Abstract
Purpose
Beta2-microglobulin (β2-M) is recognized as a surrogate marker relating to the mechanisms of dialysis-associated amyloidosis. Few studies have evaluated the association of serum β2-M with clinical outcome in hemodialysis patients using high-flux type. However, study on patients using low-flux dialyzer reuse has not been done yet.
Patients and methods
Using serum β2-M level on predicting long-term mortality of hemodialysis patients was examined in 326 prevalent hemodialysis patients (45.59±14.46 years, hemodialysis duration of 47.5 (26–79) months, 186 males and 140 females). The patients were divided into 3 groups with equal number of patients, according to their serum β2-M levels: group A (n=109, serum β2-M concentration ≤55.7 mg/L), group B (n=109, serum β2-M level from 55.8 mg/L to 75.4 mg/L) and group C (n=108, serum β2-M concentration >75.4 mg/L).
Results
During the follow-up period of 5 years, there were 75 all-cause deaths (23.0%). Kaplan–Meier analysis revealed that all-cause mortality in the higher β2-M group was significantly higher compared to that in the lower β2-M groups (p<0.001). Serum β2-M level was a significant predictor for all-cause mortality (AUC =0.898; p<0.001; Cut-off value: 74.9 mg/L, Se=93.3%, Sp=92.9%).
Conclusion
Serum β2-M levels were a significant predictor of long-term mortality in hemodialysis patients, who use only low-flux dialyzers and reuse 6 times.
Keywords: Beta2-microglobulin, mortality, hemodialysis
Introduction
Beta2-microglobulin (β2-M) is a middle-molecular-weight protein with 11,800 Dal, which is produced by all cells expressing the major histocompatibility class I. β2-M is filtered by the glomerulus and is degenerated in the proximal tubules through a megalin-dependent pathway.1 In patients with a reduced glomerular filtration rate, circulating β2-M levels are elevated.1 In dialysis patients, in whom the glomerular filtration rate is almost completely abolished, β2-M accumulates in the circulation far above its levels in normal subjects and difficult to dialyze by use of low-flux membrane.2 The deposition of β2-microglobulin induced by reactive inflammation causing carpal tunnel syndrome is one of the complications of dialysis-related amyloidosis (DRA) in maintaining hemodialysis patients.3 In recent years, the role of β2-M as a marker of cardiovascular and/or mortality risk has grown.4–6 Thus, removal of circulating β2-M during hemodialysis has been considered to be beneficial. Several methods to reduce plasma β2-M levels have been used in dialyzed patients, such as the use of high-flux membrane,7–9 hemodiafiltration.10–13 Role of β2-M in predicting outcome and mortality in both hemodialysis and peritoneal dialysis patients was published in few previous papers.14–16 In Vietnam, due to paucity of indigenous research, β2-M levels in hemodialysis patients are not known. Hence, in this study, we examined the levels of serum β2-M as well as the relationship between serum β2-M and survival of hemodialysis patients in a single dialysis center, where low-flux membranes are used exclusively as a standard approach and the reuse of membranes is done at all.
Subjects and methods
Subjects
There were 514 patients on prevalent hemodialysis (hemodialysis duration >3 months) who joined in our study at Hemodialysis Center, Bach Mai Hospital, Hanoi, Vietnam, as of February 2011. Of these, patients with acute illness, significant infection, malignancy, or used high-flux dialyzer were excluded. The remaining patients, 326 prevalent hemodialysis patients, were provided informed consent prior to participation in our study. The enrolled patients were treated with stable, regular hemodialysis, using bicarbonate dialysate. Our dialysis program used low-flux membrane as a standard reuse of dialyzer. Kt/V was calculated according to the formula of Daugirdas.17 Each dialysis session was between 3.5 and 4.5 hrs, in order to achieve the target total Kt/V of around 1.2 per session for three times weekly treatment. Reuse of dialyzer was performed for 6 times in all patients. The clinical diagnoses of primary renal disease were chronic glomerulonephritis, hypertensive nephropathy, chronic pyelo-nephritis, diabetic nephropathy, polycystic kidney disease, gout.
The clinical diagnoses of pain of shoulder, carpal tunnel syndrome were performed. Number of died patients with all-causes was collected during 5 years.
To achieve the goal that whether serum β2-M as marker predicting mortality, we arranged 326 patients in increasing order of concentration. The patients were divided into 3 groups with equal number of patients, according to the concentration of serum β2-M: group A with 109 patients (serum β2-M concentration ≤55.7 mg/L), group B with 109 patients (serum β2-M level from 55.8 mg/L to 75.4 mg/L) and group C with 108 patients (serum β2-M concentration >75.4 mg/L).
Also, this study was approved by the ethics review committee of the hospital.
Biochemical assays and other measurements
Blood was drawn just before the start of a dialysis session in a non-fasting state, to measure serum albumin, creatinine, blood urea nitrogen, C-reactive protein (CRP) and hematocrit, using routine laboratory methods, one a month as a routine clinical care as performed in most dialysis facilities in Vietnam. Serum β2-M concentration was measured using latex immunoassay principle at the time of enrolment.
Statistical methods
All the continuous data were represented by mean and standard deviation and were analyzed by ANOVA and Student t-test. Categorical data were presented by frequency with percentage and were analyzed using Chi-square test. Receiver operating characteristic (ROC) curves with the area under the curve (AUC) was calculated to predict mortality from patients after 5 years follow-up. Multivariate regression analysis was performed to identify the predictors of hospital mortality. Survival curves were assessed using the Kaplan–Meier analysis and evaluated by the log-rank test. Statistical analysis was done using Statistical Package for Social Science (SPSS) version 20.0 (Chicago, IL, USA). A p-value<0.05 was considered as significant.
Results
The baseline demographic and laboratory characteristics in patients are shown in Table 1. In our study, we found no difference in age, sex, BMI, rate of hypertension, causes of chronic renal failure, serum creatinine, serum albumin, hemoglobin level and anemia rate in groups A, B and C.
Table 1.
Clinical characteristics and laboratory parameters of the studied patients (n=326)
| Total (n=326) | Group A (β2-M ≤55.7 mg/L), (n=109) | Group B (β2-M from 55.8–75.4 mg/L), (n=109) | Group C (β2-M >75.4 mg/L), (n=108) | P for trend | |
|---|---|---|---|---|---|
| Ages (years) | 45.59±14.46 | 45.39±14.56 | 46.64±15.5 | 44.73±13.29 | 0.614 |
| Male, n (%) | 186 (57.1) | 66 (60.6) | 60 (55) | 60 (55.6) | 0.663 |
| Duration of hemodialysis (month) | 47.5 (26–79) | 28 (16–40) | 53 (27–73) | 81 (53.25–108.75) | <0.001 |
| BMI | 19.17±2.35 | 19.18±2.59 | 19.17±2.05 | 19.17±2.41 | 0.998 |
| Hypertension, n (%) | 244 (74.8) | 83 (76.1) | 79 (72.5) | 82 (75.9) | 0.783 |
| Pain of shoulder, n (%) | 102 (31.3) | 16 (14.7) | 36 (33) | 50 (46.3) | <0.001 |
| Carpal tunnel syndrome, n (%) | 40 (12.3) | 4 (3.7) | 10 (9.2) | 36 (24.1) | <0.001 |
| Etiology, n (%) | 0.063 | ||||
|
230 (70.6) | 88 (80.7) | 67 (61.5) | 75 (69.4) | |
|
43 (13.2) | 10 (9.2) | 19 (17.4) | 14 (13) | |
|
32 (9.8) | 9 (8.3) | 13 (11.9) | 10 (9.3) | |
|
21 (6.4) | 2 (1.8) | 10 (9.2) | 9 (8.3) | |
| Residual kidney function, n(%) | 63 (19.3) | 39 (35.8) | 16 (14.7) | 8 (7.4) | <0.001 |
| HBV and/or HCV (+), n (%) | 140 (42.9) | 25 (22.9) | 38 (34.9) | 77 (71.3) | <0.001 |
| Lipid disorder, n (%) | 213 (65.3) | 58 (53.2) | 76 (69.7) | 79 (73.1) | 0.004 |
| Urea (mmol/L) | 28.8 (25.1–33.9) | 27.4 (24–31.2) | 28.2 (24.95–32.7) | 30.9 (26.4–37.5) | <0.001 |
| Creatinine (µmol/L) | 824 (656.5–986) | 824 (666–982) | 837 (666.5–987) | 824 (643–978.5) | 0.954 |
| Albumin (g/L) | 38.71±3.56 | 38.73±3.1 | 39.16±3.96 | 38.24±3.56 | 0.168 |
| Hs-CRP (mg/L) | 0.4 (0.1–0.7) | 0.2 (0.1–0.3) | 0.4 (0.2–0.6) | 0.6 (0.4–1.17) | <0.001 |
| Hemoglobin (g/L) | 103.19±18.32 | 101.5±18.59 | 105.78±16.97 | 102.28±19.22 | 0.185 |
| Anemia, n (%) | 271 (83.1) | 93 (85.3) | 89 (81.7) | 89 (82.4) | 0.747 |
| β2-M (mg/L) | 66.75 (48.42–81.05) | 40.4 (32.7–48.55) | 66.8 (61.2–71.6) | 85.4 (81.05–92.17) | <0.001 |
| Mortality for all causes, n (%) | 75 (23.0) | 3 (2.8) | 4 (3.7) | 68 (63) | <0.001 |
Abbreviations: BMI, Body Mass Index; CGN, Chronic Glomerulonephritis; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; hs-CRP, high sensitive C Reactive Protein.
However, the results of our study showed that in patient group with higher serum β2-M, there was a long duration of hemodialysis, the rate of shoulder pain, tunnel syndrome, hepatitis virus infection, and especially the rate mortality was higher than in patients with lower serum β2-M concentrations, p<0.001.
In addition, patients with higher serum β2-M concentrations had a lower proportion of patients with residual renal function and higher median hs-CRP levels than patients with lower serum β2-M level, p<0.001.
There was a positive correlation, moderate level of serum β2-M concentration with duration of hemodialysis and serum hs-CRP concentration, correlation coefficients of 0.641; 0.506, respectively, p<0.001 (Table 2).
Table 2.
Correlation between serum β2-M level and duration and hs-CRP
| Variables | β2-M (mg/L) | Correlation equation | |
|---|---|---|---|
| r | p | ||
| Duration of hemodialysis | 0.641 | <0.001* | β2-M =0.359*Duration of hemodialysis +44.43 |
| Serum hs-CRP (mg/L) | 0.506 | <0.001* | β2-M =23.14*hs-CRP +52.97 |
Note: *Statistical significance.
Abbreviation: Hs-CRP: high sensitive C Reactive Protein.
Using multivariate logistic regression analysis, hemodialysis patients with long duration of hemodialysis and serum β2-M were independent risk factors for long-term mortality (Table 3).
Table 3.
Result of multivariate logistic regression analysis showing predictors of hospital mortality of hemodialysis patients after 5 years
| Variable | Adjusted hazard ratio | 95% Cl | p |
|---|---|---|---|
| Age | 0.994 | 0.963–1.026 | 0.709 |
| Sex: male | 1.204 | 0.522–2.777 | 0.664 |
| Duration of hemodialysis | 1.037 | 1.022–1.051 | <0.001* |
| HBV and/or HCV (+) | 1.992 | 0.86–4.616 | 0.108 |
| Albumin | 0.898 | 0.801–1.007 | 0.066 |
| Serum hs-CRP (mg/L) | 0.433 | 0.157–1.193 | 0.106 |
| Hemoglobin (g/l) | 0.996 | 0.977–1.016 | 0.726 |
| β2-M (mg/L) | 1.093 | 1.052–1.135 | <0.001* |
Note: *Statistical significance.
Abbreviations: HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; hs-CRP, high sensitive C Reactive Protein.
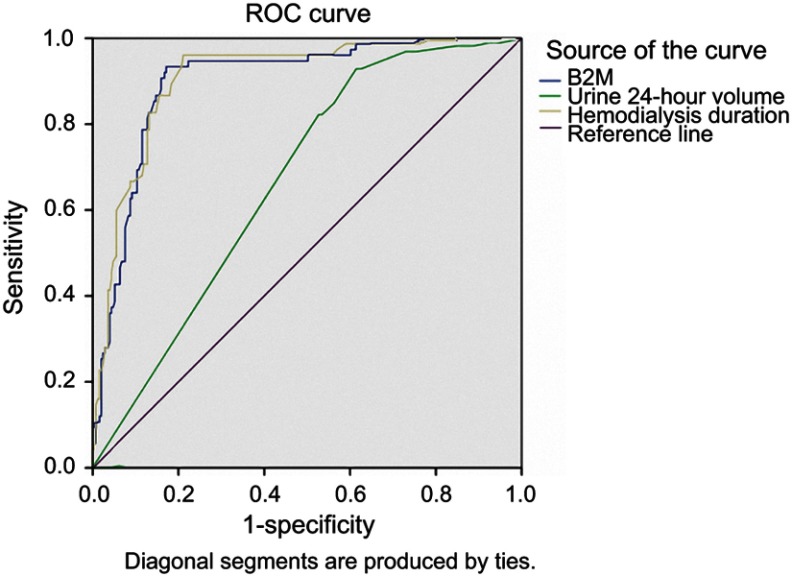
Using ROC curve model to predict mortality in maintenance hemodialysis patients for 5 years, we realized that serum β2-M concentration has an equal predictive value of mortality compared with hemodialysis duration and had a better predictive value than renal residual function (Figure 1).
Figure 1.
Receiver operating characteristics (ROC) curves of serum β2-M, hemodialysis duration and residual kidney function for prediction of hospital mortality of hemodialysis patients with all-causes. β2-M: AUC =0.898; p<0.001; Cut-off value: 74.9 mg/L, Se=93.3%, Sp=92.9%. Hemodialysis duration: AUC =0.907; p<0.001; Cut-off value: 63 months, Se=96%, Sp=78.9%. Urine 24 hrs volume: AUC =0.669; p<0.001; Cut-off value: 225 mL, Se=93.3%, Sp=38.2%. Serum β2-M concentration has an equal predictive value of mortality compared with hemodialysis duration and had a better predictive value than renal residual function in maintenance hemodialysis patients for 5 years.
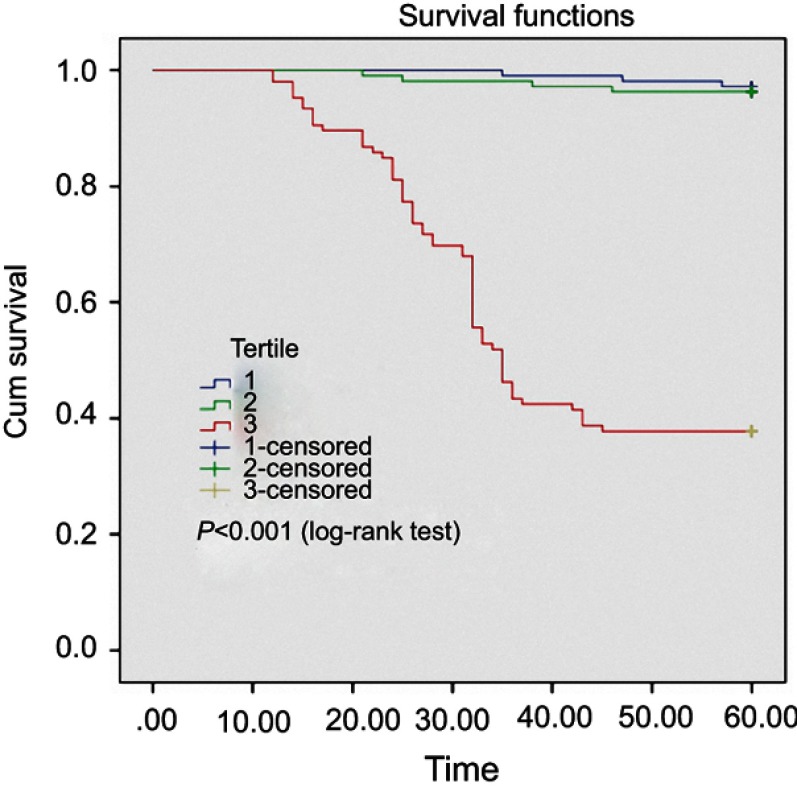
The Kaplan–Meier analysis model showed that patients with higher β2-M concentrations had a significant higher death rate than those with lower β2-M concentrations (Figure 2).
Figure 2.
Kaplan–Meier analysis of all-causes mortality of 326 hemodialysis patients, classified according to β2-M concentrations in 3 groups A, B and C. Patients with higher β2-M concentrations (red line – group C) exhibited a significantly higher death rate compared to those with lower serum β2-M concentrations (blue line – group B and violet line – group A) (log-rank test, p<0.001).
Discussion
Level of serum β2-M hemodialysis patients
Our results show that 100% of patients had elevated serum concentrations of β2-M compared to that of normal people (normal value <3 mg/L). Median β2-M level of serum was 66.75 mg/L (Table 1). There were many studies in the world that reported serum β2-M concentrations in patients with chronic renal failure treating with maintaining hemodialysis. In 1999, Dixit MP et al18 studied levels of serum β2-M in 30 young dialysis patients (mean age was 18.7±0.9 years old) who used cellulose membrane dialyzer; the result showed that level of serum β2-M was 49.7±3.9 mg/L. Okuno S et al15 studied serum β2-M levels in 490 dialysis patients, with an average age of 60.1 years, mean dialysis duration was 87.4 months. The patients were treated with high-flux dialyzers; that is, reuse not done at all. The results show that mean level of serum β2-M was 32.2 mg/L. The study by Mumtaz A et al19 in 50 patients use low-flux dialyzer; the concentration of B2-M was 92.6 mg/L. The level of serum β2-M in study of Traut et al20 in 20 patients using low-flux dialyzer was 42.0 mg/L. Thus, increase of serum β2-M was common in chronic renal failure patients treated with maintaining hemodialysis. However, the increase of serum β2-M concentrations in each study was different, suggesting that the serum β2-M concentration in hemodialysis depends on many patient characteristics. First, residual kidney function is related to serum β2-M levels in this patient group. Beta 2-microglobulin is found on the surface of all nucleated cells and plays a central role in cellular immunology. Its synthesis rate normally ranges from 2 to 4 mg/kg/day with a half life of 2–5 hrs.1 In healthy individuals, the plasma concentration varies from 1 to 3 mg/L, which varies inversely with the glomerular filtration rate. More than 95% of β2-M is eliminated by degradation in the proximal tubule. Since this compound cannot be removed from the serum by the kidney or certain dialysis membranes in patients with renal dysfunction on dialysis, β2-M concentration is increased by up to 60-fold in patients with end-stage renal disease.1 In patients with remaining residual kidney function, level of serum β2-M will be lower than that of the patients without residual kidney function, because an amount of serum β2-M will still be excreted by the kidney in dialysis patients. Results of our study also show this in Table 1: In patients with higher serum β2-M concentration, the proportion of patients still having residual renal function was lower (groups A, B, C: 35.8%, 14.7% and 7.4%, respectively), p<0.001.
The major reason for such a high level of β2-M in this study was that the dialyzer used for hemodialysis in our patients was of the low-flux dialyzer for a long time. As β2-M is a middle molecule of molecular weight, conventional, low-flux dialyzers do not clear these molecules which lead to accumulation of this silent killer in the body. Financial constraints are the major reason for using low-flux dialyzers in our patients. The same group of dialysis patients, but in our study of Okuno S et al,15 show that β2-M levels were lower (32.3 mg/L versus 64.74 mg/L) while our dialysis time was lower (87.4 months versus 47.5 months). The role of high-flux types in decreasing serum β2-M level has been published by some authors.7,21,22 In addition, long-term dialysis is also a cause of elevated serum β2-M concentrations (results are shown in Tables 1 and 2).
In Vietnam, the number of patients with end-stage chronic kidney disease is increasing, due to complications of diabetes and hypertension. Hemodialysis is still a major treatment for end-stage renal disease patients. Patients receive dialysis three times a week, 3.5 hrs to 4 hrs per time. However, patients must re-use the dialyzers 6 times. The washing, soaking, sterilizing dialyzers were done following strictly compliant with the standards of the Vietnam Ministry of Health. Although the quality of dialysis with re-used dialyzers is safe, the potential for infection with hepatitis virus and other infection is present in dialysis patients. This is evident in Table 1, with 42.9% of patients infected with hepatitis B/C virus in our study. Patients with high β2-M levels had a higher prevalence of hepatitis virus infection than those with lower β2-M level (Ratio of hepatitis virus infection in groups A, B, C: 22.9%, 34.9% and 71.3%, respectively), p<0.001. Especially in patients with higher β2-M levels, the median value of CRP-hs was higher than that of lower β2-M group (0.6 mg/L versus 0.4 mg/L versus 0.2 mg/L), p<0.001. β2-M is accumulated in the circulation of dialysis patients and its role on immunity and inflammation has been already reported.23 The results of our study also showed the relationship (Tables 1 and 2). Dialysis treatment per se has been considered to be an inflammatory stimulus, inducing cytokine production (such as interleukin-1, tumor necrosis factor-α, interleukin-6) and complement activation. The released cytokines are thought to stimulate the synthesis and release of β2-M by the macrophages and/or augment the expression of human leukocyte antigens (class I), increasing β2-M expression. Based on the findings of previous studies and our study, we could support that the elevated β2-M serum concentrations predispose to an up-regulation of the inflammatory procedure in dialysis patients.
DRA is a serious complication of long-term dialysis therapy and is characterized by the deposition of amyloid fibrils, principally composed of β2-M, in the osteoarticular structures and viscera. The duration on hemodialysis treatment plays an important role in the development of DRA.24–26 Carpal tunnel syndrome and pain of shoulder are two of the clinical manifestations of the DRA. Numerous studies have also demonstrated the role of β2-M in the pathogenesis of DRA and related clinical manifestations of carpal tunnel syndrome and pain of shoulder in maintaining dialysis patients.2,27 Our results also show that the ratio of patients with carpal tunnel syndrome and/or pain of shoulder in group with high β2-M levels were significantly higher than those of group with low β2-M levels, p<0.001 (Table 1).
Predictive value of mortality of serum β2-M
Although there were 514 patients in our dialysis center, however, only 326 patients met the criteria chosen in this study: patients who had had dialysis at the center for >3 months, using low-flux dialyzer with reuse 6 times, were monitored continuously for 5 years. In 5 years of follow-up, from February 2011 to February 2016, up to 75 patients died from all causes, accounting for 23% (Table 1). Multiple regression analysis revealed that duration of dialysis and serum β2-M levels were independent risk factors predicting of death in 5 years in this study (Table 3). In particular, serum β2-M level ≥74.9 mg/L was also an independent predictor of mortality in our study group (AUC =0.898; p<0.001; Se=93.3%, Sp=92.9%). Compared with hemodialysis duration and residual kidney function, serum β2-M concentration had an equal predictive value of mortality compared with hemodialysis duration and had a better predictive value than renal residual function in maintenance of hemodialysis patients for 5 years (Figure 1).
In Vietnam, although many studies have been conducted in patients with end-stage renal disease, treatment with maintaining dialysis, however, no studies have been done to assess the role of serum β2-M in the predicting mortality in dialysis patients. Kaplan–Meier analysis was performed to examine the univariate association between the 3 groups based on the β2-M concentrations and the outcomes of the cohort (Figure 2). Patients with higher β2 -M concentrations exhibited a significantly higher death rate than those with lower β2-M concentrations (log-rank test, p<0.001).
In the world, there were some studies referring to this issue. In 2006, Cheung AK et al14 in the HEMO study reported that the pre-dialysis serum β2-M level predicted mortality. In 2009, Okuno S et al15 confirmed that β2-M level at cut-off 32.2 mg/L, predicting mortality in dialysis patients, who follow-up for 50 months. When comparing the cut-off point for β2-M level predicting mortality in dialysis patients, we found that the study of Okuno S et al can predict with the lowest β2-M level, followed by Chung, while our study predicting with the highest β2-M level. This difference can be explained by the fact that Okuno’s subjects are patients who use high-flux dialyzer, and Cheung’s subjects are patients who use both low-flux and high-flux types, but our patients use only low-flux dialyzers.
Although studies have shown that serum β2-M level has a value predicting mortality in dialysis patients, however, in clinical practice there are many well-known factors such as cardiovascular events, hemodialysis effect, malnutrition which also have this value.
Conclusion
In conclusion, we demonstrated that serum β2-M levels were a significant predictor of mortality in hemodialysis patients, who use only low-flux dialyzers and reuse 6 times with relatively longer hemodialysis durations of 47.5 (26–79) months.
Acknowledgments
In this study, we had been strongly supported by clinical application funding of our local hospital and university to complete our research.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Vietnam Military Medical University (No.2134/QĐ/HVQY). All patients provided written informed consent.
Human and animal rights
Animals were not used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Schardijn GH, Statius van Eps LW. Beta 2-microglobulin: its significance in the evaluation of renal function. Kidney Int. 1987;32(5):635–641. [DOI] [PubMed] [Google Scholar]
- 2.Scarpioni R, Ricardi M, Albertazzi V, et al. Dialysis-related amyloidosis: challenges and solutions. Int J Nephrol Renovasc Dis. 2016;9:319–328. doi: 10.2147/IJNRD.S84784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng CH, Hu CC, Yen TH, et al. Association between environmental particulate matter and carpal tunnel syndrome in patients undergoing hemodialysis. Kidney Blood Press Res. 2017;42(5):827–836. doi: 10.1159/000484422 [DOI] [PubMed] [Google Scholar]
- 4.Zumrutdal A. Role of β2-microglobulin in uremic patients may be greater than originally suspected. World J Nephrol. 2015;4(1):98–104. doi: 10.5527/wjn.v4.i1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–1303. doi: 10.1038/ki.2012.301 [DOI] [PubMed] [Google Scholar]
- 6.Sedighi O, Abediankenari S, Omranifar B. Association between plasma Beta-2 microglobulin level and cardiac performance in patients with chronic kidney disease. Nephrourol Mon. 2014;7(1):e23563. doi: 10.5812/numonthly [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argyropoulos C, Roumelioti ME, Sattar A, et al. Dialyzer reuse and outcomes of high flux dialysis. PLoS One. 2015;10(6):e0129575. doi: 10.1371/journal.pone.0129575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topçiu-Shufta V, Miftari R, Haxhibeqiri V, et al. Association of Beta-2 microglobulin with inflammation and dislipidemia in high-flux membrane hemodialysis patients. Med Arch. 2016;70(5):348–350. doi: 10.5455/medarh.2016.70.348-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donadio C, Tognotti D, Caponi L, et al. β-trace protein is highly removed during haemodialysis with high-flux and super high-flux membranes. BMC Nephrol. 2017;18(1):68. doi: 10.1186/s12882-017-0489-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maduell F, Sánchez-Canel JJ, Blasco JA, et al. [Middle molecules removal. Beyond beta2-microglobulin]. Nefrologia. 2006;26(4):469–475. [PubMed] [Google Scholar]
- 11.Penne EL, van der Weerd NC, Blankestijn PJ, et al. Role of residual kidney function and convective volume on change in beta2-microglobulin levels in hemodiafiltration patients. Clin J Am Soc Nephrol. 2010;(1):80–86. doi: 10.2215/CJN.03340509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakurai K. Biomarkers for evaluation of clinical outcomes of hemodiafiltration. Blood Purif. 2013;35(Suppl 1):64–68. doi: 10.1159/000346364 [DOI] [PubMed] [Google Scholar]
- 13.Jean G, Hurot JM, Deleaval P, et al. Online-haemodiafiltration vs. conventional haemodialysis: a cross-over study. BMC Nephrol. 2015;16:70. doi: 10.1186/s12882-015-0062-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–555. doi: 10.1681/ASN.2005020132 [DOI] [PubMed] [Google Scholar]
- 15.Okuno S, Ishimura E, Kohno K, et al. Serum beta2-microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol Dial Transplant. 2009;24(2):571–577. doi: 10.1093/ndt/gfn521 [DOI] [PubMed] [Google Scholar]
- 16.Koh ES, Lee K, Kim SH, et al. Serum β2-microglobulin predicts mortality in peritoneal dialysis patients: a prospective cohort study. Am J Nephrol. 2015;42(2):91–98. doi: 10.1159/000439060 [DOI] [PubMed] [Google Scholar]
- 17.Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–1213. [DOI] [PubMed] [Google Scholar]
- 18.Dixit MP, Cabansag MR, Piscitelli J, et al. Serum beta2-microglobulin and immunoglobulin levels in young hemodialysispatients. Pediatr Nephrol. 1999;13(2):139–142. [DOI] [PubMed] [Google Scholar]
- 19.Mumtaz A, Anees M, Bilal M, et al. Beta-2 microglobulin levels in hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21(4):701–706. [PubMed] [Google Scholar]
- 20.Traut M, Haufe CC, Eismann U, et al. Increased binding of Beta-2 microglobulin to blood cells in dialysis patients treated with high-flux dialyzers compared with low-flux membranes contributed to reduced Beta2-microglobulin concentrations. Blood Purif. 2007;25:432–440. doi: 10.1159/000110069 [DOI] [PubMed] [Google Scholar]
- 21.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583 [DOI] [PubMed] [Google Scholar]
- 22.Kim HW, Kim SH, Kim YO, et al. Comparison of the impact of high-flux dialysis on mortality in hemodialysispatients with and without residual renal function. PLoS One. 2014;9(6):e97184. doi: 10.1371/journal.pone.0097184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzoulaki I, Murray GD, Lee AJ, et al. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh artery study. Circulation. 2005;112:976–983. doi: 10.1161/CirculationNaha.104.513085 [DOI] [PubMed] [Google Scholar]
- 24.Al-Taee IK, Al-Safar JJ, Al-Falahi YS, AlShamma IA. The clinical significance of β2-microglobulin in end-stage renal disease. Saudi J Kidney Dis Transpl. 2003;14(4):492–496. [PubMed] [Google Scholar]
- 25.Celik G, Capraz I, Yontem M, et al. The relationship between the antioxidant system, oxidative stress and dialysis-related amyloidosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2013;24(6):1157–1164. doi: 10.4103/1319-2442.121272 [DOI] [PubMed] [Google Scholar]
- 26.Tsai TT, Kaliya-Perumal AK, Jenq CC, et al. The unresolved problem of beta-2 microglobulin amyloid deposits in the intervertebral discs of long-term dialysis patients. J Orthop Surg Res. 2017;12(1):194. doi: 10.1186/s13018-017-0697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin J, Nishioka M, Shinko S, et al. Carpal tunnel syndrome and plasma beta2-microglobulin concentration in hemodialysis patients. Ther Apher Dial. 2008;12(1):62–66. doi: 10.1111/j.1744-9987.2007.00542.x [DOI] [PubMed] [Google Scholar]