Abstract
Varicocele is a common finding in men. Varicocele correction has been advocated for young patients with testicular hypotrophy, but there is a lack of morphofunctional follow-up data. We assessed whether percutaneous treatment of left varicocele is associated with testicular “catch-up growth” in the following 12 months by retrospectively reviewing data from an electronic database of 10 656 patients followed up in our clinic between 2006 and 2016. We selected all young adults (<35 years) with left varicocele who underwent percutaneous treatment, had a minimum of 12 months’ ultrasound imaging follow-up, and had no other conditions affecting testicular volume. One hundred and fourteen men (mean±standard deviation [s.d.] of age: 22.8 ± 5.4 years) met the inclusion and exclusion criteria. Left testicular hypotrophy (LTH), defined as a ≥20% difference between left and right testicular volume at baseline, was observed in 26 (22.8%) men. Participants with LTH (mean±s.d.: 14.5 ± 2.7 ml) had lower baseline testicular volume compared to those without LTH (mean±s.d.: 15.7 ± 3.8 ml; P = 0.032). Repeated measures mixed models showed a significant interaction between LTH and time posttreatment when correcting for baseline left testicular volume (β = 0.114, 95% confidence interval [CI]: 0.018–0.210, P = 0.020), resulting in a catch-up growth of up to 1.37 ml per year (95% CI: 0.221–2.516). Age at intervention was also associated with reduced testicular volume (−0.072 ml per year, 95% CI: −0.135–−0.009; P = 0.024). Percutaneous treatment of left varicocele in young adults with LTH can result in catch-up growth over 1 year of follow-up. The reproductive and psychological implications of these findings need to be confirmed in longer and larger prospective studies.
Keywords: catch-up growth, percutaneous treatment, testicular hypotrophy, testicular ultrasound, testicular volume, varicocele, young adults
INTRODUCTION
Varicocele, the excessive dilatation of the pampiniform venous plexus of the spermatic cord,1 is a common finding in adolescent males. Its prevalence is debated, as estimates from different populations yield different results;2 however, it is usually reported that varicocele may occur in up to 15% of all males between 15 and 19 years old, with a much lower prevalence before puberty.3 Testicular damage resulting from varicocele has been variably associated with reduced testicular volume, impaired spermatogenesis, and increased sperm DNA damage,4,5 although the mechanisms involved are still not completely understood.6 It is therefore unsurprising that it is listed as one of the most frequent causes of male infertility and that its prevalence is much higher in infertile men, at 30%–40% in primary and 80%–85% in secondary infertility.7,8
Existing guidelines propose different approaches on when and how to treat varicocele.9 Reduced ipsilateral testicular size is one of the indications for treatment, above all in adolescents. As 80%–90% of testicular volume is made up of germ cells within seminiferous tubules, an asymmetrical reduction in testicular volume is universally accepted as a sign of testicular damage. Improvements in testicular volume,10 sperm parameters,11 and DNA fragmentation12 have been variably reported following surgical treatment of varicocele; however, to our knowledge, no study has yet described the long-term effects of percutaneous varicocele embolization on testicular volume. As this treatment is regularly used in clinical practice,8 we retrospectively assessed its effects on testicular volume catch-up during a 12-month follow-up in a single-center protocol-driven study.
PATIENTS AND METHODS
Patients
We retrospectively assessed data in an electronic database of all patients followed up for varicocele in our clinic (Section of Medical Pathophysiology, Food Science and Endocrinology, Department of Experimental Medicine, Rome, Italy) between 2006 and 2016 (Supplementary Figure 1 (1,002.8KB, tif) ). Analysis was limited to young adults (age <35 years, Supplementary Figure 2 (1.3MB, tif) ) as we considered that a change in testicular volume following treatment is less likely in patients with long-standing varicocele. An increase in testicular size is more commonly observed in young patients, although it has also been reported in adults undergoing surgical varicocele repair.13 Patients with right or bilateral varicocele, or with any diseases known to influence testicular volume (orchitis, testicular neoplasms, cryptorchidism, hypogonadism, genetic disorders, and testicular torsion), were excluded from the analysis. We also excluded patients, who refused treatment, as well as those who were considered ineligible for treatment or those whose spermatic veins could not be accessed during the procedure, from further analysis. This study was approved by the Ethical Committee of Sapienza – University of Rome (Rome, Italy). Overall, 114 patients met the inclusion and follow-up criteria (Supplementary Figure 1 (1,002.8KB, tif) ); all patients received and signed informed consent. These patients were followed for up to 12 months: 36 patients completed all visits (3, 6, and 12 months), while 40 and 38 patients skipped one or two intermediate follow-up visits (Table 1). The recruitment strategy took advantage of the “Amico Andrologo” permanent nationwide surveillance program of male students in their final grade of high school conducted by the Italian Society of Andrology and Sexual Medicine (SIAMS) and supported by the Italian Ministry of Health.
Table 1.
Demographics of the study population
| Characteristics | Total (n=114) | No-LTH (n=88) | LTH (n=26) |
|---|---|---|---|
| Age (year) | |||
| Median (IQR) | 20.5 (19–27.5) | 20.5 (19–28) | 20.5 (20–25.2) |
| Mean (s.d.) | 22.8 (5.4) | 22.8 (5.6) | 22.9 (4.8) |
| Grade of left varicocele, n (%) | |||
| Grade 2 | 27 (23.7) | 20 (22.7) | 7 (26.9) |
| Grade 3 | 55 (48.2) | 42 (47.7) | 13 (50.0) |
| Grade 4 | 32 (28.1) | 26 (29.6) | 6 (23.1) |
LTH: left testicular hypotrophy, defined as a difference between left and right testicular volume ≥20%; IQR: interquartile range; s.d.: standard deviation
Protocol
Since the year 2004, all patients admitted to our unit for varicocele treatment have followed a fixed internal protocol. At the time of admission and at all follow-up visits (3, 6, and 12 months), patients undergo collection of medical history, physical examination, color Doppler ultrasound (US) to assess testicular volume and grade of varicocele, and blood sampling for hormone evaluation. During the first visit, after sample collection, the patients undergo percutaneous treatment of their varicocele, as described below. All US examinations are performed using a Philips IU22 unit (Philips, Bothell, WA, USA) with a 7–15 MHz wideband linear transducer. Standardized protocols with axial and transverse examinations of the testes are routinely performed.14 Testicular volume (in ml) is calculated using an ellipsoid formula as follows: length (L) × width (W) × height (H) × 0.52. Rather than using a clinical classification for varicocele, a US-based staging system15 is used in order to provide the most important information for clinicians while at the same time reducing the risk of skewness toward a higher or lower grade (Supplementary Table 1).
Supplementary Table 1.
Ultrasound classification of varicocele according to the ultrasound of the testis for the andrologist – morphological and Functional Atlas15 (modified with permission of the authors)
| B-mode | Revisited classification (Dubin–Solbiati) | Corresponding to Dubin | Solbiati | Corresponding to Dubin | |
|---|---|---|---|---|---|
| Reflux | Reflux | ||||
| Grade 1 | Dilated vessel (>2.5 mm) in inguinal region only | Inguinal reflux only during Valsalva maneuver (lasting 2–3 s) | Grade 1 | Inguinal reflux only during Valsalva maneuver | Grade 1 |
| Grade 2 | Supratesticular vessel dilation (>3 mm) | Supratesticular reflux only during Valsalva maneuver, lasting more than 3 s | Supratesticular reflux only during Valsalva maneuver | ||
| Grade 3 | Supra- and peri-testicular vessel dilation (>3 mm) | Supra- and peri-testicular reflux at rest which increases during Valsalva maneuver, lasting more than 3 s | Grade 2 | Peritesticular reflux only during Valsalva maneuver | |
| Grade 4 | Peritesticular vessel dilation with further dilation during functional maneuver, testicular hypotrophy | Peritesticular reflux at rest which may or may not increase during Valsalva maneuver | Grade 3 | Testicular reflux at rest which increases during Valsalva maneuver | Grade 2 |
| Grade 5 | Peritesticular vessel dilation that does not increase with functional maneuver or intratesticular vessels and testicular hypotrophy | Peritesticular reflux at rest which increases minimally during Valsalva maneuver or dilated intratesticular vessels which refill with Valsalva maneuver | Peritesticular reflux at rest which increases minimally during Valsalva maneuver | Grade 3 |
Percutaneous treatment of varicocele
All procedures were performed in an outpatient setting using a 4-Fr introducer sheath (Terumo, Tokyo, Japan) positioned in a right brachial vein. Catheterization of the left spermatic vein was performed using a standard 180-cm long, 0.035-inch angled glide-wire (Terumo) with a different 4-Fr angiographic catheter (Simmons 1, Cobra 2, vertebral) selected as indicated by the patient's individual anatomy. The aim of the catheterization procedure was to position the distal tip of the catheter within the internal inguinal ring. Angiography was then performed to exclude the presence of collaterals or shunting (e.g., with the ipsilateral iliac vein). Manual compression was applied to the distal inguinal channel prior to embolization to prevent distal nontarget embolization. Once the target vein was completely filled with pure contrast agent, the embolization was performed by gently withdrawing the catheter while injecting a mousse of two vials of 1% Lauromacrogol 400 (Kreussler & Co. Chemische Fabrik GmBH, Wiesbaden, DE, USA). Manual compression was then performed to seal the brachial access.
Statistical analyses
The statistical analysis was performed by R software (version 3.4.2; R Core Team, Vienna, Austria). Numerical variables were summarized as the median (interquartile range [IQR]) and mean (standard deviation [s.d.]) as appropriate. Normal distribution of data was assessed via the Shapiro–Wilk test of normality. The left varicocele grade was expressed as absolute and percent frequency of distribution. Random intercept models were assessed with the linear and nonlinear mixed effects models(nlme) package (R package version 3.1-137; https://CRAN.R-project.org/package=nlme) to assess changes in left testicular volume during follow-up. Significance was set at P < 0.05.
RESULTS
Baseline characteristics
The baseline characteristics of the caseload are presented in Table 1. As reported above, 26 patients (22.8%) had left testicular hypotrophy (LTH); there was no difference in the mean age between the LTH and no-LTH groups (total: 22.8 [s.d.: 5.4] years; LTH: 22.9 [s.d.: 4.8] years, and no-LTH: 22.8 [s.d.: 5.6] years, P = 0.953). Grade 3 varicocele was the most prevalent in our study population (55 patients, 48.2%), while similar numbers of patients had Grade 2 (27 patients, 23.7%) and Grade 4 (32 patients, 28.1%) varicocele. Left testicular volumes at the baseline and during follow-up are shown in Table 2.
Table 2.
Left testicular volume as assessed by testicular ultrasound at baseline and during follow-up
| Follow-up | Total | No-LTH | LTH |
|---|---|---|---|
| Baseline (ml, n=114), mean (s.d.) | 15.4 (3.6) | 15.7 (3.8) | 14.5 (2.7) |
| 3 months (ml, n=91), mean (s.d.) | 15.9 (3.2) | 16.2 (3.4) | 14.9 (2.3) |
| 6 months (ml, n=82), mean (s.d.) | 16.9 (3.8) | 17.2 (3.8) | 15.0 (2.6) |
| 12 months (ml, n=69), mean (s.d.) | 16.6 (3.0) | 16.5 (3.1) | 16.9 (3.0) |
LTH: left testicular hypotrophy, defined as a difference between left and right testicular volume ≥20%; s.d.: standard deviation
Assessment of treatment effects on testicular volume
Linear mixed effect (random intercept) models were used to assess the effects of percutaneous treatment of left varicocele on left testicular volume. The response variable (i.e., left testicular volume) was normally distributed, as assessed by Shapiro–Wilk test of normality. The first variables were used as covariates in the first model: grade of varicocele, age at treatment, and duration of follow-up. We also included the presence of LTH in order to evaluate different growth rates between the two groups. Based on current literature,15,16,17 LTH was defined on as a baseline difference ≥20% between left and right testicular volumes. The results are shown in Table 3.
Table 3.
Random intercept model for testicular volume increase
| Parameter | β | P |
|---|---|---|
| Intercept | 11.657 | <0.001 |
| Age (per 1 year) | 0.039 | 0.506 |
| Grade of left varicocele | 1.210 | 0.180 |
| LTH (yes) | −1.388 | 0.088 |
| Post-treatment time (per 1 month) | −0.018 | 0.448 |
| LTH (yes): post-treatment time (per 1 month) | 0.107 | 0.035* |
*P < 0.05. LTH: left testicular hypotrophy, defined as a difference between left and right testicular volume ≥20%; β: regression coefficient
There was a statistically significant difference in mean baseline left testicular volume, which was smaller in the LTH group compared to that in the no-LTH group (14.5 [s.d.: 2.7] ml vs 15.7 [s.d.: 3.8] ml, P = 0.032). No significant increase was observed in left testicular volume after treatment (P = 0.448). The grade of varicocele and age at intervention also had nonsignificant effects on testicular volume (P = 0.180 and P = 0.506, respectively). However, the interaction analysis showed that testicular volume increased significantly more in LTH (+0.107 ml per month, P = 0.035) than in no-LTH patients.
To exclude potential bias, the model was adjusted for the baseline left testicular volume. The results of the second model are reported in Table 4. This model confirmed that during follow-up, testicular volume increased at a significantly higher rate in patients with LTH (+0.114 ml per month, P = 0.020) than in those without LTH, independently of baseline testicular volume. Furthermore, a significant negative effect of age was observed in the expanded model (−0.072 ml per year, P = 0.024). No significant effects were observed for grade of varicocele: when stratifying, nonsignificant effects were confirmed for more severe degrees of varicocele (Grade 3, P = 0.604 and Grade 4, P = 0.955). At the end of follow-up, as described in Table 2, the mean left testicular volume in the LTH group was similar to that observed in the no-LTH group (LTH: 16.9 [s.d.: 3.0] ml, no-LTH: 16.5 [s.d.: 3.1] ml, P = 0.565; Figure 1) and in fact had significantly increased since the baseline (baseline: 14.5 [s.d.: 2.7] ml, at 12 months: 16.9 [s.d.: 3.0] ml, P = 0.023; Figure 2).
Table 4.
Expanded random intercept model for testicular volume increase
| Parameter | β | P |
|---|---|---|
| Intercept | 5.908 | <0.001 |
| Baseline left testicular volume (per 1 ml) | 0.784 | <0.001* |
| Age (per 1 year) | −0.072 | 0.024* |
| Grade of left varicocele | ||
| Grade 2 | Reference | |
| Grade 3 | −0.216 | 0.604 |
| Grade 4 | 0.027 | 0.955 |
| LTH (yes) | −0.426 | 0.409 |
| Post-treatment time (per 1 month) | −0.020 | 0.395 |
| LTH (yes): post-treatment time (per 1 month) | 0.114 | 0.020* |
*P < 0.05. LTH: left testicular hypotrophy, defined as a difference between left and right testicular volume ≥20%; β: regression coefficient
Figure 1.
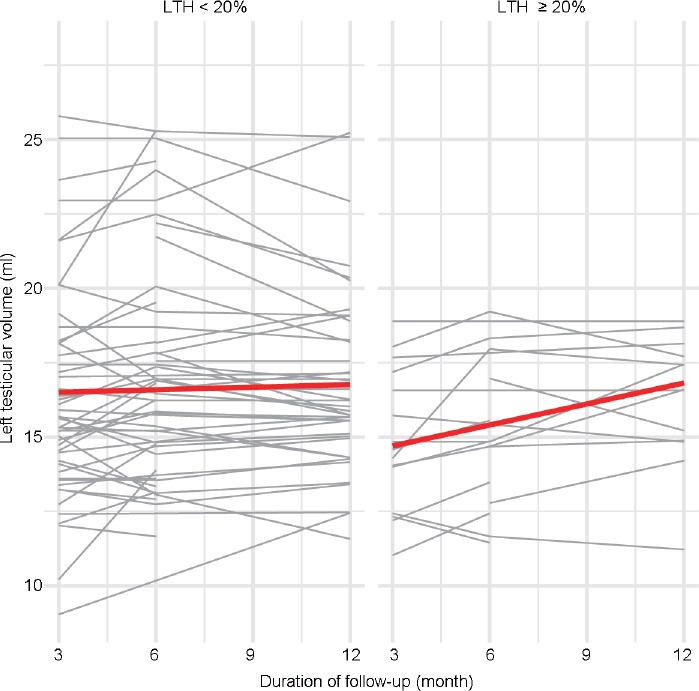
Changes in left testicular volume following percutaneous treatment of left varicocele. Each line represents a single patient; the red line describes the mean. LTH: left testicular hypotrophy.
Figure 2.
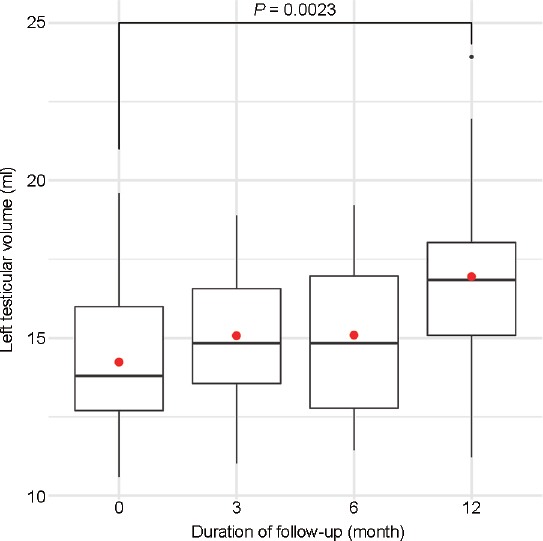
Graphical representation of changes in left testicular volume following percutaneous treatment of left varicocele in patients with left testicular hypotrophy. Boxplots describe the median and interquartile range; the red dot is the mean. Wilcoxon rank-sum test was used to assess the statistical difference between the means.
Complications, failure rate, and radiation exposure
No significant complications were reported in our population following the varicocele repair. While occasional testicular pain and transitory edema were reported, these data are not mentioned in the US report and probably have no bearing on the study outcome.
When technical problems occurred during the procedure, members of the Vascular and Interventional Radiology Unit usually re-scheduled the intervention for a later date. In these cases, we considered the date of the first complete embolization as the baseline for our study. If the Radiology Unit had reasons for doubting the efficacy of re-scheduling intervention, the patient was either transferred to Urology Unit for surgical treatment or, for more severe cases, closely monitored for clinical outcomes. These patients are listed in the flowchart as “Treatment not suggested”, Recurrence of varicocele was found in 151 of the 838 men who underwent treatment. These patients are listed in the flowchart as “Treatment failed” (Supplementary Figure 1 (1,002.8KB, tif) ).
Radiation exposure was not measured directly. However, the mean duration of exposure to radiation was minimized to the extent possible (mean: 6.2 min, range: 3–9 min) in order to reduce the risk for the patients undergoing the procedure.
DISCUSSION
The present study is the first to provide a quantitative analysis of catch-up growth in testicular volume after percutaneous varicocele embolization in a large homogeneous cohort of young adults followed up constantly for 1 year after treatment.
Whether and when to treat varicocele is a recurring dilemma for physicians due to inconsistencies in the scientific literature, with guidelines from different scientific societies suggesting different approaches to and indications for treatment.9 The risk of overtreatment should not be underestimated.18 Furthermore, improvements in sperm parameters following treatment often reach statistical significance, but have little clinical relevance; sperm quality fluctuates with time and, except for those seeking immediate conception, an objective clinical result of the treatment is lacking. A surrogate marker of improved testicular function is therefore needed. As reduced testicular volume is universally accepted as an indication for treatment, we considered left testicular volume as one of the most reliable and objective parameters to be affected by left varicocele, consistently with the most recent literature.18
Our study suggests that patients with a difference of at least 20% between left and right testicular volume are more likely to benefit from treatment of left varicocele, with a monthly increase of 0.114 ml (95% CI: 0.018–0.210) during follow-up. This increase would therefore result in an almost 1.37 ml improvement over 1 year (95% CI: 0.221–2.516), corresponding to an approximately 9.5% increase over the mean baseline volume. Graphical representations of the effects on left testicular volume are shown in Figure 1 and 2.
Our results also show that age is significantly associated with reduced left testicular volume in these patients (−0.072 ml per year, P = 0.020), possibly suggesting that early intervention should be attempted in order to prevent testicular damage. However, despite its statistical significance, whether this finding actually has any clinical bearing is a matter of debate.
Testicular hypotrophy is associated with worse outcomes in terms of sperm quality,19 and endocrine impairments have been described in patients with varicocele.16 The significant increase in testicular volume observed in our study warrants further investigation, as catch-up growth might offer a better view of subsequent long-term improvement in the spermatogenetic function of the testes.16 It could also have a positive psychological effect.
Most reports describe catch-up growth in children only. Ours is the first study to report an increase in testicular volume following percutaneous treatment of varicocele. Testicular hypoxia and hyperthermia, which have been described in this condition, could increase the production of reactive oxygen species; several studies suggest that oxidative stress is associated with germ cell apoptosis and is a marker of testicular dysfunction.20,21,22,23,24
Our study has some limitations, including its retrospective nature and absence of a control group. However, it also offers some unique advantages: just two, highly qualified clinicians performed the US in all patients and all the percutaneous embolization procedures were performed in a single center, leading to the highly consistent assessment and treatment of the caseload. In addition, the enrolled population had a relatively narrow age range (Supplementary Figure 2 (1.3MB, tif) ) and other conditions affecting testicular volumes were excluded. As previously stated, many young adults were diagnosed with varicocele during the “Amico Andrologo” permanent nationwide surveillance program for students in the last year of Italian high school.
Prospective studies assessing the effects of percutaneous varicocele repair should also consider several other features, such as number and diameter of varicose veins, changes in testicular echotexture, and circulating inflammatory markers, as any improvements in sperm parameters and endocrine function might actually be secondary, rather than a direct consequence of treatment.
CONCLUSIONS
Percutaneous treatment of left varicocele leads to a significant increase in ipsilateral testicular volume, but only in patients with a difference of at least 20% between right and left testicular volumes. Whether this improved testicular size is associated with better outcomes in terms of endocrine and reproductive function remains to be established. In any case, early intervention should be suggested in order to maximize the improvement. A 12-month follow-up is recommended for all young patients undergoing varicocele repair, as the most clinically evident effects on testicular catch-up growth take place in this period.
AUTHOR CONTRIBUTIONS
All authors contributed to the study. Initial outpatient visits were performed by AS, CP, GF, RL, MM, FR, FL, AL, and DG. US examinations were performed by CP and GF under the direct supervision of DG. Angiography and percutaneous treatments were performed by PL, MC, and MB. The study was designed by AS and DG. The first manuscript draft was written by AS, DAF, PL, and DG; AS and DAF also performed the statistical analysis. The manuscript was reviewed and critically appraised by RL, MM, FR, FL, and AL. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Flowchart detailing the process of patient retrieval from our electronic database. Several patients had more than one condition affecting testicular volume. DB: database; US: ultrasound.
Histogram detailing age distribution in the study population (n = 114).
ACKNOWLEDGMENTS
The authors wish to thank Marie-Hélène Hayes for her assistance in the English revision of the manuscript.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Serefoglu EC, Saitz TR, La Nasa JA, Jr, Hellstrom WJ. Adolescent varicocele management controversies. Andrology. 2013;1:109–15. doi: 10.1111/j.2047-2927.2012.00004.x. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson DL, Johnson EK. Varicoceles in the pediatric and adolescent population: threat to future fertility? Fertil Steril. 2017;108:370–7. doi: 10.1016/j.fertnstert.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Kolon TF. Evaluation and management of the adolescent varicocele. J Urol. 2015;194:1194–201. doi: 10.1016/j.juro.2015.06.079. [DOI] [PubMed] [Google Scholar]
- 4.Cortes-Gutierrez EI, Davila-Rodriguez MI, Fernandez JL, Lopez-Fernandez C, Aragon-Tovar AR, et al. DNA damage in spermatozoa from infertile men with varicocele evaluated by sperm chromatin dispersion and DBD-FISH. Arch Gynecol Obstet. 2016;293:189–96. doi: 10.1007/s00404-015-3822-y. [DOI] [PubMed] [Google Scholar]
- 5.Pallotti F, Paoli D, Carlini T, Vestri AR, Martino G, et al. Varicocele and semen quality: a retrospective case-control study of 4230 patients from a single centre. J Endocrinol Invest. 2018;41:185–92. doi: 10.1007/s40618-017-0713-z. [DOI] [PubMed] [Google Scholar]
- 6.Sheehan MM, Ramasamy R, Lamb DJ. Molecular mechanisms involved in varicocele-associated infertility. J Assist Reprod Genet. 2014;31:521–6. doi: 10.1007/s10815-014-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavijo RI, Carrasquillo R, Ramasamy R. Varicoceles: prevalence and pathogenesis in adult men. Fertil Steril. 2017;108:364–9. doi: 10.1016/j.fertnstert.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Johnson D, Sandlow J. Treatment of varicoceles: techniques and outcomes. Fertil Steril. 2017;108:378–84. doi: 10.1016/j.fertnstert.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Roque M, Esteves SC. A systematic review of clinical practice guidelines and best practice statements for the diagnosis and management of varicocele in children and adolescents. Asian J Androl. 2016;18:262–8. doi: 10.4103/1008-682X.169559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Chiba K, Yamaguchi K, Okada K, Matsushita K, et al. Effect of varicocelectomy on testicular volume in children and adolescents: a meta-analysis. Urology. 2012;79:1340–5. doi: 10.1016/j.urology.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Nork JJ, Berger JH, Crain DS, Christman MS. Youth varicocele and varicocele treatment: a meta-analysis of semen outcomes. Fertil Steril. 2014;102:381–7.e6. doi: 10.1016/j.fertnstert.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2013;189:S146–50. doi: 10.1016/j.juro.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto H, Saito K, Ogawa Y, Yoshida H. Effects of varicocele repair in adults on ultrasonographically determined testicular volume and on semen profile. Urology. 2008;71:485–9. doi: 10.1016/j.urology.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 14.Oyen RH. Scrotal ultrasound. Eur Radiol. 2002;12:19–34. doi: 10.1007/s00330-001-1224-y. [DOI] [PubMed] [Google Scholar]
- 15.Isidori AM, Lenzi A. Ultrasound of the testis for the andrologist – morphological and functional atlas. Cham: Springer. 2017:214. [Google Scholar]
- 16.Garcia-Roig ML, Kirsch AJ. The dilemma of adolescent varicocele. Pediatr Surg Int. 2015;31:617–25. doi: 10.1007/s00383-015-3698-8. [DOI] [PubMed] [Google Scholar]
- 17.Glassberg KI. My indications for treatment of the adolescent varicocele (and why.)? Transl Androl Urol. 2014;3:402–12. doi: 10.3978/j.issn.2223-4683.2014.12.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sack BS, Schafer M, Kurtz MP. The dilemma of adolescent varicoceles: do they really have to be repaired? Curr Urol Rep. 2017;18:38. doi: 10.1007/s11934-017-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzel O, Aslan Y, Balci M, Tuncel A, Unal B, et al. Significant worsening sperm parameters are associated to testicular hypotrophy in patients with a high grade varicocele. Actas Urol Esp. 2015;39:392–5. doi: 10.1016/j.acuro.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Lombardo F, Sansone A, Romanelli F, Paoli D, Gandini L, et al. The role of antioxidant therapy in the treatment of male infertility: an overview. Asian J Androl. 2011;13:690–7. doi: 10.1038/aja.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, et al. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem J. 2002;365:849–56. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbonetti A, Castellini C, Di Giammarco N, Santilli G, Francavilla S, et al. In vitro exposure of human spermatozoa to bisphenol A induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod Toxicol. 2016;66:61–7. doi: 10.1016/j.reprotox.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Micillo A, Vassallo MR, Cordeschi G, D’Andrea S, Necozione S, et al. Semen leukocytes and oxidative-dependent DNA damage of spermatozoa in male partners of subfertile couples with no symptoms of genital tract infection. Andrology. 2016;4:808–15. doi: 10.1111/andr.12188. [DOI] [PubMed] [Google Scholar]
- 24.Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl. 2016;18:186–93. doi: 10.4103/1008-682X.170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart detailing the process of patient retrieval from our electronic database. Several patients had more than one condition affecting testicular volume. DB: database; US: ultrasound.
Histogram detailing age distribution in the study population (n = 114).


