Significance
VEGF signaling mediated by the NRPs impacts tumor cells, independently of its function in angiogenesis and vascular permeability. VEGF–NRP2 signaling in tumor cells is associated with poor prognosis and therapy resistance in TNBC patients. In light of these observations the question arises, How does VEGF–NRP2 contribute to therapy resistance in TNBC? We discovered that autocrine VEGF–NRP2 signaling contributes to HR DNA repair and therapy resistance in TNBC cells by promoting YAP/TAZ-dependent Rad51 transcription. We also demonstrated that Rad51 is a YAP/TAZ target gene and that VEGF–NRP2–YAP/TAZ–mediated cisplatin resistance occurs through downstream Rad51 expression. These observations establish functions of VEGF–NRP2 signaling and YAP/TAZ in tumor biology and provide an integrated mechanism that governs Rad51 expression and HR in TNBC.
Keywords: breast cancer, VEGF–neuropilin, YAP/TAZ, DNA repair
Abstract
Vascular endothelial growth factor (VEGF) signaling in tumor cells mediated by neuropilins (NRPs) contributes to the aggressive nature of several cancers, including triple-negative breast cancer (TNBC), independently of its role in angiogenesis. Understanding the mechanisms by which VEGF–NRP signaling contributes to the phenotype of such cancers is a significant and timely problem. We report that VEGF–NRP2 promote homologous recombination (HR) in BRCA1 wild-type TNBC cells by contributing to the expression and function of Rad51, an essential enzyme in the HR pathway that mediates efficient DNA double-strand break repair. Mechanistically, we provide evidence that VEGF–NRP2 stimulates YAP/TAZ-dependent Rad51 expression and that Rad51 is a direct YAP/TAZ–TEAD transcriptional target. We also discovered that VEGF–NRP2–YAP/TAZ signaling contributes to the resistance of TNBC cells to cisplatin and that Rad51 rescues the defects in DNA repair upon inhibition of either VEGF–NRP2 or YAP/TAZ. These findings reveal roles for VEGF–NRP2 and YAP/TAZ in DNA repair, and they indicate a unified mechanism involving VEGF–NRP2, YAP/TAZ, and Rad51 that contributes to resistance to platinum chemotherapy.
The role of vascular endothelial growth factor (VEGF) in cancer is not limited to angiogenesis and vascular biology (1–4). Tumor cells express VEGF receptors, and VEGF signaling in these cells has been implicated in the aggressive nature and chemoresistance of many cancers, independently of its function in angiogenesis (5). In addition to tyrosine kinase VEGF receptors (VEGFR1 and VEGFR2), tumor cells express neuropilins (NRPs), another family of VEGF receptors. Although NRPs have the ability to interact with and regulate VEGFR1 and VEGFR2 (6, 7), they can also mediate VEGF signaling in tumor cells independently of these tyrosine kinase receptors (8–12). The fact that VEGF–NRP signaling is characteristic of more aggressive tumors that often respond poorly to therapy has profound clinical implications, and it heightens the importance of understanding how VEGF–NRP signaling promotes resistance. This problem is exemplified in aggressive breast cancers, such as the triple-negative subtype (TNBC), that manifest VEGF–NRP2 signaling (13) and are resistant to standard therapy (14).
One potentially promising area that has not been explored rigorously with respect to VEGF–NRP signaling in breast and other cancers is its contribution to DNA repair pathways. The integrity of such pathways is a major reason for resistance to therapy. Specifically, the ability to execute efficient homologous recombination (HR) DNA repair is considered to be critical for the ability of tumors to resist platinum-based chemotherapies, poly (ADP ribose) polymerase (PARP) inhibitors, and radiation therapy (15). Conversely, HR deficiency, which is most often attributed to germ line BRCA1/2 mutations or loss of BRCA1 expression through promoter hypermethylation in breast cancer, provides an Achilles heel that renders sensitivity to these agents. For example, clinical studies have demonstrated favorable outcomes of TNBC patients with HR deficiency treated with neoadjuvant platinum chemotherapy (16–20). However, only 11 to 15% of TNBC patients harbor germ line BRCA mutations (21, 22), a fact that indicates that many TNBCs are HR proficient and, consequently, resistant to therapies that induce DNA damage. In this study, we investigated the potential contribution of VEGF–NRP2 signaling to HR in breast cancer cells, and we pursued the mechanism involved. The results obtained validate our hypothesis, and they reveal that VEGF–NRP2 signaling regulates Rad51, a central HR enzyme that catalyzes homology strand exchange and facilitates the repair of damaged DNA (23). Importantly, we also made the observation that the ability of VEGF–NRP2 signaling to regulate Rad51 is mediated by YAP/TAZ and that Rad51 is a YAP/TAZ target gene.
Results
VEGF–NRP2 Promotes Protection from DNA-Damaging Agents.
Initially, we assessed the potential contribution of NRP2 and VEGF in the response of TNBC cells to DNA damage. We focused our attention on cisplatin because platinum chemotherapy is particularly effective for tumors with HR deficiency (16–20). For this purpose, we depleted NRP2 and VEGF with short hairpin RNAs (shRNAs) in MDA-MB-231 cells, a BRCA1 wild-type TNBC cell line (24) that exhibits VEGF–NRP2 signaling (8, 25), and assessed DNA damage by measuring γH2AX levels. We observed that NRP2 and VEGF depletion resulted in increased DNA damage in comparison with cisplatin-treated control cells (Fig. 1A). Similar results were obtained with NRP2 depletion in response to cisplatin in Hs578t cells, which are another BRCA1 wild-type TNBC cell line (24) that expresses VEGF and NRP2 (Fig. 1B). Negligible γH2AX was detected under baseline conditions in control and NRP2 and VEGF-depleted MDA-MB-231 cells (SI Appendix, Fig. S1A).
Fig. 1.
VEGF–NRP2 promotes protection from cisplatin-induced DNA damage. (A) Expression of NRP2 and VEGF was diminished with shRNAs in MDA-MB-231 cells. Subsequently, cells were treated with cisplatin and processed for γH2AX immunofluorescence microscopy. (Scale bar, 50 µm.) (B) Expression of NRP2 was diminished in Hs578t cells. Subsequently, cells were treated with cisplatin, and the impact on γH2AX abundance was quantified by immunoblotting. Densitometry was assessed using ImageJ (Right bar graph). (C) Hs578t cells were treated with a control immunoglobulin G (IgG), a NRP2 function antibody, or bevacizumab in the presence of cisplatin, and the impact on γH2AX abundance was quantified by immunoblotting. Densitometry was assessed using ImageJ (Right bar graph). (D) Cell viability in BRCA-proficient mouse mammary tumor organoids treated with a control IgG, a NRP2 function-blocking antibody, or bevacizumab with and without cisplatin was assessed. Dot plots (mean ± SD) represent 3 independent experiments. *P ≤ 0.05, **P ≤ 0.005 by 2-tailed t test.
VEGF–NRP signaling can function in TNBC and other cancer cells independently of the VEGFRs (8–12). This observation is significant because bevacizumab, the most common anti-VEGF therapy, blocks VEGF binding to VEGFRs, but it does not disrupt the VEGF–NRP association or signaling (26). To assess the relative contribution of NRP2 and VEGFRs to the protection of TNBC cells from cisplatin-induced DNA damage, we treated Hs578t cells with cisplatin in the presence of a NRP2 function-blocking antibody (27) or bevacizumab and observed that NRP2 inhibition resulted in increased γH2AX abundance relative to cisplatin-treated control cells but that bevacizumab did not (Fig. 1C). We substantiated these results by treating BRCA-proficient mouse mammary tumor organoids, which were established to exhibit resistance to HR-inducing agents (28), with either cisplatin, the NRP2 function-blocking antibody, bevacizumab, or combinations of these reagents, and observed that NRP2 inhibition sensitizes these tumor organoids to cisplatin but that bevacizumab does not (Fig. 1D). These findings indicate that VEGF–NRP2 signaling mediates protection from cisplatin-induced DNA damage independently of the VEGFRs.
Following the results obtained with cisplatin, we next sought to determine if VEGF–NRP2 mediates resistance to a broader variety of agents used in TNBC. We focused our attention on PARP inhibition and ionizing radiation (IR). Indeed, we observed that NRP2 depletion sensitizes Hs578t cells to olaparib (SI Appendix, Fig. S1B) and IR (SI Appendix, Fig. S1C). Similar to our results with cisplatin, treatment of Hs578t cells with IR and the NRP2 function-blocking antibody resulted in increased γH2AX abundance compared with radiation-treated control cells (SI Appendix, Fig. S1D).
YAP/TAZ Are Necessary for VEGF–NRP2 Protection from Cisplatin-Induced DNA Damage.
The Hippo pathway transducers YAP and TAZ are critical downstream effectors of VEGF signaling in several distinct cell types (29). Moreover, VEGF–NRP2 activation of YAP/TAZ in TNBC cells occurs through a VEGFR-independent mechanism (8). For these reasons, we hypothesized that VEGF–NRP2 promotes genomic integrity and cisplatin resistance through downstream YAP/TAZ activation. To test this hypothesis, we assessed DNA damage in response to cisplatin in NRP2-depleted Hs578t cells expressing the S89A TAZ mutant, which is resistant to inhibitory phosphorylation at that site (30), or empty vector and found that S89A TAZ significantly diminished the increase in γH2AX observed upon NRP2 depletion (Fig. 2A and SI Appendix, Fig. S2). Importantly, S89A TAZ also rescued cell viability in NRP2-depleted Hs578t cells treated with cisplatin (Fig. 2B). Similar results were obtained in Hs578t cells expressing S127A YAP, which is resistant to inhibitory phosphorylation at a homologous phosphorylation site (Fig. 2 C and D) (30). Together, these data provide evidence that VEGF–NRP2 signaling protects the genome from DNA damage caused by cisplatin by a mechanism that involves downstream YAP/TAZ activation.
Fig. 2.
YAP/TAZ are necessary for VEGF–NRP2 protection from cisplatin-induced DNA damage. NRP2-depleted Hs578t cells expressing S89A TAZ, S127A YAP, or a control vector were treated with cisplatin, and the impact on γH2AX abundance (A and C) and cell viability (B and D) was assessed. Dot plots (mean ± SD) represent 3 independent experiments. *P ≤ 0.05 by 2-tailed t test.
YAP/TAZ Facilitate Homologous Recombination and Contribute to Rad51 Expression.
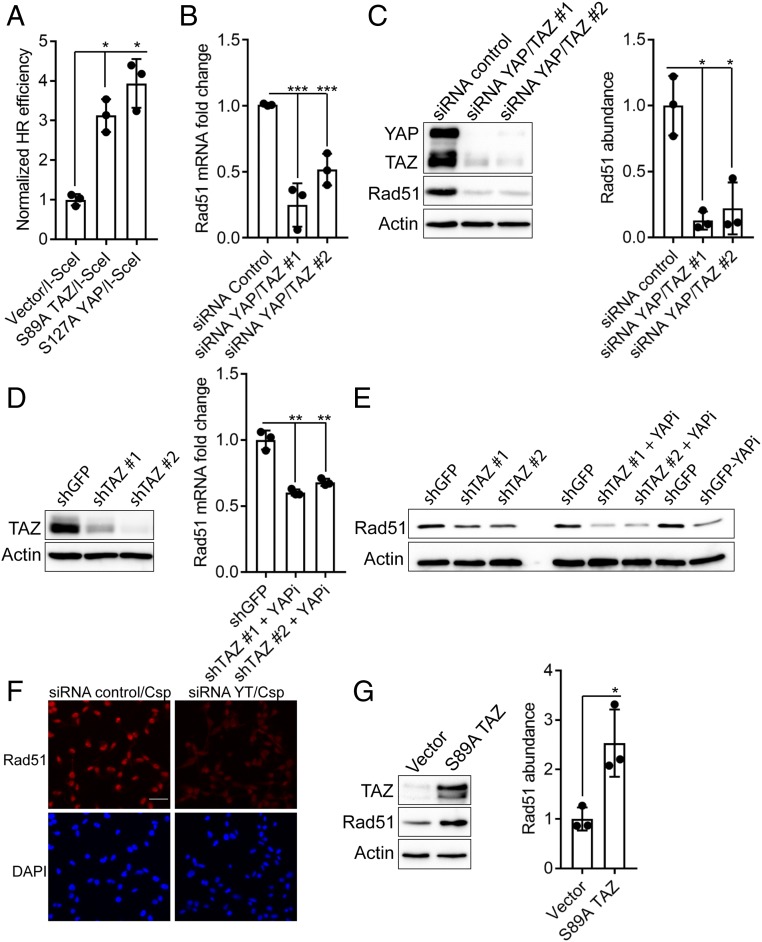
Given that YAP/TAZ contribute to NRP2-mediated DNA repair and cell viability in response to cisplatin (Fig. 2), we assessed the potential contribution of YAP and TAZ to HR. For this purpose, we utilized the well-established HR reporter assay (DR-green fluorescent protein [GFP]) (31). This assay is based on the expression of functional GFP as a result of HR in response to a double-strand break induced by the I-SceI endonuclease, which can be quantified by flow cytometry. For this assay, we used MCF7 cells, a BRCA1 wild-type estrogen receptor (ER)+ breast cancer cell line (24), engineered to stably express DR-GFP because they exhibit low YAP/TAZ activity. Consequently, expression of YAP/TAZ in these cells provides a robust system to study their role in HR. To ensure that the expressed YAP/TAZ were active, we used the S89A TAZ and S127A YAP mutants, which are resistant to inhibitory phosphorylation at those sites (30). Expression of either of these mutants in DR-GFP MCF-7 cells resulted in a significant increase in the number of GFP-positive cells following a double-strand break by I-SceI compared with control cells, providing evidence that YAP/TAZ contribute to HR (Fig. 3A and SI Appendix, Fig. S3 A and B).
Fig. 3.
YAP/TAZ promote homologous recombination and contribute to Rad51 expression. (A) DR-GFP MCF7 cells engineered to express S89A TAZ, S127A YAP, or empty vector were transfected with I-SceI and processed for flow cytometry to quantify GFP-positive cells, which were normalized to 1 and are depicted as HR efficiency. (B) Expression of YAP/TAZ was diminished with siRNAs in MDA-MB-231 cells, and the impact on Rad51 mRNA expression was quantified by qPCR. (C) Rad51 abundance was quantified by immunoblotting in YAP/TAZ-depleted Hs578t cells. Densitometry was assessed using ImageJ (Right bar graph). (D) Knockdown of TAZ was quantified by immunoblotting in MDA-MB-231 cells (Left). These cells were subsequently treated with verteporfin to also inhibit YAP, and Rad51 mRNA expression was quantified by qPCR (Right). (E) Rad51 abundance was quantified by immunoblotting in TAZ-depleted MDA-MB-231 cells, verteporfin-treated MDA-MB-231 cells, and the combination. (F) YAP/TAZ-depleted MDA-MB-231 cells were treated with cisplatin, and the impact on Rad51 was assessed by immunofluorescence microscopy. (Scale bar, 50 µm.) (G) DR-GFP MCF7 cells were transfected with either S89A TAZ or empty vector, and Rad51 abundance was quantified by immunoblotting. Densitometry was assessed using ImageJ (Right bar graph). Dot plots (mean ± SD) represent 3 independent experiments. *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005 by 2-tailed t test.
To identify the mechanism by which YAP/TAZ promote HR, we analyzed published microarray data (GSE59230) (32) derived from YAP/TAZ depletion in MDA-MB-231 cells for significant alterations in the expression of genes that could contribute to HR. Most notably, the mRNA expression of Rad51, a central HR enzyme that catalyzes homology strand exchange and facilitates the repair of damaged DNA (23), was reduced in this microarray dataset upon YAP/TAZ depletion to a degree similar to the established YAP/TAZ target genes CTGF and Cyr61 (SI Appendix, Fig. S4A). Rad51 mediates resistance of TNBC cells to therapy (33), and its expression is up-regulated in breast and other cancer cells (33, 34), which may result from increased activity at its promoter (35, 36). However, mechanisms that control Rad51 transcription and function in specific tumors are poorly understood. Given this information, we developed the hypothesis that VEGF–NRP2 facilitates YAP/TAZ-mediated Rad51 expression and HR in TNBC.
Initially, we assessed whether a correlation exists between enhanced YAP/TAZ activity and Rad51 expression in patient samples. A YAP/TAZ gene signature, as well as elevated TAZ mRNA expression, is associated with hormone receptor–negative, high-grade breast tumors (37, 38). Therefore, we analyzed the expression of Rad51 and compared it to markers of enhanced YAP/TAZ activity in patient tumors in the Metabric breast cancer database obtained from cBioPortal (39, 40). Specifically, we observed that Rad51 expression is higher in ER− tumors compared with ER+ tumors (SI Appendix, Fig. S4B) and that Rad51 expression correlates positively with breast tumor grade (SI Appendix, Fig. S4C), as well as with TAZ and the YAP/TAZ target genes CTGF and Cyr61 (SI Appendix, Fig. S4D). We validated a causal role for YAP/TAZ in regulating Rad51 in MDA-MB-231 cells (Fig. 3B). Similarly, we observed that small interfering RNA (siRNA) knockdown of YAP/TAZ in Hs578t cells reduced Rad51 abundance (Fig. 3C). Consistent with our results using YAP/TAZ siRNA, MDA-MB-231 cells with stable depletion of TAZ and treated with verteporfin to inhibit YAP exhibited a decrease in Rad51 mRNA expression (Fig. 3D). Similar results were obtained when assessing Rad51 protein abundance in response to shTAZ alone, verteporfin alone, or the combination in MDA-MB-231 cells (Fig. 3E). A reduction in nuclear and total Rad51 abundance was also observed by immunofluorescence microscopy in YAP/TAZ-depleted MDA-MB-231 cells treated with cisplatin to induce HR (Fig. 3F). Conversely, expression of S89A TAZ in MCF7 DR-GFP cells increased Rad51 abundance (Fig. 3G). Together, these data indicate that YAP/TAZ promote HR and contribute to Rad51 expression.
Rad51 Is a YAP/TAZ–TEAD Target Gene.
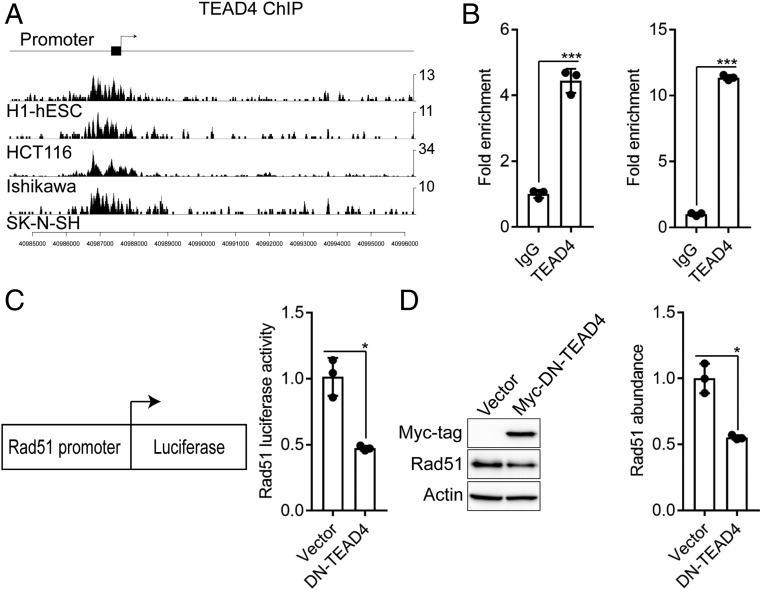
YAP/TAZ-mediated transcriptional regulation occurs through the TEAD1 to TEAD4 family of transcription factors (30, 41). TEAD4, in particular, has been shown to play a dominant role in TNBC (42, 43). In light of this information, we analyzed the encyclopedia of DNA elements (ENCODE) database for TEAD4 chromatin immunoprecipitation sequencing (ChIP-seq) experiments to determine if YAP/TAZ-dependent control of Rad51 expression occurs through a direct mechanism mediated by TEAD. We found 4 cell types in ENCODE (h1-human embryonic stem cells [hESCs], HCT116 colon cancer cells, Ishikawa endometrial adenocarcinoma cells, and SK-N-SH neuroblastoma cells), which have been previously shown to have enhanced YAP/TAZ activity (44, 45), where TEAD4 bound directly to the promoter region of Rad51 (Fig. 4A). Subsequently, we performed ChIP in MDA-MB-231 and Hs578t cells to validate direct binding of TEAD4 to the Rad51 promoter in TNBC cells (Fig. 4B). To obtain additional evidence to implicate TEAD4 in Rad51 transcription, we used a Rad51 promoter luciferase assay (36). We expressed this reporter construct in MDA-MB-231 cells stably expressing dominant negative TEAD4 and observed a reduction in luciferase activity at the Rad51 promoter (Fig. 4C). Dominant negative TEAD4 also caused a decrease in Rad51 abundance (Fig. 4D). These results provide evidence that a YAP/TAZ-TEAD transcriptional program governs Rad51 expression in TNBC.
Fig. 4.
Rad51 is a direct YAP/TAZ-TEAD target gene. (A) TEAD4 binding signals from ENCODE were analyzed in ChIP-seq datasets from h1-hESCs (human embryonic stem cells), HCT116 (colon cancer), Ishikawa (endometrial adenocarcinoma), and SK-N-SH (neuroblastoma) cells in the promoter region of the Rad51 gene. (B) Binding of TEAD4 on the Rad51 promoter was analyzed using ChIP in MDA-MB-231 (Left) and Hs578t (Right) cells. (C) MDA-MB-231 cells expressing dominant-negative TEAD4 were transfected with pRad51-Luc that utilizes the Rad51 promoter to control expression of firefly luciferase and assayed for Rad51 transcriptional activity. (D) Rad51 abundance was quantified by immunoblotting in MDA-MB-231 cells expressing dominant-negative TEAD4. Densitometry was assessed using ImageJ (Right bar graph). Dot plots (mean ± SD) represent 3 independent experiments. *P ≤ 0.05, ***P ≤ 0.0005 by 2-tailed t test.
Rad51 Mediates VEGF–NRP2–YAP/TAZ–Dependent DNA Repair.
A key issue that emerges from these findings is the role of YAP/TAZ-mediated Rad51 expression in DNA repair in the cellular response to cisplatin. As shown in Fig. 5 A and B, YAP/TAZ depletion in Hs578t cells treated with cisplatin resulted in increased DNA damage as measured by γH2AX in comparison with cisplatin-treated control cells. Importantly, reexpression of Rad51 significantly diminished the increase in γH2AX observed upon YAP/TAZ depletion (Fig. 5 A and B and SI Appendix, Fig. S5A). These results substantiate the data shown in Fig. 3A and SI Appendix, Fig. S3 A and B, that YAP/TAZ contribute to HR, and they provide evidence that YAP/TAZ-mediated HR occurs, in part, through downstream Rad51 expression. Similar to VEGF and NRP2 down-regulation, negligible γH2AX was observed under baseline conditions in YAP/TAZ-depleted Hs578t cells (SI Appendix, Fig. S5B). We also assessed the effects of cisplatin and TAZ depletion on cell viability in MDA-MB-231 cells and observed that TAZ down-regulation promotes cisplatin sensitization, which is rescued by Rad51 reexpression (Fig. 5C and SI Appendix, Fig. S5C). Along these lines, we tested the effects of either cisplatin, verteporfin, or the combination on BRCA-proficient mouse mammary tumor organoids and observed that YAP inhibition with verteporfin sensitizes these tumor organoids to cisplatin (Fig. 5D), similar to the results obtained with NRP2 inhibition (Fig. 1D).
Fig. 5.
Rad51 mediates YAP/TAZ-dependent DNA repair. Expression of YAP/TAZ was diminished in Hs578t cells (siRNA YT). Cells were then transfected with HA-tagged Rad51 or empty vector. Subsequently, cells were treated with cisplatin and processed for (A) immunofluorescence microscopy or (B) immunoblotting to quantify γH2AX. (Scale bar, 50 µm.) (C) TAZ-depleted MDA-MB-231 cells expressing Rad51 or a control vector were treated with cisplatin, and the impact on cell viability was assessed. (D) Cell viability in BRCA-proficient mouse mammary tumor organoids treated with either verteporfin, cisplatin, or the combination was assessed. Dot plots (mean ± SD) represent 3 independent experiments. *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005 by 2-tailed t test.
Given that BRCA1 is required for Rad51-mediated HR (46), we hypothesized that YAP/TAZ should not affect DNA damage in BRCA1 mutant cells. To test this hypothesis, we assessed DNA damage in response to cisplatin in SUM-1315 cells, which are a BRCA1 mutant TNBC cell line (24). Notably, although we detected a reduction in Rad51 abundance, we did not observe an increase in γH2AX in response to cisplatin in cells depleted of TAZ and treated with verteporfin to inhibit YAP (SI Appendix, Fig. S6 A and B).
We postulated that the mechanism by which VEGF–NRP2 protects from DNA damage involves YAP/TAZ-mediated Rad51 expression. Indeed, we observed that both NRP2 and VEGF depletion in MDA-MB-231 cells reduced Rad51 abundance (Fig. 6 A and B). Stimulation of VEGF-depleted MDA-MB-231 cells with exogenous VEGF substantially diminished the increase in γH2AX and reduction in Rad51 abundance in response to cisplatin (Fig. 6C). We also observed that treating Hs578t cells with the function-blocking NRP2 antibody reduces HR as measured by DR-GFP analysis (Fig. 6D and SI Appendix, Fig. S7A). Importantly, we observed that inhibiting NRP2 in cells ectopically expressing Rad51 resulted in a significant increase in HR compared with NRP2 inhibition alone (Fig. 6D and SI Appendix, Fig. S7A). This result provides evidence that downstream Rad51 is involved in VEGF–NRP2–mediated HR. Similar to our results inhibiting YAP/TAZ (Fig. 5 C and D), Rad51 rescued cell viability in response to cisplatin in NRP2-depleted Hs578t cells (Fig. 6E and SI Appendix, Fig. S7B). Last, in support of our in vitro data, we found that Rad51 expression correlates positively with VEGF and NRP2 in breast cancer patients in the Metabric dataset obtained from cBioPortal (SI Appendix, Fig. S4D) (39, 40).
Fig. 6.
VEGF–NRP2 controls YAP/TAZ-mediated Rad51 expression and homologous recombination. Expression of (A) NRP2 and (B) VEGF was diminished in MDA-MB-231 cells, and the impact on Rad51 abundance was quantified by immunoblotting. Densitometry was assessed using ImageJ (Right bar graphs). (C) VEGF-depleted MDA-MB-231 cells were treated with 50 ng/mL of VEGF for 24 h. Medium was then replaced, and cells were treated with cisplatin and also 50 ng/mL of VEGF for VEGF-depleted cells. γH2AX and Rad51 abundance was quantified by immunoblotting. (D) Hs578t DR-GFP cells were transfected with Rad51 or empty vector. Subsequently, they were treated with a control IgG or a NRP2 function-blocking antibody and processed for flow cytometry to quantify GFP-positive cells. GFP-positive cells were normalized to 1 and are depicted as HR efficiency. (E) NRP2-depleted Hs578t cells expressing Rad51 or a control vector were treated with cisplatin, and the impact on cell viability was assessed. Dot plots (mean ± SD) represent 3 independent experiments. *P ≤ 0.05 by 2-tailed t test.
Discussion
The results of this study establish a significant role for VEGF–NRP2 signaling in HR by promoting the expression and function of Rad51. Importantly, we also demonstrate that this mechanism is mediated by YAP/TAZ and that Rad51 is a YAP/TAZ target gene. These findings integrate salient characteristics of aggressive breast tumors, dependence on VEGF–NRP2 signaling (8, 13), hyperactivation of YAP/TAZ (37, 38), and high Rad51 expression (33, 47), into a unified mechanism that accounts for their therapy resistance. They also provide one mechanism for how Rad51 transcription is regulated in cancer, an area that is poorly understood.
Although many studies have revealed the importance of VEGF–NRP signaling in tumor cells, independently of its role in angiogenesis (5), its contribution to DNA repair mechanisms is significant. Of note, VEGF–NRP signaling has been implicated in drug resistance in multiple tumors, but satisfying mechanisms have been elusive (9, 48–50). Given that efficient HR is a key determinant of such resistance, our results implicating this signaling in HR-directed repair provides one such mechanism as exemplified by the data we obtained with cisplatin, olaparib, and IR in breast cancer cells and organoids. Clearly, VEGF–NRP2–mediated regulation of HR probably functions in concert with other mechanisms of HR regulation in breast cancer. Moreover, our results are timely because platinum chemotherapy has garnered interest in recent years as a therapeutic option for TNBC patients, especially those with loss of BRCA function and/or features of genomic instability (16–20). However, the majority of TNBC patients do not have germ line BRCA mutations, and accordingly, platinum analogs do not provide these patients with significant clinical benefits over mechanistically distinct drugs (20). We provide evidence for the causality of NRP signaling in this resistance by demonstrating that HR-inducing agents are more efficacious in killing breast tumor cells and organoids when simultaneously inhibiting NRP2 function.
The second major advance provided by our data is that the Hippo pathway transcriptional effectors YAP and TAZ contribute to HR by regulating Rad51 transcription. Although considerable evidence indicates that these transcriptional coactivators contribute to the aggressive behavior and therapy resistance of TNBC and other cancers (38, 51), much remains to be learned about their transcriptional targets and how they function. From this perspective, our implication of their involvement in HR by regulating Rad51 is significant. Our previous work established that VEGF–NRP2 signaling activates YAP/TAZ (8), but the contribution of these critical Hippo effectors to DNA repair mechanisms was not known. Our findings mesh with the emerging view that YAP/TAZ mediate the transcriptional addiction of cancer cells, a process implicated in drug resistance (51). The implications of our data for therapy are potentially substantial because targeted inhibition of YAP/TAZ increases the sensitivity of breast cancer cells and organoids to cisplatin, which is similar to blocking NRP2. Moreover, the selectivity that either NRP2 or YAP/TAZ inhibition is likely to display toward transcriptional-addicted tumors is a viable experimental approach, and it has the potential to limit toxicities that may be associated with Rad51 inhibition (52).
Materials and Methods
See SI Appendix. Cisplatin was used at a concentration of 10 µM for 24 h, and verteporfin was used at a concentration of 2 µM for 24 h.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821194116/-/DCSupplemental.
References
- 1.Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N., Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N., VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2, 795–803 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Senger D. R., et al. , Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 (1983). [DOI] [PubMed] [Google Scholar]
- 4.Tischer E., et al. , Vascular endothelial growth factor: A new member of the platelet-derived growth factor gene family. Biochem. Biophys. Res. Commun. 165, 1198–1206 (1989). [DOI] [PubMed] [Google Scholar]
- 5.Goel H. L., Mercurio A. M., VEGF targets the tumour cell. Nat. Rev. Cancer 13, 871–882 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neufeld G., Kessler O., Herzog Y., The interaction of neuropilin-1 and neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv. Exp. Med. Biol. 515, 81–90 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Sulpice E., et al. , Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 111, 2036–2045 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Elaimy A. L., et al. , VEGF-neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP β2-chimaerin. Sci. Signal. 11, eaao6897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel H. L., et al. , P-Rex1 promotes resistance to VEGF/VEGFR-targeted therapy in prostate cancer. Cell Rep. 14, 2193–2208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaqoob U., et al. , Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res. 72, 4047–4059 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snuderl M., et al. , Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 152, 1065–1076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grun D., Adhikary G., Eckert R. L., NRP-1 interacts with GIPC1 and α6/β4-integrins to increase YAP1/∆Np63α-dependent epidermal cancer stem cell survival. Oncogene 37, 4711–4722 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goel H. L., et al. , GLI1 regulates a novel neuropilin-2/α6β1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol. Med. 5, 488–508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchini G., Balko J. M., Mayer I. A., Sanders M. E., Gianni L., Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 13, 674–690 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helleday T., Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis 31, 955–960 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Byrski T., et al. , Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol. 28, 375–379 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Isakoff S. J., et al. , TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J. Clin. Oncol. 33, 1902–1909 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silver D. P., et al. , Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J. Clin. Oncol. 28, 1145–1153 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Telli M. L., et al. , Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin. Cancer Res. 22, 3764–3773 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tutt A., et al. , Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT trial. Nat. Med. 24, 628–637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couch F. J., et al. , Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J. Clin. Oncol. 33, 304–311 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma P., et al. , Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: Implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res. Treat. 145, 707–714 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godin S. K., Sullivan M. R., Bernstein K. A., Novel insights into RAD51 activity and regulation during homologous recombination and DNA replication. Biochem. Cell Biol. 94, 407–418 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elstrodt F., et al. , BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res. 66, 41–45 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Goel H. L., et al. , Regulated splicing of the α6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep. 7, 747–761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geretti E., et al. , A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Mol. Cancer Res. 8, 1063–1073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caunt M., et al. , Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 13, 331–342 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Duarte A. A., et al. , BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance. Nat. Methods 15, 134–140 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Elaimy A. L., Mercurio A. M., Convergence of VEGF and YAP/TAZ signaling: Implications for angiogenesis and cancer biology. Sci. Signal. 11, eaau1165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varelas X., The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614–1626 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Pierce A. J., Johnson R. D., Thompson L. H., Jasin M., XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 13, 2633–2638 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enzo E., et al. , Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 34, 1349–1370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., et al. , RAD51 mediates resistance of cancer stem cells to PARP inhibition in triple-negative breast cancer. Clin. Cancer Res. 23, 514–522 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Raderschall E., et al. , Elevated levels of Rad51 recombination protein in tumor cells. Cancer Res. 62, 219–225 (2002). [PubMed] [Google Scholar]
- 35.Hine C. M., et al. , Regulation of Rad51 promoter. Cell Cycle 13, 2038–2045 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hine C. M., Seluanov A., Gorbunova V., Use of the Rad51 promoter for targeted anti-cancer therapy. Proc. Natl. Acad. Sci. U.S.A. 105, 20810–20815 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordenonsi M., et al. , The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Zanconato F., Cordenonsi M., Piccolo S., YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerami E., et al. , The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao J., et al. , Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng Z., Moroishi T., Guan K. L., Mechanisms of Hippo pathway regulation. Genes Dev. 30, 1–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adélaïde J., et al. , Integrated profiling of basal and luminal breast cancers. Cancer Res. 67, 11565–11575 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Wang C., et al. , The interplay between TEAD4 and KLF5 promotes breast cancer partially through inhibiting the transcription of p27Kip1. Oncotarget 6, 17685–17697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X., et al. , Tead and AP1 coordinate transcription and motility. Cell Rep. 14, 1169–1180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohgushi M., Minaguchi M., Sasai Y., Rho-signaling-directed YAP/TAZ activity underlies the long-term survival and expansion of human embryonic stem cells. Cell Stem Cell 17, 448–461 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Zhao W., et al. , BRCA1-BARD1 promotes RAD51-mediated homologous DNA pairing. Nature 550, 360–365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alshareeda A. T., et al. , Clinical and biological significance of RAD51 expression in breast cancer: A key DNA damage response protein. Breast Cancer Res. Treat. 159, 41–53 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Peng K., Bai Y., Zhu Q., Hu B., Xu Y., Targeting VEGF-neuropilin interactions: A promising antitumor strategy. Drug Discov. Today 24, 656–664 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Rizzolio S., et al. , Neuropilin-1 upregulation elicits adaptive resistance to oncogene-targeted therapies. J. Clin. Invest. 128, 3976–3990 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dutta S., et al. , Neuropilin-2 regulates endosome maturation and EGFR trafficking to support cancer cell pathobiology. Cancer Res. 76, 418–428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanconato F., et al. , Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. Nat. Med. 24, 1599–1610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budke B., et al. , RI-1: A chemical inhibitor of RAD51 that disrupts homologous recombination in human cells. Nucleic Acids Res. 40, 7347–7357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.