ABSTRACT
There is some evidence supporting the beneficial effects of a Paleolithic diet (PD) on cardiovascular disease (CVD) risk factors. This diet advises consuming lean meat, fish, vegetables, fruits, and nuts and avoiding intake of grains, dairy products, processed foods, and added sugar and salt. This study was performed to assess the effects of a PD on CVD risk factors including anthropometric indexes, lipid profile, blood pressure, and inflammatory markers using data from randomized controlled trials. A comprehensive search was performed in the PubMed, Scopus, ISI Web of Science, and Google Scholar databases up to August 2018. A meta-analysis was performed using a random-effects model to estimate the pooled effect size. Meta-analysis of 8 eligible studies revealed that a PD significantly reduced body weight [weighted mean difference (WMD) = −1.68 kg; 95% CI: −2.86, −0.49 kg], waist circumference (WMD = −2.72 cm; 95% CI: −4.04, −1.40 cm), BMI (in kg/m2) (WMD = −1.54; 95% CI: −2.22, −0.87), body fat percentage (WMD = −1.31%; 95% CI: −2.06%, −0.57%), systolic (WMD = −4.75 mm Hg; 95% CI: −7.54, −1.96 mm Hg) and diastolic (WMD = −3.23 mm Hg; 95% CI: −4.77, −1.69 mm Hg) blood pressure, and circulating concentrations of total cholesterol (WMD = −0.23 mmol/L; 95% CI: −0.42, −0.04 mmol/L), triglycerides (WMD = −0.30 mmol/L; 95% CI: −0.55, −0.06 mmol/L), LDL cholesterol (WMD = −0.13 mmol/L; 95% CI: −0.26, −0.01 mmol/L), and C-reactive protein (CRP) (WMD = −0.48 mg/L; 95% CI: −0.79, −0.16 mg/L) and also significantly increased HDL cholesterol (WMD = 0.06 mmol/L; 95% CI: 0.01, 0.11 mmol/L). However, sensitivity analysis revealed that the overall effects of a PD on lipid profile, systolic blood pressure, and circulating CRP concentrations were sensitive to removing some studies and to the correlation coefficients, hence the results must be interpreted with caution. Although the present meta-analysis revealed that a PD has favorable effects on CVD risk factors, the evidence is not conclusive and more well-designed trials are still needed.
Keywords: Paleolithic diet, Paleolithic nutrition, anthropometric indexes, lipid profile, blood pressure, C-reactive protein, meta-analysis
Introduction
Cardiovascular diseases (CVDs) are a main public health concern worldwide, being the leading cause of mortality, accounting for 30% of all global deaths (1). According to the last estimation of the WHO, 7.3 million and 6.2 million died annually because of coronary artery disease and stroke, respectively (2). The development of CVDs is associated with obesity, diabetes, smoking, lack of physical activity, and harmful alcohol intake (3, 4). Furthermore, hemodynamic (hypertension) and metabolic stressors (dyslipidemia and hyperglycemia) have been established as important CVD risk factors (3–5).
Long-standing findings on the relevance of diet for CVDs have shown that diet might highly contribute to the incidence of CVDs. Many such studies have mostly focused on nutrients, foods (6, 7), and food groups (8–10), whereas less emphasis has been devoted to dietary patterns. Epidemiological research has shown that assessment of dietary patterns instead of single foods or nutrients can provide a better understanding of how dietary factors mutually affect the risk of diseases and of all-cause or CVD mortality (11, 12). Several meta-analyses have revealed beneficial effects of some dietary patterns such as the Dietary Approach to Stop Hypertension, Mediterranean diet, and Healthy Eating Index on CVD risk factors (13–17).
A Paleolithic diet (PD) is another dietary regimen based on foods commonly eaten during the Old Stone Age (18), which mostly suggests consuming lean meat, fish, eggs, fruits, vegetables, roots, and nuts but eliminating grains, dairy products, processed foods, and added sugar and salts (19). This dietary pattern contains lower sodium, whereas it has high contents of protein and some micronutrients such as vitamins C and E, carotenes, and fiber with lower caloric intake from carbohydrate and refined fat (20–22). Whereas the changes in the ancient genome over a long period during the Paleolithic era (2.6 million to 10,000 y ago) varied slightly, dietary patterns with the emergence of modern foods have faced significant changes (23).
Previous studies have reported that CVDs are less prevalent among existing hunter-gatherer tribes around the world, such as those in Papua New Guinea, than among industrialized populations (24). These findings could be due to their conventional lifestyle and their especially healthy dietary pattern (that is, PD) (25), whereas the Western diet with its high content of saturated fat, salt, and processed foods has been implicated in chronic diseases like CVDs (26–28).
Also, previous studies have reported positive effects of a PD on energy intake, body composition, insulin sensitivity, and CVD risk markers (19, 25, 29). Despite several randomized controlled trials (RCTs) investigating a PD, there is considerable controversy regarding the clinical benefits of this dietary pattern. Although a positive effect of a PD on risk factors for metabolic syndrome has been reported (30), another study found no effect of a PD on anthropometric indexes after a 24-mo follow-up (31). These controversies also exist for other CVD risk factors like lipid profile and blood pressure (19, 25).
Findings of previous RCTs are inconsistent regarding the effects of a PD on cardiometabolic markers. Therefore, the aim of the present systematic review and meta-analysis of published RCTs was to assess the effect of this dietary pattern on CVD risk factors and to quantify its possible influences on lipid profile, blood pressure, anthropometric indexes, and inflammatory markers.
Methods
Search strategy
The Preferred Reporting Items for Systematic reviews and Meta-Analyses statement was used as a guideline during all stages of the design, implementation, and reporting of the present systematic review and meta-analysis (32). Systematic computerized literature searches of PubMed (http://www.pubmed.com), ISI Web of Science (http://www.webofknowledge.com), Scopus (http://www.scopus.com), and Google Scholar (http://scholar.google.com) were performed up to August 2018 without any restrictions. The combination of medical subject headings (MeSH) and non-MeSH keywords was selected as follows: [“Diet, Paleolithic” OR (Paleolithic AND diet*) OR (Paleo AND diet*) OR (Paleo AND nutrition*) OR (Paleolithic AND nutrition) OR (“stone age” AND diet*) OR (“stone age” AND nutrition*) OR (caveman AND diet*) OR (caveman AND nutrition*) OR (“Hunter-Gatherer” AND diet*) OR (“Hunter-Gatherer” AND nutrition*) OR (“Paleolithic-type” AND diet*) OR (“Paleolithic-type” AND nutrition*)]. To find related studies, retrieved titles and abstracts were separately reviewed by 2 authors (HM and MM) and any disagreements were resolved by consultation with other investigators (AS-A, MH, and NR-J). Moreover, the references of the included literature and related reviews were screened to determine more potentially relevant studies.
Eligibility criteria
Retrieved studies were included in our review if they met the following criteria: they 1) were an original article with an RCT design; 2) evaluated the effects of a PD on human beings; and 3) assessed weight and body composition, circulating concentrations of blood lipids, blood pressure, and inflammatory markers as primary or secondary measures. Studies were excluded if they 1) were conducted among children or adolescents aged younger than 18 y; 2) reported duplicate data from other included studies; 3) evaluated single diet components rather than a whole dietary pattern; or 4) did not report the targeted outcomes.
Data extraction
Two independent researchers (HM and MM) summarized the following data which were double checked by other authors (AS-A and NR-J): first author's name, year of publication, country where the study was performed, study design, study period, participants’ characteristics (n, age, sex, and health status), components of the dietary patterns consumed in the intervention and control groups, and the mean changes with corresponding SDs of measured outcomes in the intervention and control arms. For studies in which values were presented graphically, data were extracted from figures using Plot Digitizer software (http://plotdigitizer.sourceforge.net/) (31, 33). To access the data on lipid profile and blood pressure that were not mentioned in the study of Lindeberg et al. (25), we emailed the authors and obtained the missing information.
Quality assessment
Two reviewers (HM and MM) independently evaluated the methodological quality of the eligible studies via the Cochrane Collaboration's tool, which includes 6 domains as follows: 1) random sequence generation (selection bias); 2) allocation concealment (selection bias); 3) blinding of participants and personnel (performance bias); 4) blinding of outcome assessment (detection bias); 5) incomplete outcome data (attrition bias); and 6) selective reporting (reporting bias). Blinding is not possible in dietary interventions, therefore the studies were judged regarding the other 5 items. Each domain was classified into 3 categories: low risk of bias, high risk of bias, and unclear risk of bias. According to the aforementioned domains, the overall quality of each individual study was considered as good (low risk for >2 items), fair (low risk for 2 items), or weak (low risk for <2 items) (34).
Statistical analysis
To calculate the effect sizes for each outcome parameter, the mean changes and their SDs for the intervention and control groups or periods were extracted from each study and used to estimate the mean difference and its corresponding SE. If changes from baseline to follow-up values were not provided, the correlation coefficient of 0.8 was used to compute the SD for mean change values. To check the sensitivity of meta-analyses to the correlation coefficient of 0.8, all the analyses were repeated by using r = 0.2. A random-effects model was used to compute weighted mean differences (WMDs) with 95% CIs for conducting the meta-analysis (35). Between-study heterogeneity was tested by Cochran's Q test and quantified by the I-squared (I2) statistic, where a significant Q test (P < 0.05) and a value for I2 > 75% were considered to indicate considerable heterogeneity (36). Begg's rank correlation test and Egger's regression asymmetry test were performed for detecting potential publication bias as well as observing the symmetry of the funnel plots in which mean differences were plotted against their corresponding SEs (37, 38). Sensitivity analyses were also performed by removing each study one by one and recalculating the pooled estimates. All statistical analyses were conducted using STATA version 11.2 (Stata Corp). Statistically significant values were defined as P values < 0.05.
Results
Study selection and characteristics
A total of 2979 studies were identified by the primary search of the electronic databases, and 1 additional study was found through the hand search of the citations of the included articles and related reviews. We selected 28 eligible studies which were full-text reviewed, and excluded 20 of them for the following reasons: 1) 7 studies reported no data on our target outcomes (39–45); 2) 6 studies reported duplicated results which were published in another article (46–51); 3) 5 studies had no control group (21, 29, 52–54); 4) 1 study assessed the effect of a PD along with exercise (55); and 5)1 study assessed the acute effect of a PD intake (56). In total, 8 eligible studies were included in the present systematic review and meta-analysis (19, 25, 30, 31, 33, 57–59). The study of Stomby et al. (58) is a substudy of the larger main study by Mellberg et al. (31). We thus only included the study of Mellberg et al. in our meta-analysis, but because the data on body fat percentage were not reported in this study, we extracted only body fat percentage values from the study of Stomby et al. and the other outcomes were extracted from the main study. Figure 1 shows details of the study selection process.
FIGURE 1.
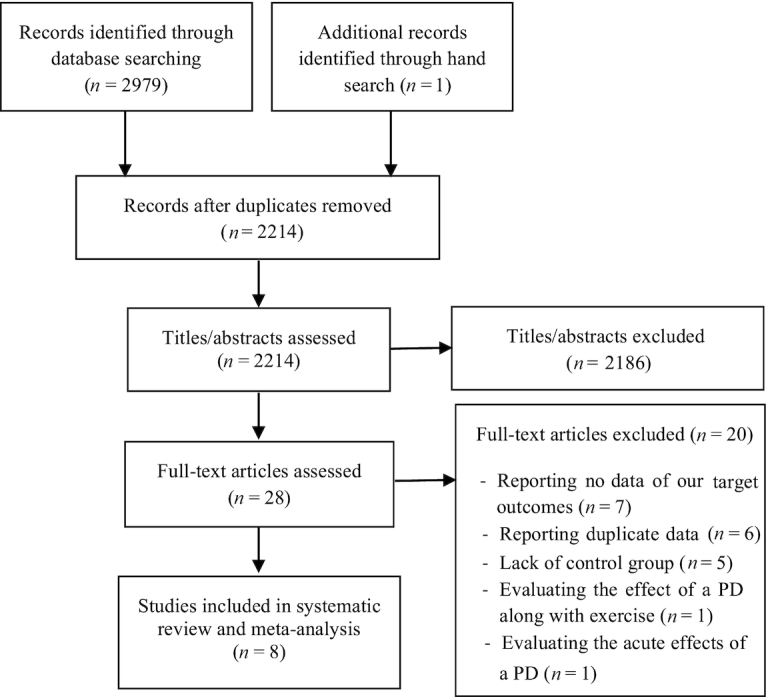
Preferred Reporting Items for Systematic reviews and Meta-Analyses flow diagram of the study selection process. PD, Paleolithic diet.
The included studies were conducted in the Netherlands (30), United States (33, 59), Sweden (19, 25, 31, 58), and Australia (57). The publication date of articles ranged from 2007 to 2017. Seven trials were designed as parallel-group studies (25, 30, 31, 33, 57–59) and 1 study used a crossover design (19), with the follow-up periods ranging from 2 wk to 6 mo.
The studies of Mellberg et al. (31) and Stomby et al. (58) had a 2-y follow-up period and reported outcomes for several follow-up periods (6, 12, 18, and 24 mo). Because the other studies had an intervention duration of <6 mo, we decided to use a 6-mo intervention period from these 2 studies, which is closer to the intervention periods of the other studies. In the studies of the present review, a PD as an intervention was compared with the usual diet of subjects (33) or other dietary patterns and guidelines such as the Nordic Nutrition Recommendations (31, 58), Australian Guide to Healthy Eating (57), dietary recommendations based on the guidelines of the American Diabetes Association (59), dietary recommendations based on the guidelines for a healthy diet of the Dutch Health Council (30), a diabetes diet designed in accordance with current diabetes dietary guidelines (19), and a Mediterranean-like diet (25).
A total of 266 subjects with a mean age of 53 y were included in the analysis. The participants had different conditions of health status such as healthy subjects, postmenopausal women, patients with type 2 diabetes, multiple sclerosis, or ischemic heart disease, and subjects with characteristics of the metabolic syndrome. Table 1 presents general characteristics of the selected trials.
TABLE 1.
Characteristics of randomized controlled clinical trials included in the systematic review1
| Study (ref.) | Country | Group: n and sex (F/M) | Mean age, y | RCT design | Period, d | Intervention diet | Control diet | Participants | Estimated effect sizes: WMD (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Irish et al. (33) | United States | Intervention: 7 F/1 M Control: 8 F/1 M |
Intervention: 35.4 Control: 37.1 |
Parallel | 90 | Modified Paleolithic diet | Usual diet | Relapsing-remitting multiple sclerosis | CRP (mg/L): −1.69 (−3.73, 0.35) |
| Genoni et al. (57) | Australia | Intervention: 22 F Control: 17 F |
Intervention: 47 Control: 47 |
Parallel | 28 | Paleolithic diet: CHO: 27.8% Fat: 39.8% Pro: 26.8% |
AGHE: CHO: 40.6% Fat: 32.6% Pro: 21.7% |
Healthy women | Weight (kg): −1.99 (−2.94, −1.04) WC (cm): −1.90 (−3.09, −0.71) Body fat (%): −1.34 (−2.48, −0.20) |
| SBP (mm Hg): −2.61 (−10.11, 4.89) DBP (mm Hg): −3.51 (−9.01, 1.99) CRP (mg/L): −0.57 (−1.62, 0.48) TC (mmol/L): −0.13 (−0.48, 0.22) TGs (mmol/L): −0.14 (−0.44, 0.16) HDL cholesterol (mmol/L): 0.04 (−0.06, 0.14) LDL cholesterol (mmol/L): −0.11 (−0.31, 0.09) |
|||||||||
| Masharani et al. (59) | United States | Intervention: 14 NR Control: 10 NR |
Intervention: 58 Control: 56 |
Parallel | 21 | Paleolithic diet: CHO: 58.2% Fat: 27% Pro: 18.5% |
ADA diet: CHO: 54.4% Fat: 28.8% Pro: 20.3% |
Patients with type 2 diabetes | Weight (kg): −0.30 (−1.38, 0.78) SBP (mm Hg): −2.00 (−12.08, 8.08) DBP (mm Hg): −1.00 (−8.27, 6.27) TC (mmol/L): −0.44 (−0.99, 0.11) TGs (mmol/L): −0.20 (−0.69, 0.29) HDL cholesterol (mmol/L): −0.05 (−0.21, 0.11) LDL cholesterol (mmol/L): −0.21 (−0.63, 0.21) |
| Stomby et al. (58) | Sweden | Intervention: 27 F Control: 22 F |
NR | Parallel | 180 | Paleolithic diet: CHO: 30% Fat: 40% Pro: 30% |
NNR: CHO: 55–60% Fat: 25–30% Pro: 15% |
Overweight postmenopausal women | Body fat (%): −1.90 (−3.64, −0.16) |
| Mellberg et al. (31) | Sweden | Intervention: 34 F Control: 27 F |
Intervention: 59.5 Control: 60.3 |
Parallel | 180 | Paleolithic diet: CHO: 30% Fat: 40% Pro: 30% |
NNR: CHO: 55–60% Fat: 25–30% Pro: 15% |
Postmenopausal nonsmoking women with a BMI ≥ 27 | Weight (kg): −4.90 (−8.30, −1.50) WC (cm): −5.30 (−8.52, −2.08) BMI: −1.80 (−2.61, −0.99) SBP (mm Hg): −3.70 (−9.57, 2.17) DBP (mm Hg): −1.60 (−5.16, 1.96) CRP (mg/L): −0.20 (−0.73, 0.33) TC (mmol/L): −0.28 (−0.67, 0.11) TGs (mmol/L): −0.26 (−0.46, −0.06) HDL cholesterol (mmol/L): −0.01 (−0.14, 0.12) LDL cholesterol (mmol/L): −0.14 (−0.43, 0.15) |
| Boers et al. (30) | Netherlands | Intervention: 13 F/5 M Control: 12 F/4 M |
Intervention: 52 Control: 55.4 |
Parallel | 14 | Paleolithic diet: CHO: 32% Fat: 41% Pro: 24% |
Guidelines for a healthy diet of the DHC: CHO: 50% Fat: 29% Pro: 17% |
Subjects with characteristics of the metabolic syndrome | Weight (kg): −1.00 (−7.82, 5.82) WC (cm): −0.10 (−4.66, 4.46) BMI: −0.80 (−3.14, 1.54) SBP (mm Hg): −4.00 (−10.21, 2.21) DBP (mm Hg): −5.00 (−9.55, −0.45) CRP (mg/L): −0.50 (−2.13, 1.13) TC (mmol/L): −0.60 (−1.09, −0.11) TGs (mmol/L): −1.00 (−1.52, −0.48) HDL cholesterol (mmol/L): 0.20 (0.03, 0.37) LDL cholesterol (mmol/L): −0.30 (−0.75, 0.15) |
| Jönsson et al. (19) | Sweden | 3 F/10 M | 64 | Crossover | 90 | Paleolithic diet: CHO: 32% Fat: 39% Pro: 24% |
Diabetes diet designed in accordance with current diabetes dietary guidelines: CHO: 42% Fat: 34% Pro: 20% |
Patients with type 2 diabetes | Weight (kg): −3.00 (−6.53, 0.53) WC (cm): −4.00 (−6.94, −1.06) BMI: −1.00 (−2.45, 0.45) SBP (mm Hg): −9.00 (−13.66, −4.34) DBP (mm Hg): −4.00 (−6.14, −1.86) CRP (mg/L): −0.60 (−1.05, −0.15) TC (mmol/L): −0.20 (−0.45, 0.05) TGs (mmol/L): −0.50 (−0.65, −0.35) HDL cholesterol (mmol/L): 0.08 (0.02, 0.14) LDL cholesterol (mmol/L): −0.10 (−0.32, 0.12) |
| Lindeberg et al. (25) | Sweden | Intervention: 14 M Control: 15 M |
Intervention: 65 Control: 57 |
Parallel | 84 | Paleolithic diet: CHO: 40.2% Fat: 26.9% Pro: 27.9% |
Mediterranean-like diet: CHO: 51.7% Fat: 24.7% Pro: 20.5% |
Ischemic heart disease patients with WC > 94 cm and increased blood glucose or known diabetes | Weight (kg): −1.20 (−3.29, 0.89) WC (cm): −2.70 (−4.86, −0.54) Body fat (%): −1.00 (−2.20, 0.20) SBP (mm Hg): 0.81 (−8.47, 10.09) DBP (mm Hg): 1.07 (−5.71, 7.85) TC (mmol/L): 0.39 (−0.27, 1.05) TGs (mmol/L): 0.28 (−0.19, 0.75) HDL cholesterol (mmol/L): 0.07 (−0.07, 0.21) |
BMI measured in kg/m2. ADA, American Diabetes Association; AGHE, Australian Guide to Healthy Eating; CHO, carbohydrate; CRP, C-reactive protein; DBP, diastolic blood pressure; DHC, Dutch Health Council; NNR, Nordic Nutrition Recommendations; NR, not reported; Pro, protein; RCT, randomized controlled trial; ref., reference; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WC, waist circumference; WMD, weighted mean difference.
Risk of bias assessment
Table 2 describes the risk of bias assessment based on different quality domains using the Cochrane Collaboration tool. After evaluating the quality of the 8 included studies, 7 of them were classified as of good quality (19, 25, 30, 31, 33, 57, 58). However, the study of Masharani et al. (59) was of fair quality and did not report their methods of allocation concealment and random sequence generation. Moreover, because blinding is impossible to perform for dietary intervention trials, the blinding of participants and investigators was not considered in all the studies.
TABLE 2.
Risk of bias assessment for included randomized controlled clinical trials1
| Irish et al. (33) | Genoni et al. (57) | Masharani et al. (59) | Stomby et al. (58) | Mellberg et al. (31) | Boers et al. (30) | Jönsson et al. (19) | Lindeberg et al. (25) | |
|---|---|---|---|---|---|---|---|---|
| Random sequence generation (selection bias) | + | + | ? | + | + | + | + | + |
| Allocation concealment (selection bias) | ? | ? | ? | ? | ? | ? | ? | ? |
| Blinding of participants and personnel (performance bias) | − | − | − | − | − | − | − | − |
| Blinding of outcome assessment (detection bias) | − | − | − | ? | + | + | + | + |
| Incomplete outcome data (attrition bias) | + | + | + | + | + | + | + | ? |
| Selective reporting (reporting bias) | + | + | + | + | + | + | + | + |
| Score | 3 | 3 | 2 | 3 | 4 | 4 | 4 | 3 |
| Overall quality | Good | Good | Fair | Good | Good | Good | Good | Good |
+, positive assessment; −, negative assessment; ?, neutral assessment.
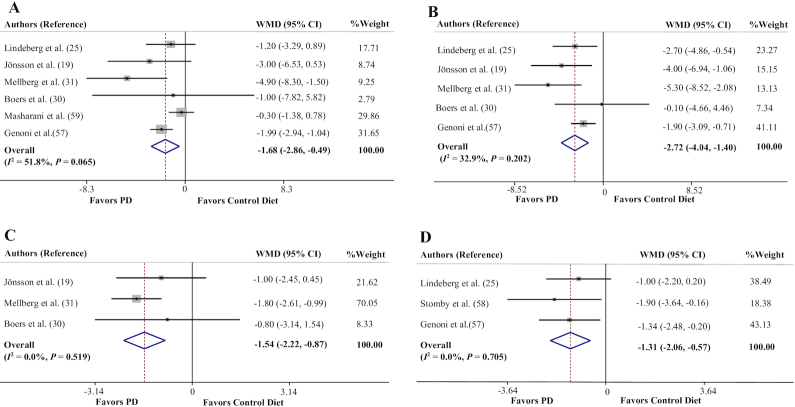
Effects of a PD on weight, waist circumference, BMI, and body fat percentage
Six trials assessed the effect of a PD on body weight measures (19, 25, 30, 31, 57, 59). The pooled effect size indicated a significant reduction in weight after a PD (WMD = −1.68 kg; 95% CI: −2.86, −0.49 kg, P = 0.005) (Figure 2A). The between-study heterogeneity was nonsignificant (Cochran's Q = 10.38, P = 0.065, I2 = 51.8%).
FIGURE 2.
Forest plots of the effect of a PD on anthropometric indexes. (A) Body weight, (B) waist circumference, (C) BMI, (D) body fat percentage. PD, Paleolithic diet; WMD, weighted mean difference.
Meta-analysis of the 5 trials which reported data on waist circumference (WC) (19, 25, 30, 31, 57) showed a significant effect of a PD on WC values (WMD = −2.72 cm; 95% CI: −4.04, −1.40 cm, P < 0.001) (Figure 2B). There was no significant heterogeneity between studies (Cochran's Q = 5.96, P = 0.202, I2 = 32.9%).
The effect of a PD on BMI measures was examined in 3 clinical trials (19, 30, 31). The results of meta-analysis showed that there was a significant effect of adherence to a PD on reduction of BMI (in kg/m2) (WMD = −1.54; 95% CI: −2.22, −0.87, P < 0.001) (Figure 2C). There was no between-study heterogeneity (Cochran's Q = 1.31, P = 0.519, I2 = 0.0%).
The overall result of meta-analysis of 3 studies (25, 57, 58) evaluating the effect of adherence to a PD on body fat percentage showed a significant reduction (WMD = −1.31%; 95% CI: −2.06%, −0.57%, P = 0.001) (Figure 2D), with no between-study heterogeneity (Cochran's Q = 0.70, P = 0.705, I2 = 0.0%).
Sensitivity analysis for all anthropometric parameters showed that the overall estimates were not influenced by elimination of any study. Moreover, no evidence of publication bias was found for weight (P = 1.00, Begg's test; P = 0.46, Egger's test), WC (P = 0.46, Begg's test; P = 0.46, Egger's test), BMI (P = 1.00, Begg's test; P = 0.23, Egger's test), and body fat percentage (P = 1.00, Begg's test; P = 0.40, Egger's test).
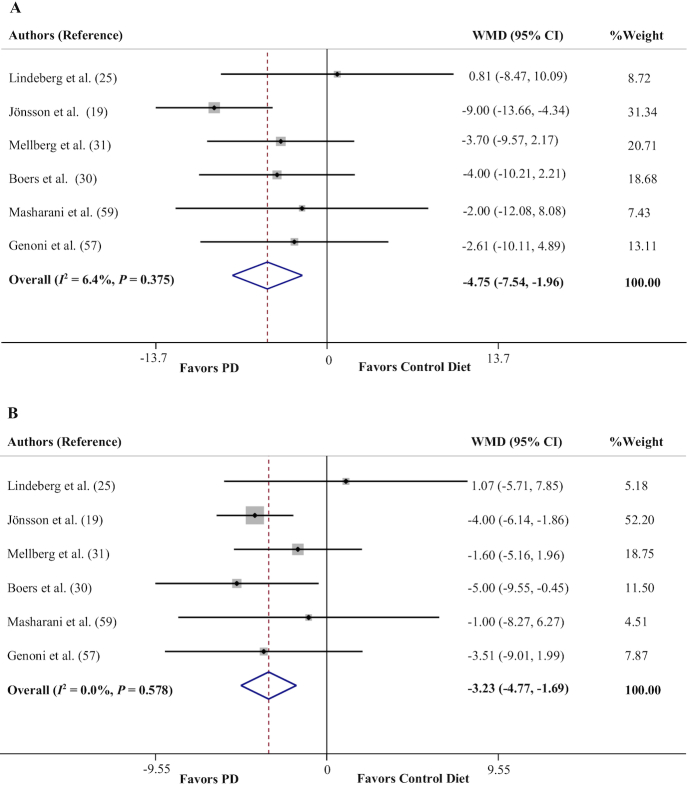
Effects of a PD on systolic blood pressure and diastolic blood pressure
Six trials assessed the impact of a PD on blood pressure changes (19, 25, 30, 31, 57, 59). Adherence to a PD was found to significantly reduce both systolic blood pressure (SBP) (WMD = −4.75 mm Hg; 95% CI: −7.54, −1.96 mm Hg, P = 0.001) (Figure 3A) and diastolic blood pressure (DBP) (WMD = −3.23 mm Hg; 95% CI: −4.77, −1.69 mm Hg, P < 0.001) (Figure 3B), with no between-study heterogeneity (Cochran's Q = 5.34, P = 0.375, I2 = 6.4% for SBP; Cochran's Q = 3.80, P = 0.578, I2 = 0.0% for DBP).
FIGURE 3.
Forest plots of the effect of a PD on blood pressure. (A) Systolic blood pressure, (B) diastolic blood pressure. PD, Paleolithic diet; WMD, weighted mean difference.
The overall estimate was not influenced by elimination of any study for DBP; however, the effect of a PD on SBP was sensitive to the study of Jönsson et al. (19), yielding an effect size equivalent to WMD = −2.84 mm Hg (95% CI: −6.10, 0.41 mm Hg). There was no evidence of publication bias for DBP (P = 0.26, Begg's test; P = 0.23, Egger's test). Although Begg's test (P = 0.13) did not indicate significant publication bias for SBP results, there was evidence of publication bias based on Egger's test (P = 0.02).
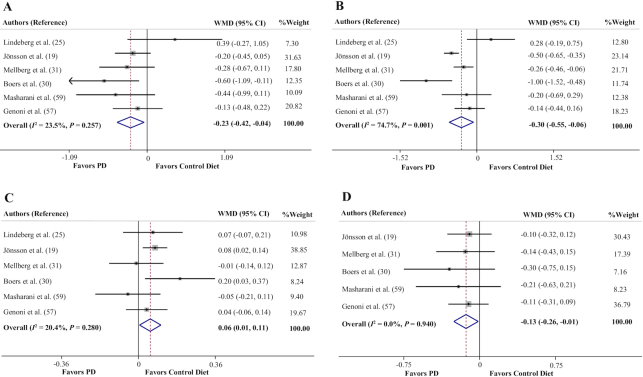
Effects of a PD on circulating concentrations of total cholesterol, triglycerides, and HDL and LDL cholesterol
The pooled effect size of 6 data sets (19, 25, 30, 31, 57, 59) represented a significant reducing effect of a PD on circulating concentrations of total cholesterol (TC) (WMD = −0.23 mmol/L; 95% CI: −0.42, −0.04 mmol/L, P = 0.017) (Figure 4A), with no significant between-study heterogeneity (Cochran's Q = 6.54, P = 0.257, I2 = 23.5%).
FIGURE 4.
Forest plots of the effect of a PD on lipid profile. (A) Total cholesterol, (B) triglycerides, (C) HDL cholesterol, (D) LDL cholesterol. PD, Paleolithic diet; WMD, weighted mean difference.
The pooled mean difference of 6 data sets (19, 25, 30, 31, 57, 59) for the effects of a PD on circulating concentrations of triglycerides (TGs) was −0.30 mmol/L (95% CI: −0.55, −0.06 mmol/L, P = 0.014) (Figure 4B). There was significant between-study heterogeneity (Cochran's Q = 19.77, P = 0.001, I2 = 74.7%). Six trials reported the effect of a PD on HDL-cholesterol concentrations (19, 25, 30, 31, 57, 59). It was observed that adherence to a PD resulted in significantly increased circulating concentrations of HDL cholesterol (WMD = 0.06 mmol/L; 95% CI: 0.01, 0.11 mmol/L, P = 0.028) (Figure 4C), with no significant between-study heterogeneity (Cochran's Q = 6.28, P = 0.280, I2 = 20.4%).
The overall result of meta-analysis of 5 studies (19, 30, 31, 57, 59) evaluating the effect of adherence to a PD on circulating concentrations of LDL cholesterol showed that there was a significant reduction (WMD = −0.13 mmol/L; 95% CI: −0.26, −0.01 mmol/L, P = 0.030) (Figure 4D). There was no significant heterogeneity between studies (Cochran's Q = 0.79, P = 0.940, I2 = 0.0%).
The results of sensitivity analysis showed that removing the studies by Jönsson et al. (19) (P = 0.073), Mellberg et al. (31) (P = 0.070), and Masharani et al. (59) (P = 0.057) changed the overall effect of a PD on circulating TC concentrations to a nonsignificant value. It was also observed that removal of the studies by Jönsson et al. (19) (P = 0.105), Mellberg et al. (31) (P = 0.063), and Boers et al. (30) (P = 0.055) altered the overall effect regarding circulating concentrations of TGs to a statistically nonsignificant result. According to the sensitivity analysis for HDL-cholesterol values, the overall effect of a PD was sensitive to the studies by Lindeberg et al. (25) (P = 0.092), Jönsson et al. (19) (P = 0.230), and Genoni et al. (57) (P = 0.081). Moreover, the sensitivity analysis indicated that except for the studies of Jönsson et al. (19) and Masharani et al. (59), exclusion of any study from the analysis changed the overall effect of a PD on circulating concentrations of LDL cholesterol to nonsignificant changes (P ≥ 0.051).
No evidence of publication bias was found from studies evaluating the effect of a PD on circulating concentrations of TC (P = 1.00, Begg's test; P = 0.96, Egger's test), TGs (P = 1.00, Begg's test; P = 0.54, Egger's test), and HDL cholesterol (P = 1.00, Begg's test; P = 0.69, Egger's test). Although Begg's test (P = 0.08) did not indicate significant publication bias for LDL cholesterol results, there was evidence of publication bias based on Egger's test (P = 0.01). However, trim-and-fill analyses yielded results similar to the original, which means it was unlikely that publication bias significantly affected the results.
Effects of a PD on circulating C-reactive protein concentrations
The quantitative analysis of C-reactive protein (CRP) values (5 trials) (19, 30, 31, 57, 33) indicated a significant effect of a PD in reduction of circulating CRP concentrations (WMD = −0.48 mg/L; 95% CI: −0.79, −0.16 mg/L, P = 0.003) (Figure 5), with no between-study heterogeneity (Cochran's Q = 2.73, P = 0.604, I2 = 0.0%). The results of sensitivity analysis showed that exclusion of the study by Jönsson et al. (19) from the analysis changed the overall effect to a nonsignificant value (P = 0.113). Begg's test (P = 0.80) and Egger's test (P = 0.41) suggested no publication bias.
FIGURE 5.
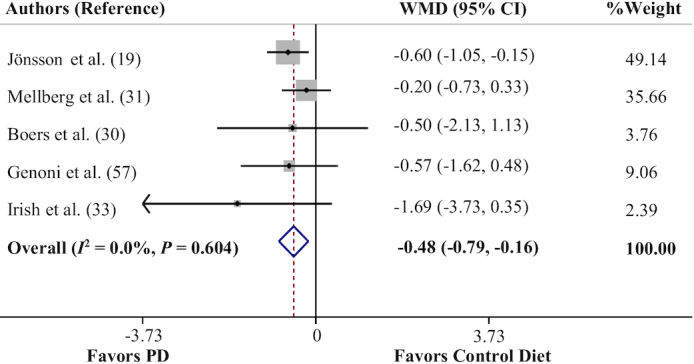
Forest plot of the effect of a PD on circulating C-reactive protein concentrations. PD, Paleolithic diet; WMD, weighted mean difference.
The overall effects of a PD on lipid profile, SBP, and circulating CRP concentrations changed to nonsignificant values when the meta-analysis was conducted on effect estimates calculated based on a correlation coefficient of 0.2.
Discussion
To the best of our knowledge, the present systematic review and meta-analysis is the first such study covering the effects of adherence to a PD on CVD risk factors. Our findings indicated that a PD could significantly decrease anthropometric indexes including weight, WC, BMI, and body fat percentage. The pooled analysis also showed that PD resulted in reduced circulating CRP concentrations, SBP, DBP, TC, LDL cholesterol, and TGs, and elevated circulating concentrations of HDL cholesterol. However, according to the sensitivity analysis, we found that the overall effects of a PD on lipid profile, SBP, and circulating CRP concentrations were sensitive to removing some studies and to the correlation coefficients; thus, the effect of a PD in this field must be interpreted with caution.
In the same vein, another meta-analysis of 4 RCTs suggested short-term improvements in metabolic syndrome components after consumption of a Paleolithic nutritional pattern; however, the beneficial changes of HDL cholesterol and fasting blood sugar values did not reach the significance level (60).
It has been observed that hunter-gatherers were generally lean and free from symptoms of chronic diseases such as CVDs owing to their diets (61, 62). In other words, their general health status got worse when their eating habits changed to an agricultural grain-based diet (62). Furthermore, after their transition to a Western diet, obesity, type 2 diabetes, atherosclerosis, and other CVDs became prevalent among them (63, 64). Considerable attempts were made to justify these findings and one of the suggested mechanisms was related to insulin sensitivity (65). Our ancestors consumed low-carbohydrate diets and their bodies became adapted to this condition. Moreover, the agricultural and industrial revolutions provided an oversupply of extra calories, especially from carbohydrates (66). Although evidence has shown that our gene-pool adapts to a novel nutritional environment (67), differences between populations as well as polymorphisms and gene variants may cause insulin resistance (60).
In addition, food-processing procedures often cause overconsumption of food additives such as salt, oils, and omega-6 fatty acids from vegetable oil, which have been described as risk factors for several chronic diseases (68–70). Furthermore, a diet rich in carbohydrate, fat, and processed foods can increase extracellular acidity, activate the zymogens, and trigger the inflammatory system (71). An acidic environment is favored by oxidative stress due to the abundance of reactive oxygen species, reactive nitrogen species, etc. Extracellular acidity also activates a wide range of enzymes involved in vesicular trafficking, autophagy, angiogenesis, proliferation, metastasis, apoptosis, etc. (72, 73).
There is little overlap between current foods and those of the Paleolithic era; our ancestors used almost no cereal grains, dairy products, oils, and processed foods. Although different PDs have been observed from the Paleolithic era and the proportions of total fat and carbohydrate varied mostly with latitude, all PDs were low in serum cholesterol–raising fat and also cereals, refined sugars, and dairy products. In essence, there is wide inconsistency in the way the modern PD is interpreted; however, this diet usually includes vegetables, fruits, nuts, roots, and meat and excludes foods such as dairy products, grains, sugar, processed oils, salt, alcohol, or coffee (74). Lindeberg et al. (25) found that a PD can improve glucose tolerance; however, this was independent of energy intake and macronutrient composition. In addition, Mellberg et al. (31) also reported that adherence to the prescribed protein intake was poor in the PD group in a 2-y RCT. Therefore, it can be mentioned that other components of a PD are of greater importance than macronutrient composition. It has also been suggested that avoidance of Western foods is more important than counting calories, or the fat, carbohydrate, or protein composition of a diet (25).
In this study, we showed that a PD can be an effective approach for weight management. It has also been reported that after administration of a PD, relative changes in the free leptin index correlated significantly with changes in WC (42), which was mainly due to the high intake of fiber as well as low intake of dairy products and refined sugars (42). Furthermore, it was proposed that a PD could raise secretion of incretin and anorectic gut hormones (glucagon-like peptide-1 and peptide YY) and they in turn improved feelings of satiety (57). The nature of this dietary pattern causes lower energy intake because the diet is satiating (75). In addition, the required energy density is lower in a PD owing to high amounts of fruits, vegetables, and protein intake (76). The water consumption in this diet is also thought to be satiating (77). We assume that alteration in the type of fiber consumed in a PD may have an important effect on the gut microbiome and this in turn can alter long-term health outcomes such as energy intake; however, this idea has not been investigated yet (78).
In addition, consumption of sugar in the Paleolithic era was considerably lower and the only natural sugars were fruits or honey. Owing to various confounding factors, evaluation of the effects of high refined-sugar intake is complicated in the long term. However, a study reported that consumption of high refined-sugar in the short term increased circulating concentrations of TGs and decreased circulating concentrations of HDL cholesterol (79). Moreover, the SFA contents of a PD are considerably lower than in the Western diet and this low intake of SFAs may partly explain our findings regarding the circulating concentrations of TC. The reduction of TG values can be due to greater loss of abdominal fat (80), lower glycemic load of the diet (81), and higher content of long-chain ω-3 fatty acids in a PD, whereas the higher dietary cholesterol content of a PD is negligible (82). Dietary SFAs are mainly found in meat, dairy products, and tropical oils; all of them are assumed to be associated with increased risk of CVDs.
Our analysis also showed a PD led to significant reduction in both SBP and DBP. Owing to high intake of fruit and vegetables, this dietary pattern is rich in potassium content (83) and therefore a PD can be effective in reducing blood pressure in hypertensive people (84).
In addition, the higher amount of phytochemicals in a PD was reported to decrease inflammation, which can explain our findings (85, 86). Indeed, it has been recommended to increase the daily consumption of fruits and vegetables as a primary preventive measure against CVDs because they can reduce circulating CRP concentrations (87).
The present meta-analysis has several limitations to be mentioned. One consideration to take into account with a PD is that it prohibits consumption of dairy products; so, it contains low calcium content that can cause reduction of bone density (88). Because the side effects of this dietary pattern were not assessed in the previous studies, recommendations for adherence to a PD with the aim of health promotion should be implemented with caution. Other limitations are that the included studies were heterogeneous regarding intervention duration (from 2 wk to 2 y), the types of dietary patterns and guidelines which were recommended for subjects in the control groups (usual diet, Nordic Nutrition Recommendations, Mediterranean-like diet, etc.), and the health status of participants (healthy subjects, postmenopausal women, patients with type 2 diabetes, multiple sclerosis, ischemic heart disease, and metabolic syndrome), and doing subgroup analysis was not possible owing to the insufficient number of eligible studies. Moreover, no data were available on the genetic background of participants and possible polymorphisms, which may have a considerable effect on results. Another problem is the fact that the results were significantly influenced by the removal of several studies in our sensitivity analysis; so, the findings should be interpreted with caution.
Conclusion
Based on our analyses, a PD decreased the anthropometric indexes (weight, BMI, WC, and body fat percentage), blood pressure (SBP and DBP), and circulating CRP concentrations, and improved the lipid profile (LDL cholesterol, TGs, and TC; HDL cholesterol increased). However, we have insufficient evidence to make solid conclusions regarding the efficacy of a PD on improving CVD risk factors, mostly owing to a lack of qualified RCTs. Thus, putative long-term useful effects of different components of a PD on CVD risk factors need to be explored in additional well-designed large trials.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—MM and NR-J: conceived and designed the research; MM and HM: conducted the systematic research and study selection, and extracted the data; MM: analyzed the data; EG, HM, and NR-J: wrote the manuscript; MH, JM, and AS-A: reviewed or edited the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (to MH).
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: CRP, C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; MeSH, medical subject headings; PD, Paleolithic diet; RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WC, waist circumference; WMD, weighted mean difference.
References
- 1. Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng Z-J, Flegal K, O'Donnell C, Kittner S et al.. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85. [DOI] [PubMed] [Google Scholar]
- 2. WHO. Global atlas of CV prevention and control. [Internet] Geneva: World Health Organization; 2011; [accessed 20 July, 2018]. Fact sheet no. 317. Available from: https://www.who.int/cardiovascular_diseases/about_cvd/en/. [Google Scholar]
- 3. Mertens E, Markey O, Geleijnse JM, Givens DI, Lovegrove JA. Dietary patterns in relation to cardiovascular disease incidence and risk markers in a middle-aged British male population: data from the Caerphilly prospective study. Nutrients. 2017;9(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perk J, De Backer G, Gohlke H, Graham I, Reiner Ž, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R et al.. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). Int J Behav Med. 2012;19(4):403–88. [DOI] [PubMed] [Google Scholar]
- 5. Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM et al.. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. Circulation. 2002;106(3):388–91. [DOI] [PubMed] [Google Scholar]
- 6. Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n−3 Fatty acids from fish or fish-oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84(1):5–17. [DOI] [PubMed] [Google Scholar]
- 7. Heianza Y, Ma W, Huang T, Wang T, Zheng Y, Smith SR, Bray GA, Sacks FM, Qi L. Macronutrient intake-associated FGF21 genotype modifies effects of weight-loss diets on 2-year changes of central adiposity and body composition: the POUNDS Lost trial. Diabetes Care. 2016;39(11):1909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhan J, Liu YJ, Cai LB, Xu FR, Xie T, He QQ. Fruit and vegetable consumption and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2017;57(8):1650–63. [DOI] [PubMed] [Google Scholar]
- 9. Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006;136(10):2588–93. [DOI] [PubMed] [Google Scholar]
- 10. Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr. 2002;76(1):93–9. [DOI] [PubMed] [Google Scholar]
- 11. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 12. Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease?. Am J Clin Nutr. 2001;73(1):1–2. [DOI] [PubMed] [Google Scholar]
- 13. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113(1):1–15. [DOI] [PubMed] [Google Scholar]
- 14. Liyanage T, Ninomiya T, Wang A, Neal B, Jun M, Wong MG, Jardine M, Hillis GS, Perkovic V. Effects of the Mediterranean diet on cardiovascular outcomes—a systematic review and meta-analysis. PLoS One. 2016;11(8):e0159252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases—incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29(4):611–18. [DOI] [PubMed] [Google Scholar]
- 16. Rashidipour-Fard N, Karimi M, Saraf-Bank S, Baghaei MH, Haghighatdoost F, Azadbakht L. Healthy eating index and cardiovascular risk factors among Iranian elderly individuals. ARYA Atheroscler. 2017;13(2):56. [PMC free article] [PubMed] [Google Scholar]
- 17. Haghighatdoost F, Sarrafzadegan N, Mohammadifard N, Sajjadi F, Maghroon M, Boshtam M, Alikhasi H, Azadbakht L. Healthy eating index and cardiovascular risk factors among Iranians. J Am Coll Nutr. 2013;32(2):111–21. [DOI] [PubMed] [Google Scholar]
- 18. Konner M, Eaton SB. Paleolithic nutrition: twenty-five years later. Nutr Clin Pract. 2010;25(6):594–602. [DOI] [PubMed] [Google Scholar]
- 19. Jönsson T, Granfeldt Y, Ahrén B, Branell UC, Pålsson G, Hansson A, Söderström M, Lindeberg S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordain L. The nutritional characteristics of a contemporary diet based upon Paleolithic food groups. J Am Nutraceutical Assoc. 2002;5(3):15–24. [Google Scholar]
- 21. Österdahl M, Kocturk T, Koochek A, Wändell P. Effects of a short-term intervention with a paleolithic diet in healthy volunteers. Eur J Clin Nutr. 2008;62(5):682–5. [DOI] [PubMed] [Google Scholar]
- 22. Jew S, AbuMweis SS, Jones PJ. Evolution of the human diet: linking our ancestral diet to modern functional foods as a means of chronic disease prevention. J Med Food. 2009;12(5):925–34. [DOI] [PubMed] [Google Scholar]
- 23. Pritchard JK. How we are evolving. Sci Am. 2010;303(4):41–7. [DOI] [PubMed] [Google Scholar]
- 24. Gurven MD, Trumble BC, Stieglitz J, Blackwell AD, Michalik DE, Finch CE, Kaplan HS. Cardiovascular disease and type 2 diabetes in evolutionary perspective: a critical role for helminths?. Evol Med Public Health. 2016(1):338–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindeberg S, Jönsson T, Granfeldt Y, Borgstrand E, Soffman J, Sjöström K, Ahrén B. A Palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50(9):1795–807. [DOI] [PubMed] [Google Scholar]
- 26. Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72(4):912–21. [DOI] [PubMed] [Google Scholar]
- 27. Sun J, Buys NJP, Shen S-YD. Dietary patterns and cardiovascular disease-related risks in Chinese older adults. Front Public Health. 2013;1:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kerver JM, Yang EJ, Bianchi L, Song WO. Dietary patterns associated with risk factors for cardiovascular disease in healthy US adults. Am J Clin Nutr. 2003;78(6):1103–10. [DOI] [PubMed] [Google Scholar]
- 29. Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC Jr, Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr. 2009;63(8):947–55. [DOI] [PubMed] [Google Scholar]
- 30. Boers I, Muskiet FA, Berkelaar E, Schut E, Penders R, Hoenderdos K, Wichers HJ, Jong MC. Favourable effects of consuming a Palaeolithic- type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, Olsson T, Lindahl B. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68(3):350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Irish AK, Erickson CM, Wahls TL, Snetselaar LG, Darling WG. Randomized control trial evaluation of a modified Paleolithic dietary intervention in the treatment of relapsing-remitting multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis. 2017;7:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: John Wiley & Sons; 2009. [Google Scholar]
- 36. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palmer TM, Peters JL, Sutton AJ, Moreno SG. Contour-enhanced funnel plots for meta-analysis. Stata J. 2008;8(2):242–54. [Google Scholar]
- 39. Singh RB, Fedacko J, Vargova V, Pella D, Niaz MA, Ghosh S. Effect of low W-6/W-3 fatty acid ratio Paleolithic style diet in patients with acute coronary syndromes: a randomized, single blind, controlled trial. World Heart J. 2012;4(1):71–84. [Google Scholar]
- 40. Lee JE, Bisht B, Hall MJ, Rubenstein LM, Louison R, Klein DT, Wahls TL. A multimodal, nonpharmacologic intervention improves mood and cognitive function in people with multiple sclerosis. J Am Coll Nutr. 2017;36(3):150–68. [DOI] [PubMed] [Google Scholar]
- 41. Jönsson T, Granfeldt Y, Lindeberg S, Hallberg AC. Subjective satiety and other experiences of a Paleolithic diet compared to a diabetes diet in patients with type 2 diabetes. Nutr J. 2013;12:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jönsson T, Granfeldt Y, Erlanson-Albertsson C, Ahrén B, Lindeberg S. A paleolithic diet is more satiating per calorie than a mediterranean-like diet in individuals with ischemic heart disease. Nutr Metab (Lond). 2010;7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baumgartner S, Imfeld T, Schicht O, Rath C, Persson RE, Persson GR. The impact of the Stone Age diet on gingival conditions in the absence of oral hygiene. J Periodontol. 2009;80(5):759–68. [DOI] [PubMed] [Google Scholar]
- 44. Chorell E, Ryberg M, Larsson C, Sandberg S, Mellberg C, Lindahl B, Antti H, Olsson T. Plasma metabolomic response to postmenopausal weight loss induced by different diets. Metabolomics. 2016;12(5):85. [Google Scholar]
- 45. Genoni A, Lo J, Lyons-Wall P, Devine A. Compliance, palatability and feasibility of PALEOLITHIC and Australian Guide to Healthy Eating diets in healthy women: a 4-week dietary intervention. Nutrients. 2016;8(8):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sandberg S, Mellberg C, Ryberg M, Olsson T, Larsson C, Lindahl B. Does a Paleolithic-type diet have a better effect than a conventional low-fat diet in achieving long-term weight loss among obese post-menopausal women?. Int J Behav Med. 2012;19:S227–S8. [Google Scholar]
- 47. Boraxbekk CJ, Stomby A, Ryberg M, Lindahl B, Larsson C, Nyberg L, Olsson T. Diet-induced weight loss alters functional brain responses during an episodic memory task. Obesity Facts. 2015;8(4):261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blomquist C, Alvehus M, Burén J, Ryberg M, Larsson C, Lindahl B, Mellberg C, Söderström I, Chorell E, Olsson T. Attenuated low-grade inflammation following long-term dietary intervention in postmenopausal women with obesity. Obesity (Silver Spring). 2017;25(5):892–900. [DOI] [PubMed] [Google Scholar]
- 49. Andersson J, Mellberg C, Otten J, Ryberg M, Rinnström D, Larsson C, Lindahl B, Hauksson J, Johansson B, Olsson T. Left ventricular remodelling changes without concomitant loss of myocardial fat after long-term dietary intervention. Int J Cardiol. 2016;216:92–6. [DOI] [PubMed] [Google Scholar]
- 50. Blomquist C, Chorell E, Ryberg M, Mellberg C, Worrsjo E, Makoveichuk E, Larsson C, Lindahl B, Olivecrona G, Olsson T. Decreased lipogenesis-promoting factors in adipose tissue in postmenopausal women with overweight on a Paleolithic-type diet. Eur J Nutr. 2018;57(8):2877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frassetto LA, Shi L, Schloetter M, Sebastian A, Remer T. Established dietary estimates of net acid production do not predict measured net acid excretion in patients with type 2 diabetes on Paleolithic–Hunter–Gatherer-type diets. Eur J Clin Nutr. 2013;67(9):899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Trexler ET, Smith MM, Sommer AJ, Starkoff BE, Devor ST. Paleolithic diet is associated with unfavorable changes to blood lipids in healthy subjects. Med Sci Sports Exerc. 2013;45(Suppl 1 5S):659. [Google Scholar]
- 53. Ryberg M, Sandberg S, Mellberg C, Stegle O, Lindahl B, Larsson C, Hauksson J, Olsson T. A Palaeolithic-type diet causes strong tissue-specific effects on ectopic fat deposition in obese postmenopausal women. J Intern Med. 2013;274(1):67–76. [DOI] [PubMed] [Google Scholar]
- 54. Smith M, Trexler E, Sommer A, Starkoff B, Devor S. Manuscript has been retracted. Int J Exerc Sci. 2014;7(2):128–39. [Google Scholar]
- 55. Otten J, Stomby A, Ryberg M, Svensson M, Hauksson J, Olsson T. Effects of a Paleolithic diet with and without supervised exercise on liver fat and insulin sensitivity: a randomised controlled trial in individuals with type 2 diabetes. Diabetologia. 2016;59:S10–S. [Google Scholar]
- 56. Bligh HFJ, Godsland IF, Frost G, Hunter KJ, Murray P, MacAulay K, Hyliands D, Talbot DCS, Casey J, Mulder TP et al.. Plant-rich mixed meals based on Palaeolithic diet principles have a dramatic impact on incretin, peptide YY and satiety response, but show little effect on glucose and insulin homeostasis: an acute-effects randomised study. Br J Nutr. 2015;113(4):574–84. [DOI] [PubMed] [Google Scholar]
- 57. Genoni A, Lyons-Wall P, Lo J, Devine A. Cardiovascular, metabolic effects and dietary composition of ad-libitum Paleolithic vs. Australian Guide to Healthy Eating diets: a 4-week randomised trial. Nutrients. 2016;8(5):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stomby A, Simonyte K, Mellberg C, Ryberg M, Stimson RH, Larsson C, Lindahl B, Andrew R, Walker BR, Olsson T. Diet-induced weight loss has chronic tissue-specific effects on glucocorticoid metabolism in overweight postmenopausal women. Int J Obes (Lond). 2015;39(5):814–19. [DOI] [PubMed] [Google Scholar]
- 59. Masharani U, Sherchan P, Schloetter M, Stratford S, Xiao A, Sebastian A, Nolte Kennedy M, Frassetto L. Metabolic and physiologic effects from consuming a hunter-gatherer (Paleolithic)-type diet in type 2 diabetes. Eur J Clin Nutr. 2015;69(8):944–8. [DOI] [PubMed] [Google Scholar]
- 60. Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. Am J Clin Nutr. 2015;102(4):922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eaton SB, Konner M, Shostak M. Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective. Am J Med. 1988;84(4):739–49. [DOI] [PubMed] [Google Scholar]
- 62. O'Keefe JH Jr, Cordain L. Cardiovascular disease resulting from a diet and lifestyle at odds with our Paleolithic genome: how to become a 21st- century hunter-gatherer. Mayo Clin Proc. 2004;79(1):101–8. [DOI] [PubMed] [Google Scholar]
- 63. Daniel M, Rowley KG, McDermott R, Mylvaganam A, O'Dea K. Diabetes incidence in an Australian aboriginal population. An 8-year follow-up study. Diabetes Care. 1999;22(12):1993–8. [DOI] [PubMed] [Google Scholar]
- 64. Ebbesson SO, Schraer CD, Risica PM, Adler AI, Ebbesson L, Mayer AM, Shubnikof EV, Yeh J, Go OT, Robbins DC. Diabetes and impaired glucose tolerance in three Alaskan Eskimo populations. The Alaska-Siberia Project. Diabetes Care. 1998;21(4):563–9. [DOI] [PubMed] [Google Scholar]
- 65. Brand-Miller JC, Griffin HJ, Colagiuri S. The carnivore connection hypothesis: revisited. J Obes. 2012:258624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cordain L, Eaton SB, Miller JB, Mann N, Hill K. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(S1):S42–52. [DOI] [PubMed] [Google Scholar]
- 67. Cochran G, Harpending H. The 10,000 year explosion: how civilization accelerated human evolution. New York: Basic Books; 2009. [Google Scholar]
- 68. Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J. 2014;13(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233(6):674–88. [DOI] [PubMed] [Google Scholar]
- 70. Meneton P, Jeunemaitre X, de Wardener HE, Macgregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85(2):679–715. [DOI] [PubMed] [Google Scholar]
- 71. Singh RK, Ishikawa S. Food additive p-80 impacts mouse gut microbiota promoting intestinal inflammation, obesity and liver dysfunction. SOJ Microbiol Infect Dis. 2016;4(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rajamäki K, Nordström T, Nurmi K, Åkerman KE, Kovanen PT, Öörni K, Eklund KK. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J Biol Chem. 2013;288(19):13410–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pan J, Sun L-C, Tao Y-F, Zhou Z, Du X-L, Peng L, Feng X, Wang J, Li Y-P, Liu L et al.. ATP synthase ecto-α-subunit: a novel therapeutic target for breast cancer. J Transl Med. 2011;9(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eaton SB, Strassman BI, Nesse RM, Neel JV, Ewald PW, Williams GC, Weder AB, Eaton SB III, Lindeberg S, Konner MJ et al.. Evolutionary health promotion. Prev Med. 2002;34(2):109–18. [DOI] [PubMed] [Google Scholar]
- 75. Jönsson T. Healthy satiety effects of paleolithic diet on satiety and risk factors for cardiovascular disease. [dissertation] Lund, Sweden: Division of Family Medicine, Department of Clinical Sciences, Lund University; 2007. [Google Scholar]
- 76. Beasley JM, Ange BA, Anderson CA, Miller ER 3rd, Erlinger TP, Holbrook JT, Sacks FM, Appel LJ. Associations between macronutrient intake and self-reported appetite and fasting levels of appetite hormones: results from the Optimal Macronutrient Intake Trial to Prevent Heart Disease. Am J Epidemiol. 2009;169(7):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Davy BM, Dennis EA, Dengo AL, Wilson KL, Davy KP. Water consumption reduces energy intake at a breakfast meal in obese older adults. J Am Diet Assoc. 2008;108(7):1236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scott KP, Duncan SH, Flint HJ. Dietary fibre and the gut microbiota. Nutr Bull. 2008;33(3):201–11. [Google Scholar]
- 79. Howard BV, Wylie-Rosett J. Sugar and cardiovascular disease: a statement for healthcare professionals from the Committee on Nutrition of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation. 2002;106(4):523–7. [DOI] [PubMed] [Google Scholar]
- 80. Ferrannini E, Balkau B, Coppack SW, Dekker JM, Mari A, Nolan J, Walker M, Natali A, Beck-Nielsen H. Insulin resistance, insulin response, and obesity as indicators of metabolic risk. J Clin Endocrinol Metab. 2007;92(8):2885–92. [DOI] [PubMed] [Google Scholar]
- 81. Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87(1):258S–68S. [DOI] [PubMed] [Google Scholar]
- 82. Weggemans RM, Zock PL, Katan MB. Dietary cholesterol from eggs increases the ratio of total cholesterol to high-density lipoprotein cholesterol in humans: a meta-analysis. Am J Clin Nutr. 2001;73(5):885–91. [DOI] [PubMed] [Google Scholar]
- 83. Palmer BF, Clegg DJ. Achieving the benefits of a high-potassium, Paleolithic diet, without the toxicity. Mayo Clin Proc. 2016;91(4):496–508. [DOI] [PubMed] [Google Scholar]
- 84. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Eaton SB, Eaton SB III. Paleolithic vs. modern diets — slected pathophysiological implications. Eur J Nutr. 2000;39(2):67–70. [DOI] [PubMed] [Google Scholar]
- 86. Hosseini B, Berthon BS, Saedisomeolia A, Starkey MR, Collison A, Wark PA, Wood LG. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. Am J Clin Nutr. 2018;108(1):136–55. [DOI] [PubMed] [Google Scholar]
- 87. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. 2006;84(6):1489–97. [DOI] [PubMed] [Google Scholar]
- 88. Obert J, Pearlman M, Obert L, Chapin S. Popular weight loss strategies: a review of four weight loss techniques. Curr Gastroenterol Rep. 2017;19(12):61. [DOI] [PubMed] [Google Scholar]