Significance
For 70 y, glucocorticoids (GCs) have been used to combat inflammatory and allergic disorders. Unfortunately, multiple undesirable side effects are associated with long-term GC treatments, thereby limiting their therapeutic usefulness. We reported that a nonsteroidal compound, CpdX, selectively activates the tethered transrepression function of the glucocorticoid receptor (GR), which contributes to the glucocorticoid beneficial antiinflammatory properties, while not inducing the GR-mediated direct transactivation nor the direct transrepression to which GC undesirable side effects are attributed. We now demonstrate that both CpdX and its deuterated form CpdX-D3 selectively trigger GR-mediated tethered transrepression, and are as efficient as the synthetic glucocorticoid Dexamethasone at curing several major inflammatory diseases in the mouse, indicating that they could be of great interest as antiinflammatory drugs.
Keywords: glucocorticoids, antiinflammatory drug, CpdX, inflammatory diseases, selective glucocorticoid receptor agonistic modulators
Abstract
We previously reported that the nonsteroidal compound CpdX, which was initially characterized 20 y ago as a possible gestagen and, shortly afterward, as a possible drug for treatments of inflammatory diseases, selectively triggers the NFκB/AP1-mediated tethered indirect transrepression function of the glucocorticoid receptor (GR), and could therefore be a selective glucocorticoid receptor agonistic modulator (SEGRAM). We now demonstrate that, upon administration to the mouse, CpdX and one of its deuterated derivatives, CpdX-D3, repress as efficiently as a synthetic glucocorticoid (e.g., Dexamethasone) an induced skin atopic dermatitis, an induced psoriasis-like inflammation, a house dust mite (HDM)-induced asthma-like allergic lung inflammation, a collagen-induced arthritis, an induced ulcerative colitis, and an ovalbumin-induced allergic conjunctivitis. Interestingly, in the cases of an HDM-induced asthma-like allergic lung inflammation and of a collagen-induced arthritis, the CpdX antiinflammatory activity was selectively exerted by one of the two CpdX enantiomers, namely, CpdX(eA) or CpdX-D3(eA).
Synthetic glucocorticoids (GCs; e.g., Dexamethasone [Dex] and Prednisolone) are widely used in clinical treatments of inflammatory and allergic disorders. However, their long-term use is associated with a variety of undesirable pathological side effects (1). Upon GC binding, the GC receptor (GR) controls the expression of target genes through 1) “direct transactivation” by direct binding to conserved “(+)GRE” DNA binding sites (DBSs) (2); 2) “direct transrepression” by binding to conserved inverted repeated (IR) negative response element “IR nGRE” DBSs (3); or 3) “tethered indirect transrepression” mediated through interaction with NFkB, AP1, or STAT3 transactivators bound to their cognate DBSs (refs. 4 and 5 and references therein). We previously reported that both the sumoylation of the GR N-terminal domain and the subsequent formation of an NCoR1/SMRT/HDAC3 repressing complex are mandatory for both GC-induced IR nGRE-mediated direct transrepression (6) and tethered indirect transrepression (7).
It is generally accepted that many beneficial antiinflammatory effects of GCs are ascribed to the tethered indirect transrepression function (1), while many of their long-term undesirable side effects are due to transactivation and/or direct transrepression (3). Interestingly, we demonstrated that a nonsteroidal compound, named CpdX, selectively lacks both the GR direct transactivation and direct transrepression activity, while still exerting its indirect transrepression activity (7). In keeping with this observation, it was previously reported that CpdX could be docked in vitro into the GR ligand binding domain using an “Agreement Docking” method (8). Intriguingly, CpdX was originally listed in a 1997 patent as a nonsteroidal gestagen (9) and, surprisingly, 1 y later, listed in another patent dealing with nonsteroidal antiinflammatory agents exhibiting little to no gestagenic action (10). These “discrepancies” led us to investigate whether CpdX could selectively exhibit the antiinflammatory activity of bona fide GCs.
We now report that, in several mouse inflammation models, the racemic CpdX represses inflammatory processes, as efficiently as the synthetic GC Dex. Of note is that, in two of these models, the antiinflammatory activity was selectively associated with one of the two CpdX enantiomers. As it was reported that replacing some hydrogen atoms by deuterium atoms may improve the efficiency of various drugs (11), we also investigated whether the antiinflammatory activity of CpdX and of its enantiomers [CpdX(eA) and CpdX(eB)] could be similarly improved by replacing some of the hydrogen atoms by deuterium, namely CpdX-D3, CpdX-D3(eA), and CpdX-D3(eB).
Results
CpdX, CpdX-D3, and Their Respective Enantiomers Do Not Exhibit Any Acute Toxicity on Mouse Behavior, Physiological Functions, and Fertility.
Before investigating the antiinflammatory activities of CpdX, as well as of its deuterated derivative CpdX-D3, and of their respective enantiomers (eA and eB) (SI Appendix, SI Materials and Methods), we tested their possible acute toxicity on behavioral and physiological functions (12). Eight-week-old male NMRI mice were intraperitoneally injected with 1 mg/kg, 3 mg/kg, or 10 mg/kg of either Dex, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB). Behavioral symptoms and physiological changes of each mouse were evaluated, during the first 15 min, 30 min, 1 h, 2 h, 3 h, and 24 h following the injection. No mice died during these experiments, and no convulsions, sedation, or excitation were observed in any of these mice. Mice did not lose their balance and motor coordination, and no abnormal gait, forepaw treading, or jump were observed. No signs of persistent pains, such as straub tail, abdominal writhes, and piloerection occurred, and no stereotypes (sniffing, chewing, or head movements), head twitches, or scratching were observed, and the respiration was normal.
We also investigated whether a long-term treatment with CpdX, its deuterated derivative CpdX-D3, and their respective enantiomers (eA and eB) could affect the mouse fertility. Two 8-wk-old C57BL/6 female mice and one 8-wk-old C57BL/6 male mouse were put in each breeding cage. Mice in the same cage were daily subcutaneously (s.c.) injected with either vehicle (NaCl 0.9%) or 1 mg/kg of Dex, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA) or CpdX-D3(eB), and two breeding cages were set for each treatment. The date of birth and the number of pups were listed during a period of 3 mo. All female mice gave birth at least once per month, and no difference was observed in the total number of pups born and surviving upon different treatments of both males and females, which indicated that daily injection of either CpdX, CpdX-D3, or their enantiomers did not affect the mouse fertility, nor the survival of their pups and their fertility.
In contrast to the Dex and Prednisolone synthetic GCs, CpdX, CpdX-D3, and their respective enantiomers selectively activate the indirect transrepression function of the GR.
To demonstrate that CpdX and CpdX-D3 and their enantiomers act similarly on GR-mediated transcription, Balb/C mouse ears were topically treated with these compounds or synthetic GCs (Dex or Prednisolone) for 18 h. RNA transcripts were extracted, and transcripts of the (+)GRE-containing gene REDD1, the IR nGRE-containing gene keratin 5 (K5), and the NFκB/AP1-containing genes IL-1β and IL-6 (whose expression was induced by 12-O-tetradecanoylphorbol-13-acetate [TPA] application) were analyzed by qRT-PCR. As expected, all three GR functions were sensitive to the administration of Dex and, to a lower extent, to Prednisolone, i.e., transactivation of the REDD1 gene (SI Appendix, Fig. S1A), direct transrepression of the K5 gene (SI Appendix, Fig. S1B), and indirect transrepression of the TPA-activated IL-1β (SI Appendix, Fig. S1C) and IL-6 (SI Appendix, Fig. S1D) genes. In marked contrast, only the indirect tethered transrepression activity was induced upon topical administration of CpdX, CpdX-D3, or any of their enantiomers (SI Appendix, Fig. S1 A–D). These results demonstrated that CpdX-D3 and its enantiomers are, like CpdX and its enantiomers, selective glucocorticoid receptor agonistic modulators (SEGRAMs). Similar conclusions were drawn from liver samples of mice after a 4-h intraperitoneal (i.p.) administration (SI Appendix, Fig. S1 E–G) (7).
CpdX, CpdX-D3, and Their Respective Enantiomers Are as Efficient as Dex at Decreasing the Skin Inflammations Generated in Mouse Models of Atopic Dermatitis, Contact Dermatitis, and Psoriasis (Fig. 1 and SI Appendix, Fig. S2).
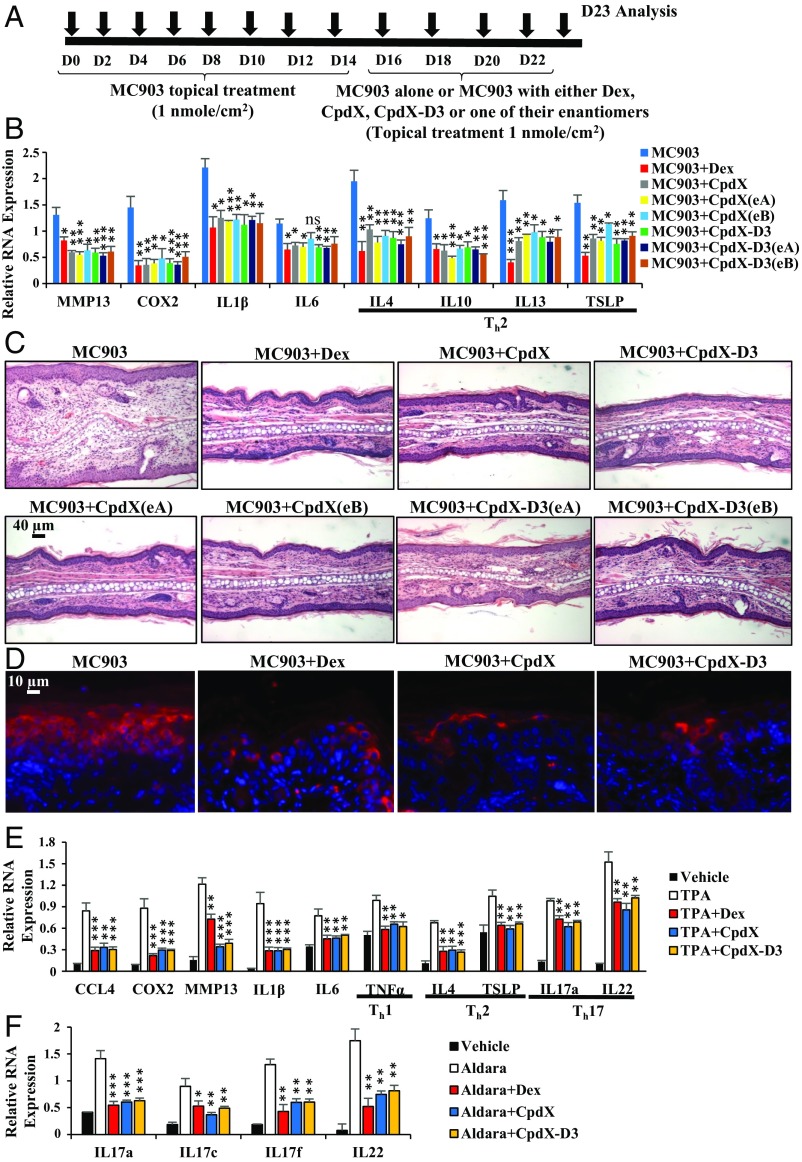
Fig. 1.
CpdX and CpdX-D3 and their respective enantiomers are as efficient as Dex at decreasing skin inflammations generated in atopic dermatitis-like and psoriasis-like models, and in a TPA-induced irritant contact dermatitis. (A) Experimental protocol for an MC903-induced atopic dermatitis-like skin inflammation. (B) The qRT-PCR for RNA transcripts of proinflammatory genes in BALB/c mouse ears treated as indicated. The statistical significance compared with the MC903 treatment was calculated by Student t test; *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant. (C) Hematoxylin and eosin staining of ear sections. (Scale bar, 40 µm.) (D) Immunohistochemistry analyses of ear sections using a TSLP-specific antibody (stained in red). (Scale bar, 20 µm.) (E) The qRT-PCR for RNA transcripts of proinflammatory genes in ears of mouse treated, as indicated. Th1-, Th2-, and Th17-specific proinflammatory interleukins are highlighted. The statistical significance compared with the TPA treatment was calculated by Student t test; *P < 0.05; **P < 0.01; ***P < 0.001. (F) The qRT-PCR for transcripts of proinflammatory genes in mouse ears treated as indicated. The statistical significance compared with the Aldara treatment was calculated by Student t test; *P < 0.05; **P < 0.01; ***P < 0.001. All data are represented as mean ± SEM of at least three independent experiments with at least three mice per treatment.
To investigate their antiinflammatory activity, CpdX, CpdX-D3, and their enantiomers were dissolved in ethanol and topically administrated on the skin of three inflammation models in 8-wk-old Balb/C female mice: 1) a Calcipotriol (MC903)-induced atopic dermatitis-like Th2 inflammation (Fig. 1A) (13), 2) a TPA-induced irritant contact dermatitis-like Th1/Th2/Th17 inflammation (SI Appendix, Fig. S2A) (14), and 3) an Aldara-induced psoriasis-like Th17 inflammation (SI Appendix, Fig. S2B) (15).
The qRT-PCR analyses of ear extracts from MC903-treated mice demonstrated that CpdX and CpdX-D3 application repressed, as efficiently as Dex, the MC903-induced transcription of a variety of proinflammatory genes (MMP13, COX2, IL-1β, IL-6, IL-4, IL-10, IL-13, and TSLP), including genes characteristic of a Th2 inflammation (IL-4, IL-10, IL-13, and TSLP) (Fig. 1B). Histological analyses revealed that an MC903-induced epidermal hyperplasia, as well as a dermal cell infiltration, was also decreased upon treatment with either Dex, CpdX, or CpdX-D3 and their respective enantiomers (Fig. 1C). Immunohistochemistry analyses of MC903-treated mouse epidermis with a TSLP-specific antibody (stained in red) showed that the expression of the TSLP lymphokine was decreased by Dex, CpdX, or CpdX-D3 treatments (Fig. 1D). Similar results were obtained with either CpdX(eA), CpdX(eB), CpdX-D3(eA), or CpdX-D3(eB) treatments.
Transcript analyses of mouse ear extracts from a TPA-induced irritant contact dermatitis-like inflammation model (14) (Fig. 1E) indicated that CpdX and CpdX-D3 applications repressed, as efficiently as Dex, the TPA-induced transcription of proinflammatory genes, including those of a Th1 inflammation (TNFα), a Th2 inflammation (IL-4, TSLP), or a Th17 inflammation (IL-17a and IL-22).
Using the Aldara-induced psoriasis-like inflammation model (15), transcript analyses revealed a similar repression of the induced expression of the Th17 inflammatory genes IL-17a, IL-17c, IL-17f, and IL-22 (Fig. 1F). Histological analyses of mouse ear skin showed that the inflammation of the Aldara-induced psoriasis-like model, and of the TPA-induced irritant dermatitis-like ear skin inflammation model, was significantly decreased by treatment with either Dex, CpdX, or deuterated CpdX-D3, compared with control mice (SI Appendix, Fig. S2C).
In all cases, similar results were obtained with a “neutral’’ cream preparation containing either 0.05% wt/wt Dex, CpdX, or CpdX-D3.
Taken together, the above results demonstrate that CpdX, its deuterated form CpdX-D3, and their enantiomers are as efficient as Dex at decreasing skin inflammations generated in atopic dermatitis-like and psoriasis-like models, as well as in a TPA-induced irritant inflammation.
CpdX, CpdX(eA), CpdX-D3, and CpdX-D3(eA), but Not CpdX(eB) or CpdX-D3(eB), Are as Efficient as Dex at Decreasing a House Dust Mite-Induced Asthma-Like Lung Allergic Th2 Inflammation.
To investigate the antiinflammatory activity of CpdX, CpdX-D3, and their enantiomers in a lung inflammation, 8-wk old Balb/C female mice were intranasally sensitized with house dust mite (HDM) (1 µg) from day zero (D0) to D4, and further intranasally sensitized with HDM (10 µg) on D14 and D21. Mice were then subdivided into eight different groups, and, on D29, D30, and D31, each mouse was again intranasally challenged with HDM (1 µg), without or together with 0.5 mg/kg of body weight of either Dex, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) (Fig. 2A).
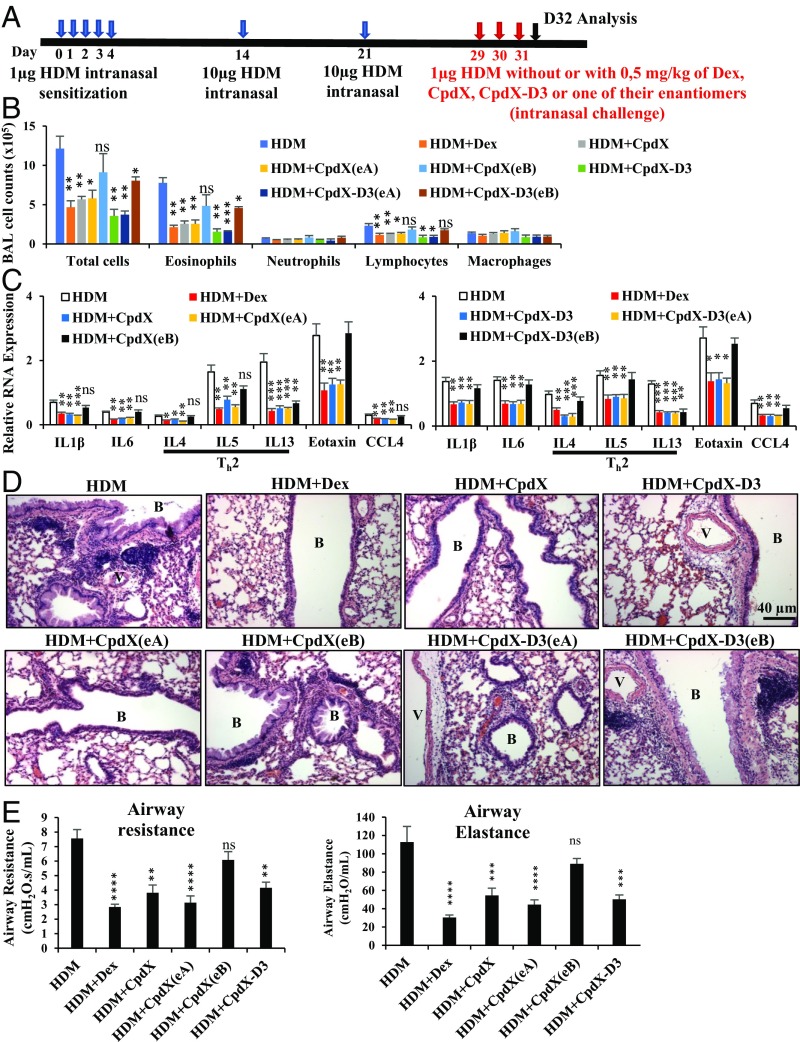
Fig. 2.
CpdX, CpdX(eA), CpdX-D3, and CpdX-D3(eA), but not CpdX(eB) or CpdX-D3(eB), are as efficient as Dex at decreasing an HDM-induced asthma-like lung allergic inflammation. (A) Experimental protocol for generating an HDM-induced asthma-like lung allergic inflammation. (B) Cell counting from the fluid of BAL. (C) The qRT-PCR for RNA transcripts of proinflammatory genes in lungs of mice treated as indicated. Th2-specific proinflammatory interleukins are highlighted. Data are represented as mean ± SEM of at least three independent experiments with at least four mice per treatment. The statistical significance, compared with the HDM treatment (for B and C), was calculated by Student t test; *P < 0.05; **P < 0.01; ***P < 0.001. (D) Hematoxylin and eosin staining of lung sections. Peribronchiolar (B) and perivascular (V) regions are indicated. (Scale bar, 40 µm.) (E) AHR determined using the FlexVent system after exposure to 50 mg/mL methacholine. Data are represented as mean ± SEM of at least two independent experiments with at least eight mice per treatment. The statistical significance compared with the HDM treatment was calculated through two-way ANOVA followed by Bonferroni multiple comparisons; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To assess the lung allergic inflammation, the fluid of bronchoalveolar lavages (BAL) was analyzed at D32. Upon treatment with either Dex, CpdX, or CpdX-D3, the numbers of BAL cells, eosinophils, and lymphocytes were significantly decreased, compared with the control group, whereas no significant change was observed in the number of neutrophils and macrophages (Fig. 2B). RT-PCR analyses of lung samples showed that the expression of IL-1β, IL-6, and the Th2 proinflammatory genes (IL-4, IL-5, IL-13), as well as those of the eosinophil chemotactic chemokines Eotaxin and CCL4, were significantly and similarly decreased by either a Dex, a CpdX, or a CpdX-D3 treatment (Fig. 2C).
Histological analyses of lung paraffin sections revealed that the HDM-induced peribronchiolar and perivascular inflammatory cell infiltrations were strongly decreased upon either a Dex, a CpdX, or a CpdX-D3 treatment (Fig. 2D). Immunohistochemistry staining using the eosinophil-specific antibody MBP confirmed that eosinophils were decreased upon Dex, CpdX, or CpdX-D3 treatment (SI Appendix, Fig. S3A), while no significant changes were observed using the neutrophil-specific antibody R14 (SI Appendix, Fig. S3B).
Pulmonary functional tests of mouse airway responsiveness were invasively performed using a computer-controlled small animal ventilator (FlexVent system; SCIREQ Technologies). Airway resistance and elastance were measured by their responses to methacholine (50 mg/mL). Upon Dex, CpdX, or CpdX-D3 administration, the HDM-induced airway hyperresponsiveness (AHR) was found similarly and significantly reduced (Fig. 2E).
Of note is that a treatment with CpdX(eA) or CpdX-D3(eA), but not with CpdX(eB) or CpdX-D3(eB), efficiently decreased the number of total BAL cells, eosinophils, and lymphocytes (Fig. 2B), as well as the expression of proinflammatory genes (Fig. 2C). Histological analyses of lung paraffin sections also revealed that the peribronchiolar and perivascular inflammatory cell infiltrations were decreased by either a CpdX(eA) or a CpdX-D3(eA) treatment, but not by a CpdX(eB) or a CpdX-D3(eB) treatment (Fig. 2D). Furthermore, immunohistochemistry staining using the eosinophil-specific antibody MBP clearly showed that eosinophils were decreased by either a CpdX(eA) or a CpdX-D3(eA) treatment, but not by CpdX(eB) or CpdX-D3(eB) treatments (SI Appendix, Fig. S3A). In keeping with these data, the mouse lung airway resistance and elastance measured by FlexVent demonstrated that the administration of CpdX(eA), but not of CpdX(eB), reduced the HDM-induced AHR (Fig. 2E).
Taken together, the above results demonstrate that CpdX and CpdX-D3 repressed as efficiently as Dex a HDM-induced lung inflammation, indicating their potential usefulness for the treatment of Th2-related inflammatory disorders, such as asthma. Of note is that CpdX(eA) and CpdX-D3(eA), but neither CpdX(eB) nor CpdX-D3(eB), did efficiently repress the HDM-induced lung inflammation, indicating a remarkable “enantiomeric” selectivity for CpdX and CpdX-D3 in their ability to “cure” an asthma-like syndrome.
CpdX, CpdX(eA), CpdX-D3, and CpdX-D3(eA), but Not CpdX(eB) or CpdX-D3(eB), Are as Efficient as Dex at Decreasing a Collagen-Induced Arthritis Th17 Inflammation.
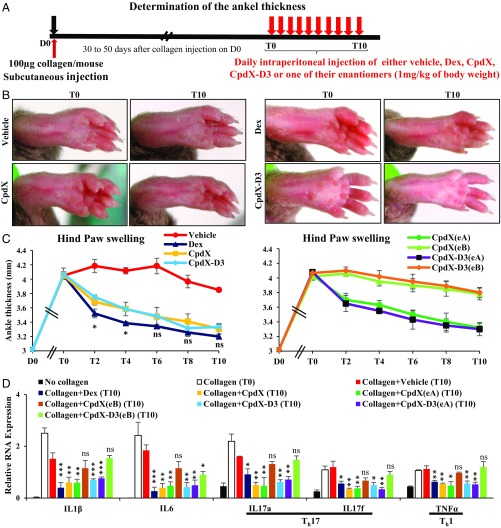
Male mice (DBA-1 strain) were s.c. injected in the tail with 100 µg of collagen per mouse on D0 to create a collagen-induced arthritis (CIA) (16). The hind paw thickness at the ankle level was measured twice a week with a caliper. When the ankle thickness had reached ∼ 4 mm (T0, i.e., 30 d to 50 d after collagen injection on D0), mice were daily i.p. injected for 10 d (T0 to T10) with either vehicle (NaCl 0.9%), Dex, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) (1 mg/kg body weight diluted in NaCl 0.9%), and the hind paw thickness was daily measured (Fig. 3A).
Fig. 3.
CpdX, CpdX(eA), CpdX-D3, and CpdX-D3(eA), but not CpdX(eB) or CpdX-D3(eB), are as efficient as Dex at decreasing a CIA Th17 inflammation. (A) Experimental protocol for CIA. (B) Photographs of the hind paws of DBA/1J mice in which a CIA-like inflammation was generated (T0, Left), and then treated for 10 d (T10) by i.p. administration (Right), as indicated. (C) Mouse hind paw swelling as determined with a caliper. Data are represented as mean ± SEM with at least six mice per treatment. Data for Vehicle, Dex, CpdX, and CpdX-D3 treatments (Left) and data for CpdX(eA), CpdX(eB), CpdX-D3(eA), and CpdX-D3(eB) (Right). The statistical significance compared with the Dex treatment was calculated by Student t test. *P < 0.05; “ns” indicates that the differences observed between Dex-treated, CpdX-treated, and CpdX-D3−treated mice are not significant. (D) The qRT-PCR for RNA transcripts of proinflammatory genes in hind paws of mice treated as indicated. Th17- and Th1-specific proinflammatory interleukins are highlighted. Data are represented as mean ± SEM of at least two independent experiments with at least six mice per treatment. The statistical significance compared with the Collagen+Vehicle (T10) treatment was calculated by Student t test; *P < 0.05; **P < 0.01; ***P < 0.001.
The initial hind paw thickness (∼ 3 mm) was increased to ∼ 4 mm within 30 d to 50 d following the collagen injection, at which time (T0) the administration of Dex, CpdX, or CpdX-D3 resulted, within 10 d, in a rapid decrease of this thickness (Fig. 3 B and C, Left). A similar decrease in hind paw thickness was observed in mice treated with CpdX(eA) or CpdX-D3(eA), whereas, in marked contrast, no such a decrease was observed upon a CpdX(eB) or a CpdX-D3(eB) treatment (Fig. 3 C, Right). Most notably, the RNA transcripts of the proinflammatory genes (IL-1β, IL-6, IL-17a, IL-17f, and TNFα) expressed in the hind paws of mice which developed a CIA were similarly repressed by either a Dex, a CpdX, a CpdX(eA), a CpdX-D3, or a CpdX-D3(eA) treatment, but, interestingly, not by a CpdX(eB) nor a CpdX-D3(eB) treatment (Fig. 3D).
These results demonstrated that both CpdX and CpdX-D3, as well as their enantiomers CpdX(eA) and CpdX-D3(eA), but not CpdX(eB) or CpdX-D3(eB), were as efficient as Dex at decreasing a rheumatoid arthritis-like Th17 inflammation.
CpdX, CpdX-D3, and Their Enantiomers Are as Efficient as Dex at Curing a Dextran Sodium Sulfate-Induced Th17 Ulcerative Colitis.
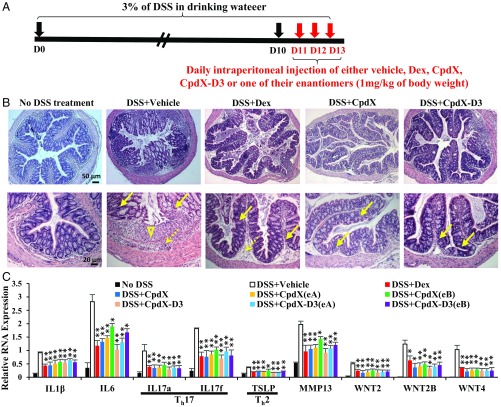
To investigate the antiinflammatory activity of CpdX and CpdX-D3 and of their respective enantiomers on a Th17 ulcerative colitis (17), Balb/C female mice were treated with 3% dextran sodium sulfate (DSS) in drinking water for 13 d, with or without an i.p. administration of either Dex, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) (1 mg/kg of body weight) on D11, D12, and D13 (Fig. 4A). At D14, RNA transcripts were extracted from mouse colons. IL-1β, IL-6, IL-17a, IL-17f, TSLP, MMP13, WNT2, WNT2B, and WNT4 transcripts were analyzed by qRT-PCR. Colon samples were also harvested for histological analyses.
Fig. 4.
CpdX, CpdX-D3, and their enantiomers are as efficient as Dex at curing a DSS-induced Th17 ulcerative colitis. (A) Experimental protocol for DSS-induced colitis in BALB/c mice. (B) Hematoxylin and eosin staining of colon sections. Solid arrows, mucosal inflammatory cell infiltration; dotted arrows, submucosal inflammatory cell infiltration; arrow head, ulceration (Scale bar, 50 µm in Upper and 20 µm in Lower.). (C) The qRT-PCR for transcripts of proinflammatory genes in colons of mice treated as indicated. Th17- and Th2-specific proinflammatory interleukins are highlighted. Data are represented as mean ± SEM of at least three independent experiments with at least four mice per treatment. The statistical significance compared with the DSS+Vehicle treatment was calculated by Student t test; *P < 0.05; **P < 0.01; ***P < 0.001.
Histological analyses (hematoxylin/eosin-stained paraffin sections; Fig. 4B) showed dramatic damages in DSS-treated mouse colon, compared with control mice (no DSS treatment): The regular colonic villus/crypt structure was highly disorganized or absent in DSS-treated mice. In addition, ulcerations (arrow heads), as well as cell infiltrations into the colonic mucosal (solid arrows) and submucosal (dotted arrows) layers, were also observed. Most notably, in mice treated for 3 d with either Dex, CpdX, CpdX-D3, or their two enantiomers CpdX(eA), CpdX(eB), CpdX-D3(eA), and CpdX-D3(eB), the colonic villus/crypt structure was almost reestablished, and both the mucosal and the submucosal cell infiltrations were significantly decreased. Transcriptional analyses showed that the proinflammatory genes, which were overexpressed in DSS-induced ulcerative colitis, were similarly repressed by either Dex, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) (Fig. 4C).
It has been shown that the expression of Wnt ligands was increased in several colitis mouse models (18, 19), and also that the Wnt−β-catenin signaling is pivotal for homeostasis of the intestinal epithelium by promoting stem cell renewal (20). In keeping with these data, the expression of WNT ligands, WNT2, WNT2B, and WNT4, was drastically increased in colon samples treated with DSS, whereas it was significantly decreased upon a treatment with either Dex, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) (Fig. 4C).
Taken together, these results demonstrated that CpdX, CpdX-D3, and their enantiomers CpdX(eA), CpdX(eB), CpdX-D3(eA), and CpdX-D3(eB) were as efficient as Dex for the treatment of a Th17-related inflammatory intestinal disorder, such as an ulcerative colitis.
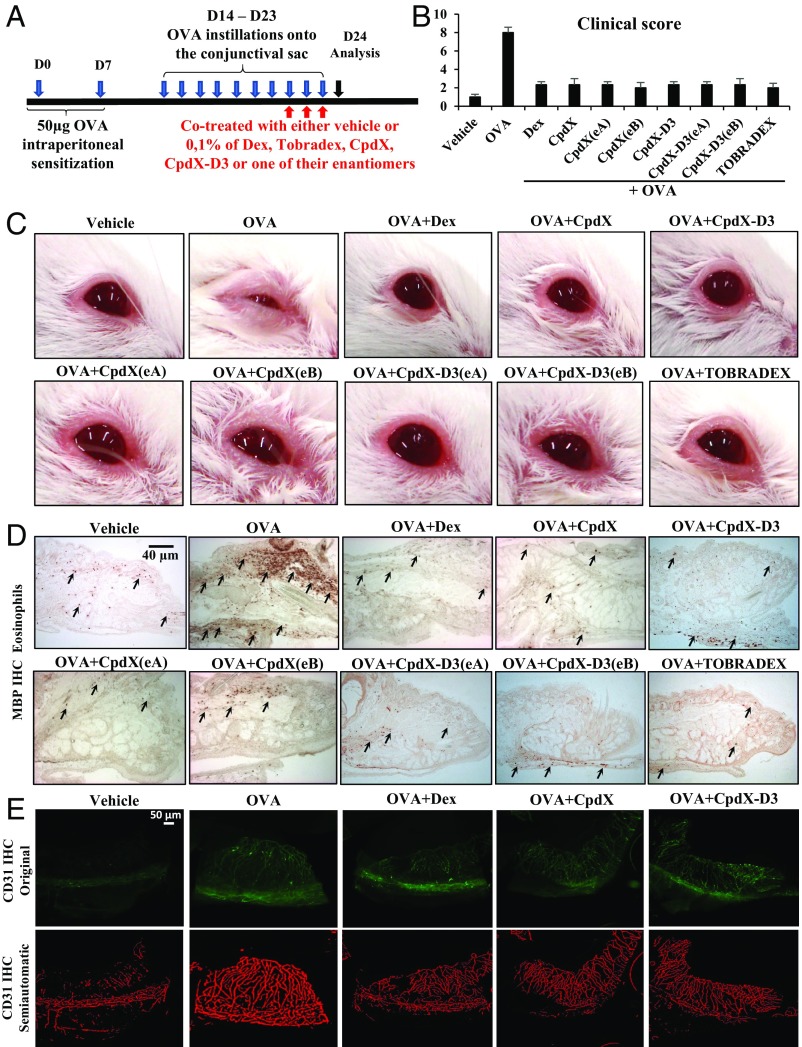
Instillations of Either CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) Alleviate, as Efficiently as Dex, an Ovalbumin-Induced Allergic Conjunctivitis.
To investigate the possible antiinflammatory effects of either CpdX or CpdX-D3 and of their respective enantiomers on an allergic conjunctivitis, Balb/C female mice were i.p. sensitized with 50 µg of ovalbumin (OVA) in alum on both D0 and D7, and then challenged from D14 to D20 with 250 µg of OVA in 5 µL of sterilized vehicle (0.9% NaCl), which were directly instilled onto the conjunctival sac. From D21 to D23, mice were divided into several groups, which received ocular instillations with either OVA alone (750 µg) or OVA (750 µg) together with 0.1% of either Dex, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) (Fig. 5A). The clinical appearance of mouse eyes was evaluated 20 min after the last instillation on D23.
Fig. 5.
CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) alleviate as efficiently as Dex an OVA-induced allergic conjunctivitis. (A) Experimental protocol for generation of an OVA-induced allergic conjunctivitis. (B) Clinical scores (conjunctival hyperemia, lid edema, and tearing) of treated mouse eyes as indicated were evaluated. The data were expressed as mean ± SEM with at least four mice per treatment. (C) Photographs of BALB/c mouse eyes taken 20 min after the last treatment on D23. (D) Immunohistochemistry analyses of eyelid sections from eyes collected on D24 using the eosinophil-specific antibody MBP. (Scale bar, 40 µm.) (E) Immunostaining of corneal flat mounts (from eyes collected on D24) using CD31/PECAM-1 antibody. (Upper) Original photos; (Lower) photos after semiautomatic normalization. (Scale bar, 50 µm.) Data are representative of at least three independent experiments with at least three mice per treatment.
Three clinical signs (conjunctival hyperemia, lid edema, and tearing) were scored, as described (21), to evaluate the occurrence and severity of the conjunctivitis. Parameters were graded on a scale ranging from 0 to 3 (0 = absence, 1 = mild, 2 = moderate, and 3 = severe symptoms), each animal receiving a total clinical score ranging from 0 to 9. Twenty minutes after the last OVA challenge, eyes from all OVA-treated mice presented obvious pathological signs of allergic conjunctivitis, compared with control mice (Vehicle) (Fig. 5 B and C). As expected, these signs were considerably reduced by instillation of Dex, as well as of a commercial collyrium (TOBRADEX; Novartis Pharma S.A.S.), and, most interestingly, similarly reduced by either CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) treatments (Fig. 5 B and C). An eosinophil infiltration is known to be characteristic of a late-phase allergic conjunctivitis (22). Immunohistochemistry analyses on D24 showed an OVA-induced eosinophil infiltration in the mouse conjunctiva, which was drastically reduced upon either Dex, TOBRADEX, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) instillations (Fig. 5D).
Severe allergic conjunctivites are also known to induce a neovascularization of the cornea (23, 24). Whole mounts of cornea collected on D24 were immunostained using the specific antibody CD31/PECAM-1 against the platelet endothelial cell adhesion molecule. OVA-treated corneas exhibited a marked angiogenesis with blood vessels sprouting into the cornea (Fig. 5E). Of note is that treatments with either Dex, TOBRADEX, CpdX, CpdX(eA), CpdX(eB), CpdX-D3, CpdX-D3(eA), or CpdX-D3(eB) significantly decreased the presence of blood vessels in the cornea (Fig. 5E).
These results demonstrated that CpdX, CpdX-D3, and their enantiomers CpdX(eA), CpdX(eB), CpdX-D3(eA), and CpdX-D3(eB) reduce, as efficiently as Dex, an OVA-induced allergic conjunctivitis by decreasing the eosinophils present in conjunctiva and the presence of blood vessels in corneas.
The Relative Antiinflammatory Activity of CpdX and CpdX-D3 Is Higher than That of Prednisolone and Lower than That of Dex.
The synthetic corticosteroids Dex and Prednisolone are widely used to treat inflammatory and allergic disorders, including skin diseases, asthma, arthritis, colitis, and conjunctivitis, the antiinflammatory activity of Dex being known to be 5 to 6 times higher than that of Prednisolone (25). To determine the relative antiinflammatory activity of CpdX and CpdX-D3 in vivo, Balb/C female mouse ears were topically treated for 18 h, and their relative antiinflammatory activity was evaluated by their capacity to repress the TPA-induced transcription of IL-1β and IL-6 genes. As shown in SI Appendix, Fig. S4, CpdX and CpdX-D3 exhibited similar relative antiinflammatory activities. Note that their potencies were lower than that of Dex, but higher than that of Prednisolone (SI Appendix, Fig. S4 A and B).
Discussion
We have demonstrated that both CpdX and its deuterated derivative CpdX-D3 repress, as efficiently as the synthetic GC Dex, an MC903-induced atopic dermatitis-like inflammation, a TPA-induced irritant contact dermatitis, an induced psoriasis-like skin inflammation, an HDM-induced asthma-like allergic lung inflammation, a CIA, a DSS-induced ulcerative colitis, and an OVA-induced allergic eye conjunctivitis.
Corticosteroids remain the mainstay treatment of frequent inflammatory diseases, such as atopic dermatitis, asthma, rheumatoid arthritis, ulcerative colitis, and allergic eye conjunctivitis (26–30), even though target-specific systemic treatments using humanized monoclonal antibodies against proinflammatory cytokines and their receptors are emerging as promising, but expensive, therapies (31). The cytokines IL-1β, TNFα, and IL-6 and their receptors are targeted for the treatment of rheumatoid arthritis (32–36), while IL-17 is targeted in the case of psoriasis and rheumatoid arthritis (37, 38), and antibodies against either TSLP, IL-4/IL-13, or IL-13 on its own are used for atopic dermatitis and asthma treatments (39–43). However, results of large-scale and multicenter clinical trials are still needed to evaluate the clinical benefit, safety, and cost-effectiveness of these immunotherapies.
Interestingly, we have shown here that CpdX and its deuterated form CpdX-D3 efficiently repress in the mouse 1) the expression of IL-1β, TNFα, IL-6, IL-17a, and IL-17f in CIA; 2) the expression of IL-4 and IL-13 in HDM-induced asthma; 3) the expression of TSLP, IL-4, and IL-13 in MC903-induced atopic dermatitis; and 4) the expression of IL-17a, IL-17c, and IL-17f in an induced psoriasis-like skin inflammation. Clearly, compared with a single antibody treatment which targets specifically one cytokine, CpdX and CpdX-D3 target a larger spectrum of inflammatory cytokines.
It is known that individual enantiomers may exhibit markedly different biological effects (44, 45). We have shown, in the present study, that two mouse models of inflammation, the HDM-induced asthma-like lung allergic inflammation and the CIA, were efficiently reduced by administration of either CpdX(eA) or CpdX-D3(eA) enantiomers, whereas, in marked contrast, the CpdX(eB) and CpdX-D3(eB) enantiomers were inefficient, clearly indicating that the CpdX(eA) or CpdX-D3(eA) enantiomers should be preferentially used in the treatment of these two diseases.
Deuterium−carbon bonds are known to be up to 10 times more stable than hydrogen−carbon bonds (46, 47). Accordingly, some deuterated compounds have been shown to exhibit a longer half-life and a significant lower rate of metabolic degradation, and therefore could be more efficient (46, 47). However, we did not observe any significant difference between the antiinflammatory properties of CpdX and its deuterated form CpdX-D3. Analyses of CpdX and CpdX-D3 in vivo metabolites might help in designing more-efficient deuterated compounds derived from CpdX and CpdX-D3.
In conclusion, we have reported here that, for both local therapy treatments (skin inflammations, asthma, and conjunctivitis) and systemic therapy (arthritis and ulcerative colitis), CpdX and its deuterated derivative CpdX-D3 repress several inflammatory processes in mouse models, as efficiently as the most currently used synthetic GC (e.g., Dex and Prednisolone). Importantly, no acute toxicity on behavioral and physiological functions, including reproduction, has been observed with these compounds, indicating that CpdX and CpdX-D3 could possibly be used as harmless antiinflammatory drugs. Upon long-term administration, the undesirable side effects of present-day GCs (e.g., Dex and Prednisolone) are the major restriction to their long-term clinical use. That CpdX and CpdX-D3 are bona fide SEGRAMs which selectively exert the beneficial antiinflammatory properties of GCs, while being devoid of their long-term detrimental side effects, is demonstrated in our accompanying report (48).
Materials and Methods
Mice.
NMRI, BALB/c female mice, DBA/1J male mice, and C57BL/6 mice were purchased from Charles River Laboratories. Breeding, maintenance, and experimental manipulation of mice were approved by the animal care and use committee of the IGBMC/Institut Clinique de la Souris (ICS).
RNA isolation and Q-PCR analyses were as in ref. 7.
SI Appendix includes SI Appendix, SI Materials and Methods, Table S1, and Figs. S1–S4 and their legends.
Supplementary Material
Acknowledgments
We thank Laetitia Paulen for excellent technical support and thank the staff of the animal and cell culture facilities of the IGBMC/ICS for efficient help. We thank Dr. Mei Li, Dr. Nelly Frossard, and Dr. Marie-France Champy for helpful discussions. The neutral cream was kindly provided by Pr. Thierry Vandamme (Faculty of Pharmacy, Strasbourg University). This work was supported by the Association pour la Recherche à l’IGBMC (ARI), the University of Strasbourg Institute for Advanced Studies, CNRS, and INSERM. G.H. was supported by a long-term ARI fellowship, and N.Z. was supported by an ARI PhD student fellowship.
Footnotes
Conflict of interest statement: An International patent application was filed on 23 October 2018 under the number PCT/EP2018/079049.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908258116/-/DCSupplemental.
References
- 1.Clark A. R., Belvisi M. G., Maps and legends: The quest for dissociated ligands of the glucocorticoid receptor. Pharmacol. Ther. 134, 54–67 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Meijsing S. H., et al. , DNA binding site sequence directs glucocorticoid receptor structure and activity. Science 324, 407–410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Surjit M., et al. , Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 145, 224–241 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Ratman D., et al. , How glucocorticoid receptors modulate the activity of other transcription factors: A scope beyond tethering. Mol. Cell. Endocrinol. 380, 41–54 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Tan C. K., Wahli W., A trilogy of glucocorticoid receptor actions. Proc. Natl. Acad. Sci. U.S.A. 113, 1115–1117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua G., Paulen L., Chambon P., GR SUMOylation and formation of an SUMO-SMRT/NCoR1-HDAC3 repressing complex is mandatory for GC-induced IR nGRE-mediated transrepression. Proc. Natl. Acad. Sci. U.S.A. 113, E626–E634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua G., Ganti K. P., Chambon P., Glucocorticoid-induced tethered transrepression requires SUMOylation of GR and formation of a SUMO-SMRT/NCoR1-HDAC3 repressing complex. Proc. Natl. Acad. Sci. U.S.A. 113, E635–E643 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker M., et al. , Design and synthesis of new nonsteroidal glucocorticoid modulators through application of an “agreement docking” method. J. Med. Chem. 48, 4507–4510 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Lehmann M., et al. , Nonsteroidal gestagens. (2002) https://patents.google.com/patent/US6344454/en. Accessed 27 March 2019.
- 10.Lehmann M., et al. , Nonsteroidal anti-inflammatory agents. (2001) https://patents.google.com/patent/US6323199/en. Accessed 27 March 2019.
- 11.Sanderson K., Big interest in heavy drugs. Nature 458, 269 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Roux S., Sablé E., Porsolt R. D., Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr Protoc Pharmacol 27, 10.10.1–10.10.23 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Li M., et al. , Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc. Natl. Acad. Sci. U.S.A. 103, 11736–11741 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley P. L., Steiner S., Havens M., Tramposch K. M., Mouse skin inflammation induced by multiple topical applications of 12-O-tetradecanoylphorbol-13-acetate. Skin Pharmacol. 4, 262–271 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Vinter H., Iversen L., Steiniche T., Kragballe K., Johansen C., Aldara®-induced skin inflammation: Studies of patients with psoriasis. Br. J. Dermatol. 172, 345–353 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Trentham D. E., Townes A. S., Kang A. H., Autoimmunity to type II collagen an experimental model of arthritis. J. Exp. Med. 146, 857–868 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chassaing B., Aitken J. D., Malleshappa M., Vijay-Kumar M., Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104, 15.25.1–15.25.14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swafford D., et al. , Canonical Wnt signaling in CD11c+ APCs regulates microbiota-induced inflammation and immune cell homeostasis in the colon. J. Immunol. 200, 3259–3268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., et al. , Wnt2 inhibits enteric bacterial-induced inflammation in intestinal epithelial cells. Inflamm. Bowel Dis. 18, 418–429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenta T., et al. , Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 15, 911–918 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Gimenes A. D., et al. , Beneficial effect of annexin A1 in a model of experimental allergic conjunctivitis. Exp. Eye Res. 134, 24–32 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Chen J., et al. , Topical application of interleukin-28A attenuates allergic conjunctivitis in an ovalbumin-induced mouse model. Invest. Ophthalmol. Vis. Sci. 57, 604–610 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Cursiefen C., et al. , VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 113, 1040–1050 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bock F., et al. , Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp. Eye Res. 87, 462–470 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Becker D. E., Basic and clinical pharmacology of glucocorticosteroids. Anesth. Prog. 60, 25–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D. E., Clark A. K., Tran K. A., Shi V. Y., New and emerging targeted systemic therapies: A new era for atopic dermatitis. J. Dermatolog. Treat. 29, 364–374 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Chung K. F., Targeting the interleukin pathway in the treatment of asthma. Lancet 386, 1086–1096 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Burmester G. R., Pope J. E., Novel treatment strategies in rheumatoid arthritis. Lancet 389, 2338–2348 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Ungaro R., Mehandru S., Allen P. B., Peyrin-Biroulet L., Colombel J.-F., Ulcerative colitis. Lancet 389, 1756–1770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Rosa M., et al. , Allergic conjunctivitis: A comprehensive review of the literature. Ital. J. Pediatr. 39, 18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotsovilis S., Andreakos E., Therapeutic human monoclonal antibodies in inflammatory diseases. Methods Mol. Biol. 1060, 37–59 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Dinarello C. A., Simon A., van der Meer J. W. M., Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Striz I., Cytokines of the IL-1 family: Recognized targets in chronic inflammation underrated in organ transplantations. Clin. Sci. (Lond.) 131, 2241–2256 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Rossi J.-F., Lu Z.-Y., Jourdan M., Klein B., Interleukin-6 as a therapeutic target. Clin. Cancer Res. 21, 1248–1257 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Jordan S. C., et al. , Interleukin-6, a cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: Therapeutic implications of IL-6 receptor blockade. Transplantation 101, 32–44 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Monaco C., Nanchahal J., Taylor P., Feldmann M., Anti-TNF therapy: Past, present and future. Int. Immunol. 27, 55–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frieder J., Kivelevitch D., Menter A., Secukinumab: A review of the anti-IL-17A biologic for the treatment of psoriasis. Ther. Adv. Chronic Dis. 9, 5–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunwar S., Dahal K., Sharma S., Anti-IL-17 therapy in treatment of rheumatoid arthritis: A systematic literature review and meta-analysis of randomized controlled trials. Rheumatol. Int. 36, 1065–1075 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Simpson E. L., et al. , Tezepelumab, an anti-TSLP monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 80, 1013–1021 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Gauvreau G. M., et al. , Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 370, 2102–2110 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Hajar T., Gontijo J. R. V., Hanifin J. M., New and developing therapies for atopic dermatitis. An. Bras. Dermatol. 93, 104–107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh G. M., Anti-IL-4/-13 based therapy in asthma. Expert Opin. Emerg. Drugs 20, 349–352 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Ohta K., Nagase H., Suzukawa M., Ohta S., Antibody therapy for the management of severe asthma with eosinophilic inflammation. Int. Immunol. 29, 337–343 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Smith S. W., Chiral toxicology: It’s the same thing...only different. Toxicol. Sci. 110, 4–30 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Chhabra N., Aseri M. L., Padmanabhan D., A review of drug isomerism and its significance. Int. J. Appl. Basic Med. Res. 3, 16–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uttamsingh V., et al. , Altering metabolic profiles of drugs by precision deuteration: Reducing mechanism-based inhibition of CYP2D6 by paroxetine. J. Pharmacol. Exp. Ther. 354, 43–54 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Mullard A., FDA approves first deuterated drug. Nat. Rev. Drug Discov. 16, 305 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Hua G., Zein N., Paulen L., Chambon P., The glucocorticoid receptor agonistic modulators CpdX and CpdX-D3 do not generate the debilitating effects of synthetic glucocorticoids. Proc. Natl. Acad. Sci. U.S.A. 116, 14200–14209.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.