Significance
To ensure our survival, the brain continuously estimates important homeostatic and physiological variables, such as our hunger, thirst, and effort levels. This estimate relies on multiple signals, most prominently on interoceptive signals coming from inside the body (e.g., cardiac and respiratory signals). Here we tested the hypothesis that providing false feedback about these signals produces an interoceptive illusion, that is a misperception of one’s own physiological state. We show that giving participants false (faster) acoustic feedback about their heartbeats during an effortful cycling task induced the illusion of making more effort. Rather, participants did not report lower effort when slower acoustic feedback was provided, suggesting that the brain considers the potential costs of underestimating effort and fatigue levels.
Keywords: interoception, interoceptive illusion, heartbeat, illusion of effort
Abstract
Interoception, or the sense of the internal state of the body, is key to the adaptive regulation of our physiological needs. Recent theories contextualize interception within a predictive coding framework, according to which the brain both estimates and controls homeostatic and physiological variables, such as hunger, thirst, and effort levels, by orchestrating sensory, proprioceptive, and interoceptive signals from inside the body. This framework suggests that providing false interoceptive feedback may induce misperceptions of physiological variables, or “interoceptive illusions.” Here we ask whether it is possible to produce an illusory perception of effort by giving participants false acoustic feedback about their heart-rate frequency during an effortful cycling task. We found that participants reported higher levels of perceived effort when their heart-rate feedback was faster compared with when they cycled at the same level of intensity with a veridical feedback. However, participants did not report lower effort when their heart-rate feedback was slower, which is reassuring, given that failing to notice one’s own effort is dangerous in ecologically valid conditions. Our results demonstrate that false cardiac feedback can produce interoceptive illusions. Furthermore, our results pave the way for novel experimental manipulations that use illusions to study interoceptive processing.
Interoception, Predictive Coding, and Illusions
Interoception, or the sense of the internal state of the body, is key to the adaptive regulation of our physiological needs and the formation of our sense of self (1). Recent theories conceptualize interoception within a predictive coding perspective, which emphasizes the predictive aspects of the processing of bodily signals and of physiological regulation (2–7), analogous to predictive perceptual processing (8, 9) and action control (10–12). These theories propose that the brain implements a process of interoceptive inference for physiological sensing and adaptive regulation. It does so by continuously generating top-down predictions about adaptive interoceptive signals (i.e., the signals that a healthy body should generate) and using prediction errors (i.e., mismatches between the expected and sensed interoceptive signals) to steer compensatory autonomic actions that restore homeostasis. Furthermore, it generates predictions about future misregulation and takes anticipatory (allostatic) actions to proactively prevent them (13).
To engage in interoceptive inference and autonomic control, the brain may use internal models (or central representations) of the physiological and homeostatic variables that it regulates, such as hunger, thirst, effort, and fatigue levels (14–17). Using an internal model for interoceptive inference and physiological regulation is analogous to using a bodily postural model (or body schema) that keeps track of spatial properties of the body, such as limb position, for movement control (18). While under normal conditions, the internal models support an accurate estimate of their relevant variables, some ambiguous conditions can lead to misperceptions.
Body illusions, such as the illusion of owning another person’s full body (19), face (20, 21), or limbs (22), are examples of experimentally induced misperceptions of one’s body representation. Note that in this context, the term “illusion” denotes a transient misperception of some variables, such as the location of one’s body or hand, as an effect of the resolution of a multisensory conflict. Thus, body illusions are markedly different from, and significantly more attenuated than, delusional beliefs, hallucinations, and the illusory perception of nonexistent entities, which are associated with clinical and psychopathological conditions (7, 17, 23–25).
In one of the most widely studied body illusions, the “rubber hand” illusion, participants misperceive the actual location of their hand (26). The classical method of inducing this illusion involves placing a rubber hand in the usual position of the participant’s real arm, which is hidden, and then gently stroking the real hand and the rubber hand simultaneously and in the same place. Participants usually report that their hand position lies in between their real hand and the rubber hand, supposedly because they integrate noisy and conflicting sources of information (e.g., visual and tactile), one of which could be considered false feedback (27).
An open question is whether a similar process may extend to the interoceptive domain as well. If the brain processes interoceptive and exteroceptive body representation in fundamentally similar ways, then providing participants with false feedback about their interoceptive streams may produce an interoceptive misperception or illusion (where illusion is used in the same sense as for body illusion, not in the clinical sense).
Aims of the Study and Hypotheses
In this study, we tested the idea that providing participants with false cardiac feedback while they performed a physical exercise would make them experience an illusion of perceived effort—that is, a misperception of their actual level of effort.
We asked participants to cycle on a stationary bicycle (cycle-ergometer) at different levels of intensity, which were unknown to them. Crucially, we provided the participants with auditory feedback, via headphones, that was either congruent with the heartbeat frequency recorded when they cycled at the same level of intensity or incongruent with it (i.e., faster or slower). After each cycling session, the participants reported their perceived level of effort using the rate of perceived exertion (RPE) (28), which is widely used in medicine and sports because it accurately indexes physiological variables, such as heart rate (HR) and exercise intensity level (29). We were interested in quantifying the participants’ misperception (or illusion) of effort as a function of their cardiac feedback, or the discrepancy between the actual physical effort that they were exerting and the perceived effort that they reported.
We predicted that participants who heard faster heartbeats (i.e., heartbeats recorded when they cycled at a higher intensity level) would experience an illusion of perceived effort and report higher scores in the RPE scale compared with a control condition in which they made the same physical effort (i.e., cycled at the same intensity level) but heard congruent (i.e., heartbeats recorded when they cycled in the same intensity level) auditory feedback or no feedback. We also reasoned that participants who heard slower heartbeats (i.e., heartbeats recorded when they cycled at a lower intensity level) would experience the opposite illusion (i.e., lower effort) in a significantly more attenuated way. This is because underestimating the effort during exertional activities such as physical exercise entails numerous risks, for example, selection of intensity levels that are excessively high. Under these conditions, a risk-avoidant strategy could be more appropriate. This idea is consistent with previous research showing that (mentally) fatigued persons overestimate their physical exertion levels, plausibly as a protection mechanism against the risks of excessive effort (30).
Finally, we hypothesized that persons with lower interoceptive awareness rely more on the false feedback and thus experience a stronger illusion of effort. This prediction is consistent with previous studies reporting that the strength of body illusions, such as the rubber hand (31) and enfacement (32) illusions, is higher in participants with low interoceptive awareness.
Results
We checked the normality of data distribution relative to each dependent variable (i.e., participants’ HR and RPE ratings) and condition using the Kolmogorov–Smirnov (K-S) test. When the normality assumption was not respected, a nonparametric approach (i.e., Friedman ANOVA followed by Bonferroni-corrected Wilcoxon matched-pairs test, and Spearman’s rank correlations) was used. Given that classical null hypothesis testing is not the ideal statistical tool for drawing conclusions about nonsignificant results (33, 34), in the event of negative results, we also calculated Bayes factors (BFs), which allows quantification of evidence in favor of the alternative or null hypothesis. All statistical analyses but BFs were run using Statistica 7 software. BFs were calculated using the open-source software JASP (35).
Preliminary Analysis
First, we checked that cycling at increasing levels of intensity increased physiological effort (as indexed by HR frequency) and perceived effort (as indexed by subjective ratings on the RPE scale) during the Baseline Exercise (BE) session, where no acoustic interoceptive feedback was provided.
Physiological Effort.
Three conditions were not normally distributed according to the K-S test [D(18) > 0.215; P < 0.028 for all]; therefore, Friedman ANOVA was performed to compare participants’ HR recorded at seven different intensity intervals: the targeted ones (60, 75, 90, 105, and 115 W) and those immediately above or below them (from 45 to 125 W). In line with our hypothesis, Friedman ANOVA was significant [χ2(6) = 108; P < 0.00000]. Bonferroni-corrected Wilcoxon matched- pair tests were all significant (Z = 3.724; P < 0.0002 for all), suggesting that, as expected, HR frequency increases when cycling at increasing levels of intensity (SI Appendix, Fig. S1a).
Perceived Effort.
RPE values were normally distributed; therefore, a parametrical approach (i.e., repeated-measures ANOVA followed by Bonferroni-corrected post hoc tests) was used. The repeated-measures ANOVA on the five levels of the targeted intensity as the sole within-subject factor was significant [F(4, 68) = 180.465; P < 0.00000]. Post hoc tests comparing the perceived effort recorded in each specific interval with the next interval were significant (P < 0.0001 for all) and showed that when the level of cycling intensity increased, participants reported higher scores of perceived effort (SI Appendix, Fig. S1b).
Main Analysis
We aimed to test whether perceived effort changes as a function of receiving incongruent auditory feedback about one’s own physiological state (i.e., HR frequency). Thus, we compared effort ratings collected after cycling at five different targeted intensities (60, 75, 90, 105, and 115 W) in a “congruent feedback” condition, in which participants listened to a heartbeat sound registered during the BE session at the same intensity level, with two “incongruent feedback” conditions, in which participants listened to faster or slower heartbeat sounds registered during the BE session at higher or lower intensity levels, respectively. To remove baseline interindividual differences, for each participant, we subtracted effort ratings at baseline from those collected after congruent and incongruent Experimental Exercise (EE) conditions. The K-S test showed that these baseline-corrected ratings of perceived effort were not normally distributed [D(18) > 0.204; P < 0.046 for all].
Friedman ANOVA comparing the baseline-corrected ratings of effort in our experimental conditions (i.e., when participants cycled at the five intensity levels receiving the three types of acoustic feedback: congruent, slower, and faster) was significant [χ2(14) = 76.321; P < 0.000], suggesting that participants perceived different levels of effort in the different experimental conditions. To see whether participants reported more (less) effort when listening to a faster (slower) HR frequency compared with the congruent condition, we performed a Bonferroni- corrected Wilcoxon matched-pairs test (threshold for statistical significance, P < 0.025).
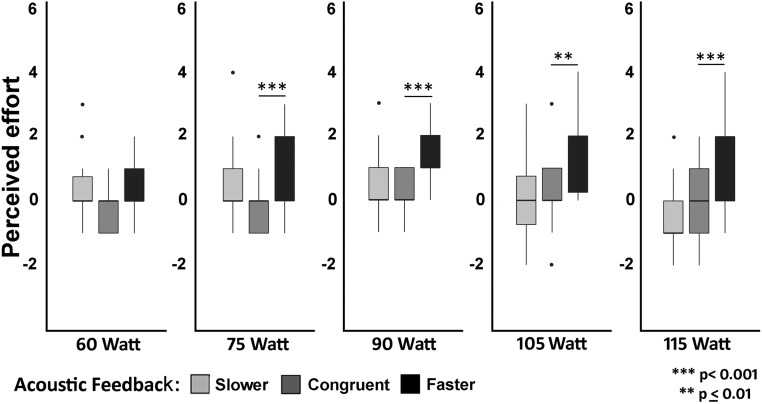
The planned Wilcoxon matched-pairs test showed that providing faster vs. congruent heartbeats significantly increased the perceived effort in all (Z > 2.982; P < 0.003 for all), with the exception of the first level of exercise intensity, which was slightly above the threshold (Z = 2.197; P = 0.028). The test also showed that providing slower vs. congruent feedback did not significantly change the perceived effort attributed to any of the exercise intensity levels (Z < 2.045; P > 0.041; BF < 0.283 for all except one value of 3.242). Results of the Wilcoxon matched- pairs tests and Bayesian paired-sample t tests are presented in Fig. 1 and SI Appendix, Table S1.
Fig. 1.
Results of the EE session. Changes in perceived effort (measured using the RPE scale) compared with the baseline session, as a function of five cycling intensity levels and with three types of acoustic feedback: slower, congruent, and faster. See the main text for explanation.
We performed an additional analysis to exclude that the illusory increase of effort was driven by an entrainment effect—that is, by a real change in HR frequency during exercise, possibly caused by the false feedback. Given that our variables were not normally distributed [D(18) > 0.219; P < 0.023 for all], we performed Friedman ANOVA on participants’ baseline-corrected HR. The Friedman ANOVA comparing the baseline-corrected HR recorded during our experimental conditions (i.e., when participants cycled at the five levels of intensity while receiving the three types of acoustic feedback: congruent; slower; faster) was not significant [χ2(14) = 18.633; P = 0.179]. Bayesian repeated-measures ANOVA confirmed these results by showing evidence in favor of the null hypothesis (types of acoustic feedback × levels of intensity interaction; BF = 0.014). Taken together, these results indicate that providing a faster (or slower) incongruent auditory heartbeat-related feedback did not increase (or decrease) participants’ HR, thus permitting the exclusion of confounding entrainment effects. Note also that during the debriefing, none of the participants reported having noticed that the acoustic feedback was manipulated.
To exclude the possibility that the illusory increase of perceived effort was due to the acoustic feedback, we compared participants’ RPE ratings collected after the congruent condition of the EE task, in which congruent acoustic feedback was provided, with those collected during the BE, in which no acoustic feedback was provided. The K-S test showed that some of the ratings of perceived effort were not normally distributed [D(18) > 0.201; P < 0.054 for all]; therefore, Friedman ANOVA was performed. Although Friedman ANOVA was significant [χ2(9) = 149.848; P < 0.000], in line with our hypothesis, the Wilcoxon matched-pairs test showed that participants perceived the same level of effort in each of the targeted frequencies when no acoustic feedback (baseline condition) was provided and when congruent HR feedback was provided (Z < 1.334; P > 0.182; 0.683 < BF > 0.243 for all). In summary, we found no evidence that providing congruent acoustic feedback changed participants’ RPE.
Finally, we tested whether interindividual differences in interoceptive awareness correlate negatively with the illusory increase of perceived fatigue when listening to faster heartbeat sounds, as was previously reported in the context of the rubber hand (31) and enfacement illusions (32). We assessed interoceptive awareness using the Multidimensional Assessment of Interoceptive Awareness (MAIA) (36, 37), focusing on the Noticing and Body Listening MAIA subscales, which relate more directly to bodily processes. For each participant, we computed a global index of illusory perceived effort, by averaging the increase of perceived effort (calculated as the difference between incongruent and congruent scores) in the four faster incongruent feedback conditions that we found to be significant. Finally, we correlated the Noticing and Body Listening scores with the illusory perceived effort index. Unexpectedly, the correlations were not statistically significant (ρ < 0.197; P > 0.05; BF < 0.210 for all). We found no significant correlations with personality traits assessed by the Body Awareness Questionnaire (38), the Body Perception Questionnaire (39), the State-Trait Anxiety Inventory (40), and the Big Five–short version (41) (SI Appendix, Table S3).
Discussion
We tested the possibility of inducing an illusion of perceived effort—that is, a misperception of one’s physiological state (here effort level)—by providing participants with false feedback about their bodily signals (here false acoustic feedback about their HR). Our results show that providing faster cardiac feedback during physical exercise consistently induces an illusory perception of increased effort.
Our results can be explained within theories of interoceptive (or embodied) inference (2–7), which assume that the brain both estimates and controls critical homeostatic and physiological variables. From this perspective, effort and other relevant interoceptive variables may be estimated on the basis of sensory (e.g., interoceptive) streams and prior information. Providing participants with false (faster) cardiac feedback may have induced them to misperceive their effort, that is, to overestimate their perceived effort compared with the actual effort that they would report based on their current exercise intensity level and physiological state (e.g., HR). This “illusion of effort” suggests that interoceptive information—perhaps made more salient by the acoustic feedback—is considered in the internal estimate of one’s own physiological state.
We found the illusion of effort to be asymmetrical. Participants reported more exertion when they received faster feedback about their HR frequency, but did not report less exertion when provided with slower feedback. At four of the five intensity levels that we tested (60–105 W), Bayesian analyses indicated strong to moderate evidence for the (null) hypothesis that the ratings of perceived effort with congruent feedback and slower feedback are the same. These findings are less conclusive at the fifth intensity level (115 W); although Bayesian analysis indicated moderate evidence for the opposite hypothesis (higher perceived effort with congruent feedback vs. slower feedback), the effect did not reach statistical significance using nonparametric analysis.
The asymmetry between effort perception with faster vs. slower cardiac feedback points toward a risk-averse strategy that takes into consideration the potential costs of a wrong estimate. Indeed, while overestimating effort level is relatively safe for an organism, underestimating it can be more maladaptive, especially during exerting tasks such as physical exercise. Several lines of research suggest that perceived levels of effort and fatigue influence the selection of exercise intensity levels and the efficacious regulation of bodily parameters (13, 42–45). Thus, it is possible that the potential costs of underestimating perceived effort (e.g., poor allostatic regulation) favor risk aversion strategies. The same form of risk aversion has been reported in the case of mentally fatigued persons who overestimate their physical exertion levels (30).
Taken together, these asymmetric results suggest that (top-down) factors, such as a concern for safety, may limit the plasticity of the interoceptive schema, preventing it from making dangerous inferences (e.g., that one is not exerted). Note that this asymmetry permits us to rule out alternative explanations for our findings—namely, that participants may have estimated their effort levels by using only the false auditory feedback or by counting their heartbeats, and also that the false feedback would have enhanced the salience of, and increased the attention to, interoceptive or exteroceptive stimuli (46, 47).
Another possible explanation for our findings is that the acoustic feedback—either congruent or incongruent—may have rendered the estimation of effort more difficult (e.g., we normally hear our heartbeats at quite a low volume) or noisier (e.g., the acoustic feedback was not synchronized with the online heartbeats), thus biasing the participants’ ratings. This interpretation is not tenable, however, given that we found no differences between the participants’ ratings during the experimental (with congruent feedback) and baseline (without feedback) sessions, and that we found an asymmetric effect of acoustic feedback (with faster but not slower feedback). Another alternative explanation for our findings is a putative entrainment effect; if participants’ heartbeats align to the false auditory feedback (i.e., become faster), then their perceived exertion cannot be considered a misperception. The fact that participants’ heartbeats do not significantly change with false feedback rules out possible entrainment effects.
A limitation of the present study is its small sample size, which may have hindered the ability to describe differences in the illusory strength of the effect in relation to interindividual differences in self-reported measures of interoceptive awareness. Future studies should examine whether the results reported here can be generalized to other populations (e.g., women, older or less fit individuals) or to other stimuli (e.g., fast or slow nonbiological rhythms), especially when participants are not induced or find it difficult to associate stimuli with their HR.
Our findings significantly extend the literature on bodily illusions, such as the rubber hand (26, 27), full body (19, 48), and enfacement (20, 21, 49) illusions, by showing that false feedback about interoceptive streams (here false cardiac feedback) can produce misperceptions of one’s own physiological state (here one’s own effort level). The false feedback mechanism has proven effective for probing interoceptive processing in different cognitive and emotional domains. A previous study that manipulated participants’ physiological feedback during exercise reported that emotional intensity and salience of neutral faces was enhanced by false feedback of increased HR (50). Other studies have reported that providing feedback on HR influences the perceived attractiveness of stimuli (51), and that increasing HR by exercise sensitizes to fearful stimuli (52). Our present study and the aforementioned studies show that providing false physiological feedback is an effective method for probing interoceptive processing and producing interoceptive illusions.
The most widely known interoceptive illusion is the thermal grill illusion, a sensation of burning heat when the arm is placed on a “grill” composed of several interleaved cold and hot metallic bars (53). As none of the bars is so hot as to produce a sensation of burning heat per se, this is considered an illusion, which may depend on the peculiar way in which our thermoreceptors integrate conflicting (cold vs. hot) peripheral information about temperature (53). The thermal grill can be conceptualized as interoceptive, if one assumes that the unmyelinated C-fibers that convey cutaneous temperature (and pain) sensations are part of the interoceptive system (1). Following similar arguments, other illusions may be considered at least partly interoceptive, such as those that use affective touch (54) or cardiovisual feedback (55, 56) to induce the illusory feeling of owning a body part or the full body (57; see also ref. 58 for different results).
While the relationships between bodily illusions and interoceptive streams remain to be fully tested, the results of the foregoing studies raise the possibility that body representations may be tightly linked to allostatic processes. Furthermore, a more integrative view should also consider proprioceptive and action-related contributions to body representations and illusions. For example, manipulating the neural command to the musculature via vibration of muscle afference induces an illusion of movement and cardiovascular and respiratory responses (59–61). The extent to which the brain maintains coherent internal models across several dimensions (e.g., postural, interoceptive) remains incompletely understood.
Several other facets of interoceptive processing are still unclear, such as the way in which the brain may combine multimodal sources of evidence to estimate physiological or homeostatic variables (e.g., hunger or effort). In principle, the information source that is temporarily more accurate may dominate and uniquely contribute to the perception of current effort and other relevant physiological variables. However, in the present study, manipulating the putatively most reliable (or at least most salient) information source—auditory feedback—produced asymmetric effects; participants reported higher effort levels with faster feedback but not lower effort levels with slower feedback. This result suggests that the brain may use more sophisticated mechanisms than selection of the most reliable source of evidence.
The optimal approach to multisensory integration involves considering prior information plus all available sources of evidence—interoceptive, proprioceptive, and exteroceptive—and weighting them based on their precision (i.e., inverse uncertainty). It has been reported that the brain can optimally weight and integrate multiple exteroceptive sources of (visual and haptic) information to form a robust percept (62). Along similar lines, it is possible to speculate that the brain maintains a central representation of physiological variables and bodily signals coming from inside the body. Such a putative interoceptive schema would be the equivalent of a body schema (i.e., an internal representation of bodily variables, such as the shape of our body, the position of our limbs) (18) for the interoceptive domain. It may support homeostatic and allostatic regulation by orchestrating and predicting interoceptive signals coming from inside the body (e.g., heartbeats, ventilation) as well as from the outside (e.g., muscular signals due to effort). However, the idea that the brain maintains an interoceptive schema for (optimal) multimodal integration is speculative and remains to be investigated in future studies. Novel experiments should assess which physiological and homeostatic variables (e.g., hunger, thirst, effort and fatigue levels) the brain estimates and controls, the relative contribution of different (interoceptive, proprioceptive, and exteroceptive) streams to these processes, and whether the different streams are integrated in an optimal way. For this, in addition to heartbeat signals, which have slow transmission and limited perceptual access, it may be worth manipulating other interoceptive signals (e.g., respiratory signals) that may be more accessible and precise. This would permit the testing of whether cross-modal conflicts arise and if so, whether they are resolved by considering the relative precision of the information sources, as is assumed by optimal integration schemes.
Another open question involves the behavioral effects of the illusion of effort (or other interoceptive illusions). Various studies in the sports literature suggest that for athletes, the selection of exercise intensity is not a simple function of muscle fatigue but rather is mediated by top-down mechanisms and perceived levels of effort and fatigue (42, 43, 63, 64). These studies suggest that an illusion of effort should induce participants (or athletes) to select lower exercise intensity levels. Similarly, one can hypothesize that participants who report being more exerted would discount future rewards more steeply if they required some effort to be secured (44). Future studies should test these predictions. Another open question concerns the relationship between implicit processes of interoceptive processing and explicit (self-reported) measures of interoceptive awareness that, according to a recent model proposed by Garfinkel and collaborators (65), may target different components of interoceptive awareness, namely interoceptive accuracy and interoceptive sensibility. Previous studies have reported that participants with lower interoceptive awareness (as assessed using the Mental Tracking Task) report weaker bodily illusions (31, 32); we did not observe a similar effect in our experiment when using an explicit self-report measure, the MAIA questionnaire.
Our results speak against the possibility of an integrative or schematic representation of effort, which combines coherently low-level interoceptive streams and higher-order interoceptive representations (putatively measured by the MAIA questionnaire). However, it remains to be tested whether objective measures of interoceptive awareness instead correlate with the strength of the illusion of effort. Preliminary evidence for this idea comes from a study reporting that participants with good heartbeat perception ability covered a significantly shorter distance when they pedaled on a bicycle ergometer for 15 min and (in contrast to the present study) were free to choose the time of their cycling (45). Notably, the same participants also exhibited significantly smaller increases in mean HR, stroke volume, and cardiac output, suggesting that by selecting a lower intensity level, they were able to improve their allostatic regulation. This finding suggests that participants with good heartbeat perception may be better at discriminating and controlling their effort levels. This prediction remains to be tested in future research.
Finally, our findings may have implications for understanding psychopathological conditions associated with failure of interoceptive processing, such as eating disorders, anxiety, depression, and chronic fatigue (7, 17, 66, 67). Some of these (and other) psychopathological conditions may stem from a failure to appropriately balance prior information and perceptual (e.g., exteroceptive and interoceptive) streams within a predictive coding architecture (3, 68). For example, excessively strong priors (e.g., that one is ill) may produce delusional beliefs or the reporting of false symptoms (24), even in the absence of perceptual evidence. Furthermore, fatigue and depression may arise from long-lasting deficits of interoceptive processing and dyshomeostasis, which are continuously monitored by a metacognitive layer. Detection of a chronic inability of the brain to regulate bodily states by the metacognitive layer can trigger early adaptive responses (fatigue) or a belief of low allostatic self-efficacy, the latter potentially resulting in depression (17). The illusion of effort described here, or other interoceptive illusions using the same logic, may provide an effective way to probe the imbalances responsible for psychopathological conditions.
Materials and Methods
Participants.
Eighteen male volunteers (mean age, 22.15 ± 2.9 y; range, 20–26 y) were recruited through posters and flyers displayed at the University Campus in Chieti, Italy for participation in this study. Additional details are provided in SI Appendix, Table S2. All were naive with respect to the purpose of the experiment and the measurements of their HR and changes with exertion. The study was approved by the Institute of Cognitive Sciences and Technologies’ Human Research Ethical Committee (Rome) (Protocol 0003871). All participants provided signed informed consent before enrolling in the study.
General Procedure.
The study was composed of a pretest and two sessions: a baseline session and an experimental session (SI Appendix, Fig. S2). All procedures were performed in the laboratory at a temperature maintained between 18 and 22 °C and a relative humidity of 45%–60%.
The pretest was conducted to assess anthropometric parameters (including age, height, and body mass index), inclusion criteria, and fitness level of the participants, including physiological parameters of maximal oxygen uptake and ventilation. Fitness level was tested to ensure that a participant could safely take part in the experiment. During the pretest, participants were familiarized with the instruments and the experimental procedures of the baseline and experimental sessions. The pretest was conducted approximately 1 wk before the baseline and experimental sessions.
The baseline and experimental sessions were conducted on the same day. During the first (baseline) session, participants’ interoceptive awareness was assessed using the Italian version of the MAIA questionnaire (36, 37). The Participants’ physiological and perceived effort were assessed by measuring HR frequency and ratings on the RPE scale, respectively, while they cycled at different levels of intensity. The HR and RPE measurements obtained during this first session were used as a baseline for our analysis, to index participants’ effort at each intensity level, and to create the acoustic stimuli to be used in the second session.
During the second (experimental) session, participants’ HR and RPE were remeasured while they cycled at different levels of intensity. However, unlike in the first (baseline) session, in this second (experimental) session, participants were provided with acoustic feedback that was either congruent or incongruent (i.e., faster or slower) with the HR frequency recorded when they cycled at the same level of intensity during the baseline condition.
First Session: BE, Without Acoustic Feedback.
At the beginning of the baseline session, we measured participants’ self-reported interoceptive awareness by asking them to complete the MAIA questionnaire (36, 37). This questionnaire comprises 32 items assessing eight dimensions of interoceptive awareness: noticing (awareness of uncomfortable, comfortable, and neutral body sensations), not-distracting (tendency not to ignore or distract oneself from sensations of pain or discomfort), not-worrying (tendency not to worry or experience emotional distress about sensations of pain or discomfort), attention regulation (ability to sustain and control attention to body sensations), emotional awareness (awareness of the connection between body sensations and emotional states), self-regulation (ability to regulate distress by paying attention to body sensations), body listening (active listening to the body for insight), and trusting (experiencing one’s body as safe and trustworthy) (69). Responses are rated on a six-point Likert scale ranging from 6-point Likert scale, ranging from 0 (never) to 5 (always).
After completing the MAIA questionnaire, participants performed the BE task. Participants performed seven submaximal 1-min trials, cycling at different levels of intensity (45, 60, 75, 90, 105, 115, and 125 W). They were instructed to maintain a cadence of 60 repetitions per minute for the entire session. During the exercise, HR frequency was recorded continuously (S610i; Polar Electro Oy, Kempele, Finland), and the average HR over the last 16 s was taken as the HR frequency of each block (70). Participants’ perceived effort was also measured during the exercise. Specifically, at the completion of each stage, participants started a 1-min recovery period. During the last 20 s of each recovery period (or when the HR returned to pre-exercise HR value), participants were asked to rate their perceived effort on the RPE scale, which ranges from 6 (no exertion at all) to 20 (maximal exertion). Participants received standardized instructions and were encouraged to focus on their overall whole-body perceptions of exertion (28).
After the BE task, participants walked to an adjacent, quiet, dimly lit room with comfortable environmental conditions, where they waited for 1 h before participating in the second session. During this time, the experimenter prepared the acoustic stimuli for use in the second session for each participant. Specifically, sounds reproducing participants’ HR frequency recorded at baseline during each block of cycling were generated using a custom sound synthesis procedure, to be used as acoustic feedback during the second session.
Second Session: EE, with Acoustic Feedback.
One hour after the baseline session, the experimental exercise session began. Participants sat quietly for 5 min while a pre-exercise HR was measured, to ensure that they had recovered sufficiently from the previous BE task. Then the EE started. Participants received the same instructions as in the BE task; however, here they were given wireless headphones and instructed that at 5 s after the start of the exercise they would hear a sound, which they were induced to believe represented their HR.
The EE consisted of 15 trials of 1 min each of cycling at five levels of cycling intensity (60, 75, 90, 105, and 115 W). Each level was associated with one of three different types of acoustic feedback (HR frequency sounds), one congruent and two incongruent (slower and faster) with the participant’s HR at the same level of cycling intensity. Specifically, in the congruent condition, participants received in their headphones the same HR frequency recorded during the baseline acquisition phase at the same level of cycling intensity. In the slower and faster conditions, participants received in their headphones the HR frequency recorded during the baseline acquisition phase at one level below or above their current cycling intensity, respectively. The recovery period between each trial was 1 min. As in the BE, participants’ physiological and perceived effort were recorded, as indexed by HR and 6–20 RPE scale ratings, respectively.
The order of the experimental conditions (five intensity intervals × three types of acoustic feedback) was randomly generated by a web-based computer program (www.randomization.com).
Supplementary Material
Acknowledgments
We thank Matthew Sims for comments on a previous version of this manuscript. G. Porciello was funded by Sapienza Progetti Ateneo (RM1161 55028E0FD5). This research has received funding from the European Union’s Horizon 2020 Framework Programme for Research and Innovation under Specific Grant Agreement 785907 (Human Brain Project SGA2, to G. Pezzulo).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821032116/-/DCSupplemental.
References
- 1.Craig A. D., How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Barrett L. F., Simmons W. K., Interoceptive predictions in the brain. Nat. Rev. Neurosci. 16, 419–429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pezzulo G., Why do you fear the bogeyman? An embodied predictive coding model of perceptual inference. Cogn. Affect. Behav. Neurosci. 14, 902–911 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Pezzulo G., Rigoli F., Friston K., Active inference, homeostatic regulation and adaptive behavioural control. Prog. Neurobiol. 134, 17–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seth A. K., Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17, 565–573 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Seth A. K., Suzuki K., Critchley H. D., An interoceptive predictive coding model of conscious presence. Front. Psychol. 2, 395 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Bergh O., Witthöft M., Petersen S., Brown R. J., Symptoms and the body: Taking the inferential leap. Neurosci. Biobehav. Rev. 74, 185–203 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Friston K., The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 11, 127–138 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Rao R. P., Ballard D. H., Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Friston K., Samothrakis S., Montague R., Active inference and agency: Optimal control without cost functions. Biol. Cybern. 106, 523–541 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Pezzulo G., Rigoli F., Friston K. J., Hierarchical active inference: A theory of motivated control. Trends Cogn. Sci. 22, 294–306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pezzulo G., Cisek P., Navigating the affordance landscape: Feedback control as a process model of behavior and cognition. Trends Cogn. Sci. 20, 414–424 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Sterling P., Allostasis: A model of predictive regulation. Physiol. Behav. 106, 5–15 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Petersen S., Schroijen M., Mölders C., Zenker S., Van den Bergh O., Categorical interoception: Perceptual organization of sensations from inside. Psychol. Sci. 25, 1059–1066 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Critchley H. D., Garfinkel S. N., Interoception and emotion. Curr. Opin. Psychol. 17, 7–14 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Tsakiris M., Preester H. D., The Interoceptive Mind: From Homeostasis to Awareness (Oxford University Press, 2018). [Google Scholar]
- 17.Stephan K. E., et al. , Allostatic self-efficacy: A metacognitive theory of Dyshomeostasis-induced fatigue and depression. Front. Hum. Neurosci. 10, 550 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Head H., Holmes G., Sensory disturbances from cerebral lesions. Brain 34, 102–254 (1911). [Google Scholar]
- 19.Blanke O., Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 13, 556–571 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Porciello G., Bufalari I., Minio-Paluello I., Di Pace E., Aglioti S. M., The “enfacement” illusion: A window on the plasticity of the self. Cortex 104, 261–275 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Bufalari I., Porciello G., Sperduti M., Minio-Paluello I., Self-identification with another person’s face: The time relevant role of multimodal brain areas in the enfacement illusion. J. Neurophysiol. 113, 1959–1962 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsakiris M., My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia 48, 703–712 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Adams R. A., Stephan K. E., Brown H. R., Frith C. D., Friston K. J., The computational anatomy of psychosis. Front. Psychiatry 4, 47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henningsen P., et al. ; EURONET-SOMA Group , Persistent physical symptoms as perceptual dysregulation: A neuropsychobehavioral model and its clinical implications. Psychosom. Med. 80, 422–431 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Sims A., Symptoms in the Mind: An Introduction to Descriptive Psychopathology (Bailliere Tindall, 1988). [Google Scholar]
- 26.Botvinick M., Cohen J., Rubber hands “feel” touch that eyes see. Nature 391, 756 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Apps M. A. J., Tsakiris M., The free-energy self: A predictive coding account of self-recognition. Neurosci. Biobehav. Rev. 41, 85–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borg G., Borg’s Perceived Exertion and Pain Scales (Human Kinetics, 1998). [Google Scholar]
- 29.Robertson R. J., Noble B. J., Perception of physical exertion: Methods, mediators, and applications. Exerc. Sport Sci. Rev. 25, 407–452 (1997). [PubMed] [Google Scholar]
- 30.Marcora S. M., Staiano W., Manning V., Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 106, 857–864 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Tsakiris M., Tajadura-Jiménez A., Costantini M., Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proc. Biol. Sci. 278, 2470–2476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tajadura-Jiménez A., Tsakiris M., Balancing the “inner” and the “outer” self: Interoceptive sensitivity modulates self-other boundaries. J. Exp. Psychol. Gen. 143, 736–744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dienes Z., Using Bayes to get the most out of non-significant results. Front. Psychol. 5, 781 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dienes Z., How Bayes factors change scientific practice. J. Math. Psychol. 72, 78–89 (2016). [Google Scholar]
- 35.Love J., et al. , JASP (Version 0.6.6, 2015).
- 36.Calì G., Ambrosini E., Picconi L., Mehling W. E., Committeri G., Investigating the relationship between interoceptive accuracy, interoceptive awareness, and emotional susceptibility. Front. Psychol. 6, 1202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehling W. E., et al. , The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS One 7, e48230 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shields S. A., Mallory M. E., Simon A., The Body Awareness Questionnaire: Reliability and validity. J. Pers. Assess. 53, 802–815 (1989). [Google Scholar]
- 39.Porges S., Body Perception Questionnaire (Laboratory of Developmental Assessment, University of Maryland, 1993). [Google Scholar]
- 40.Spielberger C. D., State-trait anxiety inventory. Corsini Encycl. Psychol. (2010). [Google Scholar]
- 41.Caprara G. V., Perugini M., Personality described by adjectives: The generalizability of the Big Five to the Italian lexical context. Eur. J. Pers. 8, 357–369 (1994). [Google Scholar]
- 42.Marcora S. M., Staiano W., The limit to exercise tolerance in humans: Mind over muscle? Eur. J. Appl. Physiol. 109, 763–770 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Tucker R., The anticipatory regulation of performance: The physiological basis for pacing strategies and the development of a perception-based model for exercise performance. Br. J. Sports Med. 43, 392–400 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Iodice P., et al. , Fatigue increases the perception of future effort during decision making. Psychol. Sport Exerc. 33, 150–160 (2017). [Google Scholar]
- 45.Herbert B. M., Ulbrich P., Schandry R., Interoceptive sensitivity and physical effort: Implications for the self-control of physical load in everyday life. Psychophysiology 44, 194–202 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Liebhart E. H., Information search and attribution: Cognitive processes mediating the effect of false autonomic feedback. Eur. J. Soc. Psychol. 9, 19–37 (1979). [Google Scholar]
- 47.Barefoot J. C., Straub R. B., Opportunity for information search and the effect of false heart-rate feedback. J. Pers. Soc. Psychol. 17, 154–157 (1971). [DOI] [PubMed] [Google Scholar]
- 48.Lenggenhager B., Tadi T., Metzinger T., Blanke O., Video ergo sum: Manipulating bodily self-consciousness. Science 317, 1096–1099 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Sforza A., Bufalari I., Haggard P., Aglioti S. M., My face in yours: Visuo-tactile facial stimulation influences sense of identity. Soc. Neurosci. 5, 148–162 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Gray M. A., Harrison N. A., Wiens S., Critchley H. D., Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS One 2, e546 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valins S., Cognitive effects of false heart-rate feedback. J. Pers. Soc. Psychol. 4, 400–408 (1966). [DOI] [PubMed] [Google Scholar]
- 52.Pezzulo G., et al. , Increased heart rate after exercise facilitates the processing of fearful but not disgusted faces. Sci. Rep. 8, 398 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Craig A. D., Bushnell M. C., The thermal grill illusion: Unmasking the burn of cold pain. Science 265, 252–255 (1994). [DOI] [PubMed] [Google Scholar]
- 54.van Stralen H. E., et al. , Affective touch modulates the rubber hand illusion. Cognition 131, 147–158 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Aspell J. E., et al. , Turning body and self inside out: Visualized heartbeats alter bodily self-consciousness and tactile perception. Psychol. Sci. 24, 2445–2453 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Suzuki K., Garfinkel S. N., Critchley H. D., Seth A. K., Multisensory integration across exteroceptive and interoceptive domains modulates self-experience in the rubber-hand illusion. Neuropsychologia 51, 2909–2917 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Morrison I., Löken L. S., Olausson H., The skin as a social organ. Exp. Brain Res. 204, 305–314 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Porciello G., Daum M. M., Menghini C., Brugger P., Lenggenhager B., Not that heart-stopping after all: Visuo-cardiac synchrony does not boost self-face attribution. PLoS One 11, e0160498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodwin G. M., McCloskey D. I., Matthews P. B., The contribution of muscle afferents to kinaesthesia shown by vibration-induced illusions of movement and by the effects of paralysing joint afferents. Brain 95, 705–748 (1972). [DOI] [PubMed] [Google Scholar]
- 60.Goodwin G. M., McCloskey D. I., Mitchell J. H., Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J. Physiol. 226, 173–190 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jennings J. R., van der Molen M. W., Brock K., Somsen R. J. M., How are tonic and phasic cardiovascular changes related to central motor command? Biol. Psychol. 35, 237–254 (1993). [DOI] [PubMed] [Google Scholar]
- 62.Ernst M. O., Banks M. S., Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415, 429–433 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Marcora S., Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. J. Appl. Physiol. 106, 2060–2062 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Noakes T. D., Fatigue is a brain-derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis. Front. Physiol. 3, 82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garfinkel S. N., Seth A. K., Barrett A. B., Suzuki K., Critchley H. D., Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 104, 65–74 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Barrett L. F., Quigley K. S., Hamilton P., An active inference theory of allostasis and interoception in depression. Phil. Trans. R Soc.Lond B Biol. Sci. 371, 20160011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pezzulo G., Barca L., Friston K. J., Active inference and cognitive-emotional interactions in the brain. Behav. Brain Sci. 38, e85 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Adams R. A., Brown H. R., Friston K. J., Bayesian inference, predictive coding and delusions. AVANT. J. Philos. Int. Vanguard 5, 51–88 (2014). [Google Scholar]
- 69.Velten J., Brotto L. A., Interoception and sexual response in women with low sexual desire. PLoS One 12, e0185979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamberts R. P., Swart J., Noakes T. D., Lambert M. I., LSCT, a novel submaximal cycle test to monitor fatigue and predict cycling performance. Br. J. Sports Med. 44, i21–i22 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.