease.
ACKNOWLEDGEMENTS
This work was supported by grants from NIDA (DA025995) and the Pennsylvania Department of Health using Tobacco Settlement Funds to RL, and a grant award of the Thomas and Kate Miller Jeffress Memorial Trust to NK.
ABBREVIATIONS
- DR
Dopamine receptor
- DzL/S
D2 long/short isoform
- GPCR
G protein coupled receptor
- D2R
D2 dopamine receptor
- NCS-l
Neuronal calcium sensor 1
- PI
Phosphoinisotol signaling
- CaMKII
Calcium-Calmodulin kinase II
- NO
Nitric Oxide
- DRIP
Dopamine receptor interacting protein
- PAR-4
Prostate apoptosis response-4
- FP
Fluorescence polarization
- FA
Fluorescence Anisotropy
- CLIC-6
Chloride intracellular channel protein-6
- Kir3
Inwardly rectifying potassium channel 3
- 4.1
Protein 4.1
- GASP
G protein coupled receptor associated sorting protein
- GIPC
GAIP (G? interacting protein) interacting protein C-terminus
- H-FABP
Heart fatty acid binding protein
- GRK2
G protein coupled receptor kinase 2
- RGSI9
Regulator of G-protein signaling 19
- CAPS
Calcium dependent activator protein for secretion
- NSF
N-ethylmaleimide sensitive factor
- DAT
Dopamine Transporter
- TEV
Tobacco etch virus
REFERENCES
- [1].Bertorello AM, Hopfield JF, Aperia A, Greengard P. Inhibition by dopamine of (Na(+)K+) ATPase activity in neostriata neurons through Dl and D2 dompamine receptor synergism. Nature 1990; 347: 386–388, [DOI] [PubMed] [Google Scholar]
- [2].Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacal 1998; 42: 707–711. [DOI] [PubMed] [Google Scholar]
- [3].Schultz W Getting formal with dopamine and reward. Neuron 2002; 36: 241–263. [DOI] [PubMed] [Google Scholar]
- [4].Albert PR, Neve KA, Bunzow JR, Civelli O. Coupling of a cloned rat dopamine D2 receptro to inhibition of adenylyl cyclase and prolactin secretion. J Bioi Chem 1990; 265: 2098–2104. [PubMed] [Google Scholar]
- [5].Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 1998; 78: 189–225. [DOI] [PubMed] [Google Scholar]
- [6].Sidhu A, Niznik HB. Coupling of dopamine receptor subtypes to multiple and diverse G proteins. Tnt J Dev Neurosci 2000; 18: 669–677. [DOI] [PubMed] [Google Scholar]
- [7].Obadian J, Avidor-Reiss T, Fishbum CS, Carmon S, Baycwitch M, Vogel Z, Fuchs S, Levavi-Sivan B. Adenylyl cyclase interaction with the D2 dopamine receptor fumily; differential coupling to Gi, Gz, and Gs. Cell Mol Neurobiol 1999; 19: 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res 2004; 24: 65–205. [DOI] [PubMed] [Google Scholar]
- [9].Nicola SM, Surmcier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 2000; 23: 185–215. [DOI] [PubMed] [Google Scholar]
- [10].Kabbani N, Hannan MA, Levenson R. Unraveling the Dopamine Receptor Signalplex by DRIPs and DRAPs. Curr Proteomics 2005; 2: 1–15. [Google Scholar]
- [11].Bockaert J, Roussignol G, Became C, Gavarini S, Joubert L, Dumuis A, Fagni L, Marin P. GPCR-interacting proteins (GIPs): nature and functions. Biochem Soc Trans 2004; 32: 85–855. [DOI] [PubMed] [Google Scholar]
- [12].Millian G, White JH. Protein-protein interactions at G-protcin-coupled receptors. Trends Pharmaco Sci 2001; 22: 513–518. [DOI] [PubMed] [Google Scholar]
- [13].Gimult JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol 2004; 61: 641–644, [DOI] [PubMed] [Google Scholar]
- [14].Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 2005; 10: 79–104. [DOI] [PubMed] [Google Scholar]
- [15].Holden C Deconstructing schizophrenia. Science 2003; 299: 333–335. [DOI] [PubMed] [Google Scholar]
- [16].Frankie WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron 2003; 39: 205–216. [DOI] [PubMed] [Google Scholar]
- [17].Seeman P, Kapur S. Schizophrenia: More dopamine more D2 receptors. Proc Natl Acad Sci USA 2000; 97:7673–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Glatt S, Faraone SV, Tsuang MT. Meta-analysis identifies an association between the dopamine D2 receptor gene and schizophrenia. Mol Psychiatry 2003; 8: 911–915. [DOI] [PubMed] [Google Scholar]
- [19].Carlsson A Antipsychotic drugs and catecholamine synapses. J Psychiatric Res 1974; 11: 57–64. [DOI] [PubMed] [Google Scholar]
- [20].Kellendonk C, Simpson EH, Polan HJ, Malleret 0, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 2006; 49: 603–615. [DOI] [PubMed] [Google Scholar]
- [21].Gao WJ, Goldman-Rakic PS. Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc Natl Acad Sci USA 2003; 100:2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bonci A, Hopf FW. The dopamine D2 receptor: New surprises from an old friend. Neuron 2005; 47:335–338. [DOI] [PubMed] [Google Scholar]
- [23].Pulver AE. Search for schizophrenia candidate genes. Bioi Psychiatry 2000; 47: 221–230. [DOI] [PubMed] [Google Scholar]
- [24].Owen MJ, Williams NM, O’Donovan MC. The molecular genetics of schizophrenia:new findings promise new insights. Mol Psychiatry 2004; 9: 14–27. [DOI] [PubMed] [Google Scholar]
- [25].Kabbani N, Levenson R. A proteomic approach to receptor signaling: Molecular mechanisms and therapeutic implications derived from discovery of the dopamine D2 receptor signalplex. Eur J Pharm 2007; 572: 83–93. [DOI] [PubMed] [Google Scholar]
- [26].Kabbani N, Negyessy L, Lin R, Goldman-Rakic P, Levenson R. Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J Neurosci 2002; 22: 8476–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Koh PO, Undie AS, Kabbani N, Goldman-Rakic PS, Levenson R, Lidow MS. Upregulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci USA 2003; 100: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bai J, He F, Novikova Sl, Undie AS, Dracheva S, Haroutunian V, Lidow MS. Abnormalities in the dopamine system in schizophrenia may lie in altered levels of dopamine receptor-interacting proteins. Bioi Psychiatry 2004; 56: 427–40. [DOI] [PubMed] [Google Scholar]
- [29].Bergson C, Levenson R, Goldman-Rakic PS, Lidow MS. Dopamine receptor interacting proteins: The calcium connection in dopamine signaling. Trends Pharmacol Sci 2003; 24: 486–492. [DOI] [PubMed] [Google Scholar]
- [30].Dedier S, Reinelt S, Rion S, Folkers G, Rognan D. Use of fluorescence polarization to monitor IvrHC-peptide interactions in solution. J Immunol Methods 2001; 255: 57–66. [DOI] [PubMed] [Google Scholar]
- [31].Jameson DM, Seifried SE. Quantification of protein-protein interactions using fluorescence polarization. Methods 1999; 19: 222–233. [DOI] [PubMed] [Google Scholar]
- [32].Harvey SC, Cheung HC. Computer simulation of fluorescence depolarization due to brownian motion. Proc Natl Acad Sci USA 1972; 69:3670–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Weber G Polarization of the nuorescence of macromolecules. I. Theory and experimental method. Biochem J 1952; 51: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Roehrl MH, Wang JY, Wagner G. A general framework for development and data analysis of competitive high-throughput screens for small-molecule inhibitors of proteinprotein interactions by nuorescence polarization. Biochemistry 2004; 43: 16056–16066. [DOI] [PubMed] [Google Scholar]
- [35].Owicki JC. Fluorescence polarization and anisotropy in high throughput screening: perspectives and primer. J Biomol Screen 2000; 5:297–306. [DOI] [PubMed] [Google Scholar]
- [36].Zhang JH, Chung TO, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 1999; 4: 67–73. [DOI] [PubMed] [Google Scholar]
- [37].Roberts E, Wendel J. Dynamic modelling of the binding of substances to the conserved membrane-adjacent heptapeptide of the 15-residue C-tem1inal cytoplasmic fragment of mammalian dopamine D2 receptors. Neurochem Res 1996; 21(2): 177–200. [DOI] [PubMed] [Google Scholar]
- [38].Skaper SO, Walsh FS. Neurotrophic molecules: strategies for designing effective thempeutic molecules in neurodegeneration. MoiCellNeurosci 1998; 12:179–193. [DOI] [PubMed] [Google Scholar]
- [39].Scarselli M, Novi F, Schallmach E, Lin R, Baragli A, Colzi A, Griffon N, Corsini GU, Sokoloff P, Levenson R, Vogel Z, Maggio R. D2/D3 dopamine receptor hcterodimers exhibit unique functional properties. J Bioi Chem 2001; 276: 30308–30314. [DOI] [PubMed] [Google Scholar]
- [40].Zou S, Li L, Pei L, Vukusic B, Van Tot HH, Lee FJ, Wan Q, Liu F. Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity.J Neurosci 2005; 25: 4385–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concunent stimulation of cannabinoid CBI and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol 2005; 67: 1697–1704. [DOI] [PubMed] [Google Scholar]
- [42].Ferre S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casado V, Hillion J, Torvinen M, Fanelli F, Benedetti P, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat Disord 2004; 10:265–271. [DOI] [PubMed] [Google Scholar]
- [43].Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 2000; 288: 154–157. [DOI] [PubMed] [Google Scholar]
- [44].Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2 receptor-NR2B interactions in response to cocaine. Neuron 2006; 52: 897–909. [DOI] [PubMed] [Google Scholar]
- [45].Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem 2002; 277: 46010–46019. [DOI] [PubMed] [Google Scholar]
- [46].Binda AV, Kabbani N, Lin R, Levenson R. D2 and D3 Dopamine Receptor Cell Surface Localization Mediated by Interaction with Protein 4.1N. Mol Pharmacol 2002; 62:507–513. [DOI] [PubMed] [Google Scholar]
- [47].Macey TA, Gurevich VV, Neve KA. Preferential Interaction between the dopamine D2 receptor and Arrestin2 in neostriatal neurons. Mol Phannacol 2004; 66: 1635–1642. [DOI] [PubMed] [Google Scholar]
- [48].Kabbani N, Jeromin A, Levenson R. Dynamin-2 associates with the dopamine receptor signalplex and regulates internalization of activated D2 receptors. Cell Signal 2004; 16:497–503. [DOI] [PubMed] [Google Scholar]
- [49].Lin R, Karpa KD, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with Filamin A. Proc Natl Acad Sci USA 2001; 98: 5258–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bartlett SE, Enquist J, Hopf FW, Lee JH, Gladher F, Kharazia V, Waldhoer M, Mailliard WS, Armstrong R, Bonci A, Whistler JL. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. ProcNatl Acad Sci USA 2005; 102: 11521–11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jeanneteau F, Diaz J, Sokoloff P, Griffon N. Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol Bioi Cell 2004; 15: 696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Takeuchi Y, Fukunaga K. Differential subcellular localization of two dopamine D2 receptor isoforms in transfected NGI 08–15 cells. J Neurochem 2003; 85: 1064–1074. [DOI] [PubMed] [Google Scholar]
- [53].Free RB, Hazelwood LA, Cabrera OM, Spalding HN, Namkung Y, Rankin ML, Sibley DR. Dl and D2 dopamine receptor expression is regulated by direct interaction with the chaperone protein calnexin. J Biol Chem 2007; 282(29): 21285–21300. [DOI] [PubMed] [Google Scholar]
- [54].Bofili-Cardona E, Kudlacek O, Yang Q, Ahom H, Freissmuth M, Nanoff C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem 2000; 275: 32672–32680. [DOI] [PubMed] [Google Scholar]
- [55].Park SK, Nguyen MD, Fischer A, Luke MP, Affar B, Dieffenbach PB, Tseng HC, Shi Y, Tsai LH. Par-4 links dopamine signaling and depression. Cell 2005; 122: 275–287. [DOI] [PubMed] [Google Scholar]
- [56].Ghavami A, Hunt RA, Olsen MA, Zhang J, Smith DL, Kalgaonkar S, Rahman Z, Young KH. Differential effects of regulator of G protein signaling (RGS) proteins on serotonin 5-HTI A, 5-HT2A, and dopamine D2 receptor-mediated signaling and adenylyl cyclase activity. Cell Signal 2004; 16:711–721. [DOI] [PubMed] [Google Scholar]
- [57].Li M, Bermak JC, Wang ZW, Zhou QY. Modulation of dopamine D(2) receptor signaling by actin-binding protein (ABP-280). Mol Phannacol 2000; 57: 446–452. [DOI] [PubMed] [Google Scholar]
- [58].Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Ce1l 2002; 111: 219–230. [DOI] [PubMed] [Google Scholar]
- [59].De Cotiis DA, Woll MP, Levenson R, Flanagan JM. Optimized Expression and Purification of Myristoylated NCS-1 in E. coli. Protein Expr Purif 2008; 61:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]