Abstract
Our previous study found that plate factor-4 variant (CXCL4L1) was downregulated in the serum of patients with prostate cancer (PCa). The aim of the present study was to investigate the prognostic value of CXCL4L1 in PCa. In total, 213 PCa patients treated with radical prostatectomy were enrolled and peripheral blood samples of all patients were collected. Expression of serum CXCL4L1 in patients with different tumor stages and grades were measured by enzyme-linked immunosorbent assay (ELISA). The Kaplan–Meier method was applied to estimate the progression to castration-resistant prostate cancer (CRPC), metastasis, biochemical recurrence (BCR)-free survival, and overall survival (OS). Prognostic factors for BCR-free survival and OS were determined by univariate and multivariate analyses using the Cox proportional hazards regression model. The expression of CXCL4L1 was significantly lower in PCa patients with advanced pathological tumor stage, high-grade Gleason score, and metastasis. Moreover, downregulation of CXCL4L1 not only strongly correlated with aggressive clinicopathological features, but also predicted tumor progression and unfavorable outcomes. Finally, multivariate Cox regression analyses identified CXCL4L1 as an independent prognostic factor for both BCR-free survival (hazard ratio [HR]: 2.03, 95% confidence interval [CI]: 1.26–3.27; P = 0.004) and OS (HR: 2.26, 95% CI: 1.07–4.79; P = 0.033). In conclusion, our results indicate that CXCL4L1 might serve as a novel and promising prognostic biomarker for patients with PCa and potential therapeutic target in the future.
Keywords: CXCL4L1, prognosis, progression, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is among the most prevalent male malignancies worldwide and the second leading cause of cancer-related death for men in the United States.1 In recent years, in China, the incidence of PCa has increased dramatically.2 In 2015, more than 60 300 new PCa cases were diagnosed and 26 600 males died from PCa in China.3
Due to its extremely heterogeneous nature, PCa varies from indolent, organ-confined to aggressive, metastatic disease. While most indolent diseases have slowly progressing tumors with little or no clinical manifestation, patients with aggressive PCa may suffer from metastatic dissemination and an invariably lethal outcome.4,5 Discrimination between indolent and aggressive disease remains a major challenge for the clinical management of PCa. Currently, risk stratification for PCa is mainly based on preoperative prostate-specific antigen (PSA), biopsy Gleason score (GS), and clinical stage, which categorizes the patients into low-, intermediate-, and high-risk groups.6,7 However, none of these parameters show optimal sensitivity or specificity for detecting, monitoring, and predicting the progression of PCa. Even within a given risk group, significant clinical heterogeneity still exists.8 Therefore, there is an urgent need for novel and accurate prognostic biomarkers, which can aid clinicians in planning appropriate treatment regimens and preventing unnecessary exposure to adverse effects associated with adjuvant and/or salvage therapies.
We previously demonstrated that the level of serum plate factor-4 variant (CXCL4L1) was downregulated in PCa patients compared to benign prostatic hyperplasia (BPH) and high-grade prostate intraepithelial neoplasia (HGPIN) patients, through a comprehensive quantitative proteomic analysis.9 Given the potential importance of CXCL4L1 in the tumorigenesis of PCa, we further wondered whether CXCL4L1 expression was linked to disease progression and outcome. In the present study, we measured CXCL4L1 expression in the serum of PCa patients treated with radical prostatectomy and examined the relationship between CXCL4L1 expression and clinicopathological characteristics of PCa patients. In addition, the predictive value of CXCL4L1 for progression to castration-resistant prostate cancer (CRPC), metastasis, biochemical recurrence (BCR)-free survival, and overall survival (OS) was also investigated.
PATIENTS AND METHODS
Patients
A total of 213 PCa patients were recruited from the Urology Department of Shengjing Hospital (Shenyang, China) and enrolled in our study between December 2010 and June 2013. The inclusion criteria included: (1) elevated serum PSA (>4 ng ml−1), (2) suspicious digital rectal examination (DRE), and (3) abnormal nodules found by ultrasonography and/or magnetic resonance imaging (MRI). Patients with acute prostatitis or other malignancies were excluded from the study. All patients initially underwent transrectal ultrasound-guided prostate needle biopsy and were treated with laparoscopic radical prostatectomy 4 weeks postbiopsy. None of the patients received neoadjuvant hormonal therapy, chemotherapy, or radiotherapy prior to surgical treatment. To confirm the diagnosis of PCa, both biopsy and surgery specimens were pathologically evaluated by a genitourinary pathologist who was blinded to clinical outcomes. To assess the clinical stage, diagnostic pelvic computed tomography (CT) or MRI was performed. Tumor stage was classified according to the 8th edition of American Joint Committee on Cancer / tumor-node-metastasis (AJCC/TNM) staging system.
Medical data for each patient including age, body mass index (BMI), PSA level, prostate volume (PV), PSA density (PSAD), biopsy/pathologic GS, tumor stage, and the Cancer of the Prostate Risk Assessment (CAPRA) score were collected.10 The clinicopathological characteristics of all patients are summarized in Table 1. The study was approved by the Ethics Committee of Shengjing Hospital, China Medical University, Shenyang, China. Written informed consent was obtained from all the participants.
Table 1.
The clinicopathological characteristics of patients with prostate cancer included in the study
| Characteristics | Value |
|---|---|
| Patients (n) | 213 |
| Age (year), median (IQR) | 64.0 (57.0−72.0) |
| BMI (kg m−2), median (IQR) | 24.7 (22.6−26.5) |
| Prostate volume (ml), median (IQR) | 59.6 (52.4−70.2) |
| PSA (ng ml−1), median (IQR) | 12.3 (8.2−19.2) |
| PSAD (ng ml−1 cm−3), median (IQR) | 0.20 (0.13−0.31) |
| Biopsy Gleason score, n (%) | |
| ≤6 | 52 (24.4) |
| 7 (3+4) | 36 (16.9) |
| 7 (4+3) | 54 (25.4) |
| ≥8 | 71 (33.3) |
| Clinical T stage, n (%) | |
| T1 | 51 (23.9) |
| T2 | 138 (64.8) |
| T3 | 24 (11.3) |
| Pathological Gleason score, n (%) | |
| ≤6 | 39 (18.3) |
| 7 (3+4) | 32 (15.0) |
| 7 (4+3) | 65 (30.5) |
| ≥8 | 77 (36.2) |
| Pathological T stage, n (%) | |
| pT2 | 145 (68.1) |
| pT3 | 45 (21.1) |
| pT4 | 23 (10.8) |
PCa: prostate cancer; IQR: interquantile range; BMI: body mass index; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; T: tumor; pT: pathological tumor
Sample collection
Blood samples were collected preoperatively on the morning of prostatic surgery and centrifuged at 3000g (Beckman Coulter Life Sciences, Indianapolis, IN, USA) for 15 min at 4°C immediately after the collection (within 30 min). The serum layer was aliquoted and stored at −80°C until assay. The specimens were discarded if jaundice or hemolysis was identified.
Enzyme-linked immunoassay (ELISA) assay
Serum CXCL4L1 levels were measured using a commercially available ELISA kit (EL017810HU, Cusabio Biotech, Wuhan, China) as previously described.9 Briefly, 100 μl serum samples were added into the corresponding wells and incubated at 37°C for 2 h with gentle shaking. The waste liquid was then discarded. Biotinylated antibody (100 μl) was added into each well, and the samples were incubated at 37°C for 1 h with gentle shaking. After three washes with washing buffer, 100 μl of streptavidin-horseradish peroxidase (avidin-HRP) was added into each well. Incubation was performed at 37°C for 1 h. The washing process was repeated as described above. Color development was achieved by adding 90 μl per well of 3,3’,55’-tetramethylbenzidine (TMB) as a substrate; sulfuric acid (50 μl) was added to stop the reaction. The optical density was measured at 450 nm on the SpectraMax 190 Microplate Reader (Molecular Devices, San Jose, CA, USA). CXCL4L1 expression was calculated with a four-parameter logistic curve and fit to the standard value. All serum specimens were measured in duplicate.
Follow-up evaluation
The patients were followed-up at intervals of 3 months during first 5 years and every 6 months thereafter. The median follow-up duration was 45 (range: 6–84) months. For disease progression, BCR was defined as two sequential PSA values ≥0.2 ng ml−1 after prostatectomy.11 The development of CRPC was judged according to the European Association of Urology (EAU) guidelines.12 Metastatic disease was confirmed by sequential imaging modalities (technetium-99 bone scan, positron emission tomography-computed tomography [PET-CT], or MRI scan). Duration of the follow-up for each outcome was assessed from the date of surgery to the date of disease progression, metastases or to the date of most recent clinical contact for those without progression. For mortality, survival time was calculated from the date of surgery to the date of death or to the date of the most recent clinical contact for censored cases.
Statistical analyses
Continuous and categorical variables were expressed as median with interquantile range and frequencies with percentages, respectively. Comparisons of CXCL4L1 expression in different tumor stages and grades were analyzed with Mann–Whitney U test. Chi-square test was used to analyze the association between CXCL4L1 expression and the clinicopathological characteristics. The time to CRPC and metastasis development, BCR-free survival, and OS curve were estimated by the Kaplan–Meier method and compared by a log-rank test. Prognostic factors for BCR-free survival and OS were identified by univariate and multivariate analyses using the Cox proportional hazards model. The hazard ratio (HR) with 95% confidence intervals (CIs) was calculated and only variables with significant values (P < 0.05) were enrolled into the multivariate analyses. All statistical analyses were performed using SPSS Statistics version 20 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). All tests were two-sided and P < 0.05 was considered statistically significant.
RESULTS
Comparison of CXCL4L1 expression in PCa with different grades and stages
The expression of CXCL4L1 was successfully detected in 213 individual serum specimens (Supplementary Table 1). We first compared CXCL4L1 levels in PCa patients with different grades and stages. The median level of CXCL4L1 in patients with GS ≥8 was 8.15 ng ml−1, which was significantly lower than that in patients with GS 7 (11.89 ng ml−1) and GS ≤6 (13.35 ng ml−1) (P < 0.001, Figure 1a). In terms of pathological tumor (pT) stage, the median level of CXCL4L1 was 9.51 ng ml−1 in patients with pT3 stage and 8.03 ng ml−1 in patients with pT4 stage, and these levels were remarkably lower than that in patients with pT2 stage (12.07 ng ml−1) (both P < 0.001; Figure 1b). However, the difference in CXCL4L1 level between patients with pT3 and pT4 stage was not statistically significant (P = 0.285; Figure 1b).
Supplementary Table 1.
Expression of serum CXCL4L1 in 213 patients with prostate cancer
| Patient number | Age (year) | BMI (kg m−2) | Prostate volume (ml) | PSA (ng ml−1) | PSAD (ng ml−1 cm−3) | CXCL4L1 expression (ng ml−1) |
|---|---|---|---|---|---|---|
| 1 | 65 | 24.2 | 63.9 | 11.9 | 0.19 | 18.62 |
| 2 | 63 | 21.5 | 60.1 | 7.8 | 0.13 | 11.43 |
| 3 | 62 | 27.8 | 44.1 | 19.2 | 0.44 | 12.31 |
| 4 | 60 | 20.4 | 56.8 | 1.2 | 0.02 | 15.78 |
| 5 | 78 | 21.6 | 65.3 | 4.3 | 0.07 | 12.31 |
| 6 | 73 | 23.5 | 63.9 | 5 | 0.08 | 12.31 |
| 7 | 91 | 21.5 | 67.5 | 29.1 | 0.43 | 9.01 |
| 8 | 67 | 23.1 | 76.3 | 9.1 | 0.12 | 7.76 |
| 9 | 64 | 32.2 | 69.1 | 7.6 | 0.11 | 12.74 |
| 10 | 56 | 30.1 | 52.4 | 5.3 | 0.10 | 15.63 |
| 11 | 55 | 29.8 | 56.5 | 4.6 | 0.08 | 9.66 |
| 12 | 54 | 26.7 | 71.2 | 10.8 | 0.15 | 6.91 |
| 13 | 47 | 24.1 | 45.3 | 11.2 | 0.25 | 14.88 |
| 14 | 38 | 23.9 | 22.6 | 8.9 | 0.39 | 10.76 |
| 15 | 50 | 20.6 | 48.5 | 21.4 | 0.44 | 9.17 |
| 16 | 59 | 31.6 | 78.1 | 20.9 | 0.27 | 15.78 |
| 17 | 69 | 30.7 | 109.8 | 14.2 | 0.13 | 10.72 |
| 18 | 79 | 25.1 | 48.8 | 32.4 | 0.66 | 2.41 |
| 19 | 71 | 32.1 | 45.6 | 20.4 | 0.45 | 8.81 |
| 20 | 61 | 19.6 | 66.4 | 3.9 | 0.06 | 7.24 |
| 21 | 54 | 22.4 | 59.6 | 7.1 | 0.12 | 17.19 |
| 22 | 58 | 23.8 | 57.2 | 98.3 | 1.72 | 4.63 |
| 23 | 72 | 24.1 | 71.1 | 12.4 | 0.17 | 14.16 |
| 24 | 77 | 22.6 | 52.5 | 7.1 | 0.14 | 13.35 |
| 25 | 63 | 22.2 | 54.4 | 6.5 | 0.12 | 11.74 |
| 26 | 64 | 21.5 | 58.3 | 6.4 | 0.11 | 8.62 |
| 27 | 61 | 21.9 | 67.3 | 7.1 | 0.11 | 16.74 |
| 28 | 82 | 22.3 | 69.2 | 2.2 | 0.03 | 13.72 |
| 29 | 88 | 21.3 | 67.3 | 3.1 | 0.05 | 15.62 |
| 30 | 76 | 28.5 | 59.1 | 4.7 | 0.08 | 11.41 |
| 31 | 62 | 28.2 | 47.4 | 7.5 | 0.16 | 22.79 |
| 32 | 72 | 29.1 | 89.2 | 6.6 | 0.07 | 11.77 |
| 33 | 82 | 21.5 | 86.3 | 9.9 | 0.11 | 11.35 |
| 34 | 89 | 18.5 | 76.6 | 10.1 | 0.13 | 16.43 |
| 35 | 45 | 17.1 | 57.7 | 14.2 | 0.25 | 11.72 |
| 36 | 48 | 20.8 | 35.2 | 11.3 | 0.32 | 10.99 |
| 37 | 49 | 26.1 | 40.1 | 17.1 | 0.43 | 7.36 |
| 38 | 50 | 31.3 | 42.7 | 19.5 | 0.46 | 12.88 |
| 39 | 51 | 30.9 | 48.3 | 22.4 | 0.46 | 10.2 |
| 40 | 57 | 25.3 | 61.2 | 23.1 | 0.38 | 14.32 |
| 41 | 53 | 24.8 | 51.2 | 29.5 | 0.58 | 9.19 |
| 42 | 54 | 24.1 | 57.6 | 7.9 | 0.14 | 14.59 |
| 43 | 51 | 24.7 | 59.6 | 9.1 | 0.15 | 22.71 |
| 44 | 50 | 24.3 | 58.7 | 8.7 | 0.15 | 6.55 |
| 45 | 69 | 23.9 | 51.4 | 6.9 | 0.13 | 10.65 |
| 46 | 66 | 23.6 | 79.1 | 9.5 | 0.12 | 16.71 |
| 47 | 86 | 23.5 | 72.4 | 9.1 | 0.13 | 7.17 |
| 48 | 61 | 26.7 | 63.6 | 7.3 | 0.11 | 10.07 |
| 49 | 71 | 25.1 | 67.2 | 3.6 | 0.05 | 16.45 |
| 50 | 72 | 27.3 | 58.2 | 3.1 | 0.05 | 10.02 |
| 51 | 64 | 28.1 | 56.1 | 2.7 | 0.05 | 11.64 |
| 52 | 69 | 26.2 | 70.2 | 11.4 | 0.16 | 16.73 |
| 53 | 60 | 26.6 | 50.1 | 12.5 | 0.25 | 9.33 |
| 54 | 50 | 26.3 | 49.6 | 17.4 | 0.35 | 10.08 |
| 55 | 49 | 26.3 | 45.8 | 11.3 | 0.25 | 9.29 |
| 56 | 90 | 25.8 | 79.3 | 15.6 | 0.20 | 12.47 |
| 57 | 88 | 25.1 | 74.1 | 16.1 | 0.22 | 6.46 |
| 58 | 82 | 24.7 | 75.2 | 20.6 | 0.27 | 9.93 |
| 59 | 68 | 23.3 | 53.3 | 56.3 | 1.06 | 8.03 |
| 60 | 69 | 25.1 | 82.5 | 71.2 | 0.86 | 8.82 |
| 61 | 71 | 25.7 | 69.3 | 44.7 | 0.65 | 12.03 |
| 62 | 53 | 26.7 | 56.1 | 45.2 | 0.81 | 9.06 |
| 63 | 54 | 25.9 | 56.5 | 14.1 | 0.25 | 1.79 |
| 64 | 57 | 26.1 | 61.1 | 11.4 | 0.19 | 7.73 |
| 65 | 59 | 27.1 | 61.2 | 15.9 | 0.26 | 17.14 |
| 66 | 60 | 27.3 | 53.3 | 16.2 | 0.30 | 9.18 |
| 67 | 60 | 28.6 | 56.2 | 9.5 | 0.17 | 13.32 |
| 68 | 61 | 30.2 | 43.8 | 9.3 | 0.21 | 28.72 |
| 69 | 62 | 20.9 | 45.1 | 8.1 | 0.18 | 10.05 |
| 70 | 66 | 31.2 | 70.1 | 8.8 | 0.13 | 10.28 |
| 71 | 68 | 21.8 | 60.9 | 7.1 | 0.12 | 9.45 |
| 72 | 63 | 21.9 | 61.3 | 7.9 | 0.13 | 14.79 |
| 73 | 75 | 27.5 | 62.1 | 8 | 0.13 | 7.48 |
| 74 | 73 | 26.5 | 72.5 | 11.1 | 0.15 | 12.04 |
| 75 | 78 | 26.7 | 63.3 | 10.3 | 0.16 | 5.32 |
| 76 | 66 | 23.8 | 64.1 | 10.6 | 0.17 | 12.57 |
| 77 | 71 | 24.1 | 58.6 | 11.7 | 0.20 | 13.2 |
| 78 | 69 | 24.2 | 58.2 | 22.5 | 0.39 | 13.69 |
| 79 | 60 | 24.5 | 57.4 | 21.6 | 0.38 | 20.67 |
| 80 | 70 | 24.8 | 64.1 | 26.4 | 0.41 | 12.59 |
| 81 | 80 | 25.3 | 54.5 | 33.3 | 0.61 | 7.03 |
| 82 | 69 | 25.9 | 59.2 | 34.6 | 0.58 | 13.44 |
| 83 | 53 | 23.7 | 43.2 | 23.1 | 0.53 | 14.33 |
| 84 | 40 | 23.4 | 41.1 | 4.5 | 0.11 | 9.26 |
| 85 | 45 | 25.9 | 56.3 | 6.7 | 0.12 | 9.96 |
| 86 | 42 | 26.1 | 50.4 | 7.3 | 0.14 | 12.46 |
| 87 | 48 | 24.2 | 59.6 | 7.2 | 0.12 | 15.15 |
| 88 | 49 | 24.7 | 64.3 | 9.3 | 0.14 | 18.83 |
| 89 | 51 | 19.5 | 63.2 | 9.2 | 0.15 | 8.15 |
| 90 | 55 | 18.3 | 78.2 | 10.5 | 0.13 | 8.29 |
| 91 | 56 | 18.6 | 46.1 | 11.4 | 0.25 | 11.62 |
| 92 | 54 | 18.2 | 57.3 | 12.3 | 0.21 | 10.92 |
| 93 | 62 | 17.6 | 66.1 | 12.9 | 0.20 | 8.14 |
| 94 | 63 | 19.1 | 66.3 | 11.6 | 0.17 | 8.46 |
| 95 | 61 | 19.5 | 50.7 | 10.1 | 0.20 | 11.53 |
| 96 | 67 | 20.5 | 50.2 | 9.7 | 0.19 | 13.37 |
| 97 | 66 | 20.8 | 71.2 | 12.5 | 0.18 | 13.42 |
| 98 | 68 | 21.6 | 73.6 | 76.3 | 1.04 | 5.66 |
| 99 | 69 | 22.4 | 50.9 | 11.9 | 0.23 | 16.71 |
| 100 | 60 | 22.3 | 71.2 | 12.7 | 0.18 | 10.36 |
| 101 | 71 | 22.5 | 75.9 | 17.1 | 0.23 | 11.04 |
| 102 | 74 | 21.4 | 81.6 | 13.2 | 0.16 | 10.82 |
| 103 | 78 | 21.6 | 39.9 | 14.6 | 0.37 | 3.61 |
| 104 | 72 | 24.9 | 61.5 | 16.2 | 0.26 | 9.64 |
| 105 | 77 | 25.2 | 52.7 | 22.8 | 0.43 | 12.78 |
| 106 | 70 | 26.2 | 77.6 | 23.1 | 0.30 | 8.78 |
| 107 | 79 | 24.7 | 63.7 | 56.3 | 0.88 | 11.36 |
| 108 | 53 | 24.6 | 54.7 | 4.8 | 0.09 | 12.27 |
| 109 | 63 | 30.1 | 45.1 | 7.6 | 0.17 | 26.71 |
| 110 | 73 | 29.6 | 51.1 | 40.9 | 0.80 | 11.93 |
| 111 | 84 | 18.3 | 64.3 | 10.3 | 0.16 | 12.09 |
| 112 | 80 | 19.6 | 59.3 | 11.4 | 0.19 | 7.75 |
| 113 | 62 | 21.4 | 60.6 | 7.8 | 0.13 | 11.54 |
| 114 | 63 | 24.2 | 71.3 | 9.3 | 0.13 | 11.89 |
| 115 | 64 | 24.1 | 59.4 | 81.3 | 1.37 | 10.61 |
| 116 | 67 | 24.8 | 58.1 | 9.1 | 0.16 | 11.37 |
| 117 | 65 | 25.6 | 57.3 | 8.2 | 0.14 | 12.78 |
| 118 | 65 | 27.2 | 53.9 | 8.4 | 0.16 | 13.33 |
| 119 | 69 | 20.1 | 64.2 | 23.5 | 0.37 | 6.71 |
| 120 | 71 | 22.5 | 72.4 | 9.5 | 0.13 | 5.17 |
| 121 | 47 | 22.9 | 73.7 | 9.3 | 0.13 | 15.43 |
| 122 | 49 | 22.6 | 49.8 | 8.1 | 0.16 | 9.81 |
| 123 | 55 | 23.1 | 44.6 | 8.8 | 0.20 | 10.74 |
| 124 | 51 | 23.8 | 43.2 | 7.1 | 0.16 | 8.13 |
| 125 | 52 | 19.8 | 34.7 | 7.9 | 0.23 | 7.47 |
| 126 | 54 | 28.4 | 28.9 | 28 | 0.97 | 14.28 |
| 127 | 64 | 27.4 | 40.3 | 11.1 | 0.28 | 9.19 |
| 128 | 74 | 26.9 | 33.4 | 10.3 | 0.31 | 9.13 |
| 129 | 73 | 26.3 | 48.6 | 10.6 | 0.22 | 9.17 |
| 130 | 83 | 27.1 | 51.2 | 11.7 | 0.23 | 6.63 |
| 131 | 82 | 27.4 | 77.5 | 22.5 | 0.29 | 12.5 |
| 132 | 60 | 19.6 | 68.9 | 21.6 | 0.31 | 17.71 |
| 133 | 59 | 24.9 | 100.4 | 26.4 | 0.26 | 13.03 |
| 134 | 52 | 21.7 | 77.4 | 33.3 | 0.43 | 18.56 |
| 135 | 57 | 26.5 | 76.1 | 34.6 | 0.45 | 17.82 |
| 136 | 66 | 26.7 | 93.1 | 23.1 | 0.25 | 16.63 |
| 137 | 68 | 23.8 | 98.5 | 4.5 | 0.05 | 14.29 |
| 138 | 69 | 24.1 | 105.1 | 6.7 | 0.06 | 7.99 |
| 139 | 70 | 24.2 | 62.4 | 7.3 | 0.12 | 8.38 |
| 140 | 71 | 24.5 | 78.9 | 17.2 | 0.22 | 8.84 |
| 141 | 72 | 24.8 | 91.9 | 9.3 | 0.10 | 9.88 |
| 142 | 74 | 25.3 | 57.4 | 13.2 | 0.23 | 6.96 |
| 143 | 49 | 25.9 | 59.4 | 4.2 | 0.07 | 7.37 |
| 144 | 51 | 23.7 | 41.1 | 5.1 | 0.12 | 6.74 |
| 145 | 55 | 23.4 | 56.3 | 5.3 | 0.09 | 12.59 |
| 146 | 56 | 25.9 | 50.4 | 6.4 | 0.13 | 13.38 |
| 147 | 54 | 26.1 | 59.6 | 6.5 | 0.11 | 10.04 |
| 148 | 62 | 24.2 | 54.3 | 7.9 | 0.15 | 6.43 |
| 149 | 63 | 24.7 | 53.2 | 12.3 | 0.23 | 13.06 |
| 150 | 61 | 19.5 | 78.2 | 13.2 | 0.17 | 15.51 |
| 151 | 67 | 18.3 | 56.1 | 15.4 | 0.27 | 12.12 |
| 152 | 66 | 25.6 | 57.3 | 32.7 | 0.57 | 9.46 |
| 153 | 68 | 25.3 | 66.1 | 65.8 | 1.00 | 12.59 |
| 154 | 69 | 26.1 | 66.3 | 46.1 | 0.70 | 11.75 |
| 155 | 60 | 25.7 | 50.7 | 9.5 | 0.19 | 11.66 |
| 156 | 71 | 25.5 | 50.2 | 9.3 | 0.19 | 8.24 |
| 157 | 74 | 26.5 | 71.2 | 28.1 | 0.39 | 9.62 |
| 158 | 78 | 25.5 | 73.6 | 8.8 | 0.12 | 13.32 |
| 159 | 72 | 24.9 | 80.9 | 7.1 | 0.09 | 7.48 |
| 160 | 77 | 23.8 | 71.2 | 7.9 | 0.11 | 9.53 |
| 161 | 70 | 26.1 | 75.9 | 8 | 0.11 | 10.37 |
| 162 | 79 | 28.3 | 61.6 | 11.1 | 0.18 | 11.71 |
| 163 | 53 | 24.7 | 49.9 | 10.3 | 0.21 | 7.24 |
| 164 | 63 | 19.1 | 91.5 | 10.6 | 0.12 | 14.2 |
| 165 | 73 | 20.3 | 42.7 | 11.7 | 0.27 | 10.74 |
| 166 | 84 | 27.4 | 77.6 | 22.5 | 0.29 | 6.71 |
| 167 | 80 | 28.1 | 63.7 | 21.6 | 0.34 | 9.51 |
| 168 | 62 | 25.5 | 54.7 | 26.4 | 0.48 | 12.07 |
| 169 | 63 | 26.6 | 65.1 | 13.3 | 0.20 | 4.36 |
| 170 | 49 | 24.4 | 64.1 | 34.6 | 0.54 | 5.26 |
| 171 | 51 | 26.5 | 59.7 | 23.1 | 0.39 | 13.49 |
| 172 | 55 | 26.7 | 56.1 | 4.5 | 0.08 | 12.24 |
| 173 | 56 | 23.8 | 57.2 | 16.7 | 0.29 | 16.67 |
| 174 | 54 | 24.1 | 45.5 | 7.3 | 0.16 | 13.11 |
| 175 | 62 | 24.2 | 67.5 | 7.2 | 0.11 | 12.25 |
| 176 | 63 | 24.5 | 59.2 | 9.3 | 0.16 | 13.36 |
| 177 | 61 | 24.8 | 46.6 | 14.3 | 0.31 | 4.31 |
| 178 | 67 | 25.3 | 58.9 | 12.5 | 0.21 | 14.27 |
| 179 | 66 | 25.9 | 67.4 | 13.5 | 0.20 | 12.28 |
| 180 | 68 | 23.7 | 77.2 | 12.9 | 0.17 | 10.67 |
| 181 | 69 | 23.4 | 44.1 | 19.4 | 0.44 | 20.49 |
| 182 | 60 | 25.9 | 39.9 | 18.4 | 0.46 | 15.78 |
| 183 | 71 | 26.1 | 42.4 | 16.4 | 0.39 | 11.59 |
| 184 | 74 | 24.2 | 79.5 | 14.3 | 0.18 | 7.31 |
| 185 | 78 | 24.7 | 41.1 | 12.9 | 0.31 | 8.55 |
| 186 | 72 | 19.5 | 56.3 | 11.9 | 0.21 | 12.51 |
| 187 | 77 | 18.3 | 50.4 | 14.8 | 0.29 | 6.22 |
| 188 | 70 | 19.6 | 59.6 | 14.6 | 0.24 | 4.28 |
| 189 | 79 | 22.6 | 64.3 | 19.7 | 0.31 | 14.87 |
| 190 | 53 | 24.7 | 63.2 | 14.2 | 0.22 | 10.06 |
| 191 | 63 | 23.8 | 78.2 | 22.6 | 0.29 | 11.63 |
| 192 | 73 | 23.9 | 46.1 | 25.1 | 0.54 | 8.61 |
| 193 | 84 | 23.6 | 57.3 | 12.9 | 0.23 | 12.01 |
| 194 | 80 | 22.2 | 66.1 | 18.9 | 0.29 | 5.11 |
| 195 | 62 | 25.3 | 66.3 | 16.4 | 0.25 | 9.18 |
| 196 | 63 | 25.1 | 50.7 | 27.6 | 0.54 | 7.14 |
| 197 | 56 | 26.1 | 50.2 | 28.1 | 0.56 | 15.32 |
| 198 | 58 | 27.2 | 71.2 | 28.5 | 0.40 | 10.06 |
| 199 | 61 | 23.3 | 73.6 | 19.5 | 0.26 | 11.67 |
| 200 | 59 | 28.8 | 80.9 | 13.8 | 0.17 | 6.26 |
| 201 | 60 | 28.1 | 71.2 | 12.5 | 0.18 | 15.44 |
| 202 | 79 | 27.1 | 75.9 | 14.6 | 0.19 | 8.92 |
| 203 | 53 | 26.9 | 81.6 | 13.6 | 0.17 | 7.99 |
| 204 | 69 | 26.5 | 59.9 | 13.2 | 0.22 | 6.01 |
| 205 | 63 | 24.6 | 81.5 | 14.8 | 0.18 | 5.37 |
| 206 | 74 | 26.6 | 52.7 | 14.7 | 0.28 | 6.11 |
| 207 | 88 | 26.7 | 77.6 | 13.2 | 0.17 | 8.81 |
| 208 | 77 | 28.5 | 63.7 | 18.6 | 0.29 | 8.49 |
| 209 | 61 | 29.1 | 54.7 | 14.7 | 0.27 | 6.53 |
| 210 | 67 | 30.7 | 65.1 | 22.6 | 0.35 | 9.01 |
| 211 | 66 | 31.1 | 59.8 | 16.8 | 0.28 | 11.07 |
| 212 | 65 | 21.2 | 57.2 | 14.6 | 0.26 | 10.62 |
| 213 | 67 | 23.6 | 64.1 | 12.5 | 0.20 | 8.75 |
Figure 1.
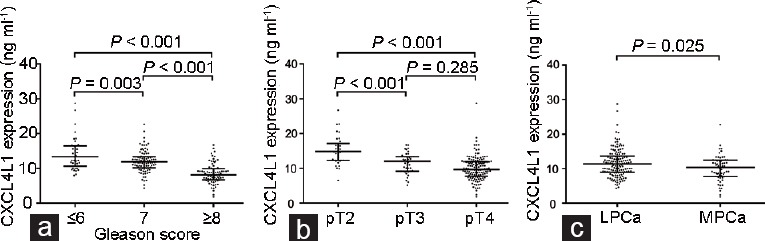
Comparisons of CXCL4L1 expression in prostate cancer patients according to different categories. The expression of CXCL4L1 in prostate cancer patients with (a) different grades of Gleason score, (b) different pathological tumor stages, and (c) localized and metastatic prostate cancer. LPCa: localized prostate cancer; MPCa: metastatic prostate cancer; CXCL4L1: plate factor-4 variant; pT: pathological tumor.
We also compared patients with localized disease and those who developed metastatic disease. During the follow-up period, a total of 63 patients developed metastatic disease, including 23 patients with bone metastases and 40 patients with pelvic lymph node metastases. Our results showed that the level of CXCL4L1 was significantly decreased in patients who subsequently developed metastases compared with patients with localized PCa (10.37 ng ml−1 vs 11.42 ng ml−1, P = 0.025; Figure 1c).
Association between CXCL4L1 expression and clinicopathological characteristics of PCa patients
We next analyzed the association between CXCL4L1 expression and clinicopathological characteristics of PCa patients (Table 2). The median level of CXCL4L1 (10.92 ng ml−1) was used as the cutoff value to classify the 213 patients into a low-expression group and a high-expression group. Our data confirmed that there was a significant correlation between low expression of CXCL4L1 and aggressive clinicopathological characteristics, including positive surgical margin (P = 0.023), tumor multifocality (P = 0.001), high GS (P < 0.001), and advanced pT stage (P = 0.002). Notably, a strong correlation was also found between CXCL4L1 expression and CAPRA score (P = 0.013). However, there were no significant differences between the low- and high-expression groups with respect to other variables, including age, BMI, preoperative PSA, and PSAD.
Table 2.
The association between CXCL4L1 expression and clinicopathological characteristics of prostate cancer
| Clinicopathological characteristics | CXCL4L1 expression | P | |
|---|---|---|---|
| Low (n=106) | High (n=107) | ||
| Age (year), median (IQR) | 67.0 (55.5–73.0) | 63.0 (58.0–70.5) | 0.332a |
| BMI (kg m−2), median (IQR) | 24.8 (23.3–26.6) | 24.5 (22.5–26.1) | 0.415a |
| PSA (ng ml−1), median (IQR) | 13.2 (8.9–18.3) | 11.3 (7.8–19.5) | 0.159a |
| PSAD (ng ml−1 cm−3), median (IQR) | 0.22 (0.15–0.31) | 0.19 (0.13–0.29) | 0.143a |
| Surgical margin, n (%) | |||
| Negative | 73 (68.9) | 88 (82.2) | 0.023b |
| Positive | 33 (31.1) | 19 (17.8) | |
| Tumor multifocality, n (%) | |||
| Unifocal | 55 (51.9) | 79 (73.8) | 0.001b |
| Multifocal | 51 (48.1) | 28 (26.2) | |
| Pathological Gleason score, n (%) | |||
| ≤6 | 10 (9.4) | 29 (27.1) | <0.001b |
| 7 (3+4) | 15 (14.2) | 17 (15.9) | |
| 7 (4+3) | 18 (17.0) | 47 (43.9) | |
| ≥8 | 63 (59.4) | 14 (13.1) | |
| Pathological T stage, n (%) | |||
| pT2 | 60 (56.6) | 85 (79.4) | 0.002b |
| pT3 | 30 (28.3) | 15 (14.1) | |
| pT4 | 16 (15.1) | 7 (6.5) | |
| CAPRA score, n (%) | |||
| ≤2 | 9 (8.5) | 20 (18.7) | 0.013b |
| 3 | 10 (9.4) | 21 (19.6) | |
| 4 | 17 (16.1) | 19 (17.8) | |
| 5 | 19 (17.9) | 12 (11.2) | |
| ≥6 | 51 (48.1) | 35 (32.7) | |
P values were obtained from the aMann–Whitney U test or bChi-square test. BMI: body mass index; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; T: tumor; pT: pathological tumor; CAPRA: Cancer of the Prostate Risk Assessment; IQR: interquantile range; CXCL4L1: plate factor-4 variant
Downregulation of CXCL4L1 correlates with poor prognosis in PCa patients
We assessed the prognostic value of CXCL4L1 expression in PCa patients. A cumulative incidence curve confirmed that PCa patients with low CXCL4L1 expression exhibited a shorter time to CRPC development compared with patients with high CXCL4L1 expression (P < 0.001; Figure 2a). A similar result was observed with regard to metastases progression (P = 0.022; Figure 2b). Overall, 36.8% of men in the CXCL4L1 low-expression cohort developed CRPC and 33.9% developed metastases, while only 15.0% and 25.2% of PCa patients in the CXCL4L1 high-expression group experienced CRPC and metastases progression, respectively.
Figure 2.
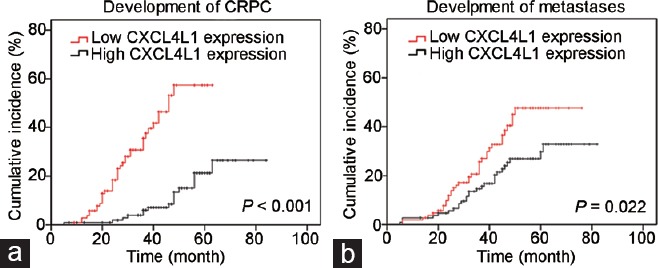
Risk of prostate cancer progression stratified by the level of CXCL4L1 expression. (a) Lower expression of CXCL4L1 was significantly associated with more rapid development of CRPC (P < 0.001). (b) Lower expression of CXCL4L1 strongly correlated with rapid progression to metastases (P = 0.002). CRPC: castration-resistant prostate cancer; CXCL4L1: plate factor-4 variant.
Kaplan–Meier analysis showed that low expression of CXCL41 was significantly associated with a shorter BCR-free survival (P < 0.001; Figure 3a). The 3- and 5-year BCR-free survival rates in patients with low expression of CXCL4L1 were 53.9% and 45.2%, respectively. In contrast, the 3- and 5-year BCR-free survival rates in patients with high expression of CXCL4L1 were 81.0% and 63.3%, respectively. The patients with low CXCL41 expression also exhibited an unfavorable OS than those with high CXCL4L1 expression (P < 0.001, Figure 3b). The 3- and 5-year OS rates were 84.2% and 54.1% for patients with low CXCL41 expression, whereas 96.7% and 85.4% for patients with high CXCL4L1 expression, respectively.
Figure 3.
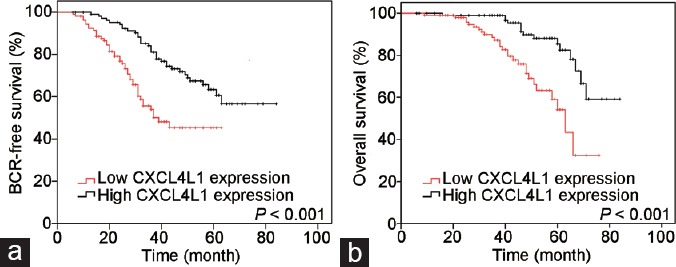
Kaplan–Meier survival analyses stratified by the level of CXCL4L1 expression. (a) Patients with low CXCL4L1 expression had significantly worse BCR-free survival compared with high CXCL4L1 expression cohorts (P < 0.001). (b) Patients with low CXCL4L1 expression were more likely to experience a shorter overall survival (P < 0.001). BCR: biochemical recurrence; CXCL4L1: plate factor-4 variant.
Finally, univariate and multivariate Cox proportional hazard analyses were performed to identify prognostic factors for PCa (Table 3). In the univariate analyses, preoperative PSA (P = 0.003), PSAD (P = 0.005), CAPRA score (P < 0.001), pT stage (P < 0.001), and CXCL4L1 expression (P < 0.001) were significantly associated with BCR-free survival of PCa patients. In terms of OS, several prognostic factors, including CAPRA score (P = 0.038), pathological GS (P < 0.001), and CXCL4L1 expression (P < 0.001), were significant predictors in PCa patients. Only the significant variables from univariate analysis were enrolled in the multivariate analysis. Among these significant prognostic factors, multivariate analysis demonstrated that pathological tumor stage (HR: 1.73; 95% CI: 1.08–2.76; P = 0.021) and CXCL4L1 expression (HR: 2.03; 95% CI: 1.26-3.27; P = 0.004) were independent prognostic factors for BCR-free survival in PCa patients, while pathological GS (HR: 2.56; 95% CI: 1.19-5.52; P = 0.016) and CXCL4L1 expression (HR: 2.13; 95% CI: 1.01-4.52; P = 0.033) were finally identified as independent prognostic factors for OS in PCa patients.
Table 3.
Univariate and multivariate Cox proportional hazard analysis for biochemical recurrence-free survival and overall survival
| Variables | BCR-free survival (univariate) | BCR-free survival (multivariate) | OS (univariate) | OS (multivariate) | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age (continuous) | 1.01 (0.99−1.03) | 0.255 | NA | NA | 1.02 (0.99−1.05) | 0.141 | NA | NA |
| BMI (continuous) | 0.98 (0.92−1.06) | 0.728 | NA | NA | 1.04 (0.95−1.14) | 0.357 | NA | NA |
| PSA (continuous) | 1.02 (1.01−1.04) | 0.003 | 1.01 (0.96−1.05) | 0.745 | 1.02 (0.98−1.02) | 0.870 | NA | NA |
| PSAD (continuous) | 3.41 (1.44−8.11) | 0.005 | 0.94 (0.08−11.67) | 0.960 | 1.66 (0.56−4.92) | 0.358 | NA | NA |
| CAPRA (continuous) | 1.22 (1.09−1.36) | <0.001 | 2.03 (1.26−3.27) | 0.058 | 1.15 (1.01−1.31) | 0.038 | 0.99 (0.86−1.16) | 0.963 |
| pGS (>7 vs ≤7) | 1.39 (0.87−2.21) | 0.161 | NA | NA | 3.71 (1.99−6.93) | <0.001 | 2.56 (1.19−5.52) | 0.016 |
| pT stage (pT2 vs pT3/pT4) | 2.25 (1.44−3.52) | <0.001 | 1.73 (1.08−2.76) | 0.021 | 1.75 (0.95−3.21) | 0.072 | NA | NA |
| CXCL4L1 expression (low vs high) | 2.50 (1.57−3.98) | <0.001 | 2.03 (1.26−3.27) | 0.004 | 3.47 (1.81−6.62) | <0.001 | 2.13 (1.01−4.52) | 0.033 |
BCR: biochemical recurrence; OS: overall survival; HR: hazard ratio; CI: confidence intervals; BMI: body mass index; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; CAPRA: Cancer of the Prostate Risk Assessment; pGS: pathological Gleason score; pT: pathological tumor; NA: not analyzed
DISCUSSION
The lack of accurate prognostic biomarkers is one of the major unmet needs in the management of men with PCa. An increasing number of researchers have attempted to clarify the prognostic factors for PCa, such as PSA derivatives (PSA velocity, PSAD), prostate volume, serum testosterone, and new simplified PCa grading system with five grades.13,14,15,16 Nevertheless, these parameters are not perfect and only achieve modest efficacy.
Recent improvements in proteomics research have led to the discovery of an abundance of new biomarkers that may be utilized in the prediction of patient outcome. Many studies have reported the successful identification of prognostic biomarkers using proteomic analysis in various types of cancers.17,18,19 Using a high-throughput quantitative proteomic analysis, we previously found that the expression of serum CXCL4L1 was decreased in patients with PCa.9 In the present study, we further investigated the role of CXCL4L1 in predicting the risk of PCa progression.
CXC chemokines have been implicated in various biological processes, including angiogenesis, anti-angiogenesis, and tumorigenesis.20 As a nonallelic gene variant of CXCL4, CXCL4L1 is one of the most important members of the angiostatic CXC chemokine family.21 CXCL4L1 has been shown to be more potent than CXCL4 at inhibiting cell proliferation and migration.22 However, the expression of CXCL4L1 in cancer still remains a controversial issue. In esophageal squamous carcinoma, CXCL4L1 exhibits a weak to negative staining in tumor cells.23 Furuya and colleagues found that CXCL4L1 expression was markedly lower in endometriosis-associated ovarian cancer compared with normal endometrium and endometriosis lesion.24 In contrast, Quemener and colleagues reported that increased CXCL4L1 expression was significantly associated with pancreatic adenocarcinoma using a tissue microarray analysis.25
The predictive value of CXCL4L1 in PCa progression had not been previously investigated. To the best of our knowledge, this is the first study on the correlation between CXCL4L1 and prognosis of PCa. Our results demonstrate that the level of serum CXCL4L1 is remarkably decreased in PCa patients with advanced pT stage and high GS. Moreover, decreased levels of CXCL4L1 were significantly correlated with an increased risk for CRPC progression, metastases, and adverse survival, suggesting that CXCL4L1 might play an important role in PCa progression. Angiogenesis is a requisite for tumor growth and progression.26 As a potent inhibitor of angiogenesis, downregulation of CXCL4L1 probably alters the delicate angiostatic balance toward angiogenesis, which subsequently leads to tumor recurrence and progression. This may partially explain why CXCL4L1 expression is downregulated in PCa. However, the exact mechanisms responsible for this observation remain unclear and warrant further investigation.
Notably, downregulation of CXCL4L1 was significantly associated with high CAPRA score, which usually predicts unfavorable clinical outcomes for PCa patients. The CAPRA score system, first established by the University of California, San Francisco (UCSF) in 2005, has been recognized as a powerful predictive tool and validated in multiple surgical cohorts.11,27 Krishnan et al.28 demonstrated the predictive ability of CAPRA score for BCR progression in PCa patients treated with external beam radiotherapy and/or brachytherapy. In accordance with previous findings, our data indicates that CAPRA score could act as a significant predictor for both BCR-free survival and OS in the univariate Cox analyses, whereas it did not achieve statistical differences in the multivariate analyses. Future studies should incorporate CXCL4L1 level and CAPRA score into current categories and examine the potential predictive value for the novel combination.
More interestingly, CXCL4L1 has recently shown potential utility in cancer treatment. Vandercappellen et al.29 showed that intratumoral injection of CXCL4L1 resulted in a significant inhibition of tumor growth and metastasis in different animal models, including B16 melanoma, A549 adenocarcinoma, and Lewis lung carcinoma. In our ongoing experiment, we found that treatment with CXCL4L1 can significantly reduce the subcutaneous and orthotopic prostate tumor size in a mouse model (data not shown), indicating that CXCL4L1 is of interest not only for the prediction of PCa prognosis, but also as a therapeutic target for drug development.
Several limitations of our study should be acknowledged.First, although all patients in our study underwent metastatic evaluation, including bone, CT, and/or MRI scans, there was no standardized protocol for monitoring patients during the follow-up period. Second, as a potential prognostic biomarker, the standardized protocol of CXCL4L1 testing at specific intervals requires further investigation. Third, all these patients were recruited from the same institute, and there were relatively limited participants included in our study. Most cases were from the past 5 years, which was not sufficient for long term follow-up. Further validation of CXCL4L1 in a large, independent, and multicenter cohort with long-term follow-up is still warranted. In addition, evaluation of the expression and validity of CXCL4L1 in a multiethnic patient group in future study is required.
CONCLUSION
Our study demonstrated that decreased CXCL4L1 expression was associated with aggressive clinicopathological features in PCa patients. Downregulation of CXCL4L1 could serve as an independent prognostic factor for unfavorable BCR-free survival and OS in PCa patients. Further studies to elucidate the molecular mechanism of CXCL4L1 in PCa will improve our understanding of disease progression and may yield a new therapeutic target.
AUTHOR CONTRIBUTIONS
MZ was responsible for the design of the study, carried out the ELISA assay, and participated in the data analysis and manuscript writing. JG contributed to the manuscript revision and participated in the data analysis. YLH reviewed the pathologic slides and evaluated the Gleason score. YSS contributed to the data collection, manuscript composition, and the funding application. LZC involved in the design of the study, the funding application, the supervision, and management of the project. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81600258 and 81802540), the Natural Science Foundation of Liaoning Province (No. 20180550985), and the Shenyang Science and Technology Program (No. F15-199-1-47).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Wang FB, Chen R, Ren SC, Shi XL, Zhu YS, et al. Prostate cancer antigen 3 moderately improves diagnostic accuracy in Chinese patients undergoing first prostate biopsy. Asian J Androl. 2017;19:238–43. doi: 10.4103/1008-682X.167715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 7.Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, et al. Prostate cancer, version 2.2014. J Natl Cancer Netw. 2014;12:686–718. doi: 10.6004/jnccn.2014.0072. [DOI] [PubMed] [Google Scholar]
- 8.Zumsteg ZS, Spratt DE, Pei I, Zhang Z, Yamada Y, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Chen L, Yuan Z, Yang Z, Li Y, et al. Combined serum and EPS-urine proteomic analysis using iTRAQ technology for discovery of potential prostate cancer biomarkers. Discov Med. 2016;22:281–95. [PubMed] [Google Scholar]
- 10.Cooperberg MR, Freedland SJ, Pasta DJ, Elkin EP, Presti JC, et al. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006;107:2384–91. doi: 10.1002/cncr.22262. [DOI] [PubMed] [Google Scholar]
- 11.Leyh-Bannurah SR, Dell’Oglio P, Zaffuto E, Briganti A, Schiffmann J, et al. Assessment of oncological outcomes after radical prostatectomy according to preoperative and postoperative cancer of the prostate risk assessment scores: results from a large, two-center experience. Eur Urol Focus. 2017 doi: 10.1016/j.euf.2017.10.015. Doi: 10.1016/j. euf.2017.10.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Fleshner NE, Lawrentschuk N. Risk of developing prostate cancer in the future: overview of prognostic biomarkers. Urology. 2009;73:S21–7. doi: 10.1016/j.urology.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Kang HW, Jung HD, Lee JY, Kwon JK, Jeh SU, et al. Prostate-specific antigen density predicts favorable pathology and biochemical recurrence in patients with intermediate-risk prostate cancer. Asian J Androl. 2016;18:480–4. doi: 10.4103/1008-682X.154313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen P, Zhao J, Sun G, Chen N, Zhang X, et al. The roles of prostate-specific antigen (PSA) density, prostate volume, and their zone-adjusted derivatives in predicting prostate cancer in patients with PSA less than 20.0 ng/ml. Andrology. 2017;5:548–55. doi: 10.1111/andr.12322. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, et al. A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69:428–35. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng L, Zhong J, He G, Li F, Li J, et al. Identification of nucleobindin-2 as a potential biomarker for breast cancer metastasis using iTRAQ-based quantitative proteomic analysis. J Cancer. 2017;8:3062–9. doi: 10.7150/jca.19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papale M, Vocino G, Lucarelli G, Rutigliano M, Gigante M, et al. Urinary RKIP/p-RKIP is a potential diagnostic and prognostic marker of clear cell renal cell carcinoma. Oncotarget. 2017;8:40412–24. doi: 10.18632/oncotarget.16341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohri Y, Toiyama Y, Kusunoki M. Progress and prospects for the discovery of biomarkers for gastric cancer: a focus on proteomics. Expert Rev Proteomics. 2016;13:1131–9. doi: 10.1080/14789450.2016.1249469. [DOI] [PubMed] [Google Scholar]
- 20.Du Q, Li E, Liu Y, Xie W, Huang C, et al. CTAPIII/CXCL7: a novel biomarker for early diagnosis of lung cancer. Cancer Med. 2018;7:325–35. doi: 10.1002/cam4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Struyf S, Salogni L, Burdick MD, Vandercappellen J, Gouwy M, et al. Angiostatic and chemotactic activities of the CXC chemokine CXCL4L1 (platelet factor-4 variant) are mediated by CXCR3. Blood. 2011;117:480–8. doi: 10.1182/blood-2009-11-253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Struyf S, Burdick MD, Proost P, Van Damme J, Strieter RM. Platelets release CXCL4L1, a nonallelic variant of the chemokine platelet factor-4/CXCL4 and potent inhibitor of angiogenesis. Circ Res. 2004;95:855–7. doi: 10.1161/01.RES.0000146674.38319.07. [DOI] [PubMed] [Google Scholar]
- 23.Verbeke H, De Hertogh G, Li S, Vandercappellen J, Noppen S, et al. Expression of angiostatic platelet factor-4var/CXCL4L1 counterbalances angiogenic impulses of vascular endothelial growth factor, interleukin-8/CXCL8, and stromal cell-derived factor 1/CXCL12 in esophageal and colorectal cancer. Hum Pathol. 2010;41:990–1001. doi: 10.1016/j.humpath.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Furuya M, Tanaka R, Miyagi E, Kami D, Nagahama K, et al. Impaired CXCL4 expression in tumor-associated macrophages (TAMs) of ovarian cancers arising in endometriosis. Cancer Biol Ther. 2012;13:671–80. doi: 10.4161/cbt.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quemener C, Baud J, Boye K, Dubrac A, Billottet C, et al. Dual roles for CXCL4 chemokines and CXCR3 in angiogenesis and invasion of pancreatic cancer. Cancer Res. 2016;76:6507–19. doi: 10.1158/0008-5472.CAN-15-2864. [DOI] [PubMed] [Google Scholar]
- 26.Van Raemdonck K, Berghmans N, Vanheule V, Bugatti A, Proost P, et al. Angiostatic, tumor inflammatory and anti-tumor effects of CXCL4(47-70) and CXCL4L1(47-70) in an EGF-dependent breast cancer model. Oncotarget. 2014;5:10916–33. doi: 10.18632/oncotarget.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brajtbord JS, Leapman MS, Cooperberg MR. The CAPRA score at 10 years: contemporary perspectives and analysis of supporting studies. Eur Urol. 2017;71:705–9. doi: 10.1016/j.eururo.2016.08.065. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan V, Delouya G, Bahary JP, Larrivee S, Taussky D. The cancer of the prostate risk assessment (CAPRA) score predicts biochemical recurrence in intermediate-risk prostate cancer treated with external beam radiotherapy (EBRT) dose escalation or low-dose rate (LDR) brachytherapy. BJU Int. 2014;114:865–71. doi: 10.1111/bju.12587. [DOI] [PubMed] [Google Scholar]
- 29.Vandercappellen J, Van Damme J, Struyf S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev. 2011;22:1–18. doi: 10.1016/j.cytogfr.2010.10.011. [DOI] [PubMed] [Google Scholar]


