Significance
Tsetse flies pose a major threat to the health and economy of sub-Saharan Africa. They transmit trypanosomes that cause African sleeping sickness in humans and a disease called nagana in livestock. Tsetse find their hosts in large part through olfactory cues, but little is known about the cellular basis of olfaction in these flies. We carried out a systematic physiological analysis of olfactory response in the tsetse antenna. We identified 7 classes of olfactory sensilla that respond to human or animal odors, CO2, pheromones, and a tsetse repellent. The functional organization of the tsetse fly is strikingly different from that of the fruit fly Drosophila. This study may be useful in devising new means of controlling tsetse.
Keywords: tsetse, olfaction, antenna, trypanosomiasis
Abstract
Tsetse flies transmit trypanosomiasis to humans and livestock across much of sub-Saharan Africa. Tsetse are attracted by olfactory cues emanating from their hosts. However, remarkably little is known about the cellular basis of olfaction in tsetse. We have carried out a systematic physiological analysis of the Glossina morsitans antenna. We identify 7 functional classes of olfactory sensilla that respond to human or animal odorants, CO2, sex and alarm pheromones, or other odorants known to attract or repel tsetse. Sensilla differ in their response spectra, show both excitatory and inhibitory responses, and exhibit different response dynamics to different odor stimuli. We find striking differences between the functional organization of the tsetse fly antenna and that of the fruit fly Drosophila melanogaster. One morphological type of sensilla has a different function in the 2 species: Trichoid sensilla respond to pheromones in Drosophila but respond to a wide diversity of compounds in G. morsitans. In contrast to Drosophila, all tested G. morsitans sensilla that show excitatory responses are excited by one odorant, 1-octen-3-ol, which is contained in host emanations. The response profiles of some classes of sensilla are distinct but strongly correlated, unlike the organization described in the Drosophila antenna. Taken together, this study defines elements that likely mediate the attraction of tsetse to its hosts and that might be manipulated as a means of controlling the fly and the diseases it transmits.
Tsetse flies transmit trypanosomiasis across much of sub-Saharan Africa. Tsetse feed exclusively on the blood of humans and animals. During feeding, the flies transmit parasites that cause African sleeping sickness in humans and that cause nagana in cattle and other livestock (1, 2). Approximately 70 million people are at risk (3), and the economic burden of nagana has been estimated to be more than $4 billion per year (4). There is no vaccine or medicine to prevent these diseases. Rather, the most effective means of controlling these diseases has been to control the flies that transmit them.
Tsetse of some species, including Glossina morsitans, find their hosts in large part through olfactory cues (5, 6). Traps or targets containing olfactory attractants have been very useful in vector control (7), and olfactory repellents have also been identified (8). To build on this precedent and devise improved control methods, it could be very helpful to understand the underlying cellular basis of olfaction in tsetse. However, remarkably little information is available on the neural basis of odor response in these flies.
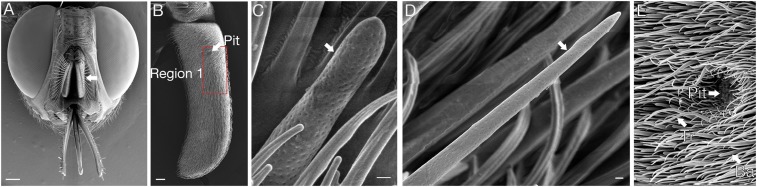
We recently carried out an anatomical and molecular analysis of the G. morsitans antenna (Fig. 1 A and B) (9). There are several morphological classes of olfactory sensilla, including basiconic sensilla that are 9–12 μm in height and have a rounded tip (Fig. 1C) and trichoid sensilla that are 22–24 μm in height and have a tapered tip (Fig. 1D). The analysis also described a sensory pit on the antenna that is lined with a type of basiconic sensilla and is surrounded on the antennal surface by a variety of sensilla (Fig. 1E). The antennal expression patterns of 8 GmmOr (Glossina morsitans morsitans Odor receptor) genes were defined. One receptor, GmmOr9, is expressed in the sensory pit and responds to several tsetse attractants used in olfactory trapping (9).
Fig. 1.
Scanning electron micrographs of the antenna of G. morsitans. (A) Head. Arrow indicates the antenna. (B) Antenna, with region 1 indicated by a red rectangle and the sensory pit indicated by the arrow. (C) Basiconic sensillum. (D) Trichoid sensillum. (E) Sensory pit. Arrows indicate the pit entrance and individual trichoid (Tr) and basiconic (Ba) sensilla. (Scale bars, 250 µm for A, 50 µm for B, 0.5 µm for C and D, and 10 µm for E.)
Physiological data on the olfactory sensilla of G. morsitans antenna are sparse. The sensilla are very dense; they protrude from the antennal surface at an acute angle, and many are difficult to penetrate with a recording electrode. These anatomical features have made it challenging to record physiologically from them. Here we have overcome this difficulty by modifying the electrophysiological methods and have tested each of 182 sensilla with an ecologically relevant panel of 16 odorants, that is, n > 2,900 recordings. The results provide an initial view of the functional organization of the G. morsitans olfactory system. The organization is similar in some respects to that of Drosophila melanogaster, the fly whose olfactory system has been best characterized. However, some of the organizational principles established in the fruit fly are violated in the tsetse fly. The different functional organization of the tsetse olfactory system may reflect its strategy of feeding on human and animal hosts and its unusual property of laying larvae rather than eggs.
Results
Trichoid Sensilla Are Diverse in Their Responses to Odorants.
We initiated an electrophysiological analysis of the olfactory responses of trichoid sensilla. We first focused on sensilla in the vicinity of the sensory pit, in part because of the relative accessibility of this region of the antenna for recording and in part because the pit provides a landmark that is useful in identifying the locations of the recordings. We subsequently expanded the region from which we recorded to the area shown in Fig. 1B, which we refer to as region 1. Recordings were made using procedures similar to those used in Drosophila (10) but modified to accommodate the different anatomy of the G. morsitans antenna (Materials and Methods).
In many insects, including Drosophila, trichoid sensilla respond to pheromones (11, 12). Accordingly, we initially tested trichoid sensilla with 6 compounds that act as pheromones in various fly species: 7(Z)-pentacosene; 7(Z),11(Z)-pentacosadiene; 7(Z)-tricosene; 7(Z),11(Z)-heptacosadiene; 7(Z),11(Z)-nonacosadiene; and methyl laurate (13–15), all tested neat. Among these pheromones, only methyl laurate elicited a strong excitatory response from the tested trichoid sensilla (see below). We therefore expanded our test odorant set to include 16 chemically diverse odorants not known to act as pheromones. These odorants include human and animal odorants and compounds previously shown either to attract (1-octen-3-ol and acetone) or repel (δ-nonalactone) G. morsitans (16). All odorants were tested at 10−2 dilutions in paraffin oil (1% vol/vol), following a precedent set in other studies (10, 17), except for methyl laurate, which was used neat.
The attractant 1-octen-3-ol elicited robust responses from many of the trichoid sensilla. An example of one such sensillum is shown in Fig. 2A. The compounds 2-pentanol and methyl laurate elicited responses from the same individual sensillum (Fig. 2 B and C), whereas stimulation with the paraffin oil diluent alone did not (Fig. 2D). In the methyl laurate trace (Fig. 2C), the action potentials that are elicited at high frequency are of smaller amplitude than those of other action potentials. Evidently, this sensillum is innervated by multiple olfactory receptor neurons (ORNs) with different response profiles, and the ORN that produces the smallest action potentials responds to methyl laurate. Although the spike amplitudes are distinguishable in this particular trace, the spikes were not easily distinguishable in many other sensilla that showed the same response profile. We have found that spike separation is more difficult in many sensilla of G. morsitans than in Drosophila, which we examined in a parallel analysis as a control (SI Appendix, Fig. S1 A–E). Accordingly, we report the responses of sensilla in terms of the total number of spikes per sensillum in this study.
Fig. 2.
Single-sensillum recordings from an at1 trichoid sensillum. (A–D) Responses to the indicated odorants and to the paraffin diluent control, all from the same individual sensillum. (E) Differing dynamics of responses to 1-octen-3-ol and methyl laurate. Baseline activity levels were not subtracted in this panel. Error bars show SEM; n = 18.
Responses differed in their dynamics. The response to 1-octen-3-ol increased quickly within the 500 ms stimulation period and then declined dramatically during the course of the next second, as illustrated by the graph shown in Fig. 2E. The response to methyl laurate also increased quickly and declined thereafter but persisted above the baseline level during the entire duration of the recording period.
We analyzed 39 trichoid sensilla in region 1 with all 17 odorants, that is, 663 recordings. We calculated the firing frequency in spikes per second during the 0.5 s stimulation period. We then carried out a hierarchical cluster analysis and found that these trichoid sensilla fell into 3 functional classes that we designate as at1 (antennal trichoid 1), at2, and at3 (Fig. 3A).
Fig. 3.
Hierarchical cluster analysis of 39 trichoid sensilla, each tested with 17 odorants. (A) Dendrogram, in which each horizontal row represents 1 of the 39 sensilla and each vertical column represents 1 of the 17 odorants. The odorants are those in D, listed in the same order. The classification was carried out with Ward’s method. (B) Response profile of at1 sensilla; mean ± SEM, n = 18 sensilla. Green = alcohols, orange = esters, blue = ketones, yellow = aldehyde, pink = aromatics, violet = lactone, gray = methyl laurate, a known insect pheromone. (C) at2; n = 12. (D) at3; n = 9. Values are in spikes per second; both the baseline activity and the response to paraffin oil have been subtracted.
at1 sensilla responded most strongly to 4 odorants of the panel: 1-octen-3-ol, 2-pentanol, and 1-hexen-3-ol (alcohols, as indicated by the color green in Fig. 3B) and methyl laurate (a pheromone that is an ester). The strongest responses observed were on the order of 100 spikes/s (Fig. 3B and SI Appendix, Table S1).
at2 sensilla, by contrast, gave no strong responses to any of the tested odorants. The strongest response was to 1-octen-3-ol: 29 ± 4 spikes/s. We suspect that ORNs in sensilla of this type respond strongly to compounds other than those of our panel.
at3 sensilla respond strongly or moderately to more compounds than either at1 or at2. Interestingly, the 4 odorants that elicited the strongest responses from at1 (the 3 alcohols, 1-octen-3-ol, 2-pentanol, and 1-hexen-ol, and the insect pheromone methyl laurate) elicited responses of similar magnitude from at3 sensilla (e.g., 1-octen-3-ol elicited a response of 102.8 ± 4.9 spikes/s from at1 and 98.3 ± 10 spikes/s from at3; SI Appendix, Table S1). One conceivable interpretation of this similarity is that at1 and at3 share a common ORN that responds to these odorants; at1 and at3 would each contain other ORNs that are not shared and that account for the differences in responses between the 2 sensilla.
These 3 types of trichoid sensilla are intermingled in region 1. Within this region, the frequency at which we detected at1 (18/39 sensilla recorded) was twice that of at3 (9/39 sensilla), with at2 occurring at an intermediate frequency (12/39).
Basiconic Sensilla Fall into Multiple Classes, of Which Some Have Correlated Response Profiles.
We next recorded from basiconic sensilla, again in region 1. There are special challenges to recording from these sensilla. They are much more densely packed than on the Drosophila antenna. In addition, they extend from the antennal surface at a more acute angle than the trichoid sensilla, such that in many cases accessibility was greatly hindered by the presence of adjacent trichoid sensilla. However, we succeeded in recording the responses of basiconic sensilla in this region (Fig. 4).
Fig. 4.
Single-unit recordings from basiconic sensilla. (A–D) Responses to the indicated odorants, all from the same individual ab3 sensillum. (E) Excitatory response to 1-octen-3-ol of an ab1 sensillum. (F) Inhibitory response to ethyl hexanoate of an ab2 sensillum. Note the relatively high spontaneous firing frequency before the stimulus in this sensillum. (G) Inhibitory response to 3-propylphenol of an ab2 sensillum.
We recorded the responses of basiconic sensilla to 16 odorants, the same odorants used in the analysis of trichoid sensilla with the exception of methyl laurate, which did not elicit a response from any tested basiconic sensilla in a preliminary analysis. These odorants had been chosen after carrying out preliminary tests on basiconic sensilla with ∼50 diverse odorants representing different chemical classes. From this initial testing we selected these 16 odorants primarily because they elicited responses from some but not all basiconic sensilla and therefore appeared informative in distinguishing different functional types. We note that in preliminary tests we did not observe major differences between males and females or between animals 1 d vs. 5 d after a blood meal in either basiconic or trichoid sensilla, although we have not explored these parameters extensively.
We recorded the responses of 136 basiconic sensilla in region 1 to 16 odorants, that is, 2,176 recordings. Three types of basiconic sensilla, which we call ab1 (antennal basiconic 1), ab2, and ab3, were identified by hierarchical cluster analysis (Fig. 5A).
Fig. 5.
Hierarchical cluster analysis of 136 basiconic sensilla, each tested with 16 odorants. (A) Dendrogram, in which each horizontal row represents 1 of the 136 sensilla, and each vertical column represents 1 of the 16 odorants. The classification was carried out with Ward’s method. The odorants are listed in D, in the same order. (B) Response profile of ab1 sensilla; mean ± SEM, n = 68. (C) ab2; n = 17. (D) ab3; n = 51.
ab1 responds to 1-octen-3-ol and a variety of other odorants. The greatest mean response was 58 ± 3 spikes/s, the response to 1-octen-3-ol.
ab2 yielded no excitatory responses to any tested odorant, even at the relatively high concentrations used in this analysis. In the absence of a strong excitatory response, it is difficult to judge whether ab2 sensilla are functionally identical; some might respond strongly to one untested odorant, while others might respond strongly to another.
Interestingly, many ab2 sensilla showed inhibitory responses. These sensilla had high spontaneous rates of firing, and ethyl hexanoate, which excited both ab1 and ab3 sensilla, elicited inhibition from at least some ab2 sensilla (Fig. 4F). Another odorant, 3-propylphenol, which also excited ab1 and ab3, inhibited ab2 for several seconds in some cases (Fig. 4G).
ab3 gives strong excitatory responses (∼100 spikes/s) to 1-octen-3-ol (Figs. 4A and 5D and SI Appendix, Table S1) and to a number of other odorants, such as 6-methyl-5-hepten-2-one (Fig. 4B), a component of human sweat that is attractive to mosquitoes (18, 19). ab3 gives weaker responses to 3-propylphenol and the repellent δ-nonalactone (Figs. 4 C and D and 5D). We note with interest that the response to 3-propylphenol showed different dynamics than the response to 1-octanol: The 3-propylphenol response was sustained throughout the recording period (Fig. 4C).
Interestingly, the response profile of ab3 looks comparable to that of ab1, except that most response magnitudes are greater. As a simple quantitative test of the similarity between the ab1 and ab3 response profiles, we examined the rank order of the responses and found that they were strongly correlated (0.88, Spearman’s rank correlation coefficient; P < 0.0001). As a further test of the distinction between these sensillum types, we examined responses across a range of 1-octen-3-ol concentrations and found greater mean responses in ab3 across a broad range (SI Appendix, Fig. S2F; P < 0.0001, 2-way ANOVA). An analysis of the dynamics of response to 1-octen-3-ol also revealed a clear difference between ab3 and ab1 (SI Appendix, Fig. S2G).
ab1, ab2, and ab3 were intermingled in region 1. They occurred at different densities within this region: ab1 were the most common (68/136 sensilla), ab2 were the sparsest (17/136), and ab3 were intermediate (51/136). Although we have not mapped the density of each type at high resolution, there appeared to be a higher concentration of ab2 near the sensory pit.
CO2-Sensitive Basiconic Sensilla.
CO2 has been found to stimulate the flight activity of tsetse (20) and to increase the catch when added to tsetse traps (21). In another species, Glossina palpalis, sensilla responding to CO2 were identified, although their morphology and location were not defined (22).
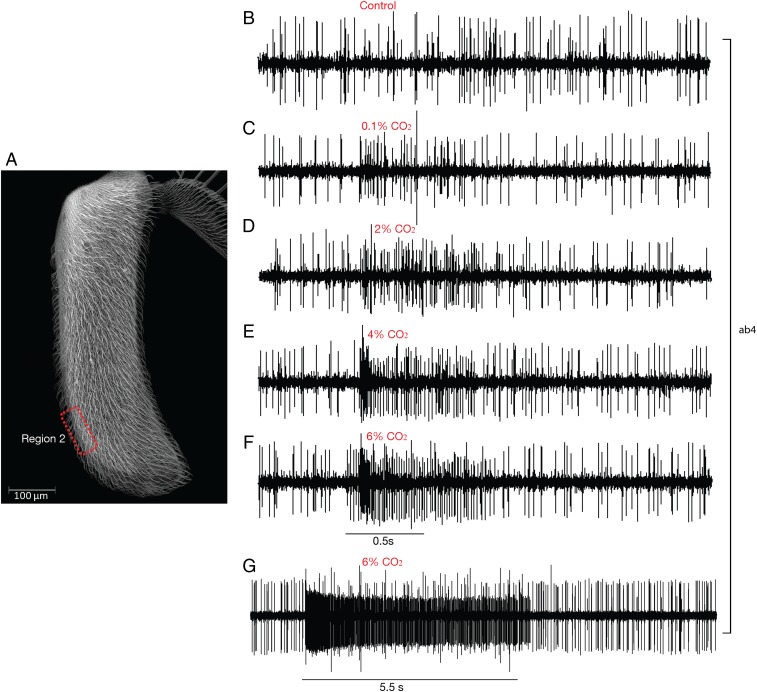
In the course of analyzing sensilla in region 1, we found only 2 sensilla that responded to 5% CO2, of ∼150 tested. We suspected that CO2-sensitive sensilla might reside preferentially in another region of the antennal surface. We tested basiconic sensilla in a more distal region (region 2; Fig. 6A) and identified 22 sensilla (of ∼50 tested) that responded to CO2 at a concentration of 5%; no CO2 responses were observed in trichoid sensilla of this region (n = 10). Testing across a range of concentrations in the CO2-sensitive basiconic sensilla shows that the mean response frequency is a monotonically increasing function of dose (Figs. 6 B–F and 7D). Longer stimulation periods resulted in longer periods of activity (Fig. 6G).
Fig. 6.
Responses to CO2 from one type of basiconic sensillum, ab4. (A) Region 2, from which recordings in this figure were made. (B–F) Responses from one individual sensillum to 500 ms pulses of increasing doses of CO2. B shows the response to a control pulse of air. (G) Response to a long CO2 stimulus.
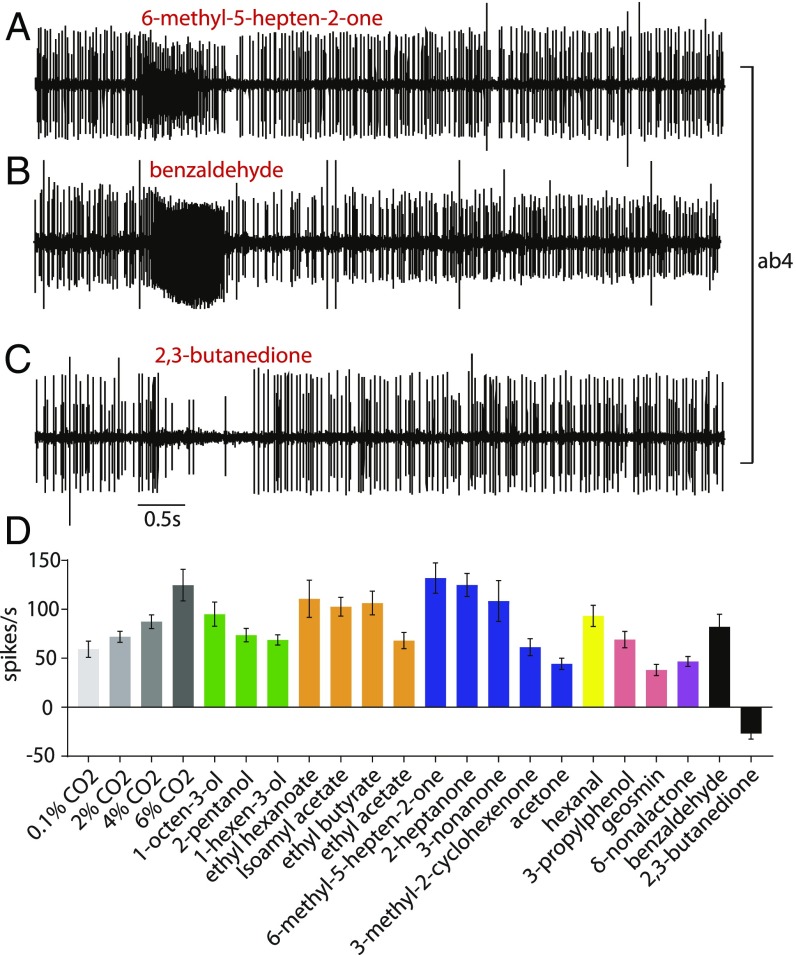
Fig. 7.
Response of CO2-sensitive basiconic sensilla to a panel of odorants. (A and B) Strong excitatory responses to 6-methyl-5-hepten-2-one and benzaldehyde. (C) Inhibitory response to 2,3-butanedione. (D) Responses to increasing doses of CO2 and to the panel of odorants used in analyzing other sensilla; responses to benzaldehyde and 2,3-butanedione are also included. Mean ± SEM; n = 11–22.
Do these sensilla also respond to other odorants? We tested some of these sensilla against all 16 odorants of our panel and found responses to many of them, including 6-methyl-5-hepten-2-one (Fig. 7). A hierarchical cluster analysis of these 16 responses revealed that these more distal CO2-sensitive sensilla form a cluster that we designate ab4, most closely related to ab3 (SI Appendix, Fig. S3).
Interestingly, the response profile of ab4 resembles that of ab3 (and, by extension, ab1) but with greater response magnitudes (Figs. 5D and 7D). The ab4 profile correlates strongly with ab3 (0.92 Spearman’s rank correlation coefficient; P < 0.0001) as well as with ab1 (0.88; P < 0.0001).
We also tested these CO2-sensitive sensilla with benzaldehyde and with 2,3-butanedione, an odorant that inhibits the baseline activity of the CO2-sensitive neuron of Drosophila as well as its CO2 response (23). Benzaldehyde excites the CO2 sensilla, and 2,3-butanedione inhibits the baseline activity of the CO2 sensilla in G. morsitans (Fig. 7 B–D).
Discussion
We have analyzed odor coding in the antenna of the tsetse fly G. morsitans. We have identified 3 functional types of trichoid sensilla and 4 types of basiconic sensilla. ORNs of tsetse respond to a variety of odorants that emanate from their human and animal hosts, including 1-octen-3-ol, ethyl hexanoate, 6-methyl-5-hepten-2-one, and CO2. We also identified sensilla that respond to an insect sex pheromone, methyl laurate (14), and an insect alarm pheromone, 2-pentanol (24).
G. morsitans is similar to Drosophila in exploiting several degrees of freedom to encode the odors of its environment. Different sensilla are differently tuned; both excitatory and inhibitory responses are elicited. Different response dynamics are elicited from the same sensillum by different odorants.
However, there are some striking differences in odor coding between G. morsitans and Drosophila. First, some trichoid sensilla of G. morsitans are broadly tuned to a chemically diverse set of host odorants. The at3 sensillum of G. morsitans, for example, responds robustly to more than half of the odorants in our test panel. Trichoid sensilla of Drosophila, by contrast, are more narrowly tuned to pheromones (12, 14, 25, 26). Thus, in the 75 million years since Drosophila diverged from tsetse flies there has been a change in the ecological functions subserved by this morphological class of sensilla.
Another surprising finding was the dearth of very strong responses. We surveyed ∼50 odorants in a preliminary screen, of which 16 were selected for detailed analysis. Among all this testing we found no responses greater than 139 spikes/s at a 10−2 dilution (SI Appendix, Table S1). Two odorants produced responses of 139 spikes/s in ab4; among the other 6 types of sensilla, the greatest mean responses were on the order of 100 spikes/s or less, and responses of such magnitude were sparse. In Drosophila, a number of odorants at a 10−2 dilution elicit responses of 180 spikes/s or more from individual ORNs in basiconic sensilla, and the summed responses from ORNs of a sensillum are even higher in some cases (10). We verified that with our electrophysiological procedures we obtained summed responses from Drosophila sensilla on the order of 180 spikes/s with some odorants (SI Appendix, Fig. S1 F and G). It is possible that the paucity of strong responses we have detected from tsetse reflects a sampling bias; perhaps tsetse ORNs respond very strongly to odorants we have not tested. Another possibility is that the dynamic range of these G. morsitans ORNs is different. We note that the CO2-sensitive ORN of Drosophila also gives a stronger response than that of G. morsitans to a CO2 stimulus (4% CO2; Fig. 7D and SI Appendix, Fig. S1F; P < 0.01, Mann-Whitney test).
Another remarkable feature was the ubiquity of responses to 1-octen-3-ol. Of 7 types of sensillum analyzed here, all showed excitatory responses to 1-octen-3-ol except ab2, which showed no excitation to any odorants; 1-octen-3-ol emanates from tsetse hosts and attracts tsetse (27, 28). We note that many antennal ORNs of 2 other tsetse species, G. fuscipes fuscipes and G. p. palpalis, also respond to 1-octen-3-ol (29–31), and 1-octen-3-ol has previously been found to elicit an increase in the field potential of the G. morsitans antenna in an electroantennogram study (32, 33). There may have been great selective pressure to detect and evaluate the level of 1-octen-3-ol during the course of tsetse evolution; evidently, 1-octen-3-ol is a salient odorant for tsetse.
The similarity between the odor response profiles of ab1 and ab3 is provocative. The hierarchical cluster analysis separated them clearly, but their response profiles are strongly correlated. The responses that we have measured are summed responses of the ORNs in the sensilla. One possible interpretation of the similarity is that ab1 and ab3 each contain a common ORN, ORNX, which is paired with ORNY in ab1 and ORNZ in ab3. This explanation would violate a fundamental pairing rule established in the olfactory system of Drosophila, in which each ORN is located uniquely in a single sensillum type, paired uniquely with one or more other ORNs.
Likewise, ab4 is closely related to ab3 and ab1 in terms of its response profile. Perhaps ab4 also contains an ORNX that accounts for many of these responses, in addition to a CO2-sensitive neuron that is not found in ab1 or ab3. A violation of the pairing rule could also provide an explanation for a commonality between at1 and at3: The odorants that elicit the strongest responses from at1, 1-octen-3-ol, 2-pentanol, 1-hexen-3-ol, and methyl laurate, elicit comparable responses from at1 and at3. Perhaps both sensilla also share a common ORN that accounts for these similar responses.
We note the formal possibility that similar response profiles could also arise in 2 sensilla if each sensillum contains an ORN that expresses a receptor OrX, but in the ORN of one sensillum OrX is coexpressed with OrY, whereas in the ORN of the other sensillum OrX it is not. Coexpression of Ors in ORNs has been observed in both Drosophila (34) and mosquitoes (35).
The differences between the functional organization of the antennae in the fruit fly and the tsetse fly may reflect differences in their ecology and life cycle. Drosophila can feed on an extraordinary diversity of food sources, whereas tsetse feed uniquely on vertebrate blood. Drosophila also depends on its olfactory system to identify oviposition sites: A female fruit fly must identify a site on which eggs can hatch and on which larvae can feed and develop. By contrast, in the female tsetse fly a fertilized egg hatches, and the larva develops, within the uterus, feeding on a milk-like fluid supplied by the mother (36). These differences in the roles served by the olfactory systems of these 2 flies may explain in part the differences in their functional organization.
It is interesting to consider the G. morsitans and Drosophila olfactory systems in the context of their mosquito counterparts. G. morsitans is similar to Drosophila in that it encodes CO2 via neurons of the antenna, whereas mosquitoes encode CO2 via the maxillary palp (37, 38). Perhaps this similarity between the 2 kinds of flies reflects their closer phylogenetic relationship. By contrast, however, G. morsitans is similar to certain mosquitoes in using sharp-tipped trichoid sensilla to encode a broad range of odorants (39); likewise, very strong responses in these mosquitoes and tsetse are sparse, although quantitative comparisons are complicated by the possibility of methodological differences in different studies. G. morsitans differs from both Drosophila and at least some mosquito species by the ubiquity of its responses to 1-octen-3-ol and perhaps by violations of a pairing rule (39, 40).
The results described here provide a foundation for further work. First, testing with additional odorants could resolve additional sensillum types. In particular, at2 and ab2 showed no strong excitatory responses to any of the tested odorants but could each consist of multiple types that are distinguishable by virtue of their responses to an untested odorant. Second, if spikes could be sorted with confidence, perhaps by alternative electrophysiological techniques, the response profiles of individual ORNs and the logic by which they are combined in sensilla could be determined at high resolution. Third, we have explored 2 regions of the antenna in depth; other regions likely contain other sensillum types. We estimate that there are on the order of 2,500 olfactory sensilla on the entire antenna. Moreover, the first bioinformatic search for GmmOrs identified ∼46 genes (41). Some of these genes may be expressed exclusively in the larva or in other tissues, but it seems likely that a number of GmmOrs are expressed in antennal sensillum types that remain to be identified. In Drosophila and many other organisms, axons from ORNs that express the same Or gene converge upon a common glomerulus in the antennal lobe or olfactory bulb (42–44). From a preliminary examination of the G. morsitans antennal lobe we estimate that there are ∼40 glomeruli in both males and females (SI Appendix, Fig. S4), consistent with the notion that the present study provides precedent for a great deal of further discovery.
Materials and Methods
Tsetse Flies.
G. morsitans morsitans were cultured in the insectary at the Yale School of Public Health. Flies received defibrinated bovine blood (100 mL for 1,500 flies, blood temperature 35 °C–37 °C, feeding time 10–15 min) through an artificial membrane feeding system every 48 h. We used male and female virgin flies aged 8–12 d. Flies were kept at 50–55% relative humidity at 24 °C on a 12 h light to 12 h dark cycle.
Scanning Electron Microscopy.
Scanning electron microscopy was performed as in ref. 9. Briefly, heads were fixed in 0.1 M sodium cacodylate, 2% paraformaldehyde, and 2.5% glutaraldehyde for 1.5 h in microporous specimen capsules (Electron Microscopy Sciences). Heads were then dehydrated in a series of ethanol washes, finally being incubated overnight in 100% ethanol. These ethanol-dehydrated heads were then dried in a critical point dryer (Leica CPD300), and then forceps were used to remove antennae. Antennae were subsequently glued to metallic pegs with a graphite conductive adhesive (Electron Microscopy Sciences). Samples were then coated in ∼6 nm of iridium with a Cressington Sputter Coater and imaged in a Hitachi SU-70 scanning electron microscope.
Electrophysiology.
Single-sensillum recordings were performed as described previously (10), but with some modifications. Briefly, a single tsetse fly was first anesthetized on ice (∼30 s) and then placed in a 200 μL plastic pipette tip with the narrow end cut to allow only the antennae to protrude. Before recording, an immobilized fly was acclimatized for 30 min in a stream of clean humidified air under the recording microscope (Olympus BX51WI).
A tungsten electrode of ∼1 μm tip diameter that had been electrolytically sharpened in 5% potassium nitrate was used as a ground electrode. However, unlike a method used commonly in Drosophila (10), it was inserted carefully into the distal tip of the antenna. Care was taken to make sure that the ground wire did not damage the antenna. Using the antenna rather than the eye as a site of the ground electrode served to stabilize the antenna. This stability greatly facilitated the insertion of a recording electrode into the olfactory sensillum and extended the period during which we could record from the antenna.
As a recording electrode, another electrically sharpened tungsten wire was inserted into an olfactory sensillum at a position midway along its length using a second micromanipulator (NMN-21, Narishige). Alternating current (AC) signals were fed from a preamplifier (ISO-DAM, World Precision Instruments) to 16-bit Digidata 1440A (Molecular Devices, Axon Instruments). Data were sampled and recorded in a gap-free mode sampled at 10 kHz, with subsequent analysis being performed using Clampfit 10.3 (Axon Instruments, Molecular Devices).
A glass tube (20 cm long, ∼4 mm inner diameter) supplied humidified air to the preparation at a flow rate of 3 mL/s. The end of the tube was placed within ∼1 cm of the preparation. As an odor source, a cellulose filter disk (∼1 cm diameter) was soaked with 25 μL of diluted odorant and placed in a disposable borosilicate glass Pasteur pipette (capacity of 2 mL, Fisher Scientific GSA). The end of the Pasteur pipette was introduced into the continuous airstream via a hole in the delivery tube. A solenoid valve (Parker Hannifin, 001-0028-900) and a Uniblitz SD-10 driver control unit were used to control the delivery of the odor stimulus: An airflow was directed through the Pasteur pipette for 500 ms. The valve system was designed such that the airflow over the odor stimulus would be accompanied by a concomitant decrease in the flow rate of the continuous airstream, thereby minimizing any change in total flow rate. Only 2 deliveries were applied from a single odor cartridge and a maximum of 2 or 3 recordings were made from a single fly. There was an interval of at least 30 s between recordings from an individual sensillum to avoid desensitization. There was a gap of ∼15 min between recordings from 2 different sensilla of the same fly.
CO2 stimuli were delivered from a gas tank using a separate valve controller (Parker Hannifin, Picospritzer III). The stimulus was delivered into the same 20 cm tube, but in the absence of a continuous airflow. The CO2 stimulus is expected to be accompanied by a small increase in pressure; however, no change in baseline current was observed in response to a control pulse of air.
Spikes were counted off-line in 500 ms periods before and after the odor stimulus. Since there is a transit time between the initiation of the odorant pulse and the time when the odorant reaches the antenna, we began counting spikes when spikes began to be produced, which was ∼100 ms after the onset of odorant delivery. The spike frequency before stimulation was subtracted from the frequency after stimulation, unless otherwise indicated. Odorants other than methyl laurate were dissolved in paraffin oil (Sigma-Aldrich) at a 10−2 concentration unless otherwise indicated; methyl laurate was used neat. With trichoid sensilla, paraffin oil alone elicited no response in any tested case; with basiconic sensilla, the diluent alone elicited a low spike frequency that was subtracted from the frequency elicited by the odorant that was diluted in paraffin oil, unless otherwise indicated. We note the formal possibility that the response elicited by methyl laurate arises at least in part from unidentified impurities.
The 50 odorants used in preliminary tests on basiconic sensilla were acetone, ethyl butyrate, 2-pentanol, δ-nonalactone, 2-butanone, butyric acid, 1-octen-3-ol, hexanal, p-cresol, geosmin, 3-propylphenol, 2-heptanone, geranyl acetate, ethyl hexanoate, 1-hexen-3-ol, 3-octanol, 2-octanol, 3-octen-1-ol, 2-acetylpyridine, 2-acetylthiazole, 2-acetylthiophene, 4-ethylphenol, 3-ethylphenol, 2-isobutylthiazole, 2-methoxy-4-methylphenol, 3-nonanone, phenoxyethyl propionate, 2,4-dimethylthiazole, 4,5-dimethylthiazole, 2,4,5-trimethylthiazole, 3-methyl-2-cyclohexenone, 3-octanone, 2-octanone, 2-ethyl-4-methylthiazole, d-carvone, l-carvone, decanal, undecanal, undecanoic acid, ethyl formate, methyl eugenol, l-fenchone, heptanal, 2-methylheptanoic acid, 2-methylhexanoic acid, indole, isoamyl acetate, butylamine, octanal, and α,α-dimethylphenethyl acetate.
Various concentrations of CO2 were prepared from cylinders containing CO2 mixed with nitrogen and from air cylinders containing dry air without CO2 (AirGas). Multivariable statistical analysis (i.e., cluster analysis) was performed using JMP version 11 (SAS Institute), and PAST, a statistics program (http://folk.uio.no/ohammer/past/). Other statistical analysis and plotting were performed using Prism 8 (GraphPad).
Immunostaining.
The heads of adult flies, aged ∼12 d, were dissected in phosphate buffer saline containing 0.1% Triton X-100 (PBS-T) on ice. Brains were then fixed in 4% formaldehyde for 20 min, followed by washes (4 × 15 min) in PBS-T. Brains were rocked gently for an hour in Western blocking solution (5%) and then incubated in mouse anti-nc82 (anti-Bruchpilot; University of Iowa Hybridoma Bank, antibody registry ID AB_2314866), diluted 1:200 in 5% Western blocking solution, at 4 °C for 48 h on a rocking plate. Brains were then washed in PBS-T (4 × 15 min) and incubated with secondary antibody (Alexa 488 goat anti-rabbit at a 1:250 dilution, Invitrogen) for 1.5 h, followed by a final wash in PBS-T (5 × 30 s). Brains were mounted with Vectashield mounting media (H-1200, Vectashield) for confocal imaging using a Zeiss LSM 880 microscope. Z stacks of antennal lobes were scanned in 2 μm intervals using 20× air and 60× oil immersion lenses for higher resolution and to achieve better separation at boundaries between glomeruli. All images were postprocessed and analyzed using ImageJ software (NIH) to count the number of glomeruli in the antennal lobes.
Supplementary Material
Acknowledgments
We thank Dr. Brian Weiss and Dr. Serap Aksoy for tsetse flies and support and Dr. Nirag Kadakia for help with statistical analysis. This work was supported by a National Research Service Award (NIH F32 DC015969); NIH U01 AI115648 to J.R.C. and Serap Aksoy; and NIH R01 DC02174, NIH R01 DC04729, and NIH R01 DC11697 (to J.R.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907075116/-/DCSupplemental.
References
- 1.Legros D., et al. , Treatment of human African trypanosomiasis—Present situation and needs for research and development. Lancet Infect. Dis. 2, 437–440 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Bruce D., Preliminary Report on the Tsetse Fly Disease or Nagana in Zululand (Bennett and Davis, Durban, 1895). [Google Scholar]
- 3.Simarro P. P., et al. , Estimating and mapping the population at risk of sleeping sickness. PLoS Negl. Trop. Dis. 6, e1859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budd L. T., DFID-Funded Tsetse and Trypanosome Research and Development Since 1980: Economic Analysis (DFID, 1999). [Google Scholar]
- 5.Torr S. J., Dose responses of tsetse flies (Glossina) to carbon dioxide, acetone and octenol in the field. Physiol. Entomol. 15, 93–103 (1990). [Google Scholar]
- 6.Torr S. J., Hall D. R., Smith J. L., Responses of tsetse flies (Diptera: Glossinidae) to natural and synthetic ox odours. Bull. Entomol. Res. 85, 157–166 (1995). [Google Scholar]
- 7.Kuzoe F. A. S., Strategic Review of Traps and Targets for Tsetse and African Trypanosomiasis Control (World Health Organization, 2005).
- 8.Saini R. K., Hassanali A., A 4-alkyl-substituted analogue of guaiacol shows greater repellency to savannah tsetse (Glossina spp.). J. Chem. Ecol. 33, 985–995 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Chahda J. S., et al. , The molecular and cellular basis of olfactory response to tsetse fly attractants. PLoS Genet. 15, e1008005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bruyne M., Foster K., Carlson J. R., Odor coding in the Drosophila antenna. Neuron 30, 537–552 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Clyne P., Grant A., O’Connell R., Carlson J. R., Odorant response of individual sensilla on the Drosophila antenna. Invert. Neurosci. 3, 127–135 (1997). [DOI] [PubMed] [Google Scholar]
- 12.van der Goes van Naters W., Carlson J. R., Receptors and neurons for fly odors in Drosophila. Curr. Biol. 17, 606–612 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everaerts C., Farine J. P., Cobb M., Ferveur J. F., Drosophila cuticular hydrocarbons revisited: Mating status alters cuticular profiles. PLoS One 5, e9607 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dweck H. K., et al. , Pheromones mediating copulation and attraction in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 112, E2829–E2835 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yew J. Y., et al. , A new male sex pheromone and novel cuticular cues for chemical communication in Drosophila. Curr. Biol. 19, 1245–1254 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mwangi M. T., Gikonyo N. K., Ndiege I. O., Repellent properties of delta-octalactone against the tsetse fly, Glossina morsitans morsitans. J. Insect Sci. 8, 1–4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey A. F., Wang G., Su C. Y., Zwiebel L. J., Carlson J. R., Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride C. S., et al. , Evolution of mosquito preference for humans linked to an odorant receptor. Nature 515, 222–227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leal H. M., Hwang J. K., Tan K., Leal W. S., Attraction of Culex mosquitoes to aldehydes from human emanations. Sci. Rep. 7, 17965 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warnes M. L., Responses of the tsetse fly, Glossina pallidipes, to ox odour, carbon dioxide and a visual stimulus in the laboratory. Entomol. Exp. Appl. 17, 19–24 (1992). [Google Scholar]
- 21.Vale G. A., Bursell E., Hargrove J. W., Catching-out the tsetse fly. Parasitol. Today 1, 106–110 (1985). [DOI] [PubMed] [Google Scholar]
- 22.Bogner F., Response properties of CO2-sensitive receptors in tsetse flies (Diptera: Glossina Palpalis). Physiol. Entomol. 17, 19–24 (1992). [Google Scholar]
- 23.Turner S. L., Ray A., Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature 461, 277–281 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Ono M., Terabe H., Hori H., Sasaki M., Insect signalling: Components of giant hornet alarm pheromone. Nature 424, 637–638 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Lin C. C., Potter C. J., Re-classification of Drosophila melanogaster trichoid and intermediate sensilla using fluorescence-guided single sensillum recording. PLoS One 10, e0139675 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtovic A., Widmer A., Dickson B. J., A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446, 542–546 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Vale G. A., Hall D. R., The use of 1-octen-3-ol, acetone and carbon dioxide to improve baits for tsetse flies, Glossina spp. (Diptera: Glossinidae). Bull. Entomol. Res. 75, 219–232 (2009). [Google Scholar]
- 28.Colvin J., Gibson G., Host-seeking behavior and management of tsetse. Annu. Rev. Entomol. 37, 21–40 (1992). [DOI] [PubMed] [Google Scholar]
- 29.van der Goes van Naters W. M., Bootsma L., den Otter C. J., Belemtougri R. G., Search for tsetse attractants: A structure-activity study on 1-octen-3-ol in Glossina fuscipes fuscipes (Diptera: Glossinidae). J. Chem. Ecol. 22, 343–355 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Voskamp K. E., Everaarts E., Den Otter C. J., Olfactory responses to attractants and repellents in tsetse. Med. Vet. Entomol. 13, 386–392 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Voskamp K. E., Van der Goes van Naters W. M., Den Otter C. J., Comparison of single cell sensitivities to attractants in the tsetse Glossina fuscipes fuscipes, G. morsitans morsitans and G. pallidipes. Med. Vet. Entomol. 13, 460–462 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Ouedraogo L., den Otter C. J., Comparison of single cell sensitivities to acetone, 1-octen-3-ol and 3-methylphenol in the riverine tsetse species Glossina fuscipes fuscipes and G. palpalis palpalis. J. Insect Physiol. 107, 144–151 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Otter C. J. D., Van der Goes van Naters W., Single cell recordings from tsetse (Glossina m.morsitans) antennae reveal olfactory, mechano- and cold receptors. Physiol. Entomol. 17, 33–42 (1992). [Google Scholar]
- 34.Goldman A. L., Van der Goes van Naters W., Lessing D., Warr C. G., Carlson J. R., Coexpression of two functional odor receptors in one neuron. Neuron 45, 661–666 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Karner T., Kellner I., Schultze A., Breer H., Krieger J., Co-expression of six tightly clustered odorant receptor genes in the antenna of the malaria mosquito Anopheles gambiae. Front. Ecol. Evol. 3, 26 (2015). [Google Scholar]
- 36.Ma W. C., Denlinger D. L., Järlfors U., Smith D. S., Structural modulations in the tsetse fly milk gland during a pregnancy cycle. Tissue Cell 7, 319–330 (1975). [DOI] [PubMed] [Google Scholar]
- 37.Lu T., et al. , Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 17, 1533–1544 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellogg F. E., Water vapour and carbon dioxide receptors in Aedes aegypti. J. Insect Physiol. 16, 99–108 (1970). [DOI] [PubMed] [Google Scholar]
- 39.Hill S. R., Hansson B. S., Ignell R., Characterization of antennal trichoid sensilla from female southern house mosquito, Culex quinquefasciatus Say. Chem. Senses 34, 231–252 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Ghaninia M., Ignell R., Hansson B. S., Functional classification and central nervous projections of olfactory receptor neurons housed in antennal trichoid sensilla of female yellow fever mosquitoes, Aedes aegypti. Eur. J. Neurosci. 26, 1611–1623 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Glossina Genome Initiative , Genome sequence of the tsetse fly (Glossina morsitans): Vector of African trypanosomiasis. Science 344, 380–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vosshall L. B., Wong A. M., Axel R., An olfactory sensory map in the fly brain. Cell 102, 147–159 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Gao Q., Yuan B., Chess A., Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat. Neurosci. 3, 780–785 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Fishilevich E., Vosshall L. B., Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548–1553 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.