Abstract
Case series
Patient: —
Final Diagnosis: Generalized myasthenia gravis
Symptoms: Muscle weakness
Medication: IVIg • eculizumab
Clinical Procedure: Transition from IVIg to eculizumab
Specialty: Neurology
Objective:
Unusual setting of medical care
Background:
Eculizumab is a terminal complement inhibitor used to treat myasthenia gravis in patients refractory (because of insufficient efficacy or intolerance) to other therapies, including intravenous immunoglobulin. However, information is lacking on how to transition patients from intravenous immunoglobulin to eculizumab, while avoiding a crossover effect of intravenous immunoglobulin and minimizing the risk of a transient worsening of symptoms if treatment that may be at least partially effective is interrupted. The aim of this study was to determine whether eculizumab can be safely initiated before complete intravenous immunoglobulin washout, using a standardized protocol.
Case Report:
A series of 13 patients with generalized treatment-refractory myasthenia gravis were transitioned to eculizumab 10–14 days after their last intravenous immunoglobulin infusion. Patients’ clinical status was assessed before and 6 weeks after transition using the Myasthenia Gravis Composite Score. Most patients (8/13; 62%) had received ≥3 immunosuppressants as well as intravenous immunoglobulin. The median (range) Myasthenia Gravis Composite Score before and 6 weeks after transition was 21 (11–29) and 12 (6–18), respectively. Clinically significant improvements (score decrease ≥3) were observed in all patients. Two patients experienced mild myalgia during transition.
Conclusions:
In this case series, patients with treatment-refractory myasthenia gravis were successfully transitioned to eculizumab 10–14 days after their last intravenous immunoglobulin infusion without any significant safety concerns.
MeSH Keywords: Complement Inactivating Agents; Immunoglobulins, Intravenous; Myasthenia Gravis
Background
Myasthenia gravis is an autoimmune condition that is mediated by autoantibodies to components of the postsynaptic muscle endplate; in approximately 85% of cases, these antibodies target the acetylcholine receptor (AChR) [1]. As AChR antibodies are highly specific for myasthenia gravis, their presence confirms the diagnosis in patients with muscle weakness [2]. Most patients with myasthenia gravis respond to symptomatic treatment with acetylcholinesterase inhibitors in combination with immunosuppressants; however, approximately 10–15% of patients are refractory to treatment and do not achieve full disease control, or they develop intolerable adverse effects from immunosuppressive therapy [2,3].
Therapeutic options for patients with treatment-refractory myasthenia gravis include corticosteroids, methotrexate, mycophenolate mofetil, azathioprine, and intravenous immunoglobulin (IVIg). IVIg has been used for many years in the treatment of myasthenia gravis, and although there is good evidence to support its short-term use in this condition, evidence for its efficacy as long-term maintenance therapy is limited to retrospective studies [4–6]. Adverse events associated with IVIg include fever, nausea, and headache; these are of moderate severity and self-limiting [5]. Rare but potentially serious adverse effects of IVIg include thrombotic events, renal dysfunction, and hemolytic anemia [6–9]. The considerable cost of IVIg therapy (US$150 000–$200 000/year) also has to be taken into account [10,11].
There is good evidence that the complement system plays an important role in the pathogenesis of myasthenia gravis [12], but none of the conventional treatments target complement directly. Eculizumab is a humanized murine monoclonal antibody that blocks formation of the terminal complement complex by binding to C5 and preventing its enzymatic cleavage to C5a and C5b [13,14]. In the USA it is currently indicated for the treatment of adult patients with generalized myasthenia gravis who are anti-AChR antibody-positive [15]. Randomized, double-blind, placebo-controlled studies have shown that eculizumab is effective for the treatment of refractory generalized myasthenia gravis in patients with antibodies to AChR [16,17]. Although there is no standard definition of “refractory” disease with regard to generalized myasthenia gravis, operational criteria in published reports generally include: the failure of multiple therapies, or the requirement for regular use of IVIg or plasma exchange to manage disease symptoms; or the presence of severe adverse reactions to conventional treatments [18]. In the Phase III eculizumab study (REGAIN), 98% of patients had received ≥2 previous immunosuppressant treatments and 28% had received long-term IVIg therapy [17]. Thus, eculizumab may be a suitable treatment for patients with generalized myasthenia gravis that is not adequately controlled by other immunosuppressants and/or IVIg. There is some concern that concurrent administration of IVIg may decrease serum eculizumab concentrations [15]. Furthermore, the administration of additional protein may increase the risk of thrombosis and renal dysfunction in patients receiving IVIg. However, data are lacking on the most appropriate means by which to transition patients from IVIg to eculizumab. In the randomized, placebo-controlled studies of eculizumab, patients were excluded if they had received IVIg in the 4 weeks before the start of the studies [16,17]. If the IVIg treatment is even partially effective, this duration of ‘washout’ may cause an interruption in treatment that results in further clinical deterioration. The current case series describes the results of a standardized protocol for patients with myasthenia gravis transitioning from IVIg to eculizumab. The aim was to determine whether eculizumab could be safely initiated before complete IVIg washout in order to prevent transient worsening during the period of onset of eculizumab’s effects.
Case Reports
This was a retrospective analysis of data from a series of patients treated at a single institution in the USA between October 2017 (following US Food and Drug Administration approval of eculizumab use in patients with generalized myasthenia gravis) and May 2018. The study was reviewed by the HonorHealth Institutional Review Board (IRB) and received an IRB exemption, as the study was a retrospective chart review.
The 13 patients (7 male, 6 female) had generalized myasthenia gravis that was classed as treatment refractory. All were receiving maintenance IVIg therapy (in combination with immunosuppressants) that did not adequately control their disease: all had significant symptoms and a Myasthenia Gravis Composite Score (MGCS) >11. Most patients (8/13; 62%) had received at least 3 different immunosuppressants as well as IVIg; 5 had also received plasmapheresis (Table 1). All patients had received mycophenolate mofetil; other commonly used immunosuppressants were prednisone (n=8) and methotrexate (n=8). At the time of the transition, all 13 patients were receiving at least 1 immunosuppressant. The median (range) age was 71 (30–78) years. Most patients (11/13; 85%) were aged >60 years; the remaining 2 patients (both female) were aged 30 and 34 years (Table 1). The median (range) time since diagnosis of myasthenia gravis was 6 (2–17) years (Table 1).
Table 1.
Demographic and clinical characteristics of patients with generalized refractory myasthenia gravis transitioned from IVIg to eculizumab.
| Patient No. | Sex | Age (years) | Time since MG diagnosis (years) | Thymectomy | Previous medication | Concomitant medication* | MGCS before eculizumab | Change in MGCS after 6 weeks’ eculizumab treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 62 | 5 | Yes | IVIg Mycophenolate mofetil Prednisone Plasmapheresis Methotrexate |
IVIg 2 g/kg/month Mycophenolate mofetil 1500 mg BID Prednisone 40 mg/day Methotrexate 15 mg/week |
29 | −17 |
| 2 | F | 74 | 2 | No | Prednisone Mycophenolate mofetil IVIg |
Prednisone 20 mg/day Mycophenolate mofetil 1500 mg BID IVIg 2 g/kg/month |
13 | −7 |
| 3 | M | 71 | 9 | No | Plasmapheresis IVIg Azathioprine Mycophenolate mofetil Methotrexate |
IVIg 2 g/kg/month Methotrexate 20 mg/week |
23 | −9 |
| 4 | F | 78 | 17 | No | Mycophenolate mofetil Methotrexate Prednisone IVIg Azathioprine |
IVIg 2 g/kg/month Azathioprine 150 mg/day |
21 | −6 |
| 5 | F | 34 | 11 | Yes | Mycophenolate mofetil IVIg Rituximab Methotrexate Cyclosporine |
Mycophenolate mofetil 1500 mg BID IVIg 2 g/kg/month Cyclosporine 25 mg BID |
25 | −7 |
| 6 | M | 74 | 10 | No | IVIg Mycophenolate mofetil Plasmapheresis |
IVIg 2 g/kg/month Mycophenolate mofetil 1000 mg BID |
17 | −8 |
| 7 | F | 68 | 17 | Yes | Prednisone Mycophenolate mofetil Cyclosporine IVIg Plasmapheresis |
Mycophenolate mofetil 1500 mg BID Cyclosporine 100 mg BID IVIg 2 g/kg/month |
20 | −10 |
| 8 | M | 72 | 7 | Yes | Prednisone IVIg Mycophenolate mofetil Plasmapheresis |
Prednisone 30 mg/day IVIg 2 g/kg/month Mycophenolate mofetil 1000 mg BID |
21 | −11 |
| 9 | F | 30 | 4 | Yes | IVIg Prednisone Mycophenolate mofetil Methotrexate |
IVIg 2 g/kg/month Prednisone 20 mg/day Mycophenolate mofetil 1500 mg BID Methotrexate 10 mg/week |
11 | −4 |
| 10 | F | 64 | 3 | No | Prednisone Mycophenolate mofetil IVIg Methotrexate Cyclosporine |
Prednisone 12.5 mg/day Mycophenolate mofetil 1500 mg BID IVIg 2 g/kg/month Cyclosporine 75 mg BID |
17 | −8 |
| 11 | M | 66 | 3 | Yes | IVIg Mycophenolate mofetil |
IVIg 2 g/kg/month Mycophenolate mofetil 1500 mg BID |
24 | −7 |
| 12 | M | 71 | 4 | No | Mycophenolate mofetil IVIg Methotrexate |
Mycophenolate mofetil 1000 mg BID IVIg 2 g/kg/month Methotrexate 20 mg/week |
20 | −7 |
| 13 | M | 75 | 6 | No | Prednisone Azathioprine Mycophenolate mofetil IVIg Methotrexate Cyclosporine |
Prednisone 30 mg/day IVIg 2 g/kg/month Cyclosporine 75 mg BID |
25 | −10 |
Information on IVIg refers to the last dose that patients received before being transitioned to eculizumab; all other drugs were continued during eculizumab treatment. BID – twice daily; F – female; IVIg – intravenous immunoglobulin; M – male; MG – myasthenia gravis; MGCS – Myasthenia Gravis Composite Score.
Patients were transitioned from IVIg to eculizumab using a standardized protocol, such that eculizumab was initiated 10–14 days after the last IVIg infusion. All patients were AChR antibody-positive and had received meningococcal vaccines at least 2 weeks before initiation of eculizumab, as recommended in the prescribing information for the product [15]. The initial eculizumab dose was 900 mg weekly for the first 4 weeks, followed by 1200 mg on Week 5, then 1200 mg every 2 weeks thereafter, in line with the prescribing information [15]. Other immunosuppressant therapies were continued at the same dose during the transition and the 6-week evaluation period.
Clinical status was assessed as part of a patient’s routine clinical care before starting eculizumab and after 6 weeks of treatment using the MGCS [19]. The results were summarized descriptively; no statistical analysis was conducted in view of the small number of patients and the aim of the study being to assess the safety of the transition to eculizumab rather than the efficacy of eculizumab. The MGCS improved in all patients in the 6 weeks after transitioning to eculizumab (Figure 1). Median (range) scores before and after the transition were 21 (11–29) and 12 (6–18), respectively. The MGCS decreased by ≥3 points, which denotes a clinically significant improvement [19], in all 13 patients; the median (range) change was 8 (4–17).
Figure 1.
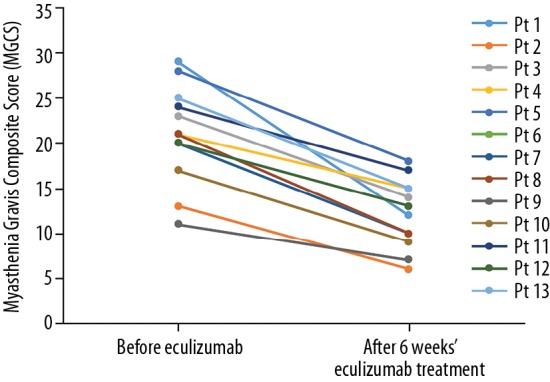
Change in Myasthenia Gravis Composite Score before and 6 weeks after transition to eculizumab in patients with generalized refractory myasthenia gravis. Pt – patient.
Two patients experienced mild myalgia after initiation of eculizumab, but no changes to the eculizumab dose were required in these patients. No other adverse events were reported.
Discussion
In this series of patients with treatment-refractory generalized myasthenia gravis, transitioning patients from IVIg to the terminal complement inhibitor eculizumab using a standardized protocol that incorporates a shorter washout period than that imposed in clinical trials was associated with clinically significant improvements (decrease in MGCS ≥3) in all patients after 6 weeks. The decrease in MGCS, a valid and reliable measure for assessing myasthenia gravis in the clinic [19], was ≥6 in 12 of the 13 patients. These improvements are particularly notable for being achieved in a group of patients for whom multiple previous therapies had proven ineffective or only partially effective. At the time of the transition, most patients had received at least 3 different immunosuppressants as well as IVIg, and 5 had also received plasmapheresis.
This is the first report describing the use of a standardized protocol to transition patients from IVIg to eculizumab. In eculizumab clinical trials, the interval between the last IVIg infusion and the start of eculizumab was ≥4 weeks, presumably to ensure that there was no crossover effect of IVIg [16,17]. In the present series, eculizumab was initiated 10–14 days after the last IVIg infusion, as it was anticipated that the beneficial effects of eculizumab would start to manifest before the effects of IVIg started to wane. In the Phase III eculizumab trial, a significant separation from placebo for myasthenia gravis outcome measures was evident as early as Week 1 and continued to accrue until approximately Week 12 [17]. In a randomized, placebo-controlled trial of IVIg in myasthenia gravis, beneficial effects were evident at Day 14 and persisted through Day 28 [20].
There were no clinically evident efficacy or safety issues associated with the overlap in the pharmacodynamic effects of eculizumab and IVIg. Only 2 patients reported adverse effects of mild myalgia, which is consistent with the known safety profile of eculizumab. Eculizumab has been associated with an increased risk of serious meningococcal infections, but in line with the prescribing information [15], all patients had been vaccinated at least 2 weeks before eculizumab initiation.
Limitations of this evaluation include the retrospective case-series design and the small number patients included, which restricts generalizability of the results. In addition, in the absence of an accepted definition of “refractory” disease, patients were treated with eculizumab on the basis of their medical history and the presence of significant symptoms and their MGCS. Nevertheless, the characteristics of the patients included were consistent with the wider treatment-refractory myasthenia gravis population, and their treatment history reflects that of patients likely to be treated with eculizumab in clinical practice. The lack of a control group limits conclusions about the efficacy of eculizumab; however, the aim of this case series was not to examine its efficacy per se, but to determine the suitability of this transition protocol, in particular, the safety and tolerability of the abbreviated washout period. Furthermore, the beneficial effects of up to 6 months’ treatment with eculizumab have been demonstrated previously in rigorously designed, randomized, double-blind, placebo-controlled studies in patients with treatment-refractory myasthenia gravis [16,17]. It is also acknowledged that the transition protocol used for the current series is not necessarily the only protocol that could be used in these patients, but the results indicate that it can be used successfully to manage the transition.
Conclusions
Data from this case series of representative patients with generalized treatment-refractory myasthenia gravis indicate that these patients can be transitioned to eculizumab 10–14 days after their last IVIg infusion, without significant safety concerns and with clinically significant improvements in outcomes.
Acknowledgments
Editorial support was provided by Dr Nicky French of Anthemis Consulting, Ltd, funded by Alexion Pharmaceuticals, Inc. Alexion Pharmaceuticals provided a medical-accuracy review of the final draft.
Footnotes
Disclosure
Dr Levine is a member of the speaker bureaux of Grifols, CSL Behring, and Alexion. He has a financial interest in Corinthian Reference Labs and Cutaneous Neurodiagnostic Labs and serves as a consultant for Nufactor.
References:
- 1.Silvestri NJ, Wolfe GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis. 2014;15:167–78. doi: 10.1097/CND.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 2.Gilhus NE, Verschuuren JJ. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14:1023–36. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 3.Suh J, Goldstein JM, Nowak RJ. Clinical characteristics of refractory myasthenia gravis patients. Yale J Biol Med. 2013;86:255–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Alabdali M, Barnett C, Katzberg H, et al. Intravenous immunoglobulin as treatment for myasthenia gravis: Current evidence and outcomes. Expert Rev Clin Immunol. 2014;10:1659–65. doi: 10.1586/1744666X.2014.971757. [DOI] [PubMed] [Google Scholar]
- 5.Gajdos P, Chevret S, Toyka KV. Intravenous immunoglobulin for myasthenia gravis. Cochrane Database Syst Rev. 2012;12:CD002277. doi: 10.1002/14651858.CD002277.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmann MA, Mosberg-Galili R, Lotan I, Steiner I. Maintenance IVIg therapy in myasthenia gravis does not affect disease activity. J Neurol Sci. 2014;338:39–42. doi: 10.1016/j.jns.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 7.Bennett CL, Hermanson T. Big data, thromboembolism, and immunoglobulin. Blood. 2016;127:171–72. doi: 10.1182/blood-2015-11-675421. [DOI] [PubMed] [Google Scholar]
- 8.Dantal J. Intravenous immunoglobulins: In-depth review of excipients and acute kidney injury risk. Am J Nephrol. 2013;38:275–84. doi: 10.1159/000354893. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;9:1299. doi: 10.3389/fimmu.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guptill JT, Marano A, Krueger A, Sanders DB. Cost analysis of myasthenia gravis from a large U.S. insurance database. Muscle Nerve. 2011;44:907–11. doi: 10.1002/mus.22212. [DOI] [PubMed] [Google Scholar]
- 11.Heatwole C, Johnson N, Holloway R, Noyes K. Plasma exchange versus intravenous immunoglobulin for myasthenia gravis crisis: An acute hospital cost comparison study. J Clin Neuromuscul Dis. 2011;13:85–94. doi: 10.1097/CND.0b013e31822c34dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard JFJ. Myasthenia gravis: The role of complement at the neuromuscular junction. Ann NY Acad Sci. 2018;1412:113–28. doi: 10.1111/nyas.13522. [DOI] [PubMed] [Google Scholar]
- 13.Davis J. Eculizumab. Am J Health Syst Pharm. 2008;65:1609–15. doi: 10.2146/ajhp080043. [DOI] [PubMed] [Google Scholar]
- 14.Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–64. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 15.Alexion Pharmaceuticals Inc Soliris (eculizumab) injection; Prescribing information. 2018. Available from: http://alexion.com/Documents/Soliris_USPI.aspx. Accessed April 24, 2019.
- 16.Howard JF, Jr, Barohn RJ, Cutter GR, et al. MG Study Group A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013;48:76–84. doi: 10.1002/mus.23839. [DOI] [PubMed] [Google Scholar]
- 17.Howard JF, Jr, Utsugisawa K, Benatar M, et al. REGAIN Study Group Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16:976–86. doi: 10.1016/S1474-4422(17)30369-1. [DOI] [PubMed] [Google Scholar]
- 18.Mantegazza R, Antozzi C. When myasthenia gravis is deemed refractory: Clinical signposts and treatment strategies. Ther Adv Neurol Disord. 2018;11 doi: 10.1177/1756285617749134. 1756285617749134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns TM, Conaway M, Sanders DB. The MG Composite: A valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74:1434–40. doi: 10.1212/WNL.0b013e3181dc1b1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinman L, Ng E, Bril V. IV immunoglobulin in patients with myasthenia gravis: A randomized controlled trial. Neurology. 2007;68:837–41. doi: 10.1212/01.wnl.0000256698.69121.45. [DOI] [PubMed] [Google Scholar]


