ABSTRACT
Limited data are available that summarize the relation between egg intake and the risk of upper aero-digestive tract (UADT) cancers. This systematic review and meta-analysis was conducted to investigate the association between egg intake and the risk of UADT cancers. Medline/PubMed, ISI web of knowledge, EMBASE, Scopus, and Google Scholar were searched using relevant keywords. Observational studies conducted on humans investigating the association between egg consumption and the risk of UADT cancers were included. Overall, 38 studies with a total of 164,241 subjects (27, 025 cases) were included. Based on 40 effect sizes from 32 case-control studies, we found a 42% increased risk of UADT cancers among those with the highest egg consumption (ranging from ≥1 meal/d to ≥1 time/mo among studies) compared to those with the lowest intake (ranging from 0–20 g/d to never consumed among studies) (overall OR: 1.42; 95% CI: 1.19, 1.68; P < 0.001). However, this association was only evident in hospital-based case-control (HCC) studies (OR = 1.50; 95% CI: 1.34, 1.68; P < 0.001 for ‘oropharyngeal and laryngeal cancer’ and OR: 1.27; 95% CI: 1.08, 1.50; P = 0.004 for esophageal cancer) and not in population-based case-control (PCC) studies (OR = 1.25; 95% CI: 0.59, 2.67; P = 0.56 for ‘oropharyngeal and laryngeal cancer’ and OR: 1.29; 95% CI: 0.92, 1.81; P = 0.13 for esophageal cancer). In addition, the association was not significant in prospective cohort studies (overall OR: 0.86; 95% CI: 0.71, 1.04; P = 0.11). Considering individual cancers, a positive association was observed between the highest egg consumption, compared with the lowest, and risk of oropharyngeal (OR: 1.88; 95% CI: 1.61, 2.20; P < 0.001), laryngeal (OR: 1.83; 95% CI: 1.45, 2.32; P < 0.001), oral & pharyngeal & laryngeal (OR: 1.37; 95% CI: 1.12, 1.67; P < 0.001), and esophageal cancers (OR: 1.28; 95% CI: 1.10,1.48; P = 0.001). We also found an inverse association between egg intake and the risk of oral cancer (OR: 0.78; 95% CI: 0.62, 0.99; P = 0.04). In conclusion, high egg consumption (ranging from ≥1 meal/d to ≥1 time/mo among studies) was associated with increased risk of UADT cancers only in HCC studies but not in PCC or prospective cohort studies. PROSPERO registration number: CRD42018102619.
Keywords: Egg, upper aero-digestive tract cancer, oral cancer, pharyngeal cancer, laryngeal cancer, esophageal cancer
Introduction
Upper aero-digestive tract (UADT) cancers, including cancer of the oral cavity, pharynx, larynx, and esophagus, are the seventh most frequent cancer type and the seventh most common cause of death from cancer worldwide (1). The number of new cases of esophageal cancer in 2018 was estimated to be 572,034, and the corresponding figures for cancers of the ‘lip and oral cavity’ and ‘oropharynx’ were estimated to be 354,864 and 92,887, respectively. The mortality rate of oral cancer is higher than that of other cancers including kidney cancer, Hodgkin's lymphoma, and skin cancer (2, 3).
Genetic and environmental factors, including tobacco smoking, alcohol consumption and betel quid chewing, human papillomavirus (HPV), poor immune system, and inadequate diet, are well-known risk factors for UADT cancers (4–6). Some studies have reported lower serum concentrations of vitamins A, B-12, C, E, and folate, beta-carotene, and zeaxanthin/lutein in subjects with oral cancer compared with controls (7, 8). Previous studies have investigated the association between egg consumption and the risk of several cancers, including UADT cancers. Some studies showed a positive association between the consumption of >3 eggs per wk and risk of UADT cancers (9, 10); however, others did not find a significant association or found an inverse relation between egg consumption and the risk of UADT cancers (11, 12).
Eggs are a good source of vitamins D, E, and B-12, lutein, and zeaxanthin, which have been previously linked with a reduced risk of UADT cancers (7, 8, 13–16). Eggs are also an excellent source of animal protein, which has been associated with increased risk of UADT cancers (15, 17). Findings on the association between egg consumption and UADT cancers are conflicting. The World Cancer Research Fund (WCRF) report in 2018, which summarized earlier prospective studies published until 2015 on diet and cancer prevention, revealed that there is ‘limited-no conclusion’ evidence with regards to egg intake and cancers of the oral cavity, larynx, pharynx, and esophagus (18, 19). However, case-control studies and those published after 2015 were not included in that report. This study aimed to systematically review the current evidence regarding egg consumption and the risk of UADT cancers and to summarize earlier findings through a meta-analysis.
Methods
Search strategy
We selected articles published up until May 2018 searching through the following databases: Medline/PubMed, ISI web of knowledge, EMBASE, Scopus, and Google Scholar. We further searched in social networks including ResearchGate and Mendeley to find additional relevant articles. The following keywords and their combinations were used in our literature search: (‘egg intake’ OR ‘ovum’ OR ‘egg consumption’ OR ‘diet cholesterol’ OR ‘meat’ OR ‘animal products’ OR ‘diet’ OR ‘food intake’ OR ‘nutrition’ OR ‘dietary indicators of’ OR ‘risk factors’ OR ‘food group’ OR ‘dietary factors’) AND (‘oropharynx’ OR ‘oral squamous cell’ OR ‘mouth’ OR ‘bucca’ OR ‘oral cavity’ OR ‘oral mucosa’ OR ‘mouth mucosa’ OR ‘intra-oral’ OR ‘head and neck’ OR ‘upper aero-digestive tract’ OR ‘oral pharyngeal’ OR ‘oral-pharyngeal’ OR ‘laryngeal’ OR ‘oral epithelial’ OR ‘intra-epithelial’ OR ‘oro-pharyngeal’ OR ‘esophageal’ OR ‘upper aero-digestive tract’) AND (‘carcinoma’ OR ‘cancer’ OR ‘tumour’ OR ‘tumor’ OR ‘carcinogen’ OR ‘neoplasm” OR ‘metastasis*’ OR ‘malignancies’ OR ‘leukoplakia’ OR ‘hyperplasia’ OR ‘biopsy’). All keywords were selected from the Medical Subject Headings (MeSH) database. No filter or limitation was used while searching the mentioned databases. We completed the search by reviewing the reference list of all relevant publications. All these steps were performed by two independent investigators (AA, RFM). Any disagreements were resolved by discussion or if necessary by the third investigator (AE). Duplicate citations were then removed. The full text of related articles was obtained, in some cases through contacting the corresponding author. The study protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO): CRD42018102619.
Eligibility criteria
Studies that fulfilled the following criteria were included in the meta-analysis: 1) conducted on humans; 2) were of observational design investigating the relation between egg consumption and the risk of oral cavity, oropharyngeal, pharyngeal, laryngeal, esophageal, and UADT cancers; 3) reported RRs or rate ratios and corresponding 95% CIs or provided figures enabling us to calculate these estimates. All potentially relevant studies were screened by two independent investigators (AA, RFM) on the basis of the study title and abstract. In the case of disagreements, the principal investigator (AE) was consulted.
Excluded studies
We excluded duplicate citations and studies that did not meet the above-mentioned inclusion criteria. In total 1673 articles were found in our initial search. Through screening for the title and abstract, 1629 articles were excluded. In addition, 6 studies were excluded because of the following reasons: the study by Bosetti et al. (20) was not included in the current analysis due to the overlap of subjects with another publication of the same group (21). Another study that had reported OR and 95% CIs for consumption of egg and dairy products together was excluded (22). Two other studies (23, 24) were excluded because of the overlap in participants with another study (10). The studies of Ren et al. (25) and Xibib et al. (26) were not included in the meta-analysis due to inadequate data. Despite our efforts to contact the authors of these publications, no results were obtained; however, we included them in our systematic review. After these exclusions, 40 studies remained for our systematic review (9–12, 15, 21, 25–58) and 38 studies for the meta-analysis (9–12, 15, 21, 27–58) (Figure 1).
FIGURE 1.
Flow diagram of study selection.
Data extraction
Required data were extracted using a standardized data collection form. The primary exposure was consumption of egg. The main outcome of interest in the current study was all cancers of the aero-digestive tract including oral, pharyngeal, oropharyngeal, laryngeal, and esophageal cancer. The following information was extracted by two independent reviewers (AA, RFM): the first author's last name, date of publication, study design, participants’ age range, gender, number of cases and controls, comparisons, method of assessment of egg intake, ascertainment of outcomes, ORs or RRs for the risk of oropharyngeal, laryngeal, esophageal, or UADT cancers, 95% CIs, and covariates controlled for. In the case of any disagreements between the two reviewers, the principal investigator (AE) was consulted.
Quality assessment of studies
The quality of studies included in this meta-analysis was assessed using the Newcastle-Ottawa Scale (59). Based on this method, a maximum of nine scores can be awarded to each study for selection of study groups (cancer patients and control group), comparability of groups, and substantiation of exposure (egg consumption). The quality score ranged from 4.0 to 8.0, with a median of 7.0. In the present analysis, we considered the quality scores of ≥7.0 as high-quality studies and those with a score of <7.0 were considered as low-quality studies.
Statistical analysis
RRs, HRs, or ORs for comparison of the highest versus the lowest categories of egg consumption were used as the measure of association between egg consumption and the risk of oropharyngeal, laryngeal, esophageal, or UADT cancers. Since the prevalence of these cancers was relatively low, ORs and HRs were directly considered as RRs. One study (35) did not report 95% CI; therefore, we calculated it using the number of patients with cancers in the highest (ranging from ≥1 meal/d to ≥1 time/mo among studies) and lowest categories of egg consumption (ranging from 0–20 g/d to never consumed among studies). For another study (48) that reported risk estimates for the lowest versus highest categories of egg consumption, the risk estimates were re-calculated for the highest versus the lowest categories of egg intake. We applied a random-effects model to compute overall RRs. In addition, Q-statistic and I2 were considered as indicators of heterogeneity. In the case of significant between-study heterogeneity, we used subgroup analysis to determine possible sources of heterogeneity. To assess publication bias, we constructed funnel plots for each outcome, in which log RRs were plotted against their SEs. We also conducted sensitivity analysis to examine the influence of any specific study on the overall estimate. Statistical analyses were conducted using STATA version 14 (STATA Corp.) and P values <0.05 were considered as statistically significant.
Results
Study characteristics
The main characteristics of included studies in our systematic review are summarized in Table 1. Out of 1,673 articles found in our initial research, 40 studies were included in the systematic review. Four cohort studies (32, 33, 40, 56), 2 nested case-control (12, 38), 10 population-based case-control (PCC) (9, 26, 28, 29, 36, 47, 50, 54, 55, 58), 22 hospital-based case-control (HCC) studies (10, 11, 15, 21, 25, 27, 30, 34, 35, 37, 39, 41–46, 49, 51–53, 57), 1 pooled analysis (31), and 1 study with both PCC and HCC design (48) met our criteria. These studies were published between 1987 and 2017. The sample size of these studies ranged from 54 to 37,257 participants. In total, 165,197 subjects (27,348 cases and 13,7849 controls) were included. Nineteen studies (9, 11, 12, 25, 26, 30, 33, 36, 38, 39, 41, 47, 48, 50, 51, 54, 55, 57, 58) were conducted in Asian countries, 12 investigations (15, 21, 27, 34, 35, 40, 42–46, 49) were from Europe, 4 (10, 37, 52, 53) were from South America, and 4 (28, 29, 32, 56) were from North America. In a pooled analysis study (31) based on 22 studies, 20 studies were from non-Asian countries, 1 was from an Asian country, and 1 study was international. None of these studies had overlap, in terms of population studied, with the other included studies in our meta-analysis. Out of 40 publications we included, 22 studies (11, 15, 21, 28–30, 32–35, 40, 42–46, 49–51, 54, 55, 58) were scored as high-quality studies, and 16 articles (9, 10, 12, 26, 27, 36–39, 41, 47, 48, 52, 53, 56, 57) were defined as low-quality studies. Due to lack of information for individual studies in the pooled analysis article (31), the Newcastle-Ottawa Scale was not completed for this case. For the study of Ren et al. (25), we could not find the full article and therefore, we failed to examine the study quality.
TABLE 1.
Characteristics of included studies in the systematic review1
| First Author, Year (Ref) | Study design | Country | Age range | Gender | Number of cases/controls | Assessment of exposure | Outcome variable | Comparison | OR or RR or HR (95% CI) | Study quality | Matching or Adjustments2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Butler et al. (28) | HCC | China and Taiwan | 18–85 | M/F | 921/806 | Questionnaire 12-item | Cancers of the oral cavity, oropharynx, hypopharynx, and larynx | ≥1 meal every d vs. never consumed | OR: 0.54 (0.26, 1.11) | 8 | 1, 2, 5, 6,7, 22, 23 24, 25 |
| Bravi et al. (15) | HCC | Italy and Switzerland | 19–79 | M/F | 768/2,078 | FFQ 78-item | Cancers of the oral cavity and pharynx | Q5 vs. Q1 | OR: 1.71 (1.14, 2.58) | 7 | 1,2,3,4,5,6,7,8 |
| Chuang et al. (29) | -3 | Multi-national study | All ages | M/F | 14,520/22,737 | Food questionnaire | Cancers of the oral cavity, pharynx, and larynx | Q4 vs. Q1 (≥ 7 vs. <1 times/wk) | OR: 1.48 (1.20, 1.82) | — | — |
| Toporcov et al. (51) | HCC | Brazil | NR | M/F | 296/296 | FFQ 41-item | Cancers of the mouth and oropharyngeal tract | ≥5 vs. <1 times/wk | OR: 22.23 (8.51, 60.17) | 5 | 1,2,7, 9,10 |
| Gao et al. (34) | PCC | China | 51–65 | M/F | 600/1,514 | Questionnaire | Esophageal cancer | Daily vs. monthly/seldom/never | OR: 1.12 (0.84, 1.49) | 6 | 1,2,13 |
| Aune et al. (10) | HCC | Uruguay | 23–89 | M/F | 283/2,032 | FFQ 60-item | Cancers of the oral cavity and pharynx | >3.5 vs. 0 eggs/wk | OR: 2.02 (1.19, 3.44) | 6 | 1,2,3,4,5,6,7,8,9,10,11,12 |
| 281/2,032 | Laryngeal cancer | OR: 1.17 (0.69, 1.97) | |||||||||
| 234/2,032 | Esophageal cancer | OR: 1.69 (0.98, 2.93) | |||||||||
| Fan et al. (31) | Cohort 20-Y | China | 45–64 | M | 101/18,244 | Questionnaire | Esophageal cancer | T3 vs. T1 | HRs: 0.83 (0.51, 1.35) | 8 | 1,2,3,4,5,6,7 |
| Wu et al. (52) | PCC | China Dafeng | Mean: 64.6 ± 8.9 | M/F | 291/291 | FFQ 90-item | Esophageal cancer | Q4 vs. Q1 | OR: 1.99 (0.72, 5.49) | 7 | 1,2,5,6,7,17,19,20,22,23 |
| China Ganyu | Mean: 65.4 ± 10.3 | 240/240 | OR: 0.95 (0.41, 2.22) | ||||||||
| Kapil et al. (37) | HCC | India | 41–80 | M/F | 305/305 | Semi-structured questionnaire | Laryngeal cancer | Consumption vs. no-consumption | OR: 2.69 (1.89, 3.85) | 6 | 1,2,13 |
| Yang et al. (53) | PCC | China | 35–85 | M/F | 185/185 | Questionnaire | Esophageal cancer | >1 vs. ≤1 times/wk | OR: 0.59 (0.25, 1.39) | 7 | 6,7,10,12,14,17,19,20 |
| Toporcov et al. (50) | HCC | Brazil | 34–81 | M/F | 31/23 | FFQ 41-item | Oral cavity cancer | ≥3 vs. <3 times per/wk | OR: 1.727 (0.781, 4.018) | 6 | 1,2,6 |
| Sanchez et al. (47) | HCC | Spain | 20–91 | M/F | 375/375 | FFQ 25-item | Cancers of the oral cavity and oropharynx | T3 vs.T1 (≥4 vs. ≤1 servings/wk) | OR: 0.97 (0.61, 1.52) | 7 | 1,2,3,6,7,16 |
| Lissowska et al. (44) | HCC | Poland | 23–80 | M/F | 122/124 | Questionnaire 25-item | Oral cavity cancer | T3 vs.T1 (≥6 vs. <3/wk) | OR: 0.51 (0.23, 1.15) | 7 | 1,2,6,13 |
| Rajkumar et al. (11) | HCC | India | 22–85 | M/F | 591/582 | Questionnaire 21-item | Oral cavity cancer | ≥3 vs. <1 servings/wk | OR:0.41 (0.25, 0.66) | 8 | 1,2,3,6,7,13 |
| Xibib et al. (24) | PCC | China | 30–75 | M/F | 211/633 | Questionnaire | Esophageal cancer | High vs. low intake | OR:0.93P = 0.98 | 5 | 1,2,3,9,13 |
| Bosetti et al. (18) | HCC | Italy | 30–79 | M/F | 527/1,297 | FFQ 78-item | Laryngeal cancer | Q5 vs. Q1 (12.5 vs. 0.4 servings/wk) | OR: 1.74 (1.07, 2.82) | 6 | 1,2,3,6,7,8,13 |
| Garrote et al. (35) | HCC | Cuba | 28–91 | M/F | 200/200 | Questionnaire 25-item | Cancers of the oral cavity and oropharynx | T3 vs.T1 (≥6 vs. <3servings/wk) | OR: 1.64 (0.91, 2.98) | 6 | 1,2,3,6,7,13 |
| Phukan et al. (39) | HCC | India | Mean: 55 ± 8.1 | M/F | 502/1,004 | Questionnaire | Esophageal cancer | Daily vs. never | OR: 1.2 (0.08, 6.30) | 4 | 1,2 |
| Takezaki et al. (48) | PCC | China | 40–79 | M/F | 199/333 | Questionnaire 152-item | Esophageal cancer | Q4 vs. Q1 (every day vs. <1 times/wk) | OR: 1.55 (0.86, 2.79) | 8 | 1,2,6,7,21 |
| Bosetti et al. (19) | HCC | Italy | 39–77 | M/F | 304/743 | Questionnaire 78-item | Esophageal cancer | Q5 vs. Q1 (>2.9 vs. ≤0.4 servings/wk) | OR: 1.86 (1.00, 3.43) | 7 | 1,2,3,4,6,7,8,13 |
| Levi et al. (43) | HCC | Italy | 34–74 | M/F | 101/327 | FFQ 79-item | Esophageal cancer | T3 vs. T1 (>2.5 vs. ≤1 serving/wk) | OR: 5.75 (2.5, 13.1) | 7 | 1,2,3,6,7,8 |
| Franceschi et al. (33) | HCC | Italy | 22–77 | M/F | 598/1,491 | FFQ 78-item | Cancers of the oral cavity and pharynx | Q5 vs. Q1 (>4 vs. ≤1 serving/wk) | OR: 2.5 (1.7, 3.7) | 7 | 1,2,3,6,7,8,13 |
| Gao et al. (9) | PCC | China | 30–79 | M/F | 81/234 | Questionnaire | Esophageal cancer | ≥3 vs. <1 times/week | OR: 3.35 (1.54, 7.30) | 5 | 1,2,13 |
| Pan et al. (12) | Nested case-control | China | Mean: 61.5 ± 7.7 | M | 125/250 | Questionnaire | Esophageal cancer | High vs. low intake | OR: 0.55 (0.36, 0.86) | 5 | 1 |
| Levi et al. (42) | HCC | Italy (Vaud, Switzerland) | 26–72 | M/F | 156/284 | FFQ 79-item | Cancers of the oral and pharynx | T3 vs. T1 (>3 vs. ≤1 serving/wk) | OR: 2.32 (1.28, 4.22) | 7 | 1,2,3,6,7,8,13 |
| Kjaerheim et al. (38) | Cohort 24-Y | Norway | Mean:59 | M | 71/10,960 | FFQ 32-item | Cancers of the oral cavity, pharynx, larynx, and esophageal | ≥6 vs. <1 times/mo | RR: 1.1 (0.3, 3.4) | 8 | 1,6,7 |
| Brown et al. (27) | PCC | USA | 30–79 | White men | 114/681 | FFQ 60-item | Esophageal cancer | Q4 vs. Q1 | OR: 1.4 (0.6, 2.9) | 8 | 1,2,6.7,8,13,15 |
| Black men | 219/557 | OR: 2.7 (1.3, 5.5) | |||||||||
| Launoy et al. (41) | HCC | France | NR | M | 208/399 | Questionnaire | Esophageal cancer | >45 vs. 0–20 g/d | OR: 1.17 (0.68, 2.08) | 7 | 1,6,7,8,13,16,18 |
| Takezaki et al. (49) | HCC | Japan | 20–79 | M/F | 266/36,527 | Questionnaire | Oral cavity cancer | T3 vs. T1 | OR: 1.4 (0.8, 2.2) | 7 | 1,2,6,7 |
| Chyou et al. (30) | Cohort 24-Y | USA (Japanese American) | 45–68 | M | 92/7,902 | FFQ 23-item | Cancers of the upper aerodigestive tract | ≥5 vs. <1 time/wk | RR: 1.33 (0.72, 2.45) | 7 | 1,6,7 |
| Guo et al. (36) | Nested case-control | China | 40–69 | M/F | 640/3,200 | Questionnaire | Esophageal cancer | >5 vs. 0 times/mo | OR: 0.8 (0.6, 1.1) | 5 | 6,17 |
| Zheng et al. (55) | HCC | China | 18–80 | M/F | 404/404 | FFQ 63-item | Oral cavity cancer | Fresh egg:≥4/wk vs. ≤2/mo | OR: 0.68 (0.35, 1.32) | 6 | 1,2,3,5,6,7 |
| Salted egg: ≥3 vs. <1/mo | OR: 0.69 (0.24, 1.79) | ||||||||||
| Yu et al. (54) | Cohort 15-Y | China | 20–67 | M/F | 1,162/12,693 | Questionnaire | Esophageal cancer | ≥1/mo vs. never | RR: 0.94 (0.83, 1.07) | 6 | 1,2 |
| Zheng et al. (56) | PCC | China | 20–75 | M | 115/269 | FFQ 42-item | Cancers of the oral cavity and pharynx | Daily vs. seldom | OR: 1.25 (0.49, 3.23) | 8 | 1,2,6,7 |
| F | 89/145 | OR: 1.25 (0.35, 4.48) | |||||||||
| La Vecchia et al. (40) | HCC | Italy | 37–74 | M/F | 105/1,169 | Questionnaire 17-item | Cancers of the oral cavity and pharynx | >1 vs. <1 portion/wk | OR: 1.5 (0.9, 2.4) | 7 | 1,2 |
| Franceschi et al. (32) | HCC | Italy | Median:Case: 59control: 58 | M/F | 302/699 | Questionnaire 40-item | Cancer of the oral cavity and pharynx | T3 vs. T1 | OR: 2.0P < 0.01 | 7 | 1,2,6,7,14 |
| Ren et al. (23) | HCC | China | — | M/F | Cases: 112 | — | Esophageal cancer | — | OR: 0.30 | — | — |
| Brown et al. (26) | PCC | USA | 30–79 | M | 207/422 | Questionnaire 65-item | Esophageal cancer | T3 vs. T1 | OR: 0.7 (0.4, 1.2) | 7 | 1,6,7,13,15,16 |
| Yu et al. (45) | PCC | USA | 20–64 | M/F | 275/275 | Questionnaire | Esophageal cancer | >5/wk vs. <1/wk | OR: 0.8 (0.5, 1.3) | 6 | 1,2,15 |
| Notani and Jayant (46) | HCC | India | All ages | M | 278/215 | Questionnaire | Oral cavity cancer | <1/wk vs. 1/wk | OR: 1.20 (0.7, 1.9) | 6 | 1,7 |
| 225/215 | Pharyngeal cancer | OR: 0.68 (0.4, 1.1) | |||||||||
| 80/215 | Esophageal cancer | OR: 1.01 (0.6, 1.7) | |||||||||
| 236/215 | Laryngeal cancer | OR: 0.97 (0.5, 2.0) | |||||||||
| PCC | 278/177 | Oral cavity cancer | OR: 0.83 (0.5, 1.5) | 7 | |||||||
| 225/177 | Pharyngeal cancer | OR: 0.43 (0.2, 0.8) | |||||||||
| 80/177 | Esophageal cancer | OR: 0.79 (0.4, 1.4) | |||||||||
| 236/177 | Laryngeal cancer | OR: 0.64 (0.3, 1.4) |
1HCC, hospital case-control; PCC, population case-control; NR, not reported; Ref, reference.
2Age (1), sex (2), education (3), year of interview (4), BMI (5), smoking (6), alcohol consumption (7), energy intake (8), income (9), food intake (vegetables, fruits, grain, dairy, meat), (10), mate (11), tea (12), area of residence (13), occupation (14), race (15), hospital (16), cancer history (17), interviewer (18), eating hot foods (19), eating speed (20), ethnicity (21), level of education (22), past economic status (23), betel quid use (24), center of data collection (25).
3Pooled analysis: both HCC and PCC studies.
Overall, almost all studies reported ORs except for 4 studies that reported HRs (33) and RRs (32, 40, 56). Ten studies (10, 15, 34, 35, 34, 42, 44, 49, 53, 58) examined oropharyngeal cancer, 6 studies (11, 46, 48, 51, 52, 57) investigated oral cavity cancer, 4 investigations (10, 27, 39, 48) provided risk estimates for laryngeal cancer, 1 article (48) reported the risk of pharyngeal cancer, 2 studies (30, 31) investigated oral cavity, pharyngeal, and laryngeal cancers, 2 articles (32, 40) evaluated the risk of UADT cancers, and 20 studies (9, 10, 12, 21, 25, 26, 28, 29, 33, 36, 38, 41, 43, 45, 47, 48, 50, 54–56) presented data for esophageal cancer.
Among included studies, 31 articles reported their findings for males and females combined (9–11, 15, 21, 25–27, 30, 31, 34–39, 41, 42, 44–47, 49–57), 9 studies for males only (12, 28, 29, 32, 33, 40, 43, 48, 58), and 1 article for females only (58). Egg consumption was assessed using an FFQ in 15 investigations (10, 15, 27, 29, 32, 35, 40, 44, 45, 49, 52–54, 57, 58) and through the use of other questionnaires in 23 studies. The mean follow-up duration for cohort and nested case-control studies ranged from 5 to 24 y (32, 38, 40). For almost all case-control studies, cases were matched with controls in terms of age and sex. One study (31) did not control for any confounder. Nine studies adjusted for total energy intake (10, 15, 21, 27, 29, 35, 43–45), others did not. Smoking/alcohol (n = 29) (10, 11, 15, 21, 27–30, 32–35, 37, 38, 40, 43–46, 48–55, 57, 58), area of residence (n = 14) (9, 11, 21, 26–29, 35–37, 39, 43, 44, 46), and BMI (n = 6) (10, 15, 30, 33, 54, 57) were also controlled for in some studies. The minimum ORs for oral cavity, oropharyngeal, laryngeal, and esophageal cancers were 0.41 (11), 0.97 (49), 0.97 (48), and 0.3 (25), respectively, and the maximum corresponding ORs were 1.72 (52), 22.23 (53), 2.96 (39), and 5.75 (45), respectively.
Findings from the meta-analysis on case-control studies
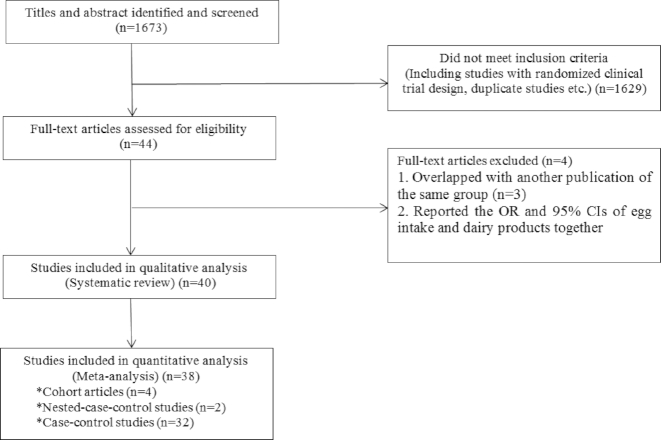
In total, out of 40 studies included in the systematic review, 38 studies (32 case-control studies, 2 nested case-control studies, and 4 cohort studies) met our criteria for meta-analysis. Total sample size enrolled in these studies was 164,241 (27,025 cases and 13,7216 controls). Case-control studies (n = 32) that examined the relation between egg consumption and a certain UADT cancer (oral cavity, pharyngeal, laryngeal, and esophageal) included a total of 108,361 subjects. The total number of cases with oral, pharyngeal, oropharyngeal, laryngeal, ‘oral & pharyngeal & laryngeal’, and esophageal cancer was 1,692, 225, 3,287, 1,349, 15,441, and 3,840, respectively. Based on 40 effect sizes from 32 case-control studies (9–11, 15, 21, 27–31, 34–37, 39, 41–55, 57, 58), we found a significant association between the highest egg consumption (ranging from ≥1 meal/d to ≥3 times/mo among studies) compared with the lowest (ranging from 0–20 g/d to never consumed among studies) and increased risk of UADT cancers (overall OR:1.42; 95% CI: 1.19,1.68; P < 0.001) (Figure 2). However, between-study heterogeneity was significant (I2 = 74.8%; P-heterogeneity <0.001). When we removed the study of Chuang et al. (31), which was a pooled analysis study, the results did not change (OR: 1.42; 95%: 1.17, 1.70; P < 0.001). In addition, excluding the study of Toporcov et al. (52), which had a small number of controls and wider ORs than other publications, did not affect the findings (OR: 1.41; 95% CI: 1.18, 1.68; P < 0.001).
FIGURE 2.
Forest plot derived from random-effects meta-analysis of case-control studies investigating the association between egg consumption and UADT cancers. UADT, upper-aero-digestive tract; ES, effect size.
To find the source of heterogeneity, we conducted subgroup analysis for two mainly reported outcomes: ‘oropharyngeal and laryngeal’ and ‘esophageal’ cancers. Combining 24 effect sizes from 20 studies (10, 11, 15, 27, 30, 31, 34, 35, 37, 39, 42, 44, 46, 48, 49, 51–53, 57, 58) on ‘oropharyngeal and laryngeal’ cancer, we found that individuals with the highest egg consumption had a 49% increased risk of these cancers (OR: 1.49; 95% CI: 1.35, 1.64; P < 0.001). When we excluded the study of Toporcov et al. (53), the same findings were obtained (OR: 1.49; 95% CI: 1.35, 1.64; P < 0.001). Based on 16 effect sizes from 14 case-control studies on esophageal cancer (9, 10, 21, 28, 29, 36, 41, 43, 45, 47, 48, 50, 54, 55), we reached an overall effect size of 1.28, meaning a 28% increased risk of esophageal cancer for individuals with the highest egg consumption compared with those of the lowest egg intake (OR: 1.28; 95% CI: 1.10, 1.48; P = 0.001).
Due to a high between-study heterogeneity in these two sets of studies (for studies on ‘oropharyngeal and laryngeal cancer’: I2 = 79.9%; P-heterogeneity <0.001 and for studies on ‘esophageal cancer’: I2 = 62.5%; P-heterogeneity <0.001), we performed subgroup analysis by outcome, study design, energy adjustment, gender, country, smoking adjustment, alcohol adjustment, and study quality (Table 2).
TABLE 2.
Results of subgroup analysis for egg consumption and risk of upper aero-digestive tract in case-control studies
| No. of effect sizes | OR (95% CI) | P-within1 | I 2 (%) | P-between2 | |
|---|---|---|---|---|---|
| Subgroup analyses for oropharyngeal and laryngeal cancers | |||||
| Outcome | < 0.001 | ||||
| Oral cancer | 6 | 0.78 (0.62, 0.99) | 0.005 | 70.2 | |
| Oropharyngeal cancer | 11 | 1.88 (1.61, 2.20) | <0.001 | 73.4 | |
| Laryngeal cancer | 4 | 1.83 (1.45, 2.32) | 0.019 | 69.9 | |
| Oral & pharyngeal & laryngeal cancer | 2 | 1.37 (1.12, 1.67) | 0.009 | 85.4 | |
| Study design | 0.893 | ||||
| Population-based case-control | 2 | 1.25 (0.59, 2.67) | 1.00 | 0.00 | |
| Hospital-based case-control | 21 | 1.50 (1.34, 1.68) | <0.001 | 82.15 | |
| Adjustment for energy intake | 0.006 | ||||
| Yes | 6 | 1.89 (1.56,2.29) | 0.301 | 17.4 | |
| No | 18 | 1.38 (1.23, 1.54) | <0.001 | 82.7 | |
| Gender | 0.102 | ||||
| Both | 19 | 1.55 (1.40, 1.72) | <0.001 | 82.8 | |
| Male | 4 | 1.10 (0.82, 1.48) | 0.456 | 0.0 | |
| Asian vs. non-Asian | 0.001 | ||||
| Asian | 10 | 1.15 (0.96, 1.37) | <0.001 | 81.7 | |
| Non-Asian | 13 | 1.76 (1.53, 2.02) | <0.001 | 75.5 | |
| Adjustment for smoking | 0.225 | ||||
| Yes | 17 | 1.39 (1.22, 1.58) | <0.001 | 75.2 | |
| No | 7 | 1.63 (1.41, 1.89) | <0.001 | 86.6 | |
| Adjustment for alcohol consumption | 0.115 | ||||
| Yes | 19 | 1.43 (1.26, 1.61) | <0.001 | 80.8 | |
| No | 5 | 1.62 (1.37, 1.90) | 0.002 | 75.9 | |
| Study quality3 | 0.73 | ||||
| High quality | 11 | 1.44 (1.24, 1.67) | <0.001 | 79.9 | |
| Low quality | 12 | 1.57 (1.33, 1.84) | <0.001 | 82.1 | |
| Subgroup analyses for esophageal cancer | |||||
| Outcome | |||||
| Study design | 0.93 | ||||
| Population-based case-control | 10 | 1.16 (0.97, 1.39) | 0.006 | 60.7 | |
| Hospital-based case-control | 6 | 1.56 (1.20, 2.02) | 0.016 | 64.1 | |
| Adjustment for energy intake | 0.001 | ||||
| Yes | 6 | 1.87 (1.44, 2.44) | 0.044 | 56.2 | |
| No | 10 | 1.08 (0.90, 1.28) | 0.048 | 47.2 | |
| Gender | 0.31 | ||||
| Both | 11 | 1.34 (1.12, 1.60) | 0.001 | 66.6 | |
| Male | 5 | 1.13 (0.837, 1.49) | 0.059 | 55.9 | |
| Asian vs. non-Asian | 0.41 | ||||
| Asian | 8 | 1.20 (0.98, 1.47) | 0.099 | 41.9 | |
| Non-Asian | 8 | 1.36 (1.10, 1.69) | <0.001 | 74.4 | |
| Adjustment for smoking | 0.085 | ||||
| Yes | 11 | 1.44 (1.18, 1.77) | 0.002 | 63.4 | |
| No | 5 | 1.11 (0.90, 1.38) | 0.045 | 58.9 | |
| Adjustment for alcohol consumption | |||||
| Yes | 12 | 1.37 (1.14, 1.66) | 0.002 | 62.2 | |
| No | 4 | 1.14 (0.90, 1.44) | 0.023 | 68.4 | |
| Study quality3 | 0.236 | ||||
| High quality | 10 | 1.41 (1.13, 1.75) | 0.001 | 66.6 | |
| Low quality | 6 | 1.18 (0.97, 1.44) | 0.040 | 57.2 |
1 P values were obtained through fixed-effects analysis.
2 P values were obtained through random-effects analysis.
3Study quality: high: score ≥7, Low: score <7.
In the subgroup analysis based on studies on ‘oropharyngeal and laryngeal cancer’, we found a positive association between the highest egg consumption compared with the lowest and risk of oropharyngeal (OR: 1.88; 95% CI: 1.61, 2.20; P < 0.001), laryngeal (OR: 1.83; 95% CI: 1.45, 2.32; P < 0.001), and ‘oral & pharyngeal & laryngeal’ cancers (OR: 1.37; 95% CI: 1.12, 1.67; P = 0.002) and an inverse association between egg intake and the risk of oral cancer (OR: 0.78; 95% CI: 0.62, 0.99; P = 0.04). In addition, a significant positive association was seen between egg intake and risk of ‘oropharyngeal and laryngeal cancer’ in HCC studies (OR = 1.50; 95% CI: 1.34, 1.68; P < 0.001), as well as in studies either adjusted (OR: 1.89; 95% CI: 1.56, 2.29 P < 0.001) or not for total energy intake (OR: 1.38; 95% CI: 1.23, 1.54; P < 0.001). This association was also seen in studies that involved both genders (OR: 1.55; 95% CI: 1.40, 1.72; P < 0.001), performed in non-Asian countries (OR: 1.76; 95% CI: 1.53, 2.02; P < 0.001) and those that adjusted (OR: 1.39; 95% CI: 1.22, 1.58; P < 0.001) or not for smoking (OR: 1.63; 95% CI: 1.41, 1.89; P < 0.001). Sensitivity analysis revealed that none of the single studies had a significant effect on the overall effect size. No evidence of publication bias was observed (PEgger's test = 0.55).
When we performed subgroup analysis based on studies on ‘esophageal cancer’, a significant positive association between the highest egg consumption compared with the lowest intake and risk of esophageal cancer was seen in HCC studies (OR: 1.56; 95% CI: 1.20, 2.02; P = 0.001), as well as in studies adjusted for energy intake (OR: 1.87; 95% CI: 1.44–2.44; P < 0.001), smoking (OR: 1.44; 95% CI: 1.18, 1.77; P < 0.001), and alcohol (OR: 1.37; 95% CI: 1.14, 1.66; P < 0.001) and those that involved both genders (OR: 1.34; 95% CI: 1.12, 1.60; P = 0.001). The same findings were obtained for studies from non-Asian countries (OR: 1.36; 95% CI: 1.10, 1.69; P = 0.005) and those of high quality (OR: 1.41; 95% CI: 1.13, 1.75; P = 0.002). No single study influenced the overall effect size in our sensitivity analysis. No evidence of publication bias was observed (PEgger's test = 0.64).
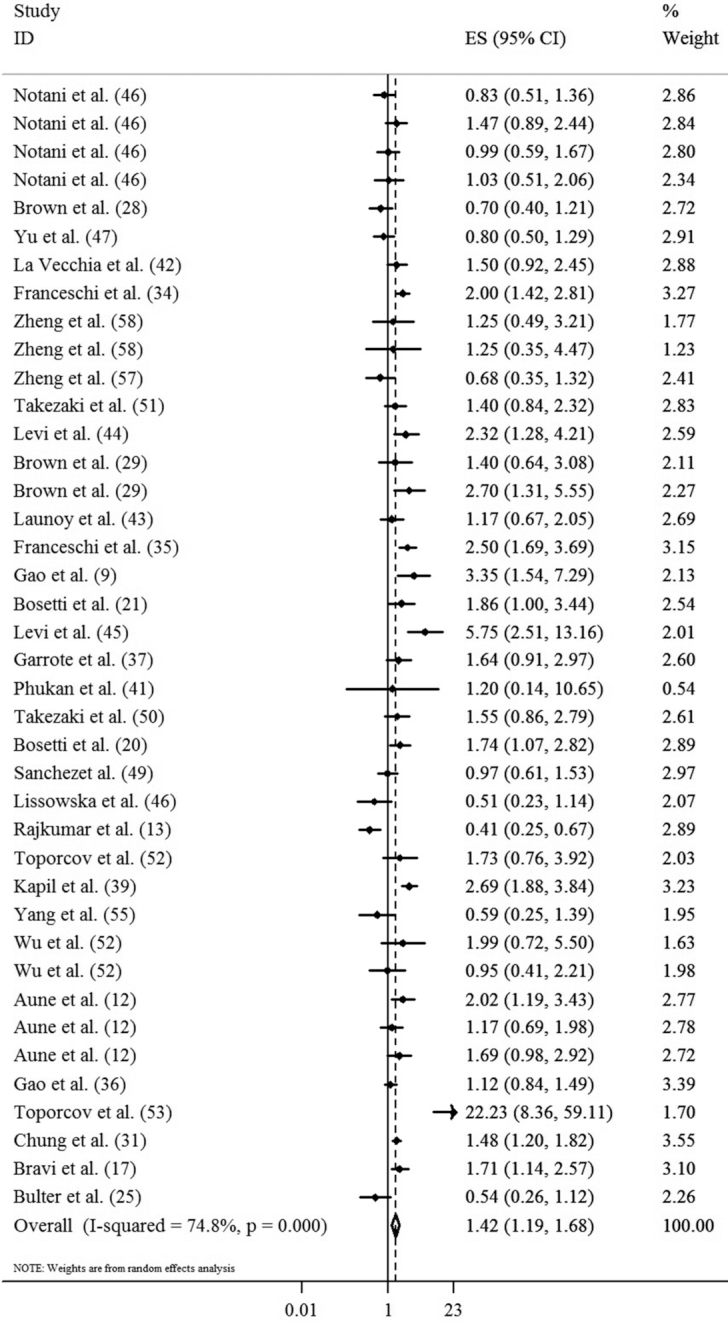
To conduct dose-response meta-analysis on egg consumption and UADT cancers, only 4 studies reported the required information; in these 4 studies, the outcome was esophageal cancer (9, 36, 41, 47). Consumption of one additional egg per wk was not associated with an increased risk of esophageal cancer (OR: 1.06; 95% CI: 0.95, 1.18; P = 0.27; I2 = 74.8%; P-heterogeneity = 0.008). No evidence of a significant nonlinear association was observed between egg intake and the risk of esophageal cancer (P = 0.35) (Figure 3). For other UADT cancers, sufficient information was not available to perform dose-response analysis.
FIGURE 3.
Nonlinear dose-response meta-analysis of case-control studies investigating the association between egg consumption and risk of esophageal cancer (P = 0.35).
Findings from the meta-analysis on cohort studies
Combining results from 4 cohorts (32, 34, 40, 56) and 2 nested case-control studies (12, 38), with 6 effect sizes, we found no significant association between the highest egg consumption (ranging from ≥5 times/wk to ≥1 time/mo among studies) compared with the lowest (ranging from <1 time/wk to never consumed among studies) and risk of UADT cancers (OR: 0.86; 95% CI: 0.71, 1.04; P = 0.11) (Figure 4). When we excluded nested case-control studies (12, 38) from the analysis, similar findings were obtained (OR: 0.95; 95% CI: 0.84, 1.07; P = 0.37; I2 = 0.0%, P-heterogeneity = 0.67). No significant between-study heterogeneity (I2 = 35.2%; P = 0.173) and publication bias (PEgger's test = 0.67) was seen. Required data were not available to perform dose-response analysis.
FIGURE 4.
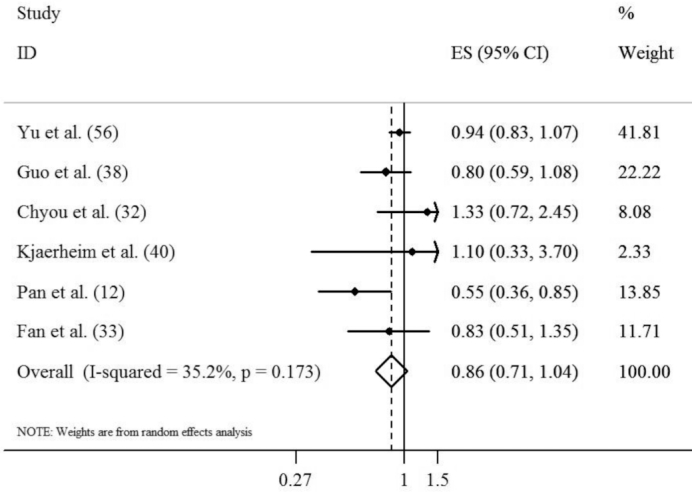
Forest plot derived from random-effects meta-analysis of cohort and nested case-control studies investigating the association between egg consumption and risk of UADT cancers. UADT, upper-aero-digestive tract; ES, effect size.
Discussion
Based on findings of the present meta-analysis, we found a positive association between egg consumption and the risk of UADT cancers in case-control studies; however, this was not confirmed by prospective data. In the subgroup analysis, we found a positive significant association between egg intake and the risk of ‘oropharyngeal’, ‘laryngeal’, ‘oral & pharyngeal & laryngeal’, and ‘esophageal’ cancers but this association was inverse for oral cancer. This study is among the first publications to examine the relation between egg consumption and the risk of UADT cancers.
UADT cancers are associated with significant morbidity and mortality (60, 61) and diet is a potentially modifiable risk factor for UADT cancers (7). We found a positive association between egg consumption and the risk of UADT cancers in case-control studies, but not in prospective cohort studies. In addition, when we considered HCC versus PCC studies separately, we found this association only in hospital-based studies. Contradictory to our findings on case-control studies, several previous studies have reported a significant positive association between egg intake and oropharyngeal cancer (10, 15, 34, 35, 44, 53), laryngeal cancer (27, 39), and oral & pharyngeal & laryngeal cancer (31). However, some others did not find any significant association (10, 30, 37, 42, 48, 49, 58). Only 1 study (11) has reported a protective relation between egg consumption and the risk of oral cancer; others found no significant association (46, 48, 51, 52, 57). Earlier studies conducted on esophageal cancer concluded there was no significant association between egg intake and the risk of esophageal cancer (10, 28, 29, 36, 41, 43, 47, 48, 50, 54, 55). The 2018 report of the World Cancer Research Fund (WCRF), which has summarized the scientific evidence on cancer prevention, concluded that there is ‘limited’ evidence with regards to egg intake and risk of mouth, pharynx, larynx, and esophageal cancers and therefore they claimed that there could be ‘no conclusion’ regarding the association (18, 19). They defined ‘limited-no conclusion’ evidence as ‘data were of too low quality, too inconsistent’, or ‘few number of studies’. However, the methodology used in that report was different from ours. They restricted their literature search to only Medline, up to April 2015, whereas in addition to Medline/PubMed, we searched several other databases including ISI Web of Knowledge, EMBASE, Scopus, and Google Scholar until May 2018. The report of the WCRF only included cohort and nested case-control studies and 1 pooled analysis, whereas we considered all observational studies examining the association between egg consumption and the risk of UADT cancers, including 4 cohort, 2 nested case-control, 1 pooled analysis, and 32 case-control studies. Furthermore, despite sufficient information on individual cancers of the mouth, pharynx, larynx, and pharyngeal cancers, they did not consider separately analyzing egg consumption in relation to these cancers.
Eggs are cooked by different methods in various countries based on food culture. Boiled or fried eggs are different in their nutrient content, in particular, in terms of fatty acids (SFA, PUFA, MUFA, trans-FA) and heterocyclic amines which are formed during high temperature frying (62). Therefore, cooking methods might be an effect modifier when investigating the association between egg and risk of diseases. However, most studies we included did not provide data on the egg preparation method. Moreover, the comparison ranges for egg intake varies across studies; the highest egg intake ranged from ≥1 meal/d to ≥1 time/mo and the range for the lowest intake varied from 0–20 g/d to never consumed. Another important point that should be considered when interpreting our findings is lack of controlling for several confounding factors in the included studies. The WCRF report listed important contributors to UADT cancers including alcohol intake, mate, body fat, physical activity, and dietary intake of coffee, vegetables, fruit, and processed meat which were not controlled in most included studies in our meta-analysis. It should also be kept in mind that the significant associations we found were confined to only HCC studies. These associations were not found in PCC studies or in prospective cohort studies. We all know that findings from case-control studies, in particular HCC, might be misleading because of several methodological limitations that have been mentioned previously (63). Recall bias and selection bias should be considered in these surveys. Although similar covariates were controlled for in HCC and PCC studies, the difference in their findings might be explained by the additional major sources of bias in hospital-based studies compared with population-based studies. For instance, dietary habits of hospital-based controls might not represent those of the general population (64, 65). In addition, the limited number of PCC studies for some cancers might also help explain this difference. In contrast to case-control studies, cohort studies have several strengths (66); however, not all cohort studies we included were of high quality. When we performed the meta-analysis on 4 cohort and 2 nested case-control studies, no significant association was observed between high egg consumption and the risk of UADT cancers. Such differences between findings of case-control and cohort studies were also seen in other investigations on diet–cancer relations (63, 67). In the dose-response meta-analysis, we found no significant association between 1 additional egg intake per wk and increased risk of esophageal cancer. Considering the limitations of case-control studies and limited number of cohort studies in this field, it seems additional data are required to shed light on this issue.
Several potential mechanisms may explain the association of egg consumption with UADT cancers. The most plausible explanation for the positive association between egg intake and the risk of UADT is the role of animal fat and protein and the high cholesterol content of eggs. Several previous investigations found a direct association between animal fats, proteins, saturated fatty acids, and cholesterol intake and risk of oropharyngeal and laryngeal cancers (15, 34, 52, 68). Fatty acids may also influence carcinogenesis, including their effect on cell membrane integrity, increasing lipid peroxidase, alteration of hormone concentrations, and impairment of nutrient metabolism (69). Moreover, high egg consumption, which is a cheap source of animal protein, may be a general indicator of low income and poor diet (44). Frequent egg intake was associated with poor and unhealthy diets and higher intakes of total, red, and processed meats (10), all of which were defined as risk factors for esophageal squamous cell carcinoma (19). On the other hand, egg contains high amounts of some micronutrients including vitamin A and riboflavin. These nutrients are involved in repairing damaged mucosal lining and might have anti-carcinogenic effects (70–72), which can in turn explain the inverse association of egg intake and oral cancer.
This meta-analysis was among the first studies to summarize the relation between egg intake and the risk of UADT cancers. A large sample size (164,241 subjects and 27,025 cases) from different geographic regions with different dietary patterns are among the strengths of this study. Furthermore, our findings were stable and robust in sensitivity analysis. We used a prospectively defined protocol, explicit study inclusion criteria, and comprehensive literature search with the least limitations; however, some limitations should be noted. Total energy intake, alcohol consumption, and smoking were not considered as confounding factors in all included studies. In addition, none of the studies considered physical activity. Residual confounding by other inadequately measured covariates could also be of concern. Self-reported egg intake through questionnaires that might inevitably result in some misclassification of participants in terms of exposure must be noted. We have considered the amount of egg intake in different categories of egg consumption if they were reported in the original articles. However, we were not able to perform a meta-regression on the actual egg intake data due to lack of such information in the included studies. In addition, we did not consider cooking methods of egg due to lack of data. Moreover, we performed dose-response meta-analysis on a limited number of studies (only 4 studies regarding esophageal cancer) that provided adequate data.
Conclusion
In conclusion, we found no significant association between egg consumption and risk of UADT cancers in PCC and prospective cohort studies; however, a positive significant relation was observed in HCC studies for egg intake and UADT cancers, except for oral cancer, which was inversely associated with egg consumption.
Acknowledgments
The authors’ responsibilities were as follows—RFM, BL, and AE: designed the research; AA, RFM, and AE: conducted the research; AA, RFM, and PS: analyzed the data; AA, BL, and AE: wrote the paper; AE: had primary responsibility for the final content; and all authors read and approved the final manuscript. All authors reported no conflict of interest.
Notes
Supported by Tehran University of Medical Sciences, Tehran, Iran.
Author disclosures: AA, RF-M, AS-M, PS, BL, and AE, no conflicts of interest.
AA and RF-M are co-first authors.
Abbreviations used: HCC, hospital-based case-control; HPV, human papillomavirus; PCC, population-based case-control; UADT, upper aero-digestive tract.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. [DOI] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer, [Internet]. Available from:. http://gco.iarc.fr/today/home. Accessed on 28 October 2018. [Google Scholar]
- 3. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. 2012 Int J Cancer. 2015;136(5):E359–E86. [DOI] [PubMed] [Google Scholar]
- 4. Helen-Ng LC, Razak IA, Ghani WMN, Marhazlinda J, Norain AT, Raja Jallaludin RL, Rahman ZAA, Abdullah N, Zain RB. Dietary pattern and oral cancer risk–a factor analysis study. Community Dent Oral Epidemiol. 2012;40(6):560–6. [DOI] [PubMed] [Google Scholar]
- 5. Guha N, Warnakulasuriya S, Vlaanderen J, Straif K. Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int J Cancer. 2014;135(6):1433–43. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Mao Y, Zhang Y, Cai S, Chen G, Ding Y, Guo J, Chen K, Jin M. Alcohol drinking and upper aerodigestive tract cancer mortality: a systematic review and meta-analysis. Oral Oncol. 2014;50(4):269–75. [DOI] [PubMed] [Google Scholar]
- 7. Zain RB. Cultural and dietary risk factors of oral cancer and precancer—a brief overview. Oral Oncol. 2001;37(3):205–10. [DOI] [PubMed] [Google Scholar]
- 8. Ramaswamy G, Rao V, Kumaraswamy S, Anantha N. Serum vitamins' status in oral leucoplakias—a preliminary study. Eur J Cancer Part B: Oral Oncology. 1996;32(2):120–2. [DOI] [PubMed] [Google Scholar]
- 9. Gao CM, Takezaki T, Ding JH, Li MS, Tajima K. Protective effect of allium vegetables against both esophageal and stomach cancer: a simultaneous case-referent study of a high-epidemic area in Jiangsu Province, China. Cancer Sci. 1999;90(6):614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aune D, De Stefani E, Ronco AL, Boffetta P, Deneo-Pellegrini H, Acosta G, Mendilaharsu M. Egg consumption and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10(5):869–76. [PubMed] [Google Scholar]
- 11. Rajkumar T, Sridhar H, Balaram P, Vaccarella S, Gajalakshmi V, Nandakumar A, Ramdas K, Jayshree R, Munoz N, Herrero R. Oral cancer in Southern India: the influence of body size, diet, infections and sexual practices. Eur J Cancer Prev. 2003;12(2):135–43. [DOI] [PubMed] [Google Scholar]
- 12. Pan G, Takahashi K, Feng Y, Liu L, Liu T, Zhang S, Liu N, Okubo T, Goldsmith DF. Nested case-control study of esophageal cancer in relation to occupational exposure to silica and other dusts. Am J Ind Med. 1999;35(3):272–80. [DOI] [PubMed] [Google Scholar]
- 13. Song WO, Kerver JM. Nutritional contribution of eggs to American diets. J Am Coll Nutr. 2000;19(sup5):556S–62S. [DOI] [PubMed] [Google Scholar]
- 14. Ribaya-Mercado JD, Blumberg JB. Lutein and zeaxanthin and their potential roles in disease prevention. J Am Coll Nutr. 2004;23(sup6):567S–87S. [DOI] [PubMed] [Google Scholar]
- 15. Bravi F, Bosetti C, Filomeno M, Levi F, Garavello W, Galimberti S, Negri E, La Vecchia C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br J Cancer. 2013;109(11):2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palladino-Davis A, Mendez B, Fisichella P, Davis C. Dietary habits and esophageal cancer. Dis Esophagus. 2015;28(1):59–67. [DOI] [PubMed] [Google Scholar]
- 17. Edefonti V, Hashibe M, Ambrogi F, Parpinel M, Bravi F, Talamini R, Levi F, Yu G, Morgenstern H, Kelsey K. Nutrient-based dietary patterns and the risk of head and neck cancer: a pooled analysis in the International Head and Neck Cancer Epidemiology consortium. Annal Oncol. 2011;23(7):1869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Dirt, nutrition, physical activity and cancer of the mouth, pharynx and larynx. Available from: dietandcancerreport.org. [Google Scholar]
- 19. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Dirt, nutrition, physical activity and oesophageal cancer. Available from: dietandcancerreport.org. [Google Scholar]
- 20. Bosetti C, Talamini R, Levi F, Negri E, Franceschi S, Airoldi L, La Vecchia C. Fried foods: a risk factor for laryngeal cancer?. Br J Cancer. 2002;87(11):1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, Trichopoulos D, Lagiou P, Bardini R, Franceschi S. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87(2):289–94. [PubMed] [Google Scholar]
- 22. Winn DM, Ziegler RG, Pickle LW, Gridley G, Blot WJ, Hoover RN. Diet in the etiology of oral and pharyngeal cancer among women from the southern United States. Cancer Res. 1984;44(3):1216–22. [PubMed] [Google Scholar]
- 23. De Stefani E, Deneo-Pellegrini H, Mendilaharsu M, Ronco A. Diet and risk of cancer of the upper aerodigestive tract—I. Foods Oral Oncol. 1999;35(1):17–21. [DOI] [PubMed] [Google Scholar]
- 24. De Stefani E, Boffetta P, Ronco AL, Correa P, Oreggia F, Deneo-Pellegrini H, Mendilaharsu M, Leiva J. Dietary patterns and risk of cancer of the oral cavity and pharynx in Uruguay. Nutr Cancer. 2005;51(2):132–9. [DOI] [PubMed] [Google Scholar]
- 25. Ren A, Han X. Dietary factors and esophageal cancer: a case-control study. Zhonghua Liu Xing Bing Xue Za Zhi. 1991;12(4):200–4. [PubMed] [Google Scholar]
- 26. Xibin S, Meilan H, Moller H, Evans HS, Dixin D, Wenjie D, Jianbang L. Risk factors for oesophageal cancer in Linzhou, China: a case-control study. Asian Pac J Cancer Prev. 2003;4(2):119–24. [PubMed] [Google Scholar]
- 27. Bosetti C, La Vecchia C, Talamini R, Negri E, Levi F, Dal Maso L, Franceschi S. Food groups and laryngeal cancer risk: a case-control study from Italy and Switzerland. Int J Cancer. 2002;100(3):355–60. [DOI] [PubMed] [Google Scholar]
- 28. Brown LM, Blot WJ, Schuman SH, Smith VM, Ershow AG, Marks RD, Fraumeni Jr JF. Environmental factors and high risk of esophageal cancer among men in coastal South Carolina. J Natl Cancer Inst. 1988;80(20):1620–5. [DOI] [PubMed] [Google Scholar]
- 29. Brown LM, Swanson CA, Gridley G, Swanson GM, Silverman DT, Greenberg RS, Hayes RB, Schoenberg JB, Pottern LM, Schwartz AG. Dietary factors and the risk of squamous cell esophageal cancer among black and white men in the United States. Cancer Causes Control. 1998;9(5):467–74. [DOI] [PubMed] [Google Scholar]
- 30. Butler C, Lee Y-CA, Li S, Li Q, Chen C-J, Hsu W-L, Lou P-J, Zhu C, Pan J, Shen H. Diet and the risk of head-and-neck cancer among never-smokers and smokers in a Chinese population. Cancer Epidemiol. 2017;46:20–6. [DOI] [PubMed] [Google Scholar]
- 31. Chuang S-C, Jenab M, Heck JE, Bosetti C, Talamini R, Matsuo K, Castellsague X, Franceschi S, Herrero R, Winn DM. Diet and the risk of head and neck cancer: a pooled analysis in the INHANCE consortium. Cancer Causes Control. 2012;23(1):69–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chyou PH, Nomura AM, Stemmermann GN. Diet, alcohol, smoking and cancer of the upper aerodigestive tract: a prospective study among Hawaii Japanese men. Int J Cancer. 1995;60(5):616–21. [DOI] [PubMed] [Google Scholar]
- 33. Fan Y, Yuan J-M, Wang R, Gao Y-T, Yu MC. Alcohol, tobacco, and diet in relation to esophageal cancer: the Shanghai Cohort Study. Nutr Cancer. 2008;60(3):354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Franceschi S, Bidoli E, Barón AE, Barra S, Talamini R, Serraino D, La Vecchia C. Nutrition and cancer of the oral cavity and pharynx in north-east Italy. Int J Cancer. 1991;47(1):20–5. [DOI] [PubMed] [Google Scholar]
- 35. Franceschi S, Favero A, Conti E, Talamini R, Volpe R, Negri E, Barzan L, La Vecchia C. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br J Cancer. 1999;80(3-4):614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, Taylor PR. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer Epidemiol. 2011;35(6):e91–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garrote LF, Herrero R, Reyes RO, Vaccarella S, Anta JL, Ferbeye L, Munoz N, Franceschi S. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo W, Blot WJ, Li J-Y, Taylor PR, Liu BQ, Wang W, Wu YP, Zheng W, Dawsey SM, Li B. A nested case-control study of oesophageal and stomach cancers in the Linxian nutrition intervention trial. Int J Epidemiol. 1994;23(3):444–50. [DOI] [PubMed] [Google Scholar]
- 39. Kapil U, Singh P, Bahadur S, Dwivedi SN, Singh R, Shukla N. Assessment of risk factors in laryngeal cancer in India: a case-control study. Asian Pac J Cancer Prev. 2005;6(2):202–7. [PubMed] [Google Scholar]
- 40. Kjærheim K, Gaard M, Andersen A. The role of alcohol, tobacco, and dietary factors in upper aerogastric tract cancers: a prospective study of 10,900 Norwegian men. Cancer Causes Control. 1998;9(1):99–108. [DOI] [PubMed] [Google Scholar]
- 41. Kumar Phukan R, Kanta Chetia C, Shahadat Ali M, Mahanta J. Role of dietary habits in the development of esophageal cancer in Assam, the north-eastern region of India. Nutr Cancer. 2001;39(2):204–9. [DOI] [PubMed] [Google Scholar]
- 42. La Vecchia C, Negri E, D'avanzo B, Boyle P, Franceschi S. Dietary indicators of oral and pharyngeal cancer. Int J Epidemiol. 1991;20(1):39–44. [DOI] [PubMed] [Google Scholar]
- 43. Launoy G, Milan C, Day NE, Pienkowski MP, Gignoux M, Faivre J. Diet and squamous-cell cancer of the oesophagus: a French multicentre case-control study. Int J Cancer. 1998;76(1):7–12. [DOI] [PubMed] [Google Scholar]
- 44. Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S, Monnier P. Food groups and risk of oral and pharyngeal cancer. Int J Cancer. 1998;77(5):705–9. [DOI] [PubMed] [Google Scholar]
- 45. Levi F, Pasche C, Lucchini F, Bosetti C, Franceschi S, Monnier P, La CV. Food groups and oesophageal cancer risk in Vaud, Switzerland. Eur J Cancer Prev. 2000;9(4):257–63. [DOI] [PubMed] [Google Scholar]
- 46. Lissowska J, Pilarska A, Pilarski P, Samolczyk-Wanyura D, Piekarczyk J, Bardin-Mikollajczak A, Zatonski W, Herrero R, Munoz N, Franceschi S. Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur J Cancer Prev. 2003;12(1):25–33. [DOI] [PubMed] [Google Scholar]
- 47. Mimi CY, Garabrant DH, Peters JM, Mack TM. Tobacco, alcohol, diet, occupation, and carcinoma of the esophagus. Cancer Res. 1988;48(13):3843–8. [PubMed] [Google Scholar]
- 48. Notani PN, Jayant K. Role of diet in upper aerodigestive tract cancers. Nutr Cancer. 1987;10(1–2):103–13. [DOI] [PubMed] [Google Scholar]
- 49. Sanchez M, Martinez C, Nieto A, Castellsague X, Quintana M, Bosch F, Munoz N, Herrero R, Franceschi S. Oral and oropharyngeal cancer in Spain: influence of dietary patterns. Eur J Cancer Prev. 2003;12(1):49–56. [DOI] [PubMed] [Google Scholar]
- 50. Takezaki T, Gao CM, Wu JZ, Ding JH, Liu YT, Zhang Y, Li SP, Su P, Liu TK, Tajima K. Dietary protective and risk factors for esophageal and stomach cancers in a low-epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high-epidemic area. Cancer Sci. 2001;92(11):1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takezaki T, Hirose K, Inoue M, Hamajima N, Kuroishi T, Nakamura S, Koshikawa T, Matsuura H, Tajima K. Tobacco, alcohol and dietary factors associated with the risk of oral cancer among Japanese. Cancer Sci. 1996;87(6):555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Toporcov TN, Antunes JLF, Tavares MR. Fat food habitual intake and risk of oral cancer. Oral Oncol. 2004;40(9):925–31. [DOI] [PubMed] [Google Scholar]
- 53. Toporcov TN, Biazevic MGH, Rotundo LDB, Andrade FPd, Carvalho MBd, Brasileiro RS, Kowalski LP, Antunes JLF. Consumo de alimentos de origem animal e câncer de boca e orofaringe. Rev Panam Salud Publica. 2012;32:185–91. [DOI] [PubMed] [Google Scholar]
- 54. Wu M, Zhao J-K, Hu X-S, Wang P-H, Qin Y, Lu Y-C, Yang J, Liu A-M, Wu D-L, Zhang Z-F. Association of smoking, alcohol drinking and dietary factors with esophageal cancer in high-and low-risk areas of Jiangsu Province, China. World J Gastroenterol. 2006;12(11):1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang C, Wang H, Wang Z, Du H, Tao D, Mu X, Chen H, Lei Y, Matsuo K, Tajima K. Risk factors for esophageal cancer: a case-control study in South-western China. Asian Pac J Cancer Prev. 2005;6(1):48–53. [PubMed] [Google Scholar]
- 56. Yu Y, Taylor PR, Li J-Y, Dawsey SM, Wang G-Q, Guo W-D, Wang W, Liu B-Q, Blot WJ, Shen Q. Retrospective cohort study of risk-factors for esophageal cancer in Linxian, People's Republic of China. Cancer Causes Control. 1993;4(3):195–202. [DOI] [PubMed] [Google Scholar]
- 57. Zheng T, Boyle P, Willett WC, Hu H, Dan J, Evstifeeva TV, Niu S, MacMahon B. A case-control study of oral cancer in Beijing, People's Republic of China. Associations with nutrient intakes, foods and food groups. Eur J Canc B Oral Oncol. 1993;29(1):45–55. [DOI] [PubMed] [Google Scholar]
- 58. Zheng W, Blot WJ, Shu X-O, Diamond EL, Gao Y-T, Ji B-T, Fraumeni J. Risk factors for oral and pharyngeal cancer in Shanghai, with emphasis on diet. Cancer Epidemiol Biomarkers Prev. 1992;1(6):441–8. [PubMed] [Google Scholar]
- 59. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, [Internet] The Ottawa Hospital Research Institute. [Cited 20 March 2019]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 60. Silverman S. Demographics and occurrence of oral and pharyngeal cancers: the outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132:7S–11S. [DOI] [PubMed] [Google Scholar]
- 61. Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LT. Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Arch Surg. 1995;130(5):502–8. [DOI] [PubMed] [Google Scholar]
- 62. Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, Felton JS. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. 1995;16(1):39–52. [DOI] [PubMed] [Google Scholar]
- 63. Willett W. Nutritional epidemiology: Oxford University Press; 2012. [Google Scholar]
- 64. Ruano-Ravina A, Pérez-Ríos M, Barros-Dios JM. Population-based versus hospital-based controls: are they comparable?. Gac Sanit. 2008;22:609–13. [DOI] [PubMed] [Google Scholar]
- 65. Gail M. Case-control study, hospital-based. Encyclopedia of Epidemiologic Methods. 2000;4:155. [Google Scholar]
- 66. Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. 2009;113(3):c214–c7. [DOI] [PubMed] [Google Scholar]
- 67. Fund WCR, Research AIfC. Food, nutrition, physical activity, and the prevention of cancer: a global perspective: Amer Inst for Cancer Research; 2007. [Google Scholar]
- 68. Bosetti C, La Vecchia C, Talamini R, Negri E, Levi F, Fryzek J, McLaughlin J, Garavello W, Franceschi S. Energy, macronutrients and laryngeal cancer risk. Annal Oncol. 2003;14(6):907–12. [DOI] [PubMed] [Google Scholar]
- 69. Greenwald P, Clifford C, Milner J. Diet and cancer prevention. Eur J Cancer. 2001;37(8):948–65. [DOI] [PubMed] [Google Scholar]
- 70. Decarli A, Liati P, Negri E, Franceschi S, La Vecchia C. Vitamin A and other dietary factors in the etiology of esophageal cancer. Nutr Cancer. 1987;10::29–37. [DOI] [PubMed] [Google Scholar]
- 71. Block G, Dresser CM, Hartman AM, Carroll MD. Nutrient sources in the American diet: quantitative data from the NHANES II survey: I. Vitamins and minerals. Am J Epidemiol. 1985;122(1):13–26. [DOI] [PubMed] [Google Scholar]
- 72. Cheng K, Day N. Nutrition and esophageal cancer. Cancer Causes Control. 1996;7(1):33–40. [DOI] [PubMed] [Google Scholar]