Significance
How growth is controlled is a crucial aspect in developmental biology. The Wnt/Wingless pathway plays central roles regulating animal growth and aberrant activity in this signaling cascade can lead to cancer. It is therefore crucial to understand the roles of the different components of this pathway in growth control and tumorigenesis. We have used the wing imaginal disk of Drosophila to study the growth regulatory roles of the transcription factor dTcf/Pangolin, the final effector of the Wnt/Wingless cascade. We have observed that dTcf/Pangolin can limit tissue growth and tumor formation, and we have identified the genes controlled by dTcf/Pangolin involved in tumor formation. This work uncovers a network of genes regulated by dTcf/Pangolin involved in tumorigenesis.
Keywords: Drosophila, TCF/LEF, cancer, Wnt, growth control
Abstract
Wnt/Wingless (Wg) signaling controls many aspects of animal development and is deregulated in different human cancers. The transcription factor dTcf/Pangolin (Pan) is the final effector of the Wg pathway in Drosophila and has a dual role in regulating the expression of Wg target genes. In the presence of Wg, dTcf/Pan interacts with β-catenin/Armadillo (Arm) and induces the transcription of Wg targets. In absence of Wg, dTcf/Pan partners with the transcriptional corepressor TLE/Groucho (Gro) and inhibits gene expression. Here, we use the wing imaginal disk of Drosophila as a model to examine the functions that dTcf/Pan plays in a proliferating epithelium. We report a function of dTcf/Pan in growth control and tumorigenesis. Our results show that dTcf/Pan can limit tissue growth in normal development and suppresses tumorigenesis in the context of oncogene up-regulation. We identify the conserved transcription factors Sox box protein 15 (Sox15) and Ftz transcription factor 1 (Ftz-f1) as genes controlled by dTcf/Pan involved in tumor development. In conclusion, this study reports a role for dTcf/Pan as a repressor of normal and oncogenic growth and identifies the genes inducing tumorigenesis downstream of dTcf/Pan.
Intercellular communication plays central roles in the development of multicellular organisms. This is controlled by a handful of conserved signaling pathways that regulate central processes, such as organ growth and patterning. Abnormal signaling can affect cellular functions, including proliferation, cell death, and control of metabolic functions, and these processes are typically deregulated in cancer (1). Deciphering the mechanisms by which signaling pathways regulate growth and tumor formation is crucial in the endeavor of understanding animal development and disease.
The Wnt signaling pathway plays central roles in embryonic development and adult tissue homeostasis, and has been associated with cancer (2–5). Hyperactivation of Wnt signaling can be tumorigenic, and activating mutations in positive regulators of this pathway and loss-of-function mutations in negative components are found in multiple cancers (3, 5, 6). Importantly, pharmacological inhibitors targeting different elements in the Wnt cascade are currently being tested for cancer treatment (7).
Wnt signals are interpreted in a tissue-specific manner, leading to changes in gene expression in responding cells. β-Catenin is crucial connecting extracellular Wnt signals with transcriptional changes in the nucleus. Those changes are mediated by T cell factor/lymphoid enhancer-binding factor (TCF/LEF) transcription factors. TCF/LEFs contain a DNA-binding high-mobility group (HMG) box and a β-catenin binding domain. In the absence of the Wnt signal, β-catenin is phosphorylated by the β-catenin destruction complex and targeted for degradation. In the presence of the ligand, β-catenin destruction complex is inhibited, resulting in stabilization of β-catenin and allowing its translocation to the nucleus, where it binds TCF/LEFs to activate gene expression. When Wnt signals are not present, TCF/LEFs bind the corepressor TLE forming a complex that represses gene transcription. Hence, TCF/LEFs can act as transcriptional activators or transcriptional repressors depending on their interaction with coactivators (β-catenin) or corepressors (TLE) respectively. As observed with other components of the Wnt signaling cascade, genetic alterations in TCF/LEF genes are related to cancer (8–11).
Most invertebrates contain a single TCF/LEF gene. In vertebrates, the TCF/LEF family has expanded to 4 genes: TCF1, LEF1, TCF3, and TCF4 (also termed TCF7, LEF1, TCF7L1, and TCF7L2, respectively) (12). Even though LEF1 is typically linked to Wnt target gene activation and TCF3 is associated with target gene repression, the functions of TCF1 and TCF4 vary in a context-dependent manner (11, 13–18). Furthermore, different TCF/LEFs are frequently expressed in overlapping patterns and studies in mice have shown that functional redundancy between these elements is common (19–21). Specialization and redundancy create heterogeneous patterns of expression and activity that allow refining Wnt signaling. However, this complicates studying the roles of these transcription factors in normal development and disease. The Drosophila genome contains a single TCF/LEF gene, known as dTcf/Pan. This transcription factor plays crucial roles in Drosophila development and mutant flies die during embryogenesis, with defects in embryonic segmentation (22, 23). The presence of a single TCF/LEF gene in Drosophila eliminates genetic redundancy, allowing investigation of the key ancestral functions of this family of transcription factors.
The components of the Wnt cascade are highly conserved through evolution. Nineteen Wnt family members are present in vertebrates. Even though 7 homologous members have been identified in Drosophila, most of our knowledge about Wnt signaling in flies derives from genetic analysis of wingless (wg), the fly ortholog of Wnt-1. Wg controls multiple processes during Drosophila development, including neurogenesis in the embryo (24, 25), patterning of the embryonic epidermis (26, 27), cell-renewal of intestine stem cells (28–31), and specification, growth, and patterning of different appendages (32–39).
The wing imaginal disk of Drosophila is a sac-like epithelial structure found inside the larva that, after metamorphosis, will develop into the adult wing and thorax of the fly. It is formed by a well-polarized epithelium that proliferates extensively during larval development and has been used as a system to study normal tissue growth, as well as tumor formation and metastasis (40, 41). Furthermore, it represents an organ for which the function of Wg signaling has been thoroughly studied (42). Numerous observations have shown that this pathway controls wing growth (32, 38, 43, 44). Low levels of Wg pathway activity are sufficient to support normal wing growth in a Wg mutant background (32), and clones of cells unable to transduce Wg signaling are eliminated by apoptosis (43, 44). It has been proposed that high levels of Wg signaling promote proliferation at early stages of wing development, and this function is restricted to the periphery of the wing pouch at later stages (43). Thus, Wg growth regulatory functions seem to differ depending on the levels of activity, the developmental stage, and the position within the wing disk. Still, how Wg regulates tissue growth in the discs is complex and remains only partially understood.
Here, we present a functional study of the Wg pathway transcription factor, dTcf/Pan, in the wing imaginal disk of Drosophila. We show that dTcf/Pan knockdown results in tissue overgrowth in a region-specific manner. We show that dTcf/Pan, in addition to repressing normal tissue growth, limits tumorigenesis in a context of oncogene activation. Down-regulation of dTcf/Pan synergizes with the oncogenes YAP/Yorkie (Yki) and epidermal growth factor receptor (EGFR) in tumor formation. Remarkably, both tumor types show different characteristics and, while YAP/Yki-driven tumors grow as big hyperplastic masses of cells, tumors induced by EGFR undergo neoplastic transformation and show malignant features. We find that the transcription factors Ftz-f1 and Sox15, and the gene CG15784, are up-regulated as a consequence of dTcf/Pan depletion, and these elements are required for tumor formation in concert with YAP/Yki and EGFR.
Results
dTcf/Pan Controls Growth in the Wing Disk in a Region-Specific Manner.
We have used the wing imaginal disk of Drosophila as a model to interrogate the role of dTcf/Pan in a proliferative epithelium. The wing disk is subdivided into different regions along its proximal–distal axis: the most proximal part is the notum that will form the thorax of the adult fly; the wing pouch is located in the most distal part and will give rise to the adult wing blade; and the part connecting these 2 regions is the wing hinge, located in a medial position. Previous studies showed that clones of cells unable to transduce Wg signaling do not grow and are eliminated from the wing epithelium (35, 43, 44). In good agreement, dTcf/Pan mutant clones in the wing pouch down-regulate Wg target genes and grow poorly (45). Although the requirement for dTcf/Pan in the wing pouch has been established, the role that dTcf/Pan plays in other regions of the wing disk has not yet been determined.
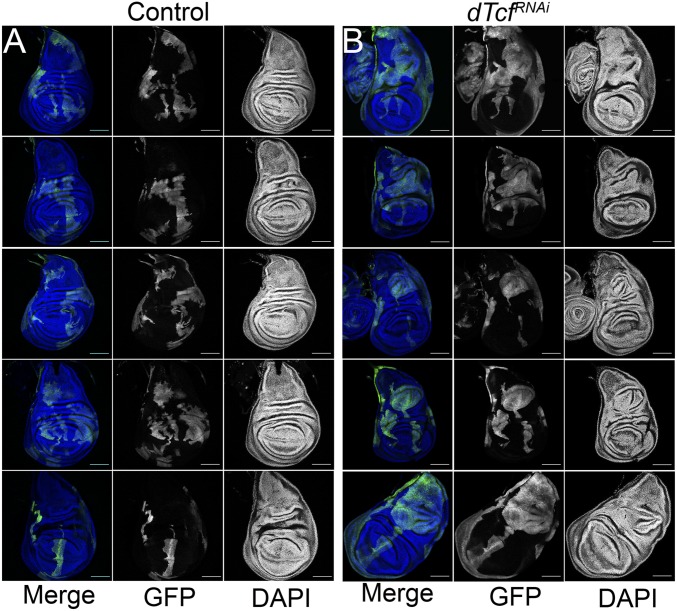
The flp-out technology allows for the generation of clones of cells labeled with a cellular marker (46). This technique can be combined with binary expression systems, such as Gal4-UAS (47), to manipulate gene activity specifically in those clones. To study the growth potential of cells with reduced dTcf/Pan in different parts of the wing disk, we generated heat shock-induced GFP-labeled clones expressing a dTcf/PanRNAi transgene. Consistent with previous observations (45), dTcf/PanRNAi-expressing clones were poorly recovered in the wing pouch (Fig. 1). This corroborates previous observations reporting that dTcf/Pan is required for normal growth in this region of the wing disk (45). Interestingly, clones of cells blind to Wg signaling are eliminated from the wing pouch by cell competition (48). Similarly, clones mutant for the zinc-finger (ZF) proteins elbow and no ocelli, which are controlled by Wg activity, are sorted out from the wing pouch (49). Taken together, these data suggest that cell competition and cell sorting might contribute to the recovery deficit detected in dTcf/PanRNAi clones. In contrast, dTcf/PanRNAi-expressing clones located out of the wing pouch grew bigger in size than control clones and led to the formation of epithelial folds resembling tissue hyperplasia (Fig. 1). This suggests that dTcf/Pan has a region-specific role in growth control: while it is required for normal growth in the wing pouch, it limits growth outside that region.
Fig. 1.
dTcf/PanRNAi-expressing clones in the wing disk. (A) Confocal micrographs of third-instar wing imaginal discs with GFP-labeled control clones. (B) Confocal micrographs of third-instar wing imaginal discs with GFP-labeled dTcf/PanRNAi-expressing clones (Scale bars, 100 μm).
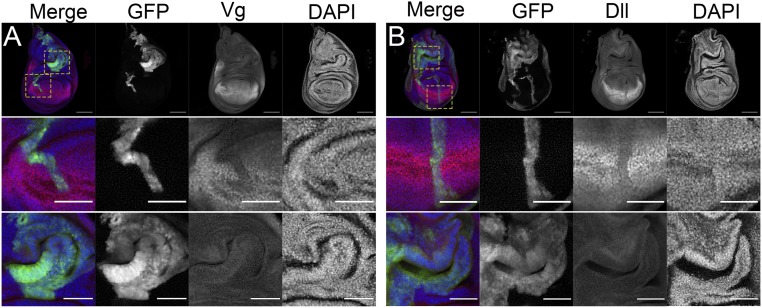
Wg signaling plays central roles in the specification of different regions of the wing disk (50) and Wg deregulation can lead to the formation of ectopic wings that create a new axis of tissue growth. Given that dTcf/Pan can antagonize Wg signaling in different developmental contexts (51, 52), we studied whether tissue hyperplasia in clones depleting dTcf/Pan resulted from the generation of ectopic wing tissue. To that end, we labeled discs containing dTcf/PanRNAi-expressing clones with antibodies against the wing specific-markers Vestigial (Vg) and Distalless (Dll). Vg and Dll are regulated by Wg signaling and control wing identity (53–55). As previously reported (45), clones of cells expressing dTcf/PanRNAi showed reduced expression of Vg and Dll in the wing pouch (Fig. 2; additional examples are provided in SI Appendix, Fig. S1). Remarkably, we did not observe ectopic Vg or Dll in clones present outside the presumptive wing region (Fig. 2; additional examples are provided in SI Appendix, Fig. S1). This suggests that tissue overgrowth in clones depleting dTcf/Pan outside the wing pouch is not a consequence of the generation of ectopic wing fields.
Fig. 2.
Wg-target genes in dTcf/PanRNAi-expressing clones. Confocal micrographs of third-instar wing imaginal discs with GFP-labeled dTcf/PanRNAi-expressing clones. Wg-target genes Vestigial (Vg) (A) and Distalless (Dll) (B) are shown, as indicated, in red and gray. GFP is shown in green and gray. Nuclei were labeled with DAPI, shown in blue and gray. The panels below show a magnification of the wing pouch and notum regions of the discs shown above. Note that clones in the wing pouch down-regulate Wg targets (Middle). Clones in the notum (Bottom) show characteristic folds of tissue hyperplasia but do not express ectopically neither Vg nor Dll. (Scale bars in the panels showing whole discs, 100 μm. Scale bars in the panels showing wing pouch and notum magnifications, 50 μm.)
dTcf/Pan Suppresses Oncogenic Growth in the Wing Epithelium.
Mutations in TCF/LEFs, as well as abnormal expression of TCF/LEF target genes, have been linked to cancer (8–11, 56). The identification of tissue hyperplasia associated to dTcf/PanRNAi-expressing clones in some regions of the wing discs suggests it might act as a tumor suppressor in those territories. We set out to determine the roles that dTcf/Pan plays in a context of tumor formation.
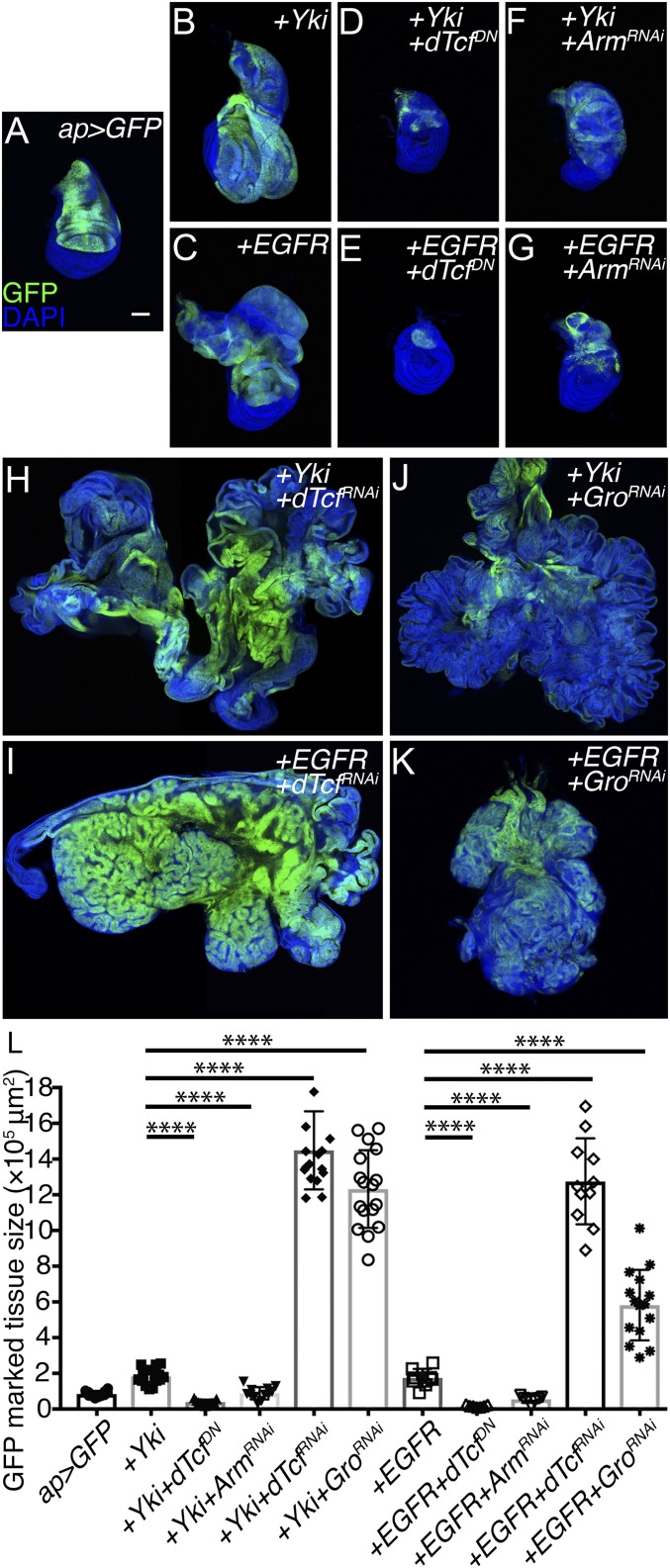
Oncogene activation in the wing disk typically results in the formation of benign tissue hyperplasia and, as observed in human cancers, oncogenes require additional cooperating factors to induce tumor formation (41). Up-regulation of EGFR or the nuclear effector of the Hippo pathway, YAP/Yki, induces the formation of tissue hyperplasia (Fig. 3 A–C and L). Previous studies have identified EGFR- and YAP/Yki-cooperating elements in the formation of neoplasia and metastasis (57–62). To determine the role of dTcf/Pan in tumor formation, we studied the consequences of manipulating dTcf/Pan in a context of oncogene up-regulation.
Fig. 3.
dTcf/Pan or TLE1/Gro depletion promotes oncogenic growth in the wing imaginal disk epithelium. (A–G) Confocal micrographs of third-instar wing imaginal discs of the following genotypes: ap-Gal4, UAS-GFP (A); ap-Gal4, UAS-GFP, UAS-Yki (B); ap-Gal4, UAS-GFP, UAS-EGFR (C); ap-Gal4, UAS-GFP, UAS-Yki, UAS-dTcf/PanDN (D); ap-Gal4, UAS-GFP, UAS-EGFR, UAS-dTcf/PanDN (E); ap-Gal4, UAS-GFP, UAS-Yki, UAS-ArmRNAi (F); and ap-Gal4, UAS-GFP, UAS-EGFR, UAS-ArmRNAi (G). (H–K) Confocal micrographs of tumorous disk of the following genotypes: ap-Gal4, UAS-GFP, UAS-Yki, UAS-dTcf/PanRNAi (H); ap-Gal4, UAS-GFP, UAS-EGFR, UAS-dTcf/PanRNAi (I); ap-Gal4, UAS-GFP, UAS-Yki, UAS-TLE/GroRNAi (J); and ap-Gal4, UAS-GFP, UAS-EGFR, UAS-TLE/GroRNAi (K). GFP is shown in green. Nuclei were labeled with DAPI (blue). All of the images were taken under the same magnification (Scale bar, 100 μm for all of the confocal micrographs). (L) Tissue-size quantification of the GFP marked area (Unpaired t test, n = 12, 15, 11, 12, 17, 17, 9, 7, 7, 11, and 15 from left to right, ****P < 0.0001).
dTcf/Pan lacking the N-terminal β-catenin/Armadillo (Arm)-interacting domain behaves as dominant-negative (dTcf/PanDN), and can be used to antagonize Wg signaling in vivo (22). Suppression of Wg signaling by expression of dTcf/PanDN or β-catenin/ArmRNAi repressed oncogene-induced tissue overgrowth in a context of YAP/Yki, and EGFR overexpression (Fig. 3 D–G and L). This indicates that Wg signaling, in addition to being required for normal growth, is required for oncogenic growth. Interestingly, expression of dTcf/PanRNAi in discs with elevated YAP/Yki or EGFR resulted in formation of massive tumors (Fig. 3 H, I, and L). Independent UAS-dTcf/PanRNAi lines led to comparable results (SI Appendix, Fig. S2). During canonical Wg signaling, the activity of nuclear β-catenin/Arm is largely mediated by its interaction with dTcf/Pan (22, 23, 63). The opposite outcomes observed in discs depleting dTcf/Pan or β-catenin/Arm suggested that dTcf/Pan might have β-catenin/Arm-independent functions in this process. Interestingly, knockdown of TLE/Gro (the dTcf/Pan transcriptional corepressor) in a context of elevated YAP/Yki or EGFR led to the formation of tumors (Fig. 3 J–L), as observed when dTcf/Pan was depleted. Altogether, these observations suggest that dTcf/Pan, independently of β-catenin/Arm, suppress oncogenic growth in the wing disk of Drosophila.
The appearance of tumors induced by TLE/Gro depletion was different to the one observed in tumors driven by dTcf/Pan knockdown. These differences might be explained by the fact that TLE/Gro can also partner and control the activity of other transcription factors mediating different signaling mechanisms in normal development and disease (64, 65).
Depletion of dTcf/Pan in Discs Expressing YAP/Yki or EGFR Leads to Tumors with Specific Characteristics.
Tumors driven by dTcf/Pan depletion in the context of EGFR or YAP/Yki up-regulation grew massively. However, those tumors presented different appearances, suggesting that they might be organized in a different manner. We used different markers to examine how these tumors were constituted.
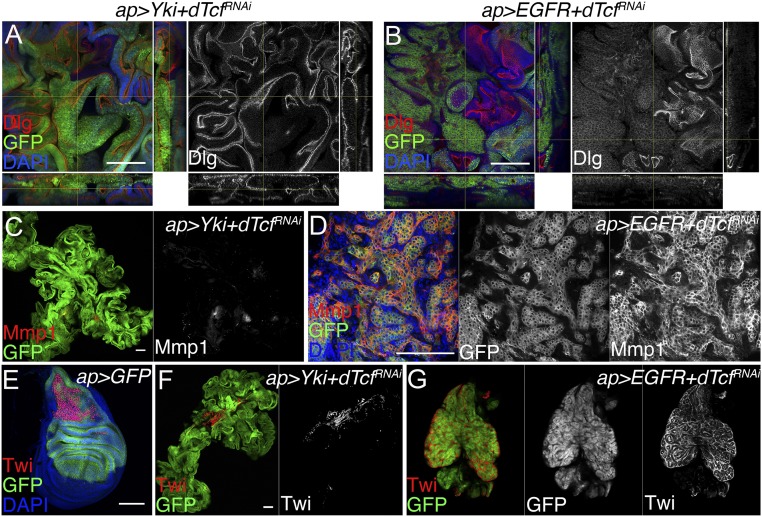
A central feature of malignancy in carcinomas is a pronounced disorganization of epithelial polarity (66). Expression of YAP/Yki or EGFR in the wing epithelium induces tissue overgrowth yet it maintains normal epithelial organization (59, 62). As revealed by the polarity marker Discs large (Dlg), epithelial polarity was not affected in tumors coexpressing YAP/Yki and dTcf/PanRNAi and grew as hyperplastic tumors (Fig. 4A). In contrast, EGFR-driven tumors depleting dTcf/Pan showed defects in polarity organization (Fig. 4B; additional examples are shown in SI Appendix, Fig. S3). This indicates that down-regulation of dTcf/Pan drives the formation of hyperplastic tumors in a context of YAP/Yki up-regulation, but it induces neoplastic transformation in EGFR-driven tumors.
Fig. 4.
Reduced dTcf/Pan promotes the formation of tumors with different characteristics in a context-dependent manner. (A and B) Confocal micrographs showing the polarity marker Discs large (Dlg, red and gray) in tumorous discs of the following genotypes: ap-Gal4, UAS-GFP, UAS-Yki, UAS-dTcf/PanRNAi (A); and ap-Gal4, UAS-GFP, UAS-EGFR, UAS-dTcf/PanRNAi (B). XZ and YZ cross-sections are shown in the bottom and right-hand side, respectively. (C and D) Confocal micrographs showing matrix metalloprotease 1 (Mmp1, red and gray) in tumorous discs of the following genotypes: ap-Gal4, UAS-GFP, UAS-Yki, UAS-dTcf/PanRNAi (C); and ap-Gal4, UAS-GFP, UAS-EGFR, UAS-dTcf/PanRNAi (D). (E) Confocal micrograph of an ap-Gal4, UAS-GFP third-instar wing imaginal disk showing the expression of Twist (Twi, red). (F and G) Confocal micrographs showing the Twist protein (red and gray) in tumorous discs of the following genotypes: ap-Gal4, UAS-GFP, UAS-Yki, UAS-dTcf/PanRNAi (F); and ap-Gal4, UAS-GFP, UAS-EGFR, UAS-dTcf/PanRNAi (G). GFP is shown in green, and green and gray in G. DAPI labels the nuclei and is shown in blue (Scale bars: 100 μm).
Neoplastic fly tumors express the secreted protein Matrix metalloproteinase 1 (Mmp1). Mmp1 degrades the basement membrane, allowing tumor cells to migrate and invade (67, 68). While tumors expressing YAP/Yki and dTcf/PanRNAi expressed low levels of Mmp1 (Fig. 4C), tumors expressing EGFR and dTcf/PanRNAi showed a robust induction in Mmp1 expression (Fig. 4D). These observations are consistent with the defects observed in cellular polarity and suggest that, while the YAP/Yki + dTcf/PanRNAi tumors grow as hyperplastic tumors, dTcf/Pan knockdown in discs expressing EGFR drives cellular transformation and neoplasia.
A population of adepithelial cells of mesenchymal origin can be observed attached to the most dorsal part of normal Drosophila wing imaginal discs. Antibodies against the protein Twist (Twi) (Fig. 4E) and Cut can be used to label specifically those cells. In a previous work, we found that depletion of the epigenetic modulator pipsqueak in disk-expressing EGFR caused the formation of neoplastic tumors composed of a mix of transformed epithelial and normal mesenchymal cells (60). We used antibodies against the adepithelial markers Twi and Cut to label the tumors depleting dTcf/Pan. Tumors expressing YAP/Yki and dTcf/PanRNAi were mostly composed of epithelial cells and contained a small group of mesenchymal cells resembling the normal adepithelial population (Fig. 4F). In contrast, tumors expressing EGFR and dTcf/PanRNAi consisted of a mix of epithelial and mesenchymal cells (Fig. 4G and SI Appendix, Fig. S4) resembling EGFR + pipsqueakRNAi neoplastic tumors (60).
Next, we used antibodies labeling different regions in the wing disk to examine the organization of those tumors. Tumors expressing YAP/Yki and depleting dTcf/Pan were mostly composed by cells positive for the hinge marker Teashirt (Tsh), suggesting that those tumors originated largely from the hinge region of the disk (SI Appendix, Fig. S5). In some cases, we observed the presence of ectopic wing tissue labeled by the wing marker Nubbin (Nub) (SI Appendix, Fig. S5), suggesting that wing duplication could partially contribute to the formation of these tumors. Other tumorous discs showed bigger wing territories but the presence of ectopic wings was not detected. The expression of those markers showed a different appearance in EGFR-driven tumors. Although ectopic Nub was observed in some cases, other tumors did not present major ectopic wings that could account for the formation of those neoplasms (SI Appendix, Fig. S5). The expression of Tsh was restricted to specific parts of the disk and, in contrast to YAP/Yki + dTcf/PanRNAi tumors, the main tumor mass was negative for wing and hinge markers (SI Appendix, Fig. S5). This, together with the observation that those tumors were composed by a mix of epithelial and adepithelial cells (normally present in the notum), suggest they derived from the notum region of the disk. Together, these results indicate that the formation of tumors induced by dTcf/Pan depletion cannot be explained solely by the formation of ectopic axis of wing growth.
In sum, these observations show that tumors knocking down dTcf/Pan present different characteristics in the context of YAP/Yki or EGFR overexpression. YAP/Yki-driven tumors grew as big and homogeneous masses of well-polarized epithelial cells. The majority of these cells were positive for the hinge marker Tsh. In contrast to that, EGFR-driven tumors showed polarity defects, expressed the malignant marker Mmp1, and were mainly composed by a mixture of transformed notum epithelial and mesenchymal-normal cells.
A Comparative Microarray Analysis Identified dTcf/Pan-Dependent Changes in Gene Expression in YAP/Yki-Driven Tumors.
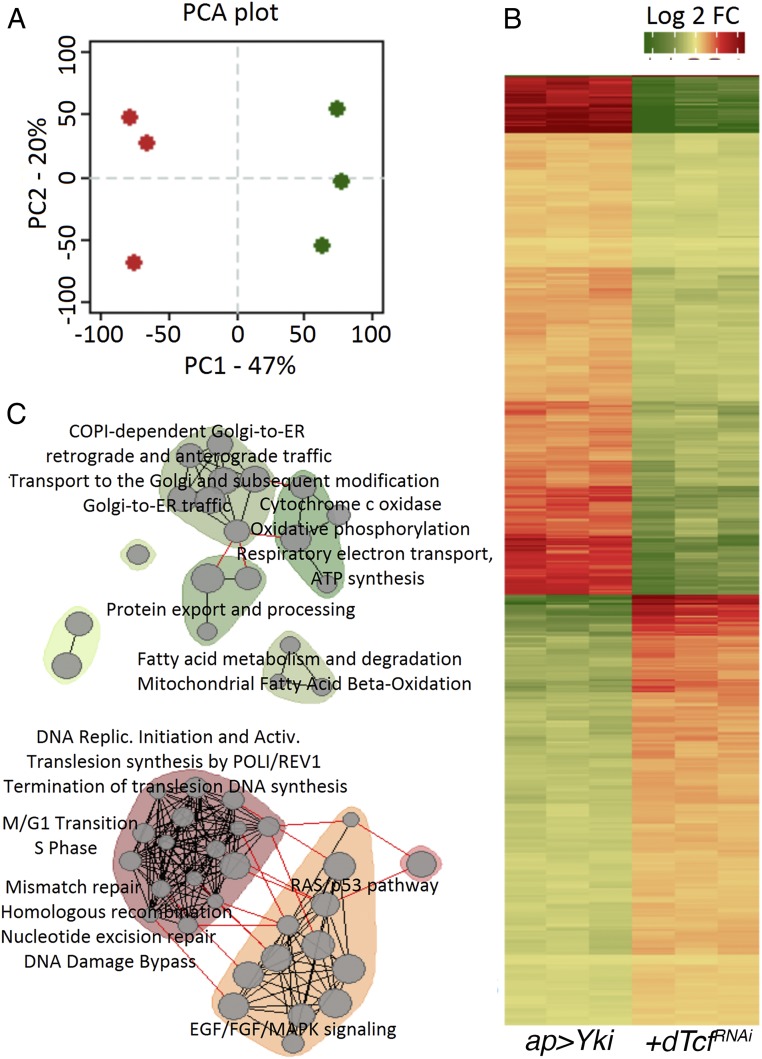
dTcf/Pan is a transcription factor and its depletion should affect the gene-expression pattern of tumor cells. We used a comparative microarray analysis to identify genes controlled by dTcf/Pan during tumorigenesis. Tumors expressing YAP/Yki and dTcf/PanRNAi were quite homogeneous and were mostly composed of epithelial cells. Tumors expressing EGFR and dTcf/PanRNAi were heterogeneous, including abundant mesenchymal cells that did not express dTcf/PanRNAi. The enrichment in those mesenchymal cells might have a profound impact on the expression profile of those tumors and might hinder the identification of genes regulated by dTcf/Pan. To bypass that issue, we decided to perform the microarray analysis in the more homogeneous YAP/Yki-expression background. Principal component analysis (PCA) revealed that the expression profiles were different between discs expressing YAP/Yki-with and without the dTcf/PanRNAi transgene (Fig. 5A).
Fig. 5.
Gene-expression analysis of YAP/Yki- versus YAP/Yki + dTcf/PanRNAi-expressing discs. (A) Loading plot of PCA. x axis, first component (accounts for 47% of variation); y axis second component (acquaints for 20% variation). Green corresponds to biological triplicates of YAP/Yki-expressing samples. Red, biological triplicates of YAP/Yki + dTcf/PanRNAi-expressing samples. (B) Relative expression heatmap of the subset of genes that that separates treatment groups according to first component. It shows relative expression centered to median of all samples. Color legend: green, lower expression; red, higher expression. (C) Enrichment map of pathway and gene set enrichment analysis. (Upper, in green) Results for genes that were down-regulated in YAP/Yki + dTcf/PanRNAi samples. (Lower, in red) Results for genes that were up-regulated in YAP/Yki + dTcf/PanRNAi samples. Gray circles represent individual terms from Reactome, Kegg, or Panther analysis. Gray lines represent similarity between terms.
We identified the relevant genes that accounted for this separation and plotted them as a heatmap. Two distinct clusters were formed. One cluster consisted of 337 genes that were mostly down-regulated in YAP/Yki + dTcf/PanRNAi tumors (Fig. 5B and Dataset S1). Pathway analysis in this group identified the presence of genes involved in oxidative phosphorylation, mitochondrial β-oxidation, protein export, and transport between the Golgi apparatus and endoplasmic reticulum (Fig. 5 C, Upper, and Dataset S1). Another cluster consisted of 255 genes that were mostly up-regulated in YAP/Yki + dTcf/PanRNAi tumors (Fig. 5B and Dataset S1). This group should include genes inhibited by the dTcf/Pan-TLE/Gro repressor complex. Genes involved in the control of the cell cycle, DNA repair, and translation were present in this set of genes. Genes involved in cellular signaling (p53/Ras; EGFR/FGF/MAPK) were also found in this group (Fig. 5 C, Lower, and Dataset S1).
Next, we narrowed our focus to genes that were strongly up-regulated in YAP/Yki + dTcf/PanRNAi tumors (SI Appendix, Fig. S6). Twenty-three protein-coding genes were up-regulated more than 2-fold [fold-change (FC) ≥ 2, adjusted P ≤ 0.05] in the YAP/Yki + dTcf/PanRNAi tumors compared with the controls. The top 15 most up-regulated genes are shown as boxplots (SI Appendix, Fig. S6 and Dataset S1); 130 protein-coding genes were down-regulated more than 2-fold (FC ≥ 2, P ≤ 0.05) (Dataset S1).
Multiple Factors Controlled by dTcf/Pan Contribute to Tumor Formation.
To identify the combination of genes controlled by dTcf/Pan involved in tumorigenesis, we focused on the 23 genes up-regulated (FC ≥ 2, P ≤ 0.05) in the microarray analysis (Dataset S1). We used UAS-RNAi transgenic flies to deplete those genes, one by one, in the YAP/Yki + dTcf/PanRNAi background and check if tumors were formed. The use of additional UAS lines in the ap-Gal4, UAS-Yki, dTcf/PanRNAi, UAS-GFP background might potentially titrate the molecules of Gal4 reducing the levels of transgene expression and affecting tumor formation. To control the potential effect that an extra UAS line might have in the YAP/Yki + dTcf/PanRNAi tumors, we added a neutral UAS transgene (UAS-mCherry) to that genetic background. We did not observe a significant size difference between tumors expressing UAS-mCherry (ap-Gal4, UAS-Yki, UAS-dTcf/PanRNAi, UAS-GFP, UAS-mCherry) and tumors without UAS-mCherry (ap-Gal4, UAS-Yki, UAS-dTcf/PanRNAi, UAS-GFP) (SI Appendix, Fig. S7). This shows that the addition of an extra UAS transgene does not affect tumor development.
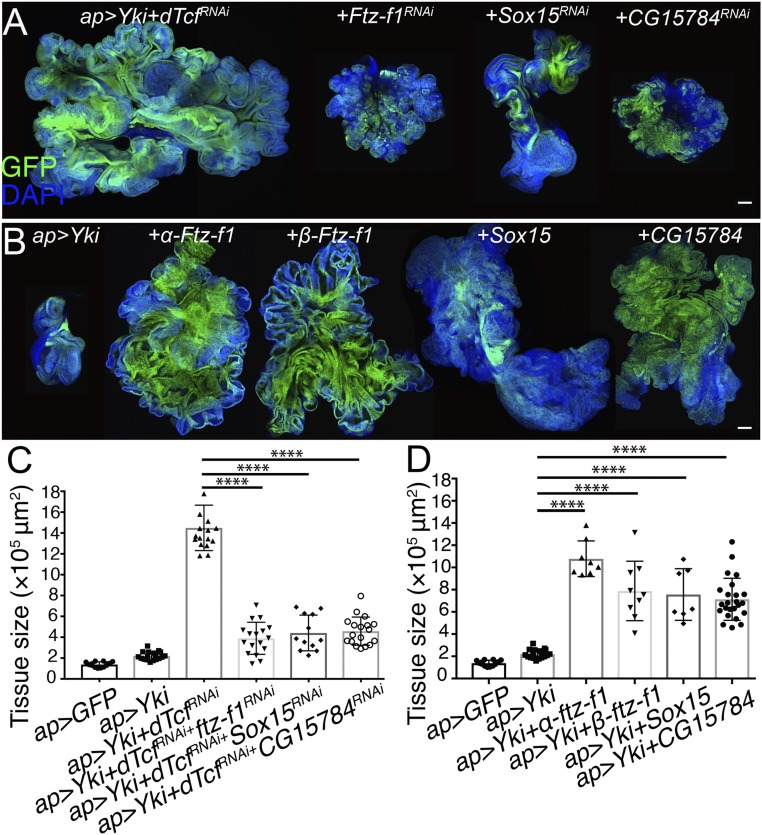
Among the genes tested, depletion of Sox15, Ftz-f1, and CG15784, significantly reduced the size of the YAP/Yki + dTcf/PanRNAi tumors (Fig. 6 A and C and Dataset S1). Notably, those were among the top up-regulated genes in a context of dTcf/Pan knockdown (SI Appendix, Fig. S6). Sox15 belongs to the family of HMG Sox transcription factors. It has been assigned to the SoxF group of this family and is the sole member of the group in Drosophila. In mammals, the SoxF group is composed Sox7, Sox17, and Sox18 (69). In Drosophila, Sox15 is the transcription factor showing the highest levels of expression in the hinge region of the wing imaginal disk (70). The nuclear receptor Ftz-f1 acts downstream of the JNK pathway and is required for neoplastic tumor development in Drosophila (71, 72). CG15784 is a protein-coding gene with unknown function (http://flybase.org/reports/FBgn0029766).
Fig. 6.
Sox15, Ftz-f1, and CG15784 contribute to YAP/Yki + dTcf/PanRNAi tumor formation. (A) Confocal micrographs of ap-Gal4, UAS-GFP, UAS-Yki, UAS-dTcf/PanRNAi tumorous disk (Left), expressing of Ftz-f1RNAi, Sox15RNAi, CG15784RNAi as indicated in the figure (panels on the Right). (B) Confocal micrographs of ap-Gal4, UAS-GFP, UAS-Yki wing imaginal disk (Left) expressing α-Ftz-f1, β-Ftz-f1, Sox15, CG15784 as indicated in the figure (panels on the Right). GFP is shown in green. Nuclei are labeled with DAPI (blue). All of the images were taken under the same magnification (Scale bars, 100 μm). (C) Tissue-size quantification of wing imaginal discs in A (Unpaired t test, n = 12, 18, 17, 17, 12, 18 from left to right, ****P < 0.0001). (D) Tissue-size quantification of wing imaginal discs in B (Unpaired t test, n = 12, 18, 8, 9, 7, 24 from left to right, ****P < 0.0001).
We set out to analyze whether up-regulation of each of those genes was sufficient to drive tumorigenesis in combination with UAS-Yki. To that end, we coexpressed UAS-Yki with UAS transgenes driving the expression of each of those candidate genes. Notably, up-regulation of Ftz-f1, Sox15, or CG15784, was sufficient to trigger tumorigenesis when expressed together with YAP/Yki (Fig. 6 B and D).
Next, we used quantitative PCR to study whether the dTcf/Pan-TLE/Gro repressor complex controlled the expression of these genes in normal discs. We compared mRNA levels of Ftz-f1, Sox15, and CG1578 in normal discs versus discs expressing dTcf/PanRNAi or TLE/GroRNAi. Ftz-f1 has 2 alternatively spliced forms, α-Ftz-f1 or β-Ftz-f1, that are differentially expressed in a stage-dependent manner (73). Both mRNAs, α-Ftz-f1 and β-Ftz-f1, as well as Sox15 and CG15784, were up-regulated in discs depleting dTcf/Pan or TLE/Gro (SI Appendix, Fig. S8). This indicates that the dTcf/Pan-TLE/Gro repressor complex modulates the expression of those genes in normal development as well as during tumor formation.
We studied the consequences of reducing each of those genes in otherwise normal discs. Expression of CG15784RNAi in normal wing discs strongly reduced tissue size. Cells expressing CG15784RNAi presented high levels of apoptosis, indicating that this gene might be normally required for cell survival (SI Appendix, Fig. S9), which might explain the effect of depleting CG15784 on tumor growth. Remarkably, knocking down Ftz-f1 or Sox15 did not have an overt impact in normal tissue growth (SI Appendix, Fig. S9). Although discs expressing Sox15RNAi were slightly smaller than normal discs, the magnitude of that effect was not comparable to the suppression of tumor growth observed in the YAP/Yki + dTcf/PanRNAi discs expressing Sox15RNAi (compare the quantification in Fig. 6C with SI Appendix, Fig. S9). We finally asked whether up-regulation of Sox15, Ftz-f1, or CG15784 can drive tissue overgrowth in otherwise normal discs. CG15784 overexpression did not alter the size of the wing disk (SI Appendix, Fig. S10). To our surprise, overexpression of Sox15 on its own resulted in smaller discs, indicating that an increase in Sox15 is not sufficient to enhance tissue growth (SI Appendix, Fig. S10). Thus, the synergy between Sox15 and YAP/Yki in tumorigenesis cannot be explained and has an additive force in tissue growth. In contrast, overexpression of α-Ftz-f1 or β-Ftz-f1 resulted in bigger discs (SI Appendix, Fig. S10), which might explain the synergy with YAP/Yki in tumor growth.
Genes Contributing to the Formation of EGFR-Driven Tumors in Discs Depleting dTcf/Pan.
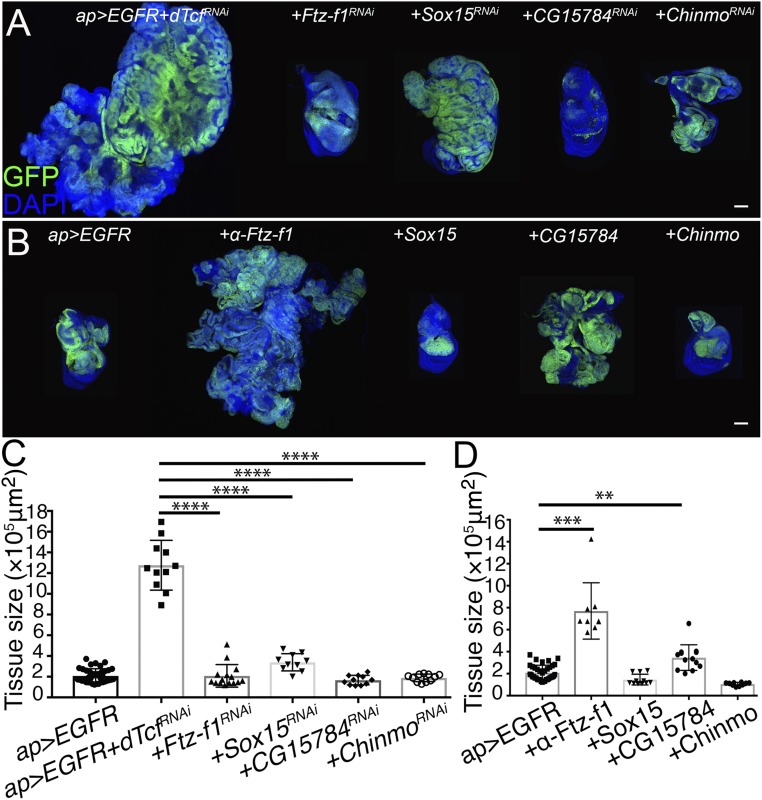
Next, we asked whether any of the 23 genes up-regulated in the YAP/Yki + dTcf/PanRNAi tumors contributed to tumor formation when dTcf/Pan was depleted in discs expressing EGFR. As observed in the tumors expressing YAP/Yki and dTcf/PanRNAi, expression of Ftz-f1RNAi, Sox15RNAi, and CG15784RNAi suppressed the formation of EGFR + dTcf/PanRNAi tumors (Fig. 7 A and C). In addition to those genes, depletion of chronologically inappropriate morphogenesis (Chinmo) suppressed the formation of the EGFR + dTcf/PanRNAi tumors (Fig. 7 A and C). Chinmo, one of the top up-regulated genes in the YAP/Yki + dTcf/PanRNAi tumors (SI Appendix, Fig. S6), encodes a BTB-ZF transcription factor that has been previously involved in the formation of epithelial and brain tumors in Drosophila (74–76). Interestingly, Chinmo appeared to be an oncogenic factor specifically in the EGFR-driven tumors because Chinmo depletion did not affect tumor formation in a YAP/Yki + dTcf/PanRNAi background (SI Appendix, Table S2). This BTB-ZF might elicit protumorigenic functions in a context-dependent manner. Next, we asked if up-regulation of Ftz-f1, Sox15, CG15784, or Chinmo, was sufficient to induce tumor formation in cooperation with EGFR. Surprisingly, among those, Ftz-f1 was the only gene that was sufficient to enhance the tumor-inducing potential of EGFR (Fig. 7 B and D).
Fig. 7.
Ftz-f1, Sox15, CG15784, and Chinmo contribute to EGFR + dTcf/PanRNAi tumor formation. (A) Confocal micrographs of ap-Gal4, UAS-GFP, UAS-EGFR, UAS-dTcf/PanRNAi tumorous disk (Left), expressing of Ftz-f1RNAi, Sox15RNAi, CG15784RNAi, and ChinmoRNAi as indicated in the figure (panels on the Right). (B) Confocal micrographs of ap-Gal4, UAS-GFP, UAS-EGFR wing imaginal disk (Left) expressing α-Ftz-f1, β-Ftz-f1, Sox15, CG15784, and Chinmo as indicated in the figure (panels on the Right). GFP is shown in green. Nuclei are labeled with DAPI (blue). All of the images were taken under the same magnification (Scale bars, 100 μm). (C) Tissue-size quantification of wing imaginal discs in A (Unpaired t test, n = 37, 11, 15, 10, 11, 11 from left to right, ****P < 0.0001). (D) Tissue-size quantification of wing imaginal discs in B (Unpaired t test, n = 37, 9, 11, 13, 10 from left to right, ***P < 0.001, **P < 0.01).
Discussion
TCF/LEF transcription factors regulate gene expression in response to Wnt signals. The 4 TCF/LEFs present in vertebrates (TCF1, LEF1, TCF3, and TCF4) show complex patterns of gene expression and different degrees of redundancy (19–21). All this generates a level of complexity that complicates the study of those transcription factors. Here, we study the functions of the sole TCF/LEF member in Drosophila, dTcf/Pan (22, 23), in a simple model organ, the wing imaginal disk of the fly. We describe a role of dTcf/Pan in limiting tissue growth during normal development and show that dTcf/Pan suppresses tumor formation in contexts of oncogene activation.
dTcf/Pan is the final effector in the Wg signaling cascade and, depending on the activity of the pathway, it can act as a transcriptional activator or repressor. In the presence of Wg, β-catenin/Arm interacts with dTcf/Pan to form a transcriptional activator complex that induces the expression of Wg target genes. Wg pathway is crucial for wing disk growth (reviewed in ref. 77) and discs depleting β-catenin/Arm are reduced in size. dTcf/Pan mutant clones show growth defects in the wing pouch of the wing disk (45). However, RNAi-mediated dTcf/Pan knockdown leads to tissue overgrowth in other regions of the wing pouch, such as the wing notum. These observations suggest that dTcf/Pan plays different growth regulatory roles in a tissue-specific manner: while the dTcf/Pan is required for normal growth in the wing pouch, it limits growth in the notum.
It has been well established that Wnt signaling has a crucial role in the growth and survival of different cancer cells. However, the functions that TCF/LEF play in cancer remain controversial and, in some cases, different studies have reached contradictory conclusions. For example, TCF4 has been shown to act as an oncogene and as a tumor suppressor in intestine and colon cancer cells (8, 11, 78). Although the roles that Wg plays in Drosophila epithelial tumors have been extensively studied (60, 79–81), the specific functions of dTcf/Pan in that context have not yet been determined. In this study, we present in vivo evidence showing that the dTcf/Pan-TLE/Gro repressor complex suppresses tumor formation in different contexts of oncogene activation.
In a previous study, we identified members of the SWI/SNF Brahma-associated protein (BAP) complex as elements that, when down-regulated, cooperate with the oncogene YAP/Yki in tumorigenesis and neoplasia (62). Remarkably, SWI/SNF proteins have been previously connected to Wnt signaling. Brg-1, a component of the SWI/SNF complex, interacts with β-catenin facilitating TCF/LEF-dependent transcriptional activation in colon carcinoma cell lines (82). In contrast to that, BAP complex limits the expression of Wg target genes in Drosophila (83). Here, we show that depletion of dTcf/Pan results in tissue hyperplasia in the notum of the wing disk and tumor formation in a context of oncogene activation. These effects are not associated with ectopic expression of Wg target genes. Furthermore, while dTcf/Pan cooperates with YAP/Yki and EGFR in tumorigenesis, BAP depletion drives neoplasia specifically in a YAP/Yki context and is not a EGFR-cooperating partner (62). Together, those results suggest that the molecular mechanisms governing the formation of tumors induced by BAP depletion and the tumors driven by dTcf/Pan knockdown might be different.
We combined the use of comparative microarray analysis and functional studies (RNAi-mediated gene knockdown), to identify the molecular elements involved in tumor formation as a consequence of dTcf/Pan depletion. We identified 3 transcription factors: Sox15, Ftz-f1, and Chinmo, and a gene with unknown function, CG15784, as elements controlled by dTcf/Pan and involved in tumorigenesis.
We showed that the nuclear receptor Ftz-f1 can promote normal and oncogenic growth in the wing epithelium, and is required for the formation of the YAP/Yki + dTcf/PanRNAi and EGFR + dTcf/PanRNAi tumors. Importantly, among the genes identified in this study, Ftz-f1 is the only element sufficient to enhance tumor growth in a context of YAP/Yki or EGFR up-regulation. The ability to induce growth and tumorigenesis is not unique to the wing epithelium and Ftz-f1 can also induce growth in the eye imaginal disk of the fly (84), and is up-regulated and required for neoplasia in Drosophila RasV12scrib− tumors induced in the eye epithelium (71, 72). In sum, the results presented here together with previous observations support the role of Ftz-f1 as an oncogene in Drosophila. Interestingly, the β-catenin–TCF3 activator complex stimulates the expression of the Ftz-f1 human ortholog, the liver receptor homolog 1 (LRH-1), to induce the expression of pluripotency genes in embryonic stem cells (85). This suggests that the regulation of Ftz-f1 by dTcf/Pan might be conserved.
Similar to Ftz-f1, Sox15 is controlled by dTcf/Pan and is required for tumor growth in the YAP/Yki + dTcf/PanRNAi and EGFR + dTcf/PanRNAi contexts. Sox15 belongs to the Sox family of sequence-specific HMG group transcription factors. Based on protein sequence comparisons, this family is divided in groups A–G in mammals, and Sox15 is the sole member of the SoxF subgroup in Drosophila. Sox genes have complex expression patterns and play essential and widespread roles during development and tumorigenesis (86–89). These proteins have been shown to regulate Wnt signaling by different mechanisms, including interaction with β-catenin and TCF/LEF transcription factors (90, 91). Sox15 is specifically expressed in the hinge region of the wing disk (70). In that region, Wg and Sox15 are embedded in a negative feedback loop that controls proliferation. Wg controls positively Sox15 expression. Sox15, in turn, represses Wg transcription and, by so doing, buffers cell proliferation and tissue growth (92). Consistently, we show here that discs with reduced dTcf/Pan or TLE/Gro up-regulate Sox15 and its overexpression potentiates the growth-promoting capacity of the oncogene YAP/Yki. Interestingly, Sox proteins can enhance YAP activity by direct repression of 2 positive regulators of the Hippo tumor suppressor pathway, Nf2 (Merlin) and WWC1 (Kibra). By regulating YAP, Sox2 maintains cancer stem cells in osteosarcomas (93).
A recent study in human HEK 293T cells lacking all 4 TCF/LEF proteins revealed the existence of a transcriptional activity of β-catenin in the absence of TCF/LE, referred to as a β-catenin–GHOST response. This transcriptional program is mediated by the interaction of β-catenin with other transcription factors, such as FOXO proteins (94). We speculate that, in a context of dTcf/Pan depletion in the wing disk, β-catenin/Arm might crosstalk with different transcription factors and regulate a different set of genes. This β-catenin–GHOST-like response might affect tissue growth and tumor formation.
Interestingly, the Ftz-f1 mammalian orthologs, LRH-1 and Steroidogenic factor 1 (SF-1) (95), have been associated with different types of human cancer (96–99). Remarkably, LRH-1 interacts with the β-catenin–TCF4 activator complex to control intestinal cell proliferation and intestinal tumorigenesis by inducing G1/S progression (98, 100, 101). Importantly, β-catenin can physically interact with the SoxF Sox17 in a mechanism analogous to the β-catenin–TCF/LEF, and this interaction potentiates the transcriptional activation of Sox17 in the regulation of endoderm development in vertebrate gastrulation (102). It would be of interest to analyze whether β-catenin/Arm can interact physically with Ftz-f1 and/or Sox15 in growth control and tumor formation in Drosophila.
Materials and Methods
Drosophila Genetics.
Transgenic fly stocks used: UAS-dTcf/PanRNAi (VDRC-3014, BDSC-40848 and 26743), UAS-dTcf/PanDN (BDSC-4784), UAS-β-catenin/ArmRNAi (VDRC-7767), UAS-GroRNAi (VDRC-6315 and 6316), UAS-Ftz-f1RNAi (VDRC-2959), UAS-Sox15RNAi (VDRC-45482), UAS-CG15784RNAi (VDRC-29599), UAS-ChinmoRNAi (BDSC-31738), UAS-α-Ftz-f1 (BDSC-64422), UAS-β-Ftz-f1 (BDSC-64289), UAS-Sox15 (FlyORF-F001698), UAS-Chinmo (BDSC-44388), ap-Gal4 (103), UAS-EGFR (104), UAS-Yki (105), UAS-mCD8:GFP (BDSC-5137), and P{XP}CG15784d07076 (BDSC-19260). Other stocks used are listed in SI Appendix, Table S2. Detailed genotypes for each figure are listed in SI Appendix, Table S1.
Clone Analysis.
UAS-dTcf/PanRNAi female flies (experimental condition) or w1118 female flies (control condition) were crossed with hs-FLP/Y; act > FRT-stop-FRT >Gal4, UAS-GFP/ST male flies at 25 °C. Flies were allowed to lay egg for 24 h at 25 °C. Second-instar larvae were induced in 37 °C water bath for 15 min. Larvae were raised at 25 °C and wandering third instar larvae were dissected for analysis.
Induction of Transgene Expression.
Crosses were set up at room temperature and flies were flipped into new vials after laying eggs for 1–2 d. The vials were kept at 18 °C for 4 d to allow the eggs to develop to the early third-instar larval stage and transferred to 29 °C to induce transgene expression.
Immunostaining and Imaging.
Primary antibodies used: rat anti-Dll (1:200) (106), rabbit anti-Vg (1:150, a gift from Sean Carroll, University of Wisconsin-Madison, Madison, WI), mouse anti-Dlg (1:200, DSHB, 4F3), mouse anti-Mmp1 (1:10, DSHB, 3A6B4/5H7B11/3B8D12 were mixed in equal amounts), rabbit anti-Twi (1:100, provided by Maria Leptin, European Molecular Biology Laboratory (EMBL), Heidelberg, Germany), mouse anti-Cut (1:100, DSHB, 2B10), and rabbit anti-cleaved Caspase-3 (1:500, Cell Signaling, 9661S). Alexa Fluor 555 secondary antibodies from Thermo Fisher Scientific were used. Immunostaining and imaging were performed as described in ref. 62.
Tissue-Size Quantification and Statistics.
Tissue-size measures were performed with Fiji software from the raw confocal images. Statistics were performed with GraphPad Prism and Excel software, statistical significance was determined by unpaired Student’s t test.
Total RNA Isolation, Whole-Transcript Expression Analysis.
Total RNA of the wing disk tissue was isolated using TRIzol reagent. Three biological replicates were prepared for each genotype. Whole-transcript expression analysis was performed by the Genomics Core Facility at the European Molecular Biology Laboratory (Heidelberg, Germany) using Affymetrix Drosophila Gene 1.1 ST Array. The microarray data have been deposited in the Gene Expression Omnibus (GEO) database with accession no. GSE120607. Detailed information can be found in SI Appendix, Supplementary Materials and Methods.
Pathway and Gene Set Enrichment Analysis.
Genes that contributed to first PCA component were split into 2 groups—up-regulated and down-regulated—and gene set enrichment analysis was performed for these 2 groups. All genes not eliminated by filtering were used to define the “gene universe” for pathway enrichment analysis. Detailed information is in SI Appendix, Supplementary Materials and Methods.
Real-Time Quantitative PCR.
Total RNA was pretreated with RQ1 DNase (Promega) and reverse transcription was performed with SuperScript III Reverse Transcriptase (Invitrogen). Real-time quantitative PCR was performed with HOT FIREPol EvaGreen reagent (Solis BioDyne) on QuantStudio 6 Flex Real-Time PCR System (ABI). Results were normalized to ribosomal protein 49 (rp49). Primers used were: rp49 (L: GGATCGATATGCTAAGCTGT; R: GATGTTGGGCATCAGATACT); Sox15 (L: CCGGTGGAGTGACTGTACT; R: CGAACATCCACATCATCTGCT); α-ftz-f1 (L: CACAGTGGCTCCAATCTCAG; R: GTCCTGGTAGCCTCCGTTAT); β-ftz-f1 (L: CGAGTAGGACGAGGAGTTGA; R: GCAGCAGCAAGAACACTACC); and CG15784 (L: CATCACTTTGGTCCACATCA; R: GTCCTTCACCGGATTCTCTT).
Supplementary Material
Acknowledgments
We thank the European Molecular Biology Laboratory genecore for performing the whole-transcript expression analysis; the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center, and the FlyORF for fly strains; and Maria Leptin, Sean Carroll, and the Developmental Studies Hybridoma Bank for antibodies. This work was supported by the Danish Council for Strategic Research grant DISC-B, Novo Nordisk Foundation Grant NNF12OC0000552, a grant from the Neye Foundation, and a grant from the Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis Legat.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE120607).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816981116/-/DCSupplemental.
References
- 1.Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Logan C. Y., Nusse R., The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 (2004). [DOI] [PubMed] [Google Scholar]
- 3.MacDonald B. T., Tamai K., He X., Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polakis P., The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 17, 45–51 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Reya T., Clevers H., Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Clevers H., Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Zhan T., Rindtorff N., Boutros M., Wnt signaling in cancer. Oncogene 36, 1461–1473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angus-Hill M. L., Elbert K. M., Hidalgo J., Capecchi M. R., T-cell factor 4 functions as a tumor suppressor whose disruption modulates colon cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 108, 4914–4919 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda H., et al. , Human sebaceous tumors harbor inactivating mutations in LEF1. Nat. Med. 12, 395–397 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Duval A., et al. , Frequent frameshift mutations of the TCF-4 gene in colorectal cancers with microsatellite instability. Cancer Res. 59, 4213–4215 (1999). [PubMed] [Google Scholar]
- 11.Tang W., et al. , A genome-wide RNAi screen for Wnt/beta-catenin pathway components identifies unexpected roles for TCF transcription factors in cancer. Proc. Natl. Acad. Sci. U.S.A. 105, 9697–9702 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadigan K. M., Waterman M. L., TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 4, a007906 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim C. H., et al. , Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407, 913–916 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kratochwil K., Galceran J., Tontsch S., Roth W., Grosschedl R., FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(-/-) mice. Genes Dev. 16, 3173–3185 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrill B. J., et al. , Tcf3: A transcriptional regulator of axis induction in the early embryo. Development 131, 263–274 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Reya T., et al. , Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 13, 15–24 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Roose J., et al. , Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285, 1923–1926 (1999). [DOI] [PubMed] [Google Scholar]
- 18.van Es J. H., et al. , A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell. Biol. 32, 1918–1927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galceran J., Fariñas I., Depew M. J., Clevers H., Grosschedl R., Wnt3a-/--like phenotype and limb deficiency in Lef1(-/-)Tcf1(-/-) mice. Genes Dev. 13, 709–717 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregorieff A., Grosschedl R., Clevers H., Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(-/-)/Tcf1(-/-) embryos. EMBO J. 23, 1825–1833 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreira S., et al. , A single TCF transcription factor, regardless of its activation capacity, is sufficient for effective trilineage differentiation of ESCs. Cell Rep. 20, 2424–2438 (2017). [DOI] [PubMed] [Google Scholar]
- 22.van de Wetering M., et al. , Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Brunner E., Peter O., Schweizer L., Basler K., Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 385, 829–833 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Chu-LaGraff Q., Doe C. Q., Neuroblast specification and formation regulated by wingless in the Drosophila CNS. Science 261, 1594–1597 (1993). [DOI] [PubMed] [Google Scholar]
- 25.Patel N. H., Schafer B., Goodman C. S., Holmgren R., The role of segment polarity genes during Drosophila neurogenesis. Genes Dev. 3, 890–904 (1989). [DOI] [PubMed] [Google Scholar]
- 26.Nüsslein-Volhard C., Wieschaus E., Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801 (1980). [DOI] [PubMed] [Google Scholar]
- 27.Bejsovec A., Martinez Arias A., Roles of wingless in patterning the larval epidermis of Drosophila. Development 113, 471–485 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Cordero J. B., Stefanatos R. K., Myant K., Vidal M., Sansom O. J., Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development 139, 4524–4535 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Lin G., Xu N., Xi R., Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119–1123 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Takashima S., Mkrtchyan M., Younossi-Hartenstein A., Merriam J. R., Hartenstein V., The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454, 651–655 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Tian A., Benchabane H., Wang Z., Ahmed Y., Regulation of stem cell proliferation and cell fate specification by Wingless/Wnt signaling gradients enriched at adult intestinal compartment boundaries. PLoS Genet. 12, e1005822 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baena-Lopez L. A., Franch-Marro X., Vincent J. P., Wingless promotes proliferative growth in a gradient-independent manner. Sci. Signal. 2, ra60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann C. J., Cohen S. M., Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development 122, 1781–1789 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Couso J. P., Bishop S. A., Martinez Arias A., The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120, 621–636 (1994). [DOI] [PubMed] [Google Scholar]
- 35.Chen C. M., Struhl G., Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126, 5441–5452 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Williams J. A., Paddock S. W., Carroll S. B., Pattern formation in a secondary field: A hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development 117, 571–584 (1993). [DOI] [PubMed] [Google Scholar]
- 37.Klein T., Arias A. M., Different spatial and temporal interactions between Notch, wingless, and vestigial specify proximal and distal pattern elements of the wing in Drosophila. Dev. Biol. 194, 196–212 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Alexandre C., Baena-Lopez A., Vincent J. P., Patterning and growth control by membrane-tethered Wingless. Nature 505, 180–185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Requena D., et al. , Origins and specification of the Drosophila wing. Curr. Biol. 27, 3826–3836.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hariharan I. K., Organ size control: Lessons from Drosophila. Dev. Cell 34, 255–265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herranz H., Eichenlaub T., Cohen S. M., Cancer in Drosophila: Imaginal discs as a model for epithelial tumor formation. Curr. Top. Dev. Biol. 116, 181–199 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Swarup S., Verheyen E. M., Wnt/Wingless signaling in Drosophila. Cold Spring Harb. Perspect. Biol. 4, a007930 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giraldez A. J., Cohen S. M., Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development 130, 6533–6543 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Johnston L. A., Sanders A. L., Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat. Cell Biol. 5, 827–833 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Schweizer L., Nellen D., Basler K., Requirement for pangolin/dTCF in Drosophila Wingless signaling. Proc. Natl. Acad. Sci. U.S.A. 100, 5846–5851 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison D. A., Perrimon N., Simple and efficient generation of marked clones in Drosophila. Curr. Biol. 3, 424–433 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Brand A. H., Perrimon N., Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993). [DOI] [PubMed] [Google Scholar]
- 48.Vincent J. P., Kolahgar G., Gagliardi M., Piddini E., Steep differences in wingless signaling trigger Myc-independent competitive cell interactions. Dev. Cell 21, 366–374 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weihe U., Dorfman R., Wernet M. F., Cohen S. M., Milán M., Proximodistal subdivision of Drosophila legs and wings: The elbow-no ocelli gene complex. Development 131, 767–774 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Wang S. H., Simcox A., Campbell G., Dual role for Drosophila epidermal growth factor receptor signaling in early wing disc development. Genes Dev. 14, 2271–2276 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavallo R. A., et al. , Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 395, 604–608 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Lawrence N., et al. , dTcf antagonises Wingless signalling during the development and patterning of the wing in Drosophila. Int. J. Dev. Biol. 44, 749–756 (2000). [PubMed] [Google Scholar]
- 53.Halder G., et al. , The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 12, 3900–3909 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neumann C. J., Cohen S. M., A hierarchy of cross-regulation involving Notch, wingless, vestigial and cut organizes the dorsal/ventral axis of the Drosophila wing. Development 122, 3477–3485 (1996). [DOI] [PubMed] [Google Scholar]
- 55.Ng M., Diaz-Benjumea F. J., Vincent J. P., Wu J., Cohen S. M., Specification of the wing by localized expression of wingless protein. Nature 381, 316–318 (1996). [DOI] [PubMed] [Google Scholar]
- 56.Roose J., Clevers H., TCF transcription factors: Molecular switches in carcinogenesis. Biochim. Biophys. Acta 1424, M23–M37 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Eichenlaub T., Cohen S. M., Herranz H., Cell competition drives the formation of metastatic tumors in a Drosophila model of epithelial tumor formation. Curr. Biol. 26, 419–427 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Gerlach S. U., Eichenlaub T., Herranz H., Yorkie and JNK control tumorigenesis in Drosophila cells with cytokinesis failure. Cell Rep. 23, 1491–1503 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Herranz H., Hong X., Hung N. T., Voorhoeve P. M., Cohen S. M., Oncogenic cooperation between SOCS family proteins and EGFR identified using a Drosophila epithelial transformation model. Genes Dev. 26, 1602–1611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herranz H., Weng R., Cohen S. M., Crosstalk between epithelial and mesenchymal tissues in tumorigenesis and imaginal disc development. Curr. Biol. 24, 1476–1484 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Sander M., Eichenlaub T., Herranz H., Oncogenic cooperation between Yorkie and the conserved microRNA miR-8 in the wing disc of Drosophila. Development 145, dev153817 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Song S., Herranz H., Cohen S. M., The chromatin remodeling BAP complex limits tumor-promoting activity of the Hippo pathway effector Yki to prevent neoplastic transformation in Drosophila epithelia. Dis. Model. Mech. 10, 1201–1209 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franz A., Shlyueva D., Brunner E., Stark A., Basler K., Probing the canonicity of the Wnt/Wingless signaling pathway. PLoS Genet. 13, e1006700 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buscarlet M., Stifani S., The ‘Marx’ of Groucho on development and disease. Trends Cell Biol. 17, 353–361 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Turki-Judeh W., Courey A. J., Groucho: A corepressor with instructive roles in development. Curr. Top. Dev. Biol. 98, 65–96 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Bilder D., Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18, 1909–1925 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Beaucher M., Hersperger E., Page-McCaw A., Shearn A., Metastatic ability of Drosophila tumors depends on MMP activity. Dev. Biol. 303, 625–634 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Uhlirova M., Bohmann D., JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25, 5294–5304 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowles J., Schepers G., Koopman P., Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 227, 239–255 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Crémazy F., Berta P., Girard F., Genome-wide analysis of Sox genes in Drosophila melanogaster. Mech. Dev. 109, 371–375 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Atkins M., et al. , An ectopic network of transcription factors regulated by Hippo signaling drives growth and invasion of a malignant tumor model. Curr. Biol. 26, 2101–2113 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Külshammer E., et al. , Interplay among Drosophila transcription factors Ets21c, Fos and Ftz-F1 drives JNK-mediated tumor malignancy. Dis. Model. Mech. 8, 1279–1293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lavorgna G., Karim F. D., Thummel C. S., Wu C., Potential role for a FTZ-F1 steroid receptor superfamily member in the control of Drosophila metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 90, 3004–3008 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doggett K., et al. , BTB-zinc finger oncogenes are required for ras and notch-driven tumorigenesis in Drosophila. PLoS One 10, e0132987 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Flaherty M. S., et al. , Chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev. Cell 18, 556–568 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Narbonne-Reveau K., et al. , Neural stem cell-encoded temporal patterning delineates an early window of malignant susceptibility in Drosophila. eLife 5, e13463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baena-Lopez L. A., Nojima H., Vincent J. P., Integration of morphogen signalling within the growth regulatory network. Curr. Opin. Cell Biol. 24, 166–172 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Korinek V., et al. , Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379–383 (1998). [DOI] [PubMed] [Google Scholar]
- 79.Dekanty A., Barrio L., Muzzopappa M., Auer H., Milán M., Aneuploidy-induced delaminating cells drive tumorigenesis in Drosophila epithelia. Proc. Natl. Acad. Sci. U.S.A. 109, 20549–20554 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geissler K., Zach O., Pathways involved in Drosophila and human cancer development: The notch, hedgehog, wingless, runt, and trithorax pathway. Ann. Hematol. 91, 645–669 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Hirabayashi S., Baranski T. J., Cagan R. L., Transformed Drosophila cells evade diet-mediated insulin resistance through wingless signaling. Cell 154, 664–675 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barker N., et al. , The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 20, 4935–4943 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collins R. T., Treisman J. E., Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev. 14, 3140–3152 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neto M., et al. , Nuclear receptors connect progenitor transcription factors to cell cycle control. Sci. Rep. 7, 4845 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wagner R. T., Xu X., Yi F., Merrill B. J., Cooney A. J., Canonical Wnt/β-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells 28, 1794–1804 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamachi Y., Kondoh H., Sox proteins: Regulators of cell fate specification and differentiation. Development 140, 4129–4144 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Kiefer J. C., Back to basics: Sox genes. Dev. Dyn. 236, 2356–2366 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Castillo S. D., Sanchez-Cespedes M., The SOX family of genes in cancer development: Biological relevance and opportunities for therapy. Expert Opin. Ther. Targets 16, 903–919 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Dong C., Wilhelm D., Koopman P., Sox genes and cancer. Cytogenet. Genome Res. 105, 442–447 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Bernard P., Harley V. R., Acquisition of SOX transcription factor specificity through protein-protein interaction, modulation of Wnt signalling and post-translational modification. Int. J. Biochem. Cell Biol. 42, 400–410 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Kormish J. D., Sinner D., Zorn A. M., Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dyn. 239, 56–68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dichtel-Danjoy M. L., Caldeira J., Casares F., SoxF is part of a novel negative-feedback loop in the wingless pathway that controls proliferation in the Drosophila wing disc. Development 136, 761–769 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Basu-Roy U., et al. , Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat. Commun. 6, 6411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doumpas N., et al. , TCF/LEF dependent and independent transcriptional regulation of Wnt/β-catenin target genes. EMBO J. 38, e98873 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu Y., Anderson W. R., Zhang H., Feng S., Pick L., Functional conservation of Drosophila FTZ-F1 and its mammalian homologs suggests ligand-independent regulation of NR5A family transcriptional activity. Dev. Genes Evol. 223, 199–205 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Straume A. H., et al. , Elevated levels of the steroidogenic factor 1 are associated with over-expression of CYP19 in an oestrogen-producing testicular Leydig cell tumour. Eur. J. Endocrinol. 166, 941–949 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lewis S. R., Hedman C. J., Ziegler T., Ricke W. A., Jorgensen J. S., Steroidogenic factor 1 promotes aggressive growth of castration-resistant prostate cancer cells by stimulating steroid synthesis and cell proliferation. Endocrinology 155, 358–369 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nadolny C., Dong X., Liver receptor homolog-1 (LRH-1): A potential therapeutic target for cancer. Cancer Biol. Ther. 16, 997–1004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bayrer J. R., Mukkamala S., Sablin E. P., Webb P., Fletterick R. J., Silencing LRH-1 in colon cancer cell lines impairs proliferation and alters gene expression programs. Proc. Natl. Acad. Sci. U.S.A. 112, 2467–2472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schoonjans K., et al. , Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc. Natl. Acad. Sci. U.S.A. 102, 2058–2062 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Botrugno O. A., et al. , Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation. Mol. Cell 15, 499–509 (2004). [DOI] [PubMed] [Google Scholar]
- 102.Sinner D., Rankin S., Lee M., Zorn A. M., Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development 131, 3069–3080 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Calleja M., Moreno E., Pelaz S., Morata G., Visualization of gene expression in living adult Drosophila. Science 274, 252–255 (1996). [DOI] [PubMed] [Google Scholar]
- 104.Buff E., Carmena A., Gisselbrecht S., Jiménez F., Michelson A. M., Signalling by the Drosophila epidermal growth factor receptor is required for the specification and diversification of embryonic muscle progenitors. Development 125, 2075–2086 (1998). [DOI] [PubMed] [Google Scholar]
- 105.Huang J., Wu S., Barrera J., Matthews K., Pan D., The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122, 421–434 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Wu J., Cohen S. M., Proximal distal axis formation in the Drosophila leg: Distinct functions of teashirt and homothorax in the proximal leg. Mech. Dev. 94, 47–56 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.