Abstract
The aim of this study was to validate the effectiveness of targeted microsurgical spermatic cord denervation (MSCD) of the trifecta nerve complex in comparison to traditional full MSCD with complete skeletonization of the spermatic cord in men with chronic orchialgia. Retrospective chart review was performed by a single fellowship-trained microsurgeon between 2011 and 2016. Patients had follow-ups at 6 weeks, 6 months, and 1 year postoperatively. Thirty-nine men with chronic orchialgia underwent full MSCD between 2011 and 2013. In July 2013, after the publication of an anatomic study with identification of Wallerian degeneration of the trifecta nerve complex in men with chronic orchialgia, the technique was changed to targeted MSCD. From July 2013 to March 2016, 43 men underwent targeted MSCD. When comparing the full MSCD group to the targeted MSCD group, there was no significant difference in resolution of pain (66.7% vs 69.8%, P = 0.88), no difference in partial relief of pain (17.9% vs 23.3%, P = 0.55), and no difference in failure to respond rates (15.4% vs 7.0%, P = 0.22) between the two groups. There was no difference in mean change of visual analog pain scale scores between the two groups (P = 0.27). Targeted MSCD had a shorter operative time (53 min vs 21 min, P = 0.0001). Targeted MSCD offers patients comparable outcomes to traditional full MSCD, with a shorter operative time, a less technically challenging surgery, and potentially less risk to cord structures which should be preserved.
Keywords: denervation, microsurgical, neurolysis, spermatic cord
INTRODUCTION
Chronic orchialgia, defined as unilateral or bilateral scrotal pain lasting at least 3 months in duration, is one of the most common complaints seen in the urologist's office and continues to be a challenging entity to diagnose and to treat.1,2 Conservative treatment is the first-line therapy; however, when conservative treatment fails, surgical intervention may be indicated. Surgical intervention for chronic orchialgia was advanced with the use of the operative microscope in the 1970s to perform microsurgical spermatic cord denervation (MSCD).3 MSCD has become the treatment of choice when an anatomic or pathologic etiology is not present and men fail conservative therapy. Conventionally, full MSCD was performed by skeletonization and dissection through the entire spermatic cord, only preserving gonadal arteries, lymphatics, and the vas deferens; while ligating all veins and microsurgically identifiable nerves throughout the spermatic cord.4 In 2013, Parekattil et al.5 described Wallerian degeneration in the trifecta nerve complex in men with chronic orchialgia in three primary sites including the cremaster muscle fibers, peri-vasal tissues and vasal sheath, and posterior lipomatous/perivessel tissues; suggesting that these nerves may explain the effect of the MSCD procedure. These three sites may be considered for a more targeted MSCD.
PATIENTS AND METHODS
Between 2011 and 2016, 82 men underwent MSCD for chronic orchialgia by a single fellowship-trained microsurgeon (PKK). All men had evaluations including normal digital rectal examinations, negative urinalysis, negative scrotal Doppler ultrasounds, had pain localized to the testicle, epididymis, or spermatic cord, without an anatomic or pathologically identifiable etiology, and failed conservative therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), heat, and scrotal elevation for at least 3 months. Men who had a history of prior hernia repair were examined to rule out the potential of recurrent hernia and were excluded if they had symptoms more consistent with mesh ilioinguinal nerve entrapment, such as paresthesia or pain solely in the distribution of the lateral scrotum and medial thigh. The men all underwent diagnostic spermatic cord block in the clinic and had to reveal at least 50% improvement from baseline pain level to complete resolution of pain by the visual analog pain scale (VAS) to be considered a candidate for MSCD. Men with bilateral orchialgia were excluded from the data to standardize for operative times. Between 2011 and 2013, 39 men underwent full traditional MSCD with skeletonization of the spermatic cord. This technique was performed by making a 1.5 cm subinguinal incision overlying the external inguinal ring, similar to the approach for subinguinal microsurgical varicocele repair, incising Scarpa's fascia, isolating the spermatic cord and then performing MSCD under microscopic magnified visualization. The cremaster muscle was divided circumferentially, all veins and microsurgically visible nerves were ligated and divided, leaving only the gonadal arteries, lymphatics, and the vas deferens intact.4 From July 2013 to March 2016, targeted MSCD was performed on 43 men with chronic orchialgia. For this technique, the spermatic cord is isolated as described for traditional MSCD, the cremaster muscle is divided circumferentially, the posterior lipomatous/perivessel tissues are fulgurated with electrocautery, and the perivessel tissues and vasal sheath are ligated under microsurgical visualization (Figure 1). Figure 1 demonstrates the microsurgical appearance of a completed full MSCD versus a targeted MSCD revealing the higher level of technical complexity of the full MSCD.
Figure 1.
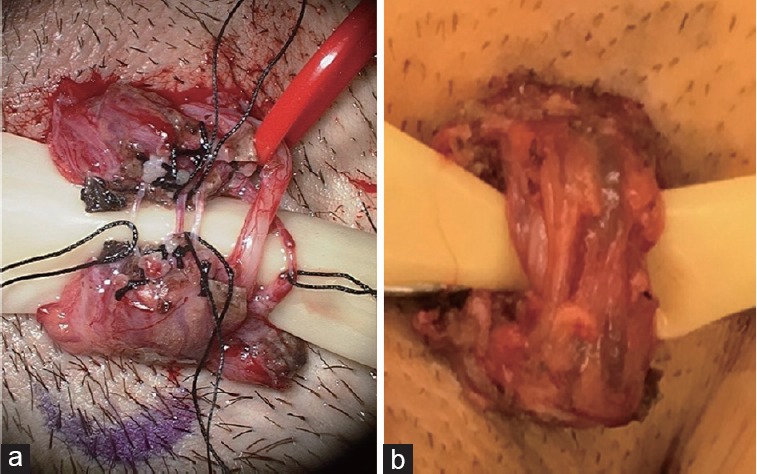
Microsurgical appearance of completed. (a) Full MSCD. (b) Targeted MSCD revealing the higher level of technical complexity of the full MSCD. Photo of the full MSCD courtesy of Daniel Williams, M.D. MSCD: microsurgical spermatic cord denervation.
A retrospective chart review was performed after obtaining exemption from St. David's Healthcare Institutional Review Board (Austin, USA). Men with idiopathic chronic orchialgia with no identifiable anatomic or pathologic etiology underwent in-office spermatic cord block. To undergo spermatic cord block they had to have a negative urologic evaluation including digital rectal examination, scrotal ultrasound, and urinalysis, and failed a trial of conservative treatment with NSAIDs, heat, and elevation for at least three months. Statistical analysis was performed using the Student's t-test with a value of P < 0.05 considered to be statistically significant.
RESULTS
Men with idiopathic chronic orchialgia with no identifiable anatomic or pathologic etiology underwent in-office spermatic cord block. To undergo spermatic cord block they had to have a negative urologic evaluation including digital rectal examination, scrotal ultrasound, and urinalysis, and failed a trial of conservative treatment with NSAIDs, heat, and elevation for at least three months. One hundred and eighteen men who either had a temporary complete resolution of pain or partial relief of pain, defined as at least a 50% improvement in pain level by VAS, with spermatic cord block, underwent MSCD between 2011 and 2016. Of those men, 82 completed follow-ups in person or by telephone at 6 weeks, 6 months, and 1 year postoperatively, whereas the remaining 36 men were excluded due to incomplete data at these time frames, 22 of which underwent targeted MSCD and 14 of which underwent complete MSCD. The first 39 men underwent traditional MSCD with complete skeletonization of the spermatic cord. After the discovery of the trifecta nerve complex, targeted MSCD of the trifecta nerve complex was performed in 43 men, from July 2013 to March 2016. All patients received a postoperative analgesic prescription of acetaminophen with codeine with a sufficient number of tablets for up to 5 days. All patients had follow-ups to evaluate their responses in person or by telephone at 6 weeks, 6 months, and 1 year postoperatively using the VAS. All in-person follow-ups and phone conversations were with the operating surgeon using the VAS. The patients with telephone follow-ups were asked to use a search engine to bring up a VAS on their computer or mobile device to refer to when responding about pain levels. Data including patient age at the time of MSCD, duration of pain before undergoing MSCD, possible etiology of orchialgia, pre-MSCD scores, and post-MSCD scores were assessed for both groups (Tables 1 and 2). Table 1 demonstrates patient demographics, VAS outcomes in traditional complete MSCD group, the age of patients at the time of MSCD, duration of pain before MSCD, possible etiology of pain, preoperative and postoperative MSCD VAS at 1 year postoperatively. Table 2 demonstrates patient demographics and outcomes, the age of the patient at the time of targeted MSCD, the duration of pain before MSCD, possible etiology of pain, preoperative and postoperative MSCD VAS at 1 year postoperatively.
Table 1.
Patient demographics/visual analog pain scale outcomes in traditional complete microsurgical spermatic cord denervation group. Age of patient at time of microsurgical spermatic cord denervation, duration of pain prior to microsurgical spermatic cord denervation, possible etiology of pain, pre- and post-microsurgical spermatic cord denervation visual analog pain scale at 1 year postoperation (postvasectomy pain syndrome)
| Patient | Age (year) | Duration (month) | Etiology | Pre-MSCD VAS | Post-MSCD VAS |
|---|---|---|---|---|---|
| 1 | 48 | 5 | Hernia repair | 8 | 0 |
| 2 | 47 | 180 | Idiopathic | 8 | 0 |
| 3 | 29 | 3 | PVPS | 8 | 0 |
| 4 | 30 | 12 | Idiopathic | 8 | 0 |
| 5 | 38 | 12 | Idiopathic | 5 | 0 |
| 6 | 24 | 8 | Idiopathic | 8 | 3 |
| 7 | 29 | 3 | Idiopathic | 6 | 5 |
| 8 | 77 | 3 | History of trauma | 7 | 0 |
| 9 | 45 | 240 | Idiopathic | 8 | 0 |
| 10 | 45 | 60 | S/p hydrocelectomy | 8 | 2 |
| 11 | 34 | 12 | History of trauma | 10 | 10 |
| 12 | 44 | 120 | PVPS | 7 | 0 |
| 13 | 64 | 360 | Idiopathic | 6 | 0 |
| 14 | 52 | 180 | Idiopathic | 5 | 0 |
| 15 | 37 | 348 | History cryptorchidism/orchiopexy | 4 | 0 |
| 16 | 42 | 12 | Idiopathic | 8 | 0 |
| 17 | 33 | 24 | PVPS | 6 | 1 |
| 18 | 49 | 24 | Idiopathic | 5 | 4 |
| 19 | 23 | 12 | Idiopathic | 8 | 8 |
| 20 | 27 | 24 | PVPS | 6 | 5 |
| 21 | 20 | 7 | Idiopathic | 7 | 0 |
| 22 | 43 | 48 | Hernia repair | 7 | 0 |
| 23 | 40 | 3 | Idiopathic | 6 | 0 |
| 24 | 21 | 84 | Idiopathic | 3 | 0 |
| 25 | 48 | 120 | PVPS | 5 | 5 |
| 26 | 28 | 3 | Idiopathic | 6 | 0 |
| 27 | 45 | 4 | Idiopathic | 7 | 2 |
| 28 | 45 | 9 | Idiopathic | 6 | 0 |
| 29 | 35 | 252 | Idiopathic | 9 | 0 |
| 30 | 47 | 6 | History of testicular torsion | 8 | 0 |
| 31 | 42 | 12 | Idiopathic | 5 | 0 |
| 32 | 38 | 24 | Idiopathic | 3 | 0 |
| 33 | 49 | 12 | PVPS | 8 | 0 |
| 34 | 19 | 12 | History of trauma | 9 | 0 |
| 35 | 36 | 36 | PVPS | 4 | 0 |
| 36 | 54 | 108 | History of trauma | 8 | 3 |
| 37 | 33 | 120 | PVPS | 8 | 1 |
| 38 | 51 | 84 | Idiopathic | 4 | 1 |
| 39 | 46 | 60 | Idiopathic | 5 | 0 |
PVPS: postvasectomy pain syndrome; MSCD: microsurgical spermatic cord denervation; VAS: visual analog pain scale; S/p: persistent pain status post hydrocelectomy
Table 2.
Patient demographics/outcomes: age of patient at time of targeted microsurgical spermatic cord denervation, duration of pain prior to microsurgical spermatic cord denervation, possible etiology of pain, pre- and post-microsurgical spermatic cord denervation visual analog pain scale at 1-year postoperative (postvasectomy pain syndrome)
| Patient | Age (year) | Duration (month) | Etiology | Pre-MSCD VAS | Post-MSCD VAS |
|---|---|---|---|---|---|
| 1 | 23 | 36 | Idiopathic | 10 | 3 |
| 2 | 43 | 264 | History of trauma | 8 | 1 |
| 3 | 44 | 6 | Idiopathic | 4 | 0 |
| 4 | 39 | 12 | PVPS | 6 | 2 |
| 5 | 67 | 12 | Idiopathic | 8 | 2 |
| 6 | 30 | 48 | History of trauma | 6 | 0 |
| 7 | 22 | 24 | Idiopathic | 5 | 0 |
| 8 | 48 | 240 | Idiopathic | 8 | 0 |
| 9 | 44 | 84 | History of trauma | 10 | 0 |
| 10 | 42 | 84 | Idiopathic | 6 | 0 |
| 11 | 43 | 36 | PVPS | 7 | 0 |
| 12 | 32 | 36 | Idiopathic | 6 | 0 |
| 13 | 31 | 96 | PVPS | 5 | 1 |
| 14 | 66 | 48 | Idiopathic | 5 | 0 |
| 15 | 30 | 12 | Idiopathic | 7 | 0 |
| 16 | 45 | 24 | Idiopathic | 6 | 0 |
| 17 | 72 | 12 | Idiopathic | 4 | 0 |
| 18 | 55 | 120 | Idiopathic | 7 | 0 |
| 19 | 38 | 6 | Idiopathic | 6 | 0 |
| 20 | 31 | 24 | Idiopathic | 8 | 0 |
| 21 | 35 | 180 | History of orchiopexy for testicular torsion | 9 | 4 |
| 22 | 58 | 12 | PVPS | 10 | 0 |
| 23 | 42 | 7 | Idiopathic | 10 | 0 |
| 24 | 24 | 24 | Idiopathic | 10 | 8 |
| 25 | 66 | 612 | Idiopathic | 5 | 0 |
| 26 | 51 | 24 | Idiopathic | 7 | 2 |
| 27 | 42 | 36 | PVPS | 4 | 4 |
| 28 | 38 | 24 | Idiopathic | 6 | 0 |
| 29 | 28 | 24 | Idiopathic | 10 | 4 |
| 30 | 39 | 24 | PVPS | 5 | 0 |
| 31 | 73 | 240 | PVPS | 6 | 2 |
| 32 | 44 | 7 | PVPS | 5 | 0 |
| 33 | 18 | 36 | Idiopathic | 9 | 0 |
| 34 | 48 | 7 | Idiopathic | 10 | 1 |
| 35 | 41 | 96 | S/p hydrocelectomy | 8 | 0 |
| 36 | 24 | 24 | Idiopathic | 5 | 0 |
| 37 | 64 | 8 | Idiopathic | 7 | 0 |
| 38 | 57 | 168 | PVPS | 4 | 4 |
| 39 | 42 | 48 | History of trauma | 3 | 0 |
| 40 | 63 | 36 | Idiopathic | 8 | 0 |
| 41 | 29 | 24 | Idiopathic | 8 | 0 |
| 42 | 17 | 3 | History of trauma | 3 | 0 |
| 43 | 43 | 6 | Idiopathic | 8 | 0 |
PVPS: postvasectomy pain syndrome; MSCD: microsurgical spermatic cord denervation; VAS: visual analog pain scale
Twenty-six out of 39 (66.7%) of the men who underwent traditional MSCD had complete resolution of pain, defined as a VAS of zero, compared to 30/43 (69.8%) of those who underwent targeted MSCD. Seven of 39 (17.9%) of men who underwent traditional MSCD had partial relief of pain, defined as a decrease in VAS score of at least 50% or more but not a VAS score of zero, in comparison to 10/43 (23.3%) of those who underwent targeted MSCD. Six of 39 (15.4%) of those who underwent traditional MSCD failed to adequately respond to surgical treatment, compared to 3/43 (7.0%) of the men who underwent targeted MSCD that failed to respond adequately. Failure to respond adequately was defined as men who did not have at least a 50% decrease in pain level using the VAS score from their preoperative pain level, including men who had no improvement. No men in either group had worsening of pain postoperatively regardless of the technique of MSCD that was performed. The mean operative time for the traditional MSCD was 53 min versus 21 min for the targeted MSCD. There was no statistically significant difference in pain response outcomes between the two techniques; however, the operative time was significantly faster for the targeted MSCD (Table 3). Operative time was defined as the time from incision to completion of closure. There was no significant difference between the changes in VAS scores between the two groups from baseline to postoperative VAS scores (Table 4). Besides typical postoperative soreness and swelling, there were no complications reported in either group such as hematoma, infection, or testicular atrophy. Table 3 demonstrates the outcomes of traditional versus targeted spermatic cord denervation and Table 4 demonstrates the mean changes in VAS scores from baseline to postoperative following MSCD.
Table 3.
Outcomes of traditional versus targeted spermatic cord denervation
| MSCD technique | Age (year) | Complete resolution, n (%) | >50% improvement (%) | Failure (%) | Operation time (min) |
|---|---|---|---|---|---|
| Traditionala | 40 | 26 (66.7) | 17.9% (7/39) | 15.4% (6/39) | 53 |
| Targetedb | 43 | 30 (69.8) | 23.3% (10/43) | 7.0% (3/43) | 21 |
| P | 0.32 | 0.88 | 0.55 | 0.22 | 0.0001 |
an=39; bn=43
Table 4.
Mean changes in visual analog pain scale scores from baseline to postmicrosurgical spermatic cord denervation
| MSCD technique | Change in VAS scores, mean (s.d.) |
|---|---|
| Traditional MSCD (n=39) | 5.3 (2.6) |
| Targeted MSCD (n=43) | 5.9 (2.3) |
| P | 0.27 |
MSCD: microsurgical spermatic cord denervation; VAS: visual analog pain scale; s.d.: standard deviation
DISCUSSION
Chronic orchialgia is a very difficult diagnostic and therapeutic challenge for the clinician. Microsurgery is the mainstay of treatment for chronic orchialgia without an identifiable etiology when conservative therapy fails. MSCD has been performed since the 1970s with relatively good response rates, without a strong microanatomic understanding of the pathology of chronic orchialgia until recently when the trifecta nerve complex demonstrated Wallerian degeneration in men with chronic orchialgia.3,5 Until the discovery of the trifecta nerve complex, there had been fairly little progress in MSCD since Devine and Schellhammer.3 first reported MSCD in 1978. Good response rates have been reported in multiple studies since that initial experience.4,6,7,8,9,10,11,12,13,14 Another anatomic study of the nerves in and in proximity to the spermatic cord has identified sensory and autonomic nerve fibers, most of which were found to be sensory and sympathetic nerve fibers, and few parasympathetic nerve fibers in the spermatic cord and surrounding tissues.15 Although this study is an elegant mapping of the nerves microanatomically, the Trifecta study is the only one to date which proposes a microanatomic pathology of nerves that may be responsible for chronic orchialgia.5 Some surgeons advocate transection of the vas deferens during MSCD to ensure ligation of the perivasal nerves. As a large population of our patients were young men who wished to maintain fertility potential, and the adventitia of the vas deferens can be meticulously dissected from the vas deferens with the additional benefit of preserving the deferential artery microsurgically, we elected to not transect the vasa.
This data reveal similar outcomes with targeted MSCD with the advantage of shorter operative times and a less technically demanding microsurgery. Although skilled microsurgeons who perform the traditional MSCD can preserve the structures which need to be preserved in men undergoing MSCD (such as the gonadal arteries, the lymphatics to prevent hydrocele formation, and the vas deferens in men who want to maintain fertility potential), the targeted MSCD puts these structures at virtually no risk.
Limitations of this study include its retrospective nature with associated biases and a 1 year follow-up period. A greater powered prospective randomized controlled trial would be helpful to elucidate the implications of this study.
CONCLUSIONS
Targeted MSCD of the trifecta nerve complex offers similar patient pain response rates with significantly shorter operative time, technically less challenging microsurgery, and the potential of lower risk of injury to intra-spermatic cord microanatomic structures which are important to preserve, in comparison to the traditional MSCD.
COMPETING INTERESTS
The author declares no competing interests.
REFERENCES
- 1.Costabile RA, Hahn M, McLeod DG. Chronic orchialgia in the pain prone patient: the clinical perspective. J Urol. 1991;146:1571–4. doi: 10.1016/s0022-5347(17)38169-7. [DOI] [PubMed] [Google Scholar]
- 2.Davis BE, Noble MJ, Weigel JW, Foret JD, Mebust WK. Analysis and management of chronic testicular pain. J Urol. 1990;143:936–9. doi: 10.1016/s0022-5347(17)40143-1. [DOI] [PubMed] [Google Scholar]
- 3.Devine CJ, Schellhammer PF. The use of microsurgical denervation of the spermatic cord for orchialgia. Trans Am Assoc Genitourin Surg. 1978;70:149–51. [PubMed] [Google Scholar]
- 4.Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol. 1996;155:1005–7. doi: 10.1016/s0022-5347(01)66369-9. [DOI] [PubMed] [Google Scholar]
- 5.Parekattil SJ, Gudeloglu A, Brahmbhatt JV, Priola KB, Vieweg J, et al. Trifecta nerve complex: potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol. 2013;190:265–70. doi: 10.1016/j.juro.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed I, Rasheed S, White C, Shaikh NA. The incidence of post-vasectomy chronic testicular pain and the role of nerve stripping (denervation) of the spermatic cord in its management. Br J Urol. 1997;79:269–70. doi: 10.1046/j.1464-410x.1997.32221.x. [DOI] [PubMed] [Google Scholar]
- 7.Levine LA, Matkov TG. Microsurgical denervation of the spermatic cord as primary surgical treatment of chronic orchialgia. J Urol. 2001;165:1927–9. doi: 10.1097/00005392-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich A, Olbert P, Engelmann UH. Management of chronic testalgia by microsurgical testicular denervation. Eur Urol. 2002;41:392–7. doi: 10.1016/s0302-2838(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 9.Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol. 2008;180:949–53. doi: 10.1016/j.juro.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Benson JS, Abern MR, Larsen S, Levine LA. Does a positive response to spermatic cord block predict response to microdenervation of the spermatic cord for chronic scrotal content pain? J Sex Med. 2013;10:876–82. doi: 10.1111/j.1743-6109.2012.02937.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsen SM, Benson JS, Levine LA. Microdenervation of the spermatic cord for chronic scrotal content pain: single institution review analyzing success rate after prior attempts at surgical correction. J Urol. 2013;189:554–8. doi: 10.1016/j.juro.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Oomen RJ, Witjens AC, van Wijck AJ, Grobbee DE, Lock TM. Prospective double-blind preoperative pain clinic screening before microsurgical denervation of the spermatic cord in patients with testicular pain syndrome. Pain. 2014;155:1720–6. doi: 10.1016/j.pain.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy DJ. Early experience with microsurgical spermatic cord denervation for chronic orchialgia at a Canadian centre. Can Urol Assoc J. 2015;9:e72–4. doi: 10.5489/cuaj.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatem A, Kovac JR. Chronic scrotal pain and microsurgical spermatic cord denervation: tricks of the trade. Transl Androl Urol. 2017;6:S30–6. doi: 10.21037/tau.2017.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka S, Shiraishi K, Matsuyama H. Microsurgical anatomy of the spermatic cord and the spermatic fascia: distriubtion of lymphatics, and sensory and autonomic nerves. J Urol. 2016;195:1841–7. doi: 10.1016/j.juro.2015.11.041. [DOI] [PubMed] [Google Scholar]


