Abstract
Vitamin D deficiency is a common health issue around the world. We therefore evaluated the associations of semen quality with both serum and seminal plasma vitamin D levels and studied the mechanisms underlying these by incubating spermatozoa with 1,25(OH)2D in vitro. Two hundred and twenty-two men were included in our study. Vitamin D was detected using an electrochemiluminescence method. Spermatozoa used for in vitro experiments were isolated by density gradient centrifugation. Positive relationships of serum 25(OH)D with semen volume and seminal plasma fructose were identified. Seminal plasma 25(OH)D level showed no relationship with serum 25(OH)D level, while it was inversely associated with sperm concentration and positively correlated with semen volume and sperm kinetic values. In vitro, sperm kinetic parameters increased after incubation with 1,25(OH)2D, especially upon incubation for 30 min with it at a concentration of 0.1 nmol l−1. Under these incubation conditions, the upward migration of spermatozoa increased remarkably with increasing adenosine triphosphate (ATP) concentration. The concentration of cyclic adenosine monophosphate (cAMP) and the activity of protein kinase A (PKA) were both elevated, and the PKA inhibitor, N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89) reversed the increase of ATP production. The concentrations of cytoplasmic calcium ions and nicotinamide adenine dinucleotide (NADH) were both enhanced, while mitochondrial calcium uniporter (MCU) inhibitor, Ruthenium 360 (Ru360) did not reverse the increase of ATP production. Therefore, seminal plasma vitamin D may be involved in regulating sperm motility, and 1,25(OH)2D may enhance sperm motility by promoting the synthesis of ATP both through the cAMP/PKA pathway and the increase in intracellular calcium ions.
Keywords: adenosine triphosphate, seminal plasma, sperm motility, vitamin D
INTRODUCTION
Vitamin D refers to a group of fat-soluble steroids that plays a pronounced role in regulating the homeostasis of calcium and phosphorus in the body.1 In humans, the most important compounds in this group of steroids are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol).2,3,4 Vitamin D2 is obtained from ultraviolet (UV) irradiation of the yeast sterol ergosterol and is found naturally in sun-exposed mushrooms.5 Vitamin D3 is synthesized in the skin and is present in oil-rich fish such as salmon, mackerel, and herring.6 Both vitamin D2 and vitamin D3 undergo their first hydroxylation in the liver to yield 25(OH)D, which is the major form of vitamin D in circulation and is the best indicator for assessing vitamin D status owing to its circulating half-life of 2–3 weeks.7,8,9,10,11 25(OH)D is transported to the kidney where, through another hydroxylation process, it becomes the biologically active form 1,25(OH)2D, which has a half-life of approximately 4 h and is tightly regulated by serum levels of parathyroid hormone (PTH), calcium, and phosphate.5,12
Vitamin D deficiency is regarded as a widespread health problem, constituting a major burden in both developing and developed countries.13,14 Deficiency in vitamin D has been linked to a series of adverse reactions including hypertension, cardiovascular disease, stroke, diabetes, and cancer.15 In recent decades, research on vitamin D has extended the spectrum of its effects to the male reproductive system.13 Dating back to the last century, the first study suggesting a role of vitamin D in reproductive function was conducted in rodents. Male rats were divided into a vitamin D-sufficient group and a vitamin D-deficient group to observe the results of mating with female rats. The results indicated that the vitamin D-deficient group had lower mating rates, fertility rates, and birth rates.16 In subsequent studies, improvements of semen parameters were identified in vitamin D-deficient rats after being fed vitamin D.17 These were the earliest reports on the relationship between vitamin D and male reproduction, which provided a new direction for the subsequent study of human reproduction. In a cross-sectional study of 300 young healthy Danish men, a positive correlation between serum vitamin D and sperm motility was shown.18 Similarly, two other studies of fertile and infertile men showed that men with vitamin D sufficiency had more motile spermatozoa than those with vitamin D deficiency.19,20 Another study of 104 patients also found a positive correlation between 25(OH)D and both total and progressive sperm motility.21 However, some other studies generated conflicting results. For example, Abbasihormozi et al.13 reported that serum vitamin D levels had no relationship with semen parameter values and reproductive hormones in a fertile population, while in patients with oligoasthenozoospermia or teratozoospermia, a positive correlation between vitamin D and sperm viability was found. Hammoud et al.22 found that excessive or insufficient levels of serum vitamin D were associated with poor semen parameters such as sperm concentration and viability. Further research highlighted the expression of vitamin D receptors in human Leydig cells, seminiferous tubules, and the epididymis and the expression of vitamin D mRNA in the head and neck of spermatozoa,23,24,25,26 suggesting that this vitamin is related to the occurrence and maturation of spermatozoa. With regard to in vitro effects, increasing evidence suggests that vitamin D has a positive impact on human spermatozoa.18,19 For example, studies have shown that vitamin D regulates human sperm cholesterol outflows, affects sperm protein serine and threonine phosphorylation, and thus increases the survival ability of spermatozoa.27 Additionally, other studies have also indicated that vitamin D enhances sperm vitality and the acrosome reaction and reduces the content of triglyceride in spermatozoa, which effectively increases their fertilization ability in the female reproductive tract.28 Nevertheless, more studies are still needed to clarify the mechanism underlying the effects of vitamin D on spermatozoa.
Spermatozoa are produced through spermatogenesis inside the testicles via meiotic division, and then mature in the epididymis and through the male reproductive tract. High adenosine triphosphate (ATP) content is crucial for mammalian spermatozoa to generate sufficient force to overcome physical barriers in the female tract, reach the oocyte, and penetrate its vestments. Mitochondria are the main site for the synthesis of ATP. As the location of energy production, the functional status of sperm mitochondria is thus closely related to sperm motility.29 Cyclic adenosine monophosphate (cAMP) is an important cellular messenger and is also known to activate a variety of downstream factors to regulate mitochondrial function. Among these factors, protein kinase A (PKA) is the most intensely studied cAMP-dependent protein kinase.30 Another molecule that plays an important role in the mitochondrial respiratory chain is nicotinamide adenine dinucleotide (NADH), which is a coenzyme present in all living cells. Four important mitochondrial dehydrogenases that can be activated by low concentrations of calcium ions have been reported to be involved in the direct supply of NADH. Among them, flavin adenine dinucleotide (FAD)-glycerol phosphate dehydrogenase is located on the outer surface of the inner mitochondrial membrane, and its activity is closely related to the cytoplasmic concentration of calcium ions. The other three enzymes are located within mitochondria and are mainly influenced by intramitochondrial calcium ions.31
Given the conflicting findings regarding the correlation between the level of serum vitamin D and sperm quality, we analyzed the associations between the level of seminal plasma vitamin D and sperm parameters. The positive relationship observed in clinical analysis prompted us to conduct in vitro experiments to further explore the mechanism underlying the effect of vitamin D on spermatozoa.
PARTICIPANTS AND METHODS
Study population
This study was approved by the Ethics Committee of Nanjing Jinling Hospital, Nanjing, China, and was performed in accordance with the national and international guidelines. A total of 222 men, referred from June 2017 to January 2018 to the Center for Reproductive Medicine, Jinling Hospital (Nanjing, China) for andrological evaluation owing to male infertility or normal physical examination, were included in our study. Informed consent to be included in this study was obtained at the clinical visit. The weight and height of all men were measured, and clinical information was requested, including age, medications, comorbidities, smoking, and abuse or use of anabolic steroids. All men were scheduled to deliver two semen samples and a blood sample. Enrollment criteria were as follows: (1) diagnosed with male infertility or oligoasthenozoospermia in accordance with the fifth edition of the World Health Organization (WHO 2010) laboratory manual for the examination and processing of human semen;32 (2) normal in semen routine examinations and volunteered to provide semen and serum samples; (3) 20–40 years old; (4) abstinence time in the range of 2–7 days; and (5) body mass index (BMI) no more than 30 kg m−2. Exclusion criteria were as follows: (1) cryptorchidism, testicular torsion, testicular cancer, or other surgical history; (2) the use of hormonal supplements in the last 3 months to treat genitourinary tract infections or other diseases; (3) a history of severe varicocele on both sides; (4) leukocyte content of >1 × 106 ml−1 or with Ureaplasma urealyticum, Chlamydia trachomatis, or Mycoplasma hominis in semen; (5) use of drugs and tobacco, or alcohol addiction; (6) severe oligoasthenozoospermia (sperm concentration <5 × 106 ml−1 or forwardly motile spermatozoa <1%) or severe teratozoospermia (morphological examination showing that the percentage of spermatozoa with normal morphology was <4%); (7) pituitary gonadal hormone secretion test showing abnormal results; (8) genetic examination revealing Y-chromosome microdeletions, including microdeletions in azoospermia factor a (AZFa), AZFb, or AZFc segments; and (9) long-term exposure to reproductive toxicity.
Biochemical analyses
Blood sampling was performed exclusively between 8 a.m. and 10 a.m. from fasting patients. Serum and semen levels of vitamin D, anti-Müllerian hormone (AMH), and inhibin B (INHB) were measured by using electrochemiluminescence immunoassay (ECLIA) kits (Roche Diagnostics GmbH, Mannheim, Germany) on an Elecsys 2010 immunoassay analyzer (Roche Diagnostics GmbH). Intra- and inter-assay coefficient of variation (CV) for vitamin D, AMH, and INHB were 7.80% and 10.70%, 5.78% and 9.73%, and 7.90% and 9.73%, respectively. Seminal plasma fructose was detected by the resorcinol method.33,34 Detection of α-glucosidase was performed in accordance with the procedure of the WHO (2010).32
Semen analyses
Semen samples were collected by masturbation. Self-reported information on the duration of ejaculation abstinence, fever, and spillage was obtained and semen analysis was conducted. Briefly, computer-assisted semen analysis (CASA, WLJY-9000, Weili New Century Technology Development Co., Ltd., Beijing, China) was used to assess sperm concentration and sperm motility.35 A Macro sperm counting chamber (Nanjing Yuancheng Company, Nanjing, China), a reusable chamber with 10-μm depth for sperm counting, was used to load semen samples. Sperm concentration was assessed by counting spermatozoa with tails; at least 200 spermatozoa were counted in each of the duplicate assessments. Nonprogressive motility (NP, all other patterns of motility with an absence of progression), progressive motility (PR, spermatozoa moving actively, either linearly or in a large circle, regardless of speed), motility (PR + NP), average path velocity (VAP, velocity over a calculated smoothed path), curvilinear velocity (VCL, velocity over the actual sperm track, including all deviations of sperm head movement), and straight-line velocity (VSL, velocity over the straight line distance between the beginning and end of the sperm track) were calculated for each group from the three recordings of at least 100 spermatozoa. Semen volume was estimated by weighing. The sperm morphology including morphologically normal rate (%) and intact acrosomes (%) was carefully evaluated following the criteria of the WHO (2010) using modified Papanicolaou staining.32
Statistical analyses
All data analyses were conducted using SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). For descriptive statistics, mean ± standard deviation (s.d.) and median (5th and 95th percentiles) were reported when appropriate.First, nonparametric tests (one-sample Kolmogorov–Smirnov test) were used to determine whether the analyzed parameters were normally distributed. If the parameter had a normal distribution, correlations were examined by Pearson's test, whereas if it had a skewed distribution, correlations were examined by Spearman's rho test. The definition of the seasons was done a priori and was based on four seasons per year: spring (March to May), summer (June to August), fall (September to November), and winter (December to February). Comparison of 25(OH)D levels among the different seasons was performed by the Kruskal–Wallis analysis of variance (ANOVA) test with Dunn's post hoc test. The differences among different groups and between two groups were assessed by one-way ANOVA with the Holm–Sidak post hoc test and paired samples t-test, respectively. For all tests, a bilateral test probability of P < 0.05 was considered to represent a statistically significant difference.
Semen collection, sperm processing, and in vitro experimental treatments
Human semen was collected in accordance with the procedure recommended by the WHO (2010) by masturbation from healthy volunteer donors of proven fertility undergoing semen analysis at our laboratory.32 An independent observer who examined several fields for each slide inspected the cells. Sperm samples with normal values for semen volume, sperm count, motility, vitality, and morphology, in accordance with the criteria of WHO (2010), were included in this study.32 The sperm samples used for each experiment were obtained by pooling the ejaculates of three normozoospermic healthy donors. After liquefaction, normal semen samples were pooled and subjected to centrifugation on a discontinuous density gradient (90%–45%) by using SpermGrad (Vitrolife, Gothenburg, Sweden). Purified spermatozoa were washed with sperm nutrition solution (Spermrinse, Vitrolife) and incubated in the same medium for 30 min at 37°C and 5% CO2, without (control) or with increasing concentrations (0.01, 0.1, 1, and 10 nmol l−1) of 1,25(OH)2D (MSDS 740578, Sigma, St. Louis, MO, USA). After incubation, the samples were centrifuged at 500 g for 6 min, and the sperm pellet was resuspended in sperm nutrition solution by gentle pipetting for subsequent experiments.
Assessment of sperm movement
The prepared reagent with no or different concentrations (0.01, 0.1, 1, and 10 nmol l−1) of 1,25(OH)2D was added to each 500-μl EP tube (Axygen, Corning, NY, USA) containing spermatozoa from the pellet, making a sperm concentration of 1 × 107 ml−1 in each tube. Spermatozoa were incubated in a 37°C water bath for 15, 30, 60, and 90 min separately. A Weili color sperm quality testing system (Weili New Century Technology Development Co., Ltd.) was used to determine sperm kinetic parameters at each time point and the results were recorded.
Upward sperm migration test
The prepared reagent with 0 or 0.1 nmol l−1 1,25(OH)2D was added to each 500-μl EP tube as described above. Spermatozoa were incubated in a 37°C water bath for 30 min in the dark; capillaries full of sperm nutrition solution were vertically inserted into the test tubes. The top of the capillary pipettes and the opening of the test tubes were sealed up with film, followed by incubation for 1 h at 37°C. In accordance with the criteria of WHO (2010), the distance moved by spermatozoa was measured under an optical microscope (CX31, Olympus, Tokyo, Japan).32
Detection of sperm ATP concentration
ATP concentration was assessed with an ATP Assay Kit (S0026, Beyotime, Nanjing, China). Briefly, 100 μl of ATP test solution was added to all wells and incubated for 5 min at room temperature for the depletion of background ATP. Then, 100 μl of sample or standard solution was added to the wells and quickly mixed with a pipette. The relative light unit (RLU) was detected with an automatic microplate reader (Synergy HTX Multi-Mode Microplate Reader, BioTek, Winooski, VT, USA). The ATP concentration of each sample was calculated from the standard curve.
Determination of sperm cAMP concentration
cAMP concentration was assessed with a Human cAMP Elisa Kit (Biocalvin, Suzhou, China). Briefly, cell lysates were added to the reaction buffer containing 100 μl of horseradish peroxidase (HRP)-conjugated reagent, and the wells were sealed and placed in a 37°C water bath for 60 min. Then, the wells were washed, and solutions A and B were added and incubated for another 15 min. The reaction was stopped by adding the termination buffer. The absorbance (A) values were detected with an automatic microplate reader. The cAMP concentration of each sample was calculated from the standard curve.
Protein kinase A catalytic activity assay
Fluorescence quantification of the PKA activity was performed by using quantification kits (Jiemei, Shanghai, China). Briefly, cell lysates were added to the reaction buffer containing a bisamide rhodamine 110 peptide substrate and were incubated for 30 min at 25°C. The reaction was stopped by adding the termination buffer, which contained a protease that removes amino acids specifically from the nonphosphorylated substrate, which results in the production of highly fluorescent rhodamine 110. Thus, the fluorescence intensity is inversely correlated with the kinase activity. PKA activity was determined from the fluorescence intensity of the nonphosphorylated substrate in an automatic microplate reader.
Intracellular calcium ion assay
The level of intracellular calcium ions of spermatozoa was determined by flow cytometry (FCM). The acetoxymethyl ester form of fluo-4 (fluo-4/AM, Keygen, Nanjing, China) was used as a fluorescent calcium ion indicator. Spermatozoa were incubated for 15 min at room temperature with 5 μmol l−1 fluo-4/AM and 0.001% Pluronic F-127 (Keygen) in a sperm nutrition solution. The cells were then washed and incubated with the same solution for 30 min. Then, flow cytometry was used to measure and capture the intensity of images of calcium-containing sperm cells.
Detection of sperm NADH concentration
The detection of the NADH concentration was performed with a Human NADH Elisa Kit (Biocalvin). Briefly, cell lysates were added to the reaction buffer containing 100 μl of HRP-conjugated reagent, and the wells were sealed and placed in a 37°C water bath for 60 min. Then, each well was washed, and chromogen solutions A and B were added and incubated for another 15 min. The reaction was stopped by adding termination buffer. The absorbance (A) value was determined with an automatic microplate reader. The NADH concentration of each sample was calculated from the standard curve.
RESULTS
Clinical observations
A total of 222 men without serious comorbidities participated in this study. These men were examined from June 2017 to January 2018. The median age of our study population was 30 (5th and 95th percentiles: 27–32) years, and the mean BMI was 22.25 (s.d.: 2.89) kg m−2. Median serum 25(OH)D level was 26.17 (5th and 95th percentiles: 19.61–31.99) ng ml−1 and it did not differ among the seasons. Median seminal plasma 25(OH)D level was 6.55 (5th and 95th percentiles: 4.30–9.62) ng ml−1 in summer (percentage of participants: 33.6%), 5.83 (5th and 95th percentiles: 3.90–8.46) ng ml−1 in fall (58.5%), and 4.98 (5th and 95th percentiles: 3.66–8.55) ng ml−1 in winter (7.9%) (P < 0.05). Supplementary Table 1 shows the samples’ basic characteristics.
Supplementary Table 1.
Characteristics of the men included (n=222)
| Variables | Number of men (n) | Median (25–75 quartile) or mean±s.d. |
|---|---|---|
| Semen volume (ml)# | 222 | 3.80 (3.10–4.80) |
| Sperm concentration (106 ml−1)# | 222 | 42.85 (21.75–62.23) |
| Sperm motility (%) | 222 | 35.27±13.60 |
| Progressive motile spermatozoa (%) | 222 | 29.97±11.31 |
| VSL (µm s−1) | 222 | 23.71±3.66 |
| VCL (µm s−1) | 222 | 31.83±4.54 |
| VAP (µm s−1) | 222 | 25.61±3.69 |
| Duration of abstinence (day)# | 222 | 4 (3–5) |
| Morphologically normal (%)# | 185 | 5.61 (4.69–6.16) |
| Intact acrosome rate (%)# | 186 | 59.82 (57.33–62.74) |
| Serum 25(OH) D (ng ml−1)# | 160 | 26.17 (19.61–31.99) |
| Seminal plasma 25(OH) D (ng ml−1)# | 192 | 6.55 (4.30–9.62) |
| Serum AMH (ng ml−1)# | 157 | 8.94 (6–14.4) |
| Seminal plasma AMH (ng ml−1)# | 92 | 0.35 (0.15–1.04) |
| Serum INHB (pg ml−1)# | 96 | 94.80 (73.50–122.50) |
| Seminal plasma INHB (pg ml−1)# | 94 | 97.45 (55.58–177.50) |
| Seminal plasma fructose (mmol l−1) | 166 | 18.39±7.70 |
| Seminal plasma α-glucosidase (U l−1) | 167 | 347.45±151.98 |
All the men lacked serious comorbidities. Data are reported as mean and s.d. for variables normally distributed or as median and interquartile range for variables not normally distributed. #Indicates that the normality test revealed a lack of conformity to a normal distribution. VSL: straight-line velocity; VCL: curvilinear velocity; VAP: average path velocity; AMH: anti-Müllerian hormone; INHB: inhibin B; s.d.: standard deviation
The associations between serum and seminal plasma 25(OH)D and semen parameter values are shown in Table 1. The Spearman's rank correlation analysis showed positive relationships of serum 25(OH)D level with semen volume (P < 0.01) and seminal plasma fructose content (P < 0.01). The Spearman's rank correlation analysis of seminal plasma 25(OH)D and sperm quality showed that seminal plasma 25(OH)D had no relationship with serum 25(OH)D (P = 0.372), while it was inversely associated with sperm concentration (P < 0.05) and positively correlated with semen volume (P < 0.01) and sperm kinetic values (VCL, VSL, and VAP) (P < 0.01). Scatterplots indicating the dispersion of data by showing the 95% confidence limits are shown in Figure 1.
Table 1.
The correlations between the level of serum or seminal plasma 25-hydroxyvitamin D and sperm parameter values (n=222)
| Variables | Serum 25(OH)D levela | Seminal plasma 25(OH)D levela | ||
|---|---|---|---|---|
| Correlation | P | Correlation | P | |
| Age (year)a | −0.034 | 0.688 | −0.037 | 0.640 |
| BMI (kg m−2) | 0.134 | 0.092 | 0.101 | 0.163 |
| Duration of abstinence (day)a | 0.066 | 0.407 | −0.028 | 0.699 |
| Semen volume (ml)a | 0.229 | 0.004** | 0.271 | 0** |
| Sperm concentration (×106 ml−1)a | −0.020 | 0.806 | −0.174 | 0.016* |
| Sperm motility (%) | −0.015 | 0.853 | 0.124 | 0.087 |
| Progressive motile spermatozoa (%) | −0.025 | 0.757 | 0.104 | 0.150 |
| VSL (µm s−1) | −0.039 | 0.624 | 0.218 | 0.002** |
| VCL (µm s−1) | −0.059 | 0.457 | 0.208 | 0.004** |
| VAP (µm s−1) | −0.060 | 0.454 | 0.222 | 0.002** |
| Morphologically normal (%)a | 0.039 | 0.633 | 0.020 | 0.799 |
| Intact acrosomes (%)a | 0.001 | 0.992 | −0.010 | 0.896 |
| Serum AMH (ng ml−1)a | 0.102 | 0.204 | 0.045 | 0.617 |
| Seminal plasma AMH (ng ml−1)a | 0.146 | 0.169 | −0.088 | 0.429 |
| Serum INHB (pg ml−1)a | 0.171 | 0.099 | 0.073 | 0.500 |
| Seminal plasma INHB (pg ml−1)a | 0.081 | 0.443 | 0.171 | 0.116 |
| Seminal plasma fructose (mmol l−1) | 0.257 | 0.002** | −0.004 | 0.963 |
| Seminal plasma a-glucosidase (U l−1) | −0.132 | 0.116 | −0.108 | 0.193 |
| Seminal plasma 25(OH)D (ng ml-1)a | 0.079 | 0.372 | - | - |
aIndicates that the normality test revealed a lack of conformity to a normal distribution. *P<0.05 and **P<0.01 for bilateral test. VSL: straight-line velocity; VCL: curvilinear velocity; VAP: average path velocity; AMH: anti-Müllerian hormone; INHB: inhibin B; 25(OH)D: 25-hydroxyvitamin D; BMI: body mass index
Figure 1.
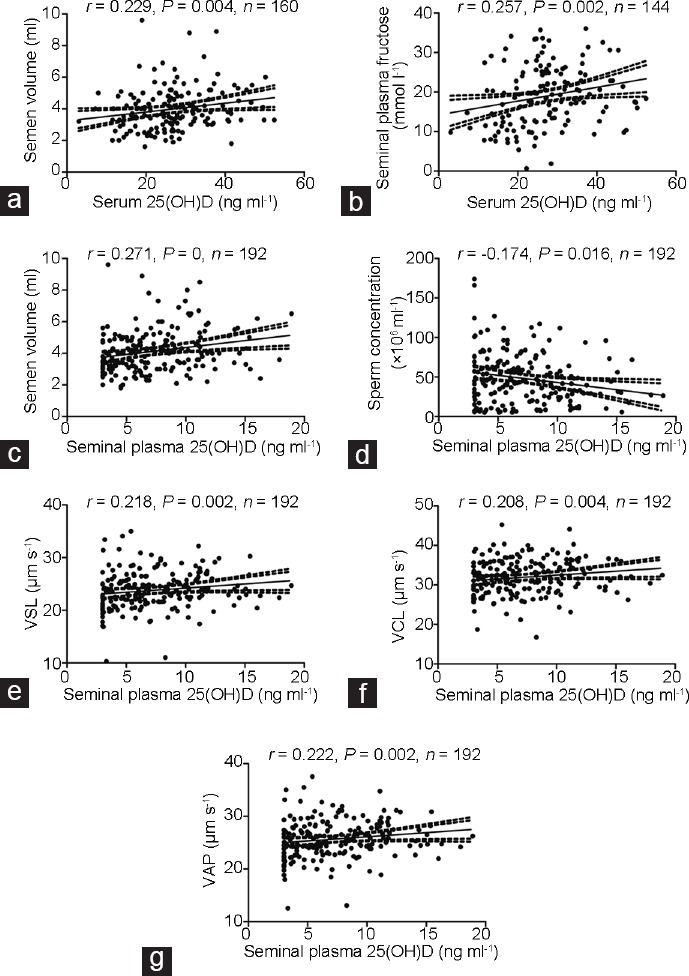
Correlation scatterplot between serum 25(OH)D and (a) semen volume and (b) seminal plasma fructose. Correlation scatterplot between seminal plasma 25(OH)D and (c) semen volume, (d) sperm concentration, (e) VSL, (f) VCL, and (g) VAP. The 95th and 99th percentiles are shown as dotted lines on either side of the solid correlation line. VSL: straight-line velocity; VCL: curvilinear velocity; VAP: average path velocity.
1,25(OH)2D and the promotion of sperm kinetic parameters in vitro
Purified spermatozoa were incubated with different concentrations (0.01, 0.1, 1, and 10 nmol l−1) of 1,25(OH)2D in a 37°C water bath. VCL, VSL, and VAP were measured at different time points (15, 30, 60, and 90 min). The results showed that, after incubating spermatozoa with 1,25(OH)2D for 30 min, there was a maximum rate of increase in each parameter. As time passed, this effect gradually weakened (Figure 2a–2c). Using 30 min as the incubation time, we compared the changes of sperm parameters among different concentrations of 1,25(OH)2D. The results showed that VSL, VCL, and VAP increased most dramatically at a concentration of 0.1 nmol l−1 (P < 0.05). Concentrations of 0.01 nmol l−1 and 1 nmol l−1 also had positive effects on sperm kinetic parameters, but they were not as pronounced as at 0.1 nmol l−1 (P < 0.05). When the concentration of 1,25(OH)2D was 10 nmol l−1, there was no obvious increase in sperm kinetic parameters (all P > 0.05) (Figure 2d–2f).
Figure 2.
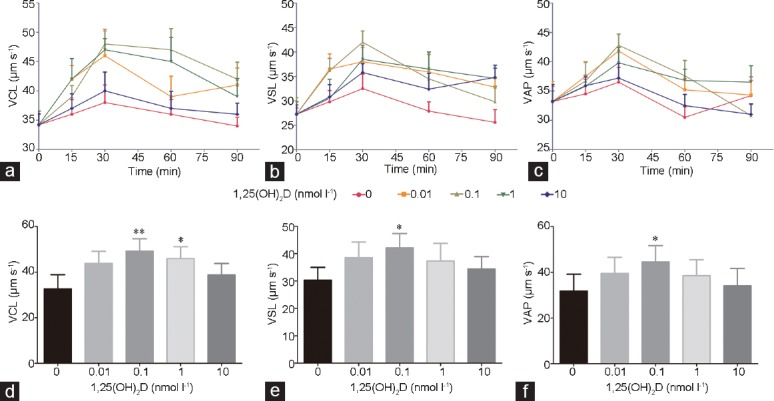
Effects of various concentrations (0.01, 0.1, 1, and 10 nmol l−1) of 1,25(OH)2D on sperm kinetic values, (a) VCL, (b) VSL, and (c) VAP at different time points (15, 30, 60, and 90 min). Effects of various concentrations (0.01, 0.1, 1, and 10 nmol l−1) of 1,25(OH)2D on sperm velocity, (d) VCL, (e) VSL, and (f) VAP after incubation for 30 min. All values are expressed as mean ± standard deviation. The sperm concentration used was 1 × 107 ml−1. The differences among different groups were assessed by one-way ANOVA with Holm–Sidak post hoc test. *P < 0.05 and **P < 0.01, groups treated with 1,25(OH)2D compared with the control group. VSL: straight-line velocity; VCL: curvilinear velocity; VAP: average path velocity; ANOVA: analysis of variance.
1,25(OH)2D and the increase of upward migration and ATP content in spermatozoa
Purified spermatozoa were incubated in the absence (control group) or presence (experimental group) of 0.1 nmol l−1 1,25(OH)2D. Upward sperm migration and sperm ATP content were assessed after incubation for 30 min in a 37°C water bath. There was remarkably more upward migration in the experimental group than in the control group (P < 0.01; Figure 3a). Moreover, the ATP content of spermatozoa in the experimental group also increased remarkably compared with that in the control group (P < 0.01; Figure 3b).
Figure 3.
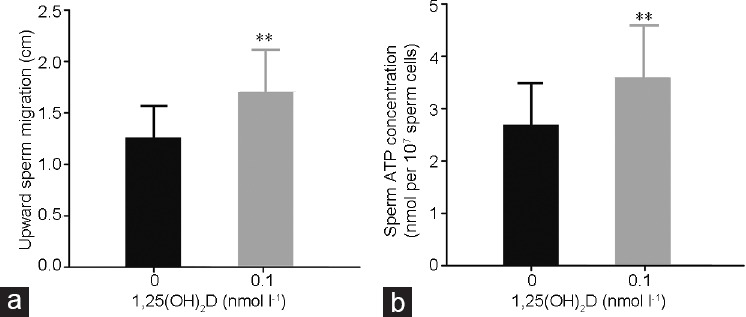
Effects of 0.1 nmol l−1 1,25(OH)2D on (a) upward sperm migration and (b) sperm ATP content after incubation for 30 min. All values are expressed as mean ± standard deviation. The sperm concentration used was 1 × 107 ml−1. The differences between two groups were assessed by paired samples t-test. **P < 0.01, the group treated with 1,25(OH)2D compared with the control group. ATP: adenosine triphosphate.
1,25(OH)2D and the promotion of cAMP/PKA pathway in spermatozoa
Purified spermatozoa were incubated in a 37°C water bath for 30 min in the absence (control group) or presence (experimental group) of 0.1 nmol l−1 1,25(OH)2D. Then, the change of sperm cAMP content and PKA activity were measured. There were notable differences of sperm cAMP content between the experimental group and the control group (P < 0.05; Figure 4a). Moreover, the PKA activity was also notably enhanced in the experimental group than that in the control group (P < 0.05; Figure 4b). Furthermore, the effect of 1,25(OH)2D on ATP production was reversed by the PKA inhibitor, N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89; S1582, Selleck, Texas, Houston, USA) at 100 μmol l−1 (P < 0.01; Figure 4c).
Figure 4.
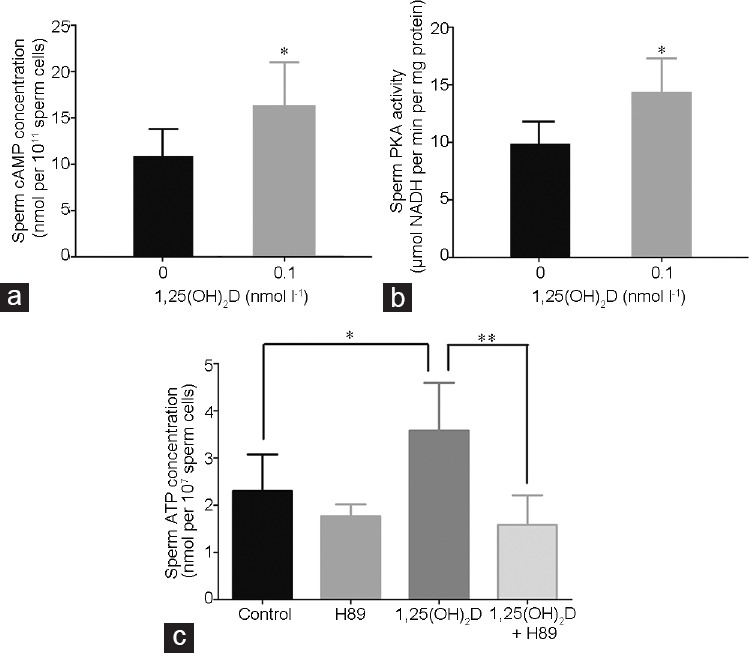
Effects of 0.1 nmol l−1 1,25(OH)2D on (a) cAMP concentration and (b) PKA activity of spermatozoa after incubation for 30 min. (c) Effects of PKA inhibitor, N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride (H89) on the ATP production of spermatozoa. All values are expressed as mean ± standard deviation. The sperm concentration used was 1 × 107 ml−1. The differences among different groups and between two groups were assessed by one-way ANOVA with Holm–Sidak post hoc test and paired samples t-test, respectively. *P < 0.05, groups treated with 1,25(OH)2D compared with the control group; **P < 0.01, the group treated with 1,25(OH)2D and H89 compared with the group treated with 1,25(OH)2D. cAMP: cyclic adenosine monophosphate; PKA: protein kinase A; ATP: adenosine triphosphate; ANOVA: analysis of variance; H89: N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochloride.
1,25(OH)2D and the increase of intracellular calcium ions and NADH content in spermatozoa
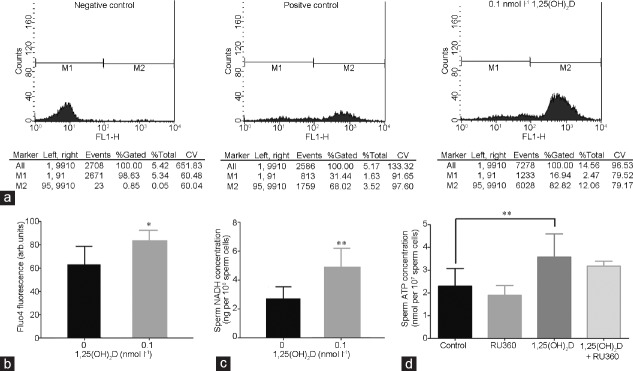
Purified spermatozoa were incubated in a 37°C water bath for 30 min in the absence (control group) or presence (experimental group) of 0.1 nmol l−1 1,25(OH)2D. Then, the change of calcium ion and NADH content in sperm cells were measured. Calcium ions were increased markedly more in the experimental group than in the control group (P < 0.05; Figure 5a and 5b). Moreover, the NADH content of spermatozoa in the experimental group was strikingly increased (P < 0.05; Figure 5c). Whereas, mitochondrial calcium uniporter (MCU) inhibitor, Ruthenium 360 (Ru360; 557440-500UG, Millipore, Burlington, MA, USA) at 10 μmol l−1 did not reverse the effect of 1,25(OH)2D on ATP production (P > 0.05; Figure 5d).
Figure 5.
(a) The FCM results of intracellular calcium ions of spermatozoa: left, sperm cells without either incubation of 1,25(OH)2D or addition of FLUO-4AM; middle, sperm cells without incubation of 1,25(OH)2D but with addition of FLUO-4AM; right, sperm cells with both incubation of 1,25(OH)2D and addition of FLUO-4AM. (b) Effects of 1,25(OH)2D on intracellular calcium ions of spermatozoa (P < 0.05). (c) Effects of 1,25(OH)2D on NADH concentration of spermatozoa (P < 0.05). (d) Effects of MCU inhibitor (Ru360) on the ATP production of spermatozoa (P > 0.05). Data for each group are expressed as mean ± standard deviation. The sperm concentration used was 1 × 107 ml−1. The differences among different groups and between two groups were assessed by one-way ANOVA with Holm–Sidak post hoc test and paired samples t-test, respectively. *P < 0.05 and **P < 0.01, groups treated with 1,25(OH)2D compared with the control group. FCM: flow cytometry; FLUO-4AM: the acetoxymethyl ester form of fluo-4; NADH: nicotinamide adenine dinucleotide; MCU: mitochondrial calcium uniporter; ATP: adenosine triphosphate; ANOVA: analysis of variance; RU360: Ruthenium 360; CV: coefficient of variation; FL1-H: FL1 pulse height; arb.units: arbitrary units.
DISCUSSION
Previous study on the relationship between serum vitamin D and male mouse reproduction showed that vitamin D-deficient mice had lower sexual desire, decreased ability to fertilize oocytes, and lower rates of birth of normal offspring than the wild type,16 which indicated that the overall level of vitamin D may reflect male sexual ability and fertility. However, many follow-up studies in humans on the relationship between the overall level of vitamin D and semen quality have presented conflicting results.18,19,21,22 Moreover, at present, there is a lack of evidence on the association between seminal plasma vitamin D and semen quality, which has prevented a consensus being established about the optimal concentration of vitamin D in a fertile reproductive system. In our research, we investigated the relationships between both serum and seminal plasma vitamin D levels and semen parameters. No direct correlation between serum vitamin D and sperm kinetic values was found. However, a positive correlation between seminal plasma vitamin D and sperm kinetic parameters was identified, which may indicate that, compared with serum vitamin D, seminal plasma vitamin D may better reflect the status of male reproduction. A study of Tak et al. has shown seasonal variation of the overall vitamin D levels.36 In our study, we collected data during summer, fall, and winter. No seasonal variations were observed in serum vitamin D levels, while they were observed for seminal plasma vitamin D levels, which may be related to the particular men who volunteered in each season.
During the process of ejaculation, spermatozoa pass through the ejaculatory ducts and mix with fluids from the testes, epididymis, and accessory sex glands to form the semen.37,38,39 Among these accessory glands, seminal vesicles account for 65%–75% of the production of semen.40 During the last decade, increasing evidence has disclosed the concomitant existence of a vitamin D receptor and metabolizing enzymes in the epithelium of human seminal vesicles.5,25 In our clinical analysis, positive correlations of serum 25(OH)D level with both semen volume and seminal plasma fructose were found, suggesting that vitamin D plays an active role in the function of the seminal vesicles. Furthermore, no statistically significant interaction was observed between seminal plasma 25(OH)D and serum 25(OH)D, while a positive relationship between seminal plasma 25(OH)D and semen volume was found, which corroborates our proposal that seminal vesicles play a critical role in the local production of vitamin D. With regard to the negative relationship found between seminal plasma 25(OH)D and sperm concentration, we assumed that the decrease of sperm concentration can be ascribed to the stimulating effect of vitamin D on semen volume. In general, despite the undoubted value of serum vitamin D in reflecting the overall status of male reproductive capacity, seminal plasma vitamin D may be more useful for the evaluation and treatment of male infertility.
Sperm motility is the main variable related to the success of fertilization,41,42 which is supported by the fact that improvements in sperm movement make it easier for spermatozoa to reach the site of fertilization, leading to higher rates of reproductive success. To address the theoretical basis for the diagnosis and treatment of male infertility, study of factors that affect sperm motility and the mechanisms underlying this is particularly needed. Given the positive relationship between locally produced vitamin D and sperm function, further study based on the mechanism underlying the effects of vitamin D should be conducted in vitro.
Consistent with previous reports,18,19,24,27,43 sperm velocity parameters and upward migration increased after incubation with 0.1 nmol l−1 1,25(OH)2D with an optimal time of 30 min. As a process that consumes energy, sperm movement is closely related to the output of ATP in mitochondria. This relationship is well supported by the observation of improvements in the functional state of the respiratory chain of sperm mitochondrial oxidation. Many substances interfere with the regulation of mitochondrial function. Being an important second messenger in the cell, it has been suggested that cAMP plays a role in modulating mitochondrial function through activating a variety of downstream factors such as PKA.44,45,46 In previous reports, increased PKA activity was shown to phosphorylate the related subunits of mitochondrial electron chain complexes I and IV as well as phosphorylating ATP synthase inhibitor 1, which in turn regulated the function of respiratory chain complex proteins, enhanced the function of oxidative phosphorylation, and promoted the production of ATP.46,47 Our observations of the elevations of cAMP content and PKA activity fit well to this notion. Furthermore, the PKA inhibitor H89 reversed the effect of 1,25(OH)2D on ATP production, which is consistent with 1,25(OH)2D functioning through the cAMP/PKA signaling pathway to enhance sperm motility.
Apart from cAMP, NADH is well known for its impact on mitochondrial function.48 It has been proven that NADH delivers hydrogen atoms that have been removed from metabolites to complex I. And then it enables them to pass through the reaction chain catalyzed by various enzymes and coenzymes. Eventually these atoms can be oxidized by electron transport chains, and generate ATP by oxidative phosphorylation.49,50,51,52 The triggering effect of calcium ions on ATP synthesis has been reported in sperm cells.53 There are consistent data to support the notion that the rise in calcium ions following vitamin D stimulation originates from intracellular stores, mainly the redundant nuclear envelope (RNE).18,24,26,54 Increased concentrations of calcium ions in the cytoplasm, which sometimes parallel increase in mitochondrial calcium, could enhance the supply of NADH to the respiratory chain through calcium-sensitive dehydrogenases.31 We examined the content of calcium ions and NADH in sperm cytoplasm after incubation with 1,25(OH)2D; the results showed clear increases of both factors. To further explore whether the calcium ions in the cytoplasm could be transferred into mitochondria and activate intramitochondrial calcium-sensitive dehydrogenases, an inhibitor of MCU, Ruthenium 360 (Ru360) was used, but did not result in any differences of ATP production. An article has reported that MCU does not transport calcium below a concentration of around 200 nmol l−1.55 Given the information above, we assume that the impact of 1,25(OH)2D on ATP production is attributable to the increase of calcium ions in the cytoplasm, rather than in the mitochondria.
In summary, seminal plasma vitamin D may be involved in regulating sperm motility. The active form of vitamin D may enhance sperm motility by promoting the activation of the sperm mitochondrial respiratory chain to produce ATP through both the cAMP/PKA pathway and the increase of intracellular calcium ion concentration.
Infertility attributable to the male partner accounts for roughly 40%–50% of all patients attending infertility clinics.41,56,57 Unfortunately, there is currently no treatment option available to improve semen quality in most men. Given that the oral supplementation of vitamin D confers benefits in health outcomes, increasing clinical trials are being conducted to evaluate the effect of vitamin D supplementation on semen quality. The studies of both Tiwari et al.58 in 2009 on patients with prostatitis and Deng et al.59 in 2014 on patients with idiopathic oligoasthenozoospermia found a positive effect of vitamin D on sperm motility in men with unknown vitamin D status, while a single-center randomized clinical trial by Blomberg et al.56 in 2017 based on 330 infertile men showed that supplementation with vitamin D and calcium had no effect on semen quality or live birth rate in men with vitamin D insufficiency. This apparent conflict may be because of the differences in the study populations, vitamin D levels, and supplementation protocols. It has been reported that the presence of vitamin D receptors (VDR) and vitamin D-metabolizing enzymes may reflect the quality of spermatozoa.20,60 Therefore, the poor response of spermatozoa to vitamin D in a previous study may be due to the loss of function of the sperm vitamin D receptor. Notably, our findings above suggest that 25(OH)D is a novel marker of semen quality, and that a new vitamin D supplementation protocol would be valuable for men with vitamin D insufficiency in seminal plasma. Consistent with previous reports,18,61 our findings also lay the foundation for adding 1,25(OH)2D to sperm capacitation solution prior to assisted reproductive techniques. However, to confirm the validity of this approach, further studies of pregnancy rates, birth rates, and other clinical outcomes are necessary.
AUTHOR CONTRIBUTIONS
KJ carried out the immunoassays, participated in the design of the study, and drafted the manuscript. ZD performed the statistical analysis and drafted the manuscript. BY conceived and designed the experiments. DDW revised the manuscript, plotted the results, and performed the statistical analysis. LPP participated in the design of the study and performed the statistical analysis. JJ and LC carried out the immunoassays. XG and XHQ performed the statistical analysis. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Research Funds for Jiangsu Province Key Research and Development Plan (Grant No. BE2016750), Research Funds for Military Family Planning (Grant No. 16JS012), Six Talent Peaks Project in Jiangsu Province (Grant No. 2017-WSW-033), Chinese Medical Association Special Fund for Clinical Medical Research (Grant No. 17020350704), Foundation for Key Medical Talents in Jiangsu Province (Grant No. ZDRCA2016096), Natural Science Foundation of Jiangsu Province (Grant No. BK20170620), China Postdoctoral Science Foundation (Grant No. 2017M613434), and National Natural Science Foundation of China (Grant No. 81701431 and 81701440).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2 vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–59. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 3.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr. 2005;135:310–6. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- 4.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–9S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MB. Vitamin D and male reproduction. Nat Rev Endocrinol. 2014;10:175–86. doi: 10.1038/nrendo.2013.262. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 7.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, et al. Clarification of DRIs for calcium and vitamin D across age groups. J Am Diet Assoc. 2011;111:1467. doi: 10.1016/j.jada.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 8.van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011;25:671–80. doi: 10.1016/j.beem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Prosser D, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–73. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Rosso A, Pansera M, Zamoner A, Zanatta L, Bouraima-Lelong H, et al. 1α,25(OH)2-vitamin D3 stimulates rapid plasma membrane calcium influx via MAPK activation in immature rat Sertoli cells. Biochimie. 2012;94:146–54. doi: 10.1016/j.biochi.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Bouillon R. [vitamin D and human health] Presse Med. 2009;38:3–6. doi: 10.1016/j.lpm.2008.11.001. [Article in French] [DOI] [PubMed] [Google Scholar]
- 13.Abbasihormozi S, Kouhkan A, Alizadeh AR, Shahverdi AH, Nasr-Esfahani MH, et al. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology. 2017;5:113–8. doi: 10.1111/andr.12280. [DOI] [PubMed] [Google Scholar]
- 14.Nikooyeh B, Neyestani TR, Tayebinejad N, Alavi-Majd H, Shariatzadeh N, et al. Daily intake of vitamin D- or calcium-vitamin D-fortified Persian yogurt drink (doogh) attenuates diabetes-induced oxidative stress: evidence for antioxidative properties of vitamin D. J Hum Nutr Diet. 2014;27(Suppl 2):276–83. doi: 10.1111/jhn.12142. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 16.Kwiecinski GG, Petrie GI, DeLuca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr. 1989;119:741–4. doi: 10.1093/jn/119.5.741. [DOI] [PubMed] [Google Scholar]
- 17.Uhland AM, Kwiecinski GG, DeLuca HF. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J Nutr. 1992;122:1338–44. doi: 10.1093/jn/122.6.1338. [DOI] [PubMed] [Google Scholar]
- 18.Blomberg Jensen M, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011;26:1307–17. doi: 10.1093/humrep/der059. [DOI] [PubMed] [Google Scholar]
- 19.Yang B, Sun H, Wan Y, Wang H, Qin W, et al. Associations between testosterone, bone mineral density, vitamin D and semen quality in fertile and infertile Chinese men. Int J Androl. 2012;35:783–92. doi: 10.1111/j.1365-2605.2012.01287.x. [DOI] [PubMed] [Google Scholar]
- 20.Blomberg Jensen M, Jorgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int J Androl. 2012;35:499–510. doi: 10.1111/j.1365-2605.2012.01256.x. [DOI] [PubMed] [Google Scholar]
- 21.Tirabassi G, Cutini M, Muscogiuri G, Delli Muti N, Corona G, et al. Association between vitamin D and sperm parameters: clinical evidence. Endocrine. 2017;58:194–8. doi: 10.1007/s12020-016-1198-9. [DOI] [PubMed] [Google Scholar]
- 22.Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, et al. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012;14:855–9. doi: 10.1038/aja.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nangia AK, Hill O, Waterman MD, Schwender CE, Memoli V. Testicular maturation arrest to testis cancer: spectrum of expression of the vitamin D receptor and vitamin D treatment in vitro . J Urol. 2007;178:1092–6. doi: 10.1016/j.juro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Aquila S, Guido C, Middea E, Perrotta I, Bruno R, et al. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol. 2009;7:140. doi: 10.1186/1477-7827-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blomberg Jensen M, Nielsen JE, Jorgensen A, Rajpert-De Meyts E, Kristensen DM, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25:1303–11. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- 26.Blomberg Jensen M, Dissing S. Non-genomic effects of vitamin D in human spermatozoa. Steroids. 2012;77:903–9. doi: 10.1016/j.steroids.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Aquila S, Guido C, Perrotta I, Tripepi S, Nastro A, et al. Human sperm anatomy: ultrastructural localization of 1alpha,25-dihydroxyvitamin D receptor and its possible role in the human male gamete. J Anat. 2008;213:555–64. doi: 10.1111/j.1469-7580.2008.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foresta C, Strapazzon G, De Toni L, Perilli L, Di Mambro A, et al. Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocr Metab. 2011;96:E646–52. doi: 10.1210/jc.2010-1628. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Pesini E, Diez-Sanchez C, Lopez-Perez MJ, Enriquez JA. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol. 2007;77:3–19. doi: 10.1016/S0070-2153(06)77001-6. [DOI] [PubMed] [Google Scholar]
- 30.Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G. cAMP and mitochondria. Physiology. 2013;28:199–209. doi: 10.1152/physiol.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–16. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. pp. 1–286. [Google Scholar]
- 33.Mann T. Fructose and fructolysis in semen in relation to fertility. Lancet. 1948;1:446–8. doi: 10.1016/s0140-6736(48)90315-8. [DOI] [PubMed] [Google Scholar]
- 34.Lu JC, Chen F, Xu HR, Huang YF, Lu NQ. Standardization and quality control for determination of fructose in seminal plasma. J Androl. 2007;28:207–13. doi: 10.2164/jandrol.106.001552. [DOI] [PubMed] [Google Scholar]
- 35.Hu YA, Lu JC, Shao Y, Huang YF, Lu NQ. Comparison of the semen analysis results obtained from two branded computer-aided sperm analysis systems. Andrologia. 2013;45:315–8. doi: 10.1111/and.12010. [DOI] [PubMed] [Google Scholar]
- 36.Tak YJ, Lee JG, Kim YJ, Park NC, Kim SS, et al. Serum 25-hydroxyvitamin D levels and testosterone deficiency in middle-aged Korean men: a cross-sectional study. Asian J Androl. 2015;17:324–8. doi: 10.4103/1008-682X.142137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kareskoski M, Katila T. Components of stallion seminal plasma and the effects of seminal plasma on sperm longevity. Anim Reprod Sci. 2008;107:249–56. doi: 10.1016/j.anireprosci.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Martinez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66(Suppl 1):11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 39.Verze P, Cai T, Lorenzetti S. The role of the prostate in male fertility, health and disease. Nat Rev Urol. 2016;13:379–86. doi: 10.1038/nrurol.2016.89. [DOI] [PubMed] [Google Scholar]
- 40.Andrade-Rocha FT. Semen analysis in laboratory practice: an overview of routine tests. J Clin Lab Anal. 2003;17:247–58. doi: 10.1002/jcla.10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skakkebaek NE, Keiding N. Changes in semen and the testis. BMJ. 1994;309:1316–7. doi: 10.1136/bmj.309.6965.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLachlan RI, Krausz C. Clinical evaluation of the infertile male: new options, new challenges. Asian J Androl. 2012;14:3–5. doi: 10.1038/aja.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blomberg Jensen M. Vitamin D metabolism, sex hormones, and male reproductive function. Reproduction. 2012;144:135–52. doi: 10.1530/REP-12-0064. [DOI] [PubMed] [Google Scholar]
- 44.Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–50. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 45.Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, et al. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A. 2004;101:13483–8. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A. 2009;106:667–8. doi: 10.1073/pnas.0811895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizrahi R, Breitbart H. Mitochondrial PKA mediates sperm motility. Biochim Biophys Acta. 2014;1840:3404–12. doi: 10.1016/j.bbagen.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Zini A, O’Bryan MK, Israel L, Schlegel PN. Human sperm NADH and NADPH diaphorase cytochemistry: correlation with sperm motility. Urology. 1998;51:464–8. doi: 10.1016/s0090-4295(97)00631-6. [DOI] [PubMed] [Google Scholar]
- 49.Vaz AD. Multiple oxidants in cytochrome P450 catalyzed reactions: implications for drug metabolism. Curr Drug Metab. 2001;2:1–16. doi: 10.2174/1389200013338847. [DOI] [PubMed] [Google Scholar]
- 50.Kurisu G, Kusunoki M, Kimata-Ariga Y, Hase T. [Structure of the electron transfer complex between plant type ferredoxin and ferredoxin dependent assimilatory enzymes] Tanpakushitsu Kakusan Koso. 2001;46:1661–7. [Article in Japanese] [PubMed] [Google Scholar]
- 51.Bakker BM, Overkamp KM, van Maris AJ, Kotter P, Luttik MA, et al. Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2001;25:15–37. doi: 10.1111/j.1574-6976.2001.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 52.Rich PR. The molecular machinery of Keilin's respiratory chain. Biochem Soc Trans. 2003;31:1095–105. doi: 10.1042/bst0311095. [DOI] [PubMed] [Google Scholar]
- 53.Lubart R, Friedmann H, Levinshal T, Lavie R, Breitbart H. Effect of light on calcium transport in bull sperm cells. J Photoch Photobio B. 1992;15:337–41. doi: 10.1016/1011-1344(92)85139-l. [DOI] [PubMed] [Google Scholar]
- 54.Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 55.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 56.Blomberg Jensen M, Gerner Lawaetz J, Andersson AM, Petersen JH, Nordkap L, et al. Vitamin D deficiency and low ionized calcium are linked with semen quality and sex steroid levels in infertile men. Hum Reprod. 2016;31:1875–85. doi: 10.1093/humrep/dew152. [DOI] [PubMed] [Google Scholar]
- 57.Huening M, Yehia G, Molina CA, Christakos S. Evidence for a regulatory role of inducible cAMP early repressor in protein kinase A-mediated enhancement of vitamin D receptor expression and modulation of hormone action. Mol Endocrinol. 2002;16:2052–64. doi: 10.1210/me.2001-0260. [DOI] [PubMed] [Google Scholar]
- 58.Tiwari A. Elocalcitol, a vitamin D3 analog for the potential treatment of benign prostatic hyperplasia, overactive bladder and male infertility. IDrugs. 2009;12:381–93. [PubMed] [Google Scholar]
- 59.Deng XL, Li YM, Yang XY, Huang JR, Guo SL, et al. [Efficacy and safety of vitamin D in the treatment of idiopathic oligoasthenozoospermia] Zhonghua Nan Ke xue. 2014;20:1082–5. [Article in Chinese] [PubMed] [Google Scholar]
- 60.Boisen IM, Bøllehuus Hansen L, Mortensen LJ, Lanske B, Juul A, et al. Possible influence of vitamin D on male reproduction. J Steriod Biochem. 2017;173:215–22. doi: 10.1016/j.jsbmb.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 61.Liu B, Tang M, Han Z, Li J, Zhang J, et al. Co-incubation of human spermatozoa with anti-VDAC antibody reduced sperm motility. Cell Physiol Biochem. 2014;33:142–50. doi: 10.1159/000356657. [DOI] [PubMed] [Google Scholar]