Significance
Dormant bacterial spores can survive long periods of time, withstanding extreme conditions, but can rapidly resume a vegetative life form once nutrients become accessible. The key event of this revival process is termed germination, the earliest phase, lasting only for a few minutes, during which cellular awakening is established. Yet, the molecular events propelling this enigmatic phase remain unknown. Here, we revealed that dephosphorylation of Arg residues on key target proteins triggers germination in the model bacterium B. subtilis. We further demonstrate that Arg dephosphorylation of a ribosome-associated factor reactivates translation, whereas dephosphorylation of the major housekeeping σ factor reinitiates transcription. Our results provide a whole mechanism for spore awakening in bacteria.
Keywords: Bacillus subtilis, spore dormancy, germination, arginine phosphorylation
Abstract
Bacterial spores can remain dormant for years but possess the remarkable ability to germinate, within minutes, once nutrients become available. However, it still remains elusive how such instant awakening of cellular machineries is achieved. Utilizing Bacillus subtilis as a model, we show that YwlE arginine (Arg) phosphatase is crucial for spore germination. Accordingly, the absence of the Arg kinase McsB accelerated the process. Arg phosphoproteome of dormant spores uncovered a unique set of Arg-phosphorylated proteins involved in key biological functions, including translation and transcription. Consequently, we demonstrate that during germination, YwlE dephosphorylates an Arg site on the ribosome-associated chaperone Tig, enabling its association with the ribosome to reestablish translation. Moreover, we show that Arg dephosphorylation of the housekeeping σ factor A (SigA), mediated by YwlE, facilitates germination by activating the transcriptional machinery. Subsequently, we reveal that transcription is reinitiated at the onset of germination and its recommencement precedes that of translation. Thus, Arg dephosphorylation elicits the most critical stages of spore molecular resumption, placing this unusual post-translational modification as a major regulator of a developmental process in bacteria.
In response to nutrient deprivation, the Gram-positive bacterium Bacillus subtilis (B. subtilis) and its relatives have the capacity to initiate a developmental process, termed sporulation, which culminates in the formation of a highly resilient durable spore (1–4). Sporulation commences with the formation of a polar septum, asymmetrically dividing the cell into a small forespore and a larger mother cell compartment. Subsequently, the forespore is engulfed by the mother cell, and through sequential activation of cell type specific σ factors, the spore matures within the mother cell (5, 6). Spore development is accompanied by the formation of a peptidoglycan layer called the cortex, surrounded by two proteinaceous coats, conferring spore robustness (7–12). The forespore core then undergoes dehydration, mainly by accumulating pyridine-2, 6-dicarboxylic acid [dipicolinic acid or (DPA)] in complex with Ca2+ (13). Eventually, the phase-bright spore is liberated by lysis of the mother cell but intriguingly can still undergo significant molecular changes, at least for a few days, influencing its emergence from quiescence (14–16).
Spores can maintain dormancy for years yet possess the remarkable ability to rapidly resume a vegetative life form once nutrients become available. This revival process includes three key phases: germination, ripening, and outgrowth (17–19). Germination, the earliest revival event, is characterized by transition from a phase-bright spore to a phase-dark cell (20), whereas no morphological changes are evident during the intermediate ripening period, exploited by the spore for molecular reorganization (18). The last phase of revival is outgrowth, in which the spore intensively synthesizes macromolecules to rebuild a rod shaped cell that emerges from the disintegrating spore shells (17, 21).
Germination is considered the most enigmatic event in the process of spore revival, lasting only for a few minutes (19). During germination, the spore undergoes rehydration, release of DPA, hydrolysis of the cortex, and coat disassembly (17, 21, 22). This phase is associated with the loss of heat resistance and a decrease in optical density (20). Recently, we demonstrated that translation is rapidly activated after DPA release and is required for the subsequent events of germination (23, 24), challenging the traditional view that germination occurs without the need for any macromolecule synthesis (13, 16, 17, 25, 26). Furthermore, we showed that protein synthesis during germination was facilitated by the bona fide translational factors RpmE, a ribosomal subunit, and Tig, a peptidyl-prolyl cis–trans isomerase, that is a ribosome-associated chaperone (23, 27–29).
Germination is triggered by binding of nutrients, termed germinants, to multiple germination receptors located in the spore membrane (30). Germinant factors include single amino acids, sugars, purine nucleosides, and cell wall muropeptides (17, 19, 31). In B. subtilis, the GerA receptor binds l-alanine (l-Ala) to induce germination, whereas GerB and GerK receptors respond jointly to a mixture of germinants consisting of aspargine (Asp), glucose, fructose, and potassium (AGFK) ions (17, 21, 22). This germinant sensing stimulates downstream effectors activating germination; however, relatively little is known about this signal transmission pathway. A potential mechanism for the fast resumption of multiple cellular processes in the germinating spore is protein phosphorylation, a ubiquitous means for mediating rapid cellular responses to various external stimuli in bacteria (32, 33). Evidence has been provided that peptidoglycan-derived muropeptides induce germination by binding to the Ser/Thr kinase PrkC, which appears to phosphorylate the translation elongation factor G (EF-G) (31, 34). However, the impact of this phosphorylation event on germination is still unexplored, and the absence of PrkC has no effect on the process of germination driven by nutrient germinants (31, 35). More recently, we described the dynamic of the Ser/Thr/Tyr phosphoproteome during germination, identifying a plethora of 155 phosphorylation sites, modified during germination, providing evidence that phosphorylation events facilitate spore revival. However, no role in promoting germination was assigned to these events (35).
Arg phosphorylation, an emerging protein modification, has been lately described to occur in B. subtilis and Staphylococcus aureus (36, 37). The kinase responsible for Arg phosphorylation in B. subtilis was shown to be McsB, which counteracts the protein Arg phosphatase YwlE (36–41). Here, we demonstrate that YwlE drives the progression of spore germination by dephosphorylating Arg phosphosites of target proteins involved in key cellular processes. Furthermore, we show that YwlE mediates the rapid reactivation of the translational machinery by dephosphorylating the translational component Tig, enabling its association with the ribosome. Surprisingly, we found that Arg dephosphorylation of the housekeeping factor SigA by YwlE is crucial for the execution of germination, and subsequently, we discovered that transcription is reestablished at the onset of the process.
Results
Spore Germination Is Driven by Arg Dephosphorylation.
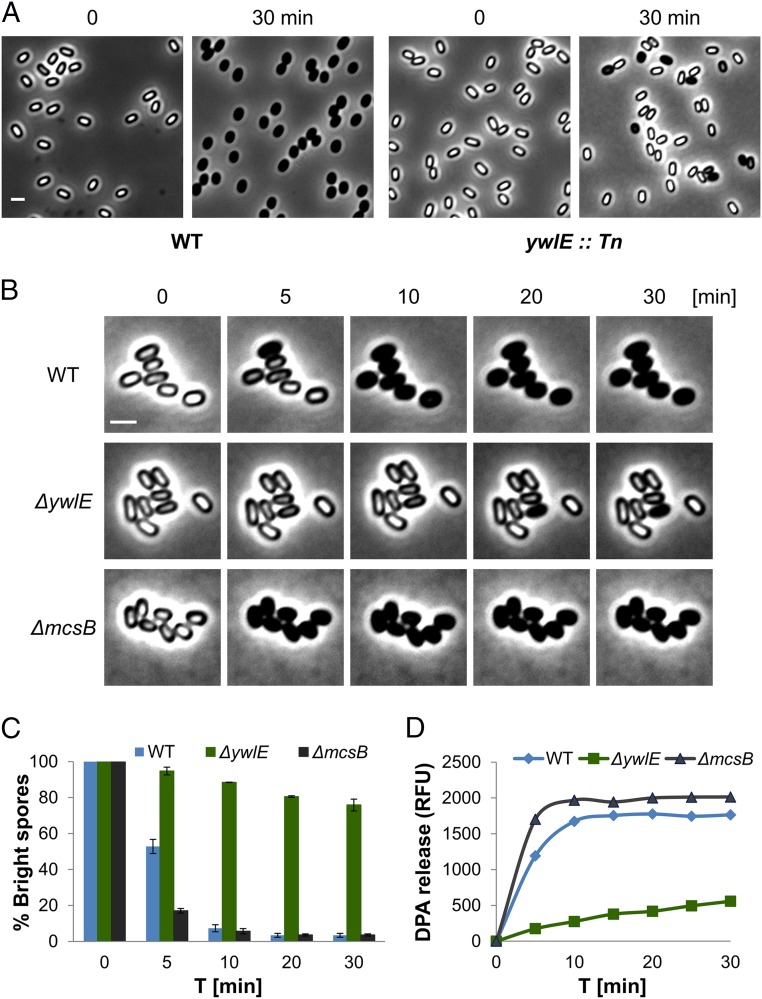
To identify genes required for spore germination, we designed a transposon-based genetic screen, searching for mutants that are able to form mature spores but deficient in the conversion from a phase-bright to a phase-dark state following germinant addition (SI Appendix, Fig. S1A). Using this approach, we identified a mutant disrupted in the ywlE gene, encoding an Arg phosphatase (39), exhibiting severe germination deficiency (Fig. 1A). Constructing a strain, fully deleted of ywlE, revealed that it was able to grow and sporulate normally but produced spores that were perturbed in germination as indicated by only a slight decrease in optical density achieved in response to the efficient l-Ala germinant (SI Appendix, Figs. S1 B and C and S2A). Monitoring germination efficiency by time lapse microscopy at a single spore resolution showed that 30 min following l-Ala addition only 20% of the ΔywlE spores could convert from a phase-bright to a phase-dark state in comparison with 96% of the wild type (WT) spores (Fig. 1 B and C). Similar results were obtained when germination was induced by supplementing the spores with AGFK and even with LB (SI Appendix, Fig. S2 B, C, and E). In line with these findings, ΔywlE mutant spores were slower to release DPA and lose their heat resistance relative to the WT spores (Fig. 1D and SI Appendix, Fig. S2D), signifying that YwlE is a key factor in prompting spore germination.
Fig. 1.
Spore germination is facilitated by Arg dephosphorylation. (A) Spores of PY79 [wild type (WT)] and ywlE::Tn were incubated with l-Ala (10 mM) and observed by light microscopy before (t = 0) and after (t = 30 min) l-Ala addition. Shown are phase contrast images from a representative experiment out of three independent biological repeats. (Scale bar: 1 μm.) (B) Spores of PY79 (WT), BZ16 (∆ywlE), and BZ129 (∆mcsB) strains were incubated on agarose supplemented with l-Ala (10 mM) and monitored by time lapse microscopy. Shown are phase contrast images from a representative experiment out of three independent biological repeats. (Scale bar: 1 μm.) (C) Spores of PY79 (WT), BZ16 (∆ywlE), and BZ129 (∆mcsB) strains were incubated on agarose supplemented with l-Ala (10 mM) and monitored by time lapse microscopy. Data are presented as percentages of the initial number of the phase-bright spores. Shown are average values and SDs obtained from three independent biological repeats (n ≥ 300 for each strain). (D) Spores of PY79 (WT), BZ16 (∆ywlE), and BZ129 (∆mcsB) strains were incubated with l-Ala to trigger germination. DPA release to the medium was determined by Tb-DPA assay. Presented are relative fluorescence units (RFUs) measured at 545 nm with excitation at 270 nm. Shown is a representative experiment out of three independent biological repeats.
The role of YwlE as an Arg phosphatase brings about the possibility that some crucial factors required for germination are phosphorylated on their Arg sites during sporulation, a modification that should be removed to allow germination. Notably, only a single kinase, McsB, is known to be responsible for Arg phosphorylation in B. subtilis (38, 40). Hence, we hypothesized that, in ΔmcsB spores, these factors would be constantly in their dephosphorylated active form, and consequently, these spores would germinate rapidly. Indeed, ΔmcsB mutant spores germinated faster than WT spores as evidenced by their remarkable rapid transition into the phase-dark state in all tested conditions (Fig. 1 B and C and SI Appendix, Fig. S2). Furthermore, germinating ΔmcsB spores turned heat sensitive and released DPA faster than WT spores (Fig. 1D and SI Appendix, Fig. S2D). Examining germination of ΔmcsB ΔywlE double mutant spores revealed kinetics similar to that of ΔmcsB spores (SI Appendix, Fig. S3). Taken together, we infer that Arg phosphorylation of target proteins is executed by McsB during sporulation and is removed by YwlE to propel spore germination.
Revealing the Arg Phosphoproteome of Dormant Spores.
Having established that Arg dephosphorylation is required for spore germination, we next sought to identify Arg-phosphorylated sites in the dormant spore proteomes that could serve as substrates for YwlE during spore germination. As Arg phosphorylation was shown to be more stable and enriched in the ywlE mutant (37, 40), we carried out Arg-phosphoproteomic analysis of ΔywlE dormant spores. In total, we identified 18 Arg-phosphorylation sites located in 18 proteins with very high confidence using stringent quality criteria for the validation of the phosphorylation sites (Table 1 and SI Appendix, Table S1). A comparison with the Arg phosphoproteome of vegetative cells (37, 40) revealed that Arg-phosphorylation events in spores are less frequent and largely differ from those of vegetative cells with 10 identified sites unique to spores (Table 1 and SI Appendix, Table S1). The detected Arg-phosphorylated proteins are involved in key biological functions, including carbon metabolism, sporulation, translation, and transcription (Table 1). Thus, we uncovered a unique set of protein Arg-phosphorylation sites existing in dormant spores that can be potentially dephosphorylated by YwlE to allow germination. Among the identified phosphorylated factors, the most intriguing were those involved in transcription and translation, raising the possibility that they provide the means for reestablishment of these basic cellular processes.
Table 1.
Proteins phosphorylated on Arg residues and their phosphosites in spores
| Protein | Description | Phosphosite |
| Translation | ||
| Tig | Trigger factor | pR45 |
| Tuf | Elongation factor G | pR265 |
| RplN | 50S ribosomal protein L17 | pR7 |
| Transcription | ||
| SigA | RNA polymerase σ factor | pR365 |
| Protein quality control | ||
| GroEL | Chaperonin | pR35 |
| ClpC | Class III stress response-related ATPase | pR70 |
| Carbon metabolism | ||
| GapA | Glyceraldehyde-3-phosphate dehydrogenase | pR199 |
| YtsJ | NADP-dependent malic enzyme | pR27 |
| MenB | Dihydroxynapthoic acid synthetase | pR269 |
| Pgi | Glucose-6-phosphate isomerase | pR5 |
| Sporulation | ||
| SspA | Small acid-soluble spore protein A | pR55 |
| SspB | Small acid-soluble spore protein B | pR65 |
| SspE | Small acid-soluble spore protein E | pR16 |
| SpoIVA | Stage IV sporulation protein A | pR454 |
| CotF | Spore coat protein | pR155 |
| YtfJ | Germination protein | pR125 |
| Unknown | ||
| YeaD | Unknown function | pR250 |
| YtxH | Unknown function | pR151 |
Dephosphorylation of an Arg Site on the Translational Factor Tig Powers Spore Germination.
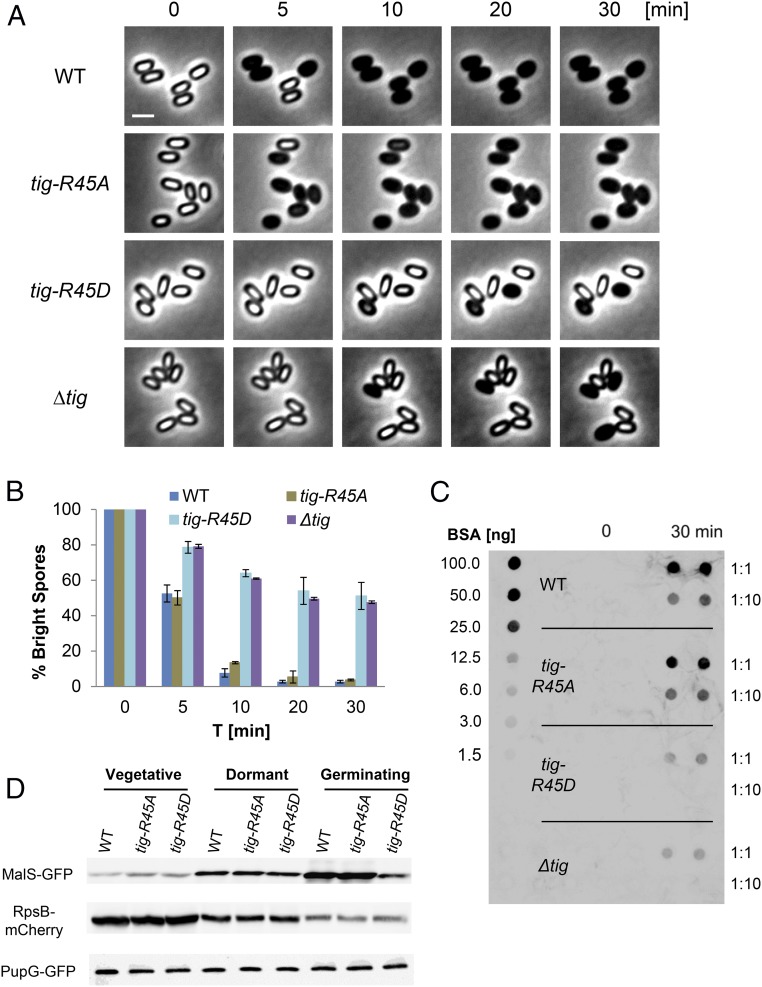
Previously, we revealed that protein synthesis takes place during germination and relies on the bona fide translational factor Tig (23, 27), identified in our phosphoproteomic analysis to harbor an Arg45-phosphorylated site (Table 1). Interestingly, the occurrence of this phosphorylation event was monitored in vegetative cells following exposure to heat stress (40). We reasoned that phosphorylation of Tig in spores blocks its activity, whereas dephosphorylation by YwlE restores the factor utility during germination. To investigate the impact of the Arg45 site on the activity of Tig, we introduced mutations that would either abolish the phosphorylation potential by replacing Arg with Ala or mimic a constitutive phosphorylation state by replacing Arg with Asp (42). Each mutation was inserted as a sole copy at the native tig chromosomal locus, replacing the original WT allele. Remarkably, tig-R45D mutant spores exhibited germination defects similar to that of ∆tig spores, whereas tig-R45A mutant spores germinated similarly to WT spores as indicated by optical density and time lapse microscopy analyses (Fig. 2 A and B and SI Appendix, Fig. S4 A–D). Consistently, mimicking constitutive phosphorylation by replacing the Arg45 site with Glu (R45E), harboring a larger side chain group, led to a germination defect similar to that of R45D protein (SI Appendix, Fig. S5). Importantly, both tig-R45A and tig-R45D mutations had no effect on vegetative growth or sporulation (SI Appendix, Fig. S6 A and B). Further analysis of the tig mutant spores showed that, upon germination induction, tig-R45D spores were capable of releasing DPA and accordingly lost their heat resistance (SI Appendix, Fig. S4 E and F). Hence, similar to ∆tig spores, tig-R45D spores initiated germination normally but were subsequently stalled in their phase-bright state.
Fig. 2.
Dephosphorylation of the translational factor Tig is required for spore germination. (A) Spores of PY79 (WT), BZ98 (tig-R45A), BZ97 (tig-R45D), and LS38 (∆tig) strains were incubated on agarose supplemented with l-Ala (10 mM) and monitored by time lapse microscopy. Shown are phase contrast images from a representative experiment out of three independent biological repeats. (Scale bar: 1 μm.) (B) Spores of PY79 (WT), BZ98 (tig-R45A), BZ97 (tig-R45D), and LS38 (∆tig) strains were incubated on agarose supplemented with l-Ala (10 mM) and monitored by time lapse microscopy. Data are presented as percentages of the initial number of the phase-bright spores. Shown are average values and SDs obtained from three independent biological repeats (n ≥ 300 for each strain). (C) Spores of LS5 (ΔmetE), BZ103 (ΔmetE, tig-R45A), BZ102 (ΔmetE, tig-R45D), and LS80 (ΔmetE, ∆tig) strains were induced to germinate with l-Ala in the presence of AHA for 30 min. Shown is a dot blot analysis of protein samples (in duplicate) that were collected before (t = 0, dormant spores) and after (t = 30 min) germination induction. Samples (1:1 and 1:10 dilution) were spotted on a membrane that was subsequently probed with antibiotin antibodies. The obtained signal was compared with known amounts of biotinylated BSA. (D) Protein extracts from vegetative cells, dormant and germinating spores of strains: (i) AR71 (WT), BZ107 (tig-R45A), and BZ106 (tig-R45D) carrying malS-gfp; (ii) BZ118 (WT), BZ120 (tig-R45A), and BZ119 (tig-R45D) carrying rpsB-mCherry; and (iii) AR68 (WT), BZ144 (tig-R45A), and BZ145(tig-R45D) carrying pupG-gfp were subjected to Western blot analysis using an antibody against GFP or mCherry, respectively. Equal amounts of protein extracts were loaded. Germination was induced by suspending the spores in 10 mM l-Ala for 10 min.
To corroborate that the Tig Arg-phosphorylation state influences the function of Tig, we examined its impact on protein synthesis during germination. We employed the BioOrthogonal Non-Canonical Amino acid Tagging (BONCAT) protein tagging technique that enables specific labeling of newly synthesized proteins due to the incorporation of an artificial amino acid termed azidohomoalanine (AHA), which is a substitute for methionine (43). This assay was utilized to establish the requirement for Tig to facilitate translation during germination (23). To this end, spores of WT, tig-R45D, and tig-R45A strains were induced to germinate with l-Ala in the presence of AHA without supplementing any additional nutrients. Then, proteins were extracted from spores at T = 0 and 30 min postgermination induction, tagged with biotin, and subjected to dot blot analysis using antibiotin antibodies. Whereas a strong signal was readily detected from WT and tig-R45A germinating spores, only a faint signal was monitored from tig-R45D germinating spores, similar to that obtained from ∆tig spores (Fig. 2C). In line with these findings, an apparent delay in protein synthesis during germination was detected in tig-R45D mutant spores by time lapse microscopy using MalS-GFP as a reporter for monitoring translation early in germination (23) (SI Appendix, Fig. S7 A and B). Finally, Western blot analysis substantiated that tig-R45D mutation influences specifically the translation of MalS during germination but did not impact translation of RpsB, an essential ribosomal subunit, and purine nucleoside phosphorylase (PupG), which were not included in the germination proteome (23, 44, 45) (Fig. 2D). Taken together, spores harboring the phosphorylated-like tig-R45D allele are impaired in protein synthesis at the course of germination, similar to ∆tig spores, highlighting the potential importance of this phosphosite for Tig activity.
Tig Association with Ribosomes Depends on Dephosphorylation of the Arg45 Site.
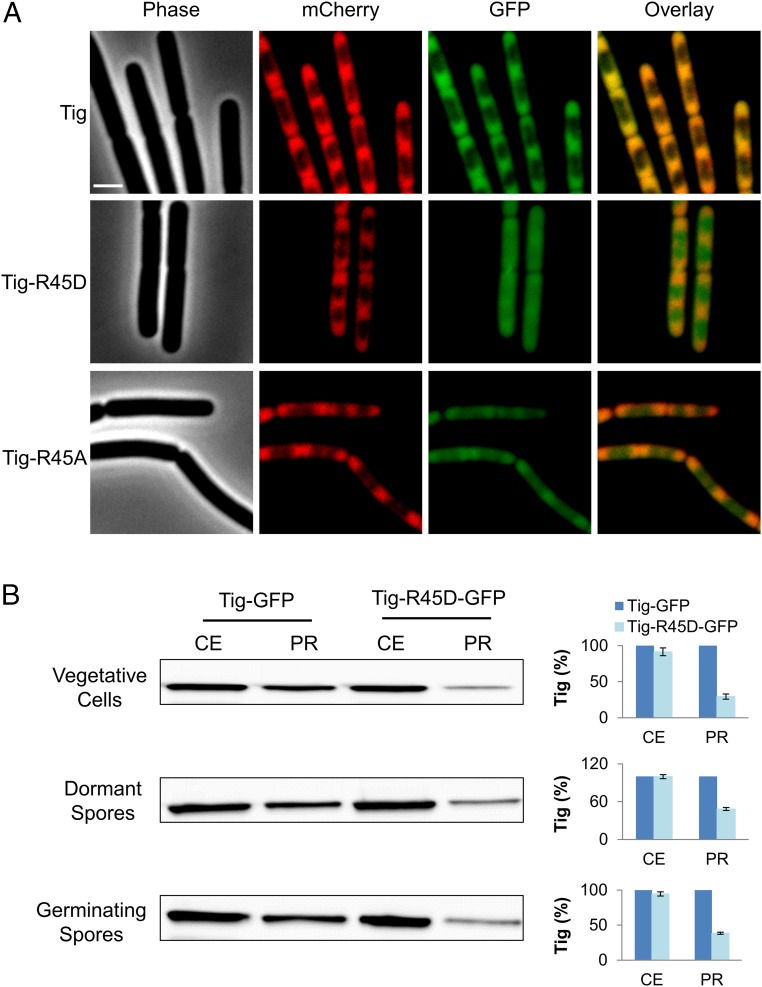
During translation, Tig associates with the ribosome and interacts with newly synthesized nascent chains to chaperone the folding of cytosolic proteins (27). Since tig-R45D mutant spores showed delayed protein synthesis during germination, we hypothesized that Arg45 phosphorylation interfered with Tig binding to ribosomes. To examine this premise, we investigated the localization of Tig and ribosomes in WT and tig mutants in vegetative growing cells whereby the typical ribosome localization to the cell circumference surrounding the nucleoid is profound (46, 47). Tig-R45D mutant protein was fused to GFP, and its localization was followed concomitantly with the RpsB-mCherry fusion, signifying ribosome position (18). Whereas Tig and RpsB perfectly colocalized, the signals from Tig-R45D and RpsB showed only marginal overlap (Fig. 3A). Furthermore, although it is challenging to covisualize RpsB-mCherry and Tig-GFP in the small sized spores, we could detect the specific localization of Tig-GFP to the spore periphery during germination, a pattern that was hardly visible in germinating tig-R45D spores (SI Appendix, Fig. S7C). Interestingly, examining the localization of Tig-R45A revealed partial association with RpsB (Fig. 3A and SI Appendix, Fig. S7C), highlighting the significance of the R45 position for proper association with the ribosome. Nevertheless, this partial association was sufficient to propel germination (Fig. 2). Of note, fusing GFP to WT Tig or to the phosphomimetic mutant proteins did not affect their germination capabilities (SI Appendix, Fig. S6C).
Fig. 3.
Tig-ribosome association is dependent on Arg dephosphorylation. (A) BZ118 (tig-gfp, rpsB-mCherry), BZ119 (tig-R45D-gfp, rpsB-mCherry), and BZ120 (tig-R45A-gfp, rpsB-mCherry) strains were grown to a mid logarithmic phase in LB at 37 °C and visualized by fluorescence microscopy. Shown are images of phase contrast (Phase), fluorescence from mCherry (red), and GFP (green), and an overlay of red and green fluorescence from a representative experiment out of three independent biological repeats. (Scale bar: 1 μm.) (B) Intact ribosomes were purified from CEs of the vegetative cells, dormant and germinating spores of BZ118 (tig-gfp, rpsB-mCherry) and BZ119 (tig-R45D-gfp, rpsB-mCherry) strains. Germination was induced by suspending the spores in 10 mM l-Ala for 10 min. Western blot analysis was carried out using an antibody against GFP, detecting the levels of Tig and TigR45D in CEs and in PRs (Left). The signal from GFP fusion proteins was quantified by MetaMorph software (version 7.7, Molecular Devices) (Right). The signal from Tig-GFP was used to normalize Tig expression levels in CEs and PRs, separately. Shown are average values and SDs obtained from two independent biological repeats.
To further substantiate the notion that Tig-R45D could not bind to ribosomes, we utilized a direct biochemical approach, purifying ribosomes from vegetative cells and spores of tig and tig-R45D strains. Subsequently, we carried out Western blot analysis to detect the levels of Tig in cell extracts (CEs) and in purified ribosomes (PRs). Although similar levels of Tig or Tig-R45D were detected in CEs, Tig-R45D levels were considerably lower in PR fractions from vegetative cells, dormant and germinating spores (Fig. 3B). Of note, the levels of the ribosomal component RpsB were relatively equal in PRs derived from tig and tig-R45D strains (SI Appendix, Fig. S7D). These results reinforce the view that phosphorylation on Arg45 of Tig causes its dissociation from ribosomes.
Germination Is Promoted by Arg Dephosphorylation of SigA.
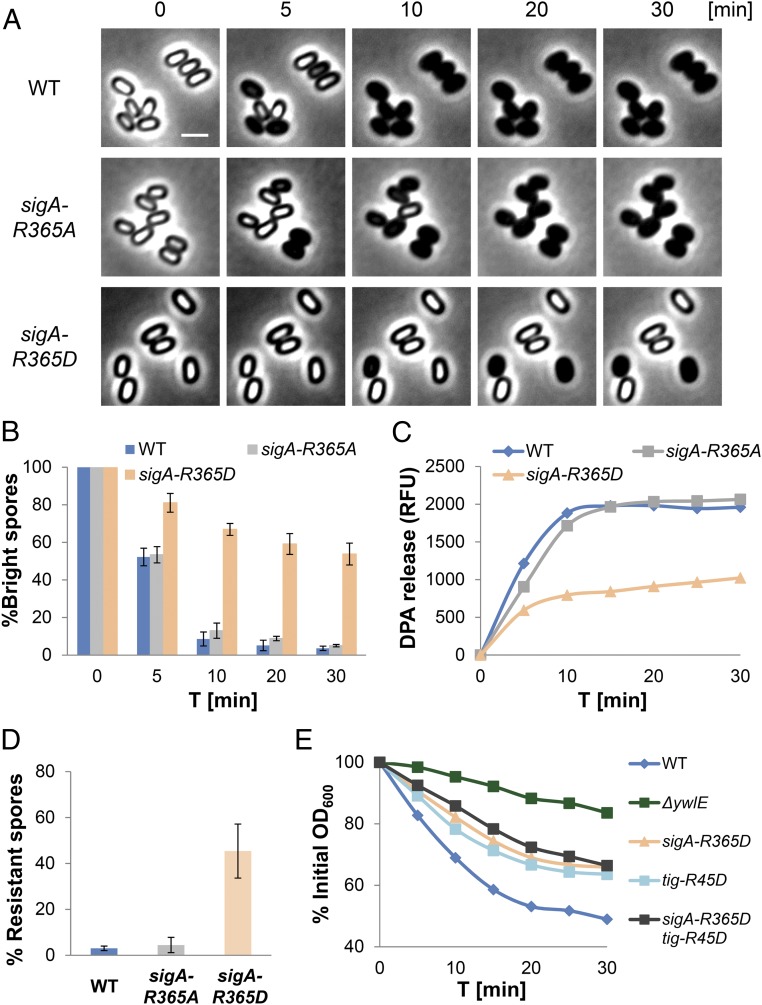
Intriguingly, Arg phosphorylation at position Arg365 of SigA, the house keeping σ factor of B. subtilis (48), was identified in our Arg-phosphoproteome analysis hinting that, similar to translation, transcription might be required to facilitate germination. Furthermore, the phosphorylation of this site appeared unique to spores and was not identified in vegetative cells (37, 40) (Table 1). To test whether the Arg365-phosphorylation state has an impact on spore germination, we constructed mutant strains in which Arg365 was replaced by either Ala or Asp and assessed their ability to germinate. The sigA-R365A mutant spores could germinate as efficiently as WT spores (Fig. 4 A and B and SI Appendix, Fig. S8). However, spores harboring the sigA-R365D mutation, mimicking the phosphorylated form of the protein, exhibited a profound germination defect with only 44% of the spores switching to phase-dark 30 min postgermination induction compared with 96% of the WT spores (Fig. 4 A and B and SI Appendix, Fig. S8). A similar germination defect was obtained in spores from a strain carrying the phosphomimetic R365E mutation (SI Appendix, Fig. S9). A slight decrease in vegetative growth could be assigned to R365D mutations but only when the cells were grown in minimal medium, although no apparent effect on sporulation could be detected (SI Appendix, Fig. S6). Additionally, monitoring the protein levels of germination receptors as well as of Tig and RpmE, required for germination (23), in sigA-R365D and WT dormant spores did not yield any significant difference (SI Appendix, Fig. S10). Examination of DPA release and heat resistance showed that germinating sigA-R365D mutant spores were halted before DPA release and remained heat resistant (Fig. 4 C and D), indicating a deficiency at an early stage of the process before protein translation (SI Appendix, Fig. S4 E and F). Thus, we surmise that the dephosphorylation of SigA promotes germination.
Fig. 4.
The dephosphorylation of SigA facilitates spore germination. (A) Spores of PY79 (WT), BZ90 (sigA-R365A), and BZ91 (sigA-R365D) strains were incubated on agarose supplemented with l-Ala (10 mM) and monitored by time lapse microscopy. Shown are phase contrast images from a representative experiment out of three independent biological repeats. (Scale bar: 1 μm.) (B) Spores of PY79 (WT), BZ90 (sigA-R365A), and BZ91 (sigA-R365D) strains were incubated on agarose supplemented with l-Ala (10 mM) and monitored by time lapse microscopy. Data are presented as percentages of the initial number of the phase-bright spores. Shown are average values and SDs obtained from three independent biological repeats (n ≥ 300 for each strain). (C) Spores of PY79 (WT), BZ90 (sigA-R365A), and BZ91 (sigA-R365D) strains were incubated with l-Ala to trigger germination. DPA release to the medium was determined by Tb-DPA assay. Presented are RFUs measured at 545 nm with excitation at 270 nm. Shown is a representative experiment out of three independent biological repeats. (D) Spores of PY79 (WT), BZ90 (sigA-R365A), and BZ91 (sigA-R365D) strains were supplemented with l-Ala for 10 min and then incubated at 80 °C for 30 min. The percentage of nongerminating heat resistant spores was determined by the number of colonies after heat treatment/number of colonies before heat treatment. (E) Spores of PY79 (WT), BZ16 (∆ywlE), BZ91 (sigA-R365D), BZ97 (tig-R45D), and BZ104 (tig-R45D, sigA-R365D) strains were incubated with l-Ala (10 mM), and OD600 was measured at the indicated time points. Data are presented as percentages of the initial OD600 of spore suspension. Germination assays were carried out in triplicate, and representative data are presented. Decreasing OD600 signifies spore germination.
Notably, combining sigA-R365D with tig-R45D mutation failed to recapitulate the extent of the ∆ywlE germination defect (Fig. 4E and SI Appendix, Fig. S11A), indicating that, as inferred by our phosphoproteomic analysis (Table 1), additional substrates are dephosphorylated by YwlE during the process. In a similar manner, spores harboring both sigA-R365A and tig-R45A mutations did not display the accelerated germination phenotype exhibited by ∆mcsB spores (SI Appendix, Fig. S11 A and B).
Evidence That the Transcriptional Machinery Is Reactivated in Germination.
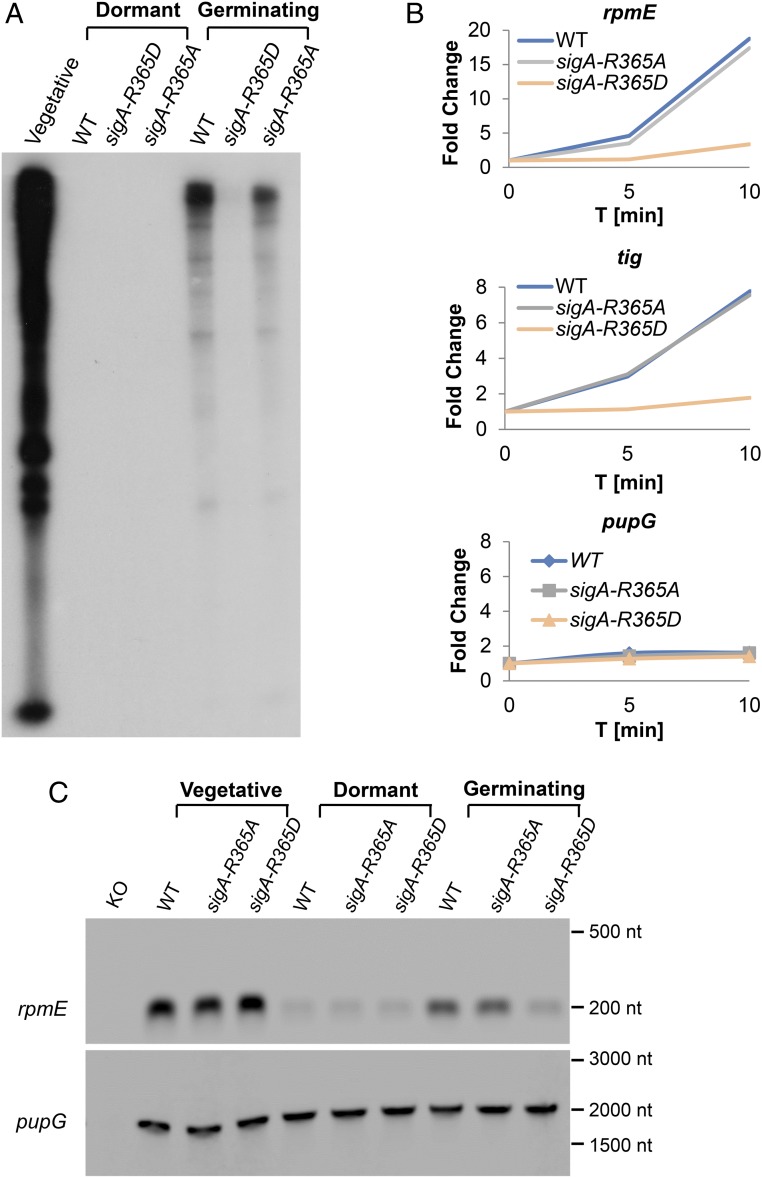
The finding that SigA dephosphorylation is necessary for prompting germination suggested that transcription is reestablished during this phase. To explore this notion, we monitored the transcriptional activity of whole extracts derived from vegetative cells, dormant or germinating spores of WT, and sigA-R365A and sigA-R365D strains by the addition of radioactively labeled ribonucleotides. Transcriptional activity was evident in the extract from vegetative cells with a minor reduction observed for sigA-R365D cells (SI Appendix, Fig. S12A). This is in line with the slight decrease in growth seen for this mutant in minimal medium (SI Appendix, Fig. S6D). Intriguingly, no detectable transcription took place in extracts of dormant spores (Fig. 5A), indicating that the transcription machinery was halted at the course of dormancy. Nonetheless, triggering germination by l-Ala was sufficient to release this inhibition and robustly reactivate the transcriptional machinery (Fig. 5A). Furthermore, although restoration of transcription was apparent in extracts from both WT and sigA-R365A germinating spores, it was nearly undetectable in extracts of sigA-R365D germinating spores (Fig. 5A), substantiating the idea that dephosphorylation of SigA mediated this molecular awakening.
Fig. 5.
Evidence that transcription occurs during germination. (A) An in vitro transcription reaction was carried out in whole extracts from vegetative cells of PY79 (WT) as well as from dormant and germinating spores of PY79 (WT), BZ90 (sigA-R365A), and BZ91 (sigA-R365D) strains and in a transcription buffer supplemented with NTPs (ATP, CTP, GTP, UTP, [α-32P]-UTP). After 40 min of incubation at 37 °C, the reaction was stopped, RNA was purified, radioactively labeled RNAs were analyzed in 8% polyacrylamide gels, and bands were visualized by autoradiography. The analysis was carried out in two biological repeats, and a representative experiment is presented. (B) Spores of PY79 (WT), BZ90 (sigA-R365A), and BZ91 (sigA-R365D) strains were incubated with 10 mM l-Ala to trigger germination, collected by centrifugation at the indicated time, and their RNAs were extracted. The mRNA levels of selective genes tig, rpmE, and pupG during germination were determined by quantitative RT-PCR. The result is presented as the fold change of target gene expression after germination relative to before germination. The assays were carried out in triplicate, and representative data are presented. (C) Total RNA was extracted from vegetative cells, dormant and germinating spores of PY79 (WT), BZ90 (sigA-R365A), and BZ91 (sigA-R365D) strains and subjected to Northern blot analysis using rpmE (201 nt) and pupG (2001 nt) specific biotinylated probes. knockout (KO), indicates control extracts derived from vegetative cells of the corresponding LS26 (∆rpmE) and LS76 (∆pupG) KO strains. The analysis was carried out in three independent biological repeats, and a representative experiment is presented. Numbers on the right correspond to RNA size marker.
Next, we wished to detect the transcription of specific genes in vivo during germination. To this end, qRT-PCR assays were performed on RNA extracted from vegetative cells, dormant and germinating spores of WT, and sigA-R365A and sigA-R365D strains, focusing on transcripts of Tig and RpmE, encoding for proteins synthesized during germination (23). Remarkably, the mRNA levels of tig and rpmE profoundly increased after germination induction in WT and sigA-R365A mutant spores (Fig. 5B and SI Appendix, Fig. S12B). Nevertheless, no significant changes in mRNA levels were observed in germinating sigA-R365D mutant spores (Fig. 5B and SI Appendix, Fig. S12B). As a control, we measured the mRNA levels of pupG (a SigA controlled gene) and yaaH (a SigB controlled gene), which their corresponding proteins are excluded from the germination proteome (23). No difference in the mRNA levels was detected in any of the tested strains (Fig. 5B and SI Appendix, Fig. S12B). Furthermore, the transcript levels of tig, rpmE, or pupG were similar among the examined strains during vegetative growth (SI Appendix, Fig. S12C). In line with these findings, Northern blot analysis designated the sigA-R365D mutation to influence specifically the transcription of rpmE during germination, but to have no impact on the transcription of pupG (Fig. 5C). These results indicate that a subset of genes is being transcribed during germination and that spores harboring the phosphorylated-like sigA-R365D allele are impaired in executing this mRNA synthesis.
Discussion
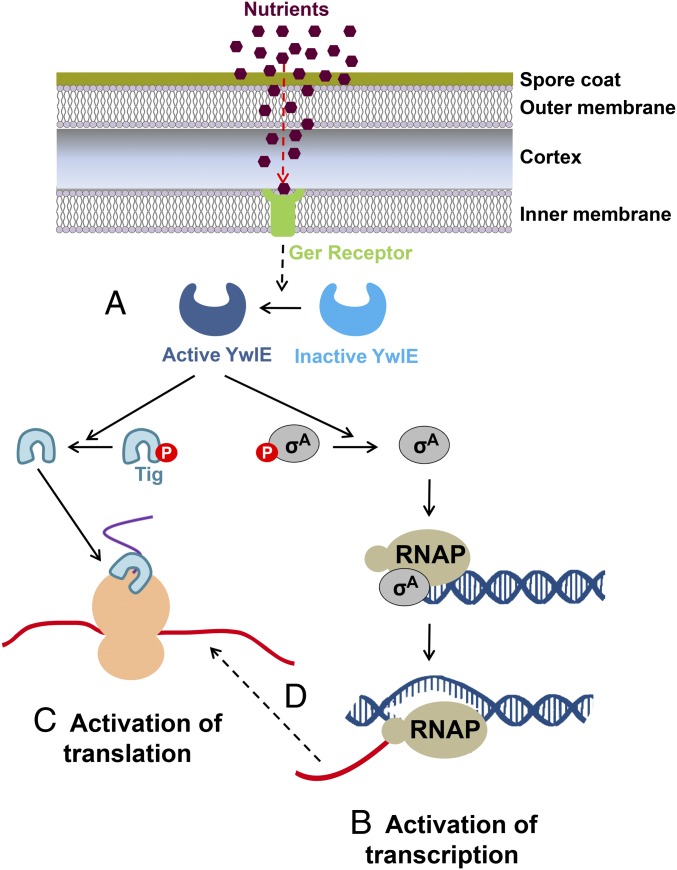
Dormant bacterial spores undergo a fascinating awakening complex process to restore active metabolism and reinitiate growth (19). However, the molecular events underlining this dramatic cellular transition are still elusive. Here, we describe that the recently identified bacterial protein modification, Arg dephosphorylation (37, 38, 40), plays a key role in facilitating the earliest molecular events, occurring during germination. We show that the Arg-phosphatase YwlE is required to prompt germination whereas the absence of the Arg kinase McsB results in accelerated germination. Based on our results, we propose the following model for spore germination (Fig. 6). The Arg-phosphatase YwlE interacts with germination receptors and is activated following the binding of a germinant to its cognate receptor, dephosphorylating its downstream substrate proteins. YwlE is a redox sensor, and thus, its activation could be achieved by switching from the disulfide-linked inactive form to the reduced active mode of the protein (49, 50). As a consequence of YwlE-phosphatase activity, transcription and translation are restored, at least in part, via Arg dephosphorylation of SigA and Tig, respectively.
Fig. 6.
A model for propelling spore germination via Arg dephosphorylation. A model depicting the events occurring during germination based on the data obtained in our analysis. Upon nutrient sensing by germination receptors, the Arg phosphatase YwlE is activated (A). Consequently, SigA is dephosphorylated by active YwlE to reactivate the transcription machinery (B). Next, translation is reestablished by the YwlE-mediated dephosphorylation of Tig (C). The source of the transcripts for protein synthesis during germination could be derived from the newly transcribed RNA (D).
Interestingly, additional factors were identified to be Arg phosphorylated in dormant spores, among them crucial enzymes involved in carbon metabolism, such as GapA (Glyceraldehyde-3-phosphate dehydrogenase) and Pgi (Glucose-6-phosphate isomerase), controlling central metabolic pathways (51, 52). The detection of Arg phosphorylation within spore specific proteins, including members of the Ssp (small, acid-soluble spore protein) family and spore coat proteins, suggests that Arg phosphomodification could mediate the degradation of spore proteins that need to be removed to enable revival (17, 53).
Arg phosphorylation was initially monitored in B. subtilis and was shown to function as part of the bacterial stress response and to mark proteins for degradation by the Clp protease complex (32, 37, 38, 40, 53). Phosphoproteomic analyses suggested that Arg phosphorylation has a regulatory effect on many cellular pathways (32, 37, 40), although the in vivo impact of the Arg-phosphorylation pathway remained limited. Here, we show that Arg dephosphorylation elicits the most critical stage of spore awakening, placing it as a major regulator of a developmental process in bacteria.
Surprisingly, our analysis revealed that not only translation, but also transcription occurs during germination and are required for its progression. The transcription machinery restoration is likely to be mediated by the switching between the phosphorylation and the dephosphorylation state of the housekeeping SigA. The Arg-365 site seems to be located in the DNA binding domain of SigA (54), suggesting that its phosphorylation might interfere with the binding of the protein to its template. The transcription machinery of dormant spores appears to be silenced regardless of the Arg-phosphporylation state, indicating that additional factors impose this arrest. It will be intriguing to elucidate at which stage of entering dormancy this halt is achieved as, according to our previous findings, this could take several days (14, 18). The activation of transcription precedes that of translation as SigA-R365D mutant spores were stalled before DPA release, whereas Tig-R45D spores could liberate DPA normally. This observation raises the possibility that the source of the transcripts required for translation during germination could be derived from newly synthesized mRNA. The RNA profile of aging spores was found to be dynamic, mainly involving RNA degradation into nucleotides, which can serve as a source for de novo germination-induced RNA synthesis (14, 15, 18).
Dephosphorylation of Tig was found to mediate its association with ribosomes to reactivate the translational machinery, reinforcing the view that translation is required to execute germination. During sporulation, Tig may be phosphorylated by McsB thereby limiting its association with the ribosomes. However, when germination is induced, Tig activity could be restored by the action of YwlE, propelling its rapid association with ribosomes and, consequently, allowing protein folding to resume. Interestingly, the Thr-phosphorylated form of the elongation factor Tu was shown to bind ribosomes during sporulation and inhibit translation elongation, hence, acting in an opposed manner to Tig (55). It is plausible that networks of co-occurring phosphorylation events could serve as biological switches functioning to control translation when entering and exiting dormancy.
The observation that not all of the SigA-R365D and Tig-R45D mutant spores are stalled in germination could reflect the differences in the levels of RNA and proteins within spores shown to affect germination capacity (14, 15, 18, 56). This bet-hedging strategy could increase population fitness to maximize survival under changeable conditions. Yet, the connection between the spore molecular reservoir and its germination propensity remains puzzling, highlighting the gaps in our understanding of spore dormancy and awakening. This suggests that the spore molecular pool plays a significant role in germination capacity of spores residing in different natural niches.
Materials and Methods
Strains and General Methods.
B. subtilis strains are derivatives of the WT PY79 and are listed in SI Appendix, Table S2. Plasmid construction is described in SI Appendix, Experimental Procedures, and primers are described in SI Appendix, Table S3. All general methods for B. subtilis were carried out as described previously (57). B. subtilis cultures were inoculated at OD600 0.05 from an overnight culture, and growth was carried out at 37 °C in LB medium (Difco). Antibiotics were used at the following concentrations: kanamycin 5 μg/mL, chloramphenicol 6 μg/mL, lincomycin 25 μg/mL, erythromycin 1 μg/mL, tetracycline 10 μg/mL, and spectinomycin 100 μg/mL. Sporulation was induced at 37 °C by suspending cells in Schaeffer's liquid medium (Difco Sporulation Medium) (58). Sporulation efficiency was evaluated by comparing the number of colony forming units before and after heat treatment. Spores were harvested, purified, and stored as described below according to (23). Purified spores were heat activated at 80 °C for 30 min before germination experiments. Spore germination is induced by l-Ala (10 mM), AGFK (2.5 mM l-Asp, 5 mg/mL d-glucose, 5 mg/mL d-fructose, and 50 mM KCl), or LB at 37 °C.
Light Microscopy.
Light microscopy was carried out as described previously (23). Briefly, bacterial cells (0.5 mL) were collected by centrifugation and resuspended in 10 μL of PBS × 1. For time lapse experiments, spores were placed on 1% agarose pads supplemented with l-Ala (10 mM) or LB and incubated in a chamber where temperature was maintained at 37 °C with a temperature controller (Pecon-Zeiss). For fluorescence measurements, the intensity of a WT (PY79) strain, lacking gfp, mCherry, or dronpa genes, was subtracted from the net average fluorescence intensity. Samples were photographed using Axio Observer Z1 (Zeiss), equipped with a CoolSnap HQII camera (Photometrics, Roper Scientific). System control, image analysis, and processing were performed using MetaMorph software (version 7.7; Molecular Devices).
DPA Measurements.
DPA release was measured as described previously (59). Spore germination was performed at 37 °C in 10 mM l-Ala suspended in 25 mM K-Hepes buffer (pH 7.4). At the indicated time, 200 µL aliquots were centrifuged, and TbCl3 was added to the supernatant fluid in a final concentration of 50 µM. Tb-DPA fluorescence was measured at a fluorescence emission of 545 nm and with excitation of 270 nm.
Quantification and Statistical Analysis.
Unless stated otherwise, bar charts display a mean ± SD from at least three independent biological experiments. MS Excel was used for all statistical analysis, data processing, and presentation.
Supplementary Material
Acknowledgments
We thank Silke Wahl for preparing samples for MS measurements. We are grateful to the members of the S.B.-Y. laboratory for valuable discussions and comments. We thank P. Setlow (University of Connecticut) for generously providing antibodies against germination receptors. This work was supported by the European Research Council Advanced Grant (339984) and the Israel Science Foundation (774/16) awarded to S.B.-Y., and by The German-Israeli Foundation (GIF) (Grant I-1464-416.13/2018) awarded to S.B.-Y. and B.M. B.Z. was funded by the Key Project of National Natural Science Foundation of China (NSFC) (31530058) and China Scholarship Council (CSC) scholarship (201606350068).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817742116/-/DCSupplemental.
References
- 1.Higgins D., Dworkin J., Recent progress in Bacillus subtilis sporulation. FEMS Microbiol. Rev. 36, 131–148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piggot P. J., Spore development in Bacillus subtilis. Curr. Opin. Genet. Dev. 6, 531–537 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Piggot P. J., Hilbert D. W., Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 7, 579–586 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Stragier P., Losick R., Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30, 297–241 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Stragier P., Losick R., Cascades of sigma factors revisited. Mol. Microbiol. 4, 1801–1806 (1990). [DOI] [PubMed] [Google Scholar]
- 6.Dworkin J., Protein targeting during Bacillus subtilis sporulation. Microbiol. Spectr. 2, TBS-0006-2012 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Driks A., Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63, 1–20 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popham D. L., Specialized peptidoglycan of the bacterial endospore: The inner wall of the lockbox. Cell. Mol. Life Sci. 59, 426–433 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriques A. O., Moran C. P. Jr, Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 61, 555–588 (2007). [DOI] [PubMed] [Google Scholar]
- 10.McKenney P. T., Driks A., Eichenberger P., The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 11, 33–44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popham D. L., Bernhards C. B., Spore peptidoglycan. Microbiol. Spectr. 3, TBS-0005-2012 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Driks A., Eichenberger P., The spore coat. Microbiol. Spectr. 4, TBS-0023-2016 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Setlow P., Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101, 514–525 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Segev E., Smith Y., Ben-Yehuda S., RNA dynamics in aging bacterial spores. Cell 148, 139–149 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S., Korza G., Maciejewski M., Setlow P., Analysis of metabolism in dormant spores of Bacillus species by 31P nuclear magnetic resonance analysis of low-molecular-weight compounds. J. Bacteriol. 197, 992–1001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korza G., Setlow B., Rao L., Li Q., Setlow P., Changes in Bacillus spore small molecules, rRNA, germination, and outgrowth after extended sublethal exposure to various temperatures: Evidence that protein synthesis is not essential for spore germination. J. Bacteriol. 198, 3254–3264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Setlow P., Spore germination. Curr. Opin. Microbiol. 6, 550–556 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Segev E., Rosenberg A., Mamou G., Sinai L., Ben-Yehuda S., Molecular kinetics of reviving bacterial spores. J. Bacteriol. 195, 1875–1882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setlow P., Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 196, 1297–1305 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moir A., Cooper G., Spore germination. Microbiol. Spectr. 3, TBS-0014-2012 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Moir A., How do spores germinate? J. Appl. Microbiol. 101, 526–530 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Setlow P., Wang S., Li Y. Q., Germination of spores of the orders Bacillales and Clostridiales. Annu. Rev. Microbiol. 71, 459–477 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Sinai L., Rosenberg A., Smith Y., Segev E., Ben-Yehuda S., The molecular timeline of a reviving bacterial spore. Mol. Cell 57, 695–707 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinai L., Ben-Yehuda S., Commentary: Changes in Bacillus spore small molecules, rRNA, germination, and outgrowth after extended sublethal exposure to various temperatures: Evidence that protein synthesis is not essential for spore germination. Front. Microbiol. 7, 2043 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg W., Halvorson H. O., Keynan A., Weinberg E., Timing of protein synthesis during germination and outgrowth of spores of Bacillus cereus strain T. Nature 208, 710 (1965). [Google Scholar]
- 26.Vinter V., Symposium on bacterial spores: V. Germination and outgrowth: Effect of inhibitors. J. Appl. Bacteriol. 33, 50–59 (1970). [DOI] [PubMed] [Google Scholar]
- 27.Kramer G., et al. , L23 protein functions as a chaperone docking site on the ribosome. Nature 419, 171–174 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Lauber M. A., Running W. E., Reilly J. P., B. subtilis ribosomal proteins: Structural homology and post-translational modifications. J. Proteome Res. 8, 4193–4206 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Göthel S. F., Scholz C., Schmid F. X., Marahiel M. A., Cyclophilin and trigger factor from Bacillus subtilis catalyze in vitro protein folding and are necessary for viability under starvation conditions. Biochemistry 37, 13392–13399 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Paredes-Sabja D., Setlow P., Sarker M. R., Germination of spores of Bacillales and Clostridiales species: Mechanisms and proteins involved. Trends Microbiol. 19, 85–94 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Shah I. M., Laaberki M. H., Popham D. L., Dworkin J., A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135, 486–496 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mijakovic I., Grangeasse C., Turgay K., Exploring the diversity of protein modifications: Special bacterial phosphorylation systems. FEMS Microbiol. Rev. 40, 398–417 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Dworkin J., Ser/Thr phosphorylation as a regulatory mechanism in bacteria. Curr. Opin. Microbiol. 24, 47–52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dworkin J., Shah I. M., Exit from dormancy in microbial organisms. Nat. Rev. Microbiol. 8, 890–896 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg A., et al. , Phosphoproteome dynamics mediate revival of bacterial spores. BMC Biol. 13, 76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bäsell K., et al. , The phosphoproteome and its physiological dynamics in Staphylococcus aureus. Int. J. Med. Microbiol. 304, 121–132 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Elsholz A. K., et al. , Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 109, 7451–7456 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuhrmann J., et al. , McsB is a protein arginine kinase that phosphorylates and inhibits the heat-shock regulator CtsR. Science 324, 1323–1327 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Fuhrmann J., et al. , Structural basis for recognizing phosphoarginine and evolving residue-specific protein phosphatases in gram-positive bacteria. Cell Rep. 3, 1832–1839 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Schmidt A., et al. , Quantitative phosphoproteomics reveals the role of protein arginine phosphorylation in the bacterial stress response. Mol. Cell. Proteomics 13, 537–550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Junker S., et al. , Spectral library based analysis of arginine phosphorylations in Staphylococcus aureus. Mol. Cell. Proteomics 17, 335–348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dephoure N., Gould K. L., Gygi S. P., Kellogg D. R., Mapping and analysis of phosphorylation sites: A quick guide for cell biologists. Mol. Biol. Cell 24, 535–542 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dieterich D. C., et al. , Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat. Protoc. 2, 532–540 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Schuch R., Garibian A., Saxild H. H., Piggot P. J., Nygaard P., Nucleosides as a carbon source in Bacillus subtilis: Characterization of the drm-pupG operon. Microbiology 145, 2957–2966 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Akanuma G., et al. , Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation. J. Bacteriol. 194, 6282–6291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis P. J., Thaker S. D., Errington J., Compartmentalization of transcription and translation in Bacillus subtilis. EMBO J. 19, 710–718 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg A., Sinai L., Smith Y., Ben-Yehuda S., Dynamic expression of the translational machinery during Bacillus subtilis life cycle at a single cell level. PLoS One 7, e41921 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haldenwang W. G., The sigma factors of Bacillus subtilis. Microbiol. Rev. 59, 1–30 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuhrmann J., Subramanian V., Kojetin D. J., Thompson P. R., Activity-based profiling reveals a regulatory link between oxidative stress and protein arginine phosphorylation. Cell Chem. Biol. 23, 967–977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuhrmann J., Subramanian V., Thompson P. R., Targeting the arginine phosphatase YwlE with a catalytic redox-based inhibitor. ACS Chem. Biol. 8, 2024–2032 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Prasad C., Freese E., Cell lysis of Bacillus subtilis caused by intracellular accumulation of glucose-1-phosphate. J. Bacteriol. 118, 1111–1122 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeLoughery A., Lalanne J. B., Losick R., Li G. W., Maturation of polycistronic mRNAs by the endoribonuclease RNase Y and its associated Y-complex in Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 115, E5585–E5594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trentini D. B., et al. , Arginine phosphorylation marks proteins for degradation by a Clp protease. Nature 539, 48–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haugen S. P., Ross W., Gourse R. L., Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat. Rev. Microbiol. 6, 507–519 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pereira S. F., Gonzalez R. L. Jr, Dworkin J., Protein synthesis during cellular quiescence is inhibited by phosphorylation of a translational elongation factor. Proc. Natl. Acad. Sci. U.S.A. 112, E3274–E3281 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mutlu A., et al. , Phenotypic memory in Bacillus subtilis links dormancy entry and exit by a spore quantity-quality tradeoff. Nat. Commun. 9, 69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harwood C. R., Cutting S. M., Molecular Biological Methods for Bacillus (Wiley, Chichester, New York, 1990). [Google Scholar]
- 58.Schaeffer P., [Problems of spore formation in bacteria]. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 1. Abt. Medizinisch-hygienische Bakteriologie, Virusforschung und Parasitologie. Originale 198, 72–75 (1965). [PubMed] [Google Scholar]
- 59.Perez-Valdespino A., et al. , Isolation and characterization of Bacillus subtilis spores that are superdormant for germination with dodecylamine or Ca2+ -dipicolinic acid. J. Appl. Microbiol. 114, 1109–1119 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Zhu B., Stülke J., SubtiWiki in 2018: from genes and proteins to functional network annotation of the model organism Bacillus subtilis. Nucleic Acids Res. 46, D743–D748 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.