Significance
Plants recognize insect-derived molecules and make the accurate defense response during herbivory. However, insects release effectors that disturb host plant defense responses for fitness. Effectors are crucial components in biotic interactions. We identified a caterpillar-derived effector (HARP1) from oral secretion of cotton bollworm, a devastating agricultural pest. HARP1 is released from larvae to plant leaves during feeding and is able to migrate from wounding site into plant cells automatically. HARP1 interacts with JASMONATE-ZIM-domain (JAZ) proteins, the suppressor of Jasmonate (JA) pathway, and blocks signaling transduction by preventing JAZ degradation. HARP1-like proteins are widely distributed and have conserved function in noctuids, and they may contribute to insect adaptation to host plants during coevolution.
Keywords: insect effector, plant defense, jasmonate signaling, coevolution
Abstract
Insects have evolved effectors to conquer plant defense. Most known insect effectors are isolated from sucking insects, and examples from chewing insects are limited. Moreover, the targets of insect effectors in host plants remain unknown. Here, we address a chewing insect effector and its working mechanism. Cotton bollworm (Helicoverpa armigera) is a lepidopteran insect widely existing in nature and severely affecting crop productivity. We isolated an effector named HARP1 from H. armigera oral secretion (OS). HARP1 was released from larvae to plant leaves during feeding and entered into the plant cells through wounding sites. Expression of HARP1 in Arabidopsis mitigated the global expression of wounding and jasmonate (JA) responsive genes and rendered the plants more susceptible to insect feeding. HARP1 directly interacted with JASMONATE-ZIM-domain (JAZ) repressors to prevent the COI1-mediated JAZ degradation, thus blocking JA signaling transduction. HARP1-like proteins have conserved function as effectors in noctuidae, and these types of effectors might contribute to insect adaptation to host plants during coevolution.
Plants and insects have developed sophisticated mechanisms to adapt to each other during coevolution. About 50% of the insect species fed on plants. To escape or survive from attacks by herbivorous insects, plants are not only equipped with physical barriers (such as cuticles, trichomes, and thorns) and toxic compounds, but also initiate an intricate network of signal recognition and transduction upon insect challenge (1). In plants, the initial signal perception and transduction are essential for an appropriate defense against biotic stress. Plants can recognize herbivore-associated molecular patterns (HAMPs) and trigger various defense signal transduction (2, 3).
The phytohormone jasmonate (JA) plays an important role in activating defense against biotic attacks including chewing insects (4). CORONATINE INSENSITIVE1 (COI1), a component of the ubiquitin E3 ligase SCFCOI1, is the first reported jasmonoyl-l-isoleucine (JA-Ile) receptor (5–7). JASMONATE-ZIM-domain (JAZ) proteins bind to transcription factors such as MYC2 to restrict JA signal output (8). The contents of JA and JA-Ile in plant cells are maintained at a low level in the absence of stress and rise rapidly upon external stimuli, such as wounding or insect herbivory (1). JA-Ile promotes COI1-JAZ interaction and triggers JAZs degradation, releasing transcription factors to activate downstream defense genes (6, 9–11).
To adapt to their host plants, insects have developed multilayered mechanisms, including a highly specialized oral cavity for feeding and complex digestive systems for enzymatic processing of toxin-laden diets (12–15). In addition, herbivorous insects contain active molecules in their oral secretion (OS), which either trigger or interfere with plant defense during herbivory (16, 17). For example, certain fatty acid conjugates (FACs) and lipases in the OS of caterpillars can be recognized by plants and act as elicitors to induce plant defense response (18, 19). Even the proteolytic fragments of chloroplastic ATP synthase γ-subunit from Fabaceae plants in insect OS are able to elicit plant defense (20). Besides elicitors, the insect-released molecules that disturb host–plant defense response are defined as insect effectors (3). The first reported effector protein in a herbivorous insect is glucose oxidase (GOX) from Helicoverpa zea, which inhibits nicotine accumulation in tobacco (21). The presence of insect effectors was further supported by the observation that the Spodoptera littoralis larvae fed on leaves pretreated with the OS gained more weight increase than the larvae fed on control leaves (22). Analysis of secretory proteins in aphids revealed multiple effector proteins in saliva of sucking insects (23–27). A set of salivary glands secreted proteins from green peach aphid (Myzus persicae) have distinct locations in its infected plants, indicating that these proteins may be directly involved in plant–aphid interaction (28, 29). The well-studied effectors in saliva, ApC002 from the pea aphid (Acyrthosiphon pisum) and its homolog in the green peach aphid (M. persicae, MpC002), were helpful for aphid fitness when reared on plants (23, 27). Recently, a new effector Bt56 from whitefly (Bemisia tabaci) was identified, which helped whitefly become more adapted to host plants (30). However, most known insect effectors are isolated from sucking insects and very few effectors other than GOX from chewing insects have been reported. Furthermore, the targets of insect effectors and the working mechanism of how insect effectors counteract host plant defense remains largely enigmatic.
Cotton bollworm (Helicoverpa armigera) is a chewing insect and one of the most devastating pests in agriculture. In this study, we identified an effector, HARP1, from H. armigera OS. HARP1 shows similarity to venom R-like proteins from the parasitoid wasp (Nasonia vitripennis) venom glands, which were proposed to interfere with the animal host immune system (31). Our investigation demonstrates that HARP1 is able to migrate into host plant cells and interact with multiple JAZ repressors. HARP1–JAZ interaction stabilizes JAZ degradation and blocks JA signaling transduction. REPAT38, a homolog in Spodoptera exigua, acts similarly as HARP1, which, together with the wide distribution of HARP1 homologs in insects, suggests a relation between the evolution of HARP1-like proteins and host adaptation in herbivorous insects.
Results
HARP1 Is an Effector in Cotton Bollworm OS Impairing Plant Wounding Response.
OS of a lepidopteran larva contains the mixture of saliva and regurgitant from the insect gut (32). When insects were fed on plants, the OS sticks to the leaf wounding sites and this made it possible for OS to directly interact with plant signals and modulate plant defense. To test the activity of insect OS on plant defense, we collected OS from cotton bollworm and painted it on the mechanically wounded sites of Arabidopsis leaves. As the JA alarm signal is rapidly triggered by wounding, we first examined the expressions of JA-responsive genes, including LOX2, MYC2, and VSP2. We found that the induction of JA-regulated genes by wounding was attenuated in the OS-treated leaves (SI Appendix, Fig. S1). We then performed proteomic analyses of the OS collected from the fourth-instar H. armigera larvae fed on artificial diet or Arabidopsis leaves by liquid chromatography-mass spectrometry (LC-MS). In total, 149 proteins were identified (Dataset S1). The accumulation of 65 proteins in the OS sample from the larvae fed on Arabidopsis leaves was increased (>1.5 fold) compared with that fed on artificial diet, among which 49 proteins were digestion related (Dataset S1). We examined the remaining 16 proteins (SI Appendix, Table S1) and found that one is similar to a probable salivary secreted protein of Spodoptera litura. Further sequence analysis revealed that this protein is also similar to venom R-like proteins from carnivorous insects, such as parasitoid wasps (N. vitripennis, Trichomalopsis sarcophagae) and assassin bug (Pristhesancus plagipennis) (SI Appendix, Table S2). We thus named it HARP1 (H. armigera R-like protein 1). HARP1 is 122 amino acids in length and contains a predicted signal peptide at the N terminus (analyzed by “SignalP 4.1 Server”, http://www.cbs.dtu.dk/services/SignalP/). As venom proteins in the carnivorous insects often target to host immune system during predation (31) and the HARP1 protein accumulation in OS increases upon herbivory, we wondered whether this class of proteins in herbivorous insects also affect the host plant defense.
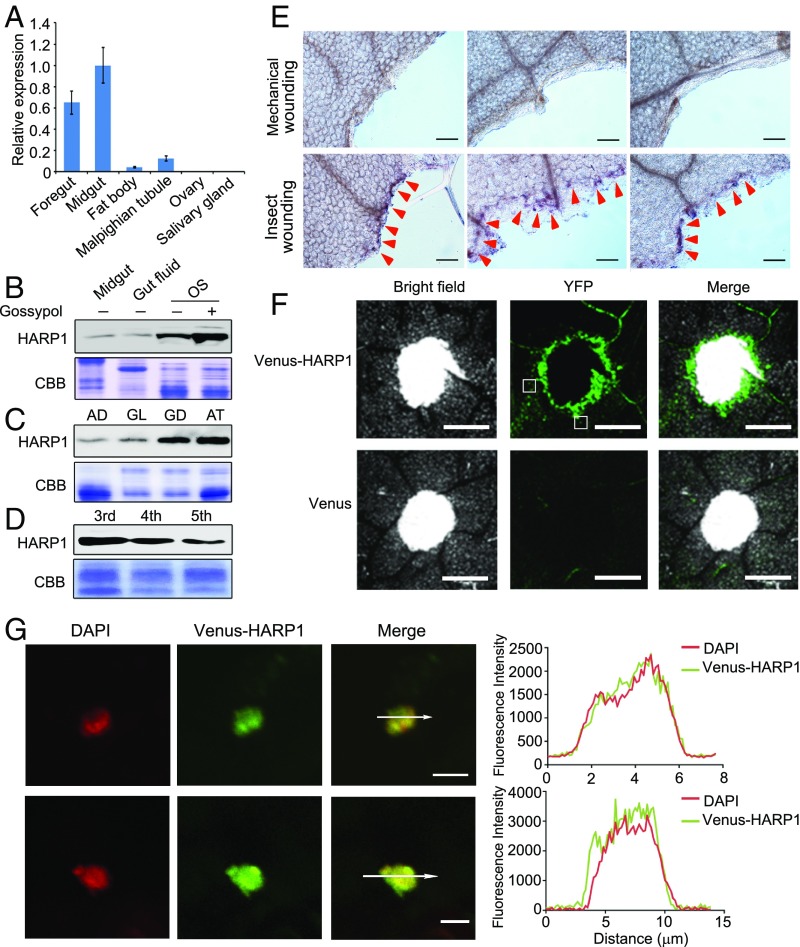
HARP1 transcripts were abundant in foregut and midgut tissues but near the limit of detection in salivary glands (Fig. 1A). Interestingly, the HARP1 protein level was much higher in OS than in midgut and gut fluid, and notably, its accumulation was increased in OS when the larvae fed on the artificial diet supplemented with 0.1% gossypol, the major defense compound in cotton plants (33) (Fig. 1B), which also stimulates the expression of defensive P450 genes in cotton bollworm midgut (12, 13). The HARP1 accumulation in OS also varied with host plants, as larvae fed on the glanded cotton cultivar with high gossypol concentrations and the nonpreferred Arabidopsis had a higher level of HARP1 than the larvae fed on the glandless cotton cultivar, which is a gossypol-free mutant (34) (Fig. 1C). It seems that HARP1 can be induced when the larvae fed on a rather toxic or unfavorable food. The HARP1 was enriched in OS from the third, fourth, and fifth instar larvae (Fig. 1D). Whole amount immunohistochemistry revealed that HARP1 was deposited to the larval injured sites of the Arabidopsis leaves during feeding (Fig. 1E). To visualize the HARP1 protein in plant tissues, we fused HARP1 with Venus, a version of the green florescence protein (GFP). After 1-h incubation of the mechanically wounded leaves with the prokaryotically expressed recombinant protein, we found that the Venus-HARP1 fusion proteins were circled around the wounded site of the leaves, but the Venus alone did not (Fig. 1F). Notably, a portion of the Venus-HARP1 was found in the nucleus (Fig. 1G). These results implied a possibility that HARP1 is able to function in plant cell.
Fig. 1.
HARP1 in H. armigera OS migrates into the leaf cells through the wounding damage sites. (A) qRT-PCR analysis of HARP1 transcripts in indicated tissues of fifth-instar larvae. The expression level in midgut was set to 1. Error bars represent ± SD (n = 3 biological replicates). (B–D) Immunoblot detection of HARP1 protein level. The protein amount in each loading was quantified by Bradford assay and visualized by Coomassie Brilliant Blue (CBB) staining. All of the experiments were repeated at least two times, and the results were consistent. In B, the fourth instar larvae were fed on artificial diet supplemented with (+) or without (−) 0.1% gossypol for 1 d, and total proteins were collected from midgut, gut fluid and OS. In C, the OS was collected from the fourth instar larvae fed on artificial diet (AD), glandless (GL), or glanded (GD) cotton and A. thaliana (AT) leaves for 1 d. In D, the OS was collected from the indicated instar larvae that were fed on AT leaves for 1 d. (E) Whole amount immunohistochemistry detection of HARP1 at the chewing sites (red arrows) of Arabidopsis leaves. The mechanical wounding leaves were used as negative control. Anti-HARP1 antibody was used to detect HARP1 in B–E. (F and G) Translocation of the Venus-HARP1 fusion protein into plant cells through damage sites. The Arabidopsis leaves were punched and incubated with the protein solutions of Venus-HARP1 or Venus for 1 h and washed for three to four times to remove the extra proteins that adhered on the leaf surface. The boxes indicate the location as shown in G. (F) Venus-HARP1 but not Venus was detected at the wounded sites. (G) A portion of Venus-HARP1 was located in the nucleus of leaf cell. Fluorescence intensity in cross-section (white arrow) is shown. (Scale bars: E, 100 μm; F, 500 μm; G, 5 μm.)
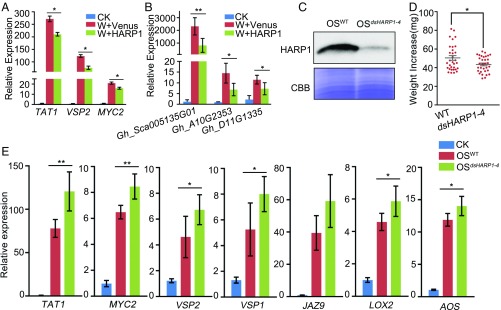
We then use the host plant cotton (Gossypium hirsutum) of the cotton bollworm as well as the nonpreferred host plant Arabidopsis to detect the effects of HARP1 on plant defense. The recombinant HARP1 protein was applied to the leaf injured sites. Similar to the OS treatment, the recombinant HARP1 reduced the wound induction of TAT1, VSP2, and MYC2 expressions in Arabidopsis leaves (Fig. 2A). Plant protease inhibitor can be induced by insect herbivory and mechanical wounding, and it was regarded as an antiinsect defense compound (35). In cotton (G. hirsutum), three genes encoding protease inhibitors (Gh_Sca005135G01, Gh_A10G2353, and Gh_D11G1335) showed a rapid response to wounding, and their wound induction in leaves was significantly suppressed by HARP1 application (Fig. 2B). We then generated transgenic Arabidopsis to produce double-stranded RNAs homologous to HARP1 (35S:dsHARP1). We selected 35S:dsHARP1-4, which had high expression of dsHARP1 by qRT-PCR detection (SI Appendix, Fig. S2A) for further investigation. When cotton bollworm larvae fed on leaves from 35S:dsHARP1-4, the larvae exhibited retarded growth and the HARP1 accumulation in OS was obviously reduced (Fig. 2 C and D). Accordingly, the defense genes had higher induction in the plants painted with the OS from the larvae fed on 35S:dsHARP1-4 (OS35S:dsHARP1-4) on the wounding sites than that painted with OS from the larvae fed on wild-type plants (OSWT) (Fig. 2E). Together, these results demonstrate that HARP1 may be one of the OS effectors which suppress plant defense signaling.
Fig. 2.
HARP1 reduces plant response to wounding. (A and B) Genes were less induced upon wounding in the presence of HARP1. Arabidopsis (A) and cotton (B) leaves were mechanically wounded and painted with the prokaryotically expressed HARP1 (W+HARP1) or Venus (W+Venus) solutions (1 mg/mL) on the wounded sites. Samples were collected 4 h later, and the gene expressions were detected by qRT-PCR. The expression in unwounded plants (CK) was set to 1. Data were analyzed by Student’s t test. *P < 0.05, **P < 0.01. Error bars represent ± SD (n = 3 biological replicates in A, n = 5 biological replicates in B). All of the experiments were repeated three times, and the results were consistent. (C–E) The impacts of HARP1 reduction in OS on the larval adaptation to plants. (C) HARP1 accumulation was reduced in OS from the larvae fed on 35S:dsHARP1 plants. Third-instar larvae were fed with wild-type (WT) and 35S:dsHARP1-4 plant leaves for 4 d. HARP1 level in OS samples from the larvae fed on the WT (OSWT) and the 35S:dsHARP1-4 (OSdsHARP1-4) Arabidopsis leaves were detected by immunoblot. The amount of total proteins in each loading was quantified by Bradford assay and visualized by CBB staining. (D) The growth of H. armigera larvae fed with 35S:dsHARP1-4 plants was inhibited compared with those fed with wild type. Weight increases of third-instar larvae fed on Arabidopsis leaves of WT and 35S: dsHARP1-4 (dsHARP1-4) for 4 d were recorded. Data were analyzed by Student’s t test. *P < 0.05. Error bars represent ± SEM (n = 32). (E) Gene expressions in plant leaves after the treatment with the OS samples as described in C. Arabidopsis leaves were wounded and painted with OSWT and OSdsHARP1-4, respectively; samples were collected 2 h after treatment. qRT-PCR was used to detect gene expressions. The gene expression in the unwounded plants (CK) was set to 1. *P < 0.05, **P < 0.01. Error bars represent ± SD (n = 5 biological replicates).
Expression of HARP1 in Arabidopsis Reduces the Insect Resistance and JA-Dependent Wounding Response.
To reveal the role of HARP1 in affecting plant defense, Arabidopsis plants were engineered to express HARP1 (35S:6MYC-HARP1), which grew normally in our greenhouse. Two lines with high (line 1 and line 7) and one line with low (line 2) HARP1 expression levels (SI Appendix, Fig. S2B) were selected for further analyses. H. armigera larvae fed on the plants with high-level HARP1 gained significantly more weight than those fed on the wild type, whereas the larval growth was not obviously affected when fed on the plants with low-level HARP1 (SI Appendix, Fig. S3 A and B). Consistent with the HARP1 painting assay, the JA-responsive genes of TAT1, MYC2, and VSP2 were less induced in 35S:6MYC-HARP1-1 plants but not in the low-level HARP1 plants 35S:6MYC-HARP1-2 by mechanical wounding than that in wild-type plants (SI Appendix, Fig. S3 C and D).
To investigate the global effects of HARP1 on plant wounding response, we performed RNA-sequencing (RNA-seq) using the wild-type and the 35S:6MYC-HARP1 (line 1) plant leaves. In the wild type, mechanical wounding led to a significant transcriptional reprogramming: 1,224 wound-induced genes (WIGs) and 1,395 wound-repressed genes (WRGs) were detected. However, the up- and down-regulated genes were reduced to 512 and 259, respectively, in the 35S:6MYC-HARP1 (SI Appendix, Fig. S4A and Dataset S2). This indicated that the plant wounding response was largely affected by the existence of HARP1. We then analyzed the expression level of 1,224 WIGs in the 35S:6MYC-HARP1 plant and found that 418 of them showed less or no induction after wounding (SI Appendix, Fig. S4B). Gene Ontology (GO) enrichment analysis revealed that these 418 HARP1-affected genes were enriched in several exogenous stimulus-related pathways, including response to JA stimulus (P = 5.5e−9) (SI Appendix, Fig. S4C). In wild-type plants, 34 genes clustered in the GO term, response to JA stimulus, were obviously induced by wounding, whereas 16 of these genes, about 47% (16/34), showed less or no induction in 35S:6MYC-HARP1 plant (SI Appendix, Fig. S4D). Further analysis by qRT-PCR confirmed 9 of the 13 selected HARP1-affected genes were obviously less induced by wounding in 35S:6MYC-HARP1 (SI Appendix, Table S3 and Fig. S5). Furthermore, the induction was JA-dependent as the wounding induction of these nine genes was largely suppressed in the JA-insensitive mutant coi1-2 (5) (SI Appendix, Fig. S6). These data demonstrate that expression of HARP1 in transgenic plants hampered the JA-Ile signal-mediated wounding response.
HARP1 Directly Interacts with JAZ Proteins from Arabidopsis, Cotton, and Tobacco.
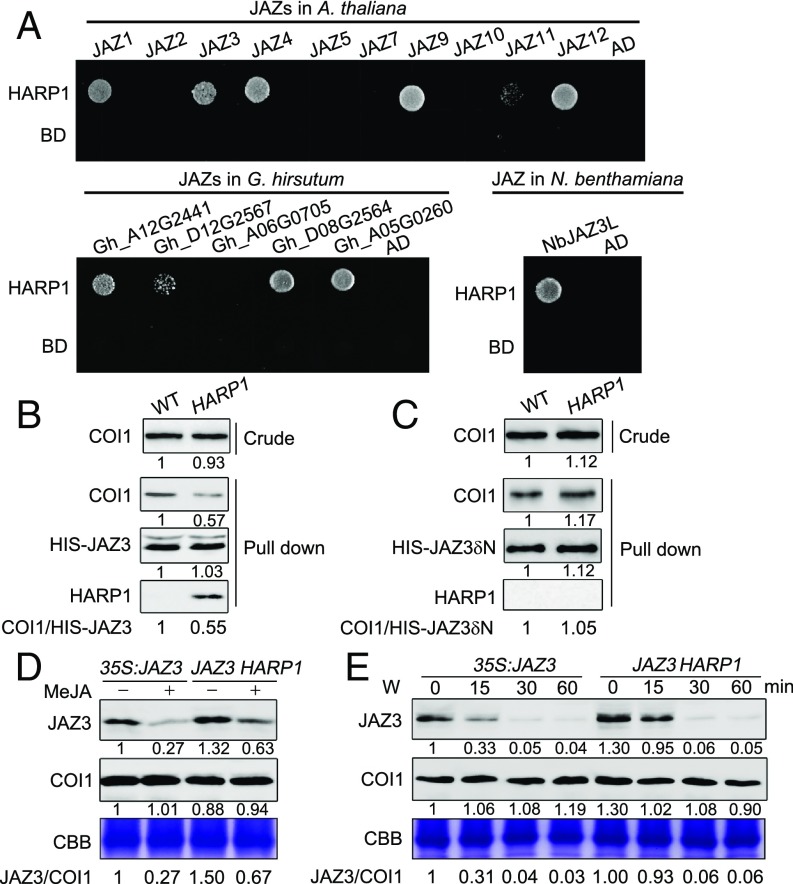
Transient expression of a GFP-HARP1 fusion protein in tobacco (Nicotiana benthamiana) leaves showed that the protein was mainly localized in nucleus (SI Appendix, Fig. S7). This finding, combined with the observation that the recombinant Venus-HARP1 is able to translocate into the nucleus of plant cell (Fig. 1G), suggests that HARP1 may affect the JA signal transduction pathway within the nucleus. As JAZ proteins are nucleus-localized and the master negative regulators of JA signaling (10), we asked whether HARP1 acted on the JAZs to intercept JA signaling. Yeast two-hybrid assay was used to examine the HARP1 interactions with JAZ proteins from the host plant cotton (G. hirsutum), as well as from the nonpreferred hosts of Arabidopsis (A. thaliana) and tobacco (N. benthamiana). We found that HARP1 exhibited a clear binding activity to multiple JAZ proteins, including 5 of the 10 Arabidopsis JAZs and four of the five cotton JAZs tested, in addition to the one tobacco JAZ (Fig. 3A and SI Appendix, Fig. S8A). The Arabidopsis JAZ3 (AT3G17860) was then used in subsequent experiments. A JAZ3 variant lacking the C-terminal Jas motif (JAZ3δC) retained the ability to bind with HARP1, whereas removal of the ZIM domain (JAZ3δZIM) or ZIM-containing N terminus (JAZ3δN) abolished the interaction (SI Appendix, Fig. S9 A and B), indicating that ZIM domain is required for JAZ3–HARP1 interaction. 35S:JAZ3δN-HA and 35S:JAZ3δC-HA plant (36) were then used for pull-down assay. JAZ3δN-HA and JAZ3δC-HA were both detectable in total protein extractions of 35S:JAZ3δN-HA and 35S:JAZ3δC-HA plant leaves but only JAZ3δC-HA could be coimmunoprecipitated with HIS-HARP1 (SI Appendix, Fig. S9C). These results indicate that HARP1 binds to the N-terminal region of JAZ3.
Fig. 3.
HARP1 interacts with and stabilizes JAZ proteins. (A) Yeast two-hybrid assay. HARP1 was fused to GAL4 DNA-binding domain (BD), JAZ proteins of Arabidopsis (A. thaliana), cotton (G. hirsutum), and tobacco (N. benthamiana) were fused to GAL4 activation domain (AD), respectively. Interactions were examined with 1 mM 3-amino-1,2,4-triazole. (B and C) HARP1 reduces COI1-JAZ3 coprecipitation. Recombinant proteins of HIS-JAZ3 (B) and HIS-JAZ3δN (C) were incubated with total leaf proteins of the wild-type (WT) and 35S:6MYC-HARP1-1 (HARP1) Arabidopsis in the presence of 50 μM Coronatine. Anti-COI1 antibody was used to detect COI1 level before (Crude) or after (Pull down) immunoprecipitation. Anti-MYC antibody was used to detect the 6MYC-HARP1 (HARP1), and Anti-HIS antibody was used to detect HIS-JAZ3 and HIS-JAZ3δN. Band intensity was quantified by ImageJ and was shown under each blot. The intensity of the WT was set to 1. The relative COI1/HIS-JAZ3 ratios were listed in the bottom. The coimmunoprecipitation of COI1 with HIS-JAZ3 but not HIS-JAZ3δN was inhibited in the presence of HARP1. (D and E) HARP1 increases JAZ3 accumulation. The JAZ3-HA level is more stable in 35S:JAZ3-HA 35S:6MYC-HARP1 than in 35S:JAZ3-HA after 50 μM MeJA (D) or wounding (E) treatment. The plant leaves were collected 45 min after MeJA treatment or at the indicated time after wounding. Anti-HA antibody was used to detect JAZ3-HA (JAZ3). Band intensity was quantified by ImageJ and was shown under each blot. The intensity of the untreated 35S:JAZ3 –HA sample was set to 1. The relative JAZ3/COI1 ratios were listed in the bottom. The amount of total proteins in each loading was quantified by Bradford assay and visualized by CBB staining.
We next examined the molecular consequences of HARP1–JAZ3 interaction. Independent pull-down assays three times revealed the consistent results that HARP1 reduced the JAZ3-COI1 coprecipitation (Fig. 3B and SI Appendix, Fig. S9D). The JAZ3 C-terminal fragment (JAZ3δN) still could interact with COI1 (37) but not HARP1 (SI Appendix, Fig. S9 A–C), and accordingly, the JAZ3δN–COI1 interaction was not affected by HARP1 (Fig. 3C). We then generated 35S:6MYC-HARP1 35S:JAZ3-HA and 35S:6MYC-HARP1 35S:JAZ3δN-HA plants by crossing. Upon wounding and MeJA treatments, the JAZ3-HA accumulated to a higher level when HARP1 was coexpressed (Fig. 3 D and E and SI Appendix, Fig. S10 A and B). However, the level of JAZ3δN-HA, which was free of HARP1 binding, was not affected by 35S:6MYC-HARP1 (SI Appendix, Fig. S10 C and D). These results indicate that HARP1 stabilizes JAZ3, likely through interfering with the COI1-mediated protein degradation.
The Effector Activity of HARP1 Is Eliminated in the jaz Quintuple Mutant.
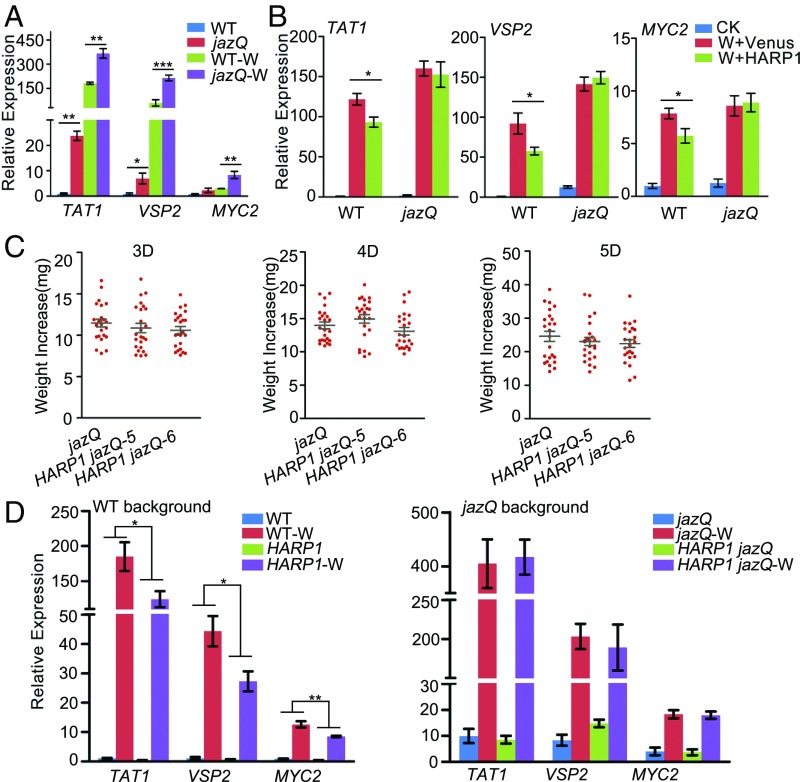
To provide further evidence that the cotton bollworm effector HARP1 attenuates the JA-mediated plant defense through JAZ proteins, we used a jaz quintuple mutant (jazQ), which is hypersensitive to JA treatment because five JAZ genes (JAZ1/3/4/9/10) were disrupted (38), and these mutated genes largely overlap with the five JAZ proteins (JAZ1/3/4/9/12) interacted with HARP1 in the yeast two-hybrid assay. In general, the larvae fed with jazQ leaves grew more slowly than those fed with wild-type leaves (SI Appendix, Fig. S11), and the JA response genes (VSP2, TAT1, MYC2) were induced to a higher degree in jazQ upon wounding treatment (Fig. 4A), consistent with the previous report (39). In contrast to the wild-type Arabidopsis, application of recombinant HARP1 to the wounded sites of jazQ leaves did not obviously repress the induction of the JA-response genes (Fig. 4B). HARP1 was then overexpressed in jazQ (35S:6MYC-HARP1 jazQ). Two independent lines (line 5 and line 6) with high and similar HARP1 level to that of the 35S:6MYC-HARP1-1 were further analyzed (SI Appendix, Fig. S2C). In the wild-type background, the high level of HARP1 led to a clear attenuation of wounding response and reduced the resistance upon cotton bollworm feeding. By contrast, no obvious difference in larval growth was observed between the jazQ and 35S:6MYC-HARP1 jazQ groups; the jazQ and 35S:6MYC-HARP1 jazQ plants responded to wounding similarly (Fig. 4 C and D). These data demonstrate that JAZ proteins are required by HARP1 to attenuate the plant JA responses and insect resistance.
Fig. 4.
Wounding responses and insect resistance were not obviously affected by HARP1 in the jaz quintuple mutant jazQ. (A) Wounding responses were higher in jazQ. The wild-type (WT) and jazQ leaves were treated with mechanical wounding; gene expressions in leaves of the unwounded and the wounded (W) plants were detected by qRT-PCR 4 h after treatment. The gene expressions in unwounded WT were set to 1. Data were analyzed by Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. Error bars represent ± SD (n = 3 biological replicates). (B) The gene inductions were not affected by HARP1 in the jazQ mutant upon wounding. The prokaryotically expressed HARP1 (W+HARP1) and Venus (W+Venus) was painted on the wounding sites of WT and jazQ plant leaves. The unwounded plants were used as control (CK). Gene expressions were detected by qRT-PCR 4 h after treatment. The gene expressions in unwounded WT were set to 1. Data were analyzed by Student’s t test. *P < 0.05. Error bars represent ± SD (n = 5 biological replicates). (C) H. armigera larvae gained similar weight increase when fed on 35S:6MYC-HARP1 jazQ (HARP1 jazQ) and jazQ leaves. The third-instar larvae were fed with plant leaves for indicated days, and the weight increases were measured (n = 24). (D) 35S:6MYC-HARP1 jazQ and jazQ plants exhibited similar gene inductions upon wounding. The HARP1 expressing plants under WT and jazQ background were treated with wounding (W) and samples of WT, 35S:6MYC-HARP1 (HARP1), jazQ, and 35S:6MYC-HARP1 jazQ (HARP1 jazQ) were collected 4 h after treatment. Gene expressions were detected by qRT-PCR. The expression in the unwounded WT was set to 1. Data were analyzed by two-way ANOVA followed by multiple comparisons (Tukey test). (*P < 0.05, **P < 0.01). Error bars represent ± SD (n = 5 biological replicates). All of the experiments were repeated at least two times, and the results were consistent.
Conserved Function of HARP1-Like Proteins in Noctuids.
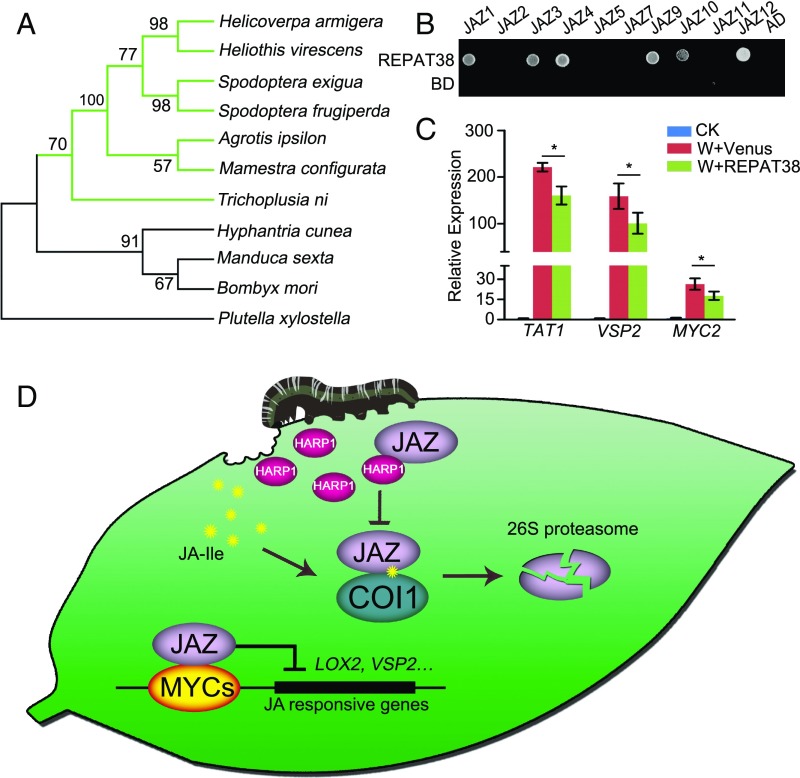
Phylogenetic analyses revealed that HARP1-like proteins are widely present in Lepidoptera and highly conserved in noctuid insects (Fig. 5A and SI Appendix, Table S4). REPAT38 (AFH57158.1) from beet armyworm (S. exigua) (40) shares 83% identity with HARP1 (SI Appendix, Table S4). Like HARP1, REPAT38 interacted with multiple JAZs of Arabidopsis (Fig. 5B and SI Appendix, Fig. S8B). Spraying the purified recombinant REPAT38 to the wounded sites of Arabidopsis leaves led to reduced inductions of VSP2, MYC2, and TAT1 (Fig. 5C). The 35S:6MYC-REPAT38 Arabidopsis plants were generated and screened by immune blot assay, and 35S:6MYC-REPAT38-4 was chosen for further assay (SI Appendix, Fig. S3C). We found that, similarly to HARP1, REPAT38 reduced the resistance to insect feeding (SI Appendix, Fig. S12A) and the JA-regulated wounding response (SI Appendix, Fig. S12B) in transgenic Arabidopsis. These results suggest that the function of HARP1-like proteins is likely conserved in noctuid insects.
Fig. 5.
HARP1-like proteins highly conserved in Noctuidae and have similar function as effectors. (A) Phylogenetic analysis of HARP1 proteins in Lepidoptera insects. HARP1 (H. armigera) and the homologous proteins in Heliothis virescens, Spodoptera frugiperda, S. exigua, Agrotis ipsilon, Mamestra configurata, Trichoplusia ni, Hyphantria cunea, Manduca sexta, Bombyx mori, and P. xylostella were analyzed. The green lines represent these insects belongs to Noctuidae. (B) Yeast two-hybrid assay. REPAT38 was fused to GAL4 DNA-binding domain (BD), and the indicated JAZs were fused to GAL4 activation domain (AD), respectively. Interactions were examined with 1 mM 3-amino-1,2,4-triazole. (C) Gene expressions were less induced upon wounding in the presence of REPAT38. Samples were collected 2 h after treatment (W), and the indicated gene expressions were detected by qRT-PCR. The gene expression in unwounded plant was set to 1. Data were analyzed by Student’s t test. *P < 0.05, Error bars represent ± SD (n = 3 biological replicates). (D) Working model of HARP1 in plant–insect interaction. When H. armigera larvae feed on plant leaves, the wounding damage triggers the JA signaling. Meanwhile, the larval effector HARP1 in OS is released into plant cells and interacts with JAZs to prevent COI1–JAZ interaction and JAZ degradation, repressing the JA-regulated defense response.
The Oligophagous Plutella xylostella Is More Adaptable to HARP1-Expressed Nonhost Plants.
Diamondback moth (P. xylostella) is a lepidopteran species specifically lived on Brassica plants (41). The less conserved HARP1 homolog from diamondback P. xylostella, PXHL1 (XP_011548876.1), is 41% identical to HARP1 (SI Appendix, Table S4). In yeast two-hybrid assay, PXHL1 did not interact with JAZs of Arabidopsis except JAZ4 (SI Appendix, Figs. S8C and S13A), which had a relative low transcript level in leaves based on our RNA-seq data (Dataset S2). GFP and 6MYC-HARP1 was transiently expressed in N. benthamiana leaves, respectively (SI Appendix, Fig. S13B). In general, the growth was largely inhibited when the P. xylostella larvae were fed with the nonhost tobacco (N. benthamiana). Interestingly, when fed on the N. benthamiana leaves expressing HARP1, the larvae gained more weight than those fed on the GFP-expressing leaves (SI Appendix, Fig. S13C). These results suggest that HARP1 could improve the performance of oligophagous insects to nonhost plants.
Discussion
Various types of insect elicitors are well studied (42), and a recent report reveals that some insect gut microbes release to OS through regurgitation and eliciting plant defense (43). Compared with the extensive studies of elicitors, relatively few studies are focused on insect effectors. In this study, we isolated a chewing insect-derived effector, HARP1, which is released from OS to plant cells and intercept JAZ degradation through interfering with COI1–JAZ3 interaction, thus attenuating JA signaling transduction triggered by insect-wounding damages (Fig. 5D). Although the binding sites of HARP1 and JAZ3 did not overlap with the jas degron, which is required for JAZ3–COI1 interaction, it was possible that the combination of HARP1 and JAZ3 affected the spatial structure of the C-terminal of JAZ3 and, thus, the JAZ3–COI1 interaction. Such regulation of JAZ stabilization was also reported in recent reports (36, 44). Insects have multiple and complex strategies adapting to their host plants. Effectors like HARP1 gives an example that insects can interfere with the plant defense response for better subsistence. Insect OS contains abundant signaling molecules including elicitors and effectors and plays important roles in insect–plant interactions (45, 46); there should be other effectors that contribute to adapting to host plant.
The phytohormone JA plays an important role in regulating the inductive defense against insect herbivores in plants (22). JAZ proteins, the main repressors of JA signaling, also serve as the molecular link in connecting to other signaling pathways (36, 44, 47, 48). In plant biotic interaction, some pathogen-derived effectors target JAZ proteins for successful infections (44, 49). Although the effectors from microbial pathogens have been extensively studied (50–53), examples of the insect effectors, especially from chewing insects, are limited, and HARP1 represents a type of insect effector that hijacks JAZ proteins. Our study reveals that microbe pathogen and insects use the similar strategy to interfere plant defense signaling for fitness.
The fourth and fifth instar larvae of cotton bollworm are large in size and have a big appetite, which causes fast biomass loss of host plants. It seems that the induced plant defense is less effective against such large insects. However, a newly hatched larva is vulnerable to plant defense, ingests less, and causes minor injuries to the host plant. Therefore, the struggle between host plant and the newborn larvae is crucial for both sides. JA-regulated defense response is conserved in higher plants. Release the effector proteins like HARP1 to weaken the JA response in the host plant during feeding seems to be an effective way for young larvae to survive at the very early life stage.
Insect herbivores have developed multiple adaptive mechanisms along with their feeding habits. Oligophagous insects evolved specific detoxification systems, which enable insects to successfully live on a defined group of plants accumulating similar specialized metabolites usually toxic to insects. For example, the diamondback moth is specified to live on Brassica plants and has developed glucosinolate sulfatases to desulfates glucosinolates, blocking the formation of cyanogenic products in Brassicas (54). However, the polyphagous insects are compatible to a broad range of plants. Since HARP1 can bind with JAZ proteins from different groups of plants (Fig. 3A), it may constitute one of the molecular basis for cotton bollworm to use a wide variety of plant species as food resources. Our findings suggest that the HARP1-type effectors may have consequences in the evolution of insect adaptation to their host. Further identification of effectors from herbivorous insects will enrich our knowledge about plant-insect coevolution and help design novel strategies to control the populations of chewing pests.
Materials and Methods
The detailed information about plant materials and treatments, insect culture and feeding test, OS collection and preparation, gene expression, immunoblot, immunohistochemistry and pull-down assays, transcriptome analysis and proteomic analyses are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Gregg A. Howe for jazQ seeds and helpful discussions and suggestions; Dao-Xin Xie for coi1-2 seeds and Anti-COI1 antibody; Jia-Wei Wang, Xiu-Fang Xin, Sheng-Yang He, and Saskia A. Hogenhout for helpful discussions and suggestions; and Wen-Juan Cai for confocal microscopy assistance. This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences Grant XDB11030000; National Natural Sciences of China Grants 31772177 and 31788103; Chinese Academy of Sciences Grant QYZDY-SSW-SMC026; and The Ministry of Agriculture of China Grant 2014ZX08009001-009.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905471116/-/DCSupplemental.
References
- 1.Erb M., Meldau S., Howe G. A., Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 17, 250–259 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boller T., Felix G., A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Hogenhout S. A., Bos J. I., Effector proteins that modulate plant–Insect interactions. Curr. Opin. Plant Biol. 14, 422–428 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Wu J., Baldwin I. T., New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 44, 1–24 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Xie D. X., Feys B. F., James S., Nieto-Rostro M., Turner J. G., COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280, 1091–1094 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Sheard L. B., et al. , Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468, 400–405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsir L., Schilmiller A. L., Staswick P. E., He S. Y., Howe G. A., COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. U.S.A. 105, 7100–7105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An C., et al. , Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc. Natl. Acad. Sci. U.S.A. 114, E8930–E8939 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F., et al. , Structural basis of JAZ repression of MYC transcription factors in jasmonate signalling. Nature 525, 269–273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chini A., et al. , The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448, 666–671 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Thines B., et al. , JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448, 661–665 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Mao Y. B., et al. , Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 25, 1307–1313 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Tao X. Y., Xue X. Y., Huang Y. P., Chen X. Y., Mao Y. B., Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide. Mol. Ecol. 21, 4371–4385 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Chen D., Chen F., Chen C., Chen X., Mao Y., Transcriptome analysis of three cotton pests reveals features of gene expressions in the mesophyll feeder Apolygus lucorum. Sci. China Life Sci. 60, 826–838 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Li X., Schuler M. A., Berenbaum M. R., Jasmonate and salicylate induce expression of herbivore cytochrome P450 genes. Nature 419, 712–715 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Stam J. M., et al. , Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 65, 689–713 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Pandey S. P., et al. , Simulated herbivory in chickpea causes rapid changes in defense pathways and hormonal transcription networks of JA/ethylene/GA/auxin within minutes of wounding. Sci. Rep. 7, 44729 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alborn H., et al. , An elicitor of plant volatiles from beet armyworm oral secretion. Science 276, 945–949 (1997). [Google Scholar]
- 19.Schäfer M., et al. , Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiol. 156, 1520–1534 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmelz E. A., et al. , Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. U.S.A. 103, 8894–8899 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musser R. O., et al. , Herbivory: Caterpillar saliva beats plant defences. Nature 416, 599–600 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Consales F., et al. , Insect oral secretions suppress wound-induced responses in Arabidopsis. J. Exp. Bot. 63, 727–737 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bos J. I., et al. , A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 6, e1001216 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elzinga D. A., Jander G., The role of protein effectors in plant-aphid interactions. Curr. Opin. Plant Biol. 16, 451–456 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Naessens E., et al. , A secreted MIF cytokine enables aphid feeding and represses plant immune responses. Curr. Biol. 25, 1898–1903 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Will T., Tjallingii W. F., Thönnessen A., van Bel A. J., Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. U.S.A. 104, 10536–10541 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutti N. S., et al. , A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc. Natl. Acad. Sci. U.S.A. 105, 9965–9969 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elzinga D. A., De Vos M., Jander G., Suppression of plant defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol. Plant Microbe Interact. 27, 747–756 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mugford S. T., Barclay E., Drurey C., Findlay K. C., Hogenhout S. A., An immuno-suppressive aphid saliva protein is delivered into the cytosol of plant mesophyll cells during feeding. Mol. Plant Microbe Interact. 29, 854–861 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Xu H. X., et al. , A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 116, 490–495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G., Schmidt O., Asgari S., A novel venom peptide from an endoparasitoid wasp is required for expression of polydnavirus genes in host hemocytes. J. Biol. Chem. 279, 41580–41585 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Vadassery J., Reichelt M., Mithöfer A., Direct proof of ingested food regurgitation by Spodoptera littoralis caterpillars during feeding on Arabidopsis. J. Chem. Ecol. 38, 865–872 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Tian X., et al. , Characterization of gossypol biosynthetic pathway. Proc. Natl. Acad. Sci. U.S.A. 115, E5410–E5418 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma D., et al. , Genetic basis for glandular trichome formation in cotton. Nat. Commun. 7, 10456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunse K. M., et al. , Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc. Natl. Acad. Sci. U.S.A. 107, 15011–15015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao Y. B., et al. , Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 8, 13925 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larrieu A., et al. , A fluorescent hormone biosensor reveals the dynamics of jasmonate signalling in plants. Nat. Commun. 6, 6043 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos M. L., et al. , Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 7, 12570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Major I. T., et al. , Regulation of growth-defense balance by the JASMONATE ZIM-DOMAIN (JAZ)-MYC transcriptional module. New Phytol. 215, 1533–1547 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro-Cerrillo G., Hernández-Martínez P., Vogel H., Ferré J., Herrero S., A new gene superfamily of pathogen-response (repat) genes in Lepidoptera: Classification and expression analysis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 164, 10–17 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Landry J. F., Hebert P. D., Plutella australiana (Lepidoptera, Plutellidae), an overlooked diamondback moth revealed by DNA barcodes. ZooKeys, 43–63 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu S., Varsani S., Louis J., Altering plant defenses: Herbivore-associated molecular patterns and effector Arsenal of chewing herbivores. Mol. Plant Microbe Interact. 31, 13–21 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Wang J., et al. , Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s). New Phytol. 214, 1294–1306 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Wu D., et al. , Viral effector protein manipulates host hormone signaling to attract insect vectors. Cell Res. 27, 402–415 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivera-Vega L. J., Acevedo F. E., Felton G. W., Genomics of Lepidoptera saliva reveals function in herbivory. Curr. Opin. Insect Sci. 19, 61–69 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Celorio-Mancera Mde. L., et al. , Sialome of a generalist lepidopteran herbivore: Identification of transcripts and proteins from Helicoverpa armigera labial salivary glands. PLoS One 6, e26676 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou X., Lee L. Y., Xia K., Yan Y., Yu H., DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 19, 884–894 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Liu L., et al. , Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 7, 13099 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang S., et al. , Bacterial effector activates jasmonate signaling by directly targeting JAZ transcriptional repressors. PLoS Pathog. 9, e1003715 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng F., et al. , A Xanthomonas uridine 5′-monophosphate transferase inhibits plant immune kinases. Nature 485, 114–118 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Xin X. F., et al. , Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539, 524–529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarris P. F., et al. , A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell 161, 1089–1100 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Plett J. M., et al. , Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proc. Natl. Acad. Sci. U.S.A. 111, 8299–8304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ratzka A., Vogel H., Kliebenstein D. J., Mitchell-Olds T., Kroymann J., Disarming the mustard oil bomb. Proc. Natl. Acad. Sci. U.S.A. 99, 11223–11228 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.