Abstract
Inhibin B is a gonadal hormone that downregulates the pituitary production of follicle-stimulating hormone (FSH). In recent years, inhibin B has proved to be an excellent marker of spermatogenesis and even a predictive factor for the recovery of fertility in patients undergoing orchiectomy and antineoplastic treatments. We propose to study inhibin B levels in orchiectomised testicular cancer patients, in order to identify a minimum value representative of normal semen quality. This retrospective study evaluates hormonal and semen parameters of 290 normozoospermic patients attending the Laboratory of Seminology - Sperm Bank “Loredana Gandini” (Rome, Italy) for cryopreservation of seminal fluid following a diagnosis of testicular cancer (TC group) and 117 healthy, normozoospermic men as a control group (CTR group). The percentile distribution of gonadotropin and inhibin B values in the TC and CTR groups was analyzed. There was a statistically significant difference between the two groups in the levels of all hormones (P ≤ 0.001) and in all semen parameters (P < 0.05). About 20% of TC patients revealed inhibin B levels below the 5th percentile of CTR group, despite normozoospermia, and 31.4% had normal spermatogenesis in the presence of FSH values >95th percentile of CTR group. Orchiectomised patients for testicular cancer presented inhibin B levels lower than healthy patients, despite normozoospermia. Our study revealed the poor sensitivity of the current inhibin B reference range when applied to monorchidic patients, suggesting the need to establish more representative ranges to enable more appropriate counseling in relation to the patient's new endocrine condition.
Keywords: inhibin B, orchiectomy, reference range, testicular cancer
INTRODUCTION
Inhibin is a gonadal glycoprotein hormone from the transforming growth factor-beta (TGF-β) superfamily. In adult men, it downregulates the production of follicle-stimulating hormone (FSH) by the gonadotropic cells of the anterior pituitary.1 In males, the predominant isoform and the only one detectable in the circulation is inhibin B.2 The main sources of inhibin B production are the Sertoli cells, which are assisted by other cell types in the assembly and secretion processes.3 Inhibin B levels vary from infancy to puberty before reaching typical adult concentrations, with a peak in the first month of life and a trough at 6–10 years. The early postnatal rise is presumably due to activation of the hypothalamic–pituitary–gonadal (HPG) axis and reflects the proliferation of the Sertoli cells at this time. In prepuberty, its levels are independent of the presence of actively proliferating germ cells and rise under the stimulation of FSH. After puberty, inhibin B secretion is mainly controlled by spermatogenesis.4 Baseline inhibin B levels are defined at the point that Sertoli cell proliferation ends and spermatogenesis begins and can be considered as a marker of Sertoli cell density.
The positive correlation of serum inhibin B levels, spermatogenesis, and testicular volume is a clearly demonstrated concept in the literature.5,6 In recent years, inhibin B has assumed particular importance in the field of andrology, proving to be an excellent marker of spermatogenesis and a predictive factor for the recovery of fertility in patients undergoing orchiectomy and antineoplastic treatments.7,8 The reference ranges for serum levels of inhibin B accepted by the international scientific community were determined on the basis of population studies of healthy men who were normozoospermic according to the semen parameters proposed by the World Health Organization (WHO).9,10,11,12 However, there are no literature reports of studies investigating the correlation between inhibin B and spermatogenesis in patients who have undergone orchiectomy for testicular cancer. As inhibin B is produced almost exclusively by the Sertoli cells, its serum concentration after removal of the affected testicle could vary in relation to the reduction in testicular mass.
While testicular tumors are rare in the general population, they are the most common cancer in men of reproductive age (second to fifth decade of life), with a prevalence of 1%–1.5%, an incidence varying by ethnic origin, and a rising trend over the last 30 years.13,14,15 The use of chemo- and radiotherapy and refinement of surgical techniques in recent decades has resulted in an excellent prognosis for testicular germ cell tumors.16 Attention to the reproductive health of these patients is therefore of paramount importance: approximately 50% of survivors state their desire for fatherhood after treatment, including 75% of patients without children at the time of diagnosis.17 Most studies show that spermatogenesis is already affected in testicular cancer (TC) patients at the time of diagnosis, in comparison with both the healthy population and patients with other types of cancer. The reduction in sperm number is accompanied by changes to the HPG axis, with a rise in FSH and luteinizing hormone (LH) and a drop in inhibin B, while testosterone levels are essentially unaffected.18,19,20 The measurement of FSH and inhibin B levels are complementary tools in andrological diagnostics and the assessment of male reproductive health. This study thus proposes to investigate inhibin B levels in TC patients who have undergone unilateral orchiectomy, in order to identify a minimum value representative of normal semen quality.
PARTICIPANTS AND METHODS
Study participants
The study was approved by Ethical Committee “Sapienza” University, Policlinico Umberto I, Rome, Italy. Written informed consent was obtained from all study participants. We conducted a retrospective study on 290 patients attending the Seminology Laboratory Sperm Bank at the Sapienza University of Rome's Department of Experimental Medicine, Medical Pathophysiology Section, between January 2012 and November 2015 for cryopreservation of seminal fluid following a diagnosis of testicular cancer (TC group). Only patients with a normal total sperm number according to the WHO 2010 reference parameters9 were selected, to ensure that the serum inhibin B levels measured were representative of normozoospermia. All patients underwent semen examination and hormone assay on the day of cryopreservation, i.e., 1 month after the orchiectomy and before beginning antineoplastic therapy. This was a single-center study. All hormone and semen analyses were carried out in the seminology and hormone laboratories of our department. The study also included a control group of 117 healthy, normozoospermic men attending our center for an andrological checkup (CTR group). Participants with sperm concentration <39 × 106 ml−1, previous anabolic steroid use, other andrological diseases affecting semen quality, hypogonadotropic hypogonadism, Klinefelter syndrome or other genetic or chromosomal disorders, and other extragonadal neoplastic diseases were excluded from the study.
Evaluation of patients
A blood sample was collected from all patients for measurement of FSH, LH, inhibin B, total testosterone, and sex hormone-binding globulin (SHBG). Samples were collected at 8 a.m. after overnight fasting. Serum FSH, LH, and testosterone were measured by chemiluminescent microparticle immunoassay (CMIA, Architect System; Abbott Laboratories, Abbott Park, IL, USA), with detection limits of 0.05 IU l−1, 0.07 IU l−1, and 0.28 nmol l−1, respectively. Intra- and inter-assay coefficients of variation were 3.1% and 7.0% at 3.2 IU l−1 (FSH), 3.6% and 5.1% at 3.3 IU l−1 (LH), and 2.1% and 3.6% at 10.08 nmol l−1 (testosterone), respectively. Normal ranges for adults were 1.38–9.58 IU l−1 (FSH), 1.80–8.16 IU l−1 (LH), and 9.4–33.5 nmol l−1 (testosterone), respectively. Serum concentrations of inhibin B were measured by enzyme-linked immunosorbent assay (ELISA; GEN II, Beckman Coulter laboratories, Brea, CA, USA). The detection limit was 7.0 pg ml−1 and the intra- and inter-assay coefficients of variation were 3.1% and 6.8%, respectively, at 126 pg ml−1. The normal postpubertal range was 80–350 pg ml−1.
Semen samples were collected by masturbation directly into a sterile plastic container after 2–7 days of sexual abstinence and examined by optical microscope (Leica DM5000B, Leica Microsystems, Wetzlar, Germany) according to the WHO criteria (2010).9 The following variables were assessed: sperm concentration (×106 ml−1), total sperm number (×106 per ejaculate), progressive motility (%), and morphology (% abnormal forms).
Statistical analyses
Continuous variables are presented as mean ± standard deviation (s.d.) or median and quartiles; categorical variables are summarized with count and percentage. Differences were evaluated by the Student's t-test or Mann–Whitney U test, depending on the shape of the distribution curve. The normality of the distribution curve was evaluated using the Kolmogorov–Smirnov test. The correlation coefficients among the variables considered were evaluated using Pearson's or Spearman's test, where appropriate. The probability values are two-sided; P < 0.05 was considered to indicate statistical significance. All computations were carried out with the SPSS 24.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The mean age of the TC group (31.0 [s.d.: 6.8] years) was similar to that of the CTR group (29.6 [s.d.: 7.4] years, P = 0.06). Clinical stage was available for 206/290 patients: 68.0% were stage I , 17.5% stage IIA, 6.8% stage IIB, and 7.8% stage IIC (Supplementary Table 1). There was a statistically significant difference between the two groups in all semen parameters (all P < 0.05, Table 1). Apart from SHBG (P = 0.088), there was a statistically significant difference between the two groups in the levels of all hormones (all P < 0.001, Table 2).
Supplementary Table 1.
Description of clinical stage and histology of the study group (TC, n=206)
| Clinical stage | Histology |
|---|---|
| Stage I (n=140) | 71.4% (seminoma) |
| 19.3% (mixed germ cell tumors) | |
| 9.3% (embryonal carcinoma) | |
| Stage IIA (n=36) | 44.4% (seminoma) |
| 33.3% (mixed germ cell tumors) | |
| 16.7% (embryonal carcinoma) | |
| 5.6% (Yolk sac tumor) | |
| Stage IIB (n=14) | 43.0% (seminoma) |
| 57.0% (mixed germ cell tumors) | |
| Stage IIC (n=16) | 56.2% (seminoma) |
| 37.5% (mixed germ cell tumors) | |
| 6.3% (embryonal carcinoma) |
Table 1.
Mean±standard deviation and median (25% quartile [Q1] –75% quartile [Q3]) of semen parameters of the testicular cancer and control groups and their statistical significance (Mann-Whitney U test)
| Group | Volume (ml) | Sperm concentration (×106 ml-1) | Total sperm number (×106 per ejaculate) | Progressive motility (%) | Abnormal forms (%) |
|---|---|---|---|---|---|
| TC | |||||
| Mean±s.d. | 3.6±1.6 | 50.6±37.7 | 172.4±139.7 | 44.4±10.3 | 81.1±5.3 |
| Median (Q1–Q3) | 3.2 (2.5–4.2) | 42.0 (27.0–60.2) | 135.0 (77.0–208.5) | 45.0 (40.0-50.0) | 80.0 (78.0–85.0) |
| CTR | |||||
| Mean±s.d. | 3.1±1.3 | 84.5±63.5 | 251.7±213.4 | 47.8±10.4 | 83.5±9.8 |
| Median (Q1–Q3) | 3.0 (2.2–4.0) | 72.0 (45.0–98.0) | 187.0 (105.0–340.0) | 50.0 (40.0–55.0) | 86.0 (80.0–88.0) |
| P | 0.008 | <0.001 | <0.001 | 0.002 | 0.015 |
s.d.: standard deviation; TC: testicular cancer; CTR: control
Table 2.
Mean±standard deviation and median (25% quartile [Q1] –75% quartile [Q3]) of hormone parameters of the testicular cancer and control groups and their statistical significance (Mann-Whitney U test)
| Group | FSH (mIU ml-1) | LH (mIU ml-1) | Inhibin B (pg ml-1) | SHBG (nmol l-1) | Testosterone (nmol l-1) |
|---|---|---|---|---|---|
| TC | |||||
| Mean±s.d. | 6.5±4.5 | 4.2±3.0 | 97.8±44.8 | 33.9±14.7 | 18.4±6.5 |
| Median (Q1–Q3) | 5.74 (4.00–8.37) | 3.93 (2.74–5.14) | 92.5 (68.3–120.0) | 32.0 (23.1–40.9) | 17.7 (13.9–21.3) |
| CTR | |||||
| Mean±s.d. | 3.2±1.9 | 3.1±1.3 | 181.8±76.9 | 42.1±49.1 | 22.0±6.8 |
| Median (Q1–Q3) | 2.70 (1.84–4.13) | 2.87 (2.11–3.99) | 172.0 (127.0–211.0) | 36.8 (27.8–46.7) | 21.1 (16.3–26.1) |
| P | <0.001 | <0.001 | <0.001 | 0.088 | <0.001 |
s.d.: standard deviation; TC: testicular cancer; CTR: control; FSH: follicle stimulating hormone; LH: luteinizing hormone; SHBG: sex hormone binding globulin
Correlations of gonadotropin, inhibin B, and semen parameters were performed for the TC group. Table 3 shows the correlation coefficient values and their significance obtained using Spearman's test. There was a positive correlation between log of absolute values of inhibin B and total sperm number and no correlation with progressive motility (Figure 1a and 1b). Then we found a negative correlation between log of absolute values of gonadotropin and total sperm number and progressive motility (Figure 1c and 1d). Moreover, multivariate analysis showed a significant association between inhibin B levels and total sperm number (P = 0.008) but not sperm progressive motility (P = 0.802); on the contrary, FSH was significantly associated with progressive motility (P = 0.009) but not with total sperm number (P = 0.162) in this particular group of patients.
Table 3.
Spearman’s correlation coefficients of semen and hormone parameters
| FSH | LH | Inhibin B | Volume | Sperm concentration | Total sperm number | Progressive motility | Abnormal forms | |
|---|---|---|---|---|---|---|---|---|
| FSH | 1.000 | 0.545** | −0.341** | −0.149* | −0.191** | −0.257** | −0.155** | 0.096 |
| LH | 0.545** | 1.000 | −0.157** | −0.029 | −0.194** | −0.205** | −0.116* | 0.068 |
| Inhibin B | −0.341** | −0.157** | 1.000 | 0.173** | 0.249** | 0.347** | 0.095 | 0.032 |
*P<0.05; **P<0.01. FSH: follicle-stimulating hormone; LH: luteinizing hormone; SHBG: sex hormone-binding globulin
Figure 1.
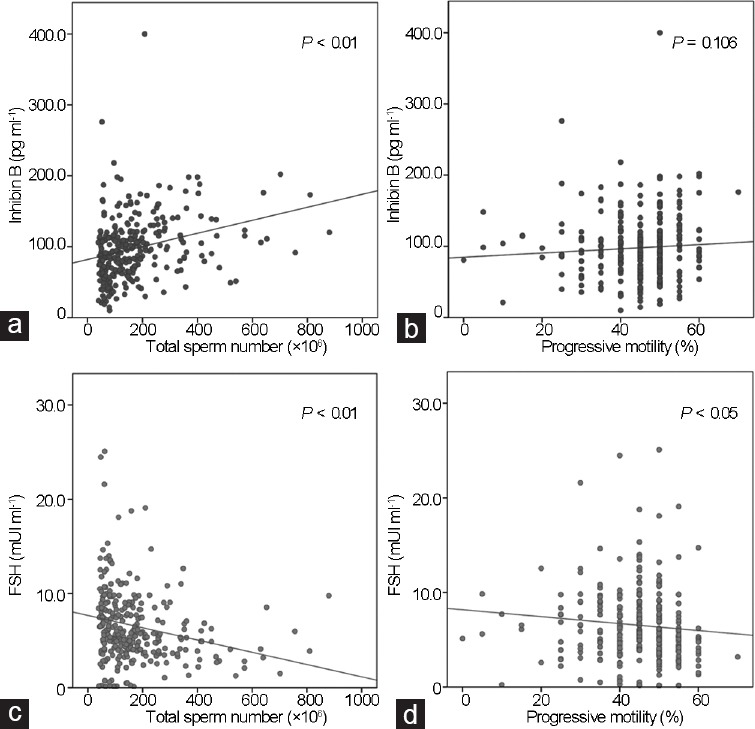
Spearman's rank correlation test in the testicular cancer (TC) patients: (a) inhibin B values and total sperm number (P < 0.01); (b) inhibin B values and percentage of progressive motility (NS, P = 0.106); (c) FSH values and total sperm number (P < 0.01); (d) FSH values and percentage progressive motility (P < 0.05). FSH: follicle-stimulating hormone; NS: not significant.
To identify an inhibin B cutoff value corresponding to normal spermatogenesis, the percentile distribution of gonadotropin and inhibin B values in the CTR group was analyzed (Table 4). The 5th percentile of the CTR group was taken as the cutoff (62.75 pg ml−1). The same analysis was then carried out for the TC group (Table 4), revealing a lower 5th percentile value, 35.52 pg ml−1, than that seen in the controls. In fact, 19.3% (56/290) of patients presented inhibin B levels below 62.75 pg ml−1 in association with normozoospermia. Evaluation of FSH values in this subgroup revealed a mean of 8.7 (s.d.: 7.7; median: 8.0) mIU ml−1, with a mean total sperm number of 119.8 × 106 (s.d.: 101.3 × 106; median: 81.3 × 106) per ejaculate (Table 5). In CTR group, FSH values were also divided into percentiles, assuming the 95th percentile (7.62 mIU ml−1) as the reference cutoff. This revealed that 31.4% (91/290) of the TC patients had normal spermatogenesis in the presence of elevated FSH values.
Table 4.
Subdivision in percentiles of follicle-stimulating hormone, luteinizing hormone, and inhibin B levels in the testicular cancer and control groups
| Percentile (%) | TC group | CTR group | ||||
|---|---|---|---|---|---|---|
| FSH (mIU ml−1) | LH (mIU ml−1) | Inhibin B (pg ml−1) | FSH (mIU ml−1) | LH (mIU ml−1) | Inhibin B (pg ml−1) | |
| 2.5 | 0.97 | 1.13 | 50.93 | 0.21 | 0.26 | 26.75 |
| 5 | 1.12 | 1.17 | 62.75 | 0.94 | 0.91 | 35.52 |
| 10 | 1.28 | 1.48 | 86.92 | 2.57 | 1.89 | 45.49 |
| 25 | 1.84 | 2.11 | 127.00 | 4.00 | 2.74 | 68.30 |
| 50 | 2.70 | 2.87 | 172.00 | 5.74 | 3.93 | 92.55 |
| 75 | 4.13 | 3.99 | 221.00 | 8.37 | 5.14 | 120.00 |
| 90 | 6.08 | 5.17 | 284.00 | 11.03 | 6.51 | 157.90 |
| 95 | 7.62 | 5.62 | 342.40 | 13.49 | 7.75 | 175.45 |
| 97.5 | 8.72 | 5.92 | 380.80 | 17.34 | 8.95 | 197.18 |
FSH: follicle-stimulating hormone; LH: luteinizing hormone; TC: testicular cancer; CTR: control
Table 5.
Mean±standard deviation and median (25% quartile [Q1] –75% quartile [Q3]) of gonadotropin and inhibin B levels, sperm concentration and total sperm number in the testicular cancer subgroup of patients with inhibin B <62.7 pg ml-1
| Subgroup | FSH (mIU ml-1) | LH (mIU ml-1) | Inhibin B (pg ml-1) | Sperm concentration (×106 ml-1) | Total sperm number (×106 ml-1) |
|---|---|---|---|---|---|
| Inhibin B <62.7 pg ml-1 | |||||
| Mean±s.d. | 8.7±7.7 | 4.4±3.5 | 43.8±13.5 | 41.3±27.6 | 119.8±101.3 |
| Median (Q1–Q3) | 8.00 (4.78–9.80) | 4.00 (2.39–5.35) | 45.0 (35.4–55.0) | 35.5 (25.0–45.0) | 81.3 (61.9–143.0) |
s.d.: standard deviation; FSH: follicle stimulating hormone; LH: luteinizing hormone
DISCUSSION
The clinical role of inhibin B
Literature evidence suggests that spermatogenesis in TC patients is already impaired at the time of diagnosis, with worsening of semen parameters after removal of the affected testicle.18 It can thus be hypothesized that impairment of spermatogenesis is not solely due to the direct effect of the cancer but is part of a preexisting gonadal dysgenesis syndrome which also affects the contralateral testicle. Testicular tumors potentially affect fertility by impairing spermatogenesis through a mechanism involving destruction of the surrounding tissue, local secretion of human chorionic gonadotropin (HCG) and other paracrine factors, elevation of intrascrotal temperature, and modification of the local blood flow.21,22,23,24 In addition to their decline in semen quality, TC patients who have undergone unilateral orchiectomy also present HPG axis modifications, with a rise in FSH and (to a lesser extent) LH and a drop in serum inhibin B concentration. Using inhibin B as a marker of semen quality, Isaksson et al.8 demonstrated that all participants with inhibin B >56 pg ml−1 12 months after the end of antineoplastic therapy maintained good semen quality at 3 years, while lower values were predictive of the risk of azoospermia. The authors thus proposed evaluation of inhibin B at 1 year after chemo- or radiotherapy as a single, reliable marker of semen quality and recovery of spermatogenesis in TC patients. The inhibin B reference ranges used internationally derive from population studies of cohorts with heterogeneous conditions. It should be stressed that most of these studies used immunoenzymatic methods which are no longer available and which suffered from poor inter-measurement precision and accuracy. The immunoenzymatic methods in use today are more refined and specific. Klingmüller and Haidl,10 in 1997, were the first authors to suggest a cutoff below which the inhibin B value was predictive of impaired spermatogenesis, on the basis of inhibin B levels above 112 pg ml−1 seen in all normozoospermic patients taken into consideration. In 1998, Jensen et al.11 identified 80 pg ml−1 as the lower reference value: below this cutoff, there was a 100% chance of having sperm concentration below 20 × 106 ml−1. The reference values used have changed over the years. In 2005, Sikaris et al.12 proposed a lower reference limit of 48 pg ml−1, in contrast with Andersson et al.'s13 proposal of 105 pg ml−1 in 2004. This remarkable difference could be due to differences in the study populations (ethnic and geographic origin), the inclusion criteria used, and the methodologies. Barbotin et al.25 recently conducted a population study with the aim of defining the concentration and reference ranges of serum inhibin B levels in normozoospermic men based on the WHO's 2010 semen parameters. That study investigated two groups, one of healthy normozoospermic participants and one with at least one impaired semen parameter. The reference range was proposed on the basis of values corresponding to the 2.5th and 97.5th percentile of the inhibin B values in the normozoospermic group, corresponding to 92 pg ml−1 and 316 pg ml−1, respectively. An inhibin B level <92 pg ml−1 was associated with increased risks of total sperm number below 39 × 106 ml−1 (odds ratio [OR]: 16.93, 95% confidential interval [CI]: 9.82–29.18), asthenozoospermia, and teratozoospermia.24 There are fewer literature reports of inhibin B levels in patients who have undergone unilateral orchiectomy for causes other than cancer (such as cryptorchidism and testicular trauma), and most studies are based on animal models.26
Inhibin B levels in our cohort of testicular cancer patients
The present study investigated the hormone and semen profile of 290 testicular cancer patients 1 month after unilateral orchiectomy and before beginning any antineoplastic treatment. This observation time was chosen to avoid any treatment-induced impairment of testicular function. Our data are consistent with literature evidence, in which we found a negative correlation between serum inhibin B and FSH levels, a positive correlation between inhibin B and total sperm number, and a negative correlation between FSH and total sperm number in both groups. The correlation coefficient values seem to confirm that inhibin B is superior to FSH as a predictive factor of normal spermatogenesis.27 A strong point of our study is its single-center nature. We analyzed the semen parameters and hormone profile of a large caseload of testicular cancer orchiectomised patients in the same laboratory, thus considerably reducing the variability of the results. Inhibin B values were analyzed to evaluate the minimum value still associated with normal semen quality, selecting only normozoospermic patients according to the WHO 2010 parameters. Enrollment was limited to monorchidic patients with semen parameters within the WHO's 2010 reference ranges. Since inhibin B is a quantitative marker of spermatogenesis, this recruitment was carried out in order to identify the minimum value of inhibin B still representative of normal semen quality. To our knowledge, this information has never been previously reported, despite its importance in the counseling of cancer patients in order to correctly interpret semen and hormone parameters and assess testicular function. In our study, approximately 68.6% (199/290) of our normozoospermic postorchiectomy TC patients had gonadotropin values within the normal reference range, comprising the 95th percentile of the control group (FSH ≤7.6 mIU ml−1). However, the remaining 31.4% presented an elevated FSH value, despite a semen quality within the reference limits. Our study revealed the poor sensitivity of the current inhibin B reference range when applied to monorchidic patients. While the literature values are heterogeneous, they identified a lower reference limit of between 80 pg ml−1 and 112 pg ml−1 for inhibin B.10,11,12 These values refer to a population of healthy men selected on the basis of a sperm concentration of >20 × 106 ml−1 and a total sperm number of ≥39 × 106. Similarly, Barbotin et al.'s25 recent study suggested an inhibin B reference range of 92–316 pg ml−1 for normozoospermic patients. In contrast, approximately 20% of the postorchiectomy patients in our caseload presented an inhibin B value below the 5th percentile of that for healthy controls (62.75 pg ml−1), despite their normal semen parameters. In fact, regardless of the statistically significant difference in the mean total sperm number between the CTR and TC groups (251.7 [s.d.: 213.4] × 106 vs 119.8 [s.d.: 101.3] × 106 per ejaculate, P ≤0.001), orchiectomised patients presented a normal semen quality. In our opinion, this is quite a high proportion which indicates the importance of establishing a new, more representative reference range for this category of patients. We therefore divided the inhibin B values in the TC group into percentiles and considered the 5th and the 95th percentiles (35.5 pg ml−1 and 175.4 pg ml−1). This lower limit is quite low in comparison with the reference value for non-orchiectomised patients, but in our pathological model, it is still indicative of normal spermatogenesis.
The mean FSH level in this subgroup was 8.7 (s.d.: 7.7; median: 8.0) mIU ml−1 and the mean total sperm number was 119.8 (s.d.: 101.3; median: 81.3) × 106 per ejaculate. In conclusion, these data suggest that even though the reduced pool of available Sertoli and germ cells in post-orchiectomy patients leads to a proportional reduction in the hormone production of these cells, in most cases, this drop does not hinder spermatogenesis in the remaining testicle. On the contrary, the reduced inhibin B levels and consequently diminished negative pituitary feedback could actually induce an increase in FSH, necessary to stimulate the remaining testicle.
Our data thus show that the hormonal assessment of monorchidic patients must necessarily take account of the different equilibrium established in their endocrine system in comparison with healthy participants. They also demonstrate the need to establish more suitable and representative ranges to enable more appropriate and specific counseling in relation to the patient's new endocrine condition.
AUTHOR CONTRIBUTIONS
Study conception and design were conceived by AP, DP, and FL. The article was written by AP, FP, and DP. Data acquisition and statistical analysis were carried out by AP and FP. Semen analysis was carried out by DP and MP. Hormone evaluations were performed by AA and AFR. Data interpretation was carried out by AP, DP, FP, FL, AFR, and AL. Critical review of the paper was performed by AP, AFR, AL, and FL. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study is dedicated to the memory of Prof. Loredana Gandini, whose observations made this work possible and who carried out the semen analyses in this study. This work was supported by a grant from the Italian Ministry of Education and Research (MIUR-PRIN 2015-2015XCR88M-006) and “Sapienza” University of Rome, Faculty of Medicine.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Meachem SJ, Nieschlag E, Simoni M. Inhibin B in male reproduction: pathophysiology and clinical relevance. Eur J Endocrinol. 2001;145:561–71. doi: 10.1530/eje.0.1450561. [DOI] [PubMed] [Google Scholar]
- 2.Illingworth PJ, Groome NP, Byrd W, Rainey WE, McNeilly AS, et al. Inhibin-B: a likely candidate for the physiologically important form of inhibin in men. J Clin Endocrinol Metab. 1996;81:1321–5. doi: 10.1210/jcem.81.4.8636325. [DOI] [PubMed] [Google Scholar]
- 3.Forti F, Vannelli GB, Barni T, Balboni GC, Orlando C, et al. Sertoli-germ cells interactions in the human testis. J Steroid Biochem Mol Biol. 1992;43:419–22. doi: 10.1016/0960-0760(92)90079-x. [DOI] [PubMed] [Google Scholar]
- 4.Radicioni AF, Anzuini A, De Marco E, Nofroni I, Castracane VD, et al. Changes in serum inhibin B during normal male puberty. Eur J Endocrinol. 2005;152:403–9. doi: 10.1530/eje.1.01855. [DOI] [PubMed] [Google Scholar]
- 5.Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, et al. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab. 1996;81:3341–5. doi: 10.1210/jcem.81.9.8784094. [DOI] [PubMed] [Google Scholar]
- 6.Bohring C, Krause W. Serum levels of inhibin B in men with different causes of spermatogenic failure. Andrologia. 1999;31:137–41. [PubMed] [Google Scholar]
- 7.Kumanov P, Nandipati K, Tomova A, Agarwal A. Inhibin B is a better marker of spermatogenesis than other hormones in the evaluation of male factor infertility. Fertil Steril. 2006;86:332–8. doi: 10.1016/j.fertnstert.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Isaksson S, Eberhard J, Ståhl O, Cavallin-Ståhl E, Cohn-Cedermark G, et al. Inhibin B concentration is predictive for long-term azoospermia in men treated for testicular cancer. Andrology. 2014;2:252–8. doi: 10.1111/j.2047-2927.2014.00182.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. pp. 669–76. [Google Scholar]
- 10.Klingmüller D, Haidl G. Inhibin B in men with normal and disturbed spermatogenesis. Hum Reprod. 1997;12:2376–8. doi: 10.1093/humrep/12.11.2376. [DOI] [PubMed] [Google Scholar]
- 11.Jensen TK, Andersson AM, Hjollund NH, Scheike T, Kolstad H, et al. Inhibin B as a serum marker of spermatogenesis: correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men. J Clin Endocrinol Metab. 1998;82:4059–63. doi: 10.1210/jcem.82.12.4456. [DOI] [PubMed] [Google Scholar]
- 12.Andersson AM, Petersen JH, Jørgensen N, Jensen TK. Serum inhibin B and follicle-stimulating hormone levels as tools in the evaluation of infertile men: significance of adequate reference values from proven fertile men. J Clin Endocrinol Metab. 2004;89:2873–9. doi: 10.1210/jc.2003-032148. [DOI] [PubMed] [Google Scholar]
- 13.Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, et al. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab. 2005;90:5928–36. doi: 10.1210/jc.2005-0962. [DOI] [PubMed] [Google Scholar]
- 14.Rosen A, Jayram G, Draze M, Eggener SE. Global trends in testicular cancer incidence and mortality. Eur Urol. 2011;60:374–9. doi: 10.1016/j.eururo.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Ghazarian AA, Trabert B, Devesa SS, McGlynn KA. Recent trends in the incidence of testicular germ cell tumors in the United States. Andrology. 2014;3:13–8. doi: 10.1111/andr.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandini L, Sgrò P, Lombardo F, Paoli D, Culasso F, et al. Effect of chemo- or radiotherapy on sperm parameters of testicular cancer patients. Hum Reprod. 2006;21:2882–9. doi: 10.1093/humrep/del167. [DOI] [PubMed] [Google Scholar]
- 17.Williamson SR, Delahunt B, Magi-Galluzzi C, Algaba F, Egevad L, et al. The World Health Organization 2016 classification of testicular germ cell tumors: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology. 2017;70:335–46. doi: 10.1111/his.13102. [DOI] [PubMed] [Google Scholar]
- 18.Petersen JH, Skakkebaek NA, Rørth M, Giwercman A. Semen quality and reproductive hormones before and after orchiectomy in men with testicular cancer. J Urol. 1999;161:822–6. [PubMed] [Google Scholar]
- 19.Vakalopoulos I, Dimou P, Anagnoustou I, Zeginiadou T. Impact of cancer and cancer treatment on male fertility. Hormone (Athens) 2015;14:579–89. doi: 10.14310/horm.2002.1620. [DOI] [PubMed] [Google Scholar]
- 20.Tvrda E, Agarwal A, Alkuhaimi N. Male reproductive cancers and infertility: a mutual relationship. Int J Mol Sci. 2015;16:7230–60. doi: 10.3390/ijms16047230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho GT, Gardner H, DeWolf WC, Loughlin K, Morgentaler A. Influence of testicular carcinoma on ipsilateral spermatogenesis. J Urol. 1992;148:821–5. doi: 10.1016/s0022-5347(17)36732-0. [DOI] [PubMed] [Google Scholar]
- 22.Rajpert-De Meyts E, Skakkebaek NA, Toppari J. Testicular cancer pathogenesis, diagnosis and endocrine aspects [Updated on 2018 Jan 07] In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc; 2000. [Last accessed on 2018 Jun 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278992/ [Google Scholar]
- 23.Giannandrea F, Paoli D, Figà-Talamanca I, Lombardo F, Lenzi A, et al. Effect of endogenous and exogenous hormones on testicular cancer: the epidemiological evidence. Int J Dev Biol. 2013;57:255–63. doi: 10.1387/ijdb.130015fg. [DOI] [PubMed] [Google Scholar]
- 24.Rajpert-De Meyts E, McGlynn KA, Okamoto K, Jewet MA, Bokemeyer C. Testicular germ cell tumors. Lancet. 2016;87:1762–74. doi: 10.1016/S0140-6736(15)00991-5. [DOI] [PubMed] [Google Scholar]
- 25.Barbotin AL, Ballot C, Sigala J, Ramdane N, Duhamel A, et al. The serum inhibin B concentration and reference ranges in normozoospermia. Eur J Endocrinol. 2015;172:669–76. doi: 10.1530/EJE-14-0932. [DOI] [PubMed] [Google Scholar]
- 26.Irkilata HC, Kibar Y, Basal S, Kurt B, Gunal A, et al. The impact of simple orchiectomy on semen quality and endocrine parameters in postpubertal cryptorchid men. Int Urol Nephrol. 2012;44:1617–22. doi: 10.1007/s11255-012-0256-3. [DOI] [PubMed] [Google Scholar]
- 27.van Beek RD, Smit M, van den Heuvel-Eibrink MM, de Jong F, Hakvoort-Cammel FG, et al. Inhibin B is superior to FSH as a serum marker for spermatogenesis in men treated for Hodgkin's lymphoma with chemotherapy during childhood. Hum Reprod. 2007;12:3215–22. doi: 10.1093/humrep/dem313. [DOI] [PubMed] [Google Scholar]


