Abstract
This study examined longitudinally the additive effect of syndemics, or co-occurring psychosocial problems, on antiretroviral treatment (ART) non-adherence among 390 HIV-positive sexual minority men. Participants completed measures of ART adherence (reduced to a non-adherence score using exploratory factor analysis) and six syndemic conditions. We employed multilevel modeling with the number of syndemics as a longitudinal predictor of non-adherence, and logistic regression with baseline syndemics predicting follow up viral load. Number of syndemics was a significant longitudinal predictor of non-adherence, with each additional syndemic associated with a 0.13 increase in non-adherence (p = 0.004). Each additional syndemic was also associated with 1.27 greater odds of detectable viral load (p = 0.002). Among HIV-positive sexual minority men in this sample, more syndemics were associated with lower ART adherence and greater odds of detectable viral load, suggesting the need for behavioral intervention to facilitate care for this population.
Keywords: HIV, sexual minority, syndemics, antiretroviral therapy/ART, adherence
Resumen
Este estudio examinó longitudinalmente el efecto aditivo de sindémicas, o problemas psicosociales concurrentes, sobre la falta de adherencia al tratamiento antirretroviral (ART por sus siglas en inglés) entre 390 hombres VIH positivos de minorías sexuales. Los participantes completaron medidas de adherencia al ART (reducidas a un puntaje de falta de adherencia usando un análisis factorial exploratorio) y seis condiciones sindémicas. Empleamos el modelado multinivel con el número de sindémicas como un predictor longitudinal de la falta de adherencia, y una regresión logística que predice la carga viral de las visitas de seguimiento usando la cita inicial como referencia. El número de sindémicas fue un predictor longitudinal significativo de la falta de adherencia, con cada sindémica adicional asociada con un aumento de 0.13 en la falta de adherencia (p = 0.004). Cada sindémica adicional también se asoció con 1.27 mayores probabilidades de carga viral detectable (p = 0.002). Entre los hombres VIH-positivos de minorías sexuales en esta muestra, más sindémicas se asociaron con menos adherencia al ART y mayores probabilidades de carga viral detectable, sugiriendo la necesidad de una intervención conductual para facilitar el cuidado de la salud de esta población.
Introduction
Among people living with HIV/AIDS (PLWHA), adequate antiretroviral treatment (ART) adherence is necessary, even with more potent ART regimens, for personal health [1] and public health, as attaining viral suppression reduces the chances of sexual transmission of HIV to uninfected partners [2,3]. Engagement in the full HIV care continuum, including attaining consistently high levels of ART adherence, is required for treatment as prevention efforts to lower individual [1] and community viral load [4].
Despite these personal and public health benefits of optimizing ART adherence, there are varying rates of adherence among sexual minority men (men who identify as gay, bisexual, or another non-heterosexual identity) living with HIV [5]. This is particularly important because men who have sex with men (MSM) are the largest group of PLWHA in the U.S. [6]. Due to environmentally based stressors (e.g. internalized, interpersonal, societal heterosexism), sexual minority men in general have a greater prevalence of psychosocial concerns [7], with this disparity more pronounced among sexual minority men living with HIV [8].
Syndemics theory holds that diseases do not occur in isolation, but rather, in the context of other diseases, psychosocial problems, and environmental conditions which interact with one another and heighten the impact of a condition such as HIV [9,10]. Among sexual minority men, researchers have largely applied syndemics theory to primary HIV-prevention. This work has revealed cross sectional [e.g. 11–14] and longitudinal [e.g. 15–17] additive associations of different syndemic conditions such as depression, childhood sexual abuse, polysubstance use, and partner violence with increased HIV acquisition risk behavior and risk of being diagnosed HIV-positive.
There are relatively fewer studies of syndemics in PLWHA versus those at risk for HIV. Cross sectional investigations include studies of PLWHA entering a depression treatment trial [18], youth living with HIV [19], and MSM living with HIV in Latin America [20]. These studies found a greater quantity of syndemics to be associated with worse ART adherence. One longitudinal study of MSM living with HIV in four U.S. cities found that a higher number of syndemics was associated with both worse ART adherence and higher viral load over a 7-year period [21].
There are several gaps in the research on the association between syndemics and HIV among sexual minority men. The majority of existing studies focus on sexual minority men at risk for contracting HIV, rather than evaluating the impact of syndemics on the health behaviors of those living with HIV. To the authors’ knowledge, only two studies have examined the relationship between syndemics and ART adherence among MSM specifically, and only one of these was longitudinal using a U.S. sample [21], which used only three indicators of syndemics (depressive symptoms, substance use, and condomless anal sex), with one of the syndemic predictors being a health behavior (condomless anal sex).
The present study therefore sought to extend these findings by examining the additive impact of psychosocial syndemics on ART non-adherence among sexual minority men within a longitudinal design. The main hypothesis was that increases in syndemic conditions over time would be associated with increases in ART non-adherence. A secondary hypothesis was that baseline syndemics would be associated with greater odds of detectable viral load during the one-year follow up period.
Method
Participants
Participants in this study included 390 sexual minority men living with HIV who participated in one of two secondary prevention trials. One of the trials was a two-arm randomized controlled trial (RCT) “Project Enhance,” with medical-social workers as interventionists in the experimental condition (N = 100) and control group participants receiving medical care as usual (N = 101; [22]) and the other was a peer-delivered demonstration project “Peer to Peer” (N = 189) of the same intervention [23]. Informed consent was obtained from all participants. The intervention is described in detail elsewhere [24] and involves sexual risk reduction by addressing stress management, sociocultural context, substance use, disclosure of HIV status, sexual risk triggers, and navigating relationships. Participants in the RCT screened in if they reported condomless sex with an HIV-negative or unknown status partner in the last three months, whereas this was not inclusion criteria for the Peer-to-Peer study. Participants were eligible to participate in either study if they were living with HIV, identified as MSM, were 18 years or older, and were a patient at Fenway Health for at least three months. Most participants identified as gay, with 15 participants identifying as bisexual (3.8%) and one identifying as “other” regarding sexual orientation identity. Participants completed a baseline assessment and follow up assessments every three months for one year, yielding a maximum of five longitudinal observations per participant.
Measures
Demographic and HIV-Related Factors.
At the baseline assessment, participants reported their age, race/ethnicity, education level, income, and relationship status. Participants also reported whether they were taking ART at baseline and all follow up assessments. Participants’ recent viral load (plasma HIV RNA concentration, copies per milliliter) and peripheral blood CD4 + T cell count (cells/mm3) were collected via medical record abstraction.
Viral Load.
Participants’ most recent viral load was collected at each follow up assessment and recoded to a binary variable (reference = undetectable viral load, < 75 copies per millimeter). Because this was collected via medical record abstraction, lab dates did not consistently align with follow up assessment dates. Therefore, the binary composite variable indicated whether participants had a detectable viral load at any point during the follow up period.
Adherence to HIV Medication.
Self-reported adherence scores were derived via factor analysis [25,26] from the ACTG Adherence Questionnaire [27]. The questionnaire elicited the number of prescribed doses missed for the prior 1, 2, 3, and 4 days. Number of doses taken divided by number of doses prescribed across each medication yielded a score from 0 (none missed) to 1 (all missed) per day. Participants were asked how closely they followed their medication schedule over the last four days (0 = never; 4 = all of the time), if they missed any medication over the last weekend (0 = no; 1 = yes), and the last time they missed medication (0 = never; 4 = within past week).
Psychosocial Assessments.
The following self-report measures were administered to assess six psychosocial conditions that were used to compute syndemics scores.
Childhood sexual abuse (CSA).
Two items, informed by the Juvenile Victimization Questionnaire [28] assessed CSA (fixed variable) at baseline. The questionnaire asked whether participants ever had a sexual experience with (1) someone at least five years older, before they reached age 13 and (2) someone at least ten years older, between the ages of 13 and 16. Endorsement of either item met criteria for CSA.
Post-traumatic stress disorder (PTSD).
Participants completed the SPAN at baseline, a brief diagnostic screening for PTSD derived from the Davidson Trauma Scale [29]. Participants who reported a PTSD qualifying event indicated frequency (0 = not at all; 4 = every day) and severity (0 = not at all distressing; 4 = extremely distressing) of four symptoms (startle, anger, numbness, and physical upset at exposure to trauma reminders). Summed severity ratings of five or higher met criteria for PTSD. Meltzer-Brody found that compared to clinician administered assessments this brief self-report measure was specific (91%) and sensitive (84%) in detecting PTSD [29].
Anxiety disorders.
Anxiety was a composite variable, made up of social anxiety disorder, panic disorder, and/or other anxiety disorder (similar to generalized anxiety disorder). Social anxiety disorder was assessed at baseline, whereas panic disorder and other anxiety disorder were assessed at all time points. The Mini Social Phobia Inventory (Mini-SPIN) screened for social anxiety disorder [30]. This three item measure assessed social anxiety symptoms (0 = not at all; 4 = extremely), with summed scores of six or higher meeting criteria for social anxiety disorder. The Mini-SPIN correlates highly with two other validated measures of social anxiety: the Social Interaction Anxiety Scale (r = 0.81) and the Social Phobia Scale (r = 0.77). It also has good test-retest reliability (r = .70) and internal consistency (α = .91) [30]. The Patient Health Questionnaire (PHQ) was used to assess for panic disorder and other anxiety disorder [31]. The PHQ’s diagnostic validity is comparable to the PRIME-MD, a clinician-administered diagnostic assessment [31]. For panic disorder, participants who endorsed experiencing a recurrent anxiety attack in the past four weeks that was unpredictable, bothersome, and causing worry about a future attack were also asked to report their panic symptoms during their last bad attack. Participants met criteria for panic if they had at least four panic symptoms during their last bad attack. Participants who endorsed feeling nervous, anxious, or general worry and three or more additional anxious symptoms in the past four weeks met criteria for other anxiety disorder.
Depression.
The PHQ was used to screen for depressive disorder at all time points [31]. Participants rated how much nine depressive symptoms bothered them for the past two weeks, using a four-point scale (0 = not at all; 3 = nearly every day). Those with loss of interest and/or depressed mood in the past two weeks, as well as at least five additional depressive symptoms met criteria for depression. Spitzer and colleagues [31] found that the sensitivity (73%) and specificity (94%) of the PHQ depression screener were high for major depressive disorder.
Alcohol abuse.
At all time-points, participants reported how often they binge drank (five or more alcoholic drinks in a single day) in the past three months. Participants who endorsed binge drinking once per week or more in the past three months met criteria for alcohol abuse.
Polysubstance and/or stimulant use.
Polysubstance and/or stimulant use was a composite variable assessed at all time-points. Participants indicated whether they sniffed, snorted, smoked, or swallowed any drugs in the past three months and/or injected any drugs in the past 30 days. Participants who endorsed drug use identified the drugs they used: (1) marijuana, (2) crack, (3) cocaine, (4) heroin, (5) methamphetamine or amphetamine, (6) ketamine, (7) opiates such as Vicodin, OxyContin, Dilaudid, Percocet, or Darvocet, (8) tranquilizers or barbiturates (e.g. Valium, Xanax, GHB), (9) hallucinogens (e.g. LSD, ecstasy), (10) inhalants (e.g. glue, poppers, nitrous oxide), (11) steroids, or (12) other drugs not listed. Participants also indicated whether they used crystal methamphetamine in the past three months in another questionnaire. Reponses to this item were merged into the substance use questionnaire. Participants met criteria for polysubstance use if they endorsed using three or more drugs and met criteria for stimulant use if they endorsed crack, cocaine, or methamphetamine/amphetamine use.
Syndemics.
Syndemics scores were computed by summing the total number of syndemics (CSA, PTSD, anxiety disorders, depression, alcohol abuse, polysubstance/stimulant use; all coded as 1 or 0) for which they met criteria at each visit, yielding syndemics scores from 0 – 6. This approach of computing syndemics is consistent with Stall and colleagues’ early syndemics work [14] and several recent syndemics studies [21,32,33]. We elected to use the additive approach based on our review of the literature as well as our goal of using a parsimonious, clinically useful model that is most relevant to our research question. In infrequent cases that participants did not complete assessments of all the syndemic conditions, their syndemics score was based on the sum of the conditions for which they were assessed, a conservative approach assuming participants did not meet criteria for non-assessed conditions.
Data Analysis
Adherence factor analysis.
To create a continuous measure of non-adherence and maximize variability for analysis, an exploratory factor analysis (EFA) was conducted using the seven items from the ACTG Adherence Questionnaire. This approach is consistent with prior work [25,26,34], which used data reduction to create a reliable and valid measure of adherence that makes use of all dimensions of adherence assessed on the questionnaire instead of averaging participant responses to a subset of items. Factor analyzing the adherence data was also useful for creating scores that are more normally distributed and have greater variability, as self-reported adherence is a naturally skewed variable.
In order to conduct the factor analysis, we first drew a “calibration sample” [35] using one observation of adherence per participant, randomly selected (n = 301) from any of the five time points. Using this sample, a maximum likelihood EFA was conducted with a promax rotation, yielding a one factor solution via examination of the scree plot, in which only one factor had an eigenvalue greater than 1, and the majority of the variance in adherence was explained by the first factor (>50%). The rotated factor loadings produced by the calibration sample EFA and participants’ z-scores on the adherence self-report items were used to compute regression-weighted factor scores for the complete dataset across all time points. This yielded factor scores ranging from −0.56 to 5.06 (higher scores reflected greater non-adherence). Some participants received a negative factor score because some of the z-scores were negative; however, this did not affect the distribution specified for non-adherence in the longitudinal models (continuous). Participants not on ART at a given time-point (nBL = 121, 31.0%; n3m = 79, 27.8%; n6m = 82, 28.3%; n9m = 71, 25.8%; n12m = 81; 25.1%) and those on ART with incomplete adherence data (nBL = 11, 4.1%; n3m = 14, 6.8%; n6m = 7, 3.4%; n9m = 9, 4.5%; n12m = 15, 6.2%) did not receive a score for that time point.
Longitudinal modeling of syndemics on non-adherence over time.
Multilevel modeling (MLM) was employed using SAS 9.4 (PROC MIXED with restricted maximum likelihood [REML], robust standard errors, and an unstructured covariance structure) to examine the effect of syndemics scores over time on non-adherence scores. In our mixed model, observations at each time point were nested within individuals. MLM was used because it affords the flexibility to include participants with missing data at particular time points [36]. Random and fixed effects were included in the model. In mixed models, fixed effects are the overall estimates of an effect across participants in the sample (e.g. overall intercept). In contrast, random effects allow an effect to vary across participants in the sample (e.g. a random effect of time allows the slope to vary across individuals). Predictors of non-adherence scores in our mixed model were time (continuous, coded 0 to 4), intervention condition (categorical, control = reference), a time by intervention condition interaction, and syndemics score (continuous, coded 0 to 6). Time, intervention condition, and intervention by time interactions were treated as covariates. Because medication non-adherence is a naturally skewed construct (i.e. many patients closely adhere to medication regimen), the factor analysis yielded non-adherence scores that were continuously but non-normally distributed. Therefore, we used robust standard errors to support the accuracy of our results [37].1 Appendix 1 in the online supplementary materials provides a detailed description of this analysis.
Prior to entering predictor variables, we ran an unconditional model (Model 1) with non-adherence as the outcome to examine the intraclass correlation (ICC), interpreted as the proportion of variability in non-adherence attributed to between-person differences. Following this, we tested an unconditional longitudinal model, adding the fixed and random effects of time (Model 2). For our final model (Model 3), we added fixed effects for intervention condition, the interaction between intervention condition and time, and syndemics scores.
We also tested a syndemics by condition interaction term in the longitudinal model, however this was non-significant (p = 0.88), therefore it was not used in the final model. In addition, lagged effects of syndemics were considered as a possible predictor, however there was high multicollinearity between the contemporaneous and lagged syndemics scores (r = 0.85), yielding biased results. Accordingly, the lagged effect of syndemics was not used.
Syndemics predicting viral load.
Binary logistic regression was employed using IBM SPSS Statistics 23 to examine the effect of baseline syndemics scores on odds of detectable viral load during the one-year follow up period, controlling for intervention condition. Additionally, we tested a second logistic regression model, adding baseline non-adherence factor scores as a covariate, in order to examine whether any observed effect of syndemics on viral load was related to non-adherence.
For both the ART adherence and viral load models, we considered including sociodemographic predictors of both outcomes (e.g. race/ethnicity, age, sexual orientation identity, and income) as covariates. Accordingly, we added each potential covariate to the model for each outcome (prior to adding syndemics), and found no significant effects of race/ethnicity, age, or sexual orientation on either outcome. There was not a significant effect of income on viral load, however we observed a significant effect of income on adherence, which was no longer significant when syndemics was added to the model. Because income did not attenuate the relationship between syndemics and adherence in the final model, we chose to exclude it (along with the other non-significant covariates) in favor of the more parsimonious model.
Results
Table I includes descriptive statistics for demographics and psychosocial problems observed at baseline and Table II presents descriptive statistics for all longitudinally observed variables. Figure 1 illustrates change in syndemics over time. Table III shows the pattern of association among individual syndemic conditions at baseline, such that the syndemic conditions were largely co-occurring.
Table I.
Participant Variables Observed at Baseline (N = 390)
| Variable | Statistic | Variable | Statistic |
|---|---|---|---|
| Primary Race or Ethnicity | Age M (SD) | 41.95 (8.20) | |
| Black/African American | 41 (10.5%) | Range |
21 – 68 |
| Hispanic/Latino | 30 (7.7%) | Relationship Status | |
| White (Not Hispanic) | 299 (76.7%) | Not in committed relationship | 232 (59.5%) |
| Asian/Pacific Islander | 8 (2.1%) | In committed relationship | 150 (38.5%) |
| American Indian/Alaskan | 3 (0.8%) | Other relationship status | 7 (1.8%) |
| Another Race/Ethnicity | 9 (2.3%) | Missing | 1 (0.3%) |
| Income | Viral Load (Log) M (SD) | 2.66 (1.14) | |
| $20,000 or less | 130 (33.3%) | Range | 1.62 – 7.13 |
| $20,001 to $40,000 | 85 (21.8%) | Missing |
8 (2.1%) |
| $40,001 to $60,000 | 69 (17.7%) | Undetectable VL N (%) | 218 (55.9%) |
| $60,001 to $80,000 | 51 (13.1%) | Missing (%) |
8 (2.1%) |
| Over $80,000 | 50 (12.8%) | CD4+T cell count M (SD) | 537.81 (289.56) |
| Missing | 5 (1.3%) | Range | 5 – 1894 |
| Education Level | CSA N (%) | 175 (44.9%) | |
| < High school | 51 (13.1%) | Missing (%) |
3 (0.8%) |
| Some or complete college | 246 (63.1%) | PTSD N (%) | 117 (30.0%) |
| Some or complete graduate | 93 (23.9%) | Missing | 18 (4.6%) |
Table II.
Participant Variables Observed Longitudinally
| Baseline |
3 months |
6 months |
9 months |
12 months |
|
|---|---|---|---|---|---|
| (n = 390) | (n = 284) | (n = 290) | (n = 275) | (n = 323) | |
| On ART | 268 (68.7%) | 205 (72.2%) | 207 (71.4%) | 201 (73.1%) | 241 (74.6%) |
| Missing | 1 (0.3%) | 0 (0.0%) | 1 (0.3%) | 3 (1.1%) | 1 (0.3%) |
| Non-Adh. Score | |||||
| n | 257 | 191 | 200 | 192 | 226 |
| M (SD) | 0.12 (0.99) | −0.06 (0.89) | 0.01 (1.01) | −0.05 (0.93) | −0.06 (0.85) |
| Range | −0.56 – 5.06 | −0.56 – 5.06 | −0.56 – 5.06 | −0.56 – 5.06 | −0.56 – 5.06 |
| Missing (%)a | 11 (4.1%) | 14 (6.8%) | 7 (3.4%) | 9 (4.5%) | 15 (6.2%) |
| Anxiety | 126 (32.3%) | 90 (31.7%) | 91 (31.4%) | 88 (32.0%) | 97 (30.0%) |
| Missing | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Depression | 54 (13.8%) | 32 (11.3%) | 38 (13.1%) | 32 (11.6%) | 41 (12.7%) |
| Missing | 1 (0.3%) | 0 (0.0%) | 2 (0.7%) | 2 (0.7%) | 0 (0.0%) |
| Polysub./Stim. | 147 (37.7%) | 75 (26.4%) | 83 (28.6%) | 64 (23.3%) | 71 (22.0%) |
| Missing | 0 (0.0%) | 0 (0.0%) | 3 (1.0%) | 2 (0.7%) | 0 (0.0%) |
| Alcohol Abuse | 79 (20.3%) | 39 (13.7%) | 50 (17.2%) | 50 (18.2%) | 48 (14.9%) |
| Missing | 2 (0.5%) | 2 (0.7%) | 3 (1.0%) | 3 (1.1%) | 4 (1.2%) |
| Total Syndemics | |||||
| M (SD) | 1.79 (1.42) | 1.54 (1.28) | 1.63 (1.31) | 1.58 (1.30) | 1.51 (1.23) |
| Range | 0 – 6 | 0 – 5 | 0 – 6 | 0 – 5 | 0 – 6 |
Missing refers to those on ART but with incomplete or missing adherence measures, therefore they did not receive a factor score. Percentages are of those on ART at that time point.
Figure 1.
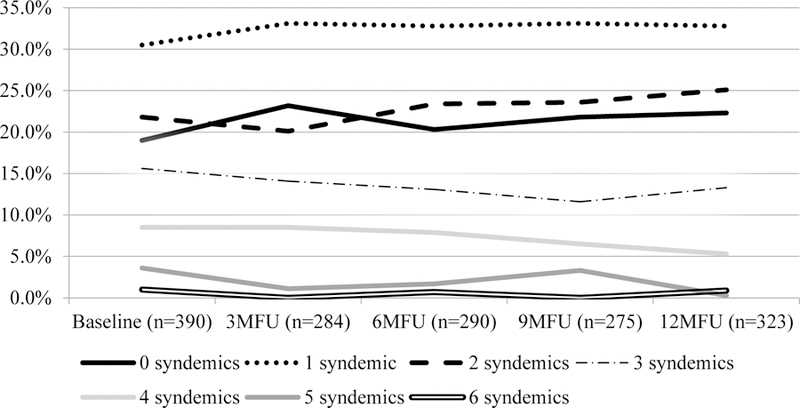
Percentage of participants endorsing syndemics by time point
Table III.
Associations of syndemic conditions at baseline (N = 390)
| Odds ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Syndemic Condition | Anxiety | Depression | PTSD | CSA | Polysub. or stimulants |
| Depression | 10.50* (5.28 – 20.88) | ||||
| PTSD | 3.13* (1.98 – 4.93) | 4.30* (2.37 – 7.80) | |||
| CSA | 1.24 (0.81 – 1.89) | 1.27 (0.72 – 2.26) | 1.52† (0.98 – 2.35) | ||
| Polysub. or stimulants | 1.45† (0.94 – 2.23) | 2.34* (1.31 – 4.19) | 1.75* (1.12 – 2.71) | 1.25 (0.83 – 1.88) | |
| Alcohol abuse | 2.20* (1.33 – 3.64) | 1.83† (0.96 – 3.48) | 1.47 (0.87 – 2.47) | 1.52† (0.92 – 2.49) | 3.55* (2.12 – 5.93) |
Note: Bivariate analyses were conducted using logistic regression between each of the six syndemic conditions. Odds ratios are reported with 95% confidence intervals reported in parentheses. Missing data, which was infrequent, was imputed with 0 (syndemic condition not present). Sensitivity tests with missing data were also conducted with the same pattern of results.
p < .10
p < .05
Missing visits varied across the five time points, with participants completing the first three follow up visits less often (n3M = 284, 72.8%; n6M = 290, 74.4%; n9M = 275, 70.5%) than the final 12 month follow up (n12M = 323, 82.8%).
Longitudinal Model of Syndemics Predicting Non-Adherence Scores (Table IV)
Table IV.
Multilevel models of non-adherence to ART
| Parameter | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Fixed effects | |||
| Intercept (γ00) | 0.03 (0.05) | 0.06 (0.05) | −0.19 (0.12) |
| Time (γ10) | −0.02 (0.02) | 0.02 (0.04) | |
| Conditionb | F(2, 513) = 0.82 | ||
| Experimental (γ10) | −0.03 (0.15) | ||
| Peer (γ01) | 0.11 (0.14) | ||
| Conditionb x Time | F(2, 513) = 2.93* | ||
| Experimental (γ11) | 0.02 (0.05) | ||
| Peer (γ12) | −0.07 (0.04) | ||
| Syndemics (γ20) | 0.13 (0.04)** | ||
| Random effects | |||
| Intercept (τ00) | 0.45 (0.05)** | 0.47 (0.07)** | 0.42 (0.07)** |
| Int/Time Cov (τ01) | −0.01 (0.02) | −0.01 (0.02) | |
| Time (τ11) | 0.01 (0.01)* | 0.01 (0.01)* | |
| Residual (σ2) | 0.47 (0.02)** | 0.44 (0.03)** | 0.44 (0.03)** |
Note. Estimates are reported followed by standard errors are in parentheses. Model 1 is the unconditional model. Model 2 includes random and fixed effects of time. Model 3 is the final model including all predictors.
bReference = control group.
p < .05
p < .01
Model 1, the unconditional model, showed that non-adherence scores varied within participants over time (σ = 0.47, SE = 0.02, p < 0.0001) and between participants (τ = 0.45, SE = 0.05; p < 0.0001). The ICC showed that about half (49.19%) of the variance in non-adherence scores was attributable to the individual (between-person variance), and the other half was attributable to time (within-person variance).
Model 2 showed that the fixed effect of time was non-significant (γ10 = −0.02, SE = 0.02, t(250) = −1.08, p = 0.28), suggesting that the average slope of the non-adherence regression line was flat, or that across all participants, there was not an average effect of time on non-adherence. The random effect of time was significant (τ11 = 0.01, SE = 0.01, p = 0.04), suggesting the slope of the trajectory varied across participants, or that there were different non-adherence regression lines across participants.
Model 3, the final model, tested the main hypothesis. In terms of the primary study hypothesis, Model 3 showed that syndemics scores over time had a significant unique effect on non-adherence scores (γ20 = 0.13, SE = 0.04, t(513) = 2.85, p = 0.004). Beyond the effects of time, intervention condition, and intervention condition by time interaction, each additional syndemic was associated with a 0.13 increase in the non-adherence score. Comparing the unconditional model to the final model shows that 7.1% of the variance in non-adherence score was explained at the person-level (Level 2; effects that varied by person, not by time) and 6.6% of the variability was explained within-person (Level 1; effects that varied across time within the individual).
As with Model 2, the fixed effect of time was non-significant (γ10 = −0.02, SE = 0.04, t(248) = 0.51, p = 0.61) and the random effect of time was significant (τ11 = 0.01, SE = 0.01, p = 0.04), again highlighting no overall effect of time across participants on non-adherence, but differential effects of time within participants. The fixed effect of intervention condition was not significant, F(2,513) = 0.82, p = 0.44, suggesting there were not baseline differences in non-adherence scores across intervention conditions. The overall condition by time interaction was marginally significant, F(2,513) = 2.93, p = 0.055, and therefore retained in the model as a control, however, the pairwise comparisons showed that neither the Peer to Peer condition nor the Enhance intervention condition had significantly different trajectories of non-adherence scores over time compared to the reference group (Enhance, control group).
To better understand the precise longitudinal relationship between syndemics and non-adherence, we re-ran Model 3 with the syndemics scores disaggregated, or, separated into two scores: the person-mean centered score and the deviation from the person-mean centered score. This allowed us to separate the within- and between-person effects of syndemics on non-adherence scores [38]. In this model, the person-mean score (the person’s average level of syndemics across the five time points) was a significant predictor of non-adherence scores (γ = 0.18, SE = 0.05, t(512) = 3.88 p = 0.0001), such that a mean increase of one syndemic was associated with a 0.18 increase in the non-adherence score. In contrast, the deviation from the person-mean (the person’s variation in syndemics scores across the five time points) was not a significant predictor of non-adherence scores (γ = 0.04, SE = 0.07, t(512) = 0.52, p = 0.60). This suggests that the effect of syndemics on non-adherence scores was largely a between person effect. In other words, a person’s average level of syndemics over the one-year observation period predicted their non-adherence scores, not the time-specific changes in their syndemics above their average level (illustrated in Figure 1).
Syndemics Predicting Viral Load
The logistic regression model with follow up viral load detectability regressed on baseline syndemics (controlling for intervention condition) was statistically significant (χ2(3) = 14.51, p = 0.002). Each additional syndemic condition for which participants met criteria at baseline was associated with 1.27 (B = 0.24, SE = 0.08, p = 0.002) greater odds of at least one detectable viral load observed during the one-year follow up period.
Following this, we added baseline non-adherence factor scores to the logistic regression model as a covariate. The overall model was again statistically significant (χ2(4) = 25.58, p < .001). In this model, syndemics was no longer a significant predictor of detectable viral load (B = 0.17, SE = 0.12, p = 0.14). Non-adherence was a significant predictor in this model (B = 0.59, SE = 0.16, p < 0.001), such that a one unit increase in the non-adherence factor score was associated with 1.80 (95% CI [1.32, 2.46]) greater odds of detectable viral load during the one-year follow up. This suggests that the effect of syndemics on viral load operated through its effect on non-adherence.
Discussion
The results of this study supported the main hypothesis. Among this sample of sexual minority men living with HIV, syndemics co-occurred and were additively and longitudinally associated with greater non-adherence to ART, even when controlling for other variables that could be related to adherence. Additionally, baseline levels of syndemics were additively associated with greater likelihood of viral non-suppression during the one-year follow up period, an effect that appears to take place through the effect of syndemics on ART non-adherence. This builds on prior research showing that syndemics are associated with HIV-related sexual risk behavior among HIV-negative men [e.g. 11–17], expands on cross-sectional findings that syndemics are associated with adherence among PLWH [18], and supports recent longitudinal research showing a relationship between syndemics and non-adherence/viral load in MSM living with HIV [21]. This study bolsters Friedman et al.’s (2015) findings by showing that the relationship between syndemics and non-adherence they observed in MSM is robust when using a larger range of syndemic conditions and a continuous outcome measure of non-adherence and extends the findings to a population of sexual minority men living in another U.S. city not observed in the earlier study.
This study also supports the expanding body of syndemics theory literature [10] in the context of HIV. Consistent with syndemics theory and research, the main findings reiterate that among sexual minority men, addressing health behaviors associated with living with HIV, such as adhering to one’s ART regimen, requires consideration of the context of the syndemic conditions that impede adherence. As the number of co-occurring psychosocial problems increases, so does non-adherence and likelihood of detectable viral load, implicating the importance of treating the overall syndemic, not just individual conditions that may interfere with ART adherence, such as depression.
In addition to this main finding, we found that the impact of syndemics on adherence in this study appears to be operating through the overall level of syndemics that a person experienced over time, not the change in their syndemics level during a one year observation period. It is possible that the relatively frequent observations of participants were not long enough to show within-person (time) effects on non-adherence, as this would be a short amount of time for some of the psychosocial problems observed to resolve fully. Relatedly, syndemics levels were relatively stable over time for participants (as shown in Figure 1). We did not observe major longitudinal shifts in participants’ syndemics levels over time, which may statistically limit the degree to which the time variable could have predictive value.
These findings have clear implications for practice. As suggested in past research focusing on HIV-prevention and syndemics, understanding HIV-related health behaviors and outcomes as impacted by psychosocial syndemics implies that interventions for health behavior change will be more successful by integrating adherence training and mental health/substance use treatment programs. Although existing adherence interventions have been shown to work [39], generally, effect sizes could be higher, and there are segments of the population of MSM living with HIV who have not reached viral suppression [5], which generally requires high levels of ART adherence despite advances in the potency of these medications [1]. Psychosocial syndemics may be heightening these health disparities. Mental health providers working with sexual minority men living with HIV may be able to enhance the effectiveness of adherence interventions by assessing the presence and functional impact of mental health and substance use concerns on their clients’ HIV-related health behaviors. By establishing integrated interventions that address multiple syndemic conditions and adherence simultaneously, these previously underserved populations may be able to achieve greater health. Some research has examined integrating cognitive behavior therapy for depression with cognitive behavioral therapy for adherence in PLWH [40–42]. Additional interventions might need to be developed to treat multiple, co-occurring psychosocial and substance use problems that interfere with ART adherence among sexual minority men in particular.
Further research is needed to explore the specific mechanisms through which syndemics impact adherence. For example, it may be that mounting stress levels associated with these psychosocial concerns decrease the availability of psychological resources to plan and engage in a daily health care behavior. It is also possible that greater syndemic conditions detract from self-efficacy regarding one’s ability to engage in HIV self-care behaviors. Understanding the pathways through which syndemics influence adherence can inform behavioral adherence interventions.
Another area of ongoing discussion and further research is how to best operationalize syndemics and their collective effects, with different syndemics researchers using different approaches. Starks and colleagues [43] compared different approaches to computing syndemics and found that latent class analysis was not a useful method of assessing syndemics in their sample of sexual minority men at risk for HIV and instead found support for additive approaches. In the present study, we used an additive approach consistent with early syndemics research and several recent syndemics studies [14,21,32,33,44]. The additive approach that we used was most parsimonious, clinically useful, and relevant to our research question. Another complex issue that has been raised in the syndemics literature is the possibility of testing interaction terms to elucidate synergistic effects of syndemics on HIV-related outcomes [45]. In our view, testing numerous interaction terms within the same model would reduce the clinical significance and utility of the outcomes. Instead of testing these interactions, we tested whether there was mutual causality, or, whether each of the syndemic conditions are predictive of one another (See Table III), another aspect of syndemics theory [10, 46]. Although this additive approach with a test of mutual causality was most appropriate to our research question and clinically significant, it will be useful for syndemics researchers to continue developing different ways of examining relationships among syndemic conditions using different statistical approaches.
Further research is also needed to examine resilience factors, such as distress tolerance or social support, which may inform interventions to prevent syndemics from having the impact observed in this study on HIV health behaviors and outcomes. Herrick et al. [47] highlight that despite exposure to adverse environmental conditions and corresponding syndemics of psychosocial problems and HIV risk, it is common to observe the resilience of sexual minority men (e.g. staying HIV negative or attaining viral suppression). Friedman and colleagues found that among HIV-positive MSM, having higher levels of social support attenuated the negative relationship between syndemics and viral load [32]. Accordingly, an area of future research is to continue exploring resilience among sexual minority men living with HIV who are at risk of worse health behaviors due to co-occurring psychosocial problems, but instead are able to remain engaged in positive self-care behaviors, including ART adherence.
Although this study presents novel research findings, there were also limitations. This study was a secondary analysis of data collected from two prior intervention studies, thus the study’s operationalization of syndemics was limited to variables in the existing datasets. We also sought to statistically control for differences among intervention arms, however we note that this was a complex and somewhat heterogeneous sample. As noted above, the self-report measures of syndemics are valid and reliable measures of the conditions that we sought to characterize. However, there may be more in depth assessments that go beyond these brief self-report measures that could further strengthen this line of research in future studies. Additionally, there has been some discussion in the literature regarding whether victimization is a syndemic condition in itself versus a life event that leads to the development of other syndemic conditions later in one’s development [48]. Consistent with several other studies examining the effect of syndemics on HIV related outcomes we included CSA as a syndemic condition [13,44]. Other syndemic conditions co-occur with CSA. Although it is possible that CSA could lead to the development of some of these other co-occurring syndemic conditions, not everyone who experiences CSA goes on to develop these outcomes. Thus, we decided to use CSA as an independent syndemic condition, which can have a unique impact on HIV-related health behaviors.
Future research may also benefit from an expansion of the variables into systemic versus individual syndemics factors, or time varying versus fixed variables, which would provide a greater understanding of how different types of syndemic conditions affect sexual minority men’s health behaviors and clarify treatment targets. For example, one study showed that a syndemic of systemic barriers (e.g. transportation, payment concerns, lack of a telephone, responsiveness of scheduling staff, convenience of appointment times, and language spoken by their physician) was associated with lower likelihood of attending clinic visits or having a suppressed viral load [49], underscoring the need for systemic interventions in addition to individual behavioral interventions. Relatedly, it may be that some syndemic indicators have a stronger association with the HIV-health outcomes than others, indicating an area for future study. It would also be useful to be able to distinguish between the impacts of fixed variables (e.g. exposure to CSA in one’s developmental history) versus time-varying variables (e.g. psychiatric diagnosis or housing status) on HIV-health behaviors and outcomes, as impairment due to time-varying issues could be remedied through behavioral and/or structural intervention.
The population studied was limited to sexual minority men living with HIV in the Northeastern U.S., with a majority of the sample identifying their primary racial/ethnic identity as white, limiting the generalizability of the findings. Racial and ethnic minority sexual minority men living with HIV may face additional stressors, such as racial minority stress, which are important to conceptualize in terms of impact on overall health behaviors. HIV health disparities have repeatedly been found, with Black and Latino MSM disproportionately impacted by HIV/AIDS [21], a disparity rooted in structural and social inequality. Future research is therefore needed to clarify the relationship between syndemics and HIV health behaviors among racial and ethnic minority sexual minority men.
Sexual minority men are the largest group living with HIV in the U.S. and have been since the start of the epidemic. Accordingly, it is important to understand predictors of the health behaviors of this population, which can in turn affect personal health (e.g. improved health quality of life, preventing resistance) and public health (e.g. reduced transmissibility via viral suppression). This longitudinal study of multiple syndemics and non-adherence and viral load among sexual minority men can be used to advance the application of syndemics theory to health behaviors among those living with HIV. To curb the impact and transmission of HIV among this already disproportionately affected population, co-occurring psychosocial problems that interfere with health behaviors and outcomes will need to be addressed through behavioral and public health intervention.
Supplementary Material
Acknowledgments
Compliance with Ethical Standards.
Funding: This study was funded in by HRSA (H97HA01293) and NIMH (5R01MH068746) awarded to Drs. Kenneth H. Mayer and Steven A. Safren. Some of the author time was supported by NIDA (9K24DA040489) awarded to Dr. Steven A. Safren.
Footnotes
Conflict of interest: All authors declare that they have no conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
A sensitivity test using the log transformed non-adherence factor scores as the outcome variable revealed the same pattern of results as the original model. Syndemics remained a significant predictor of the log transformed non-adherence scores, γ = 0.03, SE = 0.01, t(513) = 0.002. Because the pattern of the results was unchanged, we retained the original factor score distribution with robust standard errors to increase interpretability of the findings.
References
- 1.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin. Infect. Dis 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016;316:171–81. [DOI] [PubMed] [Google Scholar]
- 4.Das M, Chu PL, Santos G-M, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE 2010;5:e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazo M, Gange SJ, Wilson TE, et al. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: Longitudinal study of men and women. Clin. Infect. Dis 2007;45:1377–85. [DOI] [PubMed] [Google Scholar]
- 6.CDC. HIV Surveillance Report, 2015 [Internet] Centers for Disease Control and Prevention; 2016 Nov. Report No.: 27. Available from: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Google Scholar]
- 7.Cochran SD, Greer J, Mays VM. Prevalence of mental disorders, psychological distress, and mental health services use among lesbian, gay, and bisexual adults in the United States. J. Consult. Clin. Psychol 2003;71:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Cleirigh C, Magidson JF, Skeer MR, Mayer KH, Safren SA. Prevalence of psychiatric and substance abuse symptomatology among HIV-infected gay and bisexual men in HIV primary care. Psychosomatics 2015;56:470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer MC, Erickson PI, Badiane L, et al. Syndemics, sex and the city: Understanding sexually transmitted diseases in social and cultural context. Soc. Sci. Med 2006;63:2010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer MC, Clair S. Syndemics and public health: Reconceptualizing disease in bio-social context. Med. Anthropol. Q 2003;17:423–41. [DOI] [PubMed] [Google Scholar]
- 11.Ferlatte O, Hottes TS, Trussler T, Marchand R. Evidence of a syndemic among young Canadian gay and bisexual men: Uncovering the associations between anti-gay experiences, psychosocial issues, and HIV risk. AIDS Behav 2014;18:1256–63. [DOI] [PubMed] [Google Scholar]
- 12.Mimiaga MJ, Biello KB, Robertson AM, et al. High prevalence of multiple syndemic conditions associated with sexual risk behavior and HIV infection among a large sample of Spanish- and Portuguese-speaking men who have sex with men in Latin America. Arch. Sex. Behav 2015;44:1869–78. [DOI] [PubMed] [Google Scholar]
- 13.Parsons JT, Grov C, Golub SA. Sexual compulsivity, co-occurring psychosocial health problems, and HIV risk among gay and bisexual men: Further evidence of a syndemic. Am. J. Public Health 2012;102:156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stall R, Mills TC, Williamson J, et al. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am. J. Public Health 2003;93:939–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guadamuz TE, McCarthy K, Wimonsate W, et al. Psychosocial health conditions and HIV prevalence and incidence in a cohort of men who have sex with men in Bangkok, Thailand: Evidence of a syndemic effect. AIDS Behav 2014;18:2089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mimiaga MJ, OʼCleirigh C, Biello KB, et al. The effect of psychosocial syndemic production on 4-year HIV incidence and risk behavior in a large cohort of sexually active men who have sex with men. J. Acquir. Immune Defic. Syndr 2015;68:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustanski B, Phillips G, Ryan DT, Swann G, Kuhns L, Garofalo R. Prospective effects of a syndemic on HIV and STI incidence and risk behaviors in a cohort of young men who have sex with men. AIDS Behav 2017;21:845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blashill AJ, Bedoya CA, Mayer KH, et al. Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS Behav 2015;19:981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhns LM, Hotton AL, Garofalo R, et al. An index of multiple psychosocial, syndemic conditions is associated with antiretroviral medication adherence among HIV-positive youth. AIDS Patient Care STDs 2016;30:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biello KB, Oldenburg CE, Safren SA, et al. Multiple syndemic psychosocial factors are associated with reduced engagement in HIV care among a multinational, online sample of HIV-infected MSM in Latin America. AIDS Care 2016;28:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman MR, Stall R, Silvestre AJ, et al. Effects of syndemics on HIV viral load and medication adherence in the multicentre AIDS cohort study. AIDS 2015;29:1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safren SA, O’Cleirigh CM, Skeer M, Elsesser SA, Mayer KH. Project enhance: A randomized controlled trial of an individualized HIV prevention intervention for HIV-infected men who have sex with men conducted in a primary care setting. Health Psychol 2013;32:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safren SA, O’Cleirigh C, Skeer MR, et al. Demonstration and evaluation of a peer-delivered, individually-tailored, HIV prevention intervention for HIV-infected MSM in their primary care setting. AIDS Behav 2011;15:949–58. [DOI] [PubMed] [Google Scholar]
- 24.Knauz RO, Safren SA, O’Cleirigh C, et al. Developing an HIV-prevention intervention for HIV-infected men who have sex with men in HIV care: Project Enhance. AIDS Behav 2007;11:S117–26. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self-reported adherence with the ACTG Adherence Questionnaire: A cross-protocol analysis. J. Acquir. Immune Defic. Syndr 2007;46:402–9. [DOI] [PubMed] [Google Scholar]
- 26.Safren SA, Biello KB, Smeaton L, et al. Psychosocial predictors of non-adherence and treatment failure in a large scale multi-national trial of antiretroviral therapy for HIV: Data from the ACTG A5175/PEARLS trial. PloS One 2014;9:e104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG Adherence Instruments. AIDS Care 2000;12:255–66. [DOI] [PubMed] [Google Scholar]
- 28.Finkelhor D, Hamby SL, Ormrod R, Turner H. The Juvenile Victimization Questionnaire: Reliability, validity, and national norms. Child Abuse Negl 2005;29:383–412. [DOI] [PubMed] [Google Scholar]
- 29.Meltzer-Brody S, Churchill E, Davidson JR. Derivation of the SPAN, a brief diagnostic screening test for post-traumatic stress disorder. Psychiatry Res 1999;88:63–70. [DOI] [PubMed] [Google Scholar]
- 30.Seeley-Wait E, Abbott MJ, Rapee RM. Psychometric properties of the mini-social phobia inventory. Prim Care Companion J Clin Psychiatry 2009;11:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44. [DOI] [PubMed] [Google Scholar]
- 32.Friedman MR, Coulter RWS, Silvestre AJ, Stall R, Teplin L, Shoptaw S, Surkan PJ, Plankey MW. Someone to count on: social support as an effect modifier of viral load suppression in a prospective cohort study. AIDS Care 2017;29;469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald LR, Antoine DG, Liao C, Lee A, Wahab M, Coleman JS. Syndemic of lifetime mental illness, substance use disorders, and trauma and their association with adverse perinatal outcomes. Journal of Interpersonal Violence 2017;1–20. [DOI] [PubMed] [Google Scholar]
- 34.Safren SA, Mayer KH, Ou S, et al. Adherence to early antiretroviral therapy: Results from HPTN 052, a phase III, multinational randomized trial of ART to prevent HIV-1 sexual transmission in serodiscordant couples. J. Acquir. Immune Defic. Syndr 2015;69:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer DJ, Hussong AM. Psychometric approaches for developing commensurate measures across independent studies: Traditional and new models. Psychol. Methods 2009;14:101–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson DL. Reporting results of latent growth modeling and multilevel modeling analyses: Some recommendations for rehabilitation psychology. Rehabil. Psychol 2010;55:272–85. [DOI] [PubMed] [Google Scholar]
- 37.Maas CJM, Hox JJ. The influence of violations of assumptions on multilevel parameter estimates and their standard errors. Comput. Stat. Data Anal 2004;46:427–40. [Google Scholar]
- 38.Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Res. Hum. Dev 2009;6:97–120. [Google Scholar]
- 39.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: A review of the literature. Curr. Infect. Dis. Rep 2008;10:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol 2009;28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. J. Consult. Clin. Psychol 2012;80:404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safren SA, Bedoya CA, O’Cleirigh C, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: A three-arm randomised controlled trial. Lancet HIV 2016;3:e529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starks TJ, Millar BM, Eggleston JJ, Parsons JT. Syndemic factors associated with HIV risk for gay and bisexual men: comparing latent class and latent factor modeling. AIDS Behav 2014;18:2075–2079. [DOI] [PubMed] [Google Scholar]
- 44.Pantalone DW, Valentine SE, Woodward EN, O’Cleirigh C. Syndemic indicators predict poor medication adherence and increased healthcare utilization for urban HIV-positive men who have sex with men. Journal of Gay & Lesbian Mental Health 2018;22:71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai AC, Burns BFO. Syndemics of psychosocial problems and HIV risk: a systematic review of empirical tests of the disease interaction concept. Soc Sci Med 2015;139:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai AC, Mendenhall E, Trostle JA, Kawachi I. Co-occurring epidemics, syndemics, and population health. The Lancet 2017;389:978–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrick AL, Lim SH, Wei C, et al. Resilience as an untapped resource in behavioral intervention design for gay men. AIDS Behav 2011;15:25–9. [DOI] [PubMed] [Google Scholar]
- 48.Herrick AL, Lim SH, Plankey MW, Chmiel JS, Guadamuz TT, Kao U, Shoptaw S, Carrico A, Ostrow D, Stall R. Adversity and syndemic production among men participating in the multicenter AIDS cohort study: a life-course approach. Research and Practice 2013;103:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wawrzyniak AJ, Rodríguez AE, Falcon AE, et al. The association of individual and systemic barriers to optimal medical care in people living with HIV/AIDS (PLWHA) in Miami-Dade County. J. Acquir. Immune Defic. Syndr 2015;69:S63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


