Abstract
Background:
There is enough evidence, which suggests that nonsurgical periodontal therapy (NSPT) improved the glycemic control in patients of Type 2 diabetes mellitus (T2DM) with chronic periodontitis (CP). This meta-analysis is aimed to explore the effect of NSPT, exclusively scaling and root planing (SRP) as monotherapy on glycemic control and periodontal parameters in patients of T2DM with CP.
Materials and Methods:
Two databases, MEDLINE and EMBASE, were searched from June 2006 to June 2016. Initially, 464 potentially relevant studies were evaluated. Randomized controlled trials with duration of ≥3 months, based on the treatment group managed with SRP without any supportive use of local drug delivery or systemic antibiotics, while the control group received no periodontal therapy, were selected. This resulted in six appropriate articles with a total of 812 patients of T2DM with CP. Alteration in glycated hemoglobin (HbA1c) was the primary outcome measure, whereas the changes in clinical attachment level (CAL) and probing pocket depth (PPD) were the secondary outcome measures.
Results:
SRP treatment resulted in a decrease in HbA1c by 0.26% (P = 0.17) at 3–4 months compared to the control group. Further, on subgroup analysis, SRP therapy revealed a decrease in PPD and CAL at 3–4 months, though statistically insignificant.
Conclusion:
SRP treatment leads to modest improvement in glycemic status and periodontal parameters in T2DM patients with CP at 3–4 months.
Key words: Chronic periodontitis, clinical attachment level, glycated hemoglobin, meta-analysis, probing pocket depth, scaling and root planning, systematic review, Type 2 diabetes mellitus
INTRODUCTION
Periodontitis is a chronic and progressive inflammatory disease predominantly caused by the interaction of microorganisms and host immune response and is an important cause of tooth loss among adults.[1] Chronic periodontitis (CP) not only progressively and painlessly destroys the supporting tissues around the teeth but also has an influence on the systemic health of the individual.[2]
Many investigators have proposed a bipartisan relationship between diabetes mellitus (DM) and periodontal disease.[3] Persistent gram-negative periodontal infection leads to decline in insulin sensitivity in patients with diabetes through up-regulation of proinflammatory cytokines, and uncontrolled hyperglycemia per se increases the severity of periodontal disease by an accumulation of advanced glycation end products and subsequent destruction of periodontal supporting tissue.[3] Therefore, the strong interplay of pathogenic mechanisms between the two diseases makes their association more vicious.
It has been extensively studied that in the presence of CP, patients with DM exhibit worsening of their glycemic control.[4,5] The release of endotoxins by periodontal pathogens, and secretion of proinflammatory cytokines by the affected gingival tissue results in worsening of insulin resistance and consequent hyperglycemia.[6] Diabetes has also increasingly been viewed as chronic low-grade systemic inflammation.[7] Various studies have reported that patients with diabetes have a higher prevalence of CP.[8,9,10] Furthermore, patients with poorly controlled diabetes have a higher risk (2.9 times) of having severe periodontitis compared to the nondiabetic participants.[10] Therefore, it is imperative to consider periodontal disease as an important complication of diabetes.[11]
Both diabetes and periodontal infection are complicating and enhancing each other's magnitude and severity; therefore, it is suggested that along with the medications for diabetes, the control of active periodontal infection should also be aimed to achieve a long-term glycemic control in these patients.[12] Nonsurgical periodontal therapy (NSPT) is the initial step in the management of periodontal diseases. The target of NSPT is to modulate or abolish the microbes and other causative factors implicated in gingival and periodontal diseases.[13] The effect of NSPT on glycemic control in patients of diabetes with CP has been studied extensively in erstwhile reviews and meta-analyses.[14,15,16] However, there is a dearth of data regarding the impact of scaling and root planing (SRP) alone without the supportive use of any systemic or topical antibiotics, on glycemic control and periodontal parameters in patients with type 2 DM (T2DM) in ailing from concurrent CP. In a recent meta-analysis, selected studies mainly had an intervention in the form of subgingival and supragingival debridement or SRP with adjunctive use of topical and systemic antibiotics and surgical therapy (periodontal flap surgery) when indicated.[16] Therefore, the adjunctive use of topical and systemic antibiotics in T2DM patients with CP masked the results of SRP alone on glycemic control and periodontal status. Hence, the present study has its uniqueness in the inclusion of the most recent available data, i.e., randomized control trials (RCTs) till 2016 with an aim to accomplish a meta-analysis to redefine, whether SRP as a mono-therapy, could improve the glycemic control and periodontal parameters in these patients.
MATERIALS AND METHODS
Search strategy
Two authors independently searched the data from two databases, PubMed and EMBASE, according to the methods described in previous meta-analyses.[14,15,16] The separate search strategies were framed and used for the said databases [MEDLINE and EMBASE search strategy, Appendixes 1 and 2, respectively].
Study selection criteria
RCTs conducted from June 2006 to June 2016, were included in the present study. In view of the recent data on the effect of SRP (periodontal therapy) on the pathophysiology of T2DM, it was decided to include those articles focused exclusively on this mode of therapy, and these trials were filtered from June 2006 to June 2016 (up to 10 years). The literature search was confined to the English language only.
Inclusion and exclusion criteria
RCTs, which, enrolled the patients of T2DM with CP treated by SRP alone without any supportive use of local drug delivery and systemic antibiotics, were included in the study. The primary outcome measure was mean change in glycated hemoglobin (HbA1c) level, and the secondary outcome measures were clinical periodontal parameters, including clinical attachment levels (CAL) and/or probing pocket depth (PPD). Reviews, commentaries, case reports, cross-sectional studies, and RCTs having systemically healthy controls were excluded from the study.
If there were >1 publication from the same ethnic group, only data from the most recent report were included in the meta-analysis and remaining were excluded from the study. Criteria for T2DM [17] included a HbA1c level of ≥6.5%, or a fasting plasma glucose level ≥126 mg/dL (7 mmol/L); or a 2-h plasma glucose level ≥200 mg/dL (11.1 mmol/L) during a 75-g oral glucose tolerance test, or a random plasma glucose ≥200 mg/dL (11.1 mmol/L) in a patient with classic symptoms, including polyuria, polydipsia, polyphagia, weight loss, or hyperglycemic emergency. Criteria for CP [18] were based on the amount of CAL like this: slight = 1–2 mm CAL, moderate = 3–4 mm CAL, and severe ≥5 mm CAL.
Extraction of data
Three authors independently screened all studies to include RCTs with appropriate inclusion criteria in the present meta-analysis. Characteristics which have been extracted from each study were as follows: (i) name of the journal and year of publication (ii) last name of the first author (iii) country where the study was conducted (iv) single or multicentric trial (v) randomized or nonrandomized trial (vi) parallel or crossover study (vii) single- or double-blinded studies (viii) the mean age of the study population (ix) number of male and female participants (x) study duration (xi) definition of CP (xii) definition of T2DM (xiii) baseline HbA1c, probing depth, and CAL (xii) HbA1c, probing depth, and CAL after 3–4 months of therapy.
Quality assessment
The quality of all selected studies was evaluated using the risk of bias tool according to the Cochrane Handbook for systematic review (Version 5.1).[19] The quality of each study was assessed independently by two authors using the scale.[20] Authors graded the response options of “high risk of bias,” “low risk of bias,” and “unclear risk of bias” for each of the domains.
Statistical analysis
The absolute difference in the percentage of HbA1c in the treatment and control group was documented. For analysis of data, continuous variables were presented as means with standard deviation. In the present study, we assumed that baseline data are comparable between the treatment group and the control group. To assess the statistically significant difference in the mean values among the control and treatment group, we performed independent sample t-test. Test results showed that there was a statistically insignificant (P = 0.76) difference in the mean HbA1c level at baseline. Hence, to compare the effectiveness of the treatment, we used 3-month follow-up data for further analysis in the present study. To assess the heterogeneity among studies, we used the Cochran Q and I2 statistics; for heterogeneity; for I2, a value < 0.50% is considered an estimate of heterogeneity, and a fixed-effects model analysis was used to compute the pooled effect; otherwise, a random-effects model was employed.[19] Forest plots were drawn displaying the point estimate and confidence intervals for each study. Statistical significance was expressed as a two-tailed P < 0.05. Using RevMan version 5.1 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) the numerical data for the meta-analysis was processed.
RESULTS
Search results
The combined PubMed and the EMBASE searches resulted in retrieval of 464 potentially relevant articles [Figure 1]. Total articles after the elimination of duplicates were 409. These articles were later curtained by title and abstract for eligibility, and 300 articles were excluded. Another 53 articles were disqualified, as these were reviews, commentaries, correspondence, case-reports, protocols, and editorials. Finally, the RCTs retrieved for full-text review were 56. Out of these 56 studies, only six studies could fulfill the inclusion and exclusion criteria and have estimated the primary outcome measure HbA1c. Furthermore, only 4 and 3, included studies have evaluated the secondary outcome measures PPD and CAL, respectively. Data extraction was accomplished in these studies for final analysis [Figure 1].
Figure 1.
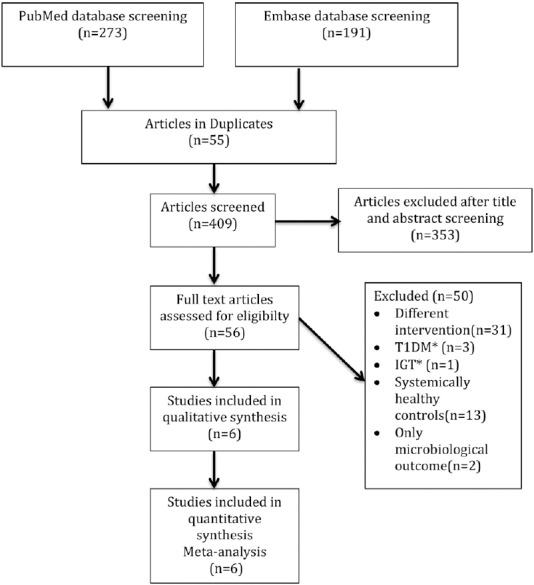
Flow diagram representing the selection process (T1DM* – Type 1 diabetes mellitus, IGT* – Impaired glucose tolerance). n – Number of articles
Study characteristics
Out of the six selected studies, two [21,22] were conducted in the USA, three [23,24,25] in Asia (one each from India, Iran, and Malaysia), and one [26] in Europe (Greece). The duration of studies was from 3 to 6 months, though the data up to 3–4 months were included for the meta-analysis. Three out of the six studies were double-blinded, while the rest of the studies did not disclose about the blinding pattern. All the studied patients had T2DM and were ailing from periodontitis. In total 410 patients were in the intervention group, and 402 patients were controls with the mean age ranging from 50 to 59 years. The baseline HbA1c levels ranged from 7.7% to 9% for the intervention group, whereas the baseline HbA1c levels for the control group ranged from 7.6% to 8.7%.
All the study participants in the intervention group underwent SRP without adjunctive use of any local or systemic antibiotics or any other therapy, whereas patients in the control group did not undergo any form of periodontal treatment. Characteristics of all six incorporated studies are shown in Table 1, and alterations in primary and secondary outcome measures are shown in Tables 2-4.
Table 1.
Study characteristics of all included randomized-control trials
| Last name of first author, years of publication, country | Total sample size | CG (n) | TG (n) | Total duration of diabetes in CG (years) | Total duration of diabetes in TG (years) | Study duration (months) | Inclusion criteria for CP | Inclusion criteria for T2DM | Intervention |
|---|---|---|---|---|---|---|---|---|---|
| Moeintaghavi A et al., 2012, Iran | 40 | 18 | 22 | NR* | NR* | 3 | Mild-to-moderate periodontitis | HbA1c levels ≥7% | 40 patients with T2DM and CP the treatment group (n=22) received full-mouth scaling and root planning, whereas the control group (n=18) received no periodontal treatment |
| Gay I et al., 2014, USA | 126 | 60 | 66 | NR* | NR* | 4 | Localized or generalized severe chronic periodontitis | HbA1c levels ≥6.5% | 126 T2DM patients with periodontal disease. The test group (n=66) was treated with scaling and root planning the control group (n=60) received oral hygiene instructions but not any periodontal treatment |
| Engebretson S et al., 2013, USA | 514 | 257 | 257 | 11.3 | 8.2 | 6 | Moderate to advanced CP defined as CAL and PD of at least >5 mm in 2 or more quadrants of the mouth | HbA1c value 7%-9% | 514 T2DM patients with CP. The treatment group (n=257) received SRP+chlorhexidine oral rinse at baseline, and supportive periodontal therapy at three and six months. The control group (n=257) received no treatment for 6 months |
| Raman RPC et al., 2014, Malaysia | 32 | 17 | 15 | NR* | NR* | 3 | Moderate-to-severe CP | HbA1c levels ≥7% | 32 T2DM patients with periodontal disease. The test group (n=15) was treated with scaling and root planning the control group (n=17) received oral hygiene instructions but not any periodontal treatment |
| Telgi RL et al., 2013, India | 40 | 20 | 20 | NR* | NR* | 3 | Mild-to-moderate CP | HbA1c levels ≥7% | 60 T2DM patients with periodontal disease. Three groups: Group A (scaling, mouthwash, and brushing), Group B (mouthwash and brushing) and Group C (brushing only). In our meta-analysis we have taken Group A data in test and Group C data in control group |
| Koromantzos PA et al., 2011, Greece | 60 | 30 | 30 | 7.84 | 4.33 | 6 | Moderate-to-severe CP | HbA1c levels 7%-10% | 60 T2DM patients with periodontal disease. The intervention group (n=30) was treated with SRP and the delayed treatment group or control group (n=30) that received periodontal care after 6 months |
CG – Control group; TG – Treatment group; CP – Chronic periodontitis; T2DM – Type 2 diabetes mellitus; HbA1c – Glycated hemoglobin; SRP – Scaling and root planning; CAL – Clinical attachment level; NR – Values are not reported in respective included study; n – Number of study subjects; PD – Probing depth
Table 2.
Changes in glycated hemoglobin levels
| Control group HbA1c (%) |
Treatment group HbA1c (%) |
|||
|---|---|---|---|---|
| Baseline | Follow-up* | Baseline | Follow up* | |
| Moeintaghavi A et al., 2012 | 8.72 (2.22) | 8.97 (1.82) | 8.15 (1.18) | 7.41 (1.18) |
| Gay I et al., 2014 | 8.40 (2.00) | 8.10 (1.80) | 9.00 (2.30) | 8.40 (1.90) |
| Engebretson S et al., 2013 | 7.78 (0.60) | 7.88 (0.60) | 7.84 (0.65) | 7.99 (0.60) |
| Raman RPC et al., 2014 | 7.60 (1.50) | 7.10 (1.20) | 7.80 (1.50) | 7.10 (1.20) |
| Telgi RL et al., 2013 | 7.74 (0.59) | 7.75 (0.58) | 7.68 (0.63) | 7.10 (0.64) |
| Koromantzos PA et al., 2011 | 7.59 (0.66) | 7.41 (0.48) | 7.87 (0.74) | 7.14 (0.54) |
*Follow-up - 3-4 months. Data were expressed as mean (SD). SD – Standard deviation; HbA1c – Glycated hemoglobin
Table 4.
Changes in clinical attachment level
| Control group CAL (mm) |
Treatment group CAL (mm) |
|||
|---|---|---|---|---|
| Baseline | Follow-up* | Baseline | Follow-up* | |
| Moeintaghavi A et al., 2012 | 3.10 (1.05) | 3.47 (1.44) | 3.14 (1.08) | 2.8 (1.09) |
| Gay I et al., 2014 | NR** | NR** | NR** | NR** |
| Engebretson S et al., 2013 | 3.49 (0.90) | 3.42 (0.90) | 3.48 (0.80) | 3.16 (0.80) |
| Raman RPC et al., 2014 | 2.79 (0.96) | 2.56 (0.97) | 3.35 (0.83) | 2.73 (0.70) |
| Telgi RL et al., 2013 | NR** | NR** | NR** | NR** |
| Koromantzos PA et al., 2011 | NR** | NR** | NR** | NR** |
*Follow-up - 3-4 months, Data were expressed as mean (SD); **NR – Values are not reported in respective included study. CAL – Clinical attachment level; SD – Standard deviation; NR – Values are not reported in respective included study
Table 3.
Changes in probing pocket depth
| Control group PPD (mm) |
Treatment group PPD (mm) |
|||
|---|---|---|---|---|
| Baseline | Follow-up* | Baseline | Follow-up* | |
| Moeintaghavi A et al., 2012 | 2.06 (0.24) | 2.33 (0.30) | 2.31 (0.65) | 2.21 (0.60) |
| Gay I et al., 2014 | NR** | NR** | NR** | NR** |
| Engebretson S et al., 2013 | 3.28 (0.70) | 3.15 (0.70) | 3.26 (0.60) | 2.82 (0.60) |
| Raman RPC et al., 2014 | 2.29 (0.69) | 2.02 (0.71) | 2.56 (0.57) | 1.76 (0.19) |
| Telgi RL et al., 2013 | 5.05 (0.69) | 5.03 (0.69) | 5.05 (0.70) | 4.59 (0.72) |
| Koromantzos PA et al., 2011 | NR** | NR** | NR** | NR** |
*Follow-up - 3-4 months, Data were expressed as mean (SD); **NR – Values are not reported in respective included study. SD – Standard deviation; PPD – Probing pocket depth; NR – Values are not reported in respective included study
Quality assessment results
Following parameters were measured for the quality assessment of included studies; selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias. Most of the studies showed a low risk of bias for almost all the parameters assessed, whereas 30% of the studies showed a high risk for performance and detection bias [Figure 2]. Further, the risk of bias was unclear for selection bias (allocation concealment).
Figure 2.
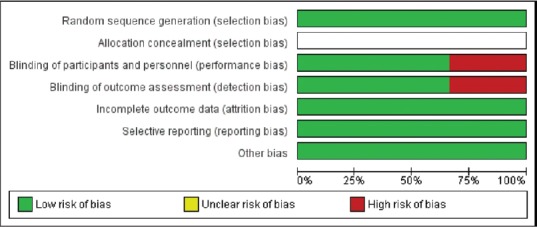
Percentages of risk of bias items across all included studies
Scaling and root planing and glycated hemoglobin
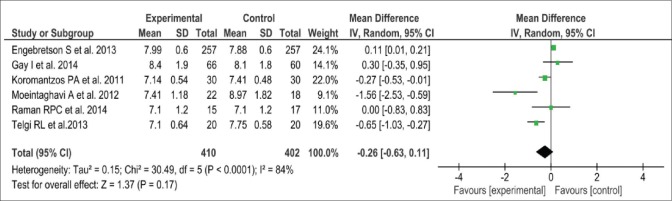
Data on alteration in HbA1c were available for analysis in 812 patients, enrolled in six studies. Three out of the six studies demonstrated substantial improvement in glycemic control after periodontal intervention as assessed by a significant reduction in HbA1c levels when compared to the control group. Overall, the mean change in HbA1c from baseline to 3 months was - 0.26% (95% CI: −0.63, 0.11) [Figure 3]. This was further illustrated in the forest plot, showing a favorable effect on glycemic control. Nevertheless, there are chances of significant heterogeneity in the change of HbA1c levels across the studies (P < 0.0001, I2 = 84%), because of the wide variations in the sample size of the included studies.
Figure 3.
Forest plot showing change in glycated hemoglobin (%) at 3-month. SD – Standard deviation; IV – Intravitreal; CI – Confidence interval
To address possible publication bias, we used the Begg's test for funnel plot asymmetry. Analysis of the funnel plot showed minimal asymmetry (data are not shown), which was further supported by the Begg's test (P = 0.04) meaning thereby that there was no publication bias in our analysis [Figure 4].
Figure 4.
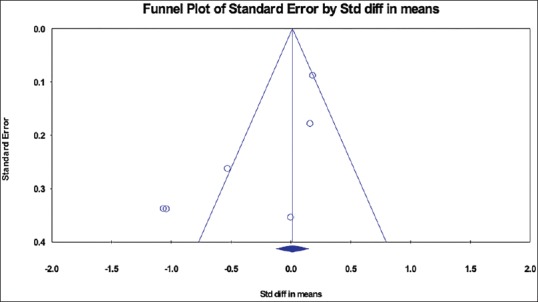
Funnel plot assessing the publication bias
Change of periodontal parameter at 3 months
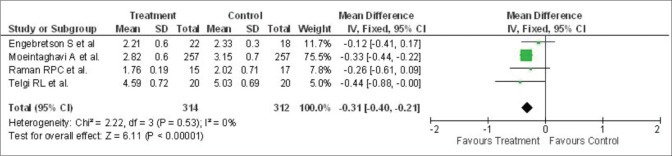
SRP demonstrated a substantial benefit on the periodontal parameters. Four studies described the mean change in PPD in 626 patients. The mean change of PPD at 3 months after periodontal therapy was - 0.31 mm (95% CI: −0.40, −0.21) [Figure 5].
Figure 5.
Forest plot showing alterations in probing pocket depth (mm) from baseline to 3 months. SD – Standard deviation; IV – Intravitreal; CI – Confidence interval
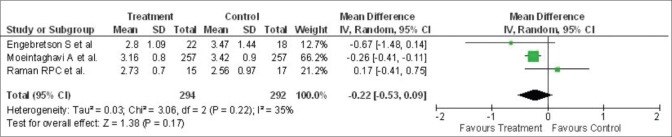
However, no heterogeneity was recorded regarding the change of PPD across studies (P = 0.53, I2 = 0%). Three studies described the mean change of CAL in 586 patients, and the mean alteration in CAL at 3 months after treatment was - 0.22 mm (95% CI: −0.53, 0.09) [Figure 6]. Further, there was minimal heterogeneity in the alteration of CAL within studies (P = 0.22, I2 = 35%).
Figure 6.
Forest plot showing change in clinical attachment level (mm) from baseline to 3-month. SD – Standard deviation; IV – Intravitreal; CI – Confidence interval
Our results demonstrated the beneficial effect of nonsurgical periodontal treatment not only on glycemic status as measured by HbA1c but also on periodontal parameters CAL and PPD.
DISCUSSION
Several earlier systematic reviews and meta-analyses have described the effect of nonsurgical periodontal treatment on glycemic control in patients of T2DM with CP.[14,15,16] Teeuw et al. in 2010, in their meta-analyses, conducted the literature search till 2009 and included five studies.[15] The type of intervention in included studies, in their meta-analyses, was NSPT, including SRP with adjunctive use of topical and systemic antibiotics.[15] Wang et al., in 2014, in their meta-analyses, conducted the literature search till January 2014 and included 10 studies.[16] Intervention in selected studies, in their meta-analysis, was mainly the NSPT, including subgingival and supragingival debridement or SRP with adjunctive use of topical and systemic antibiotics and one of the included studies also involved surgical therapy (periodontal flap surgery), when indicated.[16] Hence, in both of these previous meta-analyses, there was masking of results of SRP alone on glycemic and periodontal status, with adjunctive use of topical and systemic antibiotics, in T2DM patients with CP. The present meta-analysis focuses on the literature search to find out whether SRP as monotherapy has an effect on glycemic control of patients with T2DM. This included six RCTs conducted during the past 10 years, enrolling participants of varying ethnicity from different geographical areas, meaning thereby, the extracted data reflect the global status. The studies included in this meta-analysis have enrolled a total of 410 patients in the intervention group, and 402 control patients following a stringent inclusion criterion. The patients in the intervention group underwent only SRP as a modality of treatment to improve glycemic control and periodontal parameters in T2DM patients suffering from CP. No alterations in antidiabetic medications were made during the study period; in all the selected studies but the one by Gay et al. in 2014, which included individuals from low socioeconomic strata where twenty percentages of the study individuals were not taking any medication for the management of diabetes and patients with HbA1c levels >10% were allowed to have changes in the medical treatment.[22]
Our meta-analysis supports the evidence that SRP leads to improvement in glycemic control in the diabetic patients. The findings show that SRP as a monotherapy results in improvement in HbA1c by 0.26% in the treatment group as compared to the control group at 3 months though the values were not statistically significant. These results suggest that given treatment certainly reduces the HbA1c level, though it did not attain statistical significance. Telgi et al., in 2013, support that NSPT improves the glycemic control in diabetic patients, as there was a significant improvement in HbA1c levels after 3 months of intervention.[25] However, at the same time, the largest multicenter, randomized trial [21] conducted by Engebretson et al. in 2013, demonstrated no beneficial effect of NSPT on HbA1c after 3 and 6 months of treatment.
Two of the included RCTs [21,26] were conducted for 6 months, and one of these [26] showed that the benefit of NSPT could be extended for 6 months. This study demonstrated that there was a substantial improvement in HbA1c levels in T2DM patients suffering from moderate-to-severe periodontitis after NSPT. However, the study had a small sample size.
Some of the previous systematic reviews [14,15] have failed to demonstrate the long-term beneficial effect of NSPT on HbA1c at 6 months indicating that the beneficial effect of nonsurgical treatment is ill-sustained. The findings of the study by Raman et al. 2014, emphasized that correct brushing techniques and proper method of plaque control can also translate into a clinically relevant improvement in the metabolic control.[24] The study demonstrated a substantial decrease in HbA1c levels in the treatment group compared with the baseline values; however, there was no significant difference in reduction in HbA1c levels among the treatment and the control group at the end of their 4-month trial. Recently, an updated report of eight meta-analyses by Madianos and Koromantzos 2018, showed that effect of NSPT without the use of adjunctive use of antibiotics resulted in the statistically significant reduction in HbA1c (0.40%) at 3–4 months. However, improvement in HbA1c could not sustain for 6 months. Moreover, adjunctive use of antibiotics did not increase the effect of SRP on glycemic control.[27]
Worsening of glycemic control in the presence of CP in patients with T2DM can be attributed to the increased release of proinflammatory cytokines comprising interleukin-6 and tumor necrosis factor-α, which play an essential role in augmenting insulin resistance.[3] Therefore, the NSPT has an impact on glycemic control by reducing the systemic proinflammatory cytokines. The study by Moeintaghavi et al., in 2012, exhibited a substantial reduction in HbA1c between control and the treatment group after 3 months of completion of the study, which was attributed to decrease in proinflammatory cytokines after nonsurgical periodontal treatment.[23] However, the reduction in HbA1c was lower as shown in most of the meta-analysis.[15,16]
In the present meta-analysis, all the studies did not report the relative changes in periodontal parameters along with the change in HbA1c. The existing data retrieved from the selected studies were analyzed and only four [21,23,24,25] and three studies [21,23,24] out of six studies had recorded the periodontal parameters PPD and CAL respectively. The sub-group analysis revealed that there was an overall 0.31-mm decrease in PPD at 3 months which was not statistically significant; however, the forest plot for the same demonstrated that the effect of NSPT on PPD had a favorable outcome. The effect of SRP on CAL showed a reduction of 0.22 mm following 3 months of treatment, but it was also statistically nonsignificant. The forest plot analysis for the CAL showed that only two out of three studies showed the beneficial response for gain in CAL after the treatment, with overall results in favor of nonsurgical management.
In the light of the above discussion and the results of this meta-analysis, it can be emphasized that there is a reduction in the levels of HbA1c after SRP, but the effect is not sustained for a long period. Nevertheless, it should not be overlooked that a reduction of 0.26% as a result of SRP can contribute toward the systemic health of the diabetic patient, though for a short duration.
The strength of this meta-analysis includes a larger sample size of 812 patients, inclusion of only T2DM patients with CP, homogeneity in treatment modality, and assessment of periodontal parameters. The meta-analysis [15] which includes studies with small sample size does not provide enough power to the study for the assessment of glycemic benefits following the NSPT. Our meta-analysis included only patients with T2DM as opposed to the other study,[16] which has included patients with both T2DM and T1DM. Homogeneity in the treatment modality is the greatest strength of this meta-analysis contrary to the earlier studies, which have used systemic or local antibiotic treatment as well.[14,15,16] Any study, including intervention group using systemic or topical antibiotics as adjunctive to SRP, was not included for this meta-analysis. Administration of systemic and topical antibiotics, per se, is also instrumental in improving the HbA1c levels and systemic and local periodontal inflammatory conditions and the systemic inflammatory load. Hence, the adjunctive use of the antibiotic (topical or/and systemic) along with SRP may not depict the true relationship of the effect of SRP on HbA1c levels and periodontal parameters. However, a recent report suggests for no additional benefit of using adjunctive antibiotics along with SRP.[27]
Most importantly, the studies, which have been included for meta-analysis, recorded the PPD and CAL, the two sensitive and specific parameters for assessing periodontal status. Along with the changes in the HbA1c levels, the relative changes in PPD and CAL are also assessed which gives a substantial strength to our meta-analysis. Finally, the majority of the included studies in our meta-analysis enrolled subjects with HbA1c <8%, therefore these studies assessed the modest alteration in levels of HbA1c after conventional nonsurgical periodontal treatment.
Limitations of this meta-analysis are that the studies included were only from a limited number of databases and had a short follow-up period of 3–4 months. Further, we restricted our search to the English language only for the feasibility of data extraction. Moreover, most of the included studies enrolled small sample size except one. However, the total sample size of meta-analysis did reach a significant number of 810. Further, 30% of the studies showed a high risk of bias for blinding of participants and personnel and blinding of outcome assessment.
CONCLUSION
This meta-analysis summarized that SRP alone, as a modality of NSPT show a trend of modest (0.26%) improvement in the glycemic control and periodontal parameters in T2DM patients suffering from CP after 3–4 months of periodontal treatment. There is a significant improvement in the clinical periodontal parameter after the therapy. It is suggested that since, SRP as a monotherapy, helps in reducing general inflammatory load as well as a reduction of HbA1c levels in diabetic individuals, it should be considered as a component of the medical regime (i.e., along with other therapeutic and preventive measures) to manage T2DM patients. However, large well-conducted clinical trials with longer follow-up periods and assessing the change in immunological markers of systemic inflammation are necessary to further understand the long-term effect of SRP on glycemic indices and periodontal status.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to express our thanks to those who have helped us most, throughout this study. We are grateful to Prof. Anil Bhansali, Department of Endocrinology, Postgraduate Institute of Medical and Research, Chandigarh, and Dr. Shobhit Bhansali for their nonstop support for this study.
APPENDIXES
APPENDIX 1: MEDLINE search strategy
(Dental Prophylaxis[Mesh] OR Dental Scaling[Mesh] OR Root Planing[Mesh] OR Periodontal Debridement[Mesh] OR Subgingival Curettage[Mesh] OR ((dental[tiab] OR subgingival[tiab] OR supragingival[tiab] OR periodontal[tiab] OR pocket[tiab] OR ultrasonic[tiab] OR mechanical[tiab])
AND (scaling*[tiab] OR planing*[tiab] OR debridement*[tiab] OR curettage*[tiab] OR irrigation*[tiab] OR instrumentation*[tiab])) OR dental prophylaxis[tiab] OR periodontal therapy[tiab] OR periodontal therapeutics[tiab] OR periodontal treatment[tiab] OR periodontal intervention*[tiab] OR periodontal maintenance[tiab])
AND (Diabetes Mellitus[Mesh] OR Hemoglobin A, Glycosylated[Mesh] OR hemoglobin A1c protein, human[Supplementary Concept] OR pre-hemoglobin A, glycosylated[Supplementary Concept] OR Hb A1a + b[tiab] OR Hb A1c[tiab] OR HbA1[tiab] OR glycosylated hemoglobin*[tiab] OR Hb A1[tiab] OR glycosylated A1b[tiab] OR Hb A1b[tiab] OR Hb A1a[tiab] OR glycated hemoglobin*[tiab] OR glycosylated hemoglobin*[tiab] OR glycated hemoglobin*[tiab] OR glycemic control[tiab] OR glycemic control[tiab] OR diabetes[tiab])
APPENDIX 2: EMBASE search strategy
'periodontics'/de OR (dental: ab, ti OR subgingival: ab, ti OR supragingival: ab, ti OR periodontal: ab, ti OR pocket: ab, ti OR ultrasonic: ab, ti OR mechanical: ab, ti AND (scaling*:ab, ti OR planing*:ab, ti OR debridement*:ab, ti OR curettage*:ab, ti OR irrigation*:ab, ti OR instrumentation*:ab, ti)) OR 'dental prophylaxis':ab, ti OR 'periodontal therapy':ab, ti OR 'periodontal therapeutics':ab, ti OR 'periodontal treatment':ab, ti OR 'periodontal intervention':ab, ti OR 'periodontal interventions':ab, ti OR 'periodontal maintenance':ab, ti
AND ('glycosylated hemoglobin'/exp OR 'hb a1a + b':ab, ti OR 'hb a1c':ab, ti OR hba1:ab, ti OR 'glycosylated hemoglobin':ab, ti OR 'hb a1':ab, ti OR 'glycosylated a1b':ab, ti OR 'hb a1b':ab, ti OR 'hb a1a':ab, ti OR 'glycated hemoglobin':ab, ti OR 'glycated hemoglobins':ab, ti OR 'glycosylated haemoglobins':ab, ti OR 'glycosylated haemoglobin':ab, ti OR 'glycated haemoglobin':ab, ti OR 'glycated haemoglobins':ab, ti OR 'glycemic control':ab, ti OR 'glycaemic control':ab, ti OR diabetes: ab, ti) AND [embase]/lim
REFERENCES
- 1.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 2.Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127–53. doi: 10.1111/j.1600-0757.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 3.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: A two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 4.Hugoson A, Thorstensson H, Falk H, Kuylenstierna J. Periodontal conditions in insulin-dependent diabetics. J Clin Periodontol. 1989;16:215–23. doi: 10.1111/j.1600-051x.1989.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 5.Thorstensson H, Hugoson A. Periodontal disease experience in adult long-duration insulin-dependent diabetics. J Clin Periodontol. 1993;20:352–8. doi: 10.1111/j.1600-051x.1993.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt AM, Weidman E, Lalla E, Yan SD, Hori O, Cao R, et al. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. 1996;31:508–15. doi: 10.1111/j.1600-0765.1996.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Zamora YI, Rodriguez-Sosa M. The role of MIF in type 1 and type 2 diabetes mellitus. J Diabetes Res. 2014;2014:804519. doi: 10.1155/2014/804519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mealey BL, Oates TW. American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 9.Pucher J, Stewart J. Periodontal disease and diabetes mellitus. Curr Diab Rep. 2004;4:46–50. doi: 10.1007/s11892-004-0011-y. [DOI] [PubMed] [Google Scholar]
- 10.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 11.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–34. [PubMed] [Google Scholar]
- 12.Lösche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000;27:537–41. doi: 10.1034/j.1600-051x.2000.027008537.x. [DOI] [PubMed] [Google Scholar]
- 13.Parameters of care. American Academy of Periodontology. J Periodontol. 2000;71:i. doi: 10.1902/jop.2000.71.5-S.i. [DOI] [PubMed] [Google Scholar]
- 14.Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. J Periodontol. 2013;84:S153–69. doi: 10.1902/jop.2013.1340017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: A systematic review and meta-analysis. Diabetes Care. 2010;33:421–7. doi: 10.2337/dc09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Han X, Guo X, Luo X, Wang D. The effect of periodontal treatment on hemoglobin a1c levels of diabetic patients: A systematic review and meta-analysis. PLoS One. 2014;9:e108412. doi: 10.1371/journal.pone.0108412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basevi V, Di Mario S, Morciano C, Nonino F, Magrini N. Comment on: American diabetes association. Standards of medical care in diabetes-2011. Diabetes care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-0174. Diabetes Care 2011;34:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. The Cochrane Collaboration. 2011. [Last updated on 2011 Mar]. Available from http://handbook.cochrane.org .
- 20.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, Gelato MC, Hou W, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: A randomized clinical trial. JAMA. 2013;310:2523–32. doi: 10.1001/jama.2013.282431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gay IC, Tran DT, Cavender AC, Weltman R, Chang J, Luckenbach E, et al. The effect of periodontal therapy on glycaemic control in a hispanic population with type 2 diabetes: A randomized controlled trial. J Clin Periodontol. 2014;41:673–80. doi: 10.1111/jcpe.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moeintaghavi A, Arab HR, Bozorgnia Y, Kianoush K, Alizadeh M. Non-surgical periodontal therapy affects metabolic control in diabetics: A randomized controlled clinical trial. Aust Dent J. 2012;57:31–7. doi: 10.1111/j.1834-7819.2011.01652.x. [DOI] [PubMed] [Google Scholar]
- 24.Raman RP, Taiyeb-Ali TB, Chan SP, Chinna K, Vaithilingam RD. Effect of nonsurgical periodontal therapy verses oral hygiene instructions on type 2 diabetes subjects with chronic periodontitis: A randomised clinical trial. BMC Oral Health. 2014;14:79. doi: 10.1186/1472-6831-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telgi RL, Tandon V, Tangade PS, Tirth A, Kumar S, Yadav V. Efficacy of nonsurgical periodontal therapy on glycaemic control in type II diabetic patients: a randomized controlled clinical trial. J Periodontal Implant Sci. 2013;43:177–82. doi: 10.5051/jpis.2013.43.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN, et al. A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: Effect on periodontal status and glycaemic control. J Clin Periodontol. 2011;38:142–7. doi: 10.1111/j.1600-051X.2010.01652.x. [DOI] [PubMed] [Google Scholar]
- 27.Madianos PN, Koromantzos PA. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. J Clin Periodontol. 2018;45:188–95. doi: 10.1111/jcpe.12836. [DOI] [PubMed] [Google Scholar]