Abstract
Cell-blocks are paraffin-embedded versions of cytology specimens comparable to the formalin-fixed paraffin-embedded (FFPE) tissue from surgical pathology specimens. They allow various elective ancillary studies on a variety of specimens with enhanced cytopathologic interpretation, including opportunity to perform molecular tests. However, different dictionaries and internet search engines primarily project “cellblock” and “cell block” definition in relation to prisons. Most of the top searches lead to information related to “prison cells” followed by a few cytopathology-related searches. Due to this in the current review, it is recommended that the word for cytopathology purposes should be hyphenated and spelled as “cell-block.” Cell-blocks have been increasingly indicated on most cytology specimens. Its role is growing further with the ongoing addition of new immunohistochemistry (IHC) markers with technical advances including multicolor IHC and the SCIP (subtractive coordinate immunoreactivity pattern) approach. In addition, it is an important source of tissue for many ancillary studies even as archived material retrospectively at later stage of management if the cell-blocks are improved qualitatively and quantitatively. Because of this, the significance of cell-block is critical with the increasing number of molecular markers standardized predominantly on FFPE tissue. As compared to core biopsies, high-quality cell-blocks prepared with enhanced methodologies predominantly contain concentrated diagnostic tumor cells required for the molecular tests without significant stromal contamination. This review introduces the terminology of CellBlockistry as the science of studying chemistry and the art of achieving quantitatively and qualitatively improved cell-blocks from different types of specimens. The review addresses the cell-block making process as “cell-blocking” and discusses different historical limitations with emphasis on recent advances.
Keywords: Biopsy, cell block, cell-blocking, CellBlockistry, chromogenic in situ hybridization, cytocrit, cytology, formalin-fixed paraffin-embedded, FFPE, fixation, fine needle aspiration, immunohistochemistry, molecular pathology, subtractive coordinate immunoreactivity pattern, SCIP, tissuecrit
INTRODUCTION
The cell-blocks contain paraffin-embedded components of the specimens and allow variety of elective ancillary studies for enhanced cytopathologic interpretation. They are also an easily available tissue source for the molecular test which is increasingly becoming a part of cancer management. However, various dictionaries define “cellblock” and “cell block” as expression related to prisons. These definitions may be summed as “a unit of a prison consisting of a number of cells.”[1,2,3] If an internet search is performed with words spelled as “cellblock” or as “cell block,” most of the top searches may be related to the “prison cells” followed by a few cytopathology-related searches. For cytopathology purposes, the current review recommends to hyphenate the term and spell it as “cell-block.”
In this review, the terminology of CellBlockistry is introduced as a science of exploring the chemistry and an art for achieving the best procedural outcome after processing the micro-components present in different types of specimens into the formalin-fixed paraffin-embedded (FFPE) blocks. This science considers different issues related to the preservation of morphological and structural integrity of the components in the cell-blocks without compromising the qualitative integrity related to the various elective ancillary tests such as immunohistochemistry (IHC) and the molecular tests. In general, for appropriate comparison of results with published data, the processing should be similar to that applied for different biopsies and resections. In this review, the process of preparing the cell-block is termed “cell-blocking.”
The cell-blocks have been routinely performed on variety of specimens.[4,5,6] However, with the rapidly increasing role of molecular pathology and other ancillary tests such as multicolor IHC with the subtractive coordinate immunoreactivity pattern (SCIP) approach, the cell-blocks have been indicated more often on most of the cytology specimens. As compared to the core biopsies, the cell-blocks predominantly contain diagnostic tumor cells without significant stromal contamination. For this reason, cell-blocks should be the preferred source of tissue due to the increasing number of molecular markers standardized predominantly on FFPE tissue.[7,8,9,10,11]
Although not primary indication, the cell-block sections also allow for the benefit of improved sampling of the processed cytology specimens with an opportunity to evaluate certain architectural features such as papillary, acinar, duct-like formations, and psammoma bodies as histomorphological input.[12,13,14,15,16] The cell-blocks in this respect are particularly important while evaluating peritoneal/serous cavity washings to compare histomorphological features in the cell-block sections with the histomorphological features in the resection of the associated primary neoplasm.[17]
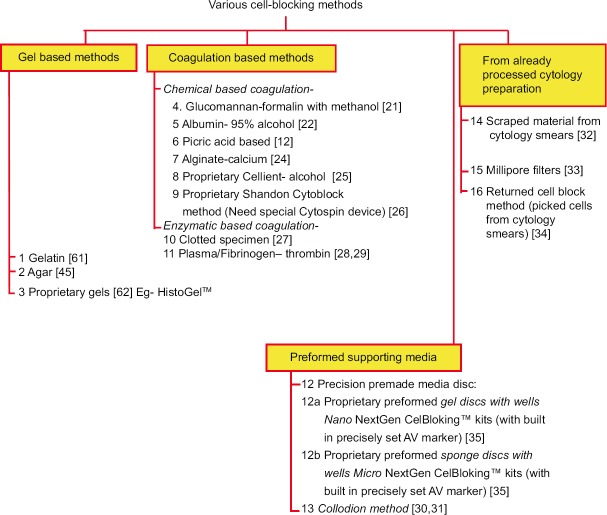
Although the cell-blocks are critical, the primary goal during the processing of cytology specimens is to apply the best techniques for preparing direct cytology smears and relevant other cytology preparations to allow for optimal cytomorphological evaluation of diagnostic components in the cytology specimens as per the institutional/local preference.[18] The residual specimen, including the clotted component, is recommended to be processed for cell-blocking. Historically, there have been many approaches applied for cell-block preparation, and some of these are summarized in Figures 1, 2, and Table 1.
Figure 1.
Different types of approaches for cell-blocking
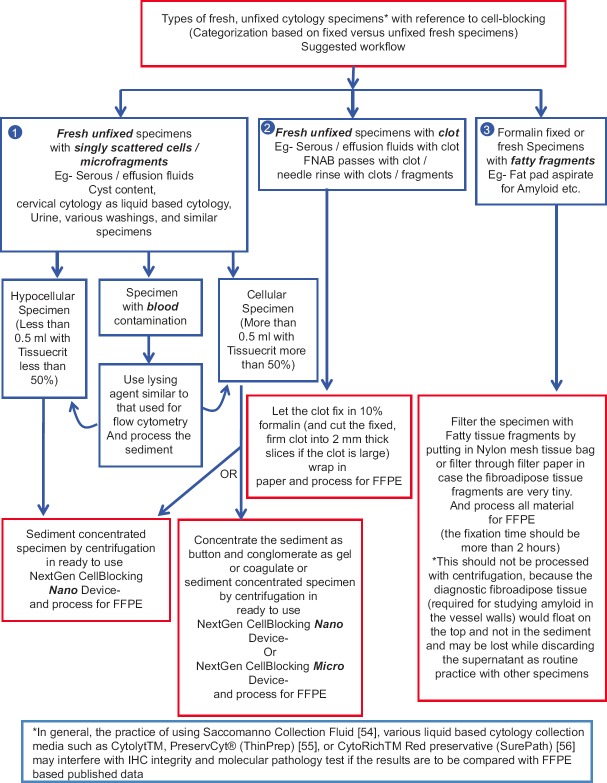
Figure 2.
The fresh, unfixed specimens may be divided into various categories for workflow
Table 1.
CellBlockistry- summary [Figure 1]
| Cell-blocking method | Limitations | Advantages | Interference with IHC and molecular Studies | Remarks (category) | |
|---|---|---|---|---|---|
| 1-2 | Gel (heat)-based methods 1. Gelatin 2 Agar[12,13,19] |
Require heating and melting of the gel with long cooling time for the medium to gel. In addition, the button formed is usually flimsy due to which centrifugation approach to align the diagnostic material precisely along the cutting surface cannot be achieved reproducibly. Cannot control the depth of cutting without including some mechanism such as AV marker. These issues lead to conventional suboptimal outcome. Overheating may compromise morphological and other integrity potentially affecting results of some IHC and other molecular tests | Low cost, can be applied to fixed or fresh specimen. Can be applied to generate FFPE which is recommended for various types of ancillary tests | No (if processed as FFPE with fixation/processing similar to the processing of surgical pathology specimens) | Less practical (random, indiscriminatory) |
| 3 | Proprietary (heat-based) gel, for example, HistoGel™ (with or without Shidham’s method)[19,20] | Higher cost. Require heating and melting of the gel with relatively shorter cooling time for the medium to gel, due to which centrifugation approach to align the diagnostic material precisely along the cutting surface may be compromised. | Firm button which allows precise alignment of the diagnostic material along the cutting surface, if done properly with Shidham’s method under hot conditions. | No (if processed as FFPE with fixation/processing similar to the processing of surgical pathology specimens) | Good method, but suboptimal nonreproducible outcome frequent |
| The reproducibility is affected by skill and other variables related to laboratory infrastructure. Cannot control the depth of cutting without including some mechanism such as AV marker Overheating may compromise morphological and other integrity potentially affecting results of some IHC and other molecular tests |
Can be applied to the fixed or fresh specimen. Can be applied to generate FFPE which is recommended for various types of ancillary tests | (random, indiscriminatory could be enhanced category if with Shidham’s method) | |||
| 4-7 | Methods based on chemically-induced coagulation 4. Glucomannan-formalin with methanol[21] 5. Albumin- 95% alcohol[22] 6. Picric acid based[12] 7. Alginate-calcium[24] |
Require special reagents and protocol to be standardized, but the button formed is usually flimsy due to which centrifugation approach to align the diagnostic material precisely along the cutting surface cannot be achieved reproducibly. Many chemicals such as picric acid and acetic acid will interfere with results of IHC and other molecular tests | No significant advantages | Possible | Less practical (random, indiscriminatory) |
| 8 | Proprietary Cellient-alcohol[25] | Require significant capital investment including dependence on proprietary special reagents. Protocol related to exposure to non-formalin tissue processing (protocol uses alcohol fixation) which may interfere with results of IHC and other molecular tests. In addition, each specimen has to be processed sequentially (with processing time 45 min for each) with practical limitations related to the high turnover laboratory. Cannot control the depth of cutting due to the inability to incorporate mechanism such as AV marker | Good morphology (However, IHC results may not be comparable to published data mostly based on FFPE) | Possible | Good method, but the suboptimal nonreproducible outcome (especially hypocellular specimens) with potential liabilities related to ancillary tests such as IHC (enhanced) |
| 9 | Proprietary Shandon Cytoblock Method (need special Cytospin device)[26] |
Require significant capital investment, including dependence on proprietary special reagents. Steps related to the instruments require the maintenance of reusable such as clips and funnels with related liability including potential for contamination The depth of initial diagnostic cells cannot be monitored by histotechnologist Contamination potential due to need to re-use some components repeatedly with extra labor to clean the re-usable |
No significant advantages | Good method, but the suboptimal nonreproducible outcome (especially hypocellular specimens) (enhanced) | |
| 10-11 | Coagulation (enzymatic) based - 10 De novo clotted specimen[27] | Need dedicated pass with a significant proportion of FNA usually with wider gauge needle.[27] The aspirate is allowed to clot with aspirated blood In effusion fluids, all specimens may not have clot. In addition, if the specimen is allowed to clot, the diagnostic cells in effusion fluid may be trapped in clot and so may not be available for cytopathologic interpretation |
Simple method | No (if processed as FFPE with fixation/processing similar to the processing of surgical pathology specimens) | Good method, but suboptimal nonreproducible outcome frequent (random, indiscriminatory) |
| 11 Plasma/fibrinogen - thrombin[28,29] | The button formed is usually flimsy due to which centrifugation approach to align the diagnostic material precisely along the cutting surface cannot be achieved reproducibly. Cannot control the depth of cutting without including some mechanism such as AV marker Cannot use specimens collected in fixative (which inhibit the thrombin enzyme) Stability of reagents and cost of thrombin may be limiting factor |
Simple method for specimens with high cytocrit/tissuecrit [Figure 3] | No (potential of nonspecific background with some immunostains) | Good method, but suboptimal nonreproducible outcome frequent. (random, indiscriminatory) | |
| 12 | Celloidin method[30,31] | Require special reagents and preparation of Collodion bags in advance with problem and risks associated with handling highly volatile and inflammable reagents. Require to standardize the protocol for each laboratory setup with proper practice and skill development. The diagnostic material cannot be aligned precisely along the cutting surface, leading to suboptimal outcome. B-5 fixative treatment mentioned in the protocol will interfere with the results of IHC and other molecular tests |
No significant advantages | Possible (especially if B5 fixative treatment step is applied) | Not recommended (enhanced) |
| 13-15 | From already processed cytology preparations 13 Scraped material from cytology smears[32] 14 Millipore filters[33] 15 Returned cell-block method (picked cells from cytology smears)[34] |
The protocol is complex and more demanding. Exposure to numerous reagents and fixatives will interfere with the results of IHC and other molecular tests | No significant advantages, except retrieval of thick tissue fragments in smears, may still be processed with potential to study histomorphological features | Yes | Not recommended (enhanced) |
| 16 | Proprietary preformed gel disc with wells: Nano NextGen cell-blocking™ kits (based on Shidham’s method with built-in precisely set AV marker)[35,36,37] | The only limitation is the availability of kit | The self-sufficient kits allow reproducibility like automation Do not need any capital investment for any special instrument other than those used routinely in usual cytoprep laboratories Relatively simple quick method with inbuilt AV marker, which extends benefits of objective control over depth of cutting by histotechs and application of SCIP approach Any type of specimen (fixed or unfixed) can be used with the application of any fixation protocol including widely used formalin fixation for FFPE |
No | Good method with many benefits [for any specimen including hypocellular specimens with lower Tissuecrit Figure 3] (enhanced) |
| 17 | Proprietary preformed sponge disc with wells: Micro NextGen Cell-blocking™ kits (with built-in precisely set AV marker)[35,38,39] | Same as #16 | No | Only for Cellular specimens with more than 1 ml concentrate with >50% Cytocrit/Tissuecrit [Figure 3] (enhanced) | |
FFPE: Formalin-fixed paraffin-embedded, IHC: Immunohistochemistry, FNA: Fine-needle aspiration, B5: A fixative, SCIP: Subtractive coordinate immunoreactivity pattern
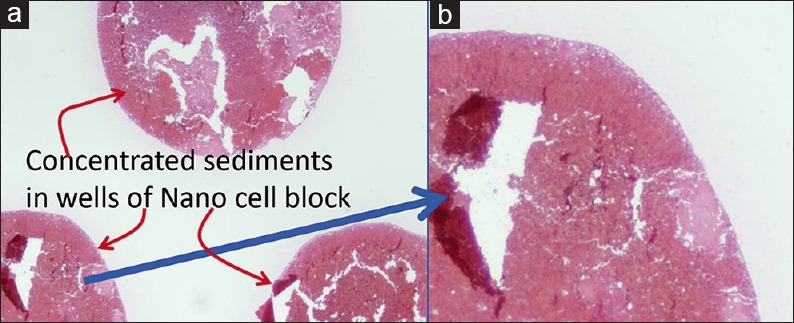
Principally, the suspended material in the cytology specimen is sedimented [Figure 3] and added with the medium or to the supporting medium that holds all the components in the sediment so that it can be handled for processing and embedding as FFPE. Depending on the methodology, there may be multiple challenges including procedure-related issues such as indiscriminate approach without proper control over the distribution of diagnostic cells in the cell-block [Figure 4] with lack of reproducibility to qualitative interference due to exposure to various fixatives/reagents [Table 2]. Because of these issues, the common random, indiscriminate methods of cell-block processing have been frequently compromising the quantitative and qualitative integrity of various components of the cell-block.[9,40,41,42,43,44]
Figure 3.
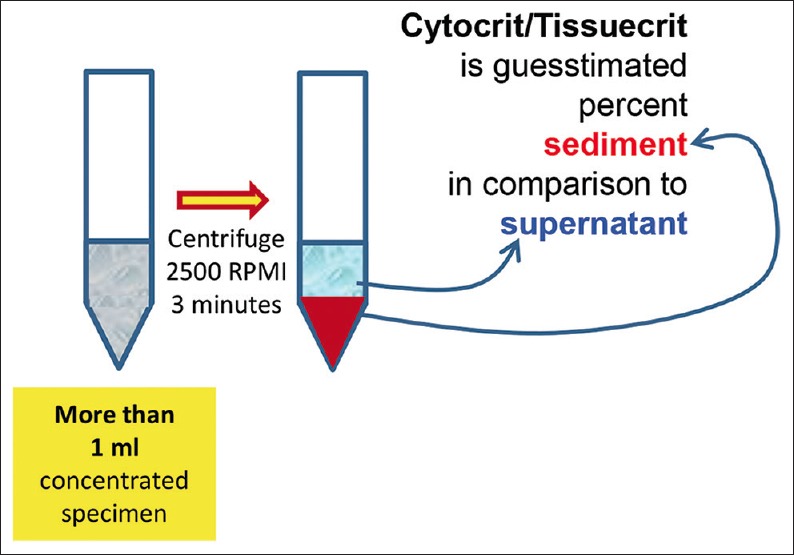
Cytocrit/Tissuecrit defined (to categorize the cytology specimens for selection of Cell-blocking protocols). For choosing Cell-blocking protocols for optimum yield, the cytology specimens may be broadly divided into two categories: (i) Hypocellular-Specimens generating <1 ml final concentrated sediment with <50% Tissuercrit. (ii) Cellular-Specimens generating >1 ml final concentrated sediment with >50% Tissuercrit
Figure 4.
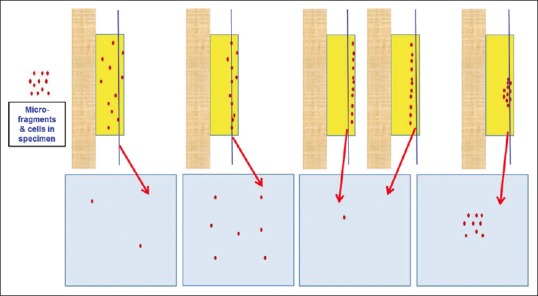
Conventional cell-blocking: Randomness of the depth of cutting, leading to suboptimum cellularity of final tissue sections
Table 2.
Issues related to fixatives in relation to cell-blocking
| Fixatives | Histology | Immunocytochemistry | Molecular testing | |
|---|---|---|---|---|
| 1 | Formalin[40] | Sections of resultant FFPE would show histomorphology comparable to that with formalin-fixed biopsies and resections | IHC results would be comparable to that with published data predominantly based on FFPE studies | The limiting factor with FFPE is fragmentation of DNA with associated artifacts during sequencing with potential interference RNA-based test (other than miRNA) may be affected due to low yield. However, most of the methodologies are standardized on FFPE |
| 2 | Chemical-based fixatives including the fixatives with heavy metal (B5, Zenker’s fixative) or Acidic solutions (Picric acid, Bouin’s fixative)[41,42] |
Histomorphology is not affected significantly and is comparable to that with formalin-fixed biopsies and resections Toxicity hazard (e.g., mercury poisoning with Zenker’s fluid) |
Morphologically good immunostaining, but results may NOT be comparable to that with FFPE with which the results will be compared. This may lead to aberrant immunoprofile with liability due to potential compromisation of patient care | Little data related to stability of nucleic acids (Some such as picric acid results in DNA damage) |
| 3 | Alcohol Methanol in PreservCyt and CytoLyt used in LBC Ethanol in SurePath LBC Cellient™ CB[43,44] |
Histomorphology is not affected significantly and is comparable that with formalin-fixed biopsies and resections Shrinkage-related artifacts may interfere |
Immunoreactivity may be affected with erroneous immunoprofiles resulting in suboptimal interpretation outcome. This is especially applicable to nuclear immunomarkers including ER/PR, Ki-67, PCNA, p53, S-100 protein, etc.,[9] | Standardized tests/protocols may be required |
B5: A fixative; IHC: Immunohistochemistry; LBC: liquid-based cytology; PCNA: Proliferating cell nuclear antigen, ER: Estrogen receptors, PR: Progesterone receptor
General review of Cell-Blocking approaches
For the purpose of general understanding, the different cell-blocking methods may be categorized as follows [Figure 1]:
-
Gel-based methods
Based on the principle that heated molten gel is allowed to solidify when cold:
-
Coagulation-based methods
These methods are based on the principle that the concentrated suspension of sediments is coagulated-
-
Chemical mechanism
-
Enzymatic coagulation (Enzyme-based methods may not work with specimens collected in fixatives such as formalin due to the interference with enzymatic activity of the enzyme)
-
-
Preformed supporting media
-
Preformed media with enhancement technique-
-
The proprietary NextGen CelBloking™ kits based on Shidham's method, preformed media discs with wells and with inbuilt AV marker to control the depth of cutting and help SCIP approach [Figure 5]:[20,35]
- Premade gel (Nano): any cytology specimen including hypocellular specimens
- Premade foam (Micro): For cellular specimens only.
-
-
Other proprietary methods:
- Cellient-alcohol[25]
-
Other methods:
-
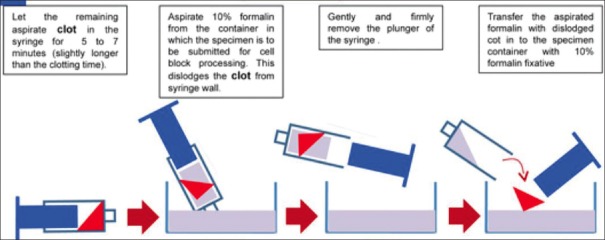
A clot method-Diagnostic cells/tissue micro-fragments may be present in the needle rinses of fine-needle aspiration (FNA) biopsy specimens including those in the dedicated passes with a wider gauge needle to aspirate many tissue fragments along with blood in the syringe.[27]
- Other cytology specimens such as effusion fluids:[12] In such cases, the clots are processed directly for cell-block preparation.
-
-
Cell-blocking of material already processed as cytology preparations
-
Combination methods: Some methods may use a combination of various methodologies such as:
Figure 5.
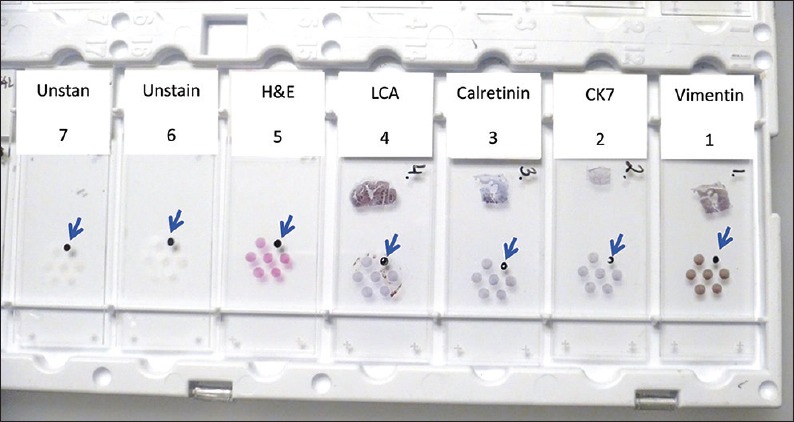
Cell-blocking and AV marker for SCIP (Subtractive Coordinate Immunoreactivity Pattern) approach.[23] Reproduced from: Shidham and Atkinson, ‘Cytopathologic Diagnosis of Serous Fluids’ Chapter #14 (Appendix 1), Elsevier (W. B. Saunders Company) First edition, 2007 (ISBN-13: 9781416001454[12]
Enhanced cell-blocks versus indiscriminate random cell-blocks [Table 3]
Table 3.
Conventional random methods versus enhanced methodologies with or without mechanism to monitor the depth at which diagnostic cells are aligned
| Cell-blocking methods | ||
|---|---|---|
| Indiscriminatory, random methods |
Enhanced methods Objective monitoring of depth of cutting mechanism |
|
| Not possible | Possible | |
| Gel-based methods | Shandon Cytoblock™ method[26] | Shidham method using gel such as Histogel[20] |
| Gelatin,[28] agar, proprietary gels such as HistoGel™[19,20] (involve heating and melting) | Cellient™ proprietary | Precision pre-made media |
| Coagulation-based methods | automated cell-block system which archives concentration, processing, and embedding of sediments in cytology specimen for making a paraffin block | Gel medium - For example, the proprietary Nano NextGen CelBloking™ kits (Device based on Shidham’s method with inbuilt AV marker[20] for any cytology specimen including hypocellular specimens)[35] |
| Egg albumin-Alcohol[22] alginate-calcium[24] picrate-based enzymatic coagulation (plasma/fibrinogen-thrombin)[25,27] | ||
| Preformed supporting media | Foam medium - For example, the proprietary[47,48] Micro NextGen CelBloking Kit™ (device based on Shidham’s method with inbuilt AV marker[20] for cellular cytology specimen only)[35] | |
| Celloidin method[30,31] Millipore filters[33] | ||
| Other methods | ||
| Clot (from FNAB specimens and in effusion fluids),[12] Scraped material from cytology smears,[32,49]Returned cell-block method (picked cells from cytology smears)[34,50] | ||
FNAB: Fine-needle aspiration biopsy
The categorization may also be approached based on the application of special efforts for quantitative and qualitative enhancements in cell-blocking.
Methodologies may be divided into [Table 3]:
Indiscriminate random cell-blocks (conventional, random, and indiscriminatory methods)
-
Enhanced cell-blocks:
With or without mechanism to monitor the depth at which the diagnostic material starts appearing in the sections while cutting the paraffin block by histotechnologist.
This approach is important for encouraging its implementation for the maximum diagnostic outcome from cytology specimens, which are usually obtained with low-cost, minimally invasive procedures. In addition, such separation endorses the extra efforts in preparation of enhanced cell-blocks so that in future, a dedicated new Current Procedural Terminology code (CPT code) with higher relative value units[52] is assigned for this category. This is strongly encouraged so that the technical component of CPT coding would award higher reimbursement than routinely processed cell-blocks and surgical biopsies due to the extra efforts and expenses in achieving this quantitative and qualitative excellence for better patient care through cytology specimens.
Which cell-blocking method to select?
The selection of the method would depend on the variety of local and institutional preferences. One should consider the nature of the specimen and status of specimen, such as fresh specimen versus fixed specimen. The specimens may be divided into various categories based on different factors related to cellularity and distribution pattern such as singly scattered cells or tissue micro-fragments [Figure 2]. Hypocellular specimens with singly scattered cells with blood contamination would require special approaches [Figures 6–8].
Figure 6.
(a) Low magnification view of the sections of cell-block produced by Nano NextGen CelBloking™ kit of blood contaminated cytology specimens. (b) Theses sections have tendency to float and fold, especially during immunostaining and other procedures requiring handling and processing through multiple reagents with problems related to floater contamination
Figure 8.
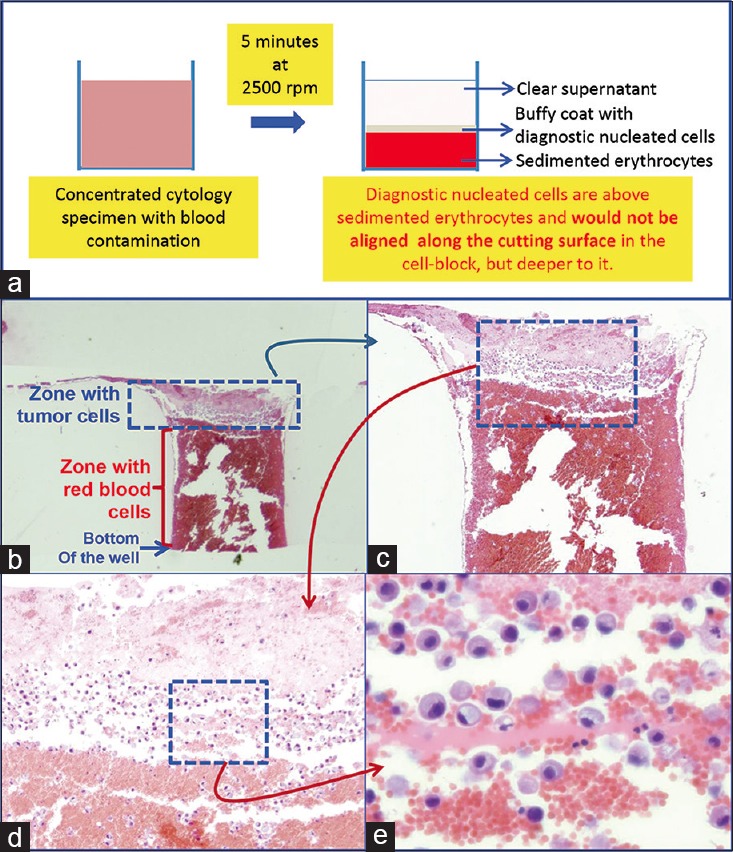
Blood contaminated cytology specimen (H and E). (a) Schematic showing result of centrifuging the blood-rich concentrated specimen with diagnostic cells which group with nucleated cells in the buffy coat area above red blood cells. (b) The longitudinal sections of one of the wells in the cell-block made with Nano NextGen CelBloking™ kit. (c) The bottom of the wells is predominantly red blood cells with tumor cells on the top which will be way deep to the actual cutting surface of usual cell blocks (H and E). (d and e) Higher magnification showing the diagnostic tumor cells in the area corresponding with the buffy coat (H and E)
Figure 7.
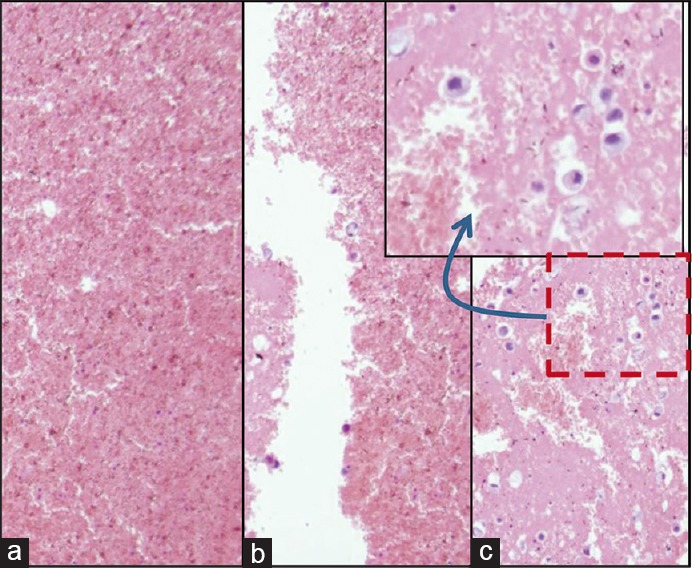
Blood interference in cell-block produced by Nano NextGen CelBloking™ kit from blood contaminated cytology specimens (same is applicable to any other method for blood rich specimens): (a and b) Section from the bottom portion of the wells with specimen sediments rich in red blood cells. The diagnostic tumor cells are missing in this zone, (c) the deeper levels showed more tumor cells, but this level cannot be predicted, and so the possibility of catching the diagnostic cells depends on chance factor with frequent sampling artifact
The cell-blocks prepared by centrifugation methods directly from blood contaminated specimens will not align the diagnostic nucleated cells along the bottoms, which would be cutting surface in final FFPE of such cell-blocks. The nucleated cells are concentrated above red blood cells like the buffy coat. Due to this, it is unpredictable to get the diagnostic cells in the paraffin sections. In addition, the paraffin sections of the blood-rich specimens interfere during immunostaining because of floating and folding tendency of such sections [Figures 6–8].
The contaminant erythrocytes should be separated out or lysed for optimal cell-blocks. Alcohol-based and acid-based lysis with acetic acid would interfere with qualitative integrity, potentially affecting the results of ancillary tests such as IHC.[9] Separation of diagnostic tumors cells and other nucleated cells may be achieved by gradient sedimentation methods comparable to separation of leucocytes as buffy coat by using media such as Ficoll-Hypaque [Figure 9]. This method is more expensive and needs significant skills with nonreproducible results.[12,53] Ammonium chloride-based erythrocyte lysing reagent, similar to that used for flow cytometry is relatively simpler and inexpensive without compromising the immunoprofile integrity.[53] Proprietary cell-blocking kits which facilitate easy implementation of the aforementioned steps of centrifugation with elective erythrocyte lysing are recently available [Figure 10].[35,58]
Figure 9.
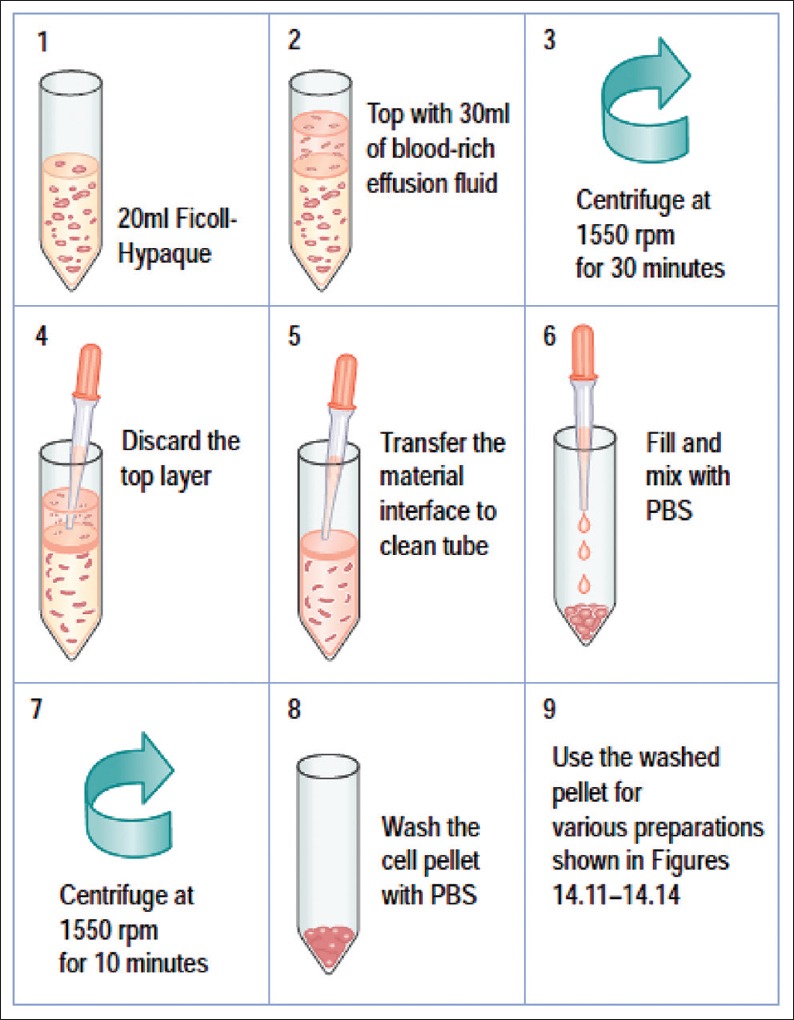
Protocol for Ficoll-Hypaque gradient separation of red blood cells. Reproduced from: Shidham and Atkinson, ‘Cytopathologic Diagnosis of Serous Fluids’ Chapter #14 (Appendix 1), Elsevier (W. B. Saunders Company) First edition, 2007 (ISBN-13: 9781416001454[12]
Figure 10.
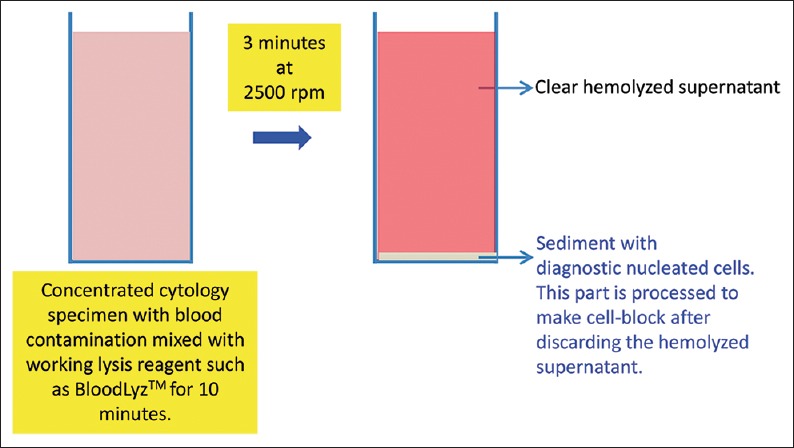
Processing of blood contaminated specimens with BloodLyz™ to nullify the problems related to red blood cell contamination[35,58]
Fresh, unfixed cytology specimens allow flexibility of practicing the best algorithm with better outcome [Figure 2]. Unfixed specimens, such as various body fluids, washings, and needle rinses in isotonic media allow lysis of erythrocytes and removal of blood contamination-related interference if indicated. Specimens collected in different fixatives and collection media may interfere with results of immunostains and other tests such as different molecular tests mostly standardized on FFPE. Collecting directly in formalin will overcome some of these limitations but will not allow making of cytology smear preparations from the same formalin-fixed cytology specimen. Formalin collected specimens will not allow for the removal of blood contamination-related interference. In general, the practice of collecting cytology specimens in weak alcohol fixatives including Saccomanno Collection Fluid,[54] various liquid-based cytology (LBC) collection media such as Cytolyt™, PreservCyt® (ThinPrep),[55] or CytoRich™ Red preservative (SurePath)[56] would interfere with the IHC integrity and the results may not be comparable with the published data on FFPE-based tissue.
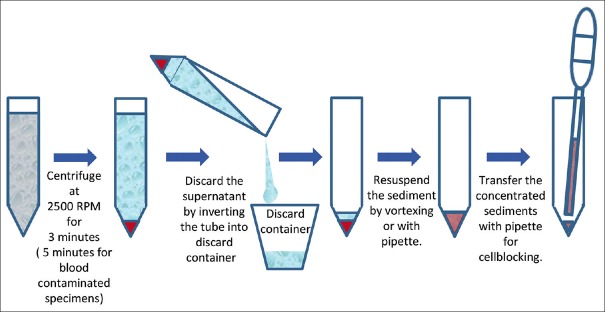
The important preliminary step is the concentration of the cytology specimen to get most of the diagnostic sediment in small volume [Figure 3]. This is usually achieved with the help of centrifugal force by centrifuging the specimen at 1250 RCF (2500 rpm on centrifuge with rotor diameter-11 cm). The sediment button should be compact enough to avoid its dislodgement while decanting the supernatant by simple inversion of the tube into discard container [Figure 11]. 3 minutes centrifugation is enough for specimen without blood contamination; however, specimens with blood contamination usually have a relatively loose button and may require 5 minutes of centrifugation as a general guideline. Individual laboratories should standardize the optimum rpm and duration for laboratory conditions and centrifuges. In general, 3–5 minutes centrifugation is suitable for most specimens at 2500 rpm.
Figure 11.
Concentration of sediments in cytology specimens as a common step for most of the cell-block preparation protocols.
(Discard the supernatant by inverting only after confirming if the sediment pellet/button is stable and compact. Specimens with blood may have loose button, in such cases the supernatant may be removed carefully by aspirating with pipette and leave small supernatant up to volume equal to the sediment volume.)
The concentrated cytology specimen is then ready to be processed for cell-blocking. The yield of cell-block would be enhanced by the sedimentation step to concentrate diagnostic material before the setting of the gel or coagulation step during cell-blocking. However, it is critical that all the diagnostic material is aligned along the potential cutting surface before gelling of the medium, which can be achieved by the Shidham's protocol reported previously.[20] This approach is not possible unless the supporting medium used for cell-blocking has the appropriate stiffness sufficient to handle and maintain the concentrated diagnostic cells in a plane parallel to the cutting surface of the final paraffin-embedded cell-block. The final processed cell-block button with appropriate stiffness would allow for orientation of the diagnostic material precisely parallel to the cutting surface.
Conventional section cutting of FFPE does not allow reproducible monitoring of the depth of cutting of cell-blocks, especially the ones prepared from hypocellular specimens. This may lead to an overcutting with permanent loss of diagnostic material from the cell-block or undercutting. In both cases, it leads to the lack of diagnostic material in the cell-block sections on the slides [Figure 4]. This random and indiscriminate approach leads to cell-block sections with nonreproducible yields, which may be improved to some extent by the simple method of prestaining the button (with eosin or hematoxylin depending on the individual institutional/laboratory protocol) after fixation and before processing for paraffin embedding. This approach may not be effective for all specimens, especially of those with low cellularity. The dark-colored, beacon-like AV marker[20] allows for precise monitoring during the section cutting process. This is the level at which the concentrated cells in the cellblock would start. Thus, the AV marker enables the reproducible objective of monitoring the depth of cutting for selecting the initial tissue section level with diagnostic cells by the histotechnologists [Figure 12].
Figure 12.
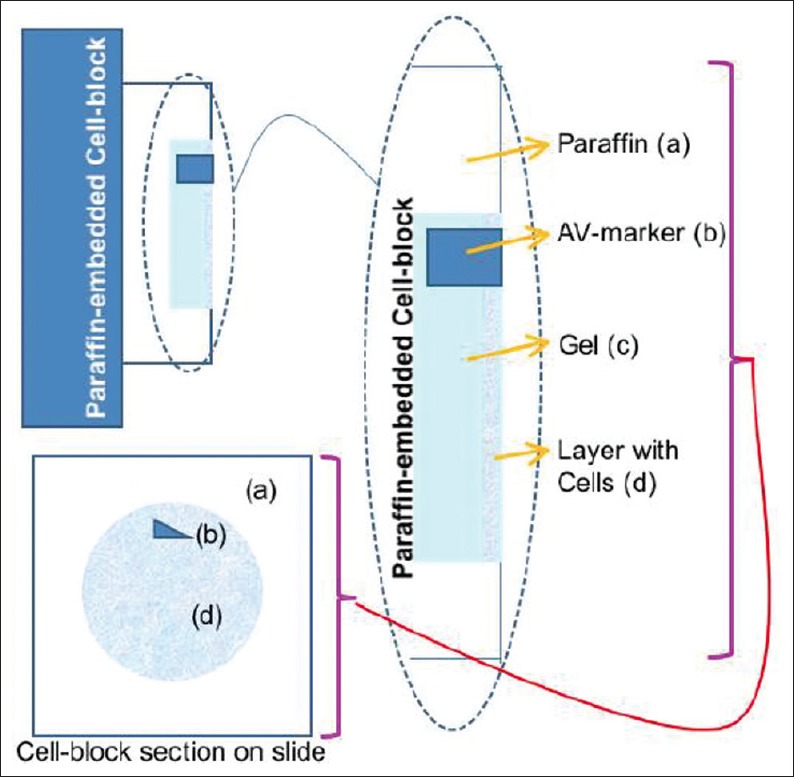
AV marker as a guide to monitor the depth of cutting .(Reproduced from Open access publication: [20] Varsegi and Shidham; Journal of Visualized Experiments; http://www. jove. com/index/Details.stp?ID=1316
In addition, the AV marker facilitates the orientation component of the SCIP approach.[23] This achieves appropriate interpretation of immunostained cell-block sections, especially those with low cellularity and singly scattered cells. AV marker thus introduces additional precision by enhancing the interpretation of the coordinate event of immunoreactivity to different immunomarkers in the identically oriented serial tissue sections like the cinematography frames in a video film [Figure 5].
It is important to note that specimens with fatty material such as loosely scattered micro-fragments in fat pad aspirates, usually performed for amyloid, should not be subjected to the centrifugation approach. The diagnostic fibro-adipose tissue fragments in such specimens would float (and would NOT sediment) with centrifugation and would be lost while discarding the supernatant. In such cases, filtering of the fat pad aspirate with dispersed fibro-adipose tissue fragments is recommended (if it could not be clumped by the clotting step) [Figure 2].[27] If the fibro-adipose tissue fragments are big enough, this may be achieved by filtering the specimen through nylon biopsy bags. This nylon bag with fibro-adipose tissue fragments may be submitted entirely into 10% formalin for fixing and further tissue processing.
The method of cell-blocking to be chosen depends on a variety of factors, but the most critical is the cellularity and qualitative integrity of the sample. For cell-blocking of cytology specimens, the best approach is to be compulsive and not to lose any cells or tissue micro-fragments in the specimen until the stage of getting tissue section on the glass slides.
Sample preparation (general approach)
For better standardization, it is important to introduce the concept of Cytocrit/Tissuecrit of final concentrated sediments [Figure 3] in a manner comparable to that of hematocrit of blood as guesstimated proportion of sediment in the final concentrated specimen.[36,57] The residual specimen after preparing required cytology preparations is concentrated as sediment. The method of cell-blocking will depend on the quantity of sediment suspension produced and its Cytocrit/Tissuecrit. The fresh, unfixed specimens may be triaged as shown in the algorithm suggested with reference to these aspects of the specimens [Figure 2].
For any method selected for cell-blocking, the first step is the concentration of the sediments from most of the specimen. This is especially true for specimens such as effusion fluids with specimen volumes >50 ml. Prepare concentrated sediment by centrifuging enough volume of specimen for 3 minutes at 2500 rpm to get >1 ml final pooled suspension with more than 50% Cytocrit/Tissuecrit.
For specimens with a significant proportion of blood such as many of the FNA needle rinses and some body fluids, the sediment button may not be compact and in such cases, the supernatant should be discarded with the help of transfer pipette without disturbing the button. Such blood contaminated specimens should be processed preferably with lysing reagent such as that used for processing for flow cytometry to nullify the erythrocyte interference [Figures 9 and 10].
Lysis of blood interference
If the specimen has a significant proportion of blood contamination as compared to the diagnostic cell-tissue component, then treat the blood contaminated concentrated specimen with lysing reagent such as proprietary lysing reagent BloodLyz™ (ammonium chloride based lysing reagent similar to that used for flow cytometry, so that immunohistochemistry results are not affected).[58] Acetic acid-based and alcohol-based lysing reagents may compromise results of ancillary tests such as immunohistochemistry and should be avoided.[9,59]
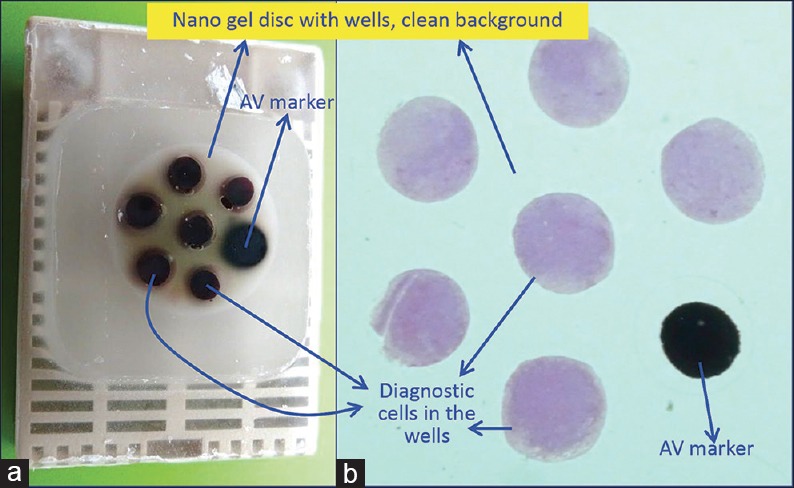
Mix the working lysing reagent with blood contaminated concentrated specimen and let the lysis be completed by keeping at room temperature for up to 10 minutes. Then, centrifuge the mixture with lysing reagent for 3 minutes at 2500 rpm to sediment the cell-tissue components in the concentrated sediment suspension. Discard the relatively clear (transparent) pink to red supernatant with lysed red blood cells and use the whitish-buff-colored sediment with concentrated nucleated diagnostic cells to make the cell-block by adding to the Nano unit [Figure 10]. The portion of this sediment with nucleated cell rich diagnostic cells may also be used for making cytology preparations.
PROCEDURES IN BRIEF FOR VARIOUS CELL-BLOCKING METHODS
Variety of cell-blocking methods are described in the literature. Some of these are described in brief in this review [Figures 13–18]. Many of these methods summarized in Tables 1 and 3 have specific limitations and benefits.
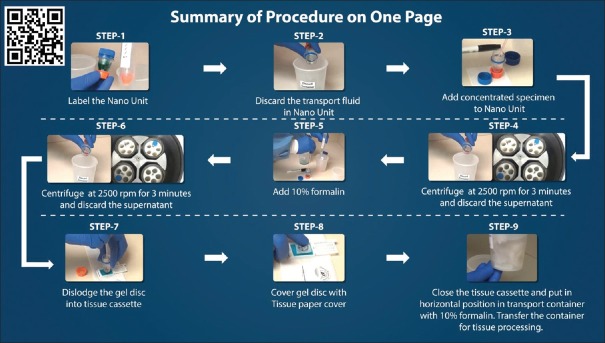
Figure 13.
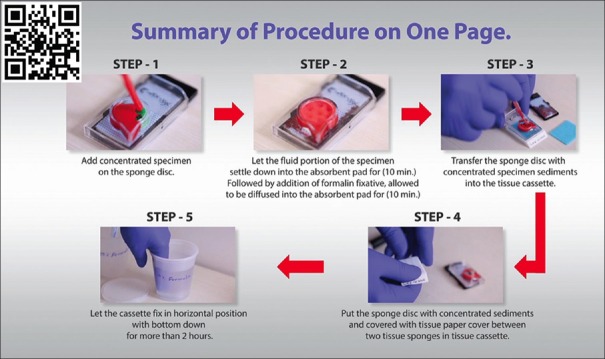
Summary of cell-block preparation protocol for Nano NextGen CelBloking™ unit.[36] The manufacturer also has a video explaining an approach for processing multiple specimens simultaneously[36] (Courtesy: www.avbioinnovation.com)
Figure 18.
Cell-blocking of clot in cytology specimen. (Reproduced from Open access publication:[27] Shidham et al.; Journal of Visualized Experiments; http://www.jove.com/index/Details. stp?ID=1747
Preparation of cell-block from cellular specimens with significant sediment (more than 1 ml sediment suspension with high Cytocrit/Tissuecrit of >50%)
In cases with scant sediments, the other centrifugation assisted precision methods[20] including proprietary kits[35] may be used (these methods may also be used in all cases routinely irrespective of the amount of sediment).
Simple sedimentation method
Spin the concentrated specimen for 3 minutes at 2500 rpm
Decant the supernatant, and add about 1 ml of 10% tinted formalin and spin again for 3 minutes at 2500 rpm
Decant the supernatant formalin and discard it appropriately. Transfer the conglomerated partially fixed sediment button on a 2 inch × 2 inch lens paper piece inside the labeled tissue cassette
Wrap the pellet gently in lens paper, prestain the button (with eosin or hematoxylin as per institutional preference), and close the tissue cassette by snapping the top cover
Transport the cassette in 10% formalin to be processed after fixing in 10% formalin for at least 2 hours with protocol for paraffin embedding.
The diagnostic cells are present as randomly and indiscriminately distributed cells and may be admixed with blood and proteinaceous material.[60]
Cell-block preparation using Celloidin (collodion) bag [Figure 17][30,45]
Figure 17.
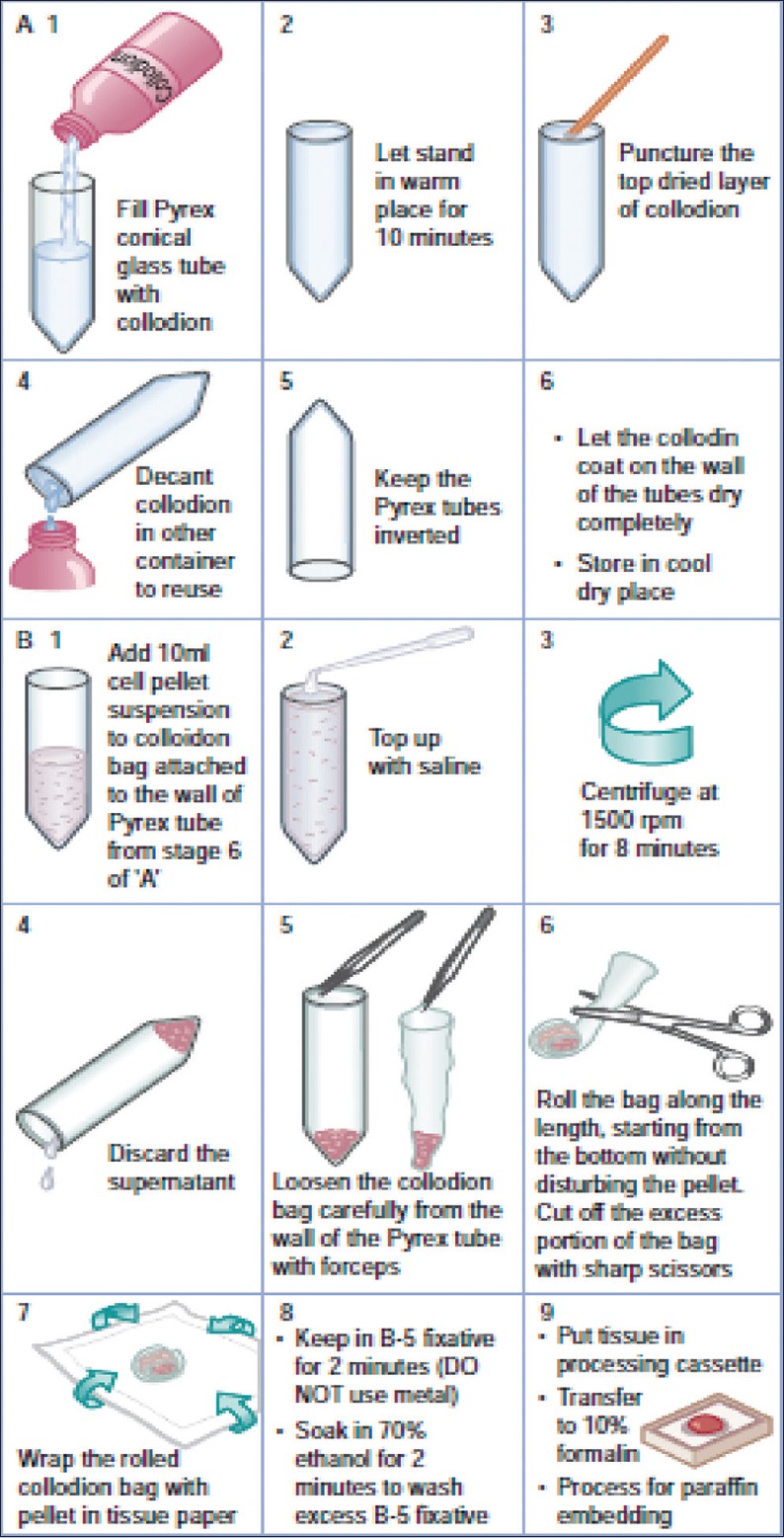
Protocol for cell-block making with collodion method
(Reproduced from: Shidham and Atkinson, ‘Cytopathologic Diagnosis of Serous Fluids’ Chapter #14 (Appendix 1), Elsevier (W. B. Saunders Company) First edition, 2007 (ISBN-13: 9781416001454[12])
B-5 fixative and alcohol exposure prior to formalin fixation would compromise the immunoreactivity of the cells in the cell-block. There are a few protocols to overcome this interference by using 10% formalin and avoid exposure to B-5 fixative and 70% ethanol prior to the fixation with 10% formalin.[51]
This method requires preparation of Celloidin bags in advance with protocol mentioned below:
Preparation of Celloidin bags (under a vented fume hood)
Material required
-
15 ml Pyrex conical glass tubes (Corning 8060, #05-505, Fisher Scientific, Pittsburgh, PA)
or 15 ml polypropylene conical plastic centrifuge tubes
-
Celloidin Flexible USP (Mallinckrodt, #4580-500-NY, Baxter Scientific, McGraw Park, Ill)
or prepare Celloidin solution (by soaking Celloidin flakes in absolute alcohol and then dissolving the soaked flakes in ether to get 10% solution in absolute alcohol-ether (1:1) (store in a well-stoppered bottle at room temperature), and
Vertical fume hood.
Method of preparation of Celloidin bags (needs experience and skill and has some risk related to the handling of inflammable, volatile material)
Place the 15 ml conical centrifuge tubes in the rack and top with Celloidin solution under a vented fume hood
Wait for 10 minutes (Celloidin bags would be thicker if this time is longer)
The top surface of Celloidin would harden with the evaporation of the alcohol-ether solvent. Puncture and remove the hardened top surface with wooden applicator stick
Pour out the Celloidin solution (back into the bottle for reusing later [Celloidin solution may be reused multiple times until the dry bags are very thick and difficult to fold]) and drain all Celloidin solution by inverting the conical tubes until the tubes dry under a vented fume hood. Initially, the tubes may appear cloudy, but final dried tubes will be clear
The celloidin tubes with thin film (about 20–50 μ thick) on the inner surface of the tubes can be stored upside down in a dry, cool condition up to 6–8 weeks until used.
Other alternative, although less practical, is to store these coated tubes (with about 20–50 μ thick layer of celloidin) partly filled with chloroform (which hardens the celloidin and prevents excessive drying of the film) and stoppered. The chloroform is discarded just before use.
Preparation of Cell-block with Celloidin bags
Add concentrated specimen to the celloidin tubes and fill the tube nearly to the top with the saline
Centrifuge the tube at 1500 rpm for 8 minutes
Discard the supernatant (with a Pasteur pipette without disturbing the cell button or by inverting the tube if the pellet is not loose)
Remove the softened Celloidin sac gently with pointed tip forceps by separating out the bag-like cast from the tube wall by peeling it away gradually and gently without significant jerking to avoid disturbing the cell button (this step needs skill and practice)
Fold the bag-like an egg role as close to the cell button as possible and trim the excess bag with scissors without disturbing the cell button
The folded bag is wrapped in lens paper and placed in B-5 fixative for 2 minutes
The wrapped Celloidin bag with concentrated cytology specimen is transferred from B-5 fixative (without exposure to any metal structure) to the labeled plastic tissue cassette
The cassette is placed in 70% ethanol for 2 minutes to rinse off excess B-5 fixative before transferring to 10% formalin for final tissue processing and paraffin embedding.
The diagnostic cells are present mostly along the wall of Celloidin bag and may be admixed with blood and proteinaceous material.[60]
Preparation of cell-blocks using various binding/supporting media with or without some proprietary methodologies
Variety of supporting/conglomerating media [Figure 1] may be used to hold together the singly scattered cells and micro-tissue fragments in the specimen for processing, paraffin embedding, and cutting. The list of different types of supporting/conglomerating media includes Gelatin,[61] Albumin,[22] Agar, proprietary gels such as HistoGel™,[62] Sodium alginate,[24] Glucomannan-formalin with methanol,[21] and plasma/fibrinogen-thrombin.[28,29] A few representative methods are described below.
Prepare respective medium according to individual published protocols or procure them from commercial sources as proprietary material.
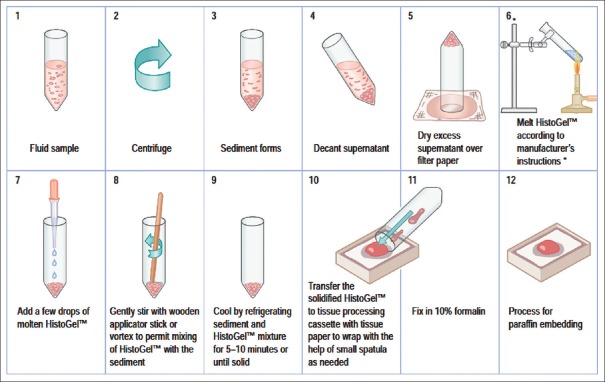
Hot methods
These are gels such as Agar, gelatin, and HistoGel™ have to be molten with heat, and the molten gel is mixed with sediment and then allowed to solidify leading to a button which can be handled for processing. Heating of gel with melting at optimum temperature may be clumsy in addition to the problems related to controlling solidification stage with proper alignment of sediments along the cutting surface of final FFPE of cell-block before solidification.
Routine indiscriminate procedures using molten gels [Figure 16][12,13,19]
Figure 16.
Protocol for cell-block making with HistoGel. *HistoGel™ may also be molten with microwave in microwave safe tubes/container
(Reproduced from: Shidham and Atkinson, ‘Cytopathologic Diagnosis of Serous Fluids' Chapter #14 (Appendix 1), Elsevier (W. B. Saunders Company) First edition, 2007 (ISBN-13: 9781416001454[12])
Specimens with relatively abundant sediments may be processed by using gels which are liquid when hot and solidify after cooling
Melt small amount of gel in a tube until it melts completely
Cool the gel to the temperature which will not be damaging to the specimen but will still keep the gel molten (e.g., −60°C for Agar and HistoGel™)
A few drops (about 1–2 drops) of molten gel are added to the sediment. The button should not have extra water (excess water may dilute the molten gel and prevent or delay solidification)
Mix by vertexing or with wooden applicator stick
Place the tube with its pellet mixed with gel into a refrigerator for 5–10 minutes or until solid
With small spatula, carefully transfer the pellet embedded in the gel from the tube onto a lens paper inside the tissue cassette
Fold the lens paper gently over the pellet. With pellet wrapped in paper, snap the top cover of tissue cassette to close it
Drop the cassette into 10% formalin and process for paraffin embedding.
Shidham's method
This method concentrates the diagnostic cells along the cutting surface with dark-colored AV marker which aligns during the procedure at the level of concentrated diagnostic cells. This addition of discriminatory component to the entire procedure results in quantitative improvement. It also introduces precision to the routine random technique of cell-block making and grants ultimate control over paraffin block sectioning by histotechnologist.[20]
Shidham's method for cell-block preparation using HG is reproduced from the original open access publication in entirety after minor modifications below.[20] This video publication in ‘Open Access’ also includes a step-by-step video demonstrating the procedure. As compared to other random approaches, the following are the two critical features of this protocol for preparing cell-blocks from relatively hypocellular specimens with singly scattered loose cells.
This protocol involves steps to concentrate the diagnostic cells along the plane parallel to the cutting surface of the cell-block
-
It also includes a beacon-like dark inclusion of AV marker, which serves two of the following purposes:
- To visualize the level at which the cells are concentrated. The area of the cell-block with the cells of interest now could be visualized by the histotechnologist when the dark-colored beacon is exposed during cutting. This ability to monitor would prevent one from cutting through the level with most of the cells, or not cutting too superficial into the level with the highest concentration of sample cells
- To provide a locator reference point in serial cell-block section on different slides. This reference point acts as a beacon to help locate particular cells or groups of cells for evaluation of a coordinate immunoreactivity pattern with the SCIP approach.
Preparation of the sample
Transfer the concentrated specimen to a flat bottom glass tube (15 mm diameter × 45 mm). Place the glass tube into a larger plastic carrier tube (28 mm × 85 mm) and centrifuge. Remove the glass bottom tube from the carrier tube and pour off the supernatant
The glass tube is then capped (to prevent spillage of heating water in the next step) and placed back inside a larger flat bottom carrier plastic tube
The carrier plastic tube containing the glass tube is then capped, placed in a centrifuge (with swiveling cups and not fixed angle cups so that the cells fall perpendicularly to the flat bottom of the glass tube), and spun at 1805 G (3000 rpms, rotor radius-17 cm) for 5 minutes
The tubes are then removed vertically from the centrifuge, and the smaller glass tube is removed with forceps from the larger carrier plastic tube without disturbing the sedimented pellet with cells
The glass tube with the specimen is uncapped, and the supernatant is poured off taking care not to disturb the flat layer of sediment cells at the bottom.
Inclusion of the reference coordinate AV marker and addition of gel
A dark beacon AV marker (about 2 mm × 2 mm in size, flat surfaced, fragment of dark-colored, sectionable material) is added as a signpost to the glass tube
Melt an aliquot of HG by melting it in the microwave for 10 s at medium power
Add 0.5 ml of molten HG to the tube, mix with the sediment quickly, and recap it (Proceed to the next step quickly without allowing the HG to begin solidifying)
Add about 2.5 ml of warm (45°C) water to the carrier plastic tube
The smaller capped glass tube is placed inside the plastic tube with warm water. (This step is necessary to keep the HG from solidifying during the next steps)
The carrier plastic tube is placed in the centrifuge (with swiveling cups and not fixed angle cups so that the cells fall perpendicularly to the flat bottom of the glass tube), and spun at 1805 G (3000 rpms, rotor radius-17 cm) for 5 minutes. The purpose of this centrifugation step is to push the AV marker and to concentrate the cells into a layer closer to the cutting surface of the final paraffin embedded cell-block
The tubes are then removed gently and vertically from the centrifuge taking care not to disturb the sedimented thin layer with sample cells at the bottom
The larger plastic tube is uncapped, and the smaller glass tube is removed vertically by a forceps without disturbing the sediment layer of specimen cells
The small glass tube is refrigerated in vertical position for 15 minutes to cool and solidify the HG.
Removal of the cell-block as a button of gel with the specimen for final processing
The solidified HG disk, with the layer of concentrated/sediment specimen at the bottom, is dislodged from the flat bottom glass tube by squirting 10% formalin through a 23 G needle with the syringe
The needle is inserted along the side of the tube at the periphery of solidified HG disc with the specimen
The needle is rotated along the side of the tube while formalin is slowly pushed through the syringe. This results in the separation of the HG button along with trapped dark-colored beacon AV marker and the concentrated specimen in it from the flat bottom of the glass tube
The cell-block (gel button with specimen cells) is then placed in a labeled cassette and submitted for tissue processing to prepare paraffin embedded cell-blocks.
Embedding and cutting of the specimen
The disk is embedded in paraffin with the dark beacon marker side down as cutting surface
The block is sectioned until the dark-colored AV marker as a beacon is exposed and clearly visible
3–4 μ sections are cut from this level which should contain most of the singly scattered cells from the specimen
The sections are collected on the glass slide for further staining, immunohistochemical staining, or other tests as indicated. The protocols for these tests including the type of slides to use for mounting the sections may vary. In general for immunostaining, coated slides are used to prevent floating and loss of sections from the slides during the immunostaining steps.
The diagnostic cells are present as randomly and indiscriminately distributed cells and may be admixed with blood and proteinaceous material with supporting gel.[20]
Cold methods
Plasma/fibrinogen-thrombin method: Plasma (pooled plasma from a blood bank may be used) and thrombin (1000 NIH U/ml) are used to prepare the cell-block. The stability of the reagents should be checked periodically by adding two drops of thrombin solution to two drops of plasma, which should clot in about 30 seconds.
Procedure for plasma/fibrinogen-thrombin method [Figure 15][28,29]
Figure 15.
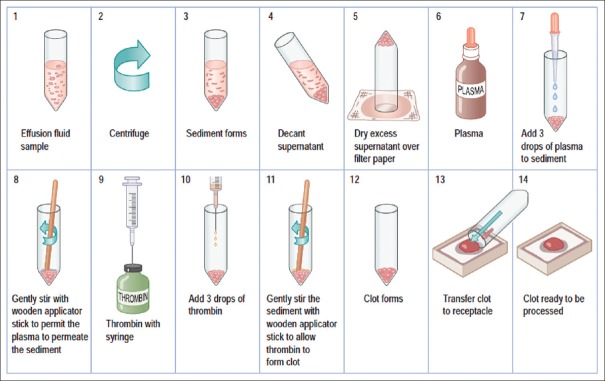
Protocol for plasma-thrombin method
(Reproduced from: Shidham and Atkinson, ‘Cytopathologic Diagnosis of Serous Fluids’ Chapter #14 (Appendix 1), Elsevier (W. B. Saunders Company) First edition, 2007 (ISBN-13: 9781416001454[12])
-
The sediment is mixed with 2–3 drops of plasma/fibrinogen solution
(If the specimen is not in isotonic medium but in fixative such as formalin, the sediment with fixative will interfere with the enzymatic activity of thrombin and may not achieve proper coagulation of plasma/fibrinogen. Such sediments should be washed in saline by centrifuging and discarding the supernatant twice. The final saline washed suspension of fixed sediments can be used similar to the sediments of the unfixed specimen)
Put 3–4 drops of thrombin solution (5000 units in 10 ml distilled water)
Mix by tapping. The mixture usually clots in a few seconds
Transfer the clot from the tube on to a 2 inch × 2 inch lens paper piece inside the labeled tissue cassette
Wrap the pellet gently in lens paper, prestain the button (with eosin or hematoxylin as per institutional preference), and close the tissue cassette by snapping the top cover of
Transport the cassette in 10% formalin to process with the protocol for paraffin embedding.
Microscopic evaluation of the HE-stained sections shows randomly and indiscriminately distributed diagnostic cells which may be admixed with blood and proteinaceous material in the background.
Procedure for cell-block preparation with proprietary instruments/methods
-
Procedure for cell-block preparation with Shandon Cytoblock™ (using proprietary reagent #1 and reagent #2. This method require Cytospin instrument with proprietary kit and reagents).[26]
- Add 10% Formal saline to 0.5 ml sediment suspension, re-suspend the sediments, and allow to be fixed for at least 30 minutes at room temperature
- Centrifuge formalized cell suspensions at 800 rpm for 5 minutes. Discard the supernatant
- Add sufficient reagent #2 to the above sediment to give a concentration of approximately 5 × 107 cells per 100 μl, and mix
- Each 100 μl cell mixture will produce one Cytoblock. Prepare the required number of Cytoblock cassettes as follows
- Apply three drops reagent #1 to the well in the Cytoblock board
- Assemble Cytoblock cassettes and cytofunnels in the cytospin clips, and load into the cytospin head
- Add 100 μl of the cell mixture from step #e to the cytofunnels and spin for 5 minutes at 1500 rpm on “Lo” acceleration
- Open the clips, and remove the Cytoblock carefully, checking that the cell button is retained in the well. Add one drop of reagent #1 to the center of the well and close cassette
- Immerse the cytospin clips and funnels in disinfectant and leave overnight, then rinse and allow to air dry to be reused
- Process the cassettes for tissue processing
- Embed the cell buttons in paraffin wax.
-
Cellient[25]
Cellient™ is proprietary automated cell-block system which achieves concentration, processing, and embedding of sediments in cytology specimen for making a paraffin block. A special cassette, used by the instrument, concentrates sediments in cytology specimen by suction. This sediment is processed by treating with alcohol followed by xylene and then infiltrated with paraffin in the same cassette.[25] Please follow the instructions from the manufacturer.
The resultant cell-block sections show good morphology, both as HE-stained sections and as immunostained sections. This approach, however, has most important limitation related to exposure and fixation with alcohol leading to potential interference with immunoprofiles affecting the final interpretation and even the results of prognostic/therapeutic tests with liability issues unless specifically validated for each test [Table 2].[9,13] Recently, a protocol for formalin fixation is available as option to overcome issue related to immunohistochemistry. However, this step cannot be matched with requirement of fixing in 10% formalin for specified duration such as 6 to 72 hours for some immunomarkers such as Her2 and some tests such as FISH per ASCO/CAP guidelines.[25a] In addition, to initial capital investment and recurring kit cost, the instrument allows processing of only one block at a time, limiting the throughput, unless laboratories with high turnover invest in multiple instruments. Similar to other random methods, the cell-blocks made with this method do not allow monitoring of the depth of section cutting by histotechnologists with potential for nonreproducible final cell-block sections due to either undercutting with lack of diagnostic material on slides or overcutting leading to loss of diagnostic material from the cell-block without diagnostic material on the slides.
The diagnostic cells are present as randomly distributed diagnostic cells admixed with blood and proteinaceous material.[60]
-
Methods using preformed supporting media such as premade gel and sponge discs with wells for the accumulation of sediments (proprietary Nano and Micro NextGen CelBloking™ kits (kits based on Shidham's method with built-in precisely set dark AV marker) [Figures 13,14,19–26]:[35]
Nano version (with premade gel discs with wells) is for specimen of any cellularity.
Micro version (with premade sponge discs with wells) is for specimens with >1 ml concentrated sediment suspension with >50% Cytocrit/Tissuecrit. The results with Micro version on low cellularity specimens may not be optimum.
- Prepare concentrated sediment button by spinning enough quantity of specimen by centrifuging for 3 minutes at 2500 rpm
- Pool all the sediments as final concentrated sediment with maximum Cytocrit/Tissuecrit and re-suspend the sediment.
- At this stage, if the final concentrated specimen is >1 ml and has >50% Cytocrit/Tissuecrit then Micro version of the NextGen CelBloking™ kits may be used. However, Nano kits[36] could be used for specimens generating final concentrated specimens with any cellularity. In case of doubt, it is recommended to choose Nano units for optimum results.
Figure 14.
Summary of cell-block preparation protocol for Micro NextGen CelBloking™ Unit.[38] The manufacturer also has a video explaining an approach for processing multiple specimens simultaneously[39] (Courtesy: www.AVBioInnovation.com)
Figure 19.
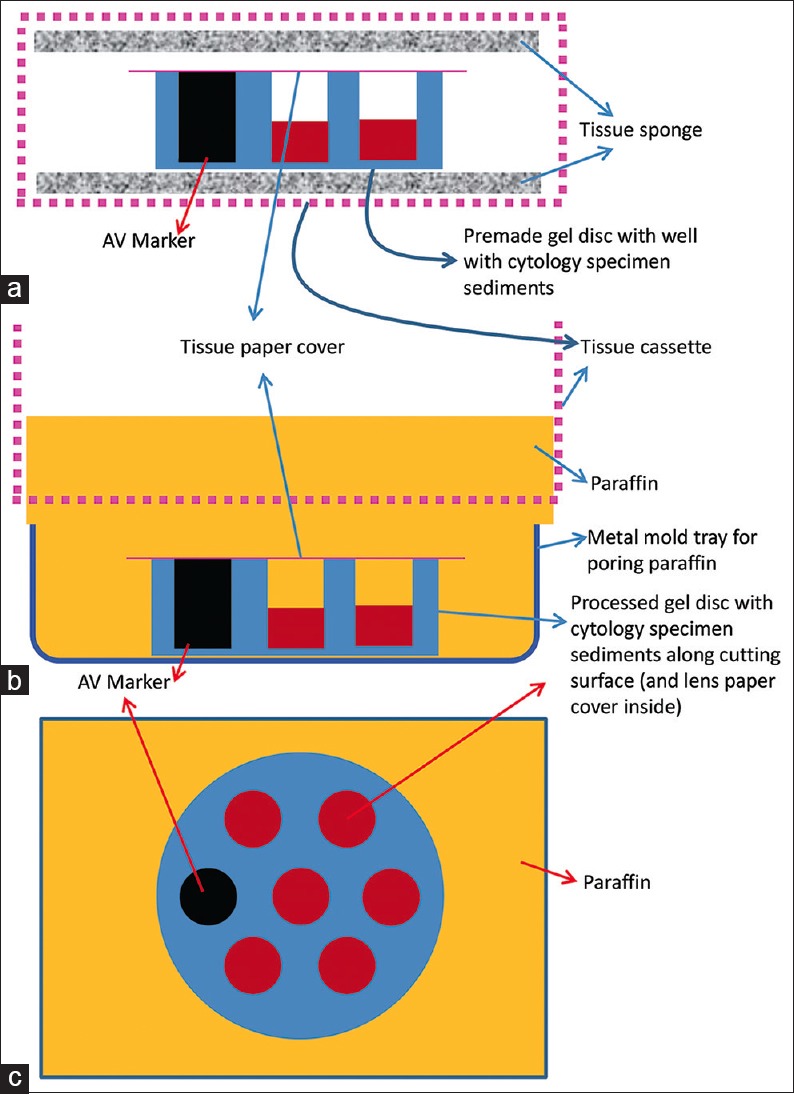
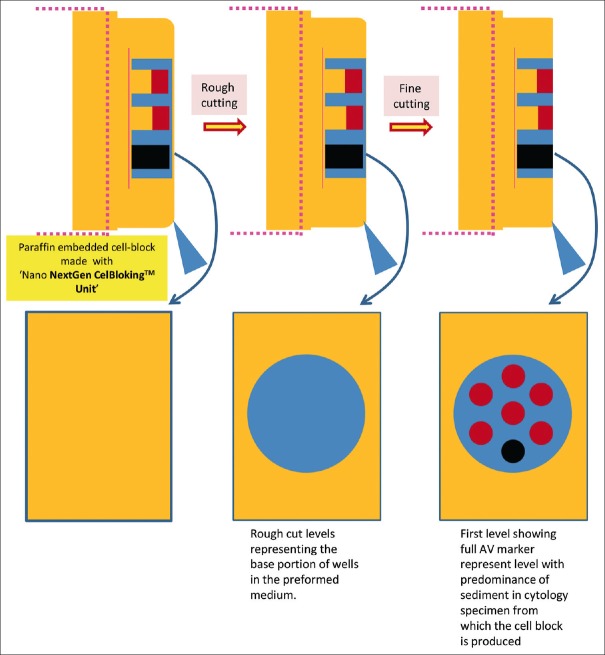
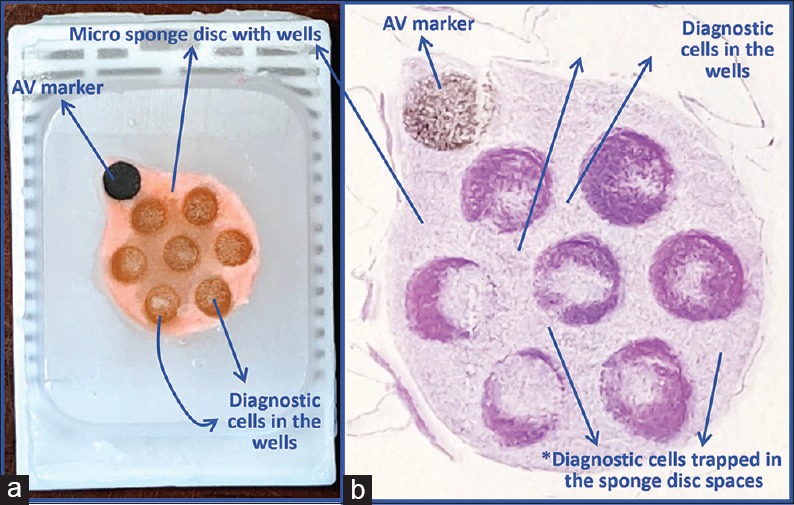
Cell-block prepared with Nano NextGen CelBloking™ unit. (a) Gel disc with preformed wells loaded with diagnostic material transferred to the tissue cassette for tissue processing. (b) Embedding of tissue processed sponge disc of Nano NextGen CelBloking™ unit with diagnostic material. The tissue paper cover is opposite the cutting surface, and the bottoms of the wells are the cutting surface. (c) The cutting surface of the final cell block with gel disc of Nano NextGen CelBloking™ unit after rough cut remove the bottom layer of the disc exposing the precisely set dark-colored AV marker which corresponds with the bottoms of the wells with concentrated diagnostic material[67]
Figure 26.
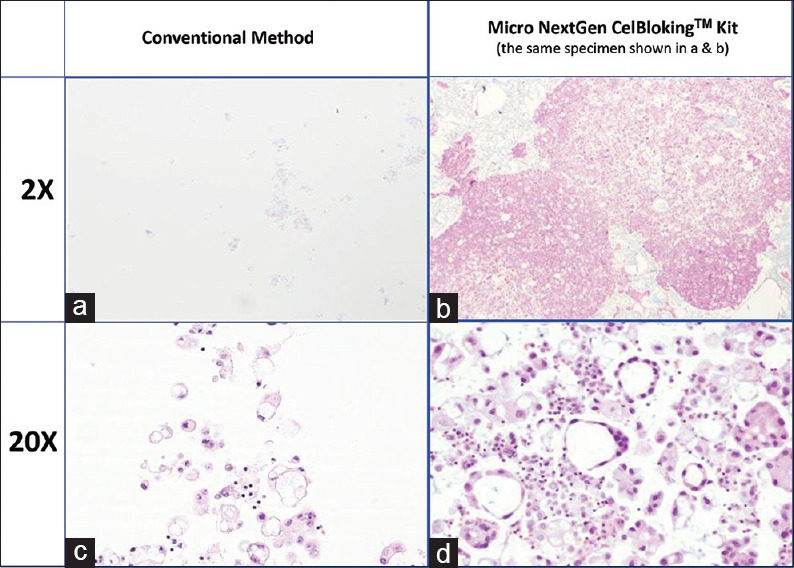
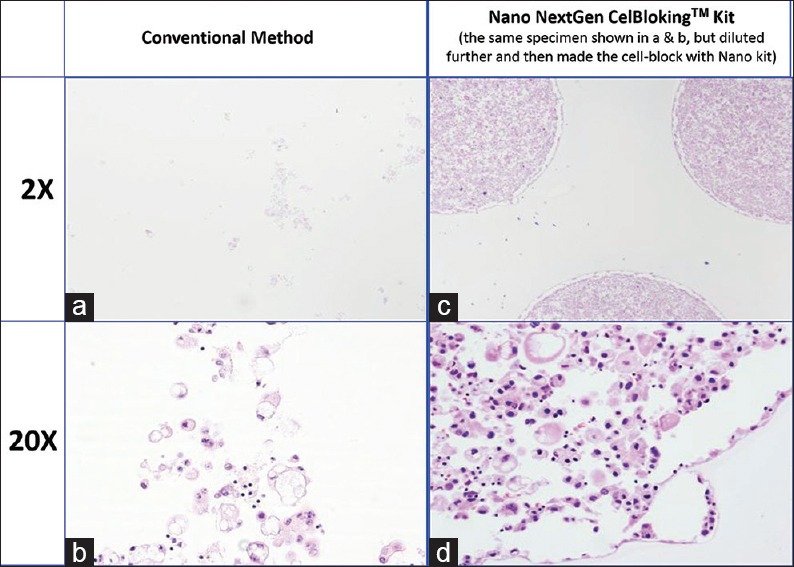
Comparison of the morphological details and quantitative enhancement by Micro NextGen CelBloking™ kit (Metastatic adenocarcinoma, pleural fluid). (a and b) Cell-block section with very scant cellularity (conventional random, indiscriminatory, plasma-thrombin method); (c and d) Relatively cellular cell-block section with many diagnostic cells in the wells and in small spaces in the sponge disc (cell-block prepared with enhancement method-Micro NextGen CelBloking™ kit
Figure 20.
Cutting of paraffin block prepared with Nano NextGen CelBloking™ unit[67]
Figure 21.
(a) Final paraffin block; (b) Scanning power view of HE-stained section of cell-block prepared with Nano NextGen CelBloking™ kit. The preformed Nano gel disc is made of the proprietary medium which allows the processing reagents to be exchanged freely, but the diagnostic cells are retained and concentrated in the wells. The gel medium has clean transparent property as a clean background. (pleural fluid)
Figure 24.
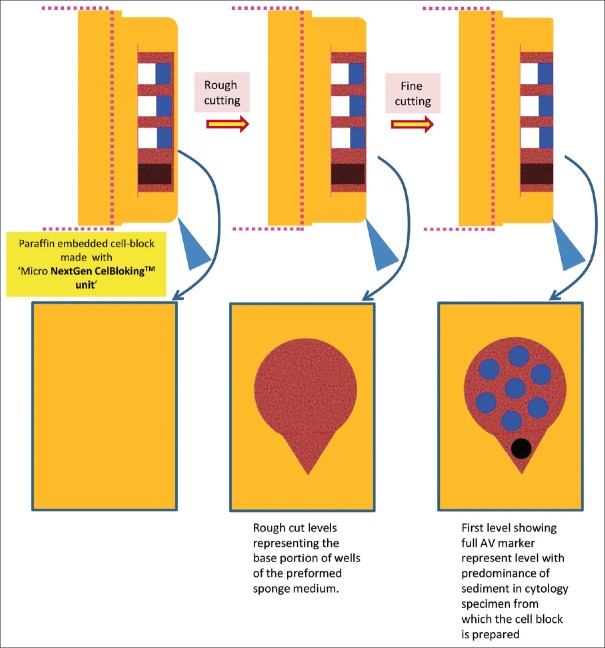
Cutting of paraffin block prepared with Micro NextGen CelBloking™ unit[67]
After this, the processing steps for both Nano and Micro kits are as mentioned below:
| Specimen with any cellularity | Only for cellular specimen |
| Nano kit [Figure 13][36,37] | Micro kit [Figure 14][38,39] |
| For videos showing the methodology in detail are available free at | For videos showing the methodology in detail are available free at |
| One specimen at a time | One specimen at a time |
| https://youtu.be/y29SS1NwO_8 | https://youtu.be/i-ZpXaljiIs |
| Multiple specimens simultaneously | Multiple specimens simultaneously |
| https://youtu.be/ZPb0nq8MsLk | https://youtu.be/TRW5Vswy6J8 |
| d. Open the caps of the Nano units.[63,64,65] Discard the transport fluid provided in the labeled Nano NextGen CelBloking™ unit by inverting the unit into the discard container. Add the concentrated suspension** in the Nano unit with preformed gel medium with wells at the bottom of the Nano NextGen CelBloking unit | d. Flood the preformed sponge disc with wells and preset black AV-marker of the Micro unit with 0.5 to 1 ml of concentrated specimen with more than 50% Cytocrit/Tissuecrit (if needed it may be done multiple times till all the wells are loaded and filled to the top with the sediments) |
| e. Centrifugeϯ the Nano unit for 3 minutes at 2500 rpm to sediment the cell-tissue components in the concentrated sediment suspension in the wells of the preformed gel medium at the bottom of unit | Micro-units generally are not suitable for blood rich specimens**, which should be used after lysing the contaminant red cells in the specimen to get the red blood cell-free concentrated specimen to be cell-blocked with Nano units |
| f. Remove the tubes from centrifuge and discard the supernatant gently by inverting the contents into the discard container after opening the bigger top cap. | e. Wait for 10 minutes to let the supernatant in the concentrated specimen be adsorbed into the absorption pad of the unit and the sediments get concentrated and flattened in the wells of the sponge disc |
| g. Add a few drops (up to 1 ml) of 10% formalin gently along the wall of the Nano unit to cover the partially compacted sediments without disturbing the compacted sediments in the gel disc wells (other fixative or reagent applicable to the individual protocol may be used instead of 10% formalin) | f, g. Add a few drops of 10% formalin gently over the Microsponge disc with concentrated specimen sediments in the wells (other fixative or reagent applicable to the individual protocol may be used instead of 10% formalin) |
| h. Centrifugeϯ the Nano unit again for 3 minutes at 2500 rpm to sediment the cell-tissue components in the concentrated sediment suspension in the wells of the preformed gel medium at the bottom of the unit | h. Wait for 10 minutes to let the 10% formalin flooded over the concentrated specimen be adsorbed into the absorption pad of the unit and all of the added formalin is adsorbed into the absorption pad |
| i. Remove the tubes from centrifuge and discard the supernatant gently by inverting the contents into the discard container after opening the bigger top cap | i, j, k. Dislodge the sponge medium disc (with wells which are now filled with sedimented cell-tissue components in the concentrated sediment suspension) by gently pulling out the black carrier plate with absorption pad of the Micro unit. If needed, the sponge disc may be pushed down with the tip of transfer pipette used for that specimen into the center of labeled tissue cassette with formalin soaked tissue sponge along the bottom. The top surface of the sponge disc with mouths of the wells should be facing up |
| j. Prepare to dislodge the bottom gel disc from the unit by gently opening the small lower cap by twist opening it counterclockwise (see the arrows on the small lower cap[63]) | |
| k. Dislodge the gel medium disc (with wells which are now filled with sedimented cell-tissue components in the concentrated sediment suspension) by gently pushing it with the tip of transfer pipette used for that specimen into the center of labeled tissue cassette with formalin soaked tissue sponge along the bottom. Avoid the wells of the disc to be poked in by the tip of the transfer pipette. Instead push at the periphery. The top surface of the gel disc with mouths of the wells would face up |
**If the specimen has a significant proportion of blood contamination as compared to the diagnostic cell-tissue component, then treat the blood contaminated concentrated specimen with lysing reagent (ammonium chloride-based lysing reagent similar to that used for flow cytometry so that immunohistochemistry results are not affected. Acetic acid-based lysing reagents may compromise results of ancillary tests such as immunohistochemistry and should be avoided. Mix the working lysing reagent with blood contaminated concentrated specimen and let the lysis be completed by keeping at room temperature for up to 10 minutes. Then centrifuge the mixture with lysing reagent for 3 minutes at 2500 rpm to sediment the cell-tissue components in the concentrated sediment suspension. Discard the supernatant with lysed red blood cells and use the sediment with concentrated nucleated diagnostic cells to make the cell-block by adding to the Nano unit [Figure 10].
ϯThe centrifuge used should have free swiveling rotor (NOT fixed angle) with cups for 50 ml tubes. If this is not available, the centrifugation step may be replaced by gravity sedimentation by leaving the units undisturbed for 30 minutes during these steps in the refrigerator (do NOT allow to freeze). Then gently discard the supernatant with the help of transfer pipette (instead of just inverting the unit after centrifugation), because the aggregation of the sediments may not be compact by gravity alone
-
l.
Cover the mouth of the wells with sediments in the gel or sponge disc with tissue paper cover provided with the kit.[66] This step minimizes the potential for cross contamination
-
m.
Then lay over this tissue paper cover, the second sheet of tissue sponge (moistened with 10% formalin) and close the labeled tissue cassette
-
n.
Transport the cassette horizontally with the bottom down in container with 10% formalin to process with the protocol for paraffin embedding after fixing in 10% formalin for at least 2 h (or for more duration as required by individual laboratory/institution protocol)
-
o.
The processed gel/sponge disc (with sediments in the wells) is embedded along with lens paper in such a way that the bottom of the discs corresponding with the bottoms of the wells will be the cutting surface in the paraffin block (and top surface with mouths of the wells covered with lens paper is deep in the paraffin block) [Figure 19 and 23][67]
-
p.
Rough cut the paraffin blocks until the dark-colored dot of AV marker is seen on the paraffin section (this is the level at which the constituent cells in the specimen have sedimented and aligned)[67]
-
q.
Then, cut the block as usual (preferably only one level at which the AV marker is initially visible). Additional levels may be cut later as indicated for elective studies after studying the HE-stained initial level with reference to clinical details and findings in cytology preparations.
Figure 23.
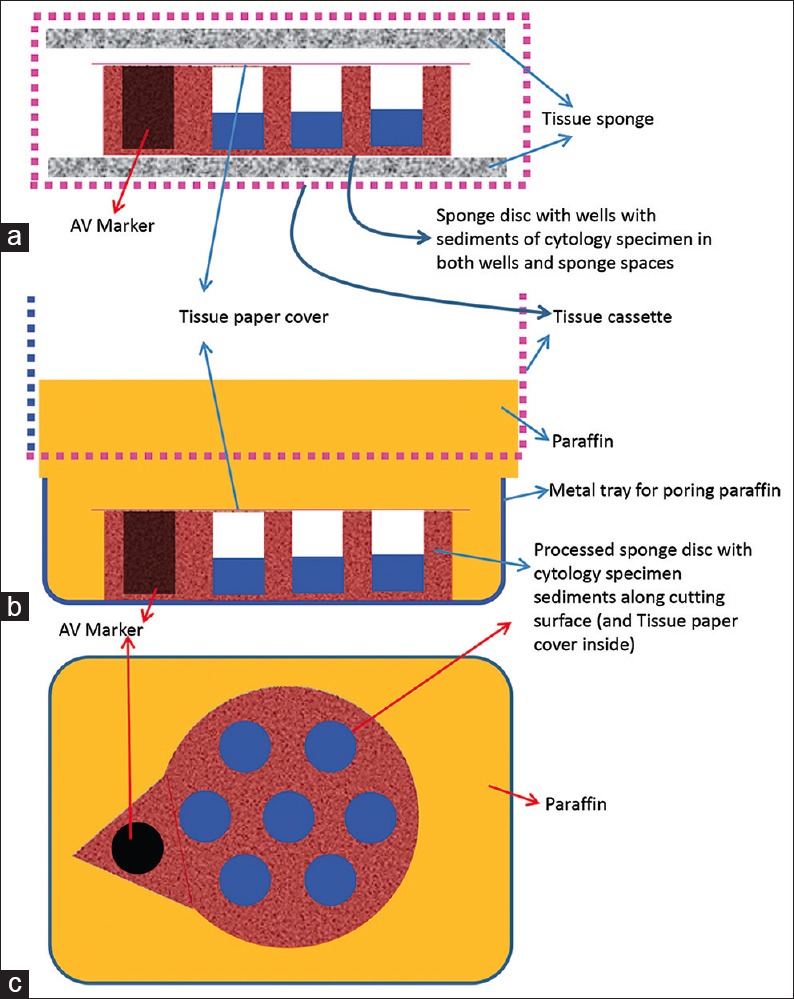
Cell-block prepared with Micro NextGen CelBloking™ unit. (a) Sponge disc with preformed wells loaded with diagnostic material transferred to the tissue cassette for tissue processing. (b) Embedding of tissue processed sponge disc of Micro NextGen CelBloking™ unit with diagnostic material. The tissue paper cover is opposite the cutting surface, and the bottoms of the wells are the cutting surface. (c) The cutting surface of the final cell block with sponge disc of Micro NextGen CelBloking™ unit after rough cut removing the bottom layer of the disc exposing the precisely set dark colored AV marker which correspond with the bottoms of the wells with concentrated diagnostic material[67]
The diagnostic cells are present as concentrated, focal accumulation in the wells of the supporting disc medium [Figures 22 and 26]. In the cell block sections prepared using Micro version of NextGen CelBloking™ kit, the cells are also present in the spaces in the sponge disc [Figures 25 and 26]. The gel disc medium in the cell block sections prepared using Nano version of NextGen CelBloking™ kits is clear and do not show cells in the areas other than in the wells. The gel disc medium in the cell block sections prepared using Micro version of NextGen CelBloking™ kits is seen as gray-bluish sponge material with intervening spaces which may have diagnostic cells in addition to those concentrated in the wells [Figures 25 and 26].
Figure 22.
Comparison of the morphological details and quantitative enhancement by Nano NextGen CelBloking™ kit (Metastatic adenocarcinoma, pleural fluid). (a and b) Cell-block section with very scant cellularity (conventional random, indiscriminatory, plasma-thrombin method); (c and d) very cellular cell-block section with many diagnostic cells in the wells (cell-block prepared with enhancement method-Nano NextGen CelBloking™ kit (AV BioInnovation, based on Shidham method http://www.jove.com/index/Details.stp?ID = 1316)
Figure 25.
(a) Final paraffin block; (b) Scanning power view of HE-stained section of cell-block prepared with Micro NextGen CelBloking™ kit. The preformed Micro sponge disc is made of the proprietary porous medium which concentrates the diagnostic cells predominantly in the wells, but the small groups of cells and singly scattered cells wandered around during concentration process may also be seen in the sponge spaces*. The sponge disc medium stains faintly. (Pleural fluid)
The precisely set, built-in AV marker, in addition to objectively guiding to select the first level with diagnostic cells, also allows orientation of individual sections on the glass slides in identical fashion in serial order for proper application of SCIP approach for the evaluation of coordinate immunoreactivity of various diagnostic components in the cell-block sections. This approach is especially critical for evaluation of immunostains on hypocellular cell-blocks.
These methods allow precise selection of wells with maximum cellularity for diagnostic material to be cored out with device similar to skin punch biopsy (trephine-like) device. Such cores could be submitted as FFPE material for ancillary tests such as molecular testing. Most of the cell-blocks are rich in diagnostic cells such as tumor cells without significant stroma as contaminant. This provides proportionately more diagnostic component without significant stromal contamination for various molecular pathology material in contrast to core biopsies, which usually have proportionately more stromal component.
Cell-blocking of material already processed as cytology preparations
Cell-blocking from already processed cytology preparations has been reported.[32,33,34] However, due to the exposure of the cells in the smears during cytology processing to various fixatives and reagents, the immunostaining pattern of the cells in these cell-blocks may be altered. Due of this, the immunoprofiles and results of various ancillary studies such as molecular tests may not be dependable and may not be in congruence with the published literature data usually based on FFPE tissue with which the results are compared for final interpretation.
Cell-block preparation from scraped material from cytology smears
Stained or unstained cytology preparations may be used to prepare cell-blocks by scrapping off the material on the slides.[32]
Cell-block preparation from Millipore filters[33]
Similarly, cell-block can be prepared from a portion of archived PAP-stained Millipore filter (Millipore Corp, Bedford, MA). In brief, remove Millipore filter from the surface of slide, process the filter with cytology material for tissue processing after fixation in 10% formalin to make FFPE.[33] This method may also be used for fresh specimens using new Millipore filter. However, exposure to various reagents prior to fixation in 10% formalin may compromise the immunoprofile.
Returned Cell-Block Method (Cell-block from a Papanicolaou stained morphologically targeted cells/micro-fragments on a glass slide)[34]
This is a time-consuming method, so it was recommended in selected situation. The method, in brief, involves marking of the targeted cells with water-based ink on the reverse side of the glass slide, followed by removal of the cover glass by overnight immersion in xylene with complete dissolution of the mounting medium. The cells of interest are cut with surgical knife and picked up under a microscope (with pipette or tweezers or needle). Transfer the picked up cell clusters into the metal mold and produce a paraffin block. The specimens processed by this protocol may not have immunoprofile comparable to that with FFPE tissue.
DISCUSSION
The cell-blocks are an essential ancillary component in cytopathologic interpretation of different types of cytology specimens. It provides FFPE which can be archived and available for multiple studies needed for various elective ancillary studies such as IHC and molecular studies including increasing number of prognostic biomarkers and markers related to targeted therapy for personalized medicine approaches.
Although the final yield in cell-block depends on the presence of diagnostic cellular/tissue components in the original specimen, the cell-blocking method has a significant impact on final quantitative and qualitative outcomes of the final cell-block as FFPE. Although almost any specimen with cellular/tissue component may be processed for cell-blocking, the commonly considered type of specimens for cell-blocking are FNA needle rinses, serous effusion fluids, different washings/lavages, brushings, and a variety of other specimens including endocervical curettage, urine, cerebrospinal fluid, cyst fluids, etc., Cell-blocks have also been reported to be relevant for processing gynecological LBC specimens equivalent to brush biopsy as additional material for performing different types of standardized/validated ancillary tests for improved interpretation of squamous and glandular lesions with the application of ancillary tests such as immunohistochemistry as needed.[20]
In some laboratories, cytology specimens including needle-rinses traditionally have been collected in weak alcohol fixatives such as Saccomanno Collection Fluid[54] and various LBC collection media such as Cytolyt™, PreservCyt® (ThinPrep),[55] or CytoRich Red preservative (SurePath).[56] However, this practice potentially interferes with the application of IHC, fluorescent in situ hybridization/chromogenic in situ hybridization, and other ancillary tests including other molecular tests, because the results would generally be compared with published data predominantly generated on FFPE tissue. This practice has led to frequent experience of not matching the IHC results on cell-block of FNAB and final resection. Continuing such practice would be a significant liability due to potential compromisation of results of the tests performed on the cell-blocks produced from such qualitatively compromised specimens which have created some historical challenges including disparity in the results of estrogen receptor, progesterone receptor, and Her2/Neu between results on cell-blocks and core/excision biopsy which may compromise patient care.[25a,68,69]
Although currently cell-blocks are not widely performed in some fields and on some specimens, recent advances in cell-blocking[20,35] extend the potential of wider application in these areas. Examples include veterinary specimens, research settings such as tissue cultures and animal experiments, FFPE positive controls from tissue cultures, and brush biopsies from variety of sites.
Unfixed cytology specimens allow for flexibility of practicing the best algorithm with better outcomes [Figure 2]. Collection of needle-rinses and other specimens directly in formalin will overcome some of these limitations but will not allow the making of cytology smear preparations from such formalin-fixed cytology specimens. Similarly, removal of blood contamination related interference will not be possible on these specimens collected in 10% formalin. For this reason, it is recommended to collect cytology specimens in isotonic media such as saline, RPMI,[70] or other isotonic mediums with protein milieu including proprietary Isotonic Medium S™.[35,58]
Collecting the fresh unfixed cells directly in plain saline without protein milieu may compromise the structural integrity of some of the cells with potential destruction/loss of some diagnostic cells. RPMI is expensive with frequent contaminant overgrowth. The better, simpler option is isotonic medium with protein milieu with preservative such as Isotonic Medium S™.[35] FNA needle rinses and other cytology specimens including brushings may be collected in such media to be submitted to the cytology lab as a fresh, unfixed specimen for processing. If delay in transport and processing is expected, the specimen may be transported on ice at cold temperature without letting it freeze. Such specimens may be processed to make cytology preparations and make cell-blocks that would be processed as formalin-fixed tissue similar to the surgical biopsy processed as FFPE.
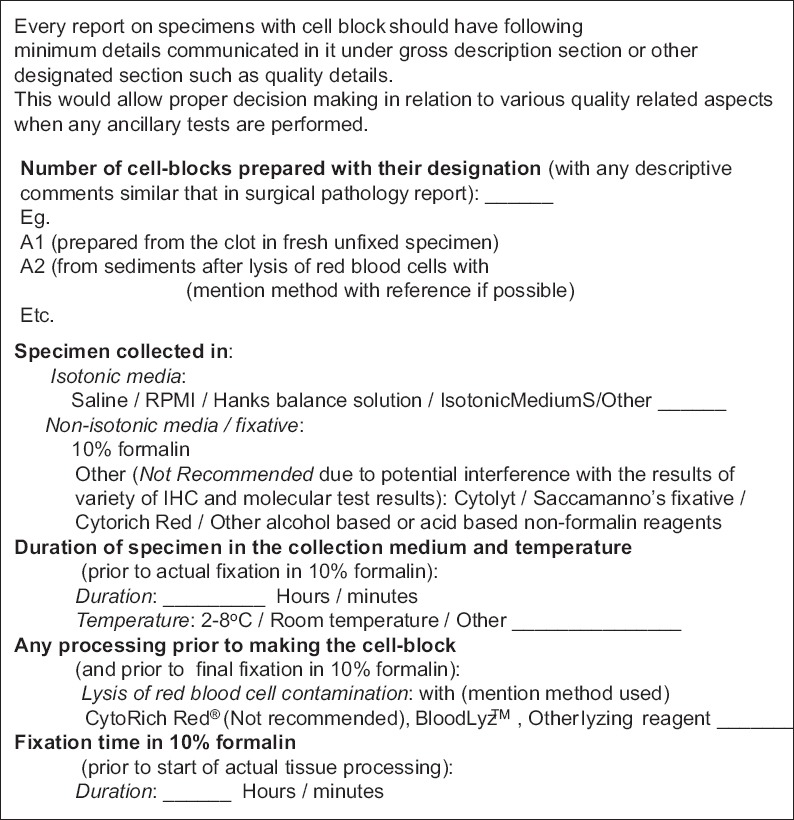
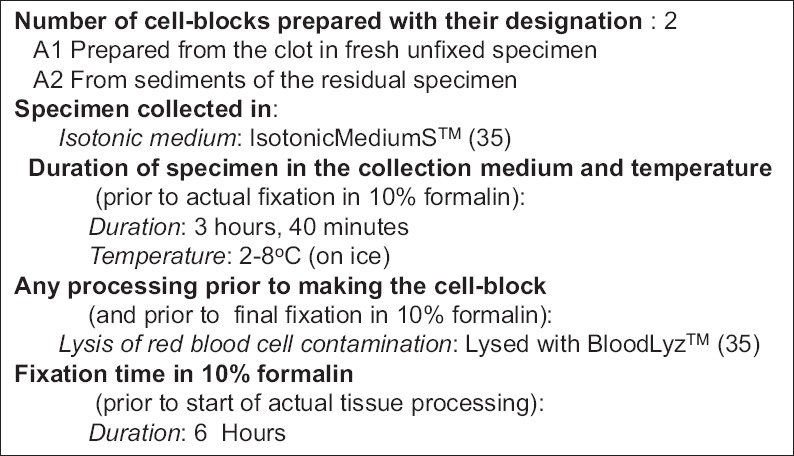
Currently, most of the cytology reports do not include quality indicators related to cell-blocks. This review recommends that if cell-blocks are prepared from any specimen, the final pathology reports should include critical information related to the cell-block. Important information suggested and listed in Figure 27 should be a part of the final report under gross description or similar section of the report. Such practice will allow proper and critical evaluation of any ancillary tests performed on such cell-blocks. As discussed previously, multiple variables could compromise the qualitative aspect of the cell-blocks in relation to currently indicated and even future indicated ancillary studies, including increasing number of molecular tests. These variables lead to frequently observed lack of consistency in results with cell-blocks from various laboratories. As Standard Optimum Cell-block Protocol (SOCP), the most appropriate approach is to follow the standards suggested in Figure 28 as an example. This would introduce reproducibility and comparability between different laboratories and ultimately matching of the results with FFPE of surgical pathology and biopsy specimens.
Figure 27.
Recommended to include details on Standardized Optimum Cell-block Protocol (SOCP) in cytology report
Figure 28.
Sample cytology report showing cell-block details
Selection of cell-blocking method depends on a variety of factors including local/institutional preferences and biases, problems related to the requirement of special machines needed in some methods (Shandon Cytoblock require Cytospin machine; Cellient™ machine with non-parallel, serial processing of one specimen at a time: each specimen about 45 minutes), cost/other resources, type of elective ancillary studies anticipated, and ultimately cellularity of the specimen with different level of Cytocrit/Tissuecrit of concentrated specimen [Figure 2]. Based on this review, most of the current methods interfere at many levels from fixation to processing (potentially compromising the commonly applied FFPE related protocols). In addition, most methods are unable to monitor the depth of cutting of the final FFPE cell-block objectively by the histotechnologist. This may lead to undercutting with lack of diagnostic material on slides or overcutting leading to loss of diagnostic material from the cell-block without diagnostic material on slides. Similarly, these methods may not allow the application of SCIP approach for orientating the serial sections identically on all the glass slides for interpretation of immunostained sections of the cell-blocks. This may compromise the final interpretation, especially for the specimens with low cellularity.
Processing of specimens with clot or collected as clot submitted directly in 10% formalin[27] is simple and straightforward. Similarly, the specimen with an abundance of sediments may also be processed by any method. However, the precautions related to interference due to fixation/processing not matching with FFPE protocol must be considered. Majority of cytology specimens require special processing for the best outcome. Many of the issues of concern are overcome at various levels with the Shidham's method reported in the Journal of Visualized Experiments.[20] However, this method is difficult to apply and include in the usual workflow of a general clinical cytology laboratory due to complexity with significant demand on skill. Recently, a commercially available alternative for the Shidham's method[20] is NextGen CelBloking™ kits. Both Nano and Micro versions of these easy- and ready-to-use relatively low-cost kits allow all the benefits of Shidham's methodology. These kits do not require any special capital investment for special machines and are simple to be used by minimally trained personnel/technologist.
SUMMARY
Almost any specimen with loosely scattered cells/tissue micro-fragments may be processed for cell-blocking. Cell-block is an excellent FFPE tissue resource required for various elective ancillary studies such as IHC and molecular tests, including prognostic biomarkers and markers related to targeted therapy.
The sediment-rich specimens may be processed by any method [Tables 1 and 3]. However, any cell-block should be prepared with precautions of preventing interference due to fixation and processing [Table 2]. The processing should simulate FFPE protocol for optimum qualitative integrity of the diagnostic cells for results to be compared with those obtained with FFPE surgical tissue. Most optimum algorithm for best outcome is with unfixed cytology specimens collected in isotonic medium with protein milieu such as Isotonic Medium S™, which allows flexibility of applying multiple enhancing alternatives including application of blood lysing step [Figure 2].
Most of the issues of concern related to qualitative and quantitative integrity of final cell-blocks are overcome by the Shidham's method.[20] However, this method as the home grown protocol is labor and skill intensive and may be difficult to practice in routine clinical cytology laboratory setting. A commercially available low cost, ready-to-use, easy alternative with many benefits including precisely set built-in AV Marker is now available as NextGen CelBloking™ kits.[35] Both Nano[36,37] and Micro[38,39] versions of the kits are simple to use without significant demand for skill. Quantitatively and qualitatively enhanced cell-blocks can be prepared easily with these kits in any standard cytology laboratory without the requirement of any special capital investment for special machines.[36,37,38,39] It is recommended to include all the critical SOCP details about the cell-blocks as quality indicators in the final pathology report [Figure 28].
COMPETING INTERESTS STATEMENT BY AUTHOR
The author's (VS) spouse has stakes in AV BioInnovation LLC product(s) cited in this review.
LIST OF ABBREVIATIONS (In alphabetic order)
CISH – Chromogenic in situ hybridization
CPT code – Current Procedural Terminology code
ER – Estrogen receptor
FFPE – Formalin-fixed paraffin-embedded
FISH – Fluorescent in situ hybridization
FNAB – Fine-needle aspiration biopsy
HG – HistoGel
IHC – Immunohistochemistry
JoVE – Journal of Visualized Experiments
LBC – Liquid based cytology
PR – Progesterone receptor
RPMI – Roswell Park Memorial Institute
RVUs – Relative value units
SCIP – Subtractive coordinate immunoreactivity pattern
SOCP – Standard Optimum Cell-block Protocol.
Glossary of terminologies:
Cell-block: Recommended to use instead of arbitrary conventional pattern as ‘cell block’ or ‘cellblock’ in an effort to separate out ‘cell block’ and ‘cellblock’ as prison related terminologies.[1,2,3]
Cell-blocking: Process of preparing cell-block
CellBlockistry: The art and chemistry of achieving capability to handle the tiny components in different types of cytology specimens
Cytocrit/Tissuecrit: Proportion of cells/micro-tissue fragments in concentrated specimen (comparable to hematocrit).[52]
Needle-rinses: Rinsing of the residual material in FNA needles after preparing direct cytology smears for cytomorphological evaluation.
ACKNOWLEDGMENTS
I thank Amy Conners, MS, MT (ASCP); Inderpreet Dhillon, MS, MT (ASCP); Yilan Li, MD, PhD (Cytopathology fellow); and Olubunmi Shoyele, MD (Cytopathology fellow) for their critical input in scientific draft editing. Their participation in improving this article is greatly appreciated.
REFERENCES
- 1.The Free Dictionary. By Farlex. [Last accessed on 2019 Jun 13]. Available from: http://www.thefreedictionary.com/cellblock .
- 2.Dictionary. [Last accessed on 2019 Jun 13]. Available from: http://com-cellblockhttp://www.dictionary.com/browse/cellblock .
- 3.Wiktionary-See also: Cell Block Cellblock. [Last accessed on 2019 Jun 13]. Available from: https://en.wiktionary.org/wiki/cellblock#English .
- 4.Saqi A. The state of cell blocks and ancillary testing: Past, present, and future. Arch Pathol Lab Med. 2016;140:1318–22. doi: 10.5858/arpa.2016-0125-RA. [DOI] [PubMed] [Google Scholar]
- 5.Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: A review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25:356–71. doi: 10.1111/cyt.12174. [DOI] [PubMed] [Google Scholar]
- 6.Nambirajan A, Jain D. Cell blocks in cytopathology: An update. Cytopathology. 2018;29:505–24. doi: 10.1111/cyt.12627. [DOI] [PubMed] [Google Scholar]
- 7.Filie AC, Copel C, Wilder AM, Abati A. Individual specimen triage of effusion samples: An improvement in the standard of practice, or a waste of resources? Diagn Cytopathol. 2000;22:7–10. doi: 10.1002/(sici)1097-0339(200001)22:1<7::aid-dc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Fetsch PA, Simsir A, Brosky K, Abati A. Comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol. 2002;26:61–6. doi: 10.1002/dc.10039. [DOI] [PubMed] [Google Scholar]
- 9.Shidham VB, Kajdacsy-Balla AA. Immunohistochemistry: Diagnostic and prognostic applications. In: Detrick B, Hamilton RG, Folds JD, editors. Manual of Molecular and Clinical Laboratory Immunology. 7th ed. Ch. 47. American Society of Microbiology Press; 2006. pp. 408–13. [Google Scholar]
- 10.Crapanzano JP, Heymann JJ, Monaco S, Nassar A, Saqi A. The state of cell block variation and satisfaction in the era of molecular diagnostics and personalized medicine. Cytojournal. 2014;11:7. doi: 10.4103/1742-6413.129187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain D, Roy-Chowdhuri S. Molecular pathology of lung cancer cytology specimens: A Concise review. Arch Pathol Lab Med. 2018;142:1127–33. doi: 10.5858/arpa.2017-0444-RA. [DOI] [PubMed] [Google Scholar]
- 12.Shidham VB, Epple J. Chapter #14 (Appendix I): Collection and processing of effusion fluids for cytopathologic evaluation. In: Shidham VB, Atkinson BF, editors. Cytopathologic Diagnosis of Serous Fluids. 1st ed. Elsevier, W. B. Saunders Company; 2007. pp. 207–35. [Google Scholar]
- 13.Shidham VB, Falzon M. Serous effusions: Reactive, benign and malignant. In: Gray W, Kocjan G, editors. Diagnostic Cytopathology. 3rd ed. Ch. 3. Churchill Livingstone; 2010. [Google Scholar]
- 14.Sidawy MK, Silverberg SG. Endosalpingiosis in female peritoneal washings: A diagnostic pitfall. Int J Gynecol Pathol. 1987;6:340–6. doi: 10.1097/00004347-198712000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Sneige N, Fernandez T, Copeland LJ, Katz RL. Müllerian inclusions in peritoneal washings. Potential source of error in cytologic diagnosis. Acta Cytol. 1986;30:271–6. [PubMed] [Google Scholar]
- 16.Sams VR, Benjamin E, Ward RH. Ectopic pancreas. A cause of false-positive peritoneal cytology. Acta Cytol. 1990;34:641–4. [PubMed] [Google Scholar]
- 17.Zuna RE. Diagnostic cytopathology of peritoneal washing. In: Shidham VB, Atkinson BF, editors. Cytopathologic Diagnosis of Serous Fluids. 1st ed. Ch. 7. Elsevier, W. B. Saunders Company; 2007. pp. 91–105. [Google Scholar]
- 18.Shidham VB, Kampalath B, England J. Routine air drying of all smears prepared during fine needle aspiration and intraoperative cytology studies. An opportunity to practice a unified protocol offering the flexibility of choosing a variety of staining methods. Acta Cytol. 2001;45:60–8. doi: 10.1159/000327188. [DOI] [PubMed] [Google Scholar]
- 19.Thermo Scientific™. Richard-Allan Scientific™ HistoGel™ Specimen Processing Gel. [Last accessed on 2019 Jun 13]. Available from: https://www.thermofisher.com/order/catalog/product/HG-4000-012 .
- 20.Varsegi GM, Shidham V. Cell block preparation from cytology specimen with predominance of individually scattered cells. J Vis Exp. 2009 doi: 10.3791/1316. pii: 1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misao Y, Taizou S, Kazuki K, Masami A. Vol. 60. Memoirs of Osaka Kyoiku University; 2011. New Cell Block Technique Using Glucomannan. Ser. 3; pp. 47–9. [Google Scholar]
- 22.Yamamoto R, Noguch S, Tatsuta M, Kasugai H, Okano Y, Wada A, et al. New cell-block method by utilizing egg albumin for fine needle aspiration biopsy. J Jpn Soc Clin Cytol. 1985;24:150–6. [Google Scholar]
- 23.Shidham VB, Atkinson BF, editors. Cytopathologic Diagnosis of Serous Fluids. 1st ed. Ch. 5. Elsevier, W. B. Saunders Company; 2007. Immunocytochemistry of effusion fluids: Introduction to the SCIP approach; pp. 55–78. [Google Scholar]
- 24.Sano J, Yoshimoto N, Mizoguchi Y, Saito M. Utility of sodium alginate cell block method. J Jpn Soc Clin Cytol. 2005;44:291–7. [Google Scholar]
- 25.Cellient Automated Cell Block System. [Last accessed on 2019 Jun 13]. Available from: https://www.hologic.com .
- 25a.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018;142:1364–82. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 26.Thermo Scientific Shandon Cytoblock Cell Block Preparation System Instructions for Use. [Last accessed on 2019 Jun 13]. Available from: https://www.thermofisher.com/order/catalog/product/7401150 .
- 27.Shidham VB, Hunt B, Jardeh SS, Barboi AC, Devata S, Hari P, et al. Performing and processing FNA of anterior fat pad for amyloid. J Vis Exp. 2010 doi: 10.3791/1747. pii: 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Girolami E. Applications of plasma-thrombin cell block in diagnostic cytology, part II: II: Digestive and respiratory tracts, breast and effusions. Pathol Annu. 1977;12(Pt 2):91–110. [PubMed] [Google Scholar]
- 29.Yang GC, Wan LS, Papellas J, Waisman J. Compact cell blocks. Use for body fluids, fine needle aspirations and endometrial brush biopsies. Acta Cytol. 1998;42:703–6. doi: 10.1159/000331830. [DOI] [PubMed] [Google Scholar]
- 30.Bussolati G. A celloidin bag for the histological preparation of cytologic material. J Clin Pathol. 1982;35:574–6. doi: 10.1136/jcp.35.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahey C, Bedrossian U. Collodion bag: A cell-block technique for enhanced cell collection. Lab Med. 1993;24:94–6. [Google Scholar]
- 32.Kulkarni MB, Prabhudesai NM, Desai SB, Borges AM. Scrape cell-block technique for fine needle aspiration cytology smears. Cytopathology. 2000;11:179–84. doi: 10.1046/j.1365-2303.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- 33.Baloch ZW, Lee A, Cobbs C, Roberts S, LiVolsi VA, Gupta PK, et al. Millipore filter cell block preparation: An alternative to cell block in nongynecologic specimens of limited cellularity. Diagn Cytopathol. 1999;20:389–92. doi: 10.1002/(sici)1097-0339(199906)20:6<389::aid-dc14>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Azami S, Aoki Y, Ogura K, Kojima K, Matsumoto T. Application of returned cell block method (Cell block from a Papanicolaou staining smear on a glass slide) for the evaluation of fine needle aspiration cytology of tumors of the breast. Diagn Cytopathol. 2016;44:505–11. doi: 10.1002/dc.23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NextGen CelBlokingTM Kits, AV BioInnovation, USA. [Last accessed on 2019 Jun 13]. Available from: http://www.AVBioInnovation.com .
- 36.Processing of Single Specimen of Any Cellularity to Make a Cell Block with Nano Unit. [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/y29SS1NwO_8 .
- 37.Simultaneous Processing of Multiple Specimens of Any Cellularity to make Cell Blocks with Nano Units. [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/ZPb0nq8MsLk .
- 38.Processing of Single Sediment Rich* Specimen at a Time to Make a Cell Block with Micro Unit. [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/i-ZpXaljiIs .
- 39.Simultaneous Processing of Multiple Concentrated Cellular Specimens (with Tissuecrit* more than 50%) for Making cell Blocks with Micro Units. [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/TRW5Vswy6J8 .
- 40.Mayall F, Darlington A. The poor man's cell block. J Clin Pathol. 2010;63:837–8. doi: 10.1136/jcp.2010.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161:1961–71. doi: 10.1016/S0002-9440(10)64472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bramwell NH, Burns BF. The effects of fixative type and fixation time on the quantity and quality of extractable DNA for hybridization studies on lymphoid tissue. Exp Hematol. 1988;16:730–2. [PubMed] [Google Scholar]
- 43.van Hemel BM, Suurmeijer AJ. Effective application of the methanol-based PreservCyt(™) fixative and the cellient(™) automated cell block processor to diagnostic cytopathology, immunocytochemistry, and molecular biology. Diagn Cytopathol. 2013;41:734–41. doi: 10.1002/dc.22963. [DOI] [PubMed] [Google Scholar]
- 44.Gorman BK, Kosarac O, Chakraborty S, Schwartz MR, Mody DR. Comparison of breast carcinoma prognostic/predictive biomarkers on cell blocks obtained by various methods: Cellient, formalin and thrombin. Acta Cytol. 2012;56:289–96. doi: 10.1159/000337436. [DOI] [PubMed] [Google Scholar]
- 45.Cattoretti G. Preparing a Cell Block from Cell Lines or Cell Suspensions. [Last accessed on 2019 Jun 13]. Available from: http://www.ihcworld.com/_protocols/histology/cell_block.htm .
- 46.Rekhtman N, Buonocore DJ, Rudomina D, Friedlander M, Dsouza C, Aggarwal G, et al. Novel modification of histoGel-based cell block preparation method: Improved sufficiency for molecular studies. Arch Pathol Lab Med. 2018;142:529–35. doi: 10.5858/arpa.2017-0030-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayall FG, Wood I. Gelatin foam cell blocks made from cytology fluid specimens. J Clin Pathol. 2011;64:818–9. doi: 10.1136/jcp.2010.088542. [DOI] [PubMed] [Google Scholar]
- 48.Mayall FG. An FNA cytology foam core device for making cell blocks. J Clin Pathol. 2012;65:959–61. doi: 10.1136/jclinpath-2012-200858. [DOI] [PubMed] [Google Scholar]
- 49.Bhatia P, Dey P, Uppal R, Shifa R, Srinivasan R, Nijhawan R, et al. Cell blocks from scraping of cytology smear: Comparison with conventional cell block. Acta Cytol. 2008;52:329–33. doi: 10.1159/000325516. [DOI] [PubMed] [Google Scholar]
- 50.Itoh O, Katoh H, Murase H, Kobayashi Y, Kondoh A, Sibata H. A study of the returned cell block method with a cell cluster picked out from a Papanicolaou smear sample. J Jpn Soc Clin Cytol. 1989;28:842–7. [Google Scholar]
- 51.Balassanian R, Wool GD, Ono JC, Olejnik-Nave J, Mah MM, Sweeney BJ, et al. Asuperior method for cell block preparation for fine-needle aspiration biopsies. Cancer Cytopathol. 2016;124:508–18. doi: 10.1002/cncy.21722. [DOI] [PubMed] [Google Scholar]
- 52.CPT® (Current Procedural Terminology) [Last accessed on 2019 Jun 13]. Available from: https://www.ama-assn.org/amaone/cpt-current-procedural-terminology .
- 53.Muirhead KA, Wallace PK, Schmitt TC, Frescatore RL, Franco JA, Horan PK, et al. Methodological considerations for implementation of lymphocyte subset analysis in a clinical reference laboratory. Ann N Y Acad Sci. 1986;468:113–27. doi: 10.1111/j.1749-6632.1986.tb42034.x. [DOI] [PubMed] [Google Scholar]
- 54.Saccomanno Fluid: Richard-Allan Scientific – Ther mo Fisher Scientific. [Last accessed on 2019 Jun 13]. Available from: https://tools.thermofisher.com .
- 55.ThinPrep Non-Gyn. [Last accessed on 2019 Jun 13]. Available from: http://www.thinprep.com/hcp/lab_professionals/thinprep_non_gyn.html .
- 56.BD CytoRich™ Non-Gyn. [Last accessed on 2019 Jun 13]. Available from: https://www.bd.com/en-us/offerings/capabilities/cervical-cancer-screening/non-gyn-cytology/cytorich-fixatives .
- 57.Hematocrit. [Last accessed on 2019 Jun 13]. Available from: https://en.wikipedia.org/wiki/Hematocrit .
- 58.BloodLyzTM. [Last accessed on 2019 Jun 13]. Available from: https://www.AVBioInnovation.com .
- 59.La Fortune KA, Randolph ML, Wu HH, Cramer HM. Improvements in cell block processing: The cell-gel method. Cancer Cytopathol. 2017;125:267–76. doi: 10.1002/cncy.21814. [DOI] [PubMed] [Google Scholar]
- 60.Rollins SD, Russell DK. Cytopathology in Focus: Cell Blocks: Getting the most from the Least Invasive Method. CAP Today. 2017. Available from: https://www.captodayonline.com/cytopathology-cell-blocks-getting-least-invasive-method/# .
- 61.Mikel UV. Advanced Laboratory Methods in Histology and Pathology. Washington, DC: Armed Forces Institute of Pathology, American Registry of Pathology; 1994. [Google Scholar]
- 62.Thermo Scientific™ Richard-Allan Scientific HistoGel™ Instructions for Use. [Last accessed on 2019 Jun 13]. Available from: https://www.thermofisher.com/order/catalog/product/HG-4000-012 .
- 63.Nano Unit Depiction of Open Arrows on Large Upper Cap and Open Reverse (anticlockwise) Arrows for Small Lower Cap. [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/1OfzS1dC28w .
- 64.How to Twist Open the Seal of Upper Large Cap of Nano Unit with Hand. [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/hlsghRI6J5I .
- 65.How to Open the Seal of Upper Large Cap of Nano Unit with Blunt Scalpel or Knife (if it could not be twist Broken with Hand) [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/RIMN8IXh4s0 .
- 66.How to retrieve ‘Tissue Paper Covers’ from its Envelop Pack. [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/oZSY0-iAy7k .
- 67.Nano & Micro Cell Block Discs After Tissue Processing Instructions to Histotechnologists. [Last accessed on 2019 Jun 13]. Available from: https://youtu.be/h2yuWg8H4pM .
- 68.Pareja F, Murray MP, Jean RD, Konno F, Friedlander M, Lin O, et al. Cytologic assessment of estrogen receptor, progesterone receptor, and HER2 status in metastatic breast carcinoma. J Am Soc Cytopathol. 2017;6:33–40. doi: 10.1016/j.jasc.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanna W, O’malley FP, Barnes P, Berendt R, Gaboury L, Magliocco A, et al. Updated recommendations from the Canadian national consensus meeting on HER2/Neu testing in breast cancer. Curr Oncol. 2007;14:149–53. doi: 10.3747/co.2007.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.RPMI 1640. [Last accessed on 2019 Jun 13]. Available from: https://en.wikipedia.org/wiki/RPMI_1640 .