Abstract
Background:
Since 2005, the Lung Allocation Score (LAS) has prioritized patient benefit and post-transplant survival, reducing waitlist to transplant time to less than 200 days and decreasing mortality on the waitlist. A current challenge is the wait for the waitlist—the time between patient’s transplant-eligible diagnosis and waitlist registration.
Methods:
We investigated whether sociodemographic (age, sex, race, insurance, marital status, median household income) and clinical (FEV1 % of predicted, body mass index, depression/anxiety, alcohol/substance misuse, absolute/relative contraindications) factors influenced referral and waitlist registration. We conducted a retrospective cohort study through chart review of hospitalized patients on the University of Chicago general medicine service from 2006–2014 meeting transplant-eligible criteria and ICD-9 billing codes for cystic fibrosis (CF) and pulmonary fibrosis (PF). We analyzed the times from transplant-eligibility to referral, work-up, and waitlist using Kaplan-Meier curves and log-rank tests.
Results:
Overall, referral rates for transplant-eligible patients were 64%. Of those referred, approximately 36% reach the lung transplant waitlist. Referred CF patients were significantly more likely to reach the transplant waitlist than PF patients (CF 60% vs. PF 22%, p<0.05). In addition, CF patients experience a shorter wait from transplant- eligibility to waitlist than PF patients (329 vs. 2369 days, respectively [25th percentile], p<0.05). Patients with PF and CF both faced delays from eligibility to referral and waitlist.
Conclusions:
Quality improvement efforts are needed to better identify and refer appropriate patients for lung transplant evaluation. Targeted interventions may facilitate more efficient evaluation completion and waitlist appearance.
Keywords: cystic fibrosis, lung transplant, pulmonary fibrosis, waitlist
Introduction
Many surgical and immunologic barriers to successful lung transplantation have been overcome in the United States since the early 1980s. System-level challenges have also been identified and improved. Prior to 2005, the Organ Procurement and Transplantation Network (OPTN) allocated deceased donor lungs to candidates based on waitlist time regardless of disease severity. Wait times averaged over 2 years and nearly 10% of candidates died on the waitlist during this time period.1,2 In 2005, the OPTN implemented reform through the Lung Allocation Score (LAS) which prioritized patient benefit and post-transplant survival, reducing waitlist to transplant time to less than 200 days and reducing mortality on the waitlist.2
Despite many advances, few studies have addressed the complicated processes involved with referring transplant-eligible patients for lung transplant evaluation and completing the evaluation process to appear on the waitlist. Prior work demonstrated that early referral for transplant evaluation improves patients’ access to appropriate specialist care, improves survival on the wait list, and mitigates the anxiety of patients and caretakers in disease management.3 Multidisciplinary transplant teams involved in the care of waitlisted patients improve patient outcomes regardless of whether the patient receives a transplant.4
In fact, the wait for the waitlist—the time spent between transplant-eligible diagnoses to waitlist appearance—may prove overwhelmingly challenging for many patients. During this time, patients must undergo a battery of more than 30 tests, screening procedures, and subspecialty consults. Additionally, patients must demonstrate a good record of medical adherence, access to consistent social support, and adequate financial resources in order to be placed on the lung transplant waitlist.4 Successful completion of these tests and appointments may hinge on the socio-demographic and clinical characteristics of the patient, as has been demonstrated in other transplant populations.5,6 Overcoming the multifaceted obstacles faced by potential candidates in accessing the waitlist requires concerted efforts by certified lung transplant programs.
Therefore, we sought to investigate the likelihood of referral, time to referral, and waitlist registration for transplant-eligible patients diagnosed with pulmonary fibrosis (PF) or cystic fibrosis (CF) hospitalized from 2006–2014 at a single academic medical transplant center. We also examine whether socio-demographic and clinical factors influence likelihood and time to referral and waitlist registration.
Methods
Study Design and Participants
Our study was a retrospective cohort study conducted at a single academic medical transplant center. We obtained data from the University of Chicago Hospitalist Project, a large-scale study of hospitalized patients’ outcomes in which participants provide written, informed consent allowing study staff to review their medical records for laboratory and health utilization information.7 Hospitalist Project participants hospitalized between 1/1/2006–1/1/2014 at the University of Chicago Medical Center (UCMC) with a diagnosis of cystic fibrosis (CF) or pulmonary fibrosis (PF) in the first 20 ICD-9-CM billing codes were assessed for a qualifying pulmonary function test (PFT) or appropriate disease documentation through chart review to create cohorts of transplant- eligible patients. This study was approved by the University of Chicago Institutional Review Board (IRB#9967).
Guidelines for lung transplantation eligibility criteria were derived through a literature review and the expertise of the UCMC transplant team. CF patients were identified using ICD-9 codes 277.0X. Transplant eligibility for CF patients was based on PFTs with a forced expiratory volume (FEV1) ≤ 30% of predicted.8 We identified patients as having PF if they had an ICD-9 diagnosis of 515.XX or 516.XX. In the UCMC Lung Transplant Program, these patients are within the LAS Lung Disease Primary Diagnostic Grouping D for restrictive lung disease and are treated similarly in terms of their eligibility and lung transplant evaluation. Transplant eligibility for PF patients was based on PFTs with a diffusing capacity of the lungs for carbon monoxide (DLCO) < 40 or confirmation of usual interstitial pneumonia (UIP).4 PF patients with UIP should be referred to a lung transplant center at the time of diagnosis due to lack of effective therapies and rapid disease progression.4
Exclusion Criteria
We excluded all patients younger than 18 or older than 65. We also excluded patients who had not been seen as outpatients at UCMC as those patients may have been evaluated and listed elsewhere. Patients with pulmonary arterial hypertension, alpha-1 antitrypsin deficiency, and sarcoidosis (without PF) were excluded from the study due to the challenges of using objective PFT-based criteria. Patients with chronic obstructive pulmonary disease (COPD) were excluded due to infeasibility of creating a well-defined cohort. Hospitalized patients with COPD comprise a large, heterogeneous sample (>600), and a low proportion of COPD patients are transplant-eligible.9,10 Figure 1 demonstrates the process of the cohort formation.
Figure 1.
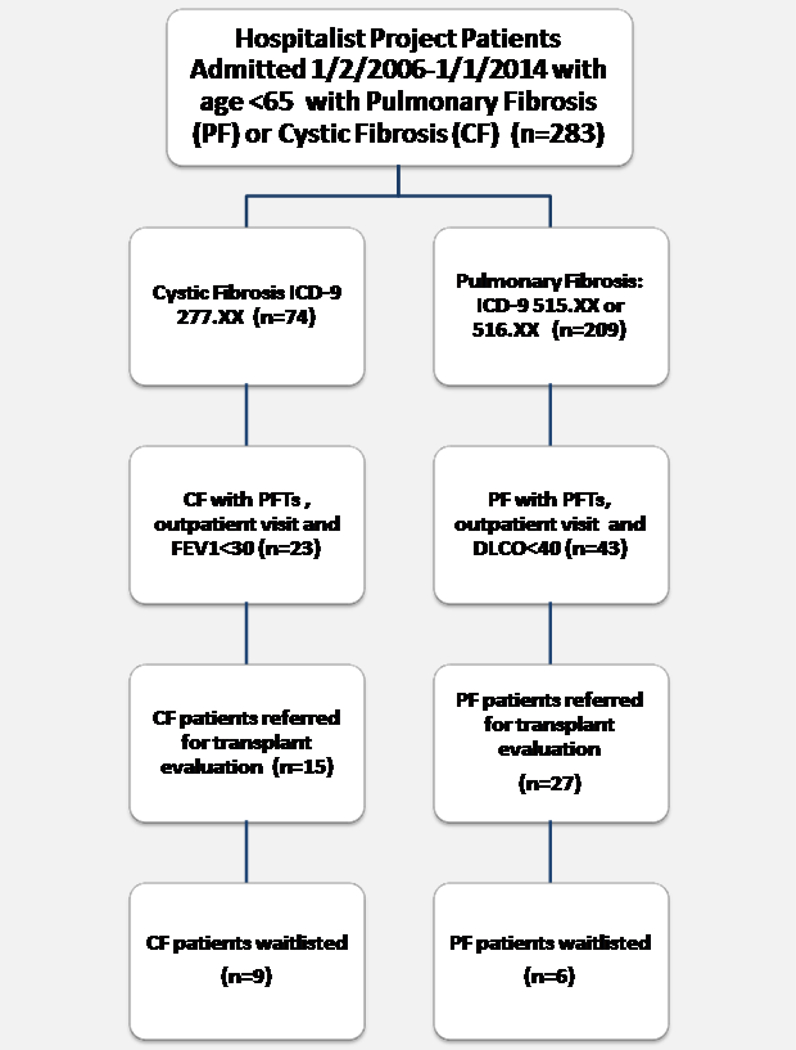
Cohort Selection Process
Data Collection
We tracked participants’ progress from initial qualifying PFT or diagnosis through chart review and administrative data to transplant waitlist appearance in the United Network of Organ Sharing (UNOS) UNet database. Patients were censored at death or determination of transplant ineligibility by the lung transplant team. Appendix Table A displays relevant electronic medical record fields from which socio-demographic and clinical information were extracted.
Variable Definition
Eligible date for CF patients was defined as the earliest date of qualifying PFT with an FEV1 ≤ 30%. Eligible date for PF patients was the earliest date of PFT with a DLCO < 40% or date of pathological diagnosis of UIP. Referral date was defined as the earliest date when chart review indicated that the patient should see lung transplant team or, alternatively, one week before work-up date for patients missing a referral date. We assumed that a patient could not appear on the waitlist without prior referral and work-up. Work-up date was defined as the earliest date that lung transplant evaluation tests (Appendix Table C) were initiated. For survival analysis, if referral or workup date were before eligibility date, the difference between dates was changed to 0.
For patients with more than one kind of insurance, only one type was entered, in the following order: private insurance, Medicare, and Medicaid. Median household income was determined from US Census data using each patient’s zip code.11 Diagnosis of depression/anxiety and alcohol/substance misuse was also included if indicated in the providers’ notes or the patient’s problem list.
Absolute contraindications were established by the UCMC Lung Transplant Program. These include medical (e.g., active malignancy) and psychosocial (e.g., lack of social support) factors that constitute an unacceptable risk for any candidate under consideration (Appendix Table B).
Within the UCMC Lung Transplant Program, each patient is assessed by the multidisciplinary team for relative contraindication(s) that may constitute an unacceptable risk. Examples include infectious (e.g., colonization with multi-resistant or virulent bacteria, fungi, or mycobacteria), clinical (e.g., body mass index or BMI < 18 or > 30), and psychosocial (e.g., psychosocial/support issues that impair compliance with medical care) risk factors (Appendix Table B). Individual risks are established at multidisciplinary consensus meetings and communicated to patients.
Statistical Analysis
STATA 14.0 was used for data analysis. Chi-squared tests were performed to compare cohort characteristics. For comparisons within cohorts, 2-sided Fisher exact tests were used due to the smaller sample size. Mann-Whitney tests were used for FEV1 comparisons. To compare the distributions of the time to various stages in the waitlist process between CF and PF patients, we created Kaplan-Meier curves for time from eligibility to referral, time from eligibility to work-up, time from eligibility to waitlist, time from referral to work-up, and time from work-up to waitlist. To test whether the survival distributions were significantly different for CF and PF patients, we performed log-rank tests.
Results
Demographic information comparing the CF and PF cohorts is displayed in Table 1. In this study, the sample was 23% white with a mean age of 45 years old. Compared to patients with PF, CF patients were younger (74% vs. 4.7% less than 35 years old), more likely to be male (61% vs. 28%) and white (59% vs. 23%), all p<0.05. Patients with CF had significantly lower FEV1 % of predicted (22% vs. 48%), lower mean BMI (20.7 kg/m2 vs. 28.1 kg/m2), and were more likely to have a diagnosis of depression or anxiety (76.2% vs. 30%) than patients with PF, all p>0.05.
Table 1.
Sample Characteristics Overall and Comparison of Cystic Fibrosis (CF) and Pulmonary Fibrosis (PF) Cohort.
| Overall n(%) |
Cystic Fibros | Pulmonary Fibrosis | P-value | |
|---|---|---|---|---|
| n=66 | n=23 | n=43 | ||
| Male | 26 (39.4) | 14 (60.9) | 12 (27.9) | 0.009* |
| White | 23 (35.4) | 13 (59.1) | 10 (23.3) | 0.004* |
| Age in years | <0.001** | |||
| < 35 years | 19 (28.8) | 17 (73.9) | 2 (4.7) | |
| 35 – 49 | 18 (27.3) | 4 (17.4) | 14 (32.6) | |
| 50 – 59 | 19 (28.8) | 1 (4.4) | 18 (41.9) | |
| > 60 years | 10 (15.2) | 1 (4.4) | 9 (20.9) | |
| Insurance Type | 0.148 | |||
| Uninsured | 4 (6.1) | 3 (13.0) | 1 (2.3) | |
| Medicare | 18 (27.3) | 4 (17.4) | 14 (32.6) | |
| Medicaid | 16 (24.2) | 4 (17.4) | 12 (27.9) | |
| Private Insurance | 28 (42.4) | 12 (52.2) | 16 (37.2) | |
| Marital Status | 0.202 | |||
| Single | 39 (60.9) | 17 (77.3) | 22 (52.4) | |
| Married | 18 (28.1) | 4 (18.2) | 14 (33.3) | |
| Divorced/Separated/Widowed | 7 (10.9) | 1 (4.6) | 6 (14.3) | |
| Median Household Income (n=56) | 0.244 | |||
| < $40,000 | 17 (30.4) | 3 (16.7) | 14 (36.8) | |
| $40,000 – $54,000 | 14 (25) | 4 (22.2) | 10 (26.3) | |
| $55,000 – $69,999 | 16 (28.6) | 11 (47.8) | 10 (26.3) | |
| > $70,000 | 9 (16.1) | 5 (21.7) | 4 (10.5) | |
| Mean FEV1 of Predicted (%) | 38.1 | 21.5 | 47.2 | <0.001** |
| Mean DLCO | --- | --- | 32.6 (1.34) | --- |
| BMI Category (n=65) | 0.004* | |||
| < 17 | 5 (7.7) | 4 (18.2) | 1 (2.3) | |
| 17 – 29.9 | 40 (61.5) | 16 (72.7) | 24 (55.8) | |
| ≥ 30 | 20 (30.8) | 2 (9.1) | 18 (41.9) | |
| Diagnosis of Depression/Anxiety | 28 (45.9) | 16 (76.2) | 12 (30.0) | 0.001* |
| EtOH/Substance Misuse | 3 (4.8) | 2 (10.5) | 1 (2.3) | 0.220 |
| Relative Contraindications | 47 (71.2) | 14 (60.9) | 33 (76.7) | 0.175 |
| Absolute Contraindications | 13 (19.7) | 3 (13.4) | 10 (23.3) | 0.320 |
| Referred | 42 (63.6) | 15 (65.2) | 27 (62.8) | 0.845 |
| Waitlisted, Overall | 15 (22.7) | 9 (39.1) | 6 (14.0) | 0.020* |
| Waitlisted, Referred (n=42) | 15 (35.7) | 9/16 (60.0) | 6/27(22.2) | 0.036* |
p<0.05
p<0.001
FEV1=Forced Expiratory Volume, BMI=Body Mass Index, EtOH=Alcohol
Of the transplant-eligible patients, 64% (42/66) were referred for transplant evaluation and only 36% (15/42) of those referred appeared on the lung transplant waitlist (Figure 1, Table 1). Of the 23 transplant-eligible CF patients, 65% (15/23) were referred for transplant evaluation; 60% (9/15) of those referred appeared on the transplant waitlist (39% [9/23] overall). Of the 43 transplant-eligible PF patients in the cohort, 63% (27/63) were referred for transplant evaluation; 22% (6/27) of those referred appeared on the transplant waitlist (14% [6/43] overall). Four CF patients were international, non-US citizens who resided permanently outside the US and came to UCMC for the purpose of pulmonary care and transplant evaluation.
When comparing patients who were referred versus not referred for lung transplant evaluation (Table 2), there were no statistically significant differences in the sociodemographic factors of sex, mean age at eligibility, type of insurance, percent uninsured, and marital status in the CF or PF cohort. Referred CF patients were more likely to have BMI < 17 than non-referred patients (p<0.05). Referred CF and PF patients were as likely to have relative and absolute contraindications as those not referred. Referred PF patients’ most common relative contraindications were clinical comorbidities, including obesity, severe reflux, and high prednisone dose; for referred CF patients, these were depression and underweight/malnutrition (results not shown).
Table 2.
Comparison of Referred by Disease Status
| Cystic Fibrosis | Pulmonary Fibrosis | |||||
|---|---|---|---|---|---|---|
| Referred | Not Referred |
P- value |
Referred | Not Referred |
P-value | |
| n = 15 | n = 8 | n = 27 | n = 16 | |||
| Male | 9 (60.0) | 5 (62.5) | 1.00 | 6 (22.2) | 6 (37.5) | 0.313 |
| White | 10 (71.4) | 3 (37.5) | 0.187 | 7 (25.9) | 3 (18.8) | 0.290 |
| Age in years | 0.337 | 0.130 | ||||
| < 35 years | 12 (80.0) | 5 (62.5) | 0 (0.0) | 2 (12.5) | ||
| 35 – 49 | 1 (6.7) | 3 (37.5) | 10 (37.0) | 4 (25.0) | ||
| 50 – 59 | 1 (6.7) | 0 (0.0) | 13 (48.2) | 5 (31.25) | ||
| > 60 years | 1 (6.7) | 0 (0.0) | 4 (14.8) | 5 (31.25) | ||
| Insurance Type | 0.250 | 1.00 | ||||
| Uninsured | 3 (20.0) | 0 (0.0) | 1 (3.7) | 0 (0.0) | ||
| Medicare | 1 (6.67) | 3 (37.5) | 9 (33.3) | 5 (31.3) | ||
| Medicaid | 3 (20.0) | 1 (12.5) | 7 (25.9) | 5 (31.3) | ||
| Private Insurance | 8 (53.3) | 4 (50.0) | 10 (37.0) | 6 (37.5) | ||
| Marital Status | 0.757 | 0.685 | ||||
| Single | 11 (78.6) | 6 (75.0) | 10 (38.5) | 4 (25.0) | ||
| Married | 2 (14.3) | 2 (25.0) | 3 (11.5) | 3 (18.8) | ||
| Divorced/Separated/Widowed | 1 (7.1) | 0 (0.0) | 13 (50.0) | 9 (56.3) | ||
| Median Household | 0.410 | 0.724 | ||||
| Income | 1 (10.0) | 2 (25.0) | 7 (30.4) | 7 (46.7) | ||
| < $40,000 | 1 (10.0) | 3 (37.5) | 6 (26.1) | 4 (26.7) | ||
| $40,000 – $54,000 | 4 (40.0) | 2 (25.0) | 7 (30.4) | 3 (20.0) | ||
| $55,000 – $69,999 | 4 (40.0) | 1 (12.5) | 3 (13.0) | 1 (6.7) | ||
| > $70,000 | ||||||
| Mean FEV1 of Predicted | 21.2 | 22 | 0.771 | 45.4 | 50.1 | 0.150 |
| (%) | ||||||
| Mean DLCO | 30.1 | 35 | 0.136 | |||
| BMI Category | 0.042* | 0.493 | ||||
| < 17 | 4 (28.6) | 0 (0.0) | 0 (0.0) | 1 (6.3) | ||
| 17 – 29.9 | 10 (71.4) | 6 (75.0) | 16 (59.3) | 8 (50.0) | ||
| ≥ 30 | 0 (0.0) | 2 (25.0) | 11 (40.7) | 7 (43.8) | ||
| Relative | 9 (60.0) | 5 (62.5) | 0.633 | 19 (70.4) | 14 (87.5) | 0.276 |
| Contraindications | ||||||
| Absolute | 1 (6.67) | 2 (25.0) | 1.00 | 5 (18.5) | 5 (31.3) | 0.460 |
| Contraindications | ||||||
| Died | 5 (33.3) | 1 (12.5) | 0.369 | 9 (33.3) | 3 (18.8) | 0.484 |
p<0.05
p<0.001
In comparing referred patients who were waitlisted to those who were not waitlisted (Table 3), overall CF patients were more likely to be waitlisted than PF (p<0.05). There is no significant difference in the socio-demographic factors of sex, race, mean age at eligibility, type of insurance, percent uninsured, marital status in either the CF or PF cohort. Waitlisted CF patients had a higher mean FEV1 than those not waitlisted (25.1% vs. 15.3%, p<0.05). Though not statistically significant, it is notable that no PF patient with Medicaid or without insurance was waitlisted. In addition, PF patients with lower household income were less likely to be waitlisted; in fact, no PF patient with median household income less $40,000 was waitlisted. Overall, waitlisted patients with PF and CF patients were significantly less likely to have relative contraindications than those not waitlisted (p<0.05).
Table 3.
Comparison of Waitlisted of those Referred by Disease Status
| Cystic Fibrosis and Referred (n=15) |
Pulmonary Fibrosis and Referred (n=27) |
|||||
|---|---|---|---|---|---|---|
| Waitlisted (n = 9) |
Not Waitlisted (n= 6) |
P- value |
Waitlisted (n=6) |
Not Waitlisted (n = 21) |
P-value | |
| Male | 6 (66.7) | 3 (50.0) | 0.622 | 2 (33.3) | 4 (19.1) | 0.588 |
| White | 2 (22.2) | 2 (40.0) | 0.580 | 3 (50.0) | 17 (81.0) | 0.290 |
| Age in years | 1.00 | 1.00 | ||||
| < 35 years | 6 (66.7) | 6 (100.0) | 0 (0.0) | 0 (0.0) | ||
| 35 – 49 | 1 (11.1) | 0 (0.0) | 2 (33.3) | 8 (38.1) | ||
| 50 – 59 | 1 (11.1) | 0 (0.0) | 3 (50.0) | 10 (47.6) | ||
| > 60 years | 1 (111) | 0 (0.0) | 1 (16.7) | 3 (14.3) | ||
| Insurance Type | 0.874 | 0.384 | ||||
| Uninsured | 2 (22.2) | 1 (16.7) | 0 (0.0) | 1 (4.8) | ||
| Medicare | 0 (0.0) | 1 (16.7) | 3 (50.0) | 6 (28.6) | ||
| Medicaid | 2 (22.2) | 1 (16.7) | 0 (0.0) | 7 (33.3) | ||
| Private Insurance | 5 (55.6) | 3 (50.0) | 3 (50.0) | 7 (33.3) | ||
| Marital Status | 0.440 | 0.328 | ||||
| Single | 7 (77.8) | 4 (80.0) | 2 (33.3) | 11 (55.0) | ||
| Married | 2 (22.2) | 0 (0.0) | 4 (66.7) | 6 (30.0) | ||
| Divorced/Separated/Widowed | 0 (0.0) | 1 (20.0) | 0 (0.0) | 3 (15.0) | ||
| Median Household | 0.133 | 0.097 | ||||
| Income | 1 (14.3) | 0 (0.0) | 0 (0.0) | 7 (38.9) | ||
| < $40,000 | 1 (14.3) | 0 (0.0) | 1 (20.0) | 5 (27.8) | ||
| $40,000 – $54,000 | 1 (14.3) | 3 (100.0) | 2 (40.0) | 5 (27.8) | ||
| $55,000 – $69,999 | 4 (57.1) | 0 (0.0) | 2 (40.0) | 1 (5.6) | ||
| > $70,000 | ||||||
| Mean FEV1 of Predicted (%) | 25.1 | 15.3 | 0.018* | 46.8 | 45.0 | 0.951 |
| Mean DLCO | -- | -- | -- | 30.8 | 31.2 | 0.939 |
| BMI Category | 0.245 | 0.350 | ||||
| < 17 | 1 (12.5) | 3 (50.0) | 0 (0.0) | 0 (0.0) | ||
| 17 – 29.9 | 7 (87.5) | 3 (50.0) | 5 (83.3) | 11 (52.4) | ||
| ≥ 30 | 0 (0.0) | 0 (0.0) | 1 (16.7) | 10 (47.6) | ||
| Relative | 3 (33.3) | 6 (100.0) | 0.028* | 2 (33.3) | 17 (81.0) | 0.044* |
| Contraindications | ||||||
| Absolute | 1 (111) | 0 (0.002) | 1.00 | 0 (0.0) | 5 (23.8) | 0.555 |
| Contraindications | ||||||
| Died | 2 (22.2) | 3 (50.0) | 0.329 | 2 (33.3) | 7 (33.3) | 1.00 |
p<0.05
p<0.001
Comparing time to referral and waitlist appearance for CF and PF patients (Table 4), there was no significant difference in the median days from eligibility to referral (210 vs. 503, p=0.62) or eligibility to work-up (483 vs. 1002, p=0.71) between the two cohorts. There was a significant difference in time from eligibility to waitlist between CF and PF patients; PF patients have a significantly longer time between eligibility and waitlist (longer “survival” without waitlist, log-rank p=0.03; 25th percentile 329 vs. 2368 days). There was no significant difference in time from referral to work-up or referral to waitlist between the two groups (p=0.92 and 0.28, respectively).
Table 4.
Median Time to Event and Log-rank test for equality of survivor function
| Cystic Fibrosis | Pulmonary Fibrosis | Log Rank Test for Equality |
|||||
|---|---|---|---|---|---|---|---|
| 25th % | Median | 75th % | 25th % | Median | 75th % | P-value | |
| Eligibility to Referrals (days) | 22 | 210 | 2463 | 148 | 503 | 2356 | 0.622 |
| Eligibility to Work-up (days) | 198 | 483 | N/A | 325 | 1002 | N/A | 0.71 |
| Eligibility to Waitlist (days) | 329 | 2682 | N/A | 2368 | N/A | N/A | 0.029* |
| Referral to Workup (days) | 1 | 34 | 157 | 24 | 45 | 260 | 0.923 |
| Referral to Waitlist (days) | 85 | 212 | 315 | 93 | 326 | N/A | 0.280 |
p<0.05
p<0.001
N/A: fewer patients with event than percentile
Discussion
Our results demonstrated no significant differences in likelihood or in time to referral or work-up between CF and PF patients. Both groups had reasonable referral rates (>60%); however, CF patients had higher waitlist rates and a shorter time from eligibility to waitlist. Nevertheless, both groups experienced long delays from eligibility to referral and to waitlist appearance.
The first delay potential candidates encounter is between initial diagnosis of end-stage lung disease and subsequent referral for transplant evaluation by a point-of-care physician. Individuals averaged 7–17 months from eligibility to referral. Patients with end-stage lung disease see many inpatient and outpatient providers and likely miss many opportunities for referral along the care continuum.12 We were unable to determine why potentially eligible patients are not referred. For PF patients, part of the delay is in diagnosing and staging PF. There may be lack of knowledge about clinical criteria for PF and CF transplant-eligibility among primary care providers, pulmonologists, and inpatient clinicians. Recent liberalization in referral criteria may have been ineffectively communicated to providers involved at various stages of patients’ disease progression.13 In addition, providers may pre-screen patients based on presumed contraindications and prognosis, and pre-screening may not be appropriately or uniformly applied. One potential solution might be a monthly case conference with general pulmonologists and transplant pulmonologists to review patients with severe or rapidly progressing lung disease. Additionally, PFT reports could include the PFT transplant-eligibility criteria for the top three transplant-qualifying conditions.
While time from clinical eligibility constitutes a large portion of the delay, time from referral to waitlist was greater than expected for both CF and PF patients (median 212 and 326 days, respectively). Our center goal from referral to waitlist is 180 days. The clinical differences between the CF and PF cohorts can help inform the specific areas of intervention and treatment for these patients throughout the referral to waitlist process.
Cystic fibrosis is a multi-system disease typically manifesting in childhood with pancreatic insufficiency and malnutrition in addition to pulmonary symptoms. All CF patients had established pulmonology care years prior to transplant eligibility. CF patients also had a lower BMI compared to their PF counterparts, likely due both to their younger age and CF-associated pancreatic insufficiency. For CF patients, being underweight was a common relative contraindication. The greater proportion of depression/anxiety and alcohol/substance misuse among our patients with CF is also worth noting and is consistent with rates published in the literature.14,15 Mental health issues may decrease adherence and increase risky behavior which are both contraindications for transplant. During work-up, patients with CF may benefit from specialized attention regarding nutrition and mental health.
Pulmonary fibrosis represents the final common pathway for a wide variety of disease processes. Patients with PF experience delays in diagnosis, referral, and completion of work-up. Patients with PF may face delays between symptom onset and diagnosis, which may contribute to delays in appropriate management and referral.16 While all PF patients were seen at UCMC as outpatients, only 74% had seen a pulmonologist. In addition, referred PF patients were more likely to have significant clinical co-morbidities such as scleroderma or obesity. The presence of these relative contraindications and associated risks may also help explain the large time gap between transplant-eligibility and waitlist appearance and the lower probability of ever appearing on the waitlist, as these conditions may overshadow their lung disease. Patients with PF related to rheumatologic disease may require an integrated systems approach to address multiple disease manifestations. Access to nutritionists, social workers, and physical therapists, in addition to medical subspecialists, may help to reduce morbidity among PF patients. These patients should receive early counseling on physical activity and weight control to reduce the incidence of obesity.17–19 Later as the candidates’ disease progresses, an integrated approach to risk reduction requires pulmonary rehabilitation and continued optimization of BMI to maximize clinical eligibility for lung transplant.
Prior work suggests that low socioeconomic status can delay or bar access to lung transplantation.20 Notably, we found that no PF patients with Medicaid or income less than $40,000 were placed on the transplant waitlist. This may be due to insurance-related problems with work-up or perhaps patients with Medicaid or low income were more likely to have other medical or psychosocial contraindications. Further work is needed to understand and reduce these disparities to ensure equitable access to lung transplantation.
In our study, uninsured patients with CF did not experience barriers to transplant compared to those with private insurance because this group included international patients, largely from the Middle East, who pay out-of-pocket for medical expenses. The growing number of international patients with CF being referred to US transplant centers for lung transplant work-up has not been well addressed in the literature as it is a recent development in an already small patient population.21 The growth of international registrants may be due, in part, to the 2012 modification of the UNOS “threshold” policy which allowed UNOS to audit centers if a specific percent of transplant recipients were non-US citizens/non-US residents. Since 2012, an ad-hoc committee reviews transplant centers’ citizenship data and can request additional information about registrations or transplant of non-US citizens/non-US residents.22
There are several limitations to our study. First, the study has limited generalizability as it relies on chart review of hospitalized patients at a single institution. The lung transplant work-up process varies significantly by transplant center. This retrospective study formed cohorts according to eligibility criteria from the literature and the internal practices of the UCMC Lung Transplant Program.4,13 Different eligibility criteria could yield different cohorts.8 Additionally, our cohorts do not include all lung transplant- eligible patients. CF patients and PF patients constitute about 43% of the total lung transplant population.23 COPD patients were excluded and may face different barriers to transplant waitlist due to their older age and co-morbidities. Second, the study has limited power due to its small sample size. Some differences between waitlisted and non-waitlisted groups, and the CF and PF groups appear substantively different, but are not statistically significant. Finally, the study depended on patients’ medical records for all extracted information. Relevant clinical and sociodemographic information may have been missing or incorrectly charted. Despite these limitations, this work addresses important issues related to access to lung transplant evaluation.
Equity of access to lung transplantation among different groups of transplant eligible patients cannot be assumed, despite the reforms of the LAS. Important barriers still remain in referral and evaluation for lung transplant. Prospective studies of patients before waitlist appearance could identify opportunities for quality improvement that lead to more timely referral and evaluation completion. Important areas for improvement gleaned from our work include increasing referral to and collaboration with pulmonologists for patients with rheumatologic-associated lung disorders; increased awareness of referral guidelines for all providers who care for CF and PF patients; emphasizing nutritionist intervention early in disease course to optimize BMI; and early mental health and social work involvement to solidify social support, encourage adherence, and treat mental health issues.
Addressing obstacles faced by potential candidates in accessing the lung transplant waitlist requires focused efforts by multi-disciplinary teams. Progression from initial referral to eventual listing should be monitored prospectively by lung transplant programs. Increasing the fairness and efficiency of the lung transplant referral and evaluation of potential candidates has the same goals as the LAS for listed candidates —to decrease patient mortality and increase patient benefit.
Supplementary Material
Acknowledgements
The authors do not have any conflicts of interest to disclose. Ms. Liu was supported by the University of Chicago Pritzker Summer Research Program (NHLBI R25HL096383). Dr. Saunders was supported by Pilot and Feasibility Funding from the Chicago Center for Diabetes Translation Research (NIDDK P30 DK092949) and by a Career Development Award from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK K23 DK103111 ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eberlein M, Garrity ER, Orens JB. Lung allocation in the United States. Clin Chest Med 2011;32(2):213–222. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Shearon TH, Qian Y, et al. Lung transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):1047–1068. [DOI] [PubMed] [Google Scholar]
- 3.Glanville AR, Estenne M. Indications, patient selection and timing of referral for lung transplantation. Eur Respir J. 2003;22(5):845–852. [DOI] [PubMed] [Google Scholar]
- 4.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update-- a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745–755. [DOI] [PubMed] [Google Scholar]
- 5.Monson RS, Kemerley P, Walczak D, Benedetti E, Oberholzer J, Danielson KK. Disparities in completion rates of the medical prerenal transplant evaluation by race or ethnicity and gender. Transplan. 2015;99(1):236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark CR, Hicks LS, Keogh JH, Epstein AM, Ayanian JZ. Promoting access to renal transplantation: the role of social support networks in completing pre-transplant evaluations. J Gen Intern Med. 2008;23(8):1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meltzer D, Manning WG, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874. [DOI] [PubMed] [Google Scholar]
- 8.Lynch JP 3rd, Saggar R, Weigt SS, Ross DJ, Belperio JA. Overview of lung transplantation and criteria for selection of candidates. Semin Respir Crit Care Med. 2006;27(5):441–469. [DOI] [PubMed] [Google Scholar]
- 9.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011. [DOI] [PubMed] [Google Scholar]
- 10.Egan TM, Trulock EP, Boychuk J, Ochoa L, Cooper JD. Analysis of referrals for lung transplantation. The Washington University Lung Transplantation Group. Chest. 1991;99(4):867–870. [DOI] [PubMed] [Google Scholar]
- 11.American FactFinder. http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed August 22, 2014, United States Census Bureau. [Google Scholar]
- 12.Bodenheimer T Coordinating care-a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064. [DOI] [PubMed] [Google Scholar]
- 13.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. [DOI] [PubMed] [Google Scholar]
- 14.Mc Ewan FA, Hodson ME, Simmonds NJ. The prevalence of “risky behavior” in adults with cystic fibrosis. J Cyst Fibros. 2012;11(1):56–58. [DOI] [PubMed] [Google Scholar]
- 15.Cruz I, Marciel KK, Quittner AL, Schechter MS. Anxiety and depression in cystic fibrosis. Semin Respir Crit Care Med. 2009; 30(5): 569–577. [DOI] [PubMed] [Google Scholar]
- 16.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed Access and Survival in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2011;184(7):842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oeser A, Chung CP, Asanuma Y, Avalos I, Stein CM. Obesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosus. Arthritis Rheum. 2005;52(11):3651–3659. [DOI] [PubMed] [Google Scholar]
- 18.Katz P, Gregorich S, Yazdany J, et al. Obesity and its measurement in a community-based sample of women with systemic lupus erythematosus. Arthritis Care Res. 2011;63(2):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gvozdenovic BS, Mihailovic-Vucinic V, Vukovic M, et al. Effect of obesity on patient-reported outcomes in sarcoidosis. Int J Tuberc Lung Dis. 2013;17(4):559–564. [DOI] [PubMed] [Google Scholar]
- 20.Quon BS, Psoter K, Mayer-Hamblett N, Aitken ML, Li CI, Goss CH. Disparities in access to lung transplantation for patients with cystic fibrosis by socioeconomic status. Am J Respir Crit Care Med. 2012;186(10):1008–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glazier A, Danovitch G, Delmonico F. Organ Transplantation for Nonresidents of the United States: A Policy for Transparency. Am J Transplant. 2014;14(8):1740–1743. [DOI] [PubMed] [Google Scholar]
- 22.Organ Procurement and Transplantation Network (OPTN) Policies: Policy 17: International Organ Transplantation. [Webpage]. 2016; https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed 4/29/2016, 2016.
- 23.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult lung and heart-lung transplant report—2011. J Heart Lung Transplant. 2011;30(10):1104–1122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


