Significance
Achieving both superstrong adhesion and reversibility is challenging, particularly for hydrogels. Here, we report a hydrogel-based, reversible, superglue-like adhesive by combining the benefits of both liquid and dry adhesives in a single material while overcoming their respective limitations. When hydrated, the softened gel conformally adapts to the target surface by low-energy deformation, which is then locked upon drying as the elastic modulus is raised from hundreds of kilopascals to a few gigapascals, analogous to the action of the epiphragm of snails. We show that reversible superstrong adhesion could be achieved from a nonstructured material when the criterion of shape adaption is met, with minimal residual strain energy stored in the system.
Keywords: polymer gels, superstrong adhesion, shape adaptation, intrinsically reversible, snail epiphragm
Abstract
Adhesives are ubiquitous in daily life and industrial applications. They usually fall into one of two classes: strong but irreversible (e.g., superglues) or reversible/reusable but weak (e.g., pressure-sensitive adhesives and biological and biomimetic surfaces). Achieving both superstrong adhesion and reversibility has been challenging. This task is particularly difficult for hydrogels that, because their major constituent is liquid water, typically do not adhere strongly to any material. Here, we report a snail epiphragm-inspired adhesion mechanism where a polymer gel system demonstrates superglue-like adhesion strength (up to 892 N⋅cm−2) that is also reversible. It is applicable to both flat and rough target surfaces. In its hydrated state, the softened gel conformally adapts to the target surface by low-energy deformation, which is locked upon drying as the elastic modulus is raised from hundreds of kilopascals to ∼2.3 GPa, analogous to the action of the epiphragm of snails. We show that in this system adhesion strength is based on the material’s intrinsic, especially near-surface, properties and not on any near-surface structure, providing reversibility and ease of scaling up for practical applications.
Adhesion between two bodies is mediated by their surface or near-surface properties, including (i) local chemistry that affects intrinsic adhesion at the molecular level, (ii) microscopic surface roughness (topography), and (iii) macroscopic material mechanical properties (1, 2). Liquid adhesives used in large-scale applications including manufacturing, construction, and assembly offer strong adhesion after curing [e.g., ∼1,000 N⋅cm−2 from superglues with cyanoacrylate chemistry (3)] but are not reworkable. Tough hydrogels have shown promise as a new class of adhesives with high interfacial toughness (4–8), for example via double interpenetrating networks consisting of both covalent and ionic cross-links. Nevertheless, they are limited to a specific target material class, for use in a wet environment. Strong hydrogel adhesion requires synergy of chemistry, topology, and mechanics, often through covalent bonding with the substrate (8). Missing one or more factors will lead to poor adhesion. In comparison, the ability of geckos and some insects to reversibly cling to almost any surface is attributed to split contact adhesion from millions of hierarchical fibrillar structures on the attachment pads, which interact with surfaces via weak, van der Waals forces and/or capillary forces (9, 10). However, the extrinsic adhesion strength of dry adhesives relying on surface structures is rather weak (9–19), rarely more than 30 N⋅cm−2, and can be dramatically attenuated when in contact with a rough surface (20–22). Velcro shows reversible adhesion with strength as high as 120 N⋅cm−2, arising from the collective mechanical interlocking of many small hooks and loops (23, 24). However, interlocking adhesives require complementary structures on opposing surfaces to engage and interlock (25–27). Whether one can design a material that works against arbitrary surfaces and yet delivers adhesive strength comparable to liquid adhesives remains an open question (2).
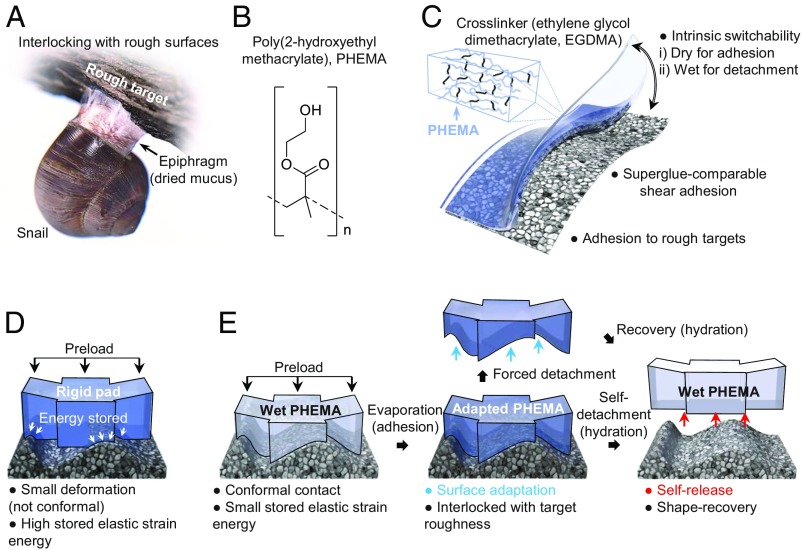
It has been shown that mucus secreted by snails allows them to maintain conformal contact with the rough surfaces of rocks or trees when they are active (28, 29). Upon drying, the shear modulus of this mucus increases from 100 Pa to ∼1 GPa, forming a stiff epiphragm that is interlocked with the target surface, rendering strong adhesion (Fig. 1A). The poly(2-hydroxyethyl methacrylate) (PHEMA) hydrogel (see chemical structures in Fig. 1B), cross-linked with ethylene glycol dimethacrylate (EGDMA), also undergoes large and reversible changes in elastic modulus, E < 200 kPa in the hydrated, rubbery state to 2.3 GPa in the dry, glassy state. We note that the hydrated modulus reported here is a near-surface property measured by atomic force microscopy (AFM) and regardless of the cross-linker concentration (2 vol % vs. 8 vol %; SI Appendix, Fig. S1) is much lower than the bulk modulus (∼34.9 MPa; see discussions in SI Appendix and SI Appendix, Fig. S2); this can be explained as a consequence of the oxygen scavenging effect near the surface of the polydimethylsiloxane (PDMS) mold (30). The surface layer of the PHEMA gel deforms readily in its hydrated state to adapt to its mating surface (i.e., shape adaption), which is subsequently locked in this configuration due to a rapid increase in the glass transition temperature (Tg) as the PHEMA gel dries, from <2 °C (hydrated state) to 104 °C (dry state) (31). Relaxation time scales far exceed those required for drying, analogous to epiphragm formation.
Fig. 1.
Design of PHEMA hydrogel superglues. (A) Illustration of epiphragm of snails, where the drying mucus facilitates its attachment to a rough surface. (B) Chemical structure of PHEMA. (C) Illustration of the major advantages of PHEMA hydrogel adhesives in overcoming challenges posed by liquid and dry adhesives. (D) Illustration of the challenge in making conformal contact between a solid adhesive that has a high modulus and a rough target surface. (E) Proposed epiphragm-like adhesion mechanism with a PHEMA gel, where shape conformability in the wet state followed by interlocking upon drying facilitates adhesion. The deformed configuration of the hydrogel can be retained after drying and forced detachment (shown by the blue arrows). Upon rehydration, the wet hydrogel adhesive pad returns to its original undeformed configuration and can be easily self-detached from the target substrate (shown by the red arrows).
This unique property of the PHEMA hydrogel network allows us to create intrinsically reversible, superglue-like adhesives by combining the benefits of liquid and dry adhesives in a single material, while overcoming their respective limitations (Fig. 1C). That is, a compliant solid surface can be created from PHEMA, which readily conforms to a rough surface such that the stored elastic energy accompanying deformation, which is proportional to the material’s modulus, E, is minimal. This is captured by the Dahlquist criterion for tackiness (1); that is, a solid adhesive with Eeff 100 kPa cannot easily adhere to a rough surface (Fig. 1D). A recent review (8) has recognized the significance and challenge of realizing a strong hydrogel adhesive with both reversibility and on-demand detachability. Here, we employ a hydrated PHEMA gel which is cross-linked to a degree such that it is soft enough to ensure near-surface compliance and undergoes low-energy deformation for intimate contact with a rough target surface in its wet state (Fig. 1E). Upon dehydration, the gel shrinks but with little residual stress; meanwhile, the Young’s modulus increases by three orders of magnitude, allowing for topographic interlocking with the target. The adapted configuration can be retained even after forced detachment, engendered by the hardened PHEMA. When rehydrated, the hydrogel pad can return to its original flat shape to release the small stored elastic energy [i.e., shape memory effect (31, 32)] for self-detachment from the target surface, adding the benefits of reversibility and recoverability.
Results and Discussion
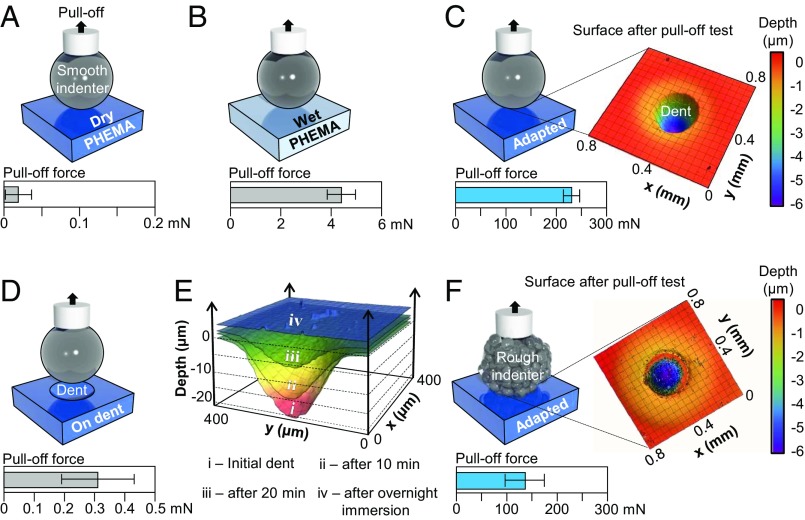
To quantitatively investigate the adhesion mechanism, we performed indentation tests (contact radius a ∼ 150 μm; SI Appendix) on PHEMA films coated on glass substrates in four configurations: (i) the pristine, dry, and stiff flat surface (Fig. 2A and SI Appendix, Fig. S3), (ii) the hydrated and compliant, flat surface (Fig. 2B and SI Appendix, Fig. S4), (iii) in situ drying of the hydrated gel with the indenter pressed into the sample under a 100-mN applied preload, followed by indenter retraction to observe shape adaptation (Fig. 2C and SI Appendix, Fig. S5), and (iv) after indenter separation in iii to repeat indentation at the same spot (i.e., dent) (Fig. 2D). The maximum pull-off forces required to separate the indenter from the respective samples are plotted in Fig. 2 A–D. For both samples i and iv, the pull-off forces were very small—with a barely measurable value (1 mN, SI Appendix, Fig. S3)—while those for ii were somewhat larger, ∼4.43 ± 0.57 mN (SI Appendix, Fig. S4). Nevertheless, indentation caused no lasting deformation of the PHEMA samples after testing. In striking contrast, the pull-off forces for samples iii were much greater, ∼232.05 24.41 mN (SI Appendix, Fig. S5). Examination of the postindented sample revealed a residual dent on the sample surface as shown in Fig. 2C, complementary to the shape of the indenter. Evidently, in the hydrated state, the solid gel behaves like a liquid: It easily conforms to a target surface as a result of the soft near-surface layer. Upon drying, the gel becomes increasingly glassy and retains this adapted shape, permitting interlocking with the target surface with a relatively small, residual stress corresponding to its rather small overall shrinkage [linear expansion ratio <1.5 (33)] compared with other conventional hydrogel systems. Studying the shape recovery kinetics upon rehydration of the PHEMA gel (Fig. 2E) confirms the shape memory effect. The liquid-like surface adaptability and shape memory effect together with the relatively small swelling/shrinkage of the PHEMA hydrogel network are key characteristics that distinguish our system from both conventional liquid glues and dry adhesives. The gel’s adaptability is further evidenced when repeating the indentation tests with a rough indenter tip (root mean square roughness ∼1.8 μm), showing more variable and somewhat reduced, yet comparable, pull forces (135.80 ± 39.60 mN; Fig. 2F) to that from the smooth tip (Fig. 2C) and a residual dent that is complementary to the rough surface of the indenter (Fig. 2F). Repeated indentation tests (up to 12 cycles with various wetting and drying times) on the same spot of the sample confirm the reversibility of adhesion (SI Appendix, Table S1).
Fig. 2.
Indentation tests using a glass indenter on PHEMA hydrogel pads. (A and B) Schematic illustrations of the indentation tests performed with a smooth glass indenter to measure the pull-off forces from (A) dry and (B) wet PHEMA gel pads. (C) Indentation in the wet state, holding the indenter in place as the sample dries. Shape adaptation of the gel to the indenter results in a 50-fold increase in pull-off force and leaves a dent in the adhesive layer. (D) Repeated indentation of the dry and dented sample at the same spot as seen in C. (E) Shape recovery kinetic process upon rehydration of a 22-µm-deep dent left behind in a PHEMA sample. (F) Indentation with a rough indenter showing the same shape adaption of the gel albeit with somewhat attenuated pull-off forces compared with the smooth indenter in (C).
Little adhesion from samples i and iv can be attributed to the lack of conformal contact between two stiff bodies, the indenter and the dry PHEMA gel. In contrast, the pull-off force increases by nearly a factor of 50 between samples ii and iii. Indentation of samples ii in the soft, hydrated state is interpreted using the Johnson–Kendall–Roberts (JKR) (34) model given that deformation of the gel is reversible. Accordingly, the pull-off force, is
| [1] |
where R (∼3.025 mm) is the radius of the indenter and is the work of adhesion between the indenter and the hydrated sample. In SI Appendix and SI Appendix, Fig. S4, we show that for the tests on hydrated samples the effect of finite substrate thickness can be neglected. For sample iii, we approximate the deformed shape attained upon indentation in the wet state (ii) as the reference unstrained configuration. Upon indenter retraction, the sample contact region (radius a) is subjected to a uniform vertical displacement. Therefore, the corresponding stress state can be approximated as that obtained upon pulling on a flat circular punch adhered to the material. The interfacial stress has a singularity at the contact edge. Applying the fracture-mechanics condition that failure occurs when energy release rate equals the work of adhesion of that interface, we predict the pull-off force (34),
| [2] |
where E is the Young’s modulus, is the Poisson’s ratio (here, 0.5), Wdry is the work of adhesion between the indenter and the dried sample, and is a correction factor for finite substrate thickness (its value is estimated to be ∼1.54; SI Appendix). The ratio of the pull-off force in the dry (iii) and wet (ii) states is
| [3] |
If we assume Wdry and Wwet have similar values, say, 300 mJ⋅m−2, Eq. 3 predicts that the postdried, pull-off force will be about 100 times greater than that under wet conditions. Alternatively, we can use Eqs. 1 and 2 to estimate work of adhesion, obtaining Wwet ∼ 300 mJ⋅m−2 and Wdry ∼ 80 mJ⋅m−2, respectively.
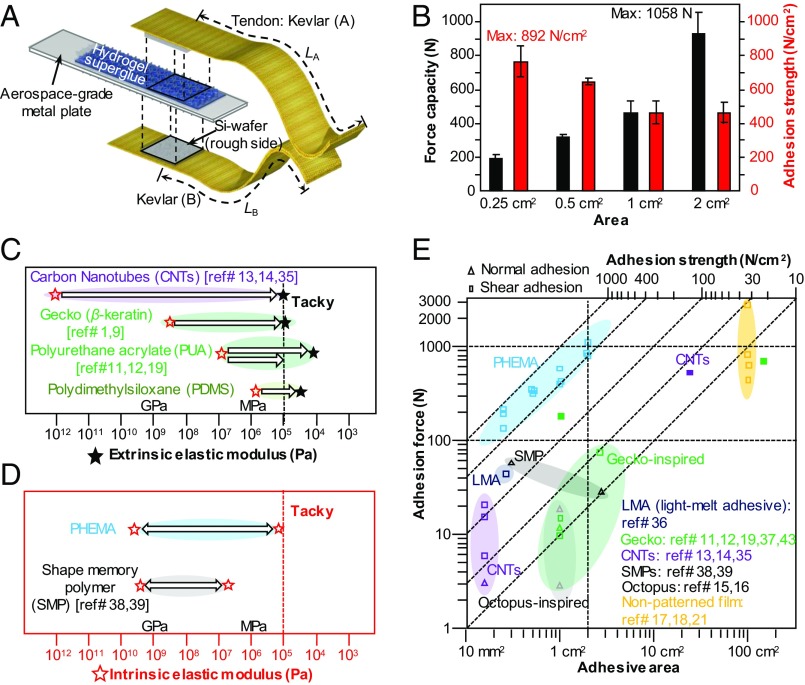
To demonstrate the utility and scalability of our PHEMA hydrogel pad as a superstrong yet reversible adhesive, we designed a double-lap jointed adhesive system consisting of the hydrogel pad on a silicon wafer that was glued on both sides of the Al plate (as the rigid backbone), and the silicon wafers glued on the Kevlar tapes (as tendons) (see Fig. 3A and the related discussions in SI Appendix). Such engineering design is important because commonly used backbones such as polyethylene terephthalate (PET) films cannot sustain the large tensile forces in our system (>200 N), which would otherwise lead to the failure of the backbone. To justify this claim, we prepared a shear adhesion test system comprising a model “rough” target surface in the form a polyurethane acrylate (PUA) micropillar array (height and diameter, 5 μm; spacing, 10 μm) supported on a PET backing layer in adhesive contact with a PHEMA film on a Si backing layer, which was then mounted on an aluminum strip. Both plastic deformation and fracture were observed in the PET film at ∼176 N, while the interface between PHEMA and PUA as well as the bulk of the PHEMA adhesive layer remained unaffected (SI Appendix, Figs. S6 and S7A). SI Appendix, Fig. S7B provides further evidence of shape adaptation and shape memory of our PHEMA adhesive. Forcible detachment of the rough target from the PHEMA film reveals an array of dented holes left behind in the latter, which are complementary to the PUA micropillar array. In comparison, tensile tests performed on Kevlar tendons of the same dimension as those utilized in our shear adhesion test setup suggest that Kevlar can sustain a much higher force capacity (up to 1,100 N of in-plane tensile force, as shown in SI Appendix, Fig. S8) than PET and is therefore appropriate for our study. The measured shear adhesion from PHEMA samples of four different sizes (0.25, 0.5, 1, and 2 cm2) is presented in Fig. 3B and SI Appendix, Fig. S9. We highlight the highest shear adhesion strength measured, 892 N⋅cm−2 from a 0.25-cm2 sample, comparable with values from superglue, and the largest shear adhesion force of 1,058 N from the 2-cm2 sample. As shown in Movie S1, we successfully demonstrate the support of an adult human subject (∼87 kg) with two 2-cm2 samples. The adhesion values are far superior to those obtained from samples with either extrinsic (via structuring) or intrinsic reversibility (see comparison in Fig. 3 C–E). In the case of fibrillar adhesives, they adhere to a target surface using short-range dispersive interactions and leverage hierarchical, slanted, high-aspect-ratio fibrillar structures, which effectively reduce the moduli of intrinsically rigid materials to the degree of self-tack (see dashed lines in Fig. 3 C and D that approximately delineate the tacky regime according to Dahlquist’s criterion), while taking advantage of the near-surface pad geometry through the contact splitting mechanism (10). Despite the extensive engineering of bioinspired dry adhesive pads over the last decade, their adhesion strength pales in comparison with liquid-based superglues, typically on the order of tens of newtons per square centimeter from structured polymers (12, 15–17, 19, 21) and ∼100 to 143 N⋅cm−2 from carbon nanotube bundles (13, 14, 35) (Fig. 3E). Higher adhesion strength has been reported from light-melting adhesives (<160 N⋅cm−2) (36) and wetting and drying of polythiophene nanotubules (∼174 N⋅cm−2) (37). By leveraging the intrinsic modulus change in shape memory polymers (38) near Tg, a high adhesion strength, up to ∼180 N⋅cm−2, has been demonstrated. However, due to the lack of conformability (the modulus at contact, ∼10 MPa, which is two orders of magnitude higher than the near-surface modulus in our system), a steep, negative slope in adhesion strength (5 to 30 N⋅cm−2) is observed upon scaling up (39). This is a common issue in structured adhesives as more defects appear over a larger area, thus initiating more random cracks. In sharp contrast, our PHEMA hydrogel pads demonstrate (i) an order of magnitude increment in adhesion force capacity (∼1,000 N) even with a small sample size (∼2 cm2), whereas most literature values are lower than 100 N (40–42), and (ii) a superior, positive size-scaling slope (adhesion force vs. sample area) compared with negative slopes in most structured dry adhesives including the gecko system (43).
Fig. 3.
Demonstration of superglue-like adhesion and scalability of the PHEMA hydrogel pads. (A) Illustration of the double-lap test setup. The PHEMA hydrogel adhesive pads fabricated on Si wafers (as backbones) are applied to both sides of a metal plate. Once hydrated, the hydrogel pads were brought into contact with the rough side of the silicon wafers glued to the double-lap Kevlar tapes (as tendons) to minimize opening and torsional modes for reliable measurement of the high shear adhesion. (B) The maximum force capacity of various sample sizes. (C and D) Graphs delineating various extrinsic and intrinsic strategies reported in literature, to modulate elastic moduli (red stars, intrinsic material modulus; black solid stars, extrinsic modulus), respectively. Note that, for extrinsic strategies, adhesion performances are highly coupled to the design of surface textures. The black and red dotted lines approximately delineate the tacky (∼<0.1 MPa) and nontacky regimes according to the Dahlquist criterion, for both extrinsic and intrinsic strategies, respectively. (E) Comparison of the scalability of adhesion strength of various reversible adhesives reported in literature vs. our PHEMA hydrogel pad. The dotted lines are guides for the eyes to show different regimes of adhesion strength and force capacity.
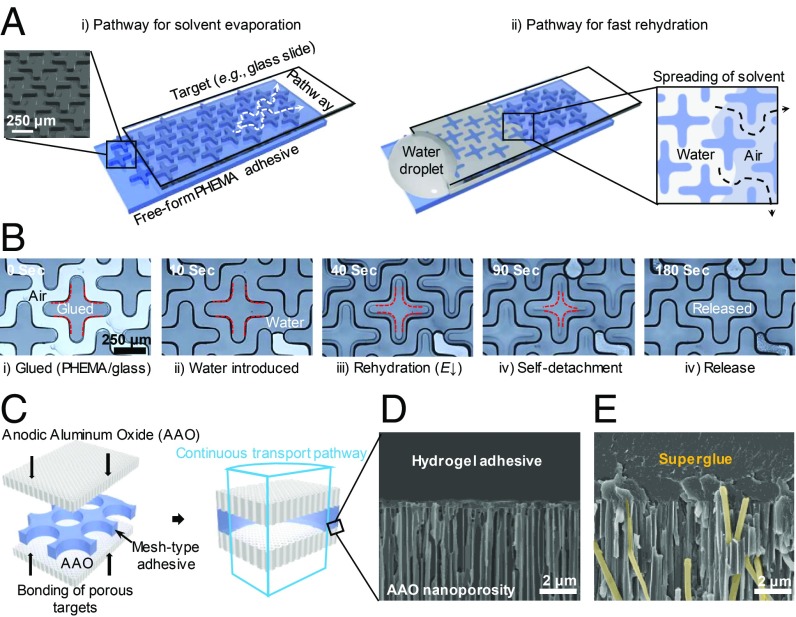
Since the adhesion force is not based on the design of near-surface structures, it affords us additional flexibility to incorporate structural designs for other functionalities. As shown in Fig. 4 A and B, we fabricate a cross-pattern to facilitate water diffusion into and out of the hydrogel pad. Upon rehydration, it takes only 170 s for the initially locked PHEMA pad to self-release from the glass slide (Fig. 4B and Movies S2 and S3). We also prepare a PHEMA mesh with through-holes (500 µm in diameter) to glue two nanoporous anodic alumina oxide (AAO) membranes, which are commonly used as filters (Fig. 4C). Fluid can freely transport across the AAO/PHEMA gel interface due to the large pore size in the PHEMA mesh, and importantly there is no hydrogel in the nanochannels of the AAO membrane to block the channels (Fig. 4D). This is because the cross-linked network has nearly infinite molecular weight and therefore individual chains cannot freely infiltrate the channels as with liquid adhesives (colored yellow in Fig. 4E and SI Appendix, Fig. S10). Last, we show the selective detachment of morpho butterfly wing scales using a patterned PHEMA gel pad without damaging the delicate scales that have hierarchical structures (SI Appendix, Fig. S11), a task that would otherwise be impossible using liquid glues, further supporting the importance of low near-surface modulus during contact.
Fig. 4.
Exemplary demonstrations of the advances of patterned PHEMA hydrogel adhesive pads. (A) Schematic of a cross-pattern design as a continuous pathway to facilitate the rapid diffusion of water within the pad. (Inset) Scanning electron microscopy image of the cross-pattern in PHEMA pad. (B) Optical microscope images showing the release of the glued PHEMA gel from the glass target upon rehydration. The red dashed lines indicate the receding contact line. (C) Illustration of a hydrogel superglue mesh sandwiched between two nanoporous AAO membranes. The clear-through holes (500-µm diameter) in the PHEMA mesh keep porous pathways open even after gluing. (D and E) Cross-sectional scanning electron microscopy images of the AAO membrane with unblocked nanopores (200 nm in diameter) in the regions in contact with PHEMA mesh pad (D) and with infiltrated liquid superglue (Gorilla Super Glue) after curing (E). A false yellow color is added to aid the eyes.
To further investigate whether PHEMA is unique, we explored the adhesive behaviors of hydrogels from PHEMA family by copolymerizing HEMA with methyl methacrylate (referred as PHEMA-co-PMMA), and acrylic acid (referred as PHEMA-co-PAA), as well as that of a commonly used hydrogel from poly(ethylene glycol) diacrylate (PEGDA). PMMA was chosen because it does not swell in water but has a high Tg and high E in the dry state, similar to PHEMA. PAA was chosen because it is highly hygroscopic but brittle, with a Tg similar to PHEMA in the dry state. It swells in water, especially with a pH above 5. Macroscopic adhesion tests (see the discussion in the SI Appendix) with these hydrogel systems, however, reveal no observable adhesion. AFM studies (SI Appendix, Figs. S12 and S13) show that the wet state near-surface elastic moduli of PHEMA-co-PMMA (5 and 10 vol % of PMMA) and PHEMA-co-PAA (5 vol % of PAA) are near (slightly above) the Dahlquist criterion for tackiness (SI Appendix, Fig. S12 A–C), which would allow for some degree of conformability upon initial contact with a target surface, whereas the PHEMA-co-PAA (10 vol % of PAA) samples have stiffer surfaces (SI Appendix, Fig. S12D) that would hinder conformal contact. All samples tested have high near-surface dry state moduli, much like that of PHEMA (SI Appendix, Fig. S13). A closer examination of these samples after the macroscopic adhesion testing suggests that large residual stress is built up upon drying, in every case. In Movie S4, we show the catastrophic failure of PEGDA on glass, including fracture and delamination due to the release of relatively large amounts of stored elastic energy. Therefore, the modulus was not measured here. Clearly PHEMA is unique; the right combination of its physical properties is key to its superstrong and reversible shear adhesion. Importantly, the decoupling of material modulus from strain and the minimization of stored elastic energy during its transition from a compliant wet state to a rigid dry state are the key characteristics of PHEMA.
In summary, we have presented an intrinsically reversible, superglue-like adhesive from the shape-adapting and shape-memorizing PHEMA hydrogel, activated by hydration and dehydration. It features structure-independent adhesion accompanied by the strong modulation of its near-surface elastic modulus, from ∼180 kPa (the hydrated state) to ∼2.3 GPa (the dry state), much like the phase transition from mucus to epiphragm in snails. In turn, we demonstrate scalability in adhesion force, as opposed to most other structure-based dry adhesives. Meanwhile, our adhesive is superior to liquid-based superglues for its reversibility and noncontaminating adhesion. Importantly, since the shape-adapting adhesion presented here does not depend on the geometry of the adhesive, it will not only ease scaling up for practical applications but also be applicable to a wide range of materials, whose elasticity can be tuned by heat, light, pH, or chemical cues in local regions (e.g., at the surface layer and via patterning), with additional functionalities.
Materials and Methods
Synthesis of Hydrogel Precursors.
The 2-hydroxyethylmethacrylate (HEMA) monomer (Sigma-Aldrich, 98%) was mixed with 1.5 vol % of a photoinitiator, Darocure 1173 (Sigma-Aldrich, 97%) and exposed to UV light (365 nm, 500 mJ/cm2) for 10-s intervals and for a total of 1.5 min to obtain a partially polymerized, viscous precursor. The sample was mixed between every 10-s exposure step using a vortexer (Barnstead Thermolyne Type 16715 mixer). After allowing the solution to rest for at least 1 h and before molding, 2 vol % or 8 vol% of cross-linker, EGDMA (Sigma-Aldrich, 98%, 90 to 110 ppm monomethyl ether hydroquinone inhibitor), was added to the obtained viscous mixture, along with an additional 1 vol % of Darocur 1173 (Sigma-Aldrich), followed by homogenization by ultrasonication (Branson 3800 Ultrasonic cleaner) to prepare PHEMA precursor. The hydrogel precursors of PHEMA-co-PMMA with 5 vol % and 10 vol % of methyl methacrylate (Sigma-Aldrich), respectively, and PHEMA-co-PAA with 5 vol % and 10 vol % of acrylic acid (Sigma-Aldrich), respectively, were prepared in the similar fashion as that of PHEMA with 2 vol % EGDMA. PEGDA hydrogel precursor was prepared by mixing PEGDA (Mn 700; Sigma-Aldrich) with total 10 vol % of Darocure 1173 then homogenized by vortexing.
Fabrication of PHEMA Hydrogel Structures.
Si masters with (i) cross-patterns (500, 100, and 50 μm in length, width, and thickness, respectively), (ii) circular pillars (150 μm in diameter and 50 μm in height), and (iii) circular holes (500 μm in diameter and 50 μm in depth), respectively, were placed in a Petri dish and covered by a mixture of PDMS and cross-linker (10:1 wt/wt, Sylgard 184; Dow Corning). The samples were cured in an oven at 70 °C for 2 h, after which the PDMS molds were peeled off from their respective master. The unpolished surface of a silicon wafer was treated with an adhesion promoter (Glass Primer; Minuta Technology) at 4,000 rpm for 30 s and baked on a hot plate (Dataplate Series 730) at 115 °C for 15 min. Next, the prepared PHEMA precursor was drop-cast on the primer-treated surface, and the respective PDMS molds with the negative patterns were placed on top. Following UV exposure at 365 nm with a dosage of 20 J⋅cm−2, the PDMS molds were gently peeled off to obtain the patterned PHEMA hydrogel adhesive pad.
Measurements of Mechanical Properties of PHEMA by AFM.
The near-surface elastic modulus of the PHEMA adhesive was characterized using an AFM (Asylum Research MFP-3D; Oxford Instruments), wherein a 0.5- 0.5-µm2 area (wet samples) and 25- 25-µm2 area (dry samples) were scanned by the AFM probe (Bruker SCANASYST-AIR and OTESPA for the wet and dry samples, respectively), in tapping mode. Values of elastic modulus were extracted from force-displacement data using the JKR model and Hertz model fits for the wet and dry samples, respectively.
Pull-Off Force Measurement by Indentation.
The adhesion of the sample was measured by indentation, following the procedure described by Lin et al. (44). The test sample was placed on an inverted optical microscope as a glass indenter tip (radius ∼3.025 mm) was brought into contact using a motor. The displacement was measured with a capacitance sensor, and the force was measured with a load cell, while videography was used to confirm the contact region. In the tests, the rough surface model was simulated by using a spherical glass indenter with a root-mean-square roughness of 1.8 μm, while the smooth model was studied using a tip of roughness ∼7.7 nm. To guarantee reproducibility, all indenter tips were treated with a hydrophobic self-assembled monolayer of n-hexadecyltrichlorosilane, following the procedure of Glassmaker et al. (45). To verify the recovery of a sample indented to a depth of 22 µm, upon rehydration a drop of water was added to the indented region and the sample was allowed to swell for 1 min before the remainder of the drop was wicked away. The sample was then allowed to dry and equilibrate for 10 min and the dented surface was optically scanned using a laser profilometer (Zegage; Zygo Corporation). This procedure was repeated another seven times and the dented surface region was scanned every time. Finally, the sample was completely immersed in water and allowed to sit overnight, dried, and scanned. Raw surface scan data were processed in MATLAB and plotted in Mathematica to show the complete recovery of the indented surface to flatness. To test the reversibility of our adhesive, 12 iterations of the indentation experiments were conducted at the same spot on the surface of a flat, 8 vol % PHEMA gel sample. The sample was affixed to the stage of the indentation apparatus with double-sided tape. Various wetting and drying times were tested for each cycle. In the case of the repeatability tests, the samples were not completely dried during each cycle, so as to avoid crack generation.
Sample Preparation for Macroscopic Adhesion Tests.
A sample for comparison and to delineate the ease-of-failure with weaker backbone materials before reaching the PHEMA adhesive’s maximum force capacity employed a PET backbone with a PUA micropillar array (height and diameter 5 μm, and spacing 10 μm) molded onto PET surface via soft lithography to serve as model roughness. To guarantee reliable measurements of high adhesion forces, aerospace-grade Al plates (2.5 cm 15 cm 2.5 mm, Al 7075-T6) and Kevlar tape strips (DuPont Kevlar 49; Fiber Glast Development Corp.) were chosen as the rigid backbones and flexible tendons for the tests, respectively. The PHEMA hydrogel pads were fabricated on Si wafers, which were cut precisely to make samples with areas of 0.25, 0.5, 1, and 2 cm2, respectively, and glued to both sides of a metal plate (the rigid backbone) using Gorilla Super Glue Gel. Another two pieces of Si wafers were each glued to the Kevlar tape using superglue. Finally, the patterned PHEMA pad and the unpolished, rough side of the Si wafer were brought into contact while the PHEMA adhesive was in the wet state and the contact was maintained during the subsequent drying process with the aid of a custom-made compression jig designed to exert a preload and ensure conformal contact (∼5 N/cm2). The sample was dried in an oven at 70 C for 4 h to ensure the complete dehydration of the hydrogel adhesive and measurement of the maximum attainable force capacity.
The hydrogel samples from PHEMA-co-PMMA and PHEMA-co-PAA with varying copolymer ratios were 180 µm thick by molding respective precursor solutions onto the rough side of a Si wafer, which was then glued on both sides of an Al plate and brought in contact with a Si wafer with the polished side glued on a Kevlar backing tendon in the wet state and in a single lap joint configuration, compressed and then dried, following the procedure for PHEMA samples as described above. The PEGDA precursor was molded with the cross pattern on a glass slide [spin-coated with the adhesion promoter Glass Primer (Minuta Technology)] using a PDMS stamp, followed by exposure to 365-nm UV light (dosage ∼20 J/cm2) to obtain a ∼180-µm-thick film.
Measurement of Shear Adhesion with Instron.
An Instron model 4206 Precision (maximum load capacity ∼134.5 kN) was employed to probe the shear adhesive properties of our samples. A strain rate of 8 mm/min and a maximum load setting of 5 kN was chosen for the tests. So as to reduce the propensity for the sample to fail via opening and out-of-plane shear modes, as well as to mitigate fixture-misalignment-related failure events, the sample was held in self-aligning grips and tested in tension with a rigid lower fixture and a universally jointed upper fixture.
Adhesion Between Porous Targets.
Anodized aluminum oxide membranes (GE Healthcare Whatman Anodisc Filter Membranes; Thermo Fisher Scientific) with a pore size of 200 nm in diameter were employed as porous targets. To obtain a free-standing, PHEMA membranous adhesive with clear-through holes (thickness ∼50 µm with 500- µm-diameter pores), the PHEMA precursor synthesized as described earlier was used in conjunction with a dewetting-based molding technique, as delineated in ref. 30. The swollen PHEMA membrane was sandwiched between two AAO targets and dried. A sample for comparison was prepared by applying Gorilla Super Glue Gel at the interface between AAO membranes. Both the PHEMA-glued and superglued samples were cleaved to image the bonded interface using a scanning electron microscope.
Selective Adhesion with Patterned PHEMA Adhesive Pads.
A PHEMA adhesive pad with a circular pillar array (150 µm in diameter, 300 µm in spacing, and 50 µm in height), while in the wet state, was pressed onto the surface of a Morpho didius butterfly wing using a jig with an applied load of 5 N, followed by drying and forcible detachment. The surfaces of the adhesive pad and the butterfly wing postdetachment were observed using a scanning electron microscope (Quanta 600 FEG Mark II; FEI).
Supplementary Material
Acknowledgments
We acknowledge partial support by the NSF DMR/Polymer program, DMR-1410253, and NSF/EFRI-ODISSEI program, EFRI-1331583 (S.Y.). The work on indentation experiments and their interpretation was supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award DE-FG02-07ER46463 (A.J.). H.C. also acknowledges support from the National Research Foundation of Korea (NRF), Grant NRF-2016M3C1B5906481. We thank Dr. Matthew Brukman for assisting with the characterization of the material modulus and Ms. Sohee Nah and Mr. Meng Li for helping with some of the indentation experiments to validate our studies.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818534116/-/DCSupplemental.
References
- 1.Autumn K., Gecko adhesion: Structure, function, and applications. MRS Bull. 32, 473–478 (2007). [Google Scholar]
- 2.Jagota A., Hui C. Y., Adhesion, friction, and compliance of bio-mimetic and bio-inspired structured interfaces. Mater. Sci. Eng. R 72, 253–292 (2011). [Google Scholar]
- 3.Henkel Corporation , “Loctite super glue professional technical data sheet” (Henkel Corporation, Rocky Hill, CT, 2018).
- 4.Li J., et al. , Tough adhesives for diverse wet surfaces. Science 357, 378–381 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J. Y., et al. , Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirthl D., et al. , Instant tough bonding of hydrogels for soft machines and electronics. Sci. Adv. 3, e1700053 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuk H., Zhang T., Lin S., Parada G. A., Zhao X., Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 15, 190–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Bai R., Chen B., Suo Z., Hydrogel adhesion: A supramolecular synergy of chemistry, topology, and mechanics. Adv. Funct. Mater., 1901693 (2019). [Google Scholar]
- 9.Autumn K., et al. , Adhesive force of a single gecko foot-hair. Nature 405, 681–685 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Arzt E., Gorb S., Spolenak R., From micro to nano contacts in biological attachment devices. Proc. Natl. Acad. Sci. U.S.A. 100, 10603–10606 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak M. K., et al. , Towards the next level of bioinspired dry adhesives: New designs and applications. Adv. Funct. Mater. 21, 3606–3616 (2011). [Google Scholar]
- 12.Jeong H. E., Lee J. K., Kim H. N., Moon S. H., Suh K. Y., A nontransferring dry adhesive with hierarchical polymer nanohairs. Proc. Natl. Acad. Sci. U.S.A. 106, 5639–5644 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu L., Dai L., Stone M., Xia Z., Wang Z. L., Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science 322, 238–242 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Xu M., Du F., Ganguli S., Roy A., Dai L., Carbon nanotube dry adhesives with temperature-enhanced adhesion over a large temperature range. Nat. Commun. 7, 13450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baik S., et al. , A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 546, 396–400 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.-C., Yang H., Octopus-inspired assembly of nanosucker arrays for dry/wet adhesion. ACS Nano 11, 5332–5338 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Bartlett M. D., et al. , Looking beyond fibrillar features to scale gecko-like adhesion. Adv. Mater. 24, 1078–1083 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Bartlett M. D., Crosby A. J., High capacity, easy release adhesives from renewable materials. Adv. Mater. 26, 3405–3409 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Kim T. I., Jeong H. E., Suh K. Y., Lee H. H., Stooped nanohairs: Geometry-controllable, unidirectional, reversible, and robust gecko-like dry adhesive. Adv. Mater. 21, 2276–2281 (2009). [Google Scholar]
- 20.Persson B. N. J., Biological adhesion for locomotion on rough surfaces: Basic principles and a theorist’s view. MRS Bull. 32, 486–490 (2007). [Google Scholar]
- 21.King D. R., Bartlett M. D., Gilman C. A., Irschick D. J., Crosby A. J., Creating gecko-like adhesives for “real world” surfaces. Adv. Mater. 26, 4345–4351 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Kim J., et al. , Nonlinear frameworks for reversible and pluripotent wetting on topographic surfaces. Adv. Mater. 29, 1605078 (2017). [DOI] [PubMed] [Google Scholar]
- 23.de Mestral G., Adhesive element in cloth form. US Patent 3,748,701 (1973).
- 24.Allyn F., Golden B., “Get a Grip” in Why Didn’t I Think of That: Bizarre Origins of Ingenious Inventions We Couldn’t Live Without (Wiley, ed. 1, 1997), pp. 99–104. [Google Scholar]
- 25.Ko H., et al. , Hybrid core-shell nanowire forests as self-selective chemical connectors. Nano Lett. 9, 2054–2058 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Chen C.-M., Chiang C.-L., Lai C.-L., Xie T., Yang S., Buckling-based strong dry adhesives via interlocking. Adv. Funct. Mater. 23, 3813–3823 (2013). [Google Scholar]
- 27.Vajpayee S., Khare K., Yang S., Hui C.-Y., Jagota A., Adhesion selectivity using rippled surfaces. Adv. Funct. Mater. 21, 547–555 (2011). [Google Scholar]
- 28.Denny M. W., Mechanical-properties of pedal mucus and their consequences for gastropod structure and performance. Am. Zool. 24, 23–36 (1984). [Google Scholar]
- 29.Giokas S., Pafilis P., Valakos E., Ecological and physiological adaptations of the land snail Albinaria caerulea (Pulmonata: Clausiliidae). J. Molluscan Stud. 71, 15–23 (2005). [Google Scholar]
- 30.Cho H., et al. , Multiplex lithography for multilevel multiscale architectures and its application to polymer electrolyte membrane fuel cell. Nat. Commun. 6, 8484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guvendiren M., Yang S., Burdick J. A., Swelling-induced surface patterns in hydrogels with gradient crosslinking density. Adv. Funct. Mater. 19, 3038–3045 (2009). [Google Scholar]
- 32.Guvendiren M., Heiney P. A., Yang S., Precipitated calcium carbonate hybrid hydrogels: Structural and mechanical properties. Macromolecules 42, 6606–6613 (2009). [Google Scholar]
- 33.Guvendiren M., Burdick J. A., Yang S., Solvent induced transition from wrinkles to creases in thin film gels with depth-wise crosslinking gradients. Soft Matter 6, 5795–5801 (2010). [Google Scholar]
- 34.Johnson K. L., Contact Mechanics (Cambridge University Press, 1987). [Google Scholar]
- 35.Ge L., Sethi S., Ci L., Ajayan P. M., Dhinojwala A., Carbon nanotube-based synthetic gecko tapes. Proc. Natl. Acad. Sci. U.S.A. 104, 10792–10795 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito S., et al. , Light-melt adhesive based on dynamic carbon frameworks in a columnar liquid-crystal phase. Nat. Commun. 7, 12094 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu G., et al. , Drying enhanced adhesion of polythiophene nanotubule arrays on smooth surfaces. ACS Nano 2, 2342–2348 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Eisenhaure J. D., Xie T., Varghese S., Kim S., Microstructured shape memory polymer surfaces with reversible dry adhesion. ACS Appl. Mater. Interfaces 5, 7714–7717 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Eisenhaure J., Kim S., An internally heated shape memory polymer dry adhesive. Polymers 6, 2274–2286 (2014). [Google Scholar]
- 40.Kim S., Sitti M., Biologically inspired polymer microfibers with spatulate tips as repeatable fibrillar adhesives. Appl. Phys. Lett. 89, 261911 (2006). [Google Scholar]
- 41.Gorb S., Varenberg M., Peressadko A., Tuma J., Biomimetic mushroom-shaped fibrillar adhesive microstructure. J. R. Soc. Interface 4, 271–275 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boesel L. F., Greiner C., Arzt E., del Campo A., Gecko-inspired surfaces: A path to strong and reversible dry adhesives. Adv. Mater. 22, 2125–2137 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Hawkes E. W., Eason E. V., Christensen D. L., Cutkosky M. R., Human climbing with efficiently scaled gecko-inspired dry adhesives. J. R. Soc. Interface 12, 20140675 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin P. C., Vajpayee S., Jagota A., Hui C. Y., Yang S., Mechanically tunable dry adhesive from wrinkled elastomers. Soft Matter 4, 1830–1835 (2008). [Google Scholar]
- 45.Glassmaker N. J., Jagota A., Hui C.-Y., Noderer W. L., Chaudhury M. K., Biologically inspired crack trapping for enhanced adhesion. Proc. Natl. Acad. Sci. U.S.A. 104, 10786–10791 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.