ABSTRACT
Background: To investigate the validity of parent reported influenza vaccination and provider reporting to the Australian Immunisation Register (AIR) in children with special risk medical conditions (SRMC).
Methods: Cross-sectional survey with parents of children with a SRMC aged ≥ 6 months and <18 years attending the Women’s and Children’s Hospital, Adelaide, Australia from September 2015 to February 2016. Children aged <7 years provided data to assess provider-AIR reporting. Influenza vaccination status was ascertained from the child’s parent, immunisation provider and the AIR. Concordance was made using the Kappa index and the sensitivity, specificity, positive predictive value and negative predictive value were calculated.
Results: 389 and 395 parent-provider influenza vaccination records were available for 2014 and 2015 respectively. 78% of parent reported vaccinations were substantiated by a provider with the kappa indicating good (κ = 0.677) to very good agreement (κ = 0.814) for 2014 and 2015 respectively. Discordance was higher in 2014, largely attributable to parents over reporting vaccination. More fathers over reported compared to mothers (Fisher’s exact = 0.052). There were 241 provider-AIR influenza vaccination records. Sensitivity of the AIR to reflect a child’s influenza immunisation status was low (32.6%).
Conclusions: Parental report over estimates confirmed influenza vaccination status and is affected by time and relationship to the child. Only a third of influenza vaccinations were reported to the AIR. Timely accurate data is critical to facilitate vaccination and evaluate program coverage.
KEYWORDS: Special risk medical condition, validity, parental report, influenza vaccination, immunisation register
Background
Many countries recommend the seasonal influenza vaccine to children with special risk medical conditions (SRMC).1–3 SRMCs include severe asthma, lung or heart disease, low immunity or diabetes and increase an individual’s risk of influenza complications or severity. In Australia, SRMCs who are at increased risk for inferior influenza outcomes are defined as per the Australian Immunisation Handbook4 which is approved by the National Health and Medical Research Council and for which the Australian Technical Advisory Group on Immunisation specifically recommends vaccination.5 Individuals with SRMCs, including children, have been funded under Australia’s National Immunisation Program (NIP) to receive the vaccine since 20105
Under the NIP, vaccines are routinely scheduled at specific ages and additionally for people at special risk or requiring catch-up according to the program and eligibility. While other vaccines may be recommended, all vaccines listed under the NIP are free. Of those routinely given to children, traditional NIP immunisation providers include, general medical practitioners (GPs) (family physicians) and practice nurses who administer the vaccines in general medical practices (78.8%), government community immunisation clinics (8.9%) and community children’s health clinics or Aboriginal Health Services (7.5%).6 Australia’s National Seasonal Influenza Vaccination Program (NSIVP) generally commences in the first month of autumn each year. Under the NSIVP, the vaccine is free to eligible people, but GPs may charge a consultation fee for the visit with non-eligible people able to obtain the vaccine privately. The influenza vaccine is widely available at general medical practices, community immunisation clinics, hospitals, community children’s health clinics and Aboriginal health centres. Additionally, travel clinics may also provide the vaccine and in South Australia, since early 2015, pharmacists, working in pharmacies (drug stores) can administer influenza vaccine to people over the age of 16 at a cost. Unlike for children’s routinely scheduled vaccines there is no information available on the distribution of provider types who administer the influenza vaccine from Australia. However, it is thought few parents would seek alternatives beyond traditional NIP immunisation providers due to the cost implications and age restrictions.
In South Australia (as in many states of Australia), legislation requires immunisation encounters to be both recorded by the provider and a handheld record given to the patient. However, there is no requirement for immunisation providers to report immunisations to the person’s primary healthcare provider (HCP), and while this is encouraged from those outside of the traditional health care delivery system, such as pharmacists, this also requires patient consent.
Ascertaining coverage of this recommendation assists in program planning and monitoring of influenza vaccination uptake over time, in line with strategic priority areas of Australia’s National Immunisation Strategy, 2013–2018.7 At the population level, a number of methods are available to determine coverage including data from healthcare providers, health insurance records, population surveys, as well as administrative and registry data.8 Population surveys can include a representative sample of the population specific target groups but rely on self-report as a proxy for the true vaccination record. Parent reported influenza vaccination status of children is thought to overestimate vaccination9-12 with suggestion that this is greater in children with SRMC.11,13,14
The use of a population registry with accurate data removes the need for data validation with multiple immunisation providers. Registry data has a use in epidemiological research and health service planning and also a role in examining vaccine effectiveness.15–17
Established in 1996, the Australian Childhood Immunisation Register (ACIR) was the first purpose-built immunisation register in the world.18 With the exception of influenza, until 2016, the ACIR routinely recorded universal and targeted vaccines given under the NIP for all children aged < 7 years of age.4,19 In September 2016, the registry became the Australian Immunisation Register (AIR) with the capability of capturing all NIP and most privately purchased vaccines, given to people of all ages.20 The AIR is linked to the Medicare enrolment register,4 and given approximately 99 per cent of children are registered with Medicare by 12 months of age and the AIR is ‘opt-out’ it is intended to constitute a nearly complete population register.19,20
However, as influenza is not required to be recorded on the AIR, and it does not currently attract notification payments for providers, as is the case for other childhood vaccines, concerns about the completeness and validity of AIR data have restricted its use in evaluating uptake of the vaccine.21 Particularly so for children with medical conditions, Indigenous children and those aged under five years for whom it is currently provided in all states in Australia.4
Given the current limitations of the AIR to identify children with SRMC, determining parent reported validity would assist in evaluation of the NIP program’s coverage in this priority group. Determining immunisation provider- AIR reporting would also provide much needed information. The aim of this study was to investigate the validity of parent-provider report and determine the accuracy of the AIR for recording provider reported influenza vaccination.
Results
A total of 443 surveys were completed; approximately 10% of those approached did not participate (Figure 1). Validation of risk status was determined for all participants with three participants included without a current SRMC.
Figure 1.
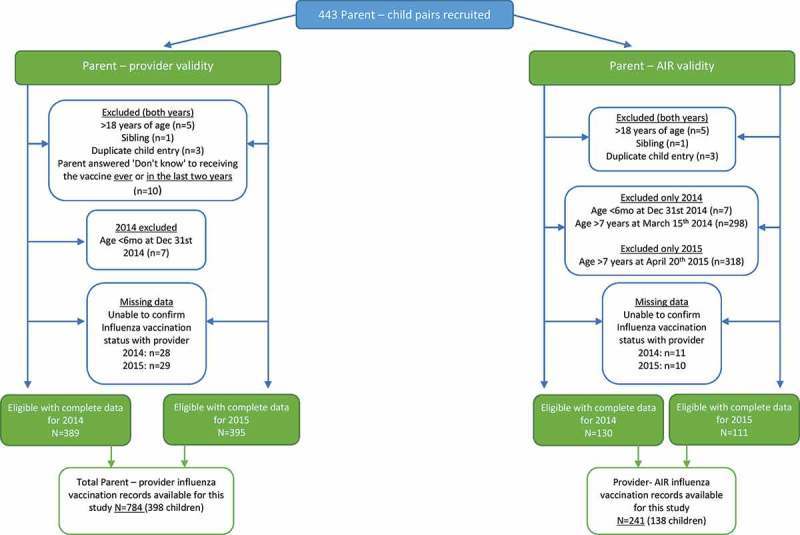
Study sample.
Parent-provider record
A total of 389 parent-provider influenza vaccination records with complete data were available in 2014 and 395 in 2015 (Figure 1). Reasons for provider non-confirmation included: not the child’s current HCP, incorrect clinic details, immunisation provider did not respond to request or would not release information, not having a GP, and mother (nurse) administered influenza vaccine.
Provider-AIR reporting
Complete data were available for 241 provider-AIR influenza vaccination records from 138 children (2014: 130 records; 2015:111 records) (Figure 1). By using the first day of the NSIVP to calculate a child’s age at that time-point, no data were included from children aged >7 years at the time of vaccination in either year; nor were data excluded from children aged <7 years at the time of vaccination. Reasons for provider non-confirmation were not current HCP, incorrect clinic details, immunisation provider did not respond to request or not having a GP. All eligible children had an AIR record.
Characteristics of study participants
Of the 398 children with parent-provider vaccination data, age at the time of the survey ranged from 10 months to 17.9 years (median 11.2 years) (Table 1).
Table 1.
Characteristics of study participants.
| Parental report |
Provider – AIR reporting |
||||
|---|---|---|---|---|---|
| Characteristic | Level | Eligible N = 424 |
Complete data n = 398 |
Eligible N = 148 |
Complete data n = 138 |
| Age of parent | 18–30 | 30 (7.1) | 25 (6.3) | 25 (16.9) | 20 (14.5) |
| 31–40 | 149 (35.1) | 137 (34.4) | 86 (58.1) | 82 (59.4) | |
| 41–50 | 206 (48.6) | 200 (50.3) | 37 (25) | 36 (26.1) | |
| >50 | 39 (9.2) | 36 (9) | - | - | |
| Place of residence | Metro | 316 (74.5) | 298 (74.9) | 104 (70.3) | 97 (70.3) |
| Rurala | 108 (25.5) | 100 (25.1) | 44 (29.7) | 41 (29.7) | |
| Relationship to child | Mother | 350 (82.5) | 329 (82.7) | 124 (83.8) | 116 (84.1) |
| Father | 66 (15.6) | 61 (15.3) | 22 (14.9) | 20 (14.5) | |
| Legal Guardian | 8 (1.9) | 8 (2) | 2 (1.4) | 2 (1.4) | |
| Parent’s highest education level | High school or less | 147 (34.7) | 138 (34.7) | 43 (29.1) | 40 (29) |
| Certificate or Diploma | 160 (37.7) | 151 (37.9) | 55 (37.2) | 53 (38.4) | |
| Bachelor | 82 (19.3) | 77 (19.3) | 33 (22.3) | 30 (21.7) | |
| Postgraduate | 35 (8.3) | 32 (8) | 17 (11.5) | 15 (10.9) | |
| Parents work status | Full time employed | 129 (30.4) | 119 (29.9) | 37 (25) | 34 (24.6) |
| Part time employed | 113 (26.7) | 109 (27.4) | 40 (27) | 39 (28.3) | |
| Casual | 45 (10.6) | 42 (10.6) | 17 (11.5) | 16 (11.6) | |
| Not working | 137 (32.3) | 128 (32.2) | 54 (36.5) | 49 (35.5) | |
| Born in Australia | 355 (83.7) | 335 (84.2) | 126 (85.1) | 119 (86.2) | |
| English is first language | 397 (93.6) | 374 (94) | 138 (93.2) | 130 (94.2) | |
| Gender of child | Male | 225 (53.1) | 209 (52.5) | 82 (55.4) | 75 (54.3) |
| Child is of Indigenous decent b | 23 (5.4) | 19 (4.8) | 8 (5.4) | 7 (5.1) | |
| Child had specified GPc | No GP | 35 (8.4) | 30 (7.6) | 14 (9.5) | 10 (7.3) |
| Specified GP | 258 (61.6) | 250 (63.1) | 87 (59.2) | 84 (61.3) | |
| Non – Specific GPd | 126 (30.1) | 116 (29.3) | 46 (31.3) | 43 (31.4) | |
| Age at survey median (IQR) | 11.2 (6.7–14.9) | 11.2 (6.7–14.9) | 5.7 (3.6–6.8) | 5.7 (3.6–6.8) | |
Footnote: a: postcodes were in defined rural areas of South Australia, New South Wales, Victoria and the Northern Territory; b: Of the eligible participants 1 participant declined to answer; c: data were missing for 1 participant; d: child was a patient of a medical practice but did not see a specific doctor at the practice; GP: general practitioner; IQR: inter quartile range.
Parents interviewed were predominately the child’s mother (83%). Six children were inpatients at the time of enrolment, but all had previously had outpatient appointments at the hospital.
Of the 138 children contributing provider-AIR reporting data, ages at the time of vaccination ranged from 10 months to 6.9 years (median 4.1 years) in 2014 and from 7 months to 6.3 years (median 3.6 years) in 2015.
Parent reported influenza vaccination uptake
Parent reported uptake of the influenza vaccine was 54.5% (212/389) for 2014 and 53% (209/395) for 2015 (Table 2). Across both years, the majority of influenza vaccinations were confirmed with the Women’s and Children’s Hospital (WCH) database (n = 162; 48%) or a medical practice (n = 164; 49%) (Table 3).
Table 2.
Comparison of parent reported influenza vaccination and AIR record with provider record, by year.
| Vaccination status |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parent report (%) | Provider report (%) | AIR | Agreement % | Kappa | Sensitivity % (95% CI) |
Specificity % (95% CI) |
Positive predictive value % (95% CI) | Negative predictive value % (95% CI) | ||
| Parent – Provider report | ||||||||||
| Overall | 421/784 (53.7) | 336/784 (42.9) | - | 87.1 | 0.745 | 97.6 (95.4–99.0) | 79.2 (75.2–82.9) | 77.9 (73.6–81.8) | 97.8 (95.7–99.0) | |
| Year | 2014a | 212/389(54.5) | 156/389(40.1) | - | 83.5 | 0.677 | 97.4 (93.6–99.3) | 74.2 (68.1–79.7) | 71.7 (65.1–77.7) | 97.7 (94.3–99.4) |
| 2015b | 209/395(52.9) | 180/395(45.6) | - | 90.6 | 0.814 | 97.8 (94.4–99.4) | 84.7 (79.1–89.2) | 84.2 (78.5–88.9) | 97.8 (94.6–99.4) | |
| Provider reporting to AIR | ||||||||||
| Overall | - | 89/241 (36.9) | 30/241(12.4) | 74.7 | 0.370 | 32.6 (23.0–43.3) | 99.3 (96.4–100) | 96.7 (82.8–99.9) | 71.6 (65.0–77.5) | |
| Year | 2014c | - | 47/130 (36.2) | 18/130 (13.8) | 77.7 | 0.442 | 38.3 (24.5–53.6) | 100 (95.7–100) | 100. (81.5–100) | 74.1 (65.0–81.9) |
| 2015d | - | 42/111 (37.8) | 12/111 (10.8) | 71.2 | 0.287 | 26.2(13.9–42.0) | 98.6(92.2–100) | 91.7(61.5–99.8) | 68.7(58.6–77.6) | |
Footnote: a: N = 389 children had complete data; b: N = 395 children had complete data; c: N = 130 encounters had complete data; d: N = 111 encounters had complete data.
Table 3.
Children’s nominated and confirmed providers of influenza vaccination in 2014 and 2015.
| Provider type | 2014 |
2015 |
||
|---|---|---|---|---|
| Parent reported (N = 212) n (%) |
Confirmed (N = 156) n (%) |
Parent reported (N = 209 n (%) |
Confirmed (N = 180) n (%) |
|
| General medical practice | 116 (54.7) | 67 (43) | 109 (52.2) | 81 (45) |
| Current HCP* | - | 6 (3.8) | - | 10 (5.6) |
| Women’s and Children’s Hospital | 88 (41.5) | 80 (51.3) | 90 (43) | 82 (45.6) |
| Other Hospital | 1 (0.5) | - | 1 (0.5) | 1 (0.6) |
| Community immunisation clinic | 6 (2.8) | 2 (1.3) | 4 (1.9) | 4 (2.2) |
| Pharmacy/drug store | - | - | 4 (1.9) | 2 (1.1) |
| Travel health clinic | 1 (0.5) | 1 (0.6) | 1 (0.5) | - |
Footnote: *: were general medical practitioners (family physicians) nominated as a child’s current healthcare provider (HCP).
Parent-provider record
A total of 78% (328/421) of parent reported vaccinations were confirmed by a provider. There was higher agreement for 2015 (90.6%) than 2014 (83.5%); with the kappa indicating good (κ = 0.677) to very good agreement (κ = 0.814) for 2014 and 2015 respectively (Table 2). The sensitivity and specificity of parental report to reflect a child’s influenza immunisation status was 97.4% and 74.2% respectively for 2014 and 97.8% and 84.7% respectively for 2015. Across both seasons, between 15.3–25.8% of children with no provider confirmed vaccination, were reported as being vaccinated by their parent.
Discordance was different across years (16.2% versus 9.4%; Fisher’s exact = 0.004). The majority of discordance resulted from parents over reporting their child was vaccinated, which was almost double in 2014 compared to 2015 (15.4% versus 8.4%; Fisher’s exact = 0.018). The inverse was true of parental relationship (the parent completing the survey). Overall, fathers were more likely to over report vaccination compared to mothers (Fisher’s exact = 0.052); which was more likely in 2015 (Fisher’s exact p = 0.020) than in 2014 (Fisher’s exact p = 0.483). There were no other differences associated with agreement observed including place of residence, parents’ age, education level, work status, place of birth, first language being English and child’s gender, indigeneity or having a specific GP (data not reported).
Reporting of the influenza vaccine to the AIR
Confirmed influenza vaccination was 36.2% (47/130) for 2014 and 37.8% (42/111) for 2015; with 38.3% (18/47) and 26.2% (11/111) of these reported to the AIR respectively. There was only one first dose recorded on the AIR that had not been confirmed by an immunisation provider; which incidentally had been given on the same day/month as the previous year. The majority of influenza vaccinations were administered by WCH immunisation providers (2014: 61.7%; 2015: 54.7%), compared to medical practices (2014: 29.8%; 2015: 42.8%), with others provided by a travel health clinic and community immunisation clinics.
Second dose
In 2014, there were four second dose provider confirmed vaccinations, with 2/4 reported to the AIR; while an additional two second dose records were identified on the AIR only. For 2015, five second dose vaccinations were provider reported, with 3/5 reported to the AIR; while one second dose record was identified on the AIR only.
Provider-AIR agreement
There was fair agreement overall (κ = 0.3701) with higher agreement for 2014 (κ = 0.442) than 2015 (κ = 0.287) (Table 2). In total, a quarter of cases (25.4%) were discordant, with almost all discordance a result of vaccinations not reported to the AIR. The sensitivity and specificity of AIR to reflect a child’s influenza immunisation status was 32.6% and 99.3% respectively. There was slightly higher sensitivity in 2014 (38.3%) than 2015 (26.2%). Across both years, 67.4% of children with a provider confirmed influenza vaccination were not reported to the AIR. Between the two highest providers, medical practices and the WCH, a much higher proportion of influenza vaccinations given at medical practices (48.6%; 17/35) compared to the WCH (23.1%; 12/52) were reported to the AIR (= 6.12; p = 0.013).
While there was no difference between years (2014–2015) in the proportion of vaccinations reported to the AIR by medical practices (range reported, 41.2–55.6%), significantly less vaccinations administered at the WCH were reported to the AIR in 2015 (4.3%; 1/23) compared to 2014 (38%; 11/29) (Fisher’s exact p = 0.007).
Discussion
At the population level, accurate influenza vaccination data are required to determine coverage, as well as guide and evaluate future programs. For children in special risk groups, such as those with underlying medical conditions, an accurate vaccination status has a role in the provision of healthcare at the individual level. Our data suggests that in children with SRMC, parents tend to over report influenza vaccination with 15–26% of vaccinations unconfirmed. AIR coverage is also not an accurate reflection of a child’s influenza vaccination status, with almost 70% of encounters in our study not reported to the AIR. Our finding of parental over reporting of influenza vaccination status is consistent with previous studies of children and adolescents, both of children in general (specificity range: 86– 92%)9,12 and in those with SRMCs, where specificity ranged from 68 to 82.3%.11,13,14 We found two characteristics to be associated with over reporting: time and parental relationship. In regards to parental relationship, we speculate that it is not fathers solely who over report but any ‘parent’ who is not the child’s primary carer, particularly when children may have complex medical conditions and multiple appointments. The misclassification (parental over reporting) could also be due to the fact that in addition to multiple medical treatments, parents can confuse the many different vaccines offered to children in general and are likely to be influenced by social desirability bias if they can’t recall. The finding that between 15 to 26% of parents over report influenza vaccination is important as it identifies that a proportion of parents incorrectly believe their child is protected against influenza when they are not. The effect of time on recall has previously been demonstrated in a study of self-reported influenza vaccination in healthcare workers that found accuracy decreased with increasing time since vaccination.22
While the accuracy of the AIR to capture additional NIP vaccines has previously been highlighted and under reporting suspected,18,21,23 to our knowledge this is the first study to investigate the AIR in terms of accurate reporting on influenza for children. Of the two major providers, there was low reporting (WCH: 18%; medical practices: 35%) of influenza vaccination encounters suggesting that barriers to reporting are likely to be common across all provider types. As children may see multiple medical practitioners including specialists, and as influenza vaccines become more available outside of traditional settings, such as in pharmacies and travel health clinics, the requirement for reporting to a centralised register (the AIR) becomes paramount.
Unlike the national Danish and Norwegian vaccination registers and some state based registers in the USA in which reporting of all vaccines is mandatory,24–26 the AIR relies on the passive reporting for some NIP vaccines, particularly those used for targeted programs. While provider incentives have previously been shown to improve reporting and data accuracy, this method requires ongoing financial support.17,23 The methods used to report to the AIR have changed over time with increasing numbers electronically reporting21,23 and taking advantage of Medical Practice Management Software (PMS) that directly uploads to the AIR.
Aside from countries that link national or state-wide registers to health data,27,28 evaluating the uptake of influenza vaccination in at risk groups is a problem worldwide, with considerable gaps in monitoring coverage. In a recent report into seasonal influenza vaccination recommendations and coverage in Europe, only 9 of 32 European Member States were able to provide data on uptake in people with chronic medical conditions; with a previous report indicating even less reliability for children.3,29
Whilst in 2016, the AIR transitioned to a registry that captures all age groups, identifying priority groups targeted for influenza vaccination with the current socio-demographic data collected remains a considerable obstacle that limits the evaluation of all current NIP programs. Establishing a way that target groups can be identified on the AIR would enable timely estimates of coverage and enhance program planning for these special vaccination groups.
In our study, the influenza vaccine was predominately delivered through medical practices or hospitals and less frequently by pharmacies/drug stores and community or travel health clinics. In comparison with delivery of the routinely scheduled NIP vaccines in which the majority (78%) (nationally) are received in medical practices, less of our study participants received them in this way (49%)6 However, this is likely to reflect the SRMC status of these children and availability of the vaccine in their specialist treatment centre (WCH).
One of the strengths of this study was the comprehensive method used to determine vaccination status. A child’s influenza vaccine status was initially confirmed with the child’s nominated provider. If this was negative other health care providers, (current HCP, WCH) were contacted to determine whether influenza vaccine had been administered and the date. We accept the possibility that some parents may not have accurately supplied immunisation provider details to us and these children could be incorrectly classified. However, we expect that only small numbers would be vaccinated outside of the traditional influenza vaccination delivery system and so being able to contact each child’s current HCP was a strength of the study.
Our study also identified an issue with recommendations in relation to immunisation providers and age restrictions for administering vaccines. In particular, all children (n = 4) reported to have received the vaccine at a pharmacy/drug store in 2015 were aged <14 years. Two children’s vaccinations were confirmed at separate pharmacies, with their age at administration below recommended practice (≥16 years for administration in a pharmacy in South Australia). Additionally, these children or their parents would have needed to pay for the vaccine, rather than receive it free as per recommendations.
There are several limitations to our study. In regards to parental report, individual years were analysed separately because of the difference in discordance. However, we acknowledge the fact that parents may report similarly from one year to the next, although if this were the case we would have expected similar discordance between years. We also did not account for the fact that some children attended the same medical practice each year or that multiple children attended the same medical practice when examining provider-AIR reporting; yet this is reflective of real world immunisation practice and we accept that our sample may limit generalizability to all immunisation providers. Our study data limited exploration of possible reasons for low reporting at the provider level. As these data came from a parent-based survey, possible confounders at the provider level were not collected, such as method of reporting to the AIR (PMS, Medicare Australia website or paper encounter forms) and size of the practice. Additionally, as almost all children in our study had a SRMC and were eligible for funded influenza vaccine, this may have prompted a higher level of provider reporting to the AIR compared to children not eligible. However, we cannot see any reason this would occur, given those who administer the vaccine are often different to those who oversee practice reporting. Whilst only undertaken in one Australian jurisdiction (South Australia), we believe the results would be applicable to other regions of Australia as the AIR is a national database.
Conclusion
Fundamental to having a vaccination program targeting children at increased risk of severe influenza is the ability to evaluate it. Parental report overestimates provider confirmed influenza vaccination status and this should be taken into account if using parental report as a proxy in population surveys. Influenza vaccination is significantly underreported to the AIR. Besides encouraging and potentially funding providers to report influenza vaccinations to the AIR, future research should focus on investigating provider level barriers in order to address them.
Methods
Study design
We report study findings for an observational cross-sectional study with consideration of the STROBE statement.30
Study setting
The study population was recruited from September 2015 to February 2016 at a paediatric hospital in Adelaide. The Women’s and Children’s Hospital (WCH) is the major provider of tertiary healthcare services for children with acute and chronic conditions in South Australia.
Study recruitment
Parents or guardians, referred to hereafter as parents, were approached at the outpatient’s department or in hospital wards at the WCH. Exclusion criteria for this study included: age < 6 months or ≥ 18 years on the day of recruitment or children without a SRMC as defined by the AIH;4 parent unable to provide written informed consent or understand English without a translator. If multiple children of the same family were eligible, the eldest child was enrolled. For both analyses, parental validity and AIR reporting, a child’s data were ineligible in a given year if they were aged <6 months’ old (prior to December 31st in that year). Additionally, the provider–AIR analyses were restricted to a subset of children from the parental validity analyses. This was due to the limited capability of the ACIR at the time of the study to record only the vaccinations of children aged less than 7 years at the time of vaccination. In order to capture those children aged less than 7 years at the time of vaccination (due to the capability of the ACIR at the time of the study), we used the first day of the National Seasonal Influenza Vaccination Program (NSIVP) in each year. A child’s data were ineligible in a given year if they were >7 years old on the first day of the NSIVP. In 2014, this was March 15th, while in 2015 this was April 20th, due to a delay in vaccine availability as a result of multiple strain changes in the vaccine.31
Parental survey questionnaire
Following parental consent, data were collected using a predominately closed-ended questionnaire in a face-to-face interview. Parents were asked questions related to influenza vaccination, including vaccination in 2014 and 2015. More specifically, we asked, “Has your child ever received a seasonal influenza vaccine?” If yes, “Have they received a seasonal influenza vaccine in the last two years?” If yes, this was followed by, “Has your child received the vaccine this year?” and “Did your child receive the vaccine last year? (2014)” with additional questions asked to extract reasons for receipt/non-receipt of the vaccine in either or both years. To confirm vaccination status we collected the child’s immunisation provider for each year along with details of their current primary HCP. For some participants this was the name of the medical practice only, while others provided the details of a specific general medical practitioner (GP) within the practice. The questionnaire was completed in the waiting area of the outpatient clinic or hospital ward. Medical case notes were reviewed to confirm risk status.
Influenza vaccination status
Provider report of influenza vaccination was defined as receipt of at least one dose of the vaccine verified by the child’s nominated immunisation provider, current HCP or WCH immunisation database. Nominated immunisation providers included general medical practitioners/medical practices, pharmacies (drug stores), community immunisation clinics, travel health clinics and hospitals. While the purpose of this study was not to consider a “gold standard”, we considered that if their nominated provider had vaccinated a child, then in keeping with relevant legislation they (the nominated immunisation provider) should be able to verify a child’s immunisation record. When contacting the child’s nominated immunisation provider four attempts were made to establish contact with the provider before recording as unable to confirm and if we could not verify receipt of the vaccine elsewhere (current HCP, WCH) then these cases were excluded. The AIR was used to confirm influenza vaccination status for 2014 and 2015. Additionally, since children aged 6 months to <9 years receiving influenza vaccine for the first time are recommended to receive 2 doses4 we examined provider and AIR record of a 2nd dose. In line with AIR coverage calculations, we allowed a minimum 3-month delay for late notification of influenza vaccinations to the AIR.32
Statistical analysis
The sample for both analyses was derived from the recruited sample. Influenza vaccination status from parent-provider record and provider-AIR record were compared in each year. The Kappa was used to measure the percent agreement between reporting.33 We interpreted Kappa using the classification proposed by Altman,34 where a kappa coefficient of 0.81–1.0 is considered to be very good; 0.61–0.80 good; 0.41–0.60 moderate; 0.21–0.40 fair and <0.20 poor. We examined the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Concordance of parent-provider record was investigated and discordant unvaccinated cases further examined, by year and demographic characteristics. Additionally, the effect of year and provider type on provider-AIR reporting was investigated. Stata (Version 14.1) was used for all statistical analyses (StataCorp, Texas, USA). The study was approved by the Women’s and Children’s Health Network Human Research Ethics Committee.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- AIR
Australian Immunisation Register
- HCP
healthcare provider
- GP
general practitioner
- NIP
National Immunisation Program
- NPV
negative predictive value
- NSIVP
National Seasonal Influenza Vaccination Program
- PPV
positive predictive value
- SRMC
special risk medical condition
- WCH
Women’s and Children’s Hospital
Disclosure of potential conflicts of interest
JT, NC and JL report no conflict. HM is an investigator on clinical trials of investigational vaccines sponsored by Industry. Her institution receives funding from Industry (GSK, Pfizer, Novavax) for Investigator led research. She does not receive any personal payments from Industry.
Acknowledgments
The authors would like to thank Siobhan Misan, Salma Salih, Bridget Joseph Xavier, Larissa Au, Mary Premnath, Emma Lane, Kathryn Riley, Chris Heath and Iann Homer for their assistance with data collection. HM is supported by a NHMRC CDF APP1084951.
Author contribution
JT contributed to study design, collected and analysed the data and prepared the first draft of the manuscript. HM contributed to study design, statistical analysis interpretation and critical review of the manuscript. JL and NC contributed to statistical analysis interpretation and critical review of the manuscript. The manuscript has been read and approved by all named authors.
References
- 1.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, Jernigan DB.. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2017-18 Influenza Season. MMWR Recomm Rep. 2017;66(2):1–20. doi: 10.15585/mmwr.rr6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada Canadian immunization guide chapter on influenza and statement on seasonal influenza vaccine for 2017–2018, in an advisory committee statement (ACS) national advisory committee on immunization (NACI). Canada (Ottawa, Ontario): Public Health Agency of Canada; 2017. [Google Scholar]
- 3.European Centre for Disease Prevention and Control Seasonal influenza vaccination in Europe. Vaccination recommendations and coverage rates in the EU Member States for eight influenza seasons: 2007–2008 to 2014–2015. Stockholm, Sweden: ECDC; 2017. [Google Scholar]
- 4.Australian Technical Advisory Group on Immunisation (ATAGI) The Australian Immunisation Handbook. 10th ed. Australian Government Department of Health, Canberra; [Electronic book] (2017 update) [accessed 2018 Mar]. http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-home. [Google Scholar]
- 5.Australian Technical Advisory Group on Immunisation (ATAGI) Statement on the Administration of seasonal influenza vaccines in 2018. Australian Government Department of Health, Canberra; [Guideline] accessed 2018. March 21 https://beta.health.gov.au/resources/publications/atagi-advice-on-seasonal-influenza-vaccines-in-2018. [Google Scholar]
- 6.Hull BP, Hendry AJ, Dey A, Beard FH, Brotherton JM, McIntyre PB. Annual immunisation coverage report, 2016. National Centre for Immunisation Research and Surveillance; [Online report] Last updated 2017. March 30 [accessed 2018 Aug]. http://www.ncirs.org.au/our-work/vaccine-coverage. [Google Scholar]
- 7.Australian Governement Department of Health National Immunisation Strategy for Australia 2013-2018. Australian Governement Department of Health; [Guideline] 2013 Last updated 2017. December 19, Canberra [accessed 2018 Mar]. https://beta.health.gov.au/resources/publications/national-immunisation-strategy-for-australia-2013-2018. [Google Scholar]
- 8.WHO Regional Office for Europe Methods for assessing influenza vaccination coverage in target groups. Copenhagen, Denmark: WHO Regional Office for Europe; 2016. [Google Scholar]
- 9.Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine. 2009;27(47):6546–6549. doi: 10.1016/j.vaccine.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Nowalk MP, Zimmerman RK, Lin CJ, Ko FS, Raymund M, Hoberman A, Kearney DH, Greenberg DP. Parental perspectives on influenza immunization of children aged 6 to 23 months. Am J Prev Med. 2005;29(3):210–214. doi: 10.1016/j.amepre.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Lin CJ, Zimmerman RK, Nowalk MP, Ko FS, Raymund M, Hoberman A, KearneyB DH. Block, Parental perspectives on influenza vaccination of children with chronic medical conditions. J Natl Med Assoc. 2006;98(2):148–153. [PMC free article] [PubMed] [Google Scholar]
- 12.Lu PJ, Dorell C, Yankey D, Santibanez TA, Singleton JA. A comparison of parent and provider reported influenza vaccination status of adolescents. Vaccine. 2012;30(22):3278–3285. doi: 10.1016/j.vaccine.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Brown C, Clayton-Boswell H, Chaves SS, Prill MM, Iwane MK, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Fairbrother G, et al. Validity of parental report of influenza vaccination in young children seeking medical care. Vaccine. 2011;29(51):9488–9492. doi: 10.1016/j.vaccine.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Shinall MC Jr., PlosaK EJ, Poehling A. Validity of parental report of influenza vaccination in children 6 to 59 months of age. Pediatrics. 2007;120(4):e783–7. doi: 10.1542/peds.2007-0052. [DOI] [PubMed] [Google Scholar]
- 15.Placzek HLC. Madoff, The use of immunization registry-based data in vaccine effectiveness studies. Vaccine. 2011;29(3):399–411. doi: 10.1016/j.vaccine.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen HT, SabroeJ S. Olsen, A framework for evaluation of secondary data sources for epidemiological research. Int J Epidemiol. 1996;25(2):435–442. [DOI] [PubMed] [Google Scholar]
- 17.Guzman Herrador BR, Aavitsland P, Feiring B, Riise BergsakerK MA. Borgen, Usefulness of health registries when estimating vaccine effectiveness during the influenza A(H1N1)pdm09 pandemic in Norway. BMC Infect Dis. 2012;12:63. doi: 10.1186/1471-2334-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin LK, Crawford NW, Rowles G, Buttery JP. Australian immunisation registers: established foundations and opportunities for improvement. Euro Surveill. 2012;17(16). [PubMed] [Google Scholar]
- 19.Hull BP, McIntyre PB, Heath TC, Sayer GP. Measuring immunisation coverage in Australia. A review of the Australian Childhood Immunisation Register. Aust Fam Physician. 1999;28(1):55–60. [PubMed] [Google Scholar]
- 20.Hull B. Coverage information The Australian Immunisation Register. National Centre for Immunisation Research and Surveillance; [Webpage] Last updated 2017. December 5 [accessed 2018 Mar]. http://www.ncirs.edu.au/provider-resources/coverage-information/. [Google Scholar]
- 21.Hull BP, Hendry AJ, Dey A, Beard FH, Brotherton JM, McIntyre PB. Annual immunisation coverage report, 2015. Australia: National Centre for Immunisation Research and Surveillance, Australia; 2017. [Google Scholar]
- 22.Llupia A, Garcia-Basteiro AL, Mena G, Rios J, Puig J, BayasA JM. Trilla, Vaccination behaviour influences self-report of influenza vaccination status: a cross-sectional study among health care workers. PLoS ONE. 2012;7(7):e39496. doi: 10.1371/journal.pone.0039496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hull BP, Deeks SL, McIntyre PB. The Australian childhood immunisation register-A model for universal immunisation registers? Vaccine. 2009;27(37):5054–5060. doi: 10.1016/j.vaccine.2009.06.056. [DOI] [PubMed] [Google Scholar]
- 24.Trogstad L, Ung G, Hagerup-Jenssen M, Cappelen I, HaugenB IL. Feiring, The Norwegian immunisation register–SYSVAK. Euro Surveill. 2012;17(16). [PubMed] [Google Scholar]
- 25.Grove Krause T, Jakobsen S, HaarhK M. Molbak, The Danish vaccination register. Euro Surveill. 2012;17(17). doi: 10.2807/ese.17.17.20155-en. [DOI] [PubMed] [Google Scholar]
- 26.Martin DW, Lowery NE, Brand B, GoldG R. Horlick, Immunization information systems: a decade of progress in law and policy. J Public Health Manag Pract. 2015;21(3):296–303. doi: 10.1097/PHH.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dombkowski KJ, Costello L, ShimingS.J D. Clark, using administrative claims to identify children with chronic conditions in a statewide immunization registry. Am J Managed Care. 2014;20(5):e166–e174. [PubMed] [Google Scholar]
- 28.Derrough T, Olsson K, Gianfredi V, Simondon F, Heijbel H, Danielsson N, KramarzL P. Pastore-Celentano, Immunisation Information Systems - useful tools for monitoring vaccination programmes in EU/EEA countries, 2016. Euro Surveill. 2017;22(17). doi: 10.2807/1560-7917.ES.2017.22.17.30519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Control Seasonal influenza vaccination in Europe - Overview of vaccination recommendations and coverage rates in the EU Member States for the 2012-13 influenza season. Stockholm, Sweden: ECDC; 2015. [Google Scholar]
- 30.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, SchlesselmanM JJ. Egger, strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Australian Government Department of Health - Therapeutic Goods Administration (TGA) 2015 Seasonal influenza vaccines. Australian Government Department of Health, Canberra; [Webpage] 2015 Last updated 2015. March 3 [accessed 2018 Mar]. https://www.tga.gov.au/media-release/2015-seasonal-influenza-vaccines. [Google Scholar]
- 32.Hull BP, Hendry AJ, Dey A, Beard FH, Brotherton JM, McIntyre PB. Immunisation coverage annual report, 2014. Commun Dis Intell Q Rep. 2017;41(1):E68–E90. [DOI] [PubMed] [Google Scholar]
- 33.Szklo M, Nieto. J. Epidemiology: beyond the basics. 3rd ed. Burlington, MA: Jones & Bartlett Learning; 2012. [Google Scholar]
- 34.Altman DG. Practical statistics for medical research. London; New York, NY: Chapman and Hall; 1991. [Google Scholar]


