Significance
We synthesized physiological and behavioral data to evaluate the risks of acute, lethal effects of extreme heat events versus the sublethal costs of chronic exposure to sustained hot weather for birds inhabiting southern Africa’s Kalahari Desert over the course of the 21st century. The risk of mass mortality events similar to those predicted for the American southwest and sometimes observed in Australia will remain low for Kalahari birds. However, the sublethal costs of chronic exposure, manifested as progressive loss of body condition, delayed fledging, reduced fledging size, and outright breeding failure, will likely drive major population declines. We anticipate that much of the Kalahari’s avian biodiversity will be lost by the end of the century.
Keywords: climate change, breeding, body condition, dehydration, hyperthermia
Abstract
Birds inhabiting hot, arid regions are among the terrestrial organisms most vulnerable to climate change. The potential for increasingly frequent and intense heat waves to cause lethal dehydration and hyperthermia is well documented, but the consequences of sublethal fitness costs associated with chronic exposure to sustained hot weather remain unclear. Using data for species occurring in southern Africa’s Kalahari Desert, we mapped exposure to acute lethal risks and chronic sublethal fitness costs under past, present, and future climates. For inactive birds in shaded microsites, the risks of lethal dehydration and hyperthermia will remain low during the 21st century. In contrast, exposure to conditions associated with chronic, sublethal costs related to progressive body mass loss, reduced nestling growth rates, or increased breeding failure will expand dramatically. For example, by the 2080s the region will experience 10–20 consecutive days per year on which Southern Pied Babblers (Turdoides bicolor) will lose ∼4% of body mass per day, conditions under which this species’ persistence will be extremely unlikely. Similarly, exposure to air temperature maxima associated with delayed fledging, reduced fledgling size, and breeding failure will increase several-fold in Southern Yellow-billed Hornbills (Tockus leucomelas) and Southern Fiscals (Lanius collaris). Our analysis reveals that sublethal costs of chronic heat exposure are likely to drive large declines in avian diversity in the southern African arid zone by the end of the century.
The resilience of desert avifaunal communities to climate change is limited by the harsh and unpredictable nature of the habitats that they occupy, with high temperature maxima and scarce and unpredictable water and food resources constraining birds’ survival and reproduction (1, 2). Deserts are centers of evolutionary radiation for Old World taxa such as sandgrouse (Pterocliformes) and larks (Alaudidae), and declines in arid-zone communities may have broad consequences for global avian diversity. There is already evidence for major avifaunal declines in North America’s Mojave Desert (3).
The direct effects of high environmental temperatures on arid-zone birds are manifested over multiple timescales. Over timescales of hours on extremely hot days, birds risk lethal hyperthermia if environmental temperature exceeds their heat tolerance limits, or lethal dehydration if water demands for evaporative cooling exceed their dehydration tolerance limits. Lethal dehydration during extreme heat events is anticipated to result in major declines in the avifaunas of very hot deserts such as the Sonoran over coming decades (4, 5), and similar processes are responsible for increasingly frequent catastrophic mortality events among flying foxes in Australia (6).
Over longer timescales of days to weeks, chronic exposure to heat also has severe negative effects on avian fitness components, reflecting the sublethal consequences of behavioral trade-offs. For example, in Southern Pied Babblers (Turdoides bicolor), a southern African arid-zone passerine, diurnal mass gain becomes insufficient to offset overnight mass loss when maximum air temperature exceeds 35.5 °C because of a trade-off between foraging efficiency and thermoregulatory behaviors such as panting (7). Similar trade-offs affect breeding, with reduced provisioning rates on hot days translating into slower nestling growth, delayed fledging, and/or lower body mass at fledging (8–11).
Both acute exposure to brief extreme heat events and chronic exposure to sustained hot conditions have the potential for severe negative consequences for desert birds in the face of warming projected for the 21st century. The likely effects of acute exposure to extreme heat waves are well-documented (4, 5), but the role of chronic effects of high temperatures in driving future species declines remains largely unexplored. For most of the world’s desert avifaunas, these sublethal effects of chronic heat exposure are likely to be important determinants of species’ vulnerability to warming.
Here we synthesized available data on thermal physiology and temperature-dependent behavioral trade-offs for birds inhabiting the Kalahari Desert of southern Africa. We then used these data to evaluate the risks faced by southern African arid-zone avifauna by the end of this century in terms of acute effects (i.e., lethal hyperthermia or exceedance of heat tolerance limits) versus chronic effects quantified as the frequency of exposure to threshold air temperatures above which adult body mass or nestling growth rates/nest success are negatively affected (12). Our aim was to evaluate the relative roles of these two categories of direct effects of high temperatures as drivers of declines in southern African desert bird communities during the 21st century.
Results
Acute Exposure During Extreme Heat Events.
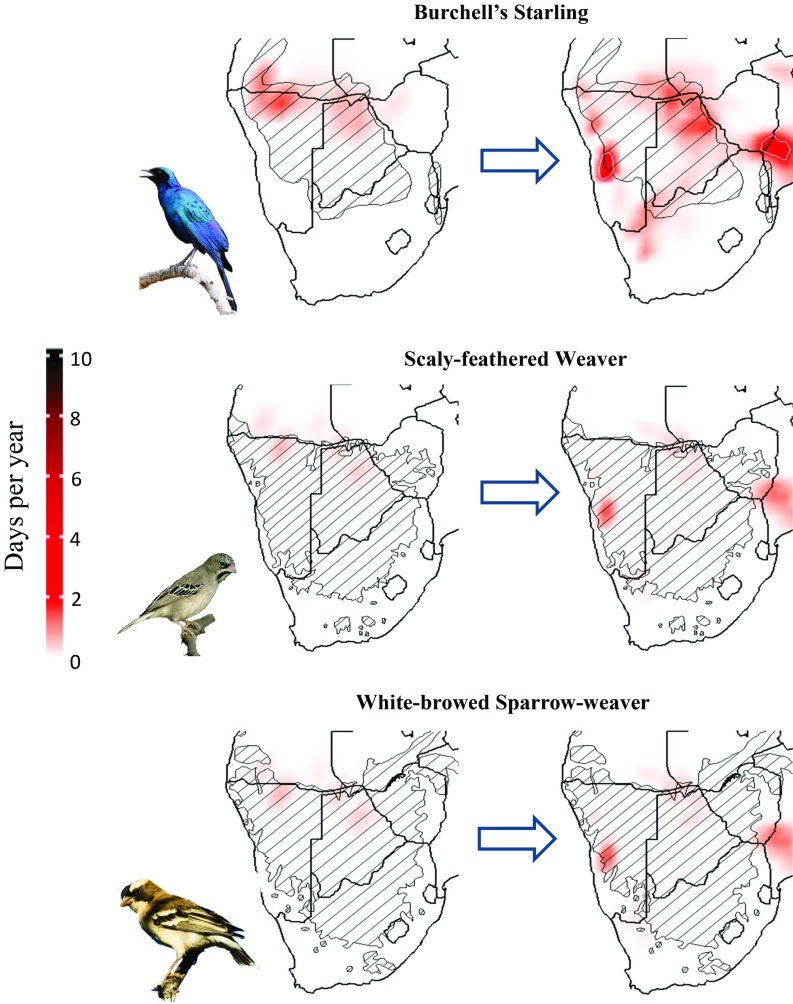
There are no areas in southern Africa where maximum daily air temperature (Tmax) is projected to exceed the heat tolerance limits [HTL, the maximum air temperature (Ta) at which body temperature (Tb) can be defended below lethal limits] for any of the 11 bird species that we examined [HTL = 48–60 °C (SI Appendix, Table S1)]. The environmental conditions experienced by these arid-zone species during the last millennium (1050–1850 CE) were not likely to induce lethal dehydration, and under present conditions a low risk exists for only three species, the Scaly-feathered Weaver, White-browed Sparrow-Weaver and Burchell’s Starling, on only a small number of days per year over limited parts of their ranges (Fig. 1). These conditions are expected to expand to include larger geographic areas and occur more frequently by 2080–2090 CE (Fig. 1). The remainder of the modeled species (Lilac-breasted Roller, African Cuckoo, Rufous-cheeked Nightjar, Burchell’s Sandgrouse, Ring-necked Dove, Laughing Dove, Namaqua Dove, and Sociable Weaver) are not likely to experience these conditions by the end of the century.
Fig. 1.
Average number of days per year with moderate dehydration risk (i.e., survival time of <5 h) across southern Africa for three arid-zone bird species: Burchell’s Starling, Scaly-feathered Weaver, and White-browed Sparrow-weaver) under current (2000–2010) and a high-risk future scenario (RCP 8.5; 2080–2090). Species ranges indicated with cross-hatching. Bird images courtesy of Warwick Tarboton (photographer).
Chronic Exposure During Sustained Hot Weather.
For all three Kalahari species for which data exist on fitness-related consequences of behavioral trade-offs between thermoregulation and foraging, the risk of chronic, sublethal effects of heat exposure will increase dramatically in the coming decades (Figs. 2–4).
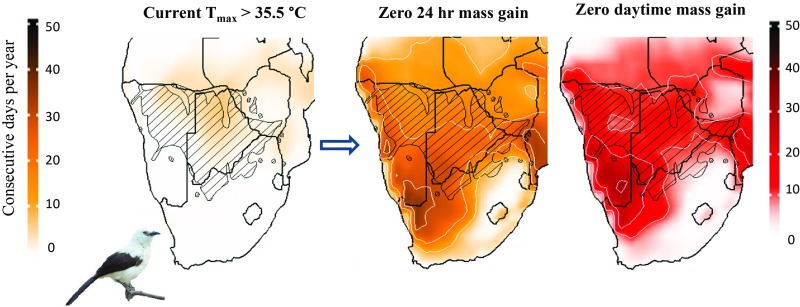
Fig. 2.
Average number of consecutive days per year on which Southern Pied Babblers are exposed to conditions of zero 24-h body mass gain and zero daytime body mass gain (i.e., ∼4% body mass loss per 24 h) under current (2000–2010) and a high-risk future scenario (RCP 8.5; 2080–2090). Species range indicated with cross-hatching. Bird image courtesy of Warwick Tarboton (photographer).
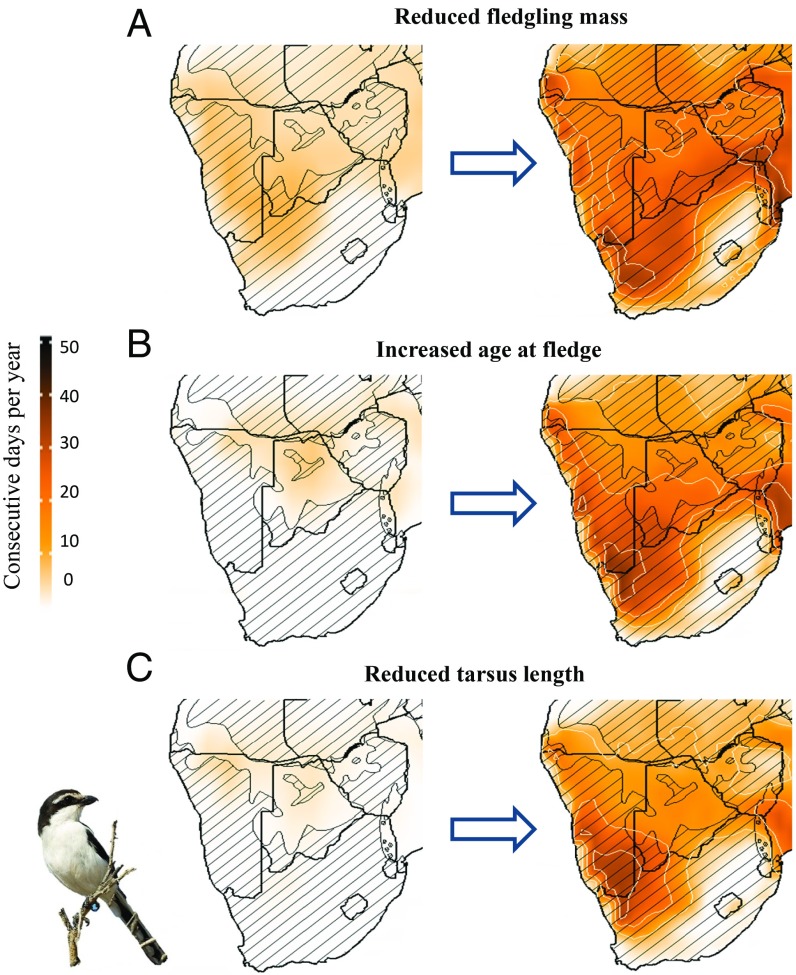
Fig. 4.
Average number of consecutive days per year on which Southern Fiscals are exposed to conditions of chronic, sublethal costs associated with (A) reduced fledgling mass (Tmax = 33 °C), (B) increased age at fledging (Tmax = 35 °C), and (C) reduced fledgling tarsus length (Tmax = 37 °C) under current (2000–2010) and a high-risk future scenario (RCP 8.5; 2080–2090). Species range is indicated with cross-hatching. Bird image courtesy of Warwick Tarboton (photographer).
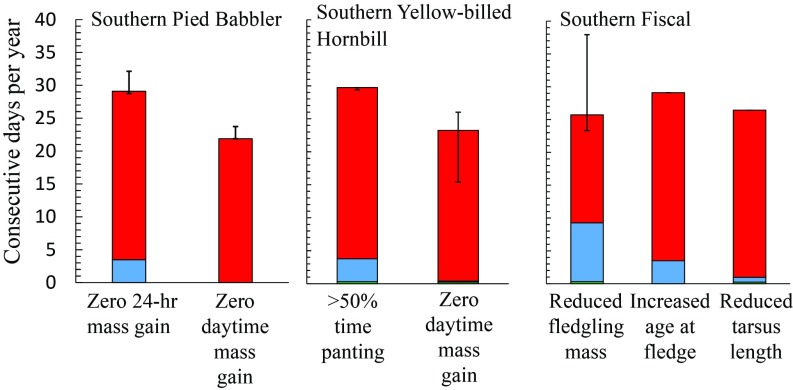
Among nonbreeding Southern Pied Babblers, when Tmax > 35.5 °C diurnal mass gain is typically inadequate to compensate for overnight mass loss, resulting in a net 24-h loss in mass (7). In the southern Kalahari, between 2000 and 2010 CE birds experienced an average of 3.5 consecutive days per austral summer with Tmax ≥ 35.5 °C (Fig. 5). Exposure will increase 7.4-fold to ∼27 consecutive days per summer by 2080–2090 (Figs. 2 and 5). The number of consecutive days per summer with Tmax ≥ 38.5 °C—the threshold associated with zero daytime mass gain and net loss of ∼4% per 24-h period (7)—was close to zero between 2000 and 2010, but will increase to ∼22 d per year by 2080–2090 (Fig. 2), conditions under which the babblers’ persistence becomes very unlikely.
Fig. 5.
Exposure of southern Kalahari birds to conditions associated with chronic, sublethal fitness costs will increase greatly during the 21st century via increases in the average number of consecutive days per year on which maximum air temperature exceeds species-specific threshold values. Past (1060–1070 CE), present (2000–2010 CE), and future (2080–2090 CE) exposures are shown as green, blue, and red, respectively. Error bars for Southern Pied Babblers and Southern Yellow-billed Hornbills show the range associated with 95% confidence intervals (CI) calculated for relationships between daily percentage of body mass gain or heat dissipation behavior and daily maximum air temperature (Tmax). In each case, the upward error bar corresponds with the Tmax value for the lower (cooler) 95% CI and the downward bar with the upper (warmer) 95% CI (in the case of zero daytime mass gain for babblers, estimating the Tmax for the upper 95% CI would require extrapolation and is hence not plotted). For Southern Fiscals, the upper bound of the error bar signifies 92.5% confidence of a negative effect of cumulative days above the threshold Tmax, and the lower bound signifies 97.5% confidence of a negative effect of cumulative days above the threshold Tmax. In the case of reduced tarsus length among the fiscals, strong nonlinearity of the effect of Tmax resulted in 92.5, 95, and 97.5% confidence in a negative effect of high Tmax being reached at ∼36.8 °C—hence the lack of visible error bars.
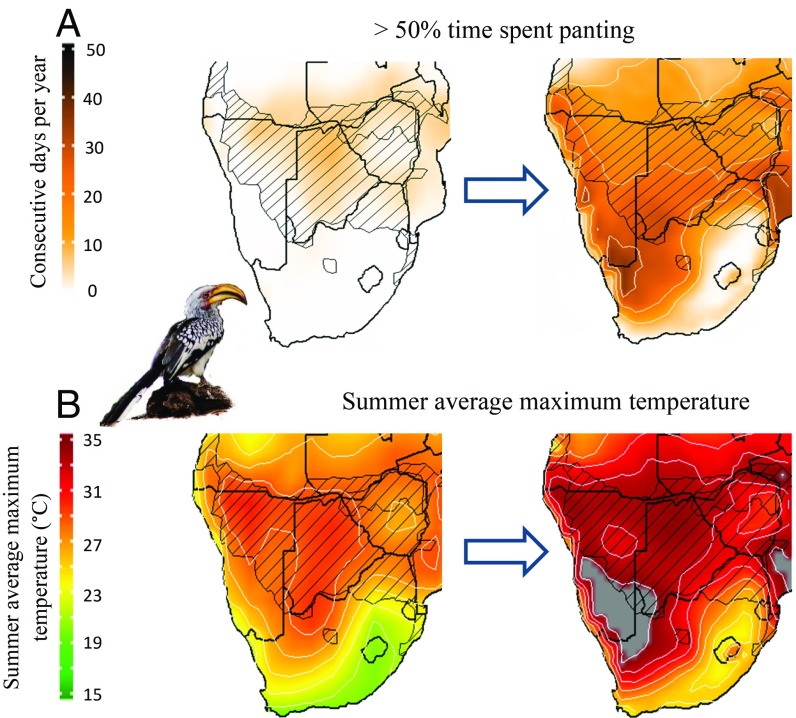
In Southern Yellow-billed Hornbills, the number of consecutive days per year on which Tmax > 34.5 °C and breeding males spend >50% of time panting is currently <10 (Figs. 3 and 5). By the end of the century, however, the corresponding value for the southern Kalahari will be ∼26 consecutive days per year (Figs. 3 and 5). The number of consecutive days per summer with Tmax ≥ 37.9 °C—the threshold associated with zero daytime mass gain in breeding adult males (7)—was close to zero between 2000 and 2010, but will increase to ∼23 by 2080–2090 (Fig. 5). The probability of breeding successfully falls below 50% when Tmax averages >35 °C during the summer nesting period, conditions unlikely under the current climate in the southern Kalahari region (Fig. 3). Under unmitigated climate change, however, parts of the southern Kalahari are likely to experience average summer temperatures of Tmax > 35 °C, and average Tmax will approach this threshold across the majority of the hornbills’ range (Fig. 3).
Fig. 3.
Average number of consecutive days per year on which Southern Yellow-billed Hornbills are exposed to conditions of chronic, sublethal costs. (A) Tmax = 34.5 °C, the threshold at which provisioning male hornbills spend 50% of their time panting) under current (2000–2010) and a high-risk future scenario (RCP 8.5; 2080–2090). (B) Summer average maximum temperature under current and future conditions, with gray indicating areas where values will exceed Ta = 35 °C, the threshold above which breeding success is <50%. Species range is indicated with cross-hatching. Bird image courtesy of Warwick Tarboton (photographer).
For Southern Fiscals, exposure to weather during which nestling growth rates are compromised will increase dramatically during the 21st century (Fig. 4). In the southern Kalahari Desert, the number of consecutive days per year with Tmax associated with reduced fledging mass will increase approximately twofold, and the number of consecutive days per year with Tmax associated with increased age at fledging (and therefore increased risk of nest loss to predation) by approximately sevenfold (Fig. 4).
Under a more conservative, moderate scenario (representative concentration pathway [RCP] 4.5), exposure of these three species to Tmax exceeding threshold values still increases dramatically in the southern Kalahari region (SI Appendix, Figs. S3–S5). The effects will be more moderate in the eastern portions of their southern African ranges.
Discussion
Our analysis reveals that risks of costly heat exposure will increase substantially for arid-zone birds over large parts of southern Africa during the course of the 21st century. The risks of lethal hyperthermia and dehydration will remain low for most species modeled here. In contrast, the risk of fitness costs of chronic exposure to sustained hot weather will increase dramatically, resulting in conditions over much of the region where birds will, at best, encounter severe challenges to maintaining body condition and/or breeding in summer.
Acute Heat Exposure During Extreme Events.
Lethal dehydration and hyperthermia risk will generally remain low for southern African arid-zone birds. Among the species for which we have risk data, we predict that Burchell’s Starling will be most severely affected, as it will experience 4–6 d per year with moderate risk of lethal dehydration in northern Namibia and southern Angola by the end of this century. For two ploceid weavers, the southern Kalahari, northern Botswana, and their western range edge in the Namib Desert will see a handful of days with a moderate risk of lethal dehydration. This outlook contrasts sharply with that projected for five passerines inhabiting the deserts of southwest North America, all of which will experience at least 1 d per year with a moderate risk of lethal dehydration by the end of the century over 50% or more of their range in the arid US Southwest (5). In some areas, lethal dehydration will become commonplace; Lesser Goldfinches, Cactus Wrens, and Curve-billed Thrashers will all experience 50 or more days per year in the Sonoran Desert in southwestern Arizona (5). The risk of lethal hyperthermia (exposure to Ta values exceeding species-specific HTL) will also remain low in southern Africa. This outlook again contrasts with the situation in North America, where for several bird species these HTLs are currently being approached or exceeded (13, 14).
Our approach to modeling lethal dehydration risk, like previous models (5, 4), is based on several assumptions. First, it assumes that when birds cease activity and seek shelter in shaded sites, water gains from food stored in the crop and/or metabolic water production are inadequate to compensate for rapid losses via evaporative cooling. Second, it assumes that laboratory data on relationships between Ta, Tb, and evaporative water loss can be extrapolated to free-ranging birds in natural environments. The available literature on hyperthermia in free-ranging birds reveals that Tb under laboratory conditions and in free-ranging birds is similar, supporting the notion that models of dehydration risk from laboratory evaporative water loss data are applicable to free-ranging birds (15–17). Our third assumption is that birds occupy completely shaded microsites when inactive during very hot weather and that Ta is a good approximation of the operative temperature (18, 19) that they actually experience. Our modeled risks of lethal effects arising from acute heat stress are likely underestimates for species that roost in partially or completely exposed microsites. In some arid-zone birds, breeding adults experience a trade-off between predation risk and nest thermal microclimate; shaded nests are cooler, but predation risk is higher because nest predators are more likely in vegetated sites (e.g., ref. 20). In desert environments, some taxa nest in exposed sites in full sunlight (20–22), and under these conditions the operative temperatures experienced by incubating birds may be 15 °C or more higher than Ta (e.g., ref. 23).
Chronic Exposure to Sustained Hot Weather.
In contrast to lethal dehydration and hyperthermia risk, our models suggest that exposure of southern African birds to temperatures associated with sublethal fitness costs will increase dramatically over the coming decades. Kalahari birds occasionally experience these temperatures currently, but they will suffer much greater severity (e.g., in terms of numbers of consecutive days of exposure) and geographical extent of exposure by the end of the century.
Sublethal costs of high temperatures affect fitness components including adult body mass (7, 10) and aspects of breeding success (9–11). These impacts are often mediated by missed-opportunity costs associated with thermoregulatory behavior. For example, shade-seeking in Southern Fiscals and Southern Yellow-billed Hornbills and panting in hornbills and Southern Pied Babblers is associated with large reductions in foraging efficiency. These reductions are driven by mechanical constraints on panting and foraging simultaneously and by lower foraging returns in shady locations (7, 10, 24). Conflicts between foraging and thermoregulation are particularly acute in arid zones, where many birds [∼50% of species in the Kalahari (25, 26)] do not drink and gain water only through food.
Associated with these foraging trade-offs, diurnal mass gain falls below zero on days when air temperatures exceed 37.9 °C (hornbills) or 38.5 °C (babblers), representing a net mass loss over 24 h [both species lose ∼4% body mass overnight during the Kalahari summer (7, 10)]. At present, days on which air temperatures exceed these thresholds are rare, and birds are likely able to recoup mass losses after heat exposure. However, our models predict that, by the end of the century, heat waves of 20 or more consecutive days above these thresholds will be part of the average Kalahari summer. Under these conditions, birds face risks of large cumulative mass losses, which may directly affect survival. Even before these scenarios are reached, difficulties maintaining body mass during increasingly hot summers is likely to compromise birds’ ability to attain breeding condition (e.g., ref. 27) or survive through the region’s dry, resource-poor winters. Such effects are already being documented in arid-zone birds on other continents (28).
In all three species that we modeled, lower foraging intake associated with thermoregulatory behaviors was correlated with lower provisioning rates and reduced offspring size and mass (9–11). Important costs are associated with even limited exposure to Tmax above critical thresholds: for Southern Fiscals, deleterious effects on nestlings (e.g., smaller size and mass at fledge and delayed fledging) are measurable after just a single day (9). Our models suggest that days above Tmax thresholds will become commonplace throughout the arid portions of the Southern Fiscal’s range by 2080–2090. Temperature has even more direct effects on breeding Southern Yellow-billed Hornbills: when average Tmax during the nestling period exceeds 35 °C (a threshold that we predict will be approached or exceeded across most of this species’ range by 2090), probability of successful breeding drops below 50%. Hornbill nestlings that do survive to fledge after such hot nestling periods are much smaller than average (10). Because future breeding success in birds correlates with both body mass at fledging and adult body condition (27, 29, 30), poor-quality offspring produced during hot summers that do survive to recruit are likely to have depressed breeding success as adults.
Under a high-risk climate change scenario, the species modeled here will experience temperature conditions associated with sublethal fitness costs across the majority of their ranges. Given the expected increases in frequency and spatial extent of high temperatures (31), the inability of adults to maintain body condition at these temperatures, and the large impacts of hot weather on offspring production, successful breeding attempts are likely to decrease by the end of the century in desert birds even if radiative forcing and greenhouse gas emission are stabilized (SI Appendix, Figs. S3–S5). By the end of this century, thermally mediated missed-opportunity costs could therefore affect avian population persistence via cascading effects on long-term adult survival and breeding success, even if temperatures do not reach levels likely to cause mass mortality events.
Assumptions and Limitations.
The analysis that we present here is based on a single future climate simulation, and other models may forecast different sensitivities of temperature change in southern Africa. A feature of the Coupled Model Intercomparison Project (CMIP) initiative is that there is strong convergence between models, and so the absolute values for changes in the future may vary slightly between simulations, but the tendency is likely to be the same as we have presented. This caveat means that we cannot predict with complete certainty the absolute values of Tmax or the number of consecutive days exceeding any particular threshold. Our analysis, however, focuses on comparing likely impacts of acute heat exposure versus chronic heat exposure, rather than precisely quantifying changes between current and future conditions. For this reason, we are confident that using a different model of future climate is unlikely to meaningfully alter our major conclusions.
One assumption implicit in our analysis is that avian heat tolerance limits and evaporative cooling capacities will remain unchanged during the 21st century. Traits related to heat tolerance do exhibit phenotypic flexibility in response to acclimation or acclimatization (32–35), and recent work has uncovered mechanisms of developmental plasticity in heat tolerance (36). Although phenotypic flexibility and developmental plasticity (37) will undoubtedly constitute important aspects of avian responses to warming, we consider it unlikely that these processes will be adequate to avoid the impacts of the rapid warming predicted for the coming decades. Two major constraints concern Tb and evaporative cooling efficiency. The maximum Tb that birds are able to tolerate before the onset of severe pathological hyperthermia associated with compromised physiological function appears to be ∼45 °C, with little evidence of meaningful variation across avian orders (15, 16, 38–42, but see also ref. 43). Evaporative cooling efficiency is largely determined by the primary avenue of evaporative heat dissipation, with taxa that rely on panting generally having a lower capacity for evaporative cooling compared with taxa in which the primary pathway is cutaneous evaporation or gular flutter (16, 39, 44). Although the cutaneous component of evaporative heat loss can be modulated in response to changing demands for evaporative cooling or water conservation (32, 34, 45, 46), the overall importance of panting, cutaneous evaporation, or gular flutter appears to be highly phylogenetically conserved across avian orders.
Breeding in arid-zone birds is typically triggered by rainfall events, which boost the availability of food (47). Under current conditions, rainfall in the southern Kalahari occurs mostly during the summer, generally constraining birds to breed during the hottest time of year [although many species will rapidly instigate breeding in response to unseasonal rainfall events outside of summer (47)]. In the absence of clear trends for change in rainfall patterns in the southern Kalahari (48), we assume that Kalahari birds will not be able to avoid negative effects of summer increases in temperature on nest outcomes by, for example, advancing their laying dates—a common response to climate warming in northern temperate species (49).
Conclusions
Our analysis of the exposure of southern African arid-zone birds to lethal and sublethal effects of heat in the coming decades reveals that sublethal fitness consequences of sustained hot weather will have a much greater impact than lethal consequences of extreme weather events. There is no reason to suspect that threshold Tmax values similar to those identified for the species that we have modeled here are do not occur in other species; indeed, quantitatively similar thresholds appear to exist in several other African taxa, at least with respect to trade-offs between foraging and thermoregulation (50, 51).
The relatively low risk of catastrophic mortality events during heat waves for southern African species contrasts with predictions for the US Southwest (5) and the scenario considered likely for large parts of Australia (52). We are not aware of data from the latter arid regions that permit a direct evaluation of the risk of sublethal fitness effects. However, many studies document changes in thermoregulatory behaviors (panting, shade seeking, and reductions in activity) analogous to those that correlate with fitness-related costs in the three species modeled here. Therefore, published data suggest species across arid zones globally are probably at risk for sublethal fitness impacts associated with curtailed foraging opportunities due to behavioral thermoregulation (e.g., refs. 53–55). Furthermore, studies from southern Europe suggest that breeding birds suffer reduced reproductive success in the face of hot temperatures in that region (e.g., refs. 8 and 56), and even birds in temperate (57) and polar (58) environments may not be immune to sublethal costs of increased temperatures. We therefore suspect that these effects may prove as severe, if not more so, for the arid-zone avifaunas of the Nearctic, Australia, and other regions.
Materials and Methods
We compiled data from physiological and behavioral studies in the southern Kalahari Desert over the past decade. The Kalahari is dominated by arid savanna vegetation (SI Appendix, Fig. S1), dry riverbeds, and dune landscapes (59). The region is characterized by cool, dry winters and hot summers, with average summer daily maximum air temperatures of 34.7 ± 0.05 °C (data for 1960–2015, Twee Rivieren, South African Weather Service). The southern Kalahari region is experiencing rapid warming; the Kgalagadi Transfrontier Park that spans South Africa and Botswana has warmed by ∼0.039 ± 0.007 °C⋅yr−1 since 1960, during which time average maximum temperatures have increased by 1.95 °C (48, 60).
To model risks of lethal dehydration and hyperthermia, we used published data on the interactions between Ta, Tb, and evaporative water loss for 11 Kalahari bird species (SI Appendix, Table S1). We modeled risk of lethal hyperthermia as maximum daytime Ta exceeding species-specific heat tolerance limits reported in previous studies (SI Appendix, Table S1). To model lethal dehydration risk, we followed the same approach as Albright et al. (5). This approach assumes that, on hot days, birds cease foraging at midday and rest in completely shaded microsites where their operative temperature (18, 19) is equivalent to Ta. Under these conditions, birds lose water via evaporative cooling, but do not gain water through drinking or foraging. Survival time during extreme heat events can thus be estimated as the time taken for cumulative evaporative water losses to exceed dehydration tolerance limits, which we assumed to be 15% of Mb, following Albright et al. (5). The only exception was the Rufous-cheeked Nightjar, for which we assumed a dehydration tolerance limit of 20% (23). Like Albright et al. (5), we used an ecologically important survival time of ≤5 h as an indicator of moderate risk of lethal dehydration. Survival times under current conditions were estimated using an average diurnal temperature profile calculated from the 10 hottest days during the past decade (2000–2010 CE), averaged over three regions across southern Africa (southern Kalahari Desert, Central Namibia, and Northern Botswana) (SI Appendix, Fig. S2). Survival times under future conditions were calculated using the same curve shifted upward to account for predicted increases in Tmax.
To model risks of chronic, sublethal fitness costs associated with exposure during sustained hot weather, we used threshold Tmax values associated with loss of body mass, reduced nestling growth rates, delayed fledging and reduced breeding success in three Kalahari species investigated so far: Southern Pied Babbler (T. bicolor), Southern Yellow-billed Hornbill (Tockus leucomelas), and Southern Fiscal (Lanius collaris) (SI Appendix, Table S2). On days when Tmax exceeds these species-specific thresholds, adults and/or nestlings experience measurable sublethal fitness costs (SI Appendix, Table S2).
Climate Models and Analyses.
We used the IPSL-CM5A-LR climate simulation (experiment r1i1p1) from PMIP (Paleoclimate Modeling Intercomparison Project III, model: CSIRO-Mk3L-1–2, https://pmip3.lsce.ipsl.fr/) to simulate historical conditions between 1050 and 1850 CE. This global dataset characterized average daily maximum air temperature for 1050–1850 CE, where the forcing fields were interpolated to 3.15° latitude by 5.63° longitude. Data for modern climate conditions (1850–2014 CE) were obtained from the Physical Sciences Division of the National Oceanic and Atmospheric Administration’s Earth Systems Research Laboratory (Boulder, CO; https://www.esrl.noaa.gov/pds/). The models selected were from the reanalyzed data from NOAA–Cooperative Institute for Research in Environmental Sciences 20th Century Reanalyses (v2c), where the forcing fields were interpolated to 1.88° latitude × 1.88° longitude. The temporal coverage was restricted to daytime values only (06:00–18:00) during the austral summer (October–March). Future climate change projections (2076–2100 CE) were obtained from the National Center for Atmospheric Research (Boulder, CO; https://esgf-node.ipsl.upmc.fr/search/cmip5-ipsl/). We used experiment r6i1p1 and RCP 8.5 scenario of the CCSM4 projection from CMIP V (https://cmip.llnl.gov/cmip5/), with forcing fields interpolated to 0.95° latitude × 1.25° longitude. This model is part of a collaborative program showing strong convergence between models, has been parameterized for biological studies, and is consistent with multimodel future climate projections (61, 62). Climate models that best simulate current conditions are increasingly found to be those projecting the greatest rate of warming for 2100 (63–65), and we used an unmitigated, business-as-usual climate change scenario (RCP 8.5) for end-of-the-century warming projections as the most likely scenario.
Species distribution data were obtained from BirdLife International and NatureServe (2013) (http://datazone.birdlife.org/species/search). These distribution data were compiled using a variety of sources, including museum data, observation records, occurrence data from Important Bird Areas, distribution atlases, and maps from surveys and field guides. The species distribution maps were checked against distribution data from the Second South African Bird Atlas Project (http://sabap2.adu.org.za/). All data analyses were conducted in the R programming environment (R Core Team 2015) using the R Studio (version 3.2.3) interface.
Supplementary Material
Acknowledgments
We thank two anonymous reviewers whose comments improved the quality of the manuscript. This work is based on research supported by the National Research Foundation of South Africa Grant 110506 (to A.E.M.) and the DST-NRF Centre of Excellence at the FitzPatrick Institute. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821312116/-/DCSupplemental.
References
- 1.Dawson W. R., Schmidt-Nielsen K., “Terrestrial animals in dry heat: desert birds” in Handbook of Physiology: Adaptation to the Environment, Dill DB, Ed. (American Physiological Society, Washington, DC, 1964), pp. 481–492. [Google Scholar]
- 2.Serventy D. L., “Biology of desert birds” in Avian Biology, Farner D. S., King J. R., Eds. (Academic Press, New York, 1971), vol. I, pp. 287–339. [Google Scholar]
- 3.Iknayan K. J., Beissinger S. R., Collapse of a desert bird community over the past century driven by climate change. Proc. Natl. Acad. Sci. U.S.A. 115, 8597–8602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKechnie A. E., Wolf B. O., Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albright T. P., et al. , Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc. Natl. Acad. Sci. U.S.A. 114, 2283–2288 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welbergen J. A., Klose S. M., Markus N., Eby P., Climate change and the effects of temperature extremes on Australian flying-foxes. Proc. Biol. Sci. 275, 419–425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.du Plessis K. L., Martin R. O., Hockey P. A. R., Cunningham S. J., Ridley A. R., The costs of keeping cool in a warming world: Implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob. Change Biol. 18, 3063–3070 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Catry I., Catry T., Patto P., Franco A. M., Moreira F., Differential heat tolerance in nestlings suggests sympatric species may face different climate change risks. Clim. Res. 66, 13–24 (2015). [Google Scholar]
- 9.Cunningham S. J., Martin R. O., Hojem C. L., Hockey P. A. R., Temperatures in excess of critical thresholds threaten nestling growth and survival in a rapidly-warming arid savanna: A study of common fiscals. PLoS One 8, e74613 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Ven T. M. F. N., “Implications of climate change on the reproductive success of the Southern Yellow-billed Hornbill Tockus leucomelas,” PhD thesis, University of Cape Town, Cape Town, South Africa (2017).
- 11.Wiley E. M., Ridley A. R., The effects of temperature on offspring provisioning in a cooperative breeder. Anim. Behav. 117, 187–195 (2016). [Google Scholar]
- 12.Cunningham S. J., Kruger A. C., Nxumalo M. P., Hockey P. A. R., Identifying biologically meaningful hot-weather events using threshold temperatures that affect life-history. PLoS One 8, e82492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor L., Death Valley records planet’s hottest month, scientists warn it may become uninhabitable. Huffington Post (2018). https://www.huffingtonpost.com/entry/death-valley-records-planets-hottest-month-again_us_5b61e5eae4b0b15aba9f183b.
- 14.Smith E. K., O’Neill J., Gerson A. R., Wolf B. O., Avian thermoregulation in the heat: Resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert doves and quail. J. Exp. Biol. 218, 3636–3646 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Whitfield M. C., Smit B., McKechnie A. E., Wolf B. O., Avian thermoregulation in the heat: Scaling of heat tolerance and evaporative cooling capacity in three southern African arid-zone passerines. J. Exp. Biol. 218, 1705–1714 (2015). [DOI] [PubMed] [Google Scholar]
- 16.McKechnie A. E., et al. , Avian thermoregulation in the heat: Efficient evaporative cooling allows for extreme heat tolerance in four southern hemisphere columbids. J. Exp. Biol. 219, 2145–2155 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Thompson M. L., Cunningham S. J., McKechnie A. E., Interspecific variation in avian thermoregulatory patterns and heat dissipation behaviours in a subtropical desert. Physiol. Behav. 188, 311–323 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Robinson D. E., Campbell G. S., King J. R., An evaluation of heat exchange in small birds. J. Comp. Physiol. B 105, 153–166 (1976). [Google Scholar]
- 19.Bakken G. S., A heat transfer analysis of animals: Unifying concepts and the application of metabolism chamber data to field ecology. J. Theor. Biol. 60, 337–384 (1976). [DOI] [PubMed] [Google Scholar]
- 20.Tieleman B. I., van Noordwijk H. J., Williams J. B., Nest site selection in a hot desert: Trade-off between microclimate and predation risk? Condor 110, 116–124 (2008). [Google Scholar]
- 21.Grant G. S., Avian incubation: Egg temperature, nest humidity, and behavioral thermoregulation in a hot environment. Ornithol. Monogr. 30, 1–100 (1982). [Google Scholar]
- 22.Cleere N., “Family Caprimulgidae (nightjars) ” in Handbook of the Birds of the World Barn-owls to Hummingbirds, del Hoyo J., Elliot A., Sargatal J., Eds. (Lynx Edicions, Barcelona, 1999), vol. 5, pp. 302–386. [Google Scholar]
- 23.O’Connor R. S., Brigham R. M., McKechnie A. E., Roosting in exposed microsites by a nocturnal bird, the rufous-cheeked nightjar: Implications for water balance under current and future climate conditions. Can. J. Zool. 96, 1122–1129 (2018). [Google Scholar]
- 24.Cunningham S. J., Martin R. O., Hockey P. A., Can behaviour buffer the impacts of climate change on an arid-zone bird? Ostrich 86, 119–126 (2015). [Google Scholar]
- 25.Abdu S., McKechnie A. E., Lee A. T., Cunningham S. J., Can providing shade at water points help Kalahari birds beat the heat? J. Arid Environ. 152, 21–27 (2018). [Google Scholar]
- 26.Smit B., Woodborne S., Wolf B. O., McKechnie A. E., Differences in the use of surface water resources by desert birds is revealed using isotopic tracers. Auk 136, 1–13 (2019). [Google Scholar]
- 27.Ridley A. R., Raihani N. J., Variable postfledging care in a cooperative bird: Causes and consequences. Behav. Ecol. 18, 994–1000 (2007). [Google Scholar]
- 28.Gardner J. L., Amano T., Sutherland W. J., Clayton M., Peters A., Individual and demographic consequences of reduced body condition following repeated exposure to high temperatures. Ecology 97, 786–795 (2016). [PubMed] [Google Scholar]
- 29.Ghalambor C. K., Martin T. E., Fecundity-survival trade-offs and parental risk-taking in birds. Science 292, 494–497 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Weimerskirch H., Prince P. A., Zimmermann L., Chick provisioning by the yellow‐nosed albatross Diomedea chlororhynchos: Response of foraging effort to experimentally increased costs and demands. Ibis 142, 103–110 (2000). [Google Scholar]
- 31.Engelbrecht F., et al. , Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ. Res. Lett. 10, 085004 (2015). [Google Scholar]
- 32.Marder J., Arieli U., Heat balance of acclimated pigeons Columba livia exposed to temperatures of up to 60°C Ta. Comp. Biochem. Physiol. 91A, 165–170 (1988). [Google Scholar]
- 33.Tieleman B. I., Williams J. B., LaCroix F., Paillat P., Physiological responses of Houbara bustards to high ambient temperatures. J. Exp. Biol. 205, 503–511 (2002). [DOI] [PubMed] [Google Scholar]
- 34.McKechnie A. E., Wolf B. O., Partitioning of evaporative water loss in white-winged doves: Plasticity in response to short-term thermal acclimation. J. Exp. Biol. 207, 203–210 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Noakes M. J., Wolf B. O., McKechnie A. E., Seasonal and geographical variation in heat tolerance and evaporative cooling capacity in a passerine bird. J. Exp. Biol. 219, 859–869 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Mariette M. M., Buchanan K. L., Prenatal acoustic communication programs offspring for high posthatching temperatures in a songbird. Science 353, 812–814 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Piersma T., Drent J., Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233 (2003). [Google Scholar]
- 38.McKechnie A. E., et al. , Avian thermoregulation in the heat: Evaporative cooling capacity in an archetypal desert specialist, Burchell’s sandgrouse (Pterocles burchelli). J. Exp. Biol. 219, 2137–2144 (2016). [DOI] [PubMed] [Google Scholar]
- 39.O’Connor R. S., Wolf B. O., Brigham R. M., McKechnie A. E., Avian thermoregulation in the heat: Efficient evaporative cooling in two southern african nightjars. J. Comp. Physiol. B 187, 477–491 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Talbot W. A., McWhorter T. J., Gerson A. R., McKechnie A. E., Wolf B. O., Avian thermoregulation in the heat: Evaporative cooling capacity of arid-zone caprimulgiformes from two continents. J. Exp. Biol. 220, 3488–3498 (2017). [DOI] [PubMed] [Google Scholar]
- 41.McWhorter T. J., et al. , Avian thermoregulation in the heat: Evaporative cooling capacity and thermal tolerance in two Australian parrots. J. Exp. Biol. 221, jeb168930 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Smit B., et al. , Avian thermoregulation in the heat: Phylogenetic variation among avian orders in evaporative cooling capacity and heat tolerance. J. Exp. Biol. 221, jeb174870 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Weathers W. W., Energetics and thermoregulation by small passerines of the humid, lowland tropics. Auk 114, 341–353 (1997). [Google Scholar]
- 44.Smith E. K., O’Neill J. J., Gerson A. R., McKechnie A. E., Wolf B. O., Avian thermoregulation in the heat: Resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert songbirds. J. Exp. Biol. 220, 3290–3300 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Muñoz-Garcia A., Cox R. M., Williams J. B., Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum in house sparrows (Passer domesticus) following acclimation to high and low humidity. Physiol. Biochem. Zool. 81, 87–96 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Hoffman T. C. M., Walsberg G. E., Inhibiting ventilatory evaporation produces an adaptive increase in cutaneous evaporation in mourning doves Zenaida macroura. J. Exp. Biol. 202, 3021–3028 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Lloyd P., Rainfall as a breeding stimulus and clutch size determinant in South African arid‐zone birds. Ibis 141, 637–643 (1999). [Google Scholar]
- 48.van Wilgen N. J., Goodall V., Holness S., Chown S. L., McGeoch M. A., Rising temperatures and changing rainfall patterns in South Africa’s national parks. Int. J. Climatol. 36, 706–721 (2016). [Google Scholar]
- 49.Crick H. Q., The impact of climate change on birds. Ibis 146, 48–56 (2004). [Google Scholar]
- 50.Olinger R., “How does temperature affect Fork-tailed Drongo, Dicrurus adsimilis, foraging effort, nestling provisioning and growth rates?” MSc thesis, University of Cape Town, Cape Town, South Africa (2017).
- 51.Pattinson N. B., Smit B., Seasonal behavioral responses of an arid-zone passerine in a hot environment. Physiol. Behav. 179, 268–275 (2017). [DOI] [PubMed] [Google Scholar]
- 52.McKechnie A. E., Hockey P. A. R., Wolf B. O., Feeling the heat: Australian landbirds and climate change. Emu 112, i–vii (2012). [Google Scholar]
- 53.Carroll J. M., Davis C. A., Elmore R. D., Fuhlendorf S. D., Thacker E. T., Thermal patterns constrain diurnal behavior of a ground‐dwelling bird. Ecosphere 6, 1–15 (2015). [Google Scholar]
- 54.Edwards E. K., Mitchell N. J., Ridley A. R., The impact of high temperatures on foraging behaviour and body condition in the Western Australian Magpie Cracticus tibicen dorsalis. Ostrich 86, 137–144 (2015). [Google Scholar]
- 55.Bladon A. J., et al. , Behavioural thermoregulation and climatic range restriction in the globally threatened Ethiopian Bush‐crow Zavattariornis stresemanni. Ibis, 10.1111/ibi.12660 (2018). [DOI] [Google Scholar]
- 56.Salaberria C., Celis P., López‐Rull I., Gil D., Effects of temperature and nest heat exposure on nestling growth, dehydration and survival in a Mediterranean hole‐nesting passerine. Ibis 156, 265–275 (2014). [Google Scholar]
- 57.Clark L., Thermal constraints on foraging in adult European starlings. Oecologia 71, 233–238 (1987). [DOI] [PubMed] [Google Scholar]
- 58.Oswald S. A., Bearhop S., Furness R. W., Huntley B., Hamer K. C., Heat stress in a high-latitude seabird: Effects of temperature and food supply on bathing and nest attendance of great skuas Catharacta skua. J. Avian Biol. 39, 163–169 (2008). [Google Scholar]
- 59.Lovegrove B. G., The Living Deserts of Southern Africa (Fernwood Press, Vlaeberg, South Africa, 1993). [Google Scholar]
- 60.Moise A., Hudson D., Probabilistic predictions of climate change for Australia and southern Africa using the reliability ensemble average of IPCC CMIP3 model simulations. J. Geophys. Res. D Atmospheres 113, D15113 (2008). [Google Scholar]
- 61.Flato G., et al. “Evaluation of climate models” in Climate Change 2013–The Physical Science Basis Working Group I Contribution to Fifth Assessment Report Intergovernmental Panel on Climate Change Stocker T. F., et al., Eds. (Cambridge Univ Press, Cambridge, 2013), pp. 741–866. [Google Scholar]
- 62.IPCC , Climate Change 2014 Synthesis Report. Contribution of working groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC, Geneva, Switzerland, 2014). [Google Scholar]
- 63.Brient F., Schneider T., Constraints on climate sensitivity from space-based measurements of low-cloud reflection. J. Clim. 29, 5821–5835 (2016). [Google Scholar]
- 64.Brown P. T., Caldeira K., Greater future global warming inferred from Earth’s recent energy budget. Nature 552, 45–50 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Zhai C., Jiang J. H., Su H., Long‐term cloud change imprinted in seasonal cloud variation: More evidence of high climate sensitivity. Geophys. Res. Lett. 42, 8729–8737 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.