Abstract
Human papillomavirus (HPV) infection appears to play an important role in the development of penile cancer (PeCa), but their relationship remains unclear. Therefore, we performed a systematic review and meta-analysis to elucidate their relationship. We systematically searched Embase, PubMed, Cochrane Library, and Web of Science for case-control studies and cross-sectional studies using polymerase chain reaction (PCR) technology on formalin-fixed paraffin-embedded (FFPE) or paraffin-embedded (PE) PeCa tissues to detect HPV (published between January 1, 2007, and December 29, 2017; no language restrictions). Twenty-two studies were identified, and 1664 cases were available for analysis. The combined HPV infectious risk of PeCa is 51.0% (95% confidence interval [CI]: 43.0%–60.0%). The three most common subtypes of HPV were HPV16 (28.5%), HPV18 (2.3%), and HPV6 (2.3%). The virus was relevantly associated with basaloid (85.5%, 95% CI: 77.2%–93.8%) and warty (50.0%, 95% CI: 35.2%–64.8%) carcinomas. The invasiveness of PeCa was not associated with HPV (χ2 = 0.181, df = 1, P < 0.671). HPV infection in PeCa tended to be moderately differentiated (54.4%, 95% CI: 47.7%–61.1%). This study found that almost half of PeCa patients are associated with HPV. The most commonly associated genotype is HPV16, but several other genotypes were also detected. In addition to types 6 and 11, other single low-risk HPV infections have been found to contribute to PeCa to a lesser degree. HPV-positive tumors tend to exhibit warty and/or basaloid features, corresponding to a moderate histological grade. The role of HPV in PeCa should be revisited to provide evidence for the development of PeCa in the presence of HPV infection.
Keywords: human papillomavirus, penile cancer, systematic review and meta-analysis
INTRODUCTION
Penile carcinoma is a rare malignant tumor, accounting for <1% of adult male cancers in Europe and North America.1 However, its incidence in South America, Africa, and some parts of Asia may be as high as 10%, and approximately 26 300 new cases are diagnosed each year in men who are older than 66 years.2 The disease is characterized by an increased incidence in older men, with an average age at diagnosis of 60 years. The peak incidence of penile cancer (PeCa) occurs at the age of 70 years.3,4 No consensus is available regarding the age distribution of PeCa cases.
Studies have identified several contributing factors for PeCa, including phimosis, smoking, and chronic inflammatory states.5 In addition, lesions on the glans are directly linked to poor hygiene.
Human papillomavirus (HPV) infection is associated with anogenital cancer (including cervical, vaginal, vulvar, penile, and anal cancers), oropharyngeal cancer, and genital warts. The HPV vaccination significantly reduces the incidence of anogenital cancer and genital warts.6 However, although the success of the quadrivalent vaccine against HPV has led to substantial decreases in HPV-associated infections and cancers in women, studies have not demonstrated similar success in men, specifically in relation to PeCa and penile precancerous lesions. Determining the pathogenesis of HPV infection may provide valuable therapeutic targets to treat this rare and difficult disease.5 The aim of this study was to evaluate the prevalence of HPV in penile malignant tumor tissue samples in the most recent decade and to determine the relationship between histological types of PeCa and HPV to further understand the development of PeCa.
MATERIALS AND METHODS
Search strategy and selection criteria
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and was registered at the International Prospective Register of Systematic Reviews (No. CRD42018086094; available at: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=86094).
An extensive literature search was conducted by two independent authors to identify all relevant studies published between January 1, 2007, and December 29, 2017, by searching Embase, PubMed, Cochrane Library, and Web of Science. With no language restrictions, we used the following combined text and Medical Subject Headings (MeSH) terms: “penile neoplasms” and “human papillomavirus.” The complete search strategy used for PubMed was as follows: (Penile Neoplasms) OR (Neoplasms, Penis) OR (Penis Neoplasms) OR (Neoplasm, Penis) OR (Penis Neoplasm) OR (Neoplasms, Penile) OR (Neoplasm, Penile) OR (Penile Neoplasm) OR (Cancer of Penis) OR (Penis Cancers) OR (Cancer of the Penis) OR (Penis Cancer) OR (Cancer, Penis) OR (Cancers, Penis) OR (Penile Cancer) OR (Cancer, Penile) OR (Cancers, Penile) OR (Penile Cancers) AND (Human Papillomavirus). We considered all potentially eligible studies for review irrespective of the primary outcome or language. We also performed a manual search using the reference lists of key articles published in English and assessed the risk for bias according to the Agency for Healthcare Research and Quality (AHRQ),7 including studies with intermediate and high grades.
Study selection and data extraction
We regarded studies as eligible for inclusion if cases of invasive PeCa had HPV data available and if HPV DNA was detected in the formalin-fixed paraffin-embedded (FFPE) or paraffin-embedded (PE) tissue samples with polymerase chain reaction (PCR) technology. The reference lists of the identified articles were also reviewed for additional publications. A comprehensive effort to exclude repetitive cases from the final analysis was undertaken. When repeated histological samples were identified, the article that used the most sensitive HPV-DNA detection technique was selected.
The authors were directly contacted if anything doubtful was found, and the article in question was excluded if no answer was received. Studies with sample sizes fewer than five were also excluded. The following information was collected: the first author, year of publication, journal title, country of origin, diagnosis date, HPV detection methods, PCR primers used for HPV DNA, sample size, sample preparation, and histological type, as well as the overall HPV DNA prevalence. Histological groups for analysis corresponded to the World Health Organization (WHO) histological classification of penile cancers.8
HPV genotypes were divided into low and high risk following the epidemiologic criteria established by Muñoz et al.9 Genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82 were classified as high risk, 26, 53, and 66 were classified as probable high-risk types, and genotypes 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and CP6108 are classified as low risk for producing malignant transformation when they invade the epithelial cells of the cervix. HPV genotype-specific contributions for the HPV 16, 18, 31, 33, 35, 45, and 56 subtypes were estimated as a more relative proportion of high-risk HPV (hrHPV) among all HPV-positive cases, and types 6 and 11 were estimated to be relatively low-risk HPV (lrHPV). Combined HPV infection samples were assigned to the appropriate group according to the original article description or classified as “other” if the article did not provide the necessary information.
Quality assessment
The methodological quality of the included studies was assessed using an 11-item checklist recommended by the AHRQ statement (available at https://www.ncbi.nlm.nih.gov/books/NBK35156/).10 The item was scored “0” if the answer was “NO” or “UNCLEAR,” and the item was scored “1” if the answer was “YES.” Article quality was assessed as follows: low quality = 0–3; moderate quality = 4–7; and high quality = 8–11.
Statistical analyses
We assessed the overall HPV prevalence with the corresponding 95% confidence interval (CI) by dividing the number of subjects for each histological group by the number of HPV-positive cases. The Chi-squared test was also used to evaluate the relationship between HPV and penile carcinoma. The statistical package Stata V.15.0 (Stata Corporation, College Station, TX, USA), Revman 5.3 (Cochrane Collaborative, Oxford, UK), SPSS 22.0 (SPSS Inc., Chicago, IL, USA), and Microsoft Office 2007 (Microsoft, Redmond, WA, USA) were used for calculations and statistical analyses. Heterogeneity among the studies was measured by a random-effects model using the Chi-squared test, P values, and I2 statistics. P < 0.05 was considered statistically significant. A previously used method of evidence-based medicine was used to produce a forest map of noncomparative binary data.11,12
RESULTS
Description of studies
A total of 22 studies4,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 complied with the inclusion criteria, which included 1664 patients with penile carcinoma (Figure 1). Through AHRQ estimation, 7 articles17,24,26,28,29,30,31 were considered high quality, and 15 articles4,13,14,15,16,18,19,20,21,22,23,25,27,32,33 were considered moderate quality (Supplementary Table 1). The overall prevalence of HPV positivity in patients with penile tumors was approximately 51% (95% CI: 43%–60%; Figure 2). Overall, 73.8% of the penile carcinoma cases were squamous cell carcinoma (SCC). The most frequently used PCR primers were PCR GP5+6+ and PCR SPF-10. The data were analyzed for differences with respect to HPV DNA detection between PCR GP5+6+ and PCR SPF-10. The results were evaluated by Pearson's Chi-squared test, and no significance (χ2 = 2.938, df = 1, P > 0.05) was detected (Supplementary Table 2).
Figure 1.
Flowchart of literature screening.
Supplementary Table 1.
Type-specific prevalence of human papillomavirus in penile carcinomas by study-relevant items and histological type
| Reference | Journal (year) | Study area | Mean age (year) | Method of detection and primers | Sample | Sample Number of case | Overall HPV positive rate Number of cases (%) | Histology vegetative and number of cases | Relative HPV Prevalence, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | J Med Virol (2017) | Brazil | 58 (26–92) | PCR MY09/11 | TFTE | 122 | 79 (64.8) | – | – | |||||
| Damásdi | Pathol Oncol Res (2015) | Hungary | 61.2 (44–87) | PCR SPF10 | FFPE | 35 | 14 (40.0) | – | ||||||
| lsaura | BMC Urol (2015) | Brazil | 66±17.10 | PCR/Nested automated sequencing | FFPE | 76 | 48 (63.2) | – | – | |||||
| Hernandez | Front Oncol (2014) | USA | PCR-based Linear Array and INNO -LiPA assays | FFPE | 79 | 50 (63.3) | Keratinizing (53) | 30 (56.6) | ||||||
| Basaloid or warty-SCC (11) | 9 (81.8) | |||||||||||||
| Other SCC (15) | 11 (73.3) | |||||||||||||
| Djajadiningrat | J Urol (2014) | Netherlands | 63 (54–71) | PCR GP5+6+ | FFPE | 212 | 53 (24.1) | SCC (183) | 44 (24.0) | |||||
| Verruceus (5) | 0 (0) | |||||||||||||
| Warty (5) | 3 (60.0) | |||||||||||||
| Basaloid (5) | 4 (80.0) | |||||||||||||
| Basaloid-SCC (2) | 1 (50.0) | |||||||||||||
| Papillar (9) | 1 (11.1) | |||||||||||||
| Others (3) | 0 (0) | |||||||||||||
| Lebelo | J Med Virol (2014) | South Africa | 49.8 (18–87) | TaqMan-based qPCR | FFPE | 44 | 35 (79.5) | Keratinizing SCC (4) | 3 (75.0) | |||||
| Verrucous (9) | 8 (88.9) | |||||||||||||
| Papillary SCC (2) | 1 (50.0) | |||||||||||||
| Others (20) | – | |||||||||||||
| Do | Br J Cancer (2013) | Vietnam | 53 (51–57) | PCR SPF10 and HPV-16 E6 | PE | 120 | 27 (22.5) | Basaloid (3) | 2 (66.7) | |||||
| Keratinising (83) | 19 (22.9) | |||||||||||||
| Non-keratinising (20) | 3 (15.0) | |||||||||||||
| Verrucous (8) | 2 (25.0) | |||||||||||||
| Warty (2) | 0 | |||||||||||||
| Undetermined (4) | 1 (25.0) | |||||||||||||
| Chaux | World J Urol (2013) | Paraguay | 62 | PCR SPF-10 | Tumor tissue | 61 | 29 (47.5) | No warty/basaloid –features (53) | 9 (17.0) | |||||
| Warty/basaloid-features (36) | 23 (63.9) | |||||||||||||
| D’Hauwers | Vaccine (2012) | Belgium | – | Real-time quantitative PCR | FFPE | 55 | 24 (43.6) | – | – | |||||
| Cubilla | Am J Surg Pathol (2010) | Paraguay | 61 (16–95) | PCR SPF-10 | 202 | 64 (31.7) | Basaloid (–) | 19 | ||||||
| Warty-basaloid (–) | 9 | |||||||||||||
| Warty (–) | 8 | |||||||||||||
| Usual (–) | 18 | |||||||||||||
| Papillary (–) | 6 | |||||||||||||
| Mixed (–) | 2 | |||||||||||||
| Krustrup | Int J Exp Pathol (2009) | Denmark | – | PCR GP5+/6+ | FFPE | 145 | 89 (61.4) | Keratinizing (68) | 31 (45.6) | |||||
| Basaloid (33) | 32 (97.0) | |||||||||||||
| Warty (11) | 7 (63.6) | |||||||||||||
| Verrucous (16) | 1 (6.3) | |||||||||||||
| Giant cell (3) | 3 (100.0) | |||||||||||||
| Combined types (12) | 10 (83.3) | |||||||||||||
| SENBA | Oncol Lett (2009) | Japan | – | PCR SPF10 | FFPE | 16 | 12 (75.0) | Keratinizing (10) | 8 (80.0) | |||||
| Nonkeratinizing (4) | 3 (75.0) | |||||||||||||
| Verrucous (2) | 1 (50.0) | |||||||||||||
| Scheiner | Int Braz J Urol (2008) | Brazil | – | PCR MY09/MY11 | FFPE | 80 | 58 (72.5) | – | – | |||||
| Guerrero | B J U Int (2008) | Spain | 67.6 | PCRGP5+/GP6+biotinylated primers | FFPE | 24 | 11 (45.8) | Squamous (17) | – | |||||
| Warty (4) | – | |||||||||||||
| Verrucous (1) | – | |||||||||||||
| Basaloid (2) | – | |||||||||||||
| Yanagawa | Pathology (2008) | Japan | 67.6 (46–87) | PCR-RFLP | FFPE | 26 | 3 (11.5) | – | – | |||||
| Tornesello | Int J Cancer (2008) | Italy | – | PCR MY09/MY11 and GP51/GP61 | FFPE | 41 | 19 (46.3) | – | – | |||||
| Madsen | Am Assoc Cancer Res (2008) | Denmark | – | PCR GP5 +/6 + | FFPE | 71 | 25 (35.2) | Keratinizing (37) | – | |||||
| Verrucous (2) | – | |||||||||||||
| Basaloid (1) | – | |||||||||||||
| Sarcomatoid (1) | – | |||||||||||||
| Prowse | Br J Dermatol (2007) | UK | – | PCR SPF10 | FFPE | 26 | 14 (53.8) | – | – | |||||
| Tornesello ML | Cancer Let (2008) | Uganda | 60.6±11.1 | semi-nested PCR | PE | 17 | 11 (64.7) | Keratinizing SCC (15) | – | |||||
| Basaloid SCC (1) | – | |||||||||||||
| Verrucous SCC (1) | – | |||||||||||||
| Warty SCC (0) | – | |||||||||||||
| Sarcomatoid (0) | – | |||||||||||||
| Italy | 61±12.6 | Semi-nested PCR | PE | 61 | 29 (47.5) | Keratinizing (54) | – | |||||||
| Basaloid SCC (3) | – | |||||||||||||
| Verrucous (2) | – | |||||||||||||
| Warty (1) | – | |||||||||||||
| Sarcomatoid (1) | – | |||||||||||||
| Heideman | J Clin Oncol (2007) | Germany | 65 (27–92) | PCR GP5 +/6 + | FFPE | 83 | 46 (55.4) | Not-Specified (72) | 40 (55.6) | |||||
| Verrucous (7) | 4 (57.1) | |||||||||||||
| Warty (2) | 1 (50.0) | |||||||||||||
| Sarcomatoid (2) | 1 (50.0) | |||||||||||||
| Protzel | Cellular Molecular Biol (2007) | Germany | 69.4 (35–89) | High Pure PCR Template |
FE | 19 | 7 (36.8) | Nonkeratinizing (4) | 2 (50.0) | |||||
| Keratinizing (9) | 2 (22.2) | |||||||||||||
| Papillary (1) | 0 (0) | |||||||||||||
| Verrucous (1) | 0 (0) | |||||||||||||
| Condylomatous (1) | 0 (0) | |||||||||||||
| Basaloid (3) | 3 (100.0) | |||||||||||||
| Pascual | Cellular Molecular Biol (2007) | Spain | – | PCR My09/My11 and GP5 +/GP6 + | 49 | 38 (77.5) | ||||||||
| Total | 1664 | 785 (47.2) | 1465 | |||||||||||
| Reference | Hr HPV | Subtotal (%) | Lr HPV | Subtotal (%) | AHRQ score | |||||||||
| 16 | 18 | 31 | 33 | 35 | 45 | 56 | Others | 6 | 11 | Others | ||||
| LA | 32 (40.5%) | 7 (8.9%) | 7 (8.9%) | 0 | 0 | 9 (11.4%) | – | 1 (1.3%) | 56 (70.9) | 13 (16.5%) | 6 (7.6%) | 4 (5.1%) | 23 (29.1) | 6 |
| Damásdi | 11 (78.6%) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (14.3%) | 13 (92.9) | 0 | 0 | 1 (7.1%) | 1 (7.1) | 4 |
| lsaura | 10 (20.8%) | 4 (8.3%) | – | – | – | 1 (2.1%) | – | – | – | – | 6 (12.5%) | – | – | 6 |
| Hernandez | 36 (72.0%) | 2 (4.0%) | 0 | 3 (6.0%) | 1 (2.0%) | 2 (4.0%) | 0 | 5 (10.0%) | 49 (98.0) | 1 (2.0%) | 0 | 0 | 1 (2.0) | 7 |
| Djajadiningrat | 42 (79.2%) | 3 (5.7%) | 1 (1.9%) | 4 (7.5%) | 0 | 2 (3.8%) | 1 (1.9%) | 0 | 53 (100) | – | – | – | – | 6 |
| Lebelo | 2 (5.7%) | 0 | 0 | 0 | 0 | 1 (2.8%) | 0 | 0 | 3 (8.6) | 0 | 0 | 0 | 0 | 8 |
| 4 (11.4%) | 4 (11.4%) | 0 | 0 | 0 | 0 | 0 | 0 | 8 (22.8) | 1 (2.8%) | 7 (20.0%) | 0 | 8 (22.8) | ||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (2.8%) | 0 | 1 (2.8) | ||
| Do | 24 (88.9%) | – | – | – | – | – | – | – | – | – | – | – | – | 5 |
| Chaux | – | – | – | – | – | – | – | – | – | – | – | – | – | 7 |
| D’Hauwers | 17 | 1 | – | 1 | 3 | 22 (91.7) | 1 | 1 | 0 | 2 (8.3) | 4 | |||
| Cubilla | 13 | 1 | – | – | 1 | 0 | – | 4 | 19 | 0 | 0 | 0 | 0 | 5 |
| 4 | 0 | – | – | 0 | 0 | – | 3 | 7 | 2 | 0 | 0 | 2 | ||
| 3 | 1 | – | – | 1 | 0 | – | 2 | 7 | 0 | 0 | 1 | 1 | ||
| 10 | 1 | – | – | 0 | 2 | – | 3 | 16 | 1 | 1 | 0 | 2 | ||
| 5 | 0 | – | – | 0 | 0 | – | 1 | 6 | 0 | 0 | 0 | 0 | ||
| 1 | 0 | – | – | 0 | 0 | – | 3 | 6 | 0 | 0 | 0 | 0 | ||
| Krustrup | 78 (87.6%) | 0 | 1 (1.1%) | 3 (3.4%) | 1 (1.1%) | 1 (1.1%) | 1 (1.1%) | 1 (1.1%) | 86 (96.6) | 5 (5.6%) | 1 (1.1%) | 1 (1.1%) | 7 (7.9) | 6 |
| SENBA | 0 | 1 (8.3%) | – | – | – | – | – | 10 (83.3%) | – | – | – | – | – | 7 |
| Scheiner | 12 (20.7%) | 1 (1.7%) | 1 (1.7%) | 1 (1.7%) | 0 | 1 (1.7%) | 0 | 0 | 16 (27.6) | 4 (6.9%) | 42 (72.4) | 8 | ||
| Guerrero | 10 (90.9%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (9.1%) | 11 (100.0) | 0 | 0 | 0 | 0 | 6 |
| Yanagawa | – | – | – | – | – | – | – | – | – | – | – | – | – | 8 |
| Tornesello | – | – | – | – | – | – | – | – | – | – | – | – | – | 5 |
| Madsen | – | – | – | – | – | – | – | – | 24 (96.0) | – | – | – | 1 (4.0) | 8 |
| Prowse | 10 (71.4%) | 1 (7.1%) | 0 | 0 | 0 | 0 | 0 | 1 (7.1%) | 12 (85.7) | 1 (7.1%) | 0 | 1 (7.1%) | 2 (14.3) | 8 |
| Tornesello ML | 7 (63.6%) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (27.3%) | 10 (90.9) | 1 (9.1%) | 0 | 0 | 1 (9.1) | 9 |
| 26 (89.6%) | 2 (6.9%) | 0 | 0 | 1 (3.4%) | 0 | 0 | 0 | 29 (100.0) | 0 | 0 | 0 | |||
| Heideman | 22 (47.8%) | 2 (4.3%) | 0 | 0 | 0 | 2 (4.3%) | 1 (2.2%) | 15 (32.6%) | 44 (95.6) | 1 (2.2%) | 0 | 0 | 2 (4.3) | 5 |
| 2 (4.3%) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (4.3%) | 0 | 0 | |||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | ||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Protzel | 1 (14.3%) | – | – | – | – | – | – | 1 (14.3) | 1 (14.3%) | 1 (14.3) | 9 | |||
| 2 (28.6%) | – | – | – | – | – | – | – | 2 (28.6) | ||||||
| 0 | – | – | – | – | – | – | – | 0 | ||||||
| 0 | – | – | – | – | – | – | – | 0 | ||||||
| 0 | – | – | – | – | – | – | – | 0 | ||||||
| 2 (28.6%) | – | – | – | – | – | – | – | 2 (28.6) | 1 (14.3%) | 1 (14.3) | ||||
| Pascual | 32 (84.2%) | 4 (10.5%) | – | – | – | – | – | 2 (5.3%) | 38 (100.0) | – | – | – | – | 6 |
| Total | 418 (28.5%) | 34 (2.3%) | 10 (0.7%) | 12 (0.8%) | 5 (0.3%) | 21 (0.1%) | 4 (0.2%) | 59 (4.0%) | 539 (36.8) | 34 (2.3%) | 23 (1.6%) | 8 (0.5%) | 97 (6.6) | |
hr HPV: high-risk Human Papillomavirus; lr HPV: low-risk Human Papillomavirus; – :no involve; PCR: polymerase chain reaction; TFTE: Tumor fragments stored in TE solution [10-mM Tris hydrochloride (pH 7.5), 1mM ethylenediaminetetraacetic acid (EDTA)]; FFPE: formalin-fixed paraffin-embedded; PE: paraffin embedded; AHRQ: The Agency for Healthcare Research and Quality; SCC: squamous cell carcinoma
Figure 2.
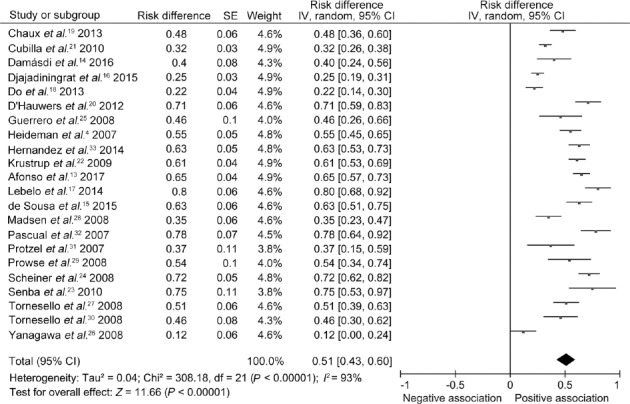
Comparison of the risk of HPV infection in penile cancer in different countries around the world. HPV: human papillomavirus; SE: standard error; IV: inverse variance methods; CI: confidence interval; df: degree of freedom.
Supplementary Table 2.
The number of human papillomavirus-positive samples detected by two different primers
| HPV | Total | ||
|---|---|---|---|
| Positive | Negative | ||
| Group | |||
| PCR G5+G6+ | 213 | 323 | 536 |
| PCR SPF10 | 146 | 279 | 425 |
| Total | 359 | 602 | 961 |
χ2=2.938, df=1, P=0.087. HPV: human papillomavirus; PCR: polymerase chain reaction
Description of HPV detection
Analyses of type-specific HPV prevalence rates were limited to 16 studies4,16,17,18,20,21,22,23,24,25,27,28,29,30,31,32,33 of SCC (n = 1270) with available data. Among these studies, approximately 19 HPV types were detected. Using all the cases tested as the denominator, the overall infection rate was 47.2% (95% CI: 31.0%–70.0%). The three most common types of HPV were HPV16 (28.5%), HPV18 (2.3%), and HPV6 (2.3%) (Supplementary Table 1). HPV18 and HPV 6 were detected in 34 (2.3%) of the 1465 cases that were tested for HPV subtypes. All other HPV subtypes had a prevalence of <2% and included the following: types 31, 33, 35, 42, 45, 52, 56, 53, 55, 58, 59, 62, 72, and 73.
HPV infection and PeCa infiltration
With respect to the correlation between HPV infection and invasive penile carcinoma, four studies15,16,22,33 were included, and no significant difference was found (Supplementary Table 3). This finding may be due to the small sample size.
Supplementary Table 3.
The invasiveness of penile carcinoma and the potential relationship with human papillomavirus infection
| HPV infection | Total | ||
|---|---|---|---|
| Positive | Negative | ||
| Group | |||
| In situ | 106 | 131 | 237 |
| Invasive | 124 | 142 | 266 |
| Total | 230 | 273 | 503 |
χ2=0.181, df=1, P=0.671. HPV: human papillomavirus
HPV infection in PeCa patients in various regions
The risks of HPV infection in patients with PeCa in disparate regions were analyzed by subgroup, and the results revealed that HPV infection rates varied on different continents. Six articles from Latin America were included and revealed that the random-effects model risk difference (RD) was 0.60 (95% CI: 0.43–0.76; P < 0.00001 for heterogeneity) (Figure 3a). The included studies consisted of 11 European articles, and the random-effects model RD was 0.50 (95% CI: 0.39–0.62; P < 0.00001 for heterogeneity) (Figure 3b). The articles from Asia demonstrated that the random-effects model RD was 0.35 (95% CI: 0.09–0.60; P < 0.00001 for heterogeneity) (Figure 3c). Significant heterogeneity was identified in the meta-analysis above. In addition, no sufficient studies from Africa or America were available for this meta-analysis. We also included three studies from Brazil, and the subgroup analysis revealed no significant change in HPV infection rates in Brazil from 2007 to 2017 (RD: 0.67, 95% CI: 0.61–0.72, χ2 = 1.68, P = 0.43) (Figure 4), indicating a lack of heterogeneity and suggesting that the prevalence of HPV infection is stable and higher than the global average rate.
Figure 3.

Subgroup analysis of the risk difference between HPV and penile cancer in different continents. (a) Subgroup analysis of the risk difference between HPV and penile cancer in Latin America. (b) Subgroup analysis of the risk difference between HPV and penile cancer in Europe. (c) Subgroup analysis of the risk difference between HPV and penile cancer in Asia. HPV: human papillomavirus; CI: confidence interval; SE: standard error; IV: inverse variance methods; df: degree of freedom.
Figure 4.
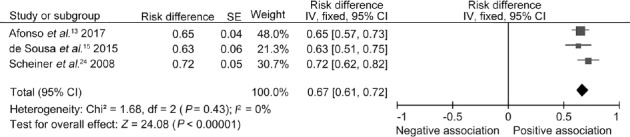
Subgroup analysis of the risk difference between HPV and penile cancer in recent years in Brazil. HPV: human papillomavirus; CI: confidence interval; SE: standard error; IV: inverse variance methods; df: degree of freedom.
Proportion of histologic types in penile carcinoma
Data from the selected studies were classified according to histological type. The overall HPV prevalence was obtained from a total of 1026 penile carcinoma cases: 597 keratinizing SCC cases (58.2%, 95% CI: 54.2%–62.2%), 28 nonkeratinizing SCC cases (2.7%, 95% CI: −3.3%–8.7%), 48 verrucous SCC cases (4.7%, 95% CI: −1.3%–10.7%), 40 warty SCC cases (3.9%, 95% CI: −2.1%–9.9%), 84 basaloid SCC cases (8.2%, 95% CI: 2.3%–14.1%), 47 cases of SCC with mixed warty and basaloid features (4.6%, 95% CI: −1.4%–10.6%), 25 papillary SCC cases (2.4%, 95% CI: −3.6%–8.4%), and 112 cases of other SCC mixed forms (10.9%, 95% CI: 5.1%–16.7%) (Supplementary Table 4). The histological subtypes of the other 481 cases were not known in the primary studies.
Supplementary Table 4.
The constituent ratio of different histological types of penile cancer
| Histological type | Constituent ratio (%) | Number of cases | 95% CI |
|---|---|---|---|
| Keratinizing SCC | 58.2 | 597 | 54.2–62.2 |
| Basaloid SCC | 8.2 | 84 | 2.3–14.1 |
| Verrucous SCC | 4.7 | 48 | −1.3–10.7 |
| Non-keratinizing | 2.7 | 28 | −3.3–8.7 |
| Warty | 3.9 | 40 | −2.1–9.9 |
| Papillary | 2.4 | 25 | −3.6–8.4 |
| Warty-basaloid | 4.6 | 47 | −1.4–10.6 |
| Combined | 4.4 | 45 | −1.6–10.4 |
| Others | 10.9 | 112 | 5.1–16.7 |
| Total | 100 | 1026 | −4.3–26.6 |
SCC: squamous cell carcinoma; CI: confidence interval
Relationship between HPV type and histology of penile carcinoma
HPV infection in penile tumors is reportedly associated with various morphological changes, and determination of the subtype association can provide a better estimate of the HPV-related cancer burden and its preventable grade. The observed specific HPV contributions by histological type were as follows: basaloid SCC 85.5% (95% CI: 77.2%–93.8%); warty SCC 50.0% (95% CI: 35.2%–64.8%); nonkeratinizing/typical SCC 28.6% (95% CI: 11.9%–45.3%); keratinizing SCC 33.8% (95% CI: 29.9%–37.7%); and verrucous SCC 32.0% (95% CI: 16.7%–44.9%) (Supplementary Table 5).
Supplementary Table 5.
The relationship between histological type and human papillomavirus infection
| Histological type | HPV positive (%) | Total | 95% CI % |
|---|---|---|---|
| Keratinizing SCC | 33.8 | 574 | 29.9–37.7 |
| Non-keratinizing | 28.6 | 28 | 11.9–45.3 |
| Basaloid SCC | 85.5 | 69 | 77.2–93.8 |
| Verrucous SCC | 32.0 | 50 | 16.7–44.9 |
| Warty | 50.0 | 44 | 35.2–64.8 |
| Papillary | 16.7 | 24 | 1.8–31.6 |
| Combined types | 28.3 | 53 | 16.2–40.4 |
HPV: human papillomavirus; SCC: squamous cell carcinoma; CI: confidence interval
Relationship between HPV infection and patient age
In our study, four studies22,25,26,33 had available information on patient age at diagnosis, which allowed us to observe the relationship between HPV infection and patient age. A total of 274 samples with HPV types detected from four studies were divided into two groups: older than 60 years and younger than 60 years (Supplementary Table 6). Pearson's Chi-squared test was used to identify a correlation between these groups and the outcome (χ2 = 22.205, df = 1, P < 0.001). However, the significance was restricted by the limited sample size, and patients may delay a visit to the doctor, thus causing a delay in diagnosis.
Supplementary Table 6.
Age at penile cancer diagnosis and the relationship with human papillomavirus infection
| HPV infection | Total | ||
|---|---|---|---|
| Positive | Negative | ||
| Age (year) | |||
| <60 | 47 | 24 | 71 |
| >60 | 102 | 101 | 203 |
| Total | 149 | 125 | 274 |
χ2=22.205, df=1, P<0.001. HPV: human papillomavirus
HPV infection and the location of PeCa
Five articles15,22,26,28,33 containing 442 cases demonstrated the incidence rates of different sites of PeCa, with glans penis carcinoma being the most common, followed by foreskin carcinoma (Supplementary Table 7). Because studies examining the correlation between HPV infection and penile carcinoma locations are lacking, we could not define this relationship.
Supplementary Table 7.
Distribution of tumor sites among penile cancers
| Region | Number of cases | Component ratio (%) |
|---|---|---|
| Glans | 201 | 45.50 |
| Foreskin | 87 | 19.70 |
| Corpus | 5 | 1.10 |
| Glans and foreskin | 29 | 6.60 |
| Non-evaluable | 120 | 27.10 |
| Total | 442 | 100 |
HPV infection and differentiation of PeCa
Various degrees of differentiation exist in cases of PeCa. We collected 408 cases from 6 articles16,17,20,24,26,33 containing the original tumor histological subtype and relevant statistics. Using Stata 15.0 for the Chi-squared test, we identified a significant statistical outcome (χ2 = 22.205, df = 2, P < 0.001; Supplementary Table 8).
Supplementary Table 8.
Constituent ratio of histological differentiation in penile tumors with human papillomavirus infection
| Differentiation | HPV-positive | Total | HPV-positive rate (%) |
|---|---|---|---|
| Well | 48 | 160 | 30.0 |
| Moderately | 117 | 215 | 54.4 |
| Poorly | 27 | 60 | 45.0 |
χ2=22.205, df=2, P<0.001 HPV: human papillomavirus
DISCUSSION
In the molecular evaluation, HPV infection was observed in 51% of lesions in the past 10 years, which is higher than the rate of 46.9% reported in earlier decades by Miralles-Guri et al.34 and the most common type found was HPV16. With respect to location, 45.50% of the tumors were located in the glans, and the most common types were squamous cell carcinoma (73.8%). These results correspond with those found in the literature.
Apart from types 6 and 11, almost no other single low-risk HPV has been found to contribute to PeCa. Previous studies have reported no significant difference in age among patients with various subtypes of SCC.21 The presence of HPV and the distribution of HPV genotypes were not associated with any single age group. However, our study found that, on average, diagnosis predominates in patients of advanced age (>60 years), which may suggest that men seek health services very late in life and that young men are also affected but in smaller percentage.
Previous research has shown no correlation between HPV status and histological subtype (P = 0.51) or between HPV status and stage stratification.22 However, our findings indicated that the basaloid (85.5%, 95% CI: 77.2%–93.8%) and warty (50.0%, 95% CI: 35.2%–64.8%) subtypes are more likely to be HPV-positive than other subtypes. These findings are consistent with the WHO classification guidelines, indicating that HPV-related carcinomas are mostly basaloid and warty SCC.8 A higher proportion of basaloid cells correspond to a higher likelihood of HPV positivity in that tumor category. This cell type is morphologically similar to the predominant cell type observed in most invasive uterine cervical carcinomas, a known etiologically HPV-related cancer.21 HPV-positive tumors tend to exhibit warty and/or basaloid features and correspond to a moderate histological grade, whereas HPV-negative carcinomas usually correspond to well-differentiated tumors. Most reports validated the association of HPV with basaloid and warty carcinomas,35,36 which is consistent with our results. Verrucous carcinoma is defined as a non-HPV-related subtype of SCC, with carcinoma cuniculatum as a variant in the WHO classification guidelines.8 However, the incidence of HPV-positive verrucous carcinoma was calculated to be 32% in our studies. Similarly, in some studies, approximately one-third of usual and verrucous carcinomas were also reported to be HPV positive.36 We consider that the differences in the prevalence of virus in penile carcinomas, either in general or special subtypes, are highly variable. In addition, non-HPV-related carcinomas may indicate no involvement of HPV in the pathogenesis, such as the P16ink4a overexpression-negative carcinomas, but such cases may not include existing HPV infection, which has no role in the formation of cancer.
At present, the pathogenesis of PeCa is mostly related to overexpression of P16ink4a.18 In addition, Sebastian et al.37 detected two genes related to the pathogenesis of PeCa by immunohistochemistry: P16ink4a overexpression identifies HPV-HR-induced penile carcinogenesis independent of the HPV-HR genotype, and positive p53 expression with P16ink4a negativity identifies HPV-negative cancers. In summary, the present study indicates that HPV plays an important role in the pathogenesis of PeCa.
The presence of metastatic disease in the inguinal lymph nodes is one of the most important prognostic factors in PeCa.38 Unfortunately, the included data regarding the relationship between lymph node metastasis and HPV were fairly limited and of little statistical value; therefore, we did not involve lymph node-related results. However, a study by Feber et al.39 in which methylation of penile oncogenes was first sequenced showed that a 4-gene epi-signature accurately predicted lymph node metastasis in an independent cohort (area under the curve [AUC] of 89%). When used as a predictive methylation index for each sample, the predictive accuracy of this signature (90% methylation array and 89% for quantitative methylation specific polymerase chain reaction [qMSP]) to identify the presence of lymph node metastasis is at least comparable to if not better than the sensitivity of sentinel lymph node biopsy. They also explored epigenetic alterations associated with PeCa-related HPV infection and defined a 30-loci lineage without an HPV-specific epi-signature or HPV16 signature that is an independent predictor of disease-free survival and suggests distinct HPV subtype-specific epigenetic alterations.
This article identified genotype-specific HPV cases from studies using more sensitive PCR measures to allow investigation of HPV type distributions in PeCa in a large sample. These data also allowed us to investigate the differences between histological subtypes that are usually limited in the number of individual publications.
The included articles all included comparisons of cross-sectional studies, which may not be of high value. Studies on PeCa are limited, and most samples rely on FFPE tissue for HPV detection. Moreover, persuading healthy people to participate in HPV detection is extremely difficult, complicating the establishment of a control group. RCTs for related research have not been found.
Because the specific phenotype of mixed HPV infection was not clear in the included original literature, the analysis effect of the data may not be optimal. Fortunately, unknown mixed HPV infections accounted for only a small proportion of the overall sample. Due to the research type, the assessment of multiple infectious contributions is limited. Our results are based on cross-sectional data, which may not reflect the natural history of the disease. However, because the incidence of PeCa is relatively low, conducting a better longitudinal study to examine disease progression is difficult. HPV testing alone is not sufficient to prove cause and effect. However, HPV has been recognized as a tumor pathogen, and HPV infection may therefore have the same effect on penile tumors, given the similar histopathological features between men and women. Merck announced the completion of an initial study, demonstrating that Gardasil has 90% efficacy in preventing external genital lesions caused by HPV types 6, 11, 16, and 18 in men aged 16–26 years.40 More extensive and effective vaccinations should be applied to prevent HPV-related malignancies. As in cervical cancer, hrHPV is also a high-risk factor for PeCa; therefore, the countries and regions with high rates of PeCa and HPV infection, such as South Africa and Brazil, should promote HPV vaccination. HPV vaccines can even be considered for infertile men with HPV infection, spouses who are HPV positive, and gay people, especially with the development of therapeutic vaccines. This study may provide a reference for clinical diagnosis and treatment and suggests that the available HPV vaccine is urgently needed in high-risk populations.
AUTHOR CONTRIBUTIONS
YBY and YHW conceived the study, participated in its design, and coordinated and drafted the manuscript. XCY and MLW collected the data. YZ performed the statistical analysis. YL and HTN participated in critical revision of the manuscript and approved the manuscript. All authors read and approved the final manuscript and agreed with the order of presentation of the authors.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
We would like to thank the authors who provided the data. This study was financially supported by the National Natural Science Foundation of China (Grant No. 81772713, No. 81472411, No. 81401899, and No. 81372752), the Taishan Scholar Program of Shandong Province (No. tsqn20161077), the Key Research and Development Program of Shandong Province (No. 2018GSF118197), the China Postdoctoral Science Foundation (No. 2017M622144), the Natural Science Foundation of Shandong Province (No. ZR2014HM088), and the Qingdao Postdoctoral Application Research Project and Qingdao Young Scientist Applied Basic Research Fund (No. 15-9-1-51-jch and No.15-9-1-105-jch).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Noble MJ. Malignancies of the penis and urethra. In: Andrew CN, Stephen JJ, Inderbir SG, Eric AK, Raymond R, et al., editors. Operative Urology at the Clevel and Clinic. Cleveland: Cleveland Clinic Foundation; 2006. pp. 415–30. [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine (Auckl) 2006;24:S11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 3.Delgado MS, Martínez FA, Márquez GP, González BB, Cosano AZ, et al. Penile cancer. Review of 18 cases. Actas Urol Esp. 2003;27:797–802. doi: 10.1016/s0210-4806(03)73017-4. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 4.Heideman DA, Waterboer T, Pawlita M, Diemen PD, Nindl I, et al. Human papillomavirus-16 is the predominant type etiologically involved in penile squamous cell carcinoma. J Clin Oncol. 2007;25:4550–6. doi: 10.1200/JCO.2007.12.3182. [DOI] [PubMed] [Google Scholar]
- 5.Diorio GJ, Giuliano AR. The role of human papilloma virus in penile carcinogenesis and preneoplastic lesions: a potential target for vaccination and treatment strategies. Urol Clin North Am. 2016;43:419–25. doi: 10.1016/j.ucl.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Immunization Expert Work Group. Committee on Adolescent Health Care. Committee Opinion No. 704: human papillomavirus vaccination. Obstet Gynecol. 2017;129:e173–8. doi: 10.1097/AOG.0000000000002052. [DOI] [PubMed] [Google Scholar]
- 7.Quality USDO. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health & Human Services. 2013. [Last accessed on 2016 Jul 21]. Available from http://pesquisa.bvsalud.org/ripsa/resource/pt/lis-LISBR1.1-20556 .
- 8.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 10.Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, et al. Celiac Disease. Evidence Report/Technology Assessment No. 104. Rockville: Agency for Healthcare Research and Quality; 2004. [Google Scholar]
- 11.Chen YH, Liang DU, Geng XY, Liu GJ. Implement meta-analysis with non-comparative binary data in RevMan software. Chin J Evid Based Med. 2014;7:889–96. Article in Chinese. [Google Scholar]
- 12.Wang PX, Li HT, Liu JM. Meta-analysis of non-comparative binary outcomes and its solution by Stata. J Evid Based Med. 2012;12:52–5. Article in Chinese. [Google Scholar]
- 13.Afonso LA, Carestiato FN, Ornellas AA, Ornellas P, Rocha WM, et al. Human papillomavirus, Epstein-Barr virus, and methylation status of p16ink4a in penile cancer. J Med Virol. 2017;89:1837–43. doi: 10.1002/jmv.24833. [DOI] [PubMed] [Google Scholar]
- 14.Damásdi M, Jakab F, Kovács K, Oldal M, Kemenesi G, et al. Prevalence and type diversity of human papillomaviruses in penile cancers in hungary. Pathol Oncol Res. 2016;22:643–6. doi: 10.1007/s12253-015-0026-5. [DOI] [PubMed] [Google Scholar]
- 15.de Sousa ID, Vidal FC, Branco Vidal JP, de Mello GC, do Desterro Soares Brandão Nascimento M, et al. Prevalence of human papillomavirus in penile malignant tumors: viral genotyping and clinical aspects. BMC Urol. 2015;15:13. doi: 10.1186/s12894-015-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djajadiningrat RS, Jordanova ES, Kroon BK, Van WE, De JJ, et al. Human papillomavirus prevalence in invasive penile cancer and association with clinical outcome. J Urol. 2015;193:526–31. doi: 10.1016/j.juro.2014.08.087. [DOI] [PubMed] [Google Scholar]
- 17.Lebelo RL, Boulet G, Nkosi CM, Bida MN, Bogers JP, et al. Diversity of HPV types in cancerous and pre-cancerous penile lesions of South African men: implications for future HPV vaccination strategies. J Med Virol. 2014;86:257–65. doi: 10.1002/jmv.23730. [DOI] [PubMed] [Google Scholar]
- 18.Do HT, Koriyama C, Khan NA, Higashi M, Kato T, et al. The etiologic role of human papillomavirus in penile cancers: a study in Vietnam. Br J Cancer. 2013;108:229–33. doi: 10.1038/bjc.2012.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaux A, Netto GJ, Rodríguez IM, Barreto JE, Oertell J, et al. Epidemiologic profile, sexual history, pathologic features, and human papillomavirus status of 103 patients with penile carcinoma. World J Urol. 2013;31:861–7. doi: 10.1007/s00345-011-0802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Hauwers KW, Depuydt CE, Bogers JJ, Noel JC, Delvenne P, et al. Human papillomavirus, lichen sclerosus and penile cancer: a study in Belgium. Vaccine. 2012;30:6573–7. doi: 10.1016/j.vaccine.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Cubilla AL, Balejo L. The basaloid cell is the best tissue marker for human papillomavirus in invasive penile squamous cell carcinoma: a study of 202 cases from Paraguay. Am J Surg Pathol. 2010;34:104–14. doi: 10.1097/PAS.0b013e3181c76a49. [DOI] [PubMed] [Google Scholar]
- 22.Krustrup D, Jensen HL, van den Brule AJ, Frisch M. Histological characteristics of human papilloma-virus-positive and -negative invasive and in situ squamous cell tumours of the penis. Int J Exp Pathol. 2009;90:182–9. doi: 10.1111/j.1365-2613.2008.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senba M, Mori N, Wada A, Fujita S, Yasunami M, et al. Human papillomavirus genotypes in penile cancers from Japanese patients and HPV-induced NF-κB activation. Oncol Lett. 2010;1:267–72. doi: 10.3892/ol_00000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheiner MA, Campos MM, Ornellas AA, Chin EW, Ornellas MH, et al. Human papillomavirus and penile cancers in Rio de Janeiro, Brazil: HPV typing and clinical features. Int Braz J Urol. 2008;34:467–76. doi: 10.1590/s1677-55382008000400009. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero D, Guarch R, Ojer A, Casas JM, Ropero S, et al. Hypermethylation of the thrombospondin-1 gene is associated with poor prognosis in penile squamous cell carcinoma. BJU Int. 2008;102:747–55. doi: 10.1111/j.1464-410X.2008.07603.x. [DOI] [PubMed] [Google Scholar]
- 26.Yanagawa N, Osakabe M, Hayashi M, Tamura G, Motoyama T. Detection of HPV-DNA, p53 alterations, and methylation in penile squamous cell carcinoma in Japanese men. Pathol Int. 2008;58:477–82. doi: 10.1111/j.1440-1827.2008.02259.x. [DOI] [PubMed] [Google Scholar]
- 27.Tornesello ML, Duraturo ML, Losito S, Botti G, Pilotti S, et al. Human papillomavirus genotypes and HPV16 variants in penile carcinoma. Int J Cancer. 2008;122:132–7. doi: 10.1002/ijc.23062. [DOI] [PubMed] [Google Scholar]
- 28.Madsen BS, van den Brule AJ, Jensen HL, Wohlfahrt J, Frisch M. Risk factors for squamous cell carcinoma of the penis – population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev. 2008;17:2683–91. doi: 10.1158/1055-9965.EPI-08-0456. [DOI] [PubMed] [Google Scholar]
- 29.Prowse DM, Ktori EN, Chandrasekaran D, Prapa A, Baithun S. Human papillomavirus-associated increase in p16INK4A expression in penile lichen sclerosus and squamous cell carcinoma. Br J Dermatol. 2008;158:261–5. doi: 10.1111/j.1365-2133.2007.08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tornesello ML, Duraturo ML, Guida V, Losito S, Botti G, et al. Analysis of TP53 codon 72 polymorphism in HPV-positive and HPV-negative penile carcinoma. Cancer Lett. 2008;269:159–64. doi: 10.1016/j.canlet.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Protzel C, Knoedel J, Zimmermann U, Woenckhaus C, Poetsch M, et al. Expression of proliferation marker Ki67 correlates to occurrence of metastasis and prognosis, histological subtypes and HPV DNA detection in penile carcinomas. Histol Histopathol. 2007;22:1197–204. doi: 10.14670/HH-22.1197. [DOI] [PubMed] [Google Scholar]
- 32.Pascual A, Pariente M, Godínez JM, Sánchez-Prieto R, Atienzar M, et al. High prevalence of human papillomavirus 16 in penile carcinoma. Histol Histopathol. 2007;22:177–83. doi: 10.14670/HH-22.177. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez BY, Goodman MT, Unger ER, Steinau M, Powers A, et al. Human papillomavirus genotype prevalence in invasive penile cancers from a registry-based United States population. Front Oncol. 2014;4:9. doi: 10.3389/fonc.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miralles-Guri C, Bruni L, Cubilla AL, Castellsague X, Bosch FX, et al. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62:870–8. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- 35.Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449–57. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- 36.Rubin MA, Kleter B, Zhou M, Ayala G, Cubilla AL, et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159:1211–8. doi: 10.1016/S0002-9440(10)62506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebastian M, Stephan S, Elke W, Sigrid R. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J Am Acad Dermatol. 2013;69:73–81. doi: 10.1016/j.jaad.2012.12.973. [DOI] [PubMed] [Google Scholar]
- 38.Horenblas S, Van TH. Squamous cell carcinoma of the penis. IV. Prognostic factors of survival: analysis of tumor, nodes and metastasis classification system. J Urol. 1994;151:1239–43. doi: 10.1016/s0022-5347(17)35221-7. [DOI] [PubMed] [Google Scholar]
- 39.Feber A, Arya M, De WP, Saqib M, Nigam R, et al. Epigenetics markers of metastasis and HPV induced tumourigenesis in penile cancer. Clin Cancer Res. 2015;21:1196–206. doi: 10.1158/1078-0432.CCR-14-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chitale R. Merck hopes to extend gardasil vaccine to men. J Natl Cancer Inst. 2009;101:222–3. doi: 10.1093/jnci/djp014. [DOI] [PubMed] [Google Scholar]


